Abstract
The Asian citrus psyllid, Diaphorina citri is the principal vector of the highly destructive citrus disease called Huanglongbing (HLB) or citrus greening, which is a major threat to citrus cultivation worldwide. More effective pest control strategies against this pest entail the identification of potential chemosensory proteins that could be used in the development of attractants or repellents. However, the molecular basis of olfaction in the Asian citrus psyllid is not completely understood. Therefore, we performed this study to analyze the antennal and abdominal transcriptome of the Asian citrus psyllid. We identified a large number of transcripts belonging to nine chemoreception-related gene families and compared their expression in male and female adult antennae and terminal abdomen. In total, 9 odorant binding proteins (OBPs), 12 chemosensory proteins (CSPs), 46 odorant receptors (ORs), 20 gustatory receptors (GRs), 35 ionotropic receptors (IRs), 4 sensory neuron membrane proteins (SNMPs) and 4 different gene families encoding odorant-degrading enzymes (ODEs): 80 cytochrome P450s (CYPs), 12 esterase (ESTs), and 5 aldehyde dehydrogenases (ADE) were annotated in the D. citri antennal and abdominal transcriptomes. Our results revealed that a large proportion of chemosensory genes exhibited no distinct differences in their expression patterns in the antennae and terminal abdominal tissues. Notably, RNA sequencing (RNA-seq) data and quantitative real time-PCR (qPCR) analyses showed that 4 DictOBPs, 4 DictCSPs, 4 DictIRs, 1 DictSNMP, and 2 DictCYPs were upregulated in the antennae relative to that in terminal abdominal tissues. Furthermore, 2 DictOBPs (DictOBP8 and DictOBP9), 2 DictCSPs (DictOBP8 and DictOBP12), 4 DictIRs (DictIR3, DictIR6, DictIR10, and DictIR35), and 1 DictCYP (DictCYP57) were expressed at higher levels in the male antennae than in the female antennae. Our study provides the first insights into the molecular basis of chemoreception in this insect pest. Further studies on the identified differentially expressed genes would facilitate the understanding of insect olfaction and their role in the interactions between olfactory system and biological processes.
Introduction
Insects use chemoreception for a variety of survival and reproductive functions, including the identification of food sources, toxic substances, and mating partners. Previous studies have shown that chemoreception-related genes in insects are highly divergent [1–4], as indicated by the presence of two major chemosensory mechanisms, olfaction and gustation, which include several multigene families. These multigene families consist of odorant-binding proteins (OBPs), chemosensory proteins (CSPs), sensory neuron membrane proteins (SNMPs), olfactory receptors (ORs), ionotropic receptors (IR), and gustatory receptors (GRs) [5], and odorant-degrading enzymes (ODEs) that are involved in peripheral olfactory processes. Specifically, the chemosensory multigene families perform diverse functions, including chemosensory as well as non-sensory functions, and are mainly expressed in the antennae or maxillary palps, which contain chemosensory sensilla with sensory neurons. These include OBPs and CSPs, which are located in the sensillar lymph, and are regarded as solubilizers and carriers of odorants and pheromones, and the chemoreceptor superfamily formed by the OR, IR, and GR families, located on the dendrites of olfactory receptor neurons (ORNs). Moreover, members of the chemosensory multigene families are expressed in other tissues and were shown to be involved in non-sensory functions. Thus far, OBPs and CSPs have been found in pheromone glands where they are involved in pheromone delivery [6–12], and in the reproductive organs and eggs where they regulate development [13–16]. Furthermore, ORs identified in testes are involved in sperm activation [17].
The Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae) is the principal vector of the fastidious bacterium Candidatus Liberibacter asiaticus (CLas), the causal agent of citrus greening disease or Huanglongbing (HLB), which is a highly destructive disease that is a major threat to citrus cultivation worldwide [18]. The psyllids are specific to citrus, on which they feed, mate, oviposit, and develop on new flush shoots [19, 20]. Currently, the control of D. citri has been recognized as the key approach to prevent the spread of HLB. Traditional D. citri management has used broad-spectrum insecticides (pyrethroid, organophosphate, and neonicotinoid classes), which has led to the evolution of pesticide-resistant strains [21–23]. Therefore, it is critical to identify potential molecular targets, particularly chemosensory proteins (binding proteins and receptors) to develop novel control strategies that interfere with olfaction [24–26]. However, the molecular components of psyllid olfaction have not been described. While the molecular basis of insect olfaction has been extensively studied in Lepidopteran and Dipteran insects, elucidation of the molecular components and mechanisms that comprise the hemipteran olfactory system is limited [27–33]. In addition, the molecular basis underlying chemical communication, including olfactory sensing, pheromone biosynthesis, and oviposition in psyllids is lacking.
In the present study, we analyzed the antennal and abdominal transcriptomes of the Asian citrus psyllid. The antenna was chosen because of its obvious involvement in the chemosensory function and the abdomen was chosen due to its role in reproduction and potential sex pheromone production. In addition, these two tissues allowed comparison of the chemosensory gene expression profiles between an olfactory and a non-olfactory tissue in males and females in order to identify olfactory genes in the Asian citrus psyllid. The expression of these chemosensory gene transcripts was analyzed based on transcriptome profiling using RNA sequencing (RNA-seq) data and validated by quantitative real time-PCR (qPCR). This is the first study on the olfactory molecular components of the Asian citrus psyllid, which provides the molecular basis to better understand psyllid chemoreception.
Methods
Ethics statement
No specific permission was required to collect insects from the locations mentioned in this article. The locations sampled were not privately owned or protected in any way, and this field study did not involve endangered or protected species.
Psyllid rearing and collection
The Asian citrus psyllid, D. citri was originally collected from the citrus groves in Fogang County, Qingyuan City, Guangdong Province, China (E113°31', N23°52'). The collected psyllids were reared in a greenhouse (21°C–26°C, 60%–80% RH) with natural lighting throughout the day. For the past 3 years, the psyllids were fed Citrus Reticulate Blanco Cv. Shatangju. To synchronize the developmental stages, a large number of eggs were collected and moved to artificial climate boxes for mass rearing. The controlled environmental conditions were 26°C ± 1°C, 70% relative humidity, and a 12 h: 12 h dark light cycle. Emerging adult psyllids were segregated by sex under a stereoscope, followed by morphological identification.
RNA isolation and Illumina sequencing
From the newly emerged adults (2-day-old), about 920 male antennae, 1,000 female antennae, 200 male terminal abdomen (cut from the 5th abdominal segments, including aedaeagus and clasper), and 200 female terminal abdomen (cut from the 5th abdominal segments, including ovipositor) were manually dissected and immediately transferred to a polypropylene tube immersed in liquid nitrogen. All frozen tissues were crushed and ground in liquid nitrogen, and total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA concentration and quality were assessed using standard procedures as recommended for Illumina (Illumina, San Diego, CA, USA) sequencing.
cDNA library preparation, including fragmentation and barcoding (ligation of specific adapters), was performed following the Illumina protocol. After quality evaluation with an Agilent 2010 Bioanalyzer, the libraries were diluted with an elution buffer and loaded on an Illumina HiSeq2500 for sequencing (paired-end, 2 × 100 bp; total, 200 cycles), and each cDNA library was deep-sequenced for 6 gigabytes. The majority of Illumina library were about 200 bp in size, and both ends were sequenced. The raw data from Illumina deep-sequencing were deposited to the NCBI Short Read Archive (SRA) database as BioProject Accession Number SRP064720.
