Abstract
The genome of a laboratory-adapted strain of Leptospira interrogans serovar Hardjo was sequenced and analyzed. Comparison of the sequenced genome with that recently published for a field isolate of the same serovar revealed relatively high sequence conservation at the nucleotide level, despite the different biological background of both samples. Conversely, comparison of both serovar Hardjo genomes with those of L. borgpetersenii serovar Hardjo showed extensive differences between the corresponding chromosomes, except for the region occupied by their rfb loci. Additionally, comparison of the serovar Hardjo genomes with those of different L. interrogans serovars allowed us to detect several genomic features that may confer an adaptive advantage to L. interrogans serovar Hardjo, including a possible integrated plasmid and an additional copy of a cluster encoding a membrane transport system known to be involved in drug resistance. A phylogenomic strategy was used to better understand the evolutionary position of the Hardjo serovar among L. interrogans serovars and other Leptospira species. The proposed phylogeny supports the hypothesis that the presence of similar rfb loci in two different species may be the result of a lateral gene transfer event.
Introduction
Leptospirosis is a bacterial zoonosis which impacts both human and animal health worldwide and is caused by pathogenic members of the genus Leptospira. The members of this genus have been classified into more than 250 serovars, grouped into 24 antigenically related serogroups [1]. This classification is based on serovar-specific antisera reacting mainly against components of the surface lipopolysaccharide (LPS). DNA-DNA hybridization has been used to classify Leptospira into species, while phylogenetic studies based mainly on 16S rRNA sequences have been used to further classify species into groups [2]. However, correlation between serological and DNA-based classification is poor, as members of the same serovar may belong to different species [3].
Some Leptospira serovars are adapted to a particular host species and asymptomatically infect renal tubules during host adaptation. Certain serovar determinants, such as the O-antigen of LPS, are thought to be involved in host selection by mechanisms that are largely unknown [4]. Serovar Hardjo belonging to L. interrogans and L. borgpetersenii are known to be adapted to cattle. L. borgpetersenii serovar Hardjo is the most common host-adapted species in cattle all over the world, while the pattern of host adaptation for L. interrogans serovar Hardjo is relatively unclear [5]. Although both belong to two different species, they were confirmed to have a very similar rfb locus, the gene cluster encoding the enzymes involved in LPS biosynthesis [6]. The presence of almost identical rfb loci can be associated with a similar LPS structure, which further explains the similar serological reaction in these rather different species. A convergent evolution of the loci due to acquisition of genes via lateral gene transfer was proposed as an explanation for this observation.
L. interrogans serovar Hardjo differs from its L. borgpetersenii counterpart in several clinical and epidemiological aspects. Infection of cattle with L. interrogans serovar Hardjo has been associated with a rate of abortion of 30%, reasonably higher than that caused by L. borgpetersenii serovar Hardjo, which is around 3–10% [7]. In addition, L. interrogans serovar Hardjo has been specifically associated with the development of milk drop syndrome in dairy cows as a consequence of acute infection [8]. The genome of a field isolate of L. interrogans serovar Hardjo (strain Norma) was recently published [9], however no functional or evolutionary analysis was provided in the article. In the present study, we sequenced, assembled and annotated the genome of a laboratory-adapted strain of L. interrogans serovar Hardjo (serogroup Sejroe, strain Hardjoprajitno). Analysis of the sequenced chromosomes allowed us to further study the genetic background of L. interrogans serovar Hardjo and its evolutionary relationship with other members of the genus Leptospira.
Materials and Methods
Bacterial Culture and DNA Extraction
The L. interrogans serovar Hardjo strain sequenced in this study was obtained from the National Veterinary Services Laboratory (Ames, Iowa, USA). This strain was originally isolated from a human patient in Indonesia and has been routinely used in veterinary diagnostic laboratories in the United States. The strain was maintained in the laboratory in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium and passaged for several generations. Genomic DNA for sequencing was isolated using the MasterPure™ Complete DNA and RNA Purification Kit (Epicentre, USA) following manufacturer’s instructions.
Genome Sequencing, Assembly and Annotation
Genomic DNA was sequenced at the Georgia Genomics Facility of the University of Georgia (UGA) by using the Illumina MiSeq technology with standard protocols. Reads were trimmed and cropped to 250 bp by using trimmomatic [10] to remove low-quality positions from the 3' end. De novo assembly was performed by using SPAdes [11], with the recommended options for MiSeq reads. Contigs were further scaffolded by using ABACAS [12] and the genome of L. interrogans serovar Lai strain 56601 [13] as a reference. Gene models annotated in the genome of serovar Lai and in that of the closely related serovar Copenhageni strain Fiocruz L1-130 [14] were transferred to the contiguated pseudochromosomes by using RATT [15]. BaSYS [16] was also used for de novo gene detection. All lines of evidence for gene models were manually revised and merged using Artemis and the Artemis Comparison Tool (ACT) [17]. To avoid the relatively high levels of over-annotation that has been reported for other Leptospira genomes when using automatic pipelines [18], we followed the guidelines described by Bulach et al. [19] for the annotation of two L. borgpetersenii serovar Hardjo genomes. Read mapping to reference genomes was performed with BWA [20] and variant calling from read alignments was done with SAMtools (v. 0.1.19) [21].