De novo assembly and sequence annotation
Clean reads were generated from raw reads by removing the adaptor sequences, duplicated sequences, ambiguous reads, and low-quality reads. De novo assembly of clean data was accomplished by the Trinity software to generate unigenes [34, 35] with min_kmer_cov set to 2 by default and all other parameters set at default. Using BLASTx, unigenes were first searched against protein databases, including NCBI-Nr, NCBI- Nt, KEGG Orthology (KO), Swiss-Prot, PFAM, Gene Ontology (GO), and euKaryotic Ortholog Groups (KOG) (significant thresholds of E-value < 10−5). Proteins with the highest sequence similarity to the given unigenes along with putative functional annotations were retrieved. BLAST results were then imported into Blast2GO pipeline for GO annotation. Protein coding region prediction was performed using the ORF Predictor according to the BLAST results. Then, the Blast2GO program was used to retrieve the GO annotation of unigenes and the GO functional classification was obtained using WEGO (http://wego.genomics.org.cn/) [36].
Identification of chemosensory genes, sequence alignment and phylogenetic analysis
The open reading frames (ORFs) of putative chemosensory genes were predicted using the ORF finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The signal peptides of OBPs and CSPs were predicted using SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/). The trans-membrane domains (TMDs) of ORs, IRs, GRs, and SNMPs were predicted using TMHMM 2.0 (http://www.cbs.dtu.dk/services/TMHMM).
Phylogenetic analyses of D. citri chemosensory genes were performed in conjunction with other insect chemosensory sequences from previously published data (S1 Text). After removal of the signal peptides from the OBP and CSP datasets, the resulting amino acid sequences of all chemosensory genes were aligned using MAFFT v.6 [37] (E-INS-I parameter set for OBPs, CSPs and SNMPs; FFT-NS-2 parameter set for ORs, GRs and IRs). Maximum-likelihood trees were constructed for OBP, CSP, OR, GR, and SNMP using MEGA6 [38] with the corresponding best substitution model, and the phylogenetic tree for IR was constructed using FastTree 2.1.7 [39]. Robustness of the branches was assessed with 1,000 bootstrap pseudo-replicates. Dendrograms were viewed and edited in FigTree (http://tree.bio.ed.ac.uk/software/figtree/). For comparative purposes, multiple putative DcitOBP and DcitCSP protein sequences (without the signal peptides), DcitIR protein sequences and DcitSNMP protein sequences were aligned using the E-INS-I strategy in MAFFT [37] and rendered in Geneious 8.1.7 (http://www.geneious.com) and Jalview 2.0.1[40].
Gene expression analysis
Clean reads were mapped back onto the assembled transcriptome and read count for each gene was obtained from the mapping results. Gene expression levels for each sample were estimated by RSEM [41] using default parameters in Bowtie2. Additionally, expression levels were assessed in terms of FPKM values (fragments per kilobase per million reads), which were calculated based on the number of mapped transcript fragments corrected for transcript length and sequencing depth [42].
Prior to differential gene expression analysis in the four transcriptomes, the read counts were adjusted by edgeR program package through one scaling normalized factor. Differential expression analysis of two samples was performed using the DEGseq R package [43]. P value was adjusted using q value [44]. A q value < 0.005 & |log2 (foldchange)| > 1 was set as the threshold for significant differential expression. Then, paired comparisons were conducted in the following manner: male antennae vs. female antennae, male terminal abdomen vs. female terminal abdomen, male antennae vs. male terminal abdomen, and female antennae vs. female terminal abdomen. Differential expression of genes was processed by following strict criteria: FPKM value differences between the two analyzed transcriptomes > 3-fold, in combination with a significant Bonferroni-corrected p-value at < 2.2 × 10−4.
Quantitative real time-PCR analysis
Quantitative real time-PCR (qPCR) was used to verify expression levels of the selected sex- and tissue-specific chemosensory gene transcripts in a Light Cycler 480 System (Roche, USA) using the SYBR Premix EX Taq (Takara, China). Total RNA isolated from the four tissues described above was used to synthesize first-strand cDNA using the First strand cDNA synthesis kit (Takara, China). Two reference genes (actin-1 and GAPDH2) were used as internal controls. Primers of the target and reference genes were designed using the Primer 3 program (http://frodo.wi.mit.edu/). Negative controls without cDNA template or transcriptase were included in each experiment. Each qPCR reaction was performed with three technical replicates and three biological replicates. All primer sequences are listed in S1 Table. The relative transcript abundance of the genes in each tissue was calculated using the comparative 2-ΔΔCT method [45]. Data analysis was performed using Prism 6.0 (GraphPad Software, CA). Statistical significance was analyzed by ANOVA, followed by a Tukey’s multiple comparison test. A value of P < 0.05 was considered statistically significant.
Results
Antennal and abdominal transcriptome
Illumina sequencing generated a total of 51,405,960 (100.00% of the total reads) and 48,894,536 (95.99%) clean reads for the female antennal and abdominal transcriptomes, and 50,686,376 (96.94%) and 55,986,086 (96.28%) clean reads for the male antennal and abdominal transcriptomes, respectively (S2 Table). The combined Trinity assembly of all above transcriptomes resulted in 78,154 unigenes from which 106,743 non-redundant putative transcripts were predicted. For annotations, unigenes longer than 200 bp were used as query in the NCBI, Swiss-PROT, KEGG, COG, and GO databases using BLASTx with a cut-off E value of 10−5. Subsequently, 16,967 (21.7%), were annotated using the NCBI-Nr database, 3,041 (3.89%) by NCBI-Nt, 5,813 (7.43%) by KO, 10,824 (13.84%) by Swiss-PROT, 14,834 (18.98%) by Pfam, 14,943 (19.11%) by GO, and 8,348 (10.68%) by KOG (S3 Table). Of the 78,154 unigenes, 14,943 (19.11%) had at least one GO term (Fig 1), and the following categories were well represented: “binding” (24574, 31.4%), “response to stimulus” (3121, 4.0%), “signal transducer activity” (371, 0.5%), “hydrolase activity” (2783, 3.6%), and “transferase activity” (2652, 3.4%).
Fig 1. GO classifications of unigenes.
Chemosensory gene expression profiles
Based on homology analysis, a total of 223 chemosensory-related transcripts were predicted. These included transcripts putatively involved in odorant binding (OBPs and CSPs, S4 Table), chemosensory reception (ORs, GRs, IRs and SNMPs, S5 Table), and odorant degradation (cytochrome P450s (CYPs), esterases (ESTs), aldehyde dehydrogenases (ADEs), S6 Table).
Odorant binding proteins
Nine candidate OBP transcripts were identified based on sequence similarity with annotated orthologous sequences and the presence of conserved cysteine residues characteristic to insect OBPs (S4 Table). Based on the hemipteran ‘Classic’ OBP cysteine motif (C1-X22–32-C2-X3- C3-X36–46-C4-X8–14-C5-X8-C6) [46], 7 DcitOBP transcripts (DcitOBP1, 3, 5–9) were classified as ‘Classic’ OBPs (S1 Fig). The remaining two DcitOBP transcripts (DcitOBP2, 4) were classified as ‘Plus-C’ (S2 Fig), which is characterized by a cysteine spacing pattern consisting of C1-X8–41-C2-X3-C3-X39–47-C4-X17–29-C4a-X9-C5-X8-C6-P-X9–11-C6a [47]. Intriguingly, DcitOBP4 more significantly deviated from the typical ‘Plus-C’ pattern with 52 residues between C1–C2, eight residues between C4–C5, a missing C6 and absence of the conserved proline residue after C6 (S2 Fig). In addition, analysis of the predicted DcitOBP amino acid sequences with signal peptide prediction algorithms suggested that all the DcitOBPs, except three (DcitOBP1, 5, and 9) had defined signal peptide sequences. Maximum-likelihood phylogenetic analysis with hemipteran OBPs showed that all DcitOBP transcripts segregated into the ‘Classic’ and ‘Plus-C’ OBP sub-families (Fig 2) based on the number of cysteines. In the ‘Classic’ OBP clade, 7 ‘Classic’ DcitOBP transcripts clustered with other classic hemipteran OBPs, which were distributed in four well distinct sub-groups.