Functional and Phylogenomic Analyses
OrthoMCL [22] was used to cluster the genes of the newly annotated serovar Hardjo genome along with those from another 23 selected Leptospira genomes previously submitted to Genbank (Table 1). Each ortholog group was tested for evidence of selection, either positive or negative, by comparing models 1 and 2 of the codeml program from the PAML 4 package [23]. Protein sequences were aligned with MAFFT [24] and the alignments were further refined by using Gblocks [25]. Phylogenetic trees were built with PhyML 3.0 [26] under the best model predicted by ProtTest3 [27], with bootstrap values for branch support resulting from 500 bootstrap replicates.
Table 1. Genomes of Leptospira species, strains and serovars selected for the functional and phylogenomic studies presented in this article.
| Species | Serovar | Strain | Locus tag prefix | NCBI BioProject |
|---|---|---|---|---|
| L. biflexa | Patoc | Patoc 1 (Ames) | LBF | PRJNA20133 |
| L. borgpetersenii | Hardjo (type A) | L550 | LBL | PRJNA16146 |
| Hardjo (type B) | JB197 | LBJ | PRJNA16148 | |
| Mini | 200901116 | LEP1GSC190 | PRJNA167259 | |
| Pomona | 200901868 | LEP1GSC133 | PRJNA167255 | |
| L. interrogans | --- | Brem 329 | LEP1GSC057 | PRJNA167229 |
| --- | FPW1039 | LEP1GSC079 | PRJNA167242 | |
| --- | FPW2026 | LEP1GSC080 | PRJNA74077 | |
| Bataviae | L1111 | LEP1GSC087 | PRJNA74089 | |
| Bulgarica | Mallika | LEP1GSC007 | PRJNA65041 | |
| Canicola | Fiocruz LV133 | LEP1GSC069 | PRJNA167235 | |
| Copenhageni | Fiocruz L1-130 | LIC | PRJNA10687 | |
| Lai | 56601 | LA | PRJNA293 | |
| Lai | IPAV | LIF | PRJNA32553 | |
| Linhai | 56609 | LIL | PRJNA217894 | |
| Hardjo | Norma | G436 | PRJNA185511 | |
| Manilae | UP-MMC-NIID LP | LIMLP | PRJNA287300 | |
| Pomona | Pomona | LEP1GSC014 | PRJNA65043 | |
| Pyrogenes | 2006006960 | LEP1GSC019 | PRJNA74039 | |
| L. kirschneri | --- | H1 | LEP1GSC081 | PRJNA74079 |
| --- | H2 | LEP1GSC082 | PRJNA167243 | |
| L. licerasiae | Varillal | VAR 010 | LEP1GSC185 | PRJNA74167 |
| L. santarosai | Shermani | LT 821 | LSS | PRJNA47139 |
| L. weilii | --- | UI 13098 | LEP1GSC108 | PRJNA74123 |
Results
Assembly and Annotation of the L. interrogans Serovar Hardjo Genome
Raw sequences of MiSeq reads generated in this study were deposited in the Sequence Read Archive (SRA) under the accession code SRX1830060. De novo assembly of the MiSeq reads with SPAdes resulted in 101 contigs larger than 500 bp, with an N50 size of 168 kb and a total size of 4.76 Mb. We were able to contiguate 71 of these contigs into two pseudomolecules corresponding to chromosomes I (4.34 Mb) and II (353 kb), by using ABACAS and the genome of L. interrogans serovar Lai as a reference. The remaining 30 contigs could not be incorporated into the contiguated pseudomolecules mainly due to their repetitive nature. This set of unplaced contigs represents a 2% of the total bases in the original de novo assembly and are available to download from our project’s website (http://bioinfo.indicasat.org.pa/lepto.html).