Fig 2. Phylogenetic relationship between DictOBPs.
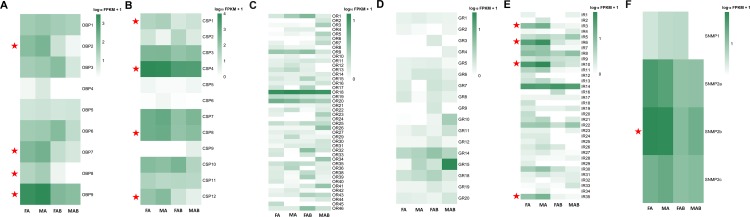
All these OBP transcripts were detected in both the antennae and terminal abdomen of both sexes. Transcripts of three Classic OBPs (OBP7, 8 and 9) and one Plus-C OBP (OBP2) were expressed significantly higher in the antennae when compared to the terminal abdomen (Red stars in Fig 3A; S7 Table). Among these, the abundance of DcitOBP9 transcript was the highest in the antennae (Fig 3A; S4 Table).
Fig 3. Expression profiles of chemosensory genes.
A: OBP; B: CSP; C: OR; D: GR; E: IR; and F: SNMP.
Chemosensory proteins
Twelve transcripts encoding candidate CSPs were identified, and they had an OS-D domain and the typical four cysteine signature (C1-X6-C2-X6-18-C3-X2-C4) of CSPs (S4 Table and S3 Fig). Three of these transcripts encoded partial proteins (DcitCSP5, 6, and 9), whereas the others were full-length CDS. All full-length DcitCSPs, but except DcitCSP4, possessed a signal peptide. Phylogenetic analysis of all DcitCSPs showed two well distinct clades (clades I and II), as seen in CSPs of other hemipteran species. Two DcitCSP transcripts (DcitCSP1 and DcitCSP9) grouped with the “divergent” clade II, whereas the remaining DcitCSP transcripts clustered with the bigger group (Fig 4).These CSP transcripts were detected in at least one of the analyzed tissues (Fig 3B; S4 Table). Transcripts of four CSPs (CSP1, 4, 8 and 12) were upregulated in the antennae when compared to the terminal abdomen (Red stars in Fig 4B; S7 Table). Among all CSP transcripts, DcitCSP4 had the highest expression.
Fig 4. Phylogenetic relationship between DictCSPs.
Odorant receptors
Analysis of the combined transcriptomes from all four D. citri tissues resulted in the identification of 46 candidate OR transcripts (S5 Table). Eleven of these had full-length CDS, and encoded proteins consisting of more than 305 amino acids. Among the full-length DcitOR transcripts, only one transcript (DcitOR1), which was an ortholog of the odorant receptor co-receptor (ORco), had the 7 transmembrane (TM) odorant receptor domains, whereas the others were predicted to have less than 7 TM domains. In the phylogenetic analysis, the co-receptor DcitOR1, grouped into a conserved clade containing ORco from D. melanogaster and A. pisum. The remaining DcitOR transcripts, except for DcitOR20, clustered together into independent species-specific clades (Fig 5).
Fig 5. Phylogenetic relationship between DictORs.
Expression levels of almost all OR transcripts (FPKM of only DcitOR18 was >10) were low in both antennae and terminal abdomen (S5 Table), and were not significantly different between these tissues (Fig 3C; S7 Table). Among them, DcitOR18 had the highest level of expression in the male antennae (FPKM: 10.32) followed by DcitOR20 for which the FPKM value in the male antennae was 4.63 (S5 Table). The Orco transcripts (DcitOR1) with very low expression (FPKM: 0.49–3.69) were observed in the antennae or terminal abdomen of both sexes (S5 Table).
Gustatory receptors
Twenty candidate GR transcripts were identified in the combined transcriptomes of D. citri tissues and had the 7TM chemosensory receptor domain (S5 Table). Except for DcitGR14, which was a full-length CDS encoding a protein with 393 amino acids, almost all DcitGR transcripts were partial fragments that encoded overlapping regions with low amino acid sequence identity, thus indicating their origin from separate genes. Interestingly, only one putative CO2 receptor (DcitGR14) ortholog [48] was found in our combined transcriptomes. A majority of the partial length DcitGR transcripts also showed high amino acid sequence similarity to known sugar receptors (S5 Table).
The range of GR transcript expression levels varied largely in the analyzed tissues with FPKM values ranging from 0.03–22.52 (S5 Table). Among them, a lower expression (FPKM: 0.03) was observed only for the putative CO2 receptor, DcitGR14, found in the antennae and the terminal abdomen (Fig 3D). Expression of DcitGR15 was significantly higher in the male terminal abdomen when compared to the other tissues (FPKM: 22.52) (Fig 3D).
Ionotropic receptors
Thirty-five candidate IR transcripts were identified in the combined transcriptomes of D. citri and were annotated as known insect IRs and ionotropic glutamate receptors (iGluRs), and as the ligand-gated ion channel family of proteins (S5 Table). Eight of the transcripts encoded full-length CDS (DcitIR3, 5, 6, 8, 10, 22, 30, and 35), whereas the remaining were partial CDS. Based on structural analysis and amino acid sequence alignments, 8 iGluRs (DcitIR3, 6, 8, 10, 11, 22, 27, and 30) were identified with all residues typical for iGluRs (R, T, and D/E) [49], and 6 IR transcripts had mismatching amino acids at one or more positions indicating variable ligand binding properties (S4 Fig). According to the ML analysis of DcitIRs with D. melanogaster IRs, five DcitIRs (DcitIR3, 6, 8, 11, and 22) grouped together with the non-NMDA iGluRs clade; three DcitIRs (DcitIR10, 30, and 35) clustered with the NMDA iGluRs clade; one DcitIR (DcitIR33) was grouped together with the divergent IRs clade; DcitIR27 grouped with the co-receptors, IR8a and IR25a; and four DcitIRs (DcitIR5, 9, 17, and 28) grouped with the antennal IRs clade (Fig 6).
Fig 6. Phylogenetic relationship between DictIRs.
Larger numbers of candidate IR transcripts were expressed in the antennae when compared to the terminal abdomen (S7 Table). Two non-NMDA iGluR orthologs (IR3 and 6) and two NMDA iGluR orthologs (IR10 and 35) were upregulated in the antennae when compared to the terminal abdomen (Fig 3E; S7 Table). Four antennal IR orthologs (IR5, 9, 17, and 28) and IR co-receptor ortholog (IR27) with low expression levels showed no significant difference in expression in the antennae and terminal abdomen.
Sensory neuron membrane proteins
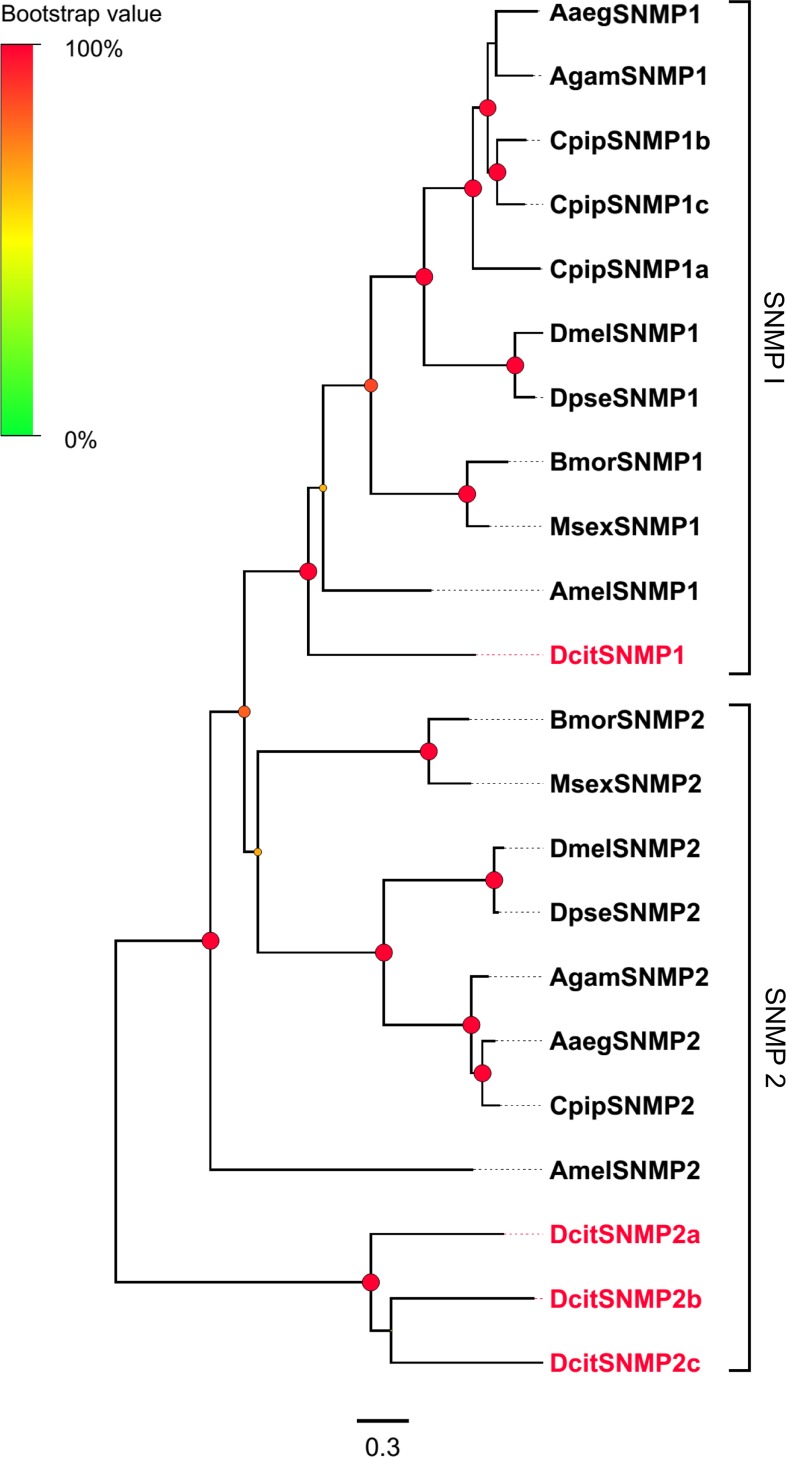
Four candidate SNMP transcripts, which were full-length genes, were identified in the combined transcriptomes from the two tissues of D. citri (S5 Table). Consistent with the characteristics of insect SNMPs, four candidate DcitSNMP transcripts contained two transmembrane domains. Phylogenetic analysis along with sequence alignment revealed the classification of four candidate DcitSNMP transcripts; one was the ortholog of SNMP1 (DcitSNMP1), and three were orthologs of SNMP2 (DcitSNMP2a, DcitSNMP2b, and DcitSNMP2c) (Fig 7).
Fig 7. Phylogenetic relationship between DictSNMPs.
All these SNMP transcripts were detected in the antennae, and three among them (except DcitSNMP1) were expressed also in male and female terminal abdomen. In addition, the expression of DcitSNMP2b was significantly higher in the male or female antennae than in the terminal abdomen (Red stars in Fig 3F; S7 Table). The remaining showed no significant difference in expression between the antennae and terminal abdomen among the sexes (S7 Table).
Odorant degrading enzymes
In the olfaction processes, previous studies have implicated odorant degrading enzymes (ODEs) such as cytochrome P450s (CYPs) [50, 51], esterases (ESTs) [52–54], and aldehyde dehydrogenases (ADEs) [55–57] involved in the rapid inactivation of signals. A large number of putative odor/xenobiotic degradation gene transcripts were detected in our transcriptomes, including 80 CYP transcripts, 12 EST transcripts, and 5 ADE transcripts (S6 Table). Additionally, 35 among the 80 CYP transcripts, 10 among the 12 EST transcripts, and three among the five ADE transcripts had full-length CDS.
Phylogenetic analysis revealed that the DictCYP transcripts clustered with four major cytochrome P450 gene families, CYP2, CYP3, CYP4, and mitochondrial clades (Fig 8). Notably, the majority of DictCYP transcripts (15 transcripts) were largely grouped into the CYP4 clade, which has previously been implicated in the metabolism of odorants or pheromones [58]. However, in the CYP4 clade, four CYP4 genes (DcitCYP18, DcitCYP66, DcitCYP76, and DcitCYP24 previously named as CYP4C67, CYP4G70, CYP4DA1, and CYP4DB1, respectively) involved in imidacloprid resistance [21] were clustered into one clade.
Fig 8. Phylogenetic relationship between DictCYPs.
In addition, 2 CYPs (CYP10 and 52) had a higher expression level in the antennae than in the terminal abdomen (Fig 9A and 9B). Among them, DcitCYP52 from the CYP4 clade was associated with metabolizing odorants and/or pheromones [58]. The antennal-dominant DcitCYP10 clustered with the NADPH CYP clade, which has been shown to be involved in insecticide resistance [59]. In the other detoxification enzyme classes including ESTs and ADEs, none of the candidates were significantly upregulated in the antennae when compared to the terminal abdomen (Fig 9C and 9D; S7 Table).
Fig 9. Expression profiles of odorant-degrading enzymes.
A: CYP; B: EST; and C: ADE.
qPCR validation
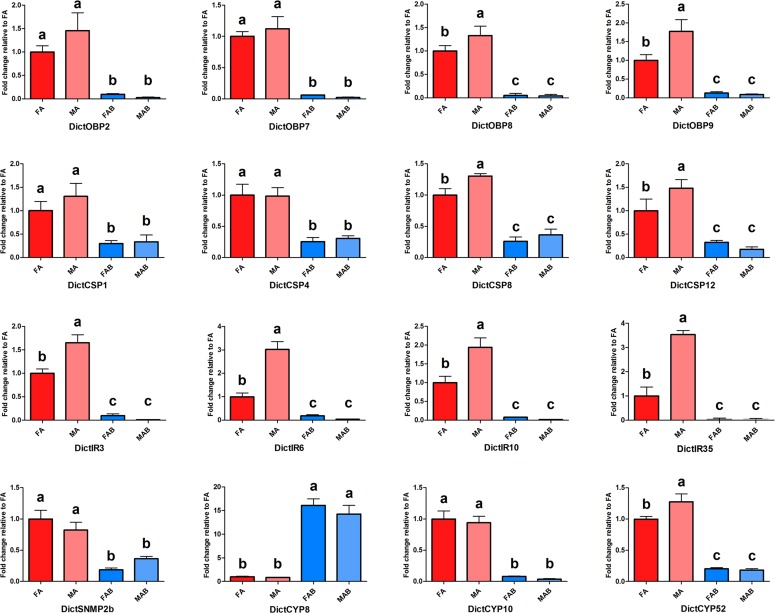
Referring to the calculated expression values determined based on the FKPM methods, the chemosensory gene transcripts, including 4 DictOBP transcripts, 4 DictCSP transcripts, 4 DictIR transcripts, 1 DictSNMP transcripts, and 3 DictCYP transcripts were differentially expressed in the antennae and terminal abdominal tissues (S7 Table, red font), and were selected for qPCR validation. The qPCR results confirmed the higher expression of the following transcripts in the antennae compared to abdomens: 4 DictOBP transcripts (DictOBP2, 7, 8, 9), 4 DictCSP transcripts (DictCSP1, 4, 8, 12), 4 DictIR transcripts (DictIR3, 6, 10, 35), 1 DictSNMP transcript (DictSNMP2b), and 2 DictCYPs transcripts (DictCYP10, 52), which were found to be enriched in the antennae by RNA-seq (Fig 10). Additionally, two DictCYP transcripts were enriched in the terminal abdominal tissues (Fig 10). In addition, the qPCR data also showed that 2 DictOBP transcripts (DictOBP8 and DictOBP9), 2 DictCSP transcripts (DictOBP8 and DictOBP12), 4 DictIR transcripts (DictIR3, DictIR6, DictIR10, and DictIR35), and 1 DictCYP transcript (DictCYP57) were expressed at higher levels in the male antennae than in the female antennae (p < 0.05; Fig 10).