RATT was able to transfer 97% and 98% of the gene models annotated in the genomes of L. interrogans serovar Lai and Copenhageni, respectively, to the contiguated pseudochromosomes. The transferred gene models were manually revised and combined with 4,739 additional ones predicted by BaSYS. Final revision of the annotation included 3,754 predicted protein-coding genes, 3,466 in chromosome I and 288 in chromosome II. We also annotated 86 suspected pseudogenes, identified on the basis of models transferred by RATT with at least one frameshift or internal stop codon, in cases where those artifacts could be confirmed in the majority of the corresponding reads. The annotated genome was deposited in GenBank under BioProject PRJNA296687 with accession numbers CP013147 (chromosome I) and CP013147 (chromosome II). General statistics regarding size and gene content of our annotated genome are roughly similar to those of serovars Lai and Copenhageni (Table 2).
Table 2. Basic statistics of the L. interrogans serovar Hardjo genome sequenced in this study, compared with those of L. interrogans serovar Lai (strain 56601), L. interrogans serovar Copenhageni (strain Fiocruz L1-130) and L. interrogans serovar Hardjo (strain Norma).
| Feature | L. interrogans sv. Lai str. 56601 | L. interrogans sv. Copenhageni str. Fiocruz L1-130 | L. interrogans sv. Hardjo str. Norma | L. interrogans sv. Hardjo str. Hardjoprajitno |
|---|---|---|---|---|
| Chromosomes | 2 | 2 | 2 | 2 |
| Chr I size (Mb) | 4.34 | 4.28 | 4.41 | 4.34 |
| Chr II size (Mb) | 0.36 | 0.35 | 0.36 | 0.35 |
| Total size (Mb) | 4.70 | 4.63 | 4.77 | 4.69 |
| GC-content (%) | 35.02 | 35.04 | 35.02 | 35.00 |
| Protein-coding genes | 3,683 | 3,793 | 4,696 | 3,754 |
| Transfer RNA | 37 | 37 | 37 | 37 |
| Ribosomal RNA | 5 | 5 | 5 | 3 |
Our genome is very similar to that recently published for a field isolate of L. interrogans serovar Hardjo (strain Norma) [9]. Regions that could be aligned on a one-to-one basis between both genomes share a 99.9% identity at the nucleotide level. These regions in turn represent a 98.4% of the strain Norma genome, with the remaining 1.6% comprising approximately 70 kb of sequence that could not be found in our assembly, 67 kb from chromosome I and 2 kb from chromosome II. These sequences roughly match to most of the contigs we were not able to contiguate into our assembled pseudochromosomes due to their repetitive nature. All of these contigs contain matches to sequences associated with repetitive elements commonly reported to be present in Leptospira genomes [4], including prophages, transposons and insertion sequence (IS) elements. Assembly of such elements is difficult, especially when using relatively short reads such as those generated by using the MiSeq platform. We assume that such sequences could be unequivocally incorporated into the strain Norma assembly because of to the larger size of reads generated by the Roche’s 454 platform. To better study sequence variation between the genomes of both strains, we aligned the raw reads from strain Hardjoprajitno to the strain Norma genome. As expected, 99.8% of the reads from strain Hardjoprajitno could be unambiguously mapped to the strain Norma genome, with a uniform coverage along both chromosomes (S1 Fig). We found 262 single nucleotide polymorphism (SNPs) located within predicted protein-coding genes between both genomes (S1 Table). This number of SNPs is relatively low when compared to those found when mapping the strain Hardjoprajitno reads to the reference genomes of L. interrogans serovars Lai and Copenhageni, which were in the order of 15,000 and 16,000, respectively (results not shown).
Except for those resulting from unplaced contigs, we found relatively few structural differences between the corresponding chromosomes from strains Norma and Hardjoprajitno (Fig 1, S2 Table). However, the number of predicted gene models is surprisingly higher in the strain Norma genome when compared to our annotation or to those of serovars Lai and Copenhageni. This unexpected level of over-annotation in the strain Norma genome is likely to be a consequence of using an automatic pipeline, a situation that has been reported for other Leptospira genomes previously sequenced [18]. Although over-annotation complicates the comparison at the level of gene content, we found no differences among those predicted genes and pseudogenes that are shared by both strains.