Fig 10. qPCR results of differentially expressed genes in the antennae and terminal abdominal tissues.
Standard error is represented by the error bar. Different letters (a, b, c) above each bar denote significant differences (p<0.05).
Discussion
The Asian citrus psyllid is one of the most important insect pests in the world. Despite its devastating impact on citrus cultivation worldwide, effective control measures including monitoring and annihilation of this insect pest are not currently available. Transcriptome analysis along with genome annotation, has paved the way to identify and characterize multiple chemoreception genes in insects [60, 61]. More importantly, as potential molecular targets, chemosensory proteins can be used to identify novel attractants or repellants for use in environment-friendly pest management strategies [24–26, 62]. However, a lack in the genetic information of chemosensory genes has limited our understanding of the chemosensory mechanisms in this species. Besides, the molecular basis of chemoreception in hemipterans is poorly understood compared to dipterans and lepidopterans. In the present study, we compared the expression profiles of chemosensory genes in an olfactory (antennae) and a non-olfactory tissue (terminal abdomen) from male and female D. citri to identify olfaction-specific genes for use as novel targets in pest control.
Odorant binding proteins and chemosensory proteins
Odorants penetrate into the sensillum lymph through pore tubules and bind to OBPs forming the odorant·OBP complex. [63] After OR activation, odorants transported to the OR by OBPs and then released [5]. Similarly, CSP are also found in olfactory and gustatory organs of insects [64–66], and are suggested to play crucial roles in chemo-detection [67]. The 9 candidate OBP transcripts (seven Classic and two Plus-C OBPs) and 12 CSP transcripts identified in D. citri were more similar to the numbers identified previously in the aphid species (A. pisum: 13 Classic and two Plus-C OBPs and 13 CSPs; A. gossypii: 9 OBPs and 9 CSPs) and another hemipteran (N. lugens: 8 Classic and two Plus-C OBPs and 11 CSPs) [27–31]. Notably, without Minus-C OBP transcripts, only Classic OBP transcripts and Plus-C OBP transcripts were found in the psyllid and other hemipterans. This may indicate their origin from a common ancestor. RNA-seq data and qPCR analyses showed that 4 DictOBP transcripts and 4 DictCSP transcripts were significantly enriched in antennae when compared to the terminal abdomen, suggesting the possible involvement of these antennal OBP and CSP proteins in odor perception. In addition, qPCR data revealed that DictOBP8, DictOBP9, DictCSP8, and DictCSP12 were expressed higher in the male antennae than in the female antennae. Male-biased expression of these genes implicates their importance in the perception of female sex pheromones [64, 68].
Odorant receptors
Insect ORX/Orco heteromers are known to function as odorant-gated ion channels, and consist of a highly conserved co-receptor (Orco) and odorants-recognizing receptor (ORX) [69–72]. In general, it is known that ORs employ a higher number of receptor protein families to detect a broad range of odors [1], and that the members of insect ORs show lineage specific expansions that might have been driven by ecological adaptation [73]. Compared to two phytophagous hemipterans with known OR sets, the number of D. citri ORs were close to that reported for the cotton aphid A. gossypii ORs (45 in genome and 36 in transcriptome of the antennae and decapitated body parts) [33], but lower than in the pea aphid A. pisum genome (79) [32]. Considering that the host range of D. citri is relatively narrow and is within the rutaceous subfamily Aurantioideae [18], we presumed that the number of candidate OR transcripts in D. citri was smaller due to lower “semiochemical diversity”. Although D. citri is a phytophagous hemipteran, D. citri ORs share low homology with the two aphids, and all but the conserved co-receptor (DcitOR1) have no homology with their ORs according to phylogeny, indicating a significant species-specific expansion and divergence in D. citri. Surprisingly, the OR repertoire of D. citri was not mainly expressed in antennae, which is not consistent with OR expression in other insect species including D. melanogaster [74], Anopheles gambiae [75], Culex quinquefasciatus [60], and Mayetiola destructor [76]. It is therefore possible that a majority of DictOR transcripts have other non-olfactory functions.
Gustatory receptors
In mosquito and Drosophila, the GR complex consists of two or three GR members that play a crucial role in CO2 detection [48, 77–80]. Only one GR homolog of insect CO2 receptors was found in D. citri and it had low expression in the antennae. In general, insect CO2 receptors are highly expressed in the antennae. We presume that an alternate organ such as the maxillary palp may be responsible for CO2 detection in this insect pest [77, 81]. In addition, we cannot rule out the possibility that sequencing depth in this study may have been the reason for identifying low abundance putative CO2 receptors in antennal transcriptomes. A large number of putative sugar receptor transcripts were also observed in the antennae and terminal abdomen. Although the exact reason for this is not known, it likely that they evolved to adapt for feeding or egg laying habits. Both male and female D. citri have a high preference for feeding on tender shoots, and females lay eggs only in the exocuticle of the earliest tender shoots [18].
Ionotropic receptors
IRs were recently identified as a novel class of insect olfactory receptors [82]. Functional studies of insect IRs expressed in olfactory neurons have demonstrated their role in the detection of amines, acids [49, 83] and DEET [62]. More recently, a few members of IRs expressed in taste neurons have also been confirmed as a new-type of larval taste receptors [84] and were reported to be involved in the detection of pheromones [85]. We identified 6 putative DcitIR transcripts and 8 iGluRs transcripts based on the amino acid sequence alignment (S4 Fig). However, the search for IR co-receptors, IR8a and IR25a, revealed that only DcitIR27 in D. citri was homologous to insect IR25a receptors. Interestingly, another homologous IR-co-receptor, IR8a, was not discovered in the four transcriptomes assembly. A similar expression pattern was also reported in Culex quinquefasciatus [60]. We therefore concluded that it is likely that DcitIR8a is not expressed in D. citri antennae and terminal abdomen. Also, this lack of identification may be related to the low sequencing depth in this study. In addition, two non-NMDA iGluR orthologs (DcitIR3 and 6), three DcitIRs (DcitIR10, 30 and 35), and two NMDA iGluRs orthologs showed a clear difference in expression level between the sexes (Figs 6, 3E and 10). In D. melanogaster, male-biased patterns of IR expression as well as functional analyses revealed that both IR52c and IR52d may determine male copulation [84]. Therefore, it is presumed that the DcitIR transcripts that showed sex-biased expression could play a role in sexual behavior.
Sensory neuron membrane proteins
SNMPs in insects, which are located in the odorant-sensitive ORNs, are a family of membrane proteins with two transmembrane domains and are presumed to be associated with pheromone reception in lepidopteran and dipteran insects [86–89]. In Lepidoptera, the SNMP family consists of two subfamilies, SNMP1 and SNMP2, which are differentially expressed in the cells of pheromone-sensitive sensilla.SNMP1 is expressed in the pheromone-specific olfactory neurons suggesting its involvement in pheromone detection [89, 90] while SNMP2 is expressed in the sensilla support cells [89]. In D. melanogaster, SNMP1 homolog is essential for the proper olfactory sensory neuron (OSN) responses to the volatile pheromone, 11-cis-vaccenyl acetate [91]. In this study, one SNMP1 and three homologs of SNMP2 were identified in D. citri, and all these SNMPs were expressed in both the antennae and terminal abdomen. Not surprisingly, the expression of SNMP1 and SNMP2 in the antennae and the remaining body parts including terminal abdominal tissues was also reported in moths and flies [76, 92]. Since SNMP1 homolog has key functions in pheromone detection in D. melanogaster, we presumed that DictSNMP1 transcripts would be expressed at the maximum levels in the antennae. However, only DictSNMP2b had highest expression in the antennae compared to terminal abdomen. This finding suggests that DictSNMP2b may play a specific role in pheromone detection, or alternatively that the role of DictSNMP1 might not be restricted to detect pheromones in psyllids.