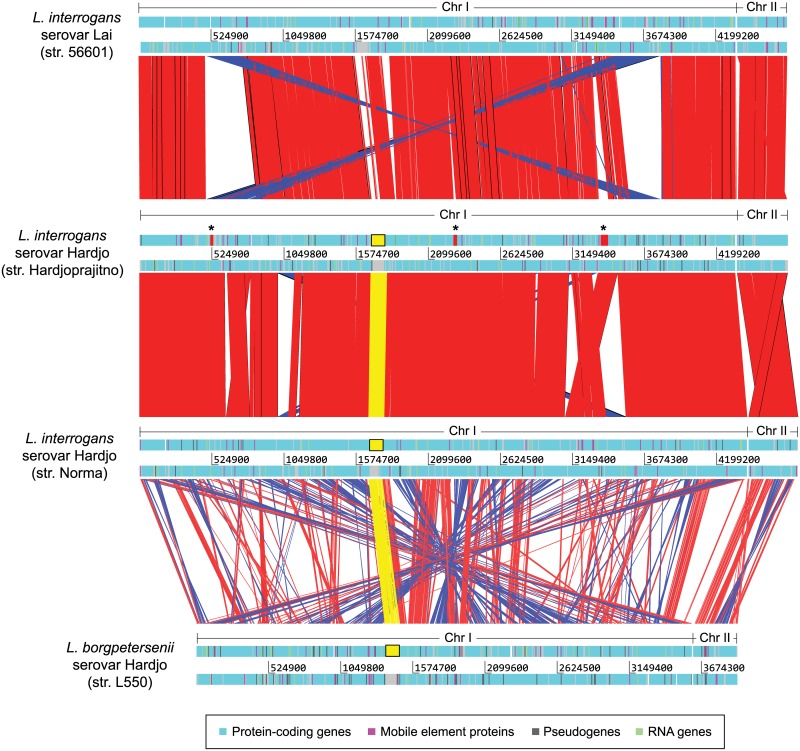
Fig 1. Comparison of the two L. interrogans serovar Hardjo genomes included in this study with those of L. interrogans serovar Lai (strain 56601) and L. borgpetersenii serovar Hardjo (strain L550).
Red bands indicate similar regions and blue bands indicate inversions. Sequences corresponding to the rfb loci typical of the Hardjo serovar are highlighted in yellow. Three regions present in the L. interrogans serovar Hardjo genome but absent from that of the Lai serovar are indicated by asterisks.
Comparative Genomics of the Hardjo Serovar
Comparison of the L. interrogans serovar Hardjo genome with those of serovars Lai and Copenhageni revealed relatively high sequence similarity, except for the region occupied by their corresponding rfb loci (Fig 1, S2 Fig). We noticed two inverted transpositions in the genomes of serovar Hardjo when compared to the genome of serovar Lai, which are located near the ends of the larger inversion previously reported between the genomes of serovar Lai and Copenhageni. In contrast, comparison of the L. interrogans serovar Hardjo genomes with those of L. borgpetersenii serovar Hardjo type A and type B [19] revealed extensive sequence and structural variation, but high conservation in the region of the rfb locus.
We also observed three regions in chromosome I of both serovar Hardjo strains that are not apparently present in the genomes of serovars Lai and Copenhageni (Fig 1, S3 Table). The first of these regions has an approximate length of 12 kb and includes 18 predicted genes (LIH_02395-LIH_02480 in strain Hardjoprajitno). The only gene in the region whose function could be predicted was one located near its beginning, putatively encoding an ISX02-like transposase.
The second region is slightly larger (~17 kb) and encompasses 27 predicted genes (LIH_09760-LIH_09890 in strain Hardjoprajitno). All these genes are encoded in the same strand and are very close together, which suggests that they may be co-transcribed as an operon. Again, functions could not be predicted for most genes in the region, except for two that appear to encode peptidases (LIH_09765 and LIH_09765) and two adjacent ones that appear to encode a PIN domain protein (LIH_09845) and a plasmid replication initiation factor (LIH_09850), respectively. The PIN domain is typically found in ribonucleases that act as the toxin component of type II toxin-antitoxin systems (TAs) [28]. Despite its relatively high sequence similarity to those described in TAs, the PIN domain protein described here does not appear to be adjacent to a gene encoding a putative antitoxin component, suggesting that if active, it may fulfill a different function.
The third region is the largest one, spanning ~37 kb and including 30 predicted genes (LIH_14055-LIH_14190 in strain Hardjoprajitno). Notably, the region contains a cluster of genes predicted to encode components of a transporter from the resistance-nodulation-cell division (RND) superfamily. Although several types of RND transporters have been described, the one we report here seems to be of the tripartite type. This type is the most common one in Gram-negative bacteria and is composed of an inner membrane exporter protein (AcrB), a periplasmic membrane fusion protein (AcrA or MFP) and an outer membrane channel protein (TolC) [29]. The cluster we found in this region includes two genes predicted to encode AcrB transporters (LIH_14115 and LIH_14120), directly adjacent to a gene encoding the AcrA subunit (LIH_14125) and a close gene encoding a TolC-like outer membrane efflux protein (LIH_14150). A similarity search with the sequences of these genes revealed the presence of a similar cluster in the genomes of most pathogenic species of Leptospira other than L. interrogans. For L. interrogans, a similar cluster could only be detected in serovars Bataviae, Canicola and Pyrogenes.