Odorant degrading enzymes
CYP4s have been linked to odorant or pheromone metabolism in some insect species [50, 51, 93–97]. DictCYP52, which aligned with the known CYP4 clade, was found to be highly expressed in the antennae than in the terminal abdomen, and higher in male antennae based on qPCR data. Thus, we presume that DictCYP52 could play a role in odorant clearance. However, further studies along this line is warranted.
Conclusions
To better understand the chemosensory genes involved in olfactory sensation, sex pheromone production and host selection behavior in D. citri, we analyzed its antennal and abdominal transcriptome in both sexes. A total of 126 chemosensory gene transcripts (excluding ODEs), including 9 OBPs, 12 CSPs, 46 ORs, 35 IRs, 20 GRs, and 4 SNMPs were identified in the present study. In addition, a group of detoxification enzymes potentially linked to odorant degradation such as 80 cytochrome P450s, 12 esterases, and 5 aldehyde dehydrogenases were also identified. Furthermore, the expression of all these chemosensory gene transcripts was analyzed based on transcriptome profiling using RNA-seq data. Both RNA-seq data and qPCR analyses revealed that a number of these newly identified genes in D. citri transcriptomes were differentially expressed in the four tissues, thus indicating their role in the interaction between the olfactory system and biological processes.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Acknowledgments
We thank Mr. Mengqiu Qu and Mr. Mei Li for insect rearing, Mr. Zhengbin Wang and Mr. Renzhao Xu for insect dissection, and Mr. Wanyu Xiao for the construction of dendrograms.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported financially by the special funds of scientific and technological development of Guangdong Province in 2016, China (Grant No. 2016B020202009 and 2016A040403118), and the Guangzhou scientific and technological project (Grant No. 201510010141)
References
- 1.Missbach C, Dweck HK, Vogel H, Vilcinskas A, Stensmyr MC, Hansson BS, et al. Evolution of insect olfactory receptors. Elife. 2014; 3:e2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vieira FG, Rozas J. Comparative Genomics of the Odorant-Binding and Chemosensory Protein Gene Families across the Arthropoda: Origin and Evolutionary History of the Chemosensory System. Genome Biol Evol. 2011; 3:476–490. 10.1093/gbe/evr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou JJ. Odorant-binding proteins in insects. Vitam Horm. 83; 2010; 241–272. 10.1016/S0083-6729(10)83010-9 [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity. 2009; 103(3):208–216. 10.1038/hdy.2009.55 [DOI] [PubMed] [Google Scholar]
- 5.Leal WS. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu Rev Entomol. 2013; 58:373–391. 10.1146/annurev-ento-120811-153635 [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Ban L, Iovinella I, Zhao L, Gao Q, Felicioli A, et al. Diversity, abundance, and sex-specific expression of chemosensory proteins in the reproductive organs of the locust Locusta migratoria manilensis. Biol Chem. 2013; 394(1):43–54. 10.1515/hsz-2012-0114 [DOI] [PubMed] [Google Scholar]
- 7.Gu SH, Wu KM, Guo YY, Pickett JA, Field LM, Zhou JJ, et al. Identification of genes expressed in the sex pheromone gland of the black cutworm Agrotis ipsilon with putative roles in sex pheromone biosynthesis and transport. BMC Genomics. 2013; 14:636 10.1186/1471-2164-14-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dani FR, Michelucci E, Francese S, Mastrobuoni G, Cappellozza S, La Marca G, et al. Odorant-binding proteins and chemosensory proteins in pheromone detection and release in the silkmoth Bombyx mori. Chem Senses. 2011; 36(4):335–344. 10.1093/chemse/bjq137 [DOI] [PubMed] [Google Scholar]
- 9.Jacquin-Joly E, Vogt RG, Francois MC, Nagnan-Le MP. Functional and expression pattern analysis of chemosensory proteins expressed in antennae and pheromonal gland of Mamestra brassicae. Chem Senses. 2001; 26(7):833–844. [DOI] [PubMed] [Google Scholar]
- 10.Sun YL, Huang LQ, Pelosi P, Wang CZ. Expression in antennae and reproductive organs suggests a dual role of an odorant-binding protein in two sibling Helicoverpa species. PLOS ONE. 2012; 7(1):e30040 10.1371/journal.pone.0030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iovinella I, Dani FR, Niccolini A, Sagona S, Michelucci E, Gazzano A, et al. Differential expression of odorant-binding proteins in the mandibular glands of the honey bee according to caste and age. J Proteome Res. 2011; 10(8):3439–3449. 10.1021/pr2000754 [DOI] [PubMed] [Google Scholar]
- 12.Li S, Picimbon JF, Ji S, Kan Y, Chuanling Q, Zhou JJ, et al. Multiple functions of an odorant-binding protein in the mosquito Aedes aegypti. Biochem Biophys Res Commun. 2008; 372(3):464–468. 10.1016/j.bbrc.2008.05.064 [DOI] [PubMed] [Google Scholar]
- 13.Cheng D, Lu Y, Zeng L, Liang G, He X. Si-CSP9 regulates the integument and moulting process of larvae in the red imported fire ant, Solenopsis invicta. Sci Rep. 2015; 5:9245 10.1038/srep09245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marinotti O, Ngo T, Kojin BB, Chou SP, Nguyen B, Juhn J, et al. Integrated proteomic and transcriptomic analysis of the Aedes aegypti eggshell. BMC Dev Biol. 2014; 14:15 10.1186/1471-213X-14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa-da-Silva AL, Kojin BB, Marinotti O, James AA, Capurro ML. Expression and accumulation of the two-domain odorant-binding protein AaegOBP45 in the ovaries of blood-fed Aedes aegypti. Parasit Vectors. 2013; 6:364 10.1186/1756-3305-6-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleszka J, Foret S, Saint R, Maleszka R. RNAi-induced phenotypes suggest a novel role for a chemosensory protein CSP5 in the development of embryonic integument in the honeybee (Apis mellifera). Dev Genes Evol. 2007; 217(3):189–196. [DOI] [PubMed] [Google Scholar]
- 17.Pitts RJ, Liu C, Zhou X, Malpartida JC, Zwiebel LJ. Odorant receptor-mediated sperm activation in disease vector mosquitoes. Proc Natl Acad Sci USA. 2014; 111(7): 2566–2571. 10.1073/pnas.1322923111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grafton-Cardwell EE, Stelinski LL, Stansly PA. Biology and Management of Asian Citrus Psyllid, Vector of the Huanglongbing Pathogens. Ann Rev Entomol. 2013; 58: 413–432. [DOI] [PubMed] [Google Scholar]
- 19.Wenninger EJ, Hall DG. Daily timing of mating and age at reproductive maturity in Diaphorina citri (Hemiptera: Psyllidae). Fla Entomol. 2007; 90: 715–722. [Google Scholar]
- 20.Yasuda K, Kawamura F, Oishi T. Location and preference of adult Asian citrus psyllid, Diaphorina citri (Homoptera: Psyllidae) on Chinese Box Orange Jasmine, Murraya exotica L. and Flat Lemon, Citrus depressa. J Appl Entomol Zool. 1997(49):146–149. [Google Scholar]
- 21.Tiwari S, Gondhalekar AD, Mann RS, Scharf ME, Stelinski LL. Characterization of five CYP4 genes from Asian citrus psyllid and their expression levels in Candidatus Liberibacter asiaticus-infected and uninfected psyllids. Insect Mol Biol. 2011; 20(6):733–744. 10.1111/j.1365-2583.2011.01103.x [DOI] [PubMed] [Google Scholar]
- 22.Tiwari S, Mann RS, Rogers ME, Stelinski LL. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag Sci. 2011; 67(10):1258–1268. 10.1002/ps.2181 [DOI] [PubMed] [Google Scholar]
- 23.Tiwari S, Pelz-Stelinski K, Stelinski LL. Effect of Candidatus Liberibacter asiaticus infection on susceptibility of Asian citrus psyllid, Diaphorina citri, to selected insecticides. Pest Manag Sci. 2011; 67(1):94–99. 10.1002/ps.2038 [DOI] [PubMed] [Google Scholar]