Similarly, genes from the other two previously described regions appear to be present only in some L. interrogans serovars and other close Leptospira species. Genes from the first region appear to be present only in L. interrogans serovar Hardjo, while those from the second region are present in L. santarosai, L. kirschneri and L. weilii, with only a few of them having detectable orthologs in serovars Bataviae and Manilae of L. interrogans.
We were also able to detect orthologs for many of these genes in genomes of unidentified serovars, submitted to Genbank as part of the Leptospira Genomics and Human Health Project sponsored by the J. Craig Venter Institute. Remarkably, almost all of these genes have collinear orthologs in the genome of a strain Brem 329 isolated from a horse in Germany (BioProject PRJNA167229). We also found a 99.9% identity at the nucleotide level between this genome and the serovar Hardjo genome sequenced in this study. The corresponding rfb loci of both genomes are also very similar. These findings suggest that the Brem 329 strain may belong to the Hardjo serovar. Similarly, several loci in these regions have detectable orthologs in the genomes of strains FPW1039 (BioProject PRJNA167242) and FPW2026 (BioProject PRJNA74077). However, these genomes share lower sequence similarity with that of serovar Hardjo, both overall and in the rfb loci, which suggests that the strains may belong to different but probably evolutionarily related serovars.
Phylogenomic Approach to Study the Evolutionary Position of the Hardjo Serovar
Given the differences observed among serovar Hardjo and other L. interrogans serovars, their relationship in the context of the evolutionary history of Leptospira species was explored. We utilized a strategy based on concatenation of sequences from orthologous genes, since commonly used phylogenetic markers such as 16S rRNA do not provide enough phylogenetic signal to properly separate serovars of the same species in phylogenies [30,31]. In this strategy, the phylogenetic signal is increased by including as many genes as possible from the sequenced genomes.
We initially performed an ortholog clustering analysis with the gene models annotated in the L. interrogans serovar Hardjo genome along with those of 23 selected Leptospira specimens. This set of genomes was selected on the basis of the differences we observed and mentioned in the previous section, and it included one strain of L. biflexa, L. licerasiae and L. santarosai, four strains of L. borgpetersenii (two of them from the Hardjo serovar), two strains of L. kirschneri and 12 strains of L. interrogans. Nine of these L. interrogans strains belong to identified serovars, two of serovar Lai and one of serovars Canicola, Bataviae, Pyrogenes, Linhai, Manilae and Pomona, respectively. The remaining three strains included in the study are those with unidentified serovar mentioned in the previous section. This analysis resulted in 8,470 ortholog groups, out of which 1,565 have only one representative member in all the genomes considered (S4 Table).
Although, as an initial approach we planned to include all of these “core” genes in the phylogenomic analysis, it has been shown that concatenating sequences from genes subjected to different evolutionary pressures may lead to erroneous phylogenetic reconstructions [32]. To avoid this, we looked for evidence of natural selection in all ortholog groups and further selected the groups suspected to have a neutral or nearly neutral evolution, that is, those with an overall dN/dS ratio between 0.2–2.0, as suggested by Massey et al. [32]. Of the 1,565 “core” genes, only 235 meet this criterion. Alignment of the concatenated amino acid sequences of those genes with MAFFT and further refinement with Gblock yielded an alignment of ~53,500 sites. A maximum likelihood tree based on this alignment was built by using PhyML and the LG+G+I model (Fig 2).
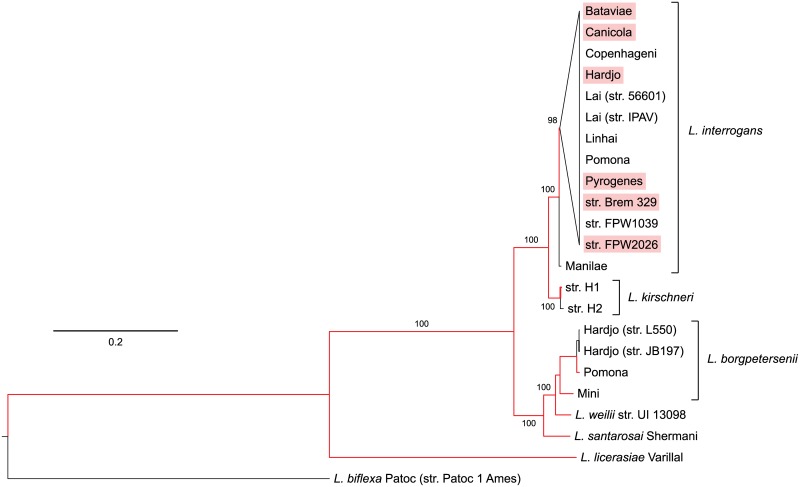
Fig 2. Maximum likelihood tree based on concatenated protein sequences from 23 Leptospira genomes.
The saprophytic non-pathogenic L. biflexa was used to root the tree. Branches highlighted in red are those leading to taxa whose genomes contain the additional cluster of RND transporter components described for serovar Hardjo (see main text). As there was not enough phylogenetic signal to separate individual L. interrogans serovars, those having this cluster are shaded in pink. Bootstrap values are shown for branches separating different species.
The topology of this tree agrees with the widely accepted phylogeny for the Leptospira genus [4]. However, individual L. interrogans serovars could not be properly separated by using only this set of genes, as there is still not enough phylogenetic signal. In an attempt to increase the signal, we repeated this analysis only on L. interrogans serovars, where the number of orthologs matching the selection criterion increased to 512. The new maximum likelihood tree was built with an alignment of ~121,000 concatenated sites (Fig 3).
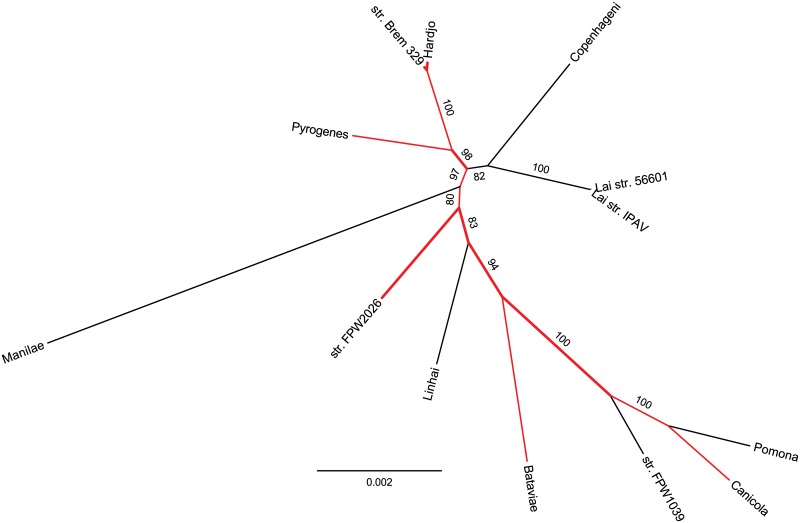
Fig 3. Unrooted tree of selected L. interrogans serovars.
This maximum likelihood tree was built following the same methodology described for the tree in Fig 2, but considering only the L. interrogans serovars. Branches highlighted in red are those corresponding to serovars whose genomes contain the additional cluster of RND transporter components. Bootstrap values are shown for branches separating different serovars.
Both trees show that Manilae is likely to be the serovar closest to the ancestral position within the L. interrogans clade. Suggestion of the relatedness between L. interrogans serovar Hardjo and the Brem 329 strain of unidentified serovar is supported by their position in the tree, which is similar to that observed for strains 56601 and IPAV, both of which belong to the same Lai serovar. The topology also shows that serovar Pyrogenes seems to be more closely related to Hardjo. Strain FPW2026 is positioned between serovars Manilae and Linhai, and strain FPW1039 is closer to serovars Pomona and Canicola.
Discussion
Whole genome sequencing allowed us to study the evolutionary relationship of L. interrogans serovar Hardjo with different serovars of L. interrogans and other species of the Leptospira genus. The suggested evolutionary position of serovar Hardjo supports the hypothesis that the convergence of the rfb loci from L. interrogans serovar Hardjo and L. borgpetersenii serovar Hardjo are likely to be the consequence of an ancestral lateral gene transfer event, as both are grouped in separate clades corresponding to their respective species.
Comparison of the genomes of L. interrogans serovar Hardjo strain Hardjoprajitno and L. interrogans serovar Hardjo strain Norma revealed relatively high sequence conservation, despite the fact that these strains have very different origins, the first one is a laboratory-adapted strain sampled from a male patient from Indonesia many years ago, while the second one is a field isolate recently sampled from infected cattle in Brazil. We found a few structural rearrangements between the corresponding chromosomes of both strains, which does not appear to affect protein-coding genes, except for some of them predicted to code for mobile element proteins. In fact, most of these rearrangements appear to be flanked by genes predicted to encode transposases, suggesting that the corresponding mobile elements may have played a role in their transposition. It is important to mention, however, that these rearrangements may ultimately be the result of assembly errors, as sequencing was in both cases performed by using next-generation sequencing techniques and no PCR or other type of experimental validation was conducted.