- 24.Jayanthi KP, Kempraj V, Aurade RM, Roy TK, Shivashankara KS, Verghese A. Computational reverse chemical ecology: virtual screening and predicting behaviorally active semiochemicals for Bactrocera dorsalis. BMC Genomics. 2014; 15:209 10.1186/1471-2164-15-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle SM, McInally S, Ray A. Expanding the olfactory code by in silico decoding of odor-receptor chemical space. Elife. 2013; 2:e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter CJ. Stop the biting: targeting a mosquito's sense of smell. Cell. 2014; 156(5):878–881. 10.1016/j.cell.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 27.Hull JJ, Perera OP, Snodgrass GL. Cloning and expression profiling of odorant-binding proteins in the tarnished plant bug, Lygus lineolaris. Insect Mol Biol. 2014; 23(1):78–97. 10.1111/imb.12064 [DOI] [PubMed] [Google Scholar]
- 28.Zhou SS, Sun Z, Ma W, Chen W, Wang MQ. De novo analysis of the Nilaparvata lugens (Stål) antenna transcriptome and expression patterns of olfactory genes. Comp Biochem Physiol Part D Genomics Proteomics. 2014; 9:31–39. 10.1016/j.cbd.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 29.He P, Zhang J, Liu NY, Zhang YN, Yang K, Dong SL. Distinct expression profiles and different functions of odorant binding proteins in Nilaparvata lugens Stal. PLOS ONE. 2011; 6(12):e28921 10.1371/journal.pone.0028921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu SH, Wu KM, Guo YY, Field LM, Pickett JA, Zhang YJ, et al. Identification and Expression Profiling of Odorant Binding Proteins and Chemosensory Proteins between Two Wingless Morphs and a Winged Morph of the Cotton Aphid Aphis gossypii Glover. PLOS ONE. 2013; 8(9):e73524 10.1371/journal.pone.0073524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou JJ, Vieira FG, He XL, Smadja C, Liu R, Rozas J, et al. Genome annotation and comparative analyses of the odorant-binding proteins and chemosensory proteins in the pea aphid Acyrthosiphon pisum. Insect Mol Biol. 2010; 19 Suppl 2:113–122. [DOI] [PubMed] [Google Scholar]
- 32.Smadja C, Shi P, Butlin RK, Robertson HM. Large Gene Family Expansions and Adaptive Evolution for Odorant and Gustatory Receptors in the Pea Aphid, Acyrthosiphon pisum. Mol Biol Evol. 2009; 26(9):2073–2086. 10.1093/molbev/msp116 [DOI] [PubMed] [Google Scholar]
- 33.Cao D, Liu Y, Walker WB, Li J, Wang G. Molecular characterization of the Aphis gossypii olfactory receptor gene families. PLOS ONE. 2014; 9(6):e101187 10.1371/journal.pone.0101187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013; 8(8):1494–1512. 10.1038/nprot.2013.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011; 29(7):644–652 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, et al. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006; 34(Web Server issue): W293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013; 30(4):772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013; 30(12): 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price MN, Dehal PS, Arkin AP: FastTree 2-approximately maximum-likelihood trees for large alignments. PLOS ONE. 2010; 5(3):e9490 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009; 25(9):1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011; 12:323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010; 28(5):511–515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Feng Z, Wang X, Wang X, Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010; 26(1):136–138. 10.1093/bioinformatics/btp612 [DOI] [PubMed] [Google Scholar]
- 44.Storey JD Tibshiran R. Statistical significance for genome-wide studies. Proc Natl Acad Sci USA. 2003(100):9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DELTADELTACT method. Methods. 2001; 25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 46.Xu YL, He P, Zhang L, Fang SQ, Dong SL, Zhang YJ, et al. Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genomics. 2009; 10:632 10.1186/1471-2164-10-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou JJ, He XL, Pickett JA, Field LM. Identification of odorant-binding proteins of the yellow fever mosquito Aedes aegypti: genome annotation and comparative analyses. Insect Mol Biol. 2008; 17(2): 147–163. 10.1111/j.1365-2583.2007.00789.x [DOI] [PubMed] [Google Scholar]
- 48.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007; 445(7123):86–90. [DOI] [PubMed] [Google Scholar]
- 49.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant Ionotropic Glutamate Receptors as Chemosensory Receptors in Drosophila. Cell. 2009; 136(1):149–162. 10.1016/j.cell.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishida Y, Leal WS. Chiral discrimination of the Japanese beetle sex pheromone and a behavioral antagonist by a pheromone-degrading enzyme. P Natl Acad Sci USA. 2008; 105(26):9076–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojtasek H, Leal WS. Degradation of an alkaloid pheromone from the pale-brown chafer, Phyllopertha diversa (Coleoptera: Scarabaeidae), by an insect olfactory cytochrome P450. FEBS Lett. 1999; 458(3):333–336. [DOI] [PubMed] [Google Scholar]
- 52.Ishida Y, Leal WS. Rapid inactivation of a moth pheromone. P Natl Acad Sci USA. 2005; 102(39):14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maibeche-Coisne M, Nikonov AA, Ishida Y, Jacquin-Joly E, Leal WS. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc Natl Acad Sci USA. 2004; 101(31):11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogt RG, Riddiford LM, Prestwich GD: Kinetic properties of a sex pheromone-degrading enzyme: the sensillar esterase of Antheraea polyphemus. P Natl Acad Sci USA. 1985; 82(24):8827–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merlin C, Francois MC, Bozzolan F, Pelletier J, Jacquin-Joly E, Maibeche-Coisne M. A new aldehyde oxidase selectively expressed in chemosensory organs of insects. Biochem Bioph Res Co. 2005; 332(1):4–10. [DOI] [PubMed] [Google Scholar]
- 56.Rybczynski R, Vogt RG, Lerner MR. Antennal-specific pheromone-degrading aldehyde oxidases from the moths Antheraea polyphemus and Bombyx mori. J Biol Chem. 1990; 265(32):19712–19715. [PubMed] [Google Scholar]
- 57.Rybczynski R, Reagan J, Lerner MR. A pheromone-degrading aldehyde oxidase in the antennae of the moth Manduca sexta. J Neurosci. 1989; 9(4):1341–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feyereisen R. Insect CYP genes and P450 enzymes In: Gilbert LI, editor. Insect molecular biology and biochemistry. San Diego: Academic; 2012. p. 236–316. [Google Scholar]
- 59.Shi L, Zhang J, Shen G, Xu Z, Wei P, Zhang Y, Xu Q, He L. Silencing NADPH-cytochrome P450 reductase results in reduced acaricide resistance in Tetranychus cinnabarinus (Boisduval). Sci Rep. 2015; 5: 15581 10.1038/srep15581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leal WS, Choo Y, Xu P, Da Silva CSB, Ueira-Vieira C. Differential expression of olfactory genes in the southern house mosquito and insights into unique odorant receptor gene isoforms. Proc Natl Acad Sci USA. 2013; 110(46):18704–18709. 10.1073/pnas.1316059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grosse-Wilde E, Kuebler LS, Bucks S, Vogel H, Wicher D, Hansson BS. Antennal transcriptome of Manduca sexta. Proc Natl Acad Sci USA. 2011; 108(18):7449–7454. 10.1073/pnas.1017963108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kain P, Boyle SM, Tharadra SK, Guda T, Christine P, Dahanukar A, et al. Odour receptors and neurons for DEET and new insect repellents. Nature. 2013; 502(7472):507–512. 10.1038/nature12594 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Laughlin JD, Ha TS, Jones DNM, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008; 133(7):1255–1265. 10.1016/j.cell.2008.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang YN, Ye ZF, Yang K, Dong SL. Antenna-predominant and male-biased CSP19 of Sesamia inferensis able to bind the female sex pheromones and host plant volatiles. Gene. 2014:279–286. [DOI] [PubMed] [Google Scholar]