Comparisons of the sequenced L. interrogans serovar Hardjo genomes and those of other Leptospira species also allowed us to identify three relatively large regions present in this serovar, with a limited distribution among other Leptospira genomes. Among these regions, the one containing the gene encoding a PIN domain protein may be reminiscent of an integrated plasmid, as such proteins and their associated TA operons are commonly found in plasmids and are thought to play a role in plasmid stability [28]. Furthermore, the region also contains a gene putatively encoding a plasmid replication initiation factor. The loss of the region from chromosome I of several other L. interrogans serovars may be explained by a large deletion event or by its re-excision from the chromosome into a plasmid, a phenomenon that has been previously described in the species [33].
Another region was found to contain an additional cluster of genes putatively encoding components of a tripartite RND transporter system. Like most Gram-negative bacteria, Leptospira has several genes encoding transporters of the RND superfamily. For example, of all the genes encoding putative membrane transporters in L. interrogans serovar Copenhageni, 11% appear to be related to the RND superfamily [14]. In the genome of L. interrogans serovar Lai there are at least 14 loci predicted to encode the inner membrane exporter protein AcrB, although only two of these loci have a structure similar to the additional cluster we described for serovar Hardjo. This particular type is composed of one or two acrB genes, which appear to form an operon with an acrA gene and a nearby gene encoding a TolC-like protein. Phylogenetic analysis shows that the genes for this additional cluster may have been acquired by an ancestor close to L. licerasiae and subsequently lost in some lineages (Fig 2). A phylogenetic tree built with the sequences of the acrA paralogs confirmed the presence of three clearly different lineages for this gene among Leptospira genomes (S3 Fig). It has been shown that RND transporters and especially their increased number of copies are actively involved in the development of drug resistance [34]. Although this analysis is preliminary, the additional copy of this cluster may represent an adaptive advantage in those lineages that have maintained the copy during their evolution.
Although functions could not be predicted for the vast majority of genes in these regions, it is likely that many of them are involved in differences in pathogenicity reported for L. interrogans serovar Hardjo and should be the target of future experimental research.
Supporting Information
Raw read depth plotted in light blue was averaged over a window of 500 bp. Vertical red bars below the coverage plot indicate SNPs located within predicted protein-coding genes.
(PDF)
Red bands indicate similar regions and blue bands indicate inversions. Sequences corresponding to the rfb loci typical of the Hardjo serovar are highlighted in yellow.
(PDF)
This tree was built with the amino acid sequences of the acrA genes from the RND transporter gene clusters present in the Leptospira genomes used in this study. The tree was built with PhyML 3.0 using the LG model and 500 bootstrap replicates. Bootstrap values are indicated for branches clustering the genes from different species. Genes from the strain sequenced in this study are indicated in bold.
(PDF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank UGA Georgia Genomics Facility for the sequencing; Dr. Walt Lorenz from the UGA Quantitative Biology Consulting Group for the initial assembly and transfer of data; Bacteriology technical staff at the UGA Tifton Veterinary Diagnostic Lab for laboratory support; and Drs. Ricardo Lleonart and Gabrielle Britton for critical review of the manuscript.
Data Availability
The sequenced genome was submitted to GenBank under BioProject PRJNA296687 with accession numbers CP013147 (chromosome I) and CP013147 (chromosome II). Raw sequences of MiSeq reads were deposited in the Sequence Read Archive (SRA) under the accession code SRX1830060.
Funding Statement
Funded by University of Georgia, Provost's Summer Research Support (http://research.uga.edu/) and Ross University School of Veterinary Medicine Intramural Grant (http://veterinary.rossu.edu/), SR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Levett PN. Leptospirosis. Clin Microbiol. 2001;14: 296–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner DJ, Kaufmann AF, Sulzer KR, Steigerwalt AG, Rogers FC, Weyant RS. Further determination of DNA relatedness between serogroups and serovars in the family Leptospiraceae with a proposal for Leptospira alexanderi sp. nov. and four new Leptospira genomospecies. Int J Syst Bacteriol. 1999;49: 839–858. [DOI] [PubMed] [Google Scholar]
- 3.Cerqueira GM, Picardeau M. A century of Leptospira strain typing. Infect Genet Evol. 2009;9: 760–8. 10.1016/j.meegid.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 4.Lehmann J, Matthias M, Vinetz J, Fouts D. Leptospiral pathogenomics. Pathogens. 2014;3: 280–308. 10.3390/pathogens3020280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis WA. Animal leptospirosis. Curr Top Microbiol Immunol. 2015;387: 99–137. 10.1007/978-3-662-45059-8_6 [DOI] [PubMed] [Google Scholar]
- 6.De La Peña-Moctezuma A, Bulach DM, Kalambaheti T, Adler B. Comparative analysis of the LPS biosynthetic loci of the genetic subtypes of serovar Hardjo: Leptospira interrogans subtype Hardjoprajitno and Leptospira borgpetersenii subtype Hardjobovis. FEMS Microbiol Lett. 1999;177: 319–326. [DOI] [PubMed] [Google Scholar]