- 65.Gu S, Wang S, Zhang X, Ji P, Liu J, Wang G, Wu K, et al. Functional Characterizations of Chemosensory Proteins of the Alfalfa Plant Bug Adelphocoris lineolatus Indicate Their Involvement in Host Recognition. PLOS ONE. 2012; 7(8):e42871 10.1371/journal.pone.0042871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu R, He X, Lehane S, Lehane M, Hertz-Fowler C, Berriman M, et al. Expression of chemosensory proteins in the tsetse fly Glossina morsitans morsitans is related to female host-seeking behaviour. Insect Mol Biol. 2012; 21:41–48. 10.1111/j.1365-2583.2011.01114.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pelosi P, Iovinella I, Felicioli A, Dani FR. Soluble proteins of chemical communication: an overview across arthropods. Front Physiol. 2014; 5:320 10.3389/fphys.2014.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gu SH, Zhou JJ, Wang GR, Zhang YJ, Guo YY. Sex pheromone recognition and immunolocalization of three pheromone binding proteins in the black cutworm moth Agrotis ipsilon. Insect Biochem Mol Biol. 2013; 43(3):237–51. 10.1016/j.ibmb.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 69.Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci USA. 2011; 108(21):8821–8825. 10.1073/pnas.1102425108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, Christie DL, et al. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Molec. 2008; 38(8):770–780. [DOI] [PubMed] [Google Scholar]
- 71.Wicher D, Schaefer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008; 452(7190):1007–1010. 10.1038/nature06861 [DOI] [PubMed] [Google Scholar]
- 72.Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008; 452(7190):1002–1009. 10.1038/nature06850 [DOI] [PubMed] [Google Scholar]
- 73.Hansson BS, Stensmyr MC. Evolution of Insect Olfaction. Neuron. 2011; 72(5):698–711. 10.1016/j.neuron.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 74.Menuz K, Larter NK, Park J, Carlson JR. An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. Plos Genet. 2014; 10(11):e1004810 10.1371/journal.pgen.1004810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitts RJ, Rinker DC, Jones PL, Rokas A, Zwiebel LJ. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics. 2011; 12:271 10.1186/1471-2164-12-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andersson MN, Videvall E, Walden KK, Harris MO, Robertson HM, Lofstedt C. Sex- and tissue-specific profiles of chemosensory gene expression in a herbivorous gall-inducing fly (Diptera: Cecidomyiidae). BMC Genomics. 2014; 15:501 10.1186/1471-2164-15-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Omondi BA, Majeed S, Ignell R. Functional development of carbon dioxide detection in the maxillary palp of Anopheles gambiae. J Exp Biol. 2015; 218(15):2482–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tauxe GM, MacWilliam D, Boyle SM, Guda T, Ray A. Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell. 2013; 155:1365–1379. 10.1016/j.cell.2013.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009; 461:159–277. [DOI] [PubMed] [Google Scholar]
- 80.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007; 104(9):3574–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grant AJ, Wigton BE, Aghajanian JG, O'Connell RJ. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol A 1995; 177(4): 389–396. [DOI] [PubMed] [Google Scholar]
- 82.Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, et al. Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction. PLoS Genet. 2010; 19; 6(8):e1001064 10.1371/journal.pgen.1001064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, et al. Acid sensing by the Drosophila olfactory system. Nature. 2010; 468(7324):691 10.1038/nature09537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, Stewart S, Carlson JR. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron. 2014; 83(4): 850–865. 10.1016/j.neuron.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stewart S, Koh TW, Ghosh AC, Carlson JR. Candidate ionotropic taste receptors in the Drosophila larva. Proc Natl Acad Sci USA. 2015, 112(14):4195–4201. 10.1073/pnas.1503292112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007; 450(7167):289 [DOI] [PubMed] [Google Scholar]
- 87.Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 2008; 105(31):10996–11001. 10.1073/pnas.0803309105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vogt RG, Miller NE, Litvack R, Fandino RA, Sparks J, Staples J, et al. The insect SNMP gene family. Insect Biochem Molec. 2009; 39(7):448–456. [DOI] [PubMed] [Google Scholar]
- 89.Forstner M, Gohl T, Gondesen I, Raming K, Breer H, Krieger J. Differential expression of SNMP-1 and SNMP-2 proteins in pheromone-sensitive hairs of moths. Chem Senses. 2008; 33(3):291–299. 10.1093/chemse/bjm087 [DOI] [PubMed] [Google Scholar]
- 90.Rogers ME, Krieger J, Vogt RG. Antennal SNMPs (Sensory Neuron Membrane Proteins) of lepidoptera define a unique family of invertebrate CD36-like proteins. J Neurobiol. 2001; 49(1): 47–61. [DOI] [PubMed] [Google Scholar]
- 91.Nichols Z, Vogt RG. The SNMP/CD36 gene family in Diptera, Hymenoptera and Coleoptera: Drosophila melanogaster, D. pseudoobscura, Anopheles gambiae, Aedes aegypti, Apis mellifera, and Tribolium castaneum. Insect Biochem Molec. 2008; 38(4):398–415. [DOI] [PubMed] [Google Scholar]
- 92.Gu SH, Sun L, Yang RN, Wu KM, Guo YY, Li XC, Zhou JJ, et al. Molecular Characterization and Differential Expression of Olfactory Genes in the Antennae of the Black Cutworm Moth Agrotis ipsilon. PLOS ONE. 2014; 9(8):e103420 10.1371/journal.pone.0103420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Younus F, Chertemps T, Pearce SL, Pandey G, Bozzolan F, Coppin CW, et al. Identification of candidate odorant degrading gene/enzyme systems in the antennal transcriptome of Drosophila melanogaster. Insect Biochem Mol Biol. 2014; 53:30–43. 10.1016/j.ibmb.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 94.Keeling CI, Henderson H, Li M, Dullat HK, Ohnishi T, Bohlmann J. CYP345E2, an antenna-specific cytochrome P450 from the mountain pine beetle, Dendroctonus ponderosae Hopkins, catalyses the oxidation of pine host monoterpene volatiles. Insect Biochem Mol Biol. 2013; 43(12):1142–51. 10.1016/j.ibmb.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 95.López MF1, Cano-Ramírez C, Cesar-Ayala AK, Ruiz EA, Zúñiga G. Diversity and expression of P450 genes from Dendroctonus valens LeConte (Curculionidae: Scolytinae) in response to different kairomones. Insect Biochem Mol Biol. 2013; 43(5):417–32. 10.1016/j.ibmb.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 96.Pottier MA, Bozzolan F, Chertemps T, Jacquin-Joly E, Lalouette L, et al. Cytochrome P450s and cytochrome P450 reductase in the olfactory organ of the cotton leafworm Spodoptera littoralis. Insect Mol Biol. 2012; 21(6):568–80. 10.1111/j.1365-2583.2012.01160.x [DOI] [PubMed] [Google Scholar]
- 97.Maïbèche-Coisne M, Nikonov AA, Ishida Y, Jacquin-Joly E, Leal WS. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc Natl Acad Sci U S A. 2004; 101(31):11459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.