- 7.Ellis WA. Leptospirosis as a cause of reproductive failure. Vet Clin North Am Food Anim Pract. 1994;10: 463–78. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi N, Yasutomi I. Prevalence of leptospirosis in farm animals. Jpn J Vet Res. 2012;60: S55–S58. [PubMed] [Google Scholar]
- 9.Cosate MR V, Soares SC, Mendes TA, Raittz RT, Moreira EC, Leite R, et al. Whole-genome sequence of Leptospira interrogans serovar Hardjo subtype Hardjoprajitno strain Norma, isolated from cattle in a leptospirosis outbreak in Brazil. Genome Announc. 2015;3: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30: 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19: 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assefa S, Keane TM, Otto TD, Newbold C, Berriman M. ABACAS: algorithm-based automatic contiguation of assembled sequences. Bioinformatics. 2009;25: 1968–9. 10.1093/bioinformatics/btp347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, Xu H, et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003;422: 888–893. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento ALTO, Ko AI, Martins EAL, Monteiro-Vitorello CB, Ho PL, Haake DA, et al. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol. 2004;186: 2164–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto TD, Dillon GP, Degrave WS, Berriman M. RATT: Rapid Annotation Transfer Tool. Nucleic Acids Res. 2011;39: e57 10.1093/nar/gkq1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Domselaar GH, Stothard P, Shrivastava S, Cruz JA, Guo AC, Dong X, et al. BASys: A web server for automated bacterial genome annotation. Nucleic Acids Res. 2005;33: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carver T, Berriman M, Tivey A, Patel C, Böhme U, Barrell BG, et al. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24: 2672–6. 10.1093/bioinformatics/btn529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ussery DW, Hallin PF. Genome update: annotation quality in sequenced microbial genomes. Microbiology. 2004;150: 2015–2017. [DOI] [PubMed] [Google Scholar]
- 19.Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, Cullen PA, et al. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci U S A. 2006;103: 14560–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26: 589–95. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25: 2078–9. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Stoeckert CJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13: 2178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24: 1586–1591. [DOI] [PubMed] [Google Scholar]
- 24.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30: 772–80. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56: 564–577. [DOI] [PubMed] [Google Scholar]
- 26.Guindon S, Dufayard JFF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59: 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 27.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27: 1164–1165. 10.1093/bioinformatics/btr088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arcus VL, Mckenzie JL, Robson J, Cook GM. The PIN-domain ribonucleases and the prokaryotic VapBC toxin-antitoxin array. Protein Eng Des Sel. 2011;24: 33–40. 10.1093/protein/gzq081 [DOI] [PubMed] [Google Scholar]
- 29.Husain F, Humbard M, Misra R. Interaction between the TolC and AcrA proteins of a multidrug efflux system of Escherichia coli. 2004;186: 8533–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzpatrick DA, Logue ME, Stajich JE, Butler G. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol. 2006;6: 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan JL, Khang TF, Ngeow YF, Choo SW. A phylogenomic approach to bacterial subspecies classification: proof of concept in Mycobacterium abscessus. BMC Genomics. 2013;14: 879 10.1186/1471-2164-14-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massey SE, Churbanov A, Rastogi S, Liberles DA. Characterizing positive and negative selection and their phylogenetic effects. Gene. 2008;418: 22–6. 10.1016/j.gene.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 33.Bourhy P, Salaun L, Lajus A, Medigue C, Boursaux-Eude C, Picardeau M. A genomic island of the pathogen Leptospira interrogans serovar Lai can excise from its chromosome. Infect Immun. 2007;75: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria. Drugs. 2009;69: 1555–1623. 10.2165/11317030-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw read depth plotted in light blue was averaged over a window of 500 bp. Vertical red bars below the coverage plot indicate SNPs located within predicted protein-coding genes.
(PDF)
Red bands indicate similar regions and blue bands indicate inversions. Sequences corresponding to the rfb loci typical of the Hardjo serovar are highlighted in yellow.
(PDF)
This tree was built with the amino acid sequences of the acrA genes from the RND transporter gene clusters present in the Leptospira genomes used in this study. The tree was built with PhyML 3.0 using the LG model and 500 bootstrap replicates. Bootstrap values are indicated for branches clustering the genes from different species. Genes from the strain sequenced in this study are indicated in bold.
(PDF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The sequenced genome was submitted to GenBank under BioProject PRJNA296687 with accession numbers CP013147 (chromosome I) and CP013147 (chromosome II). Raw sequences of MiSeq reads were deposited in the Sequence Read Archive (SRA) under the accession code SRX1830060.