Abstract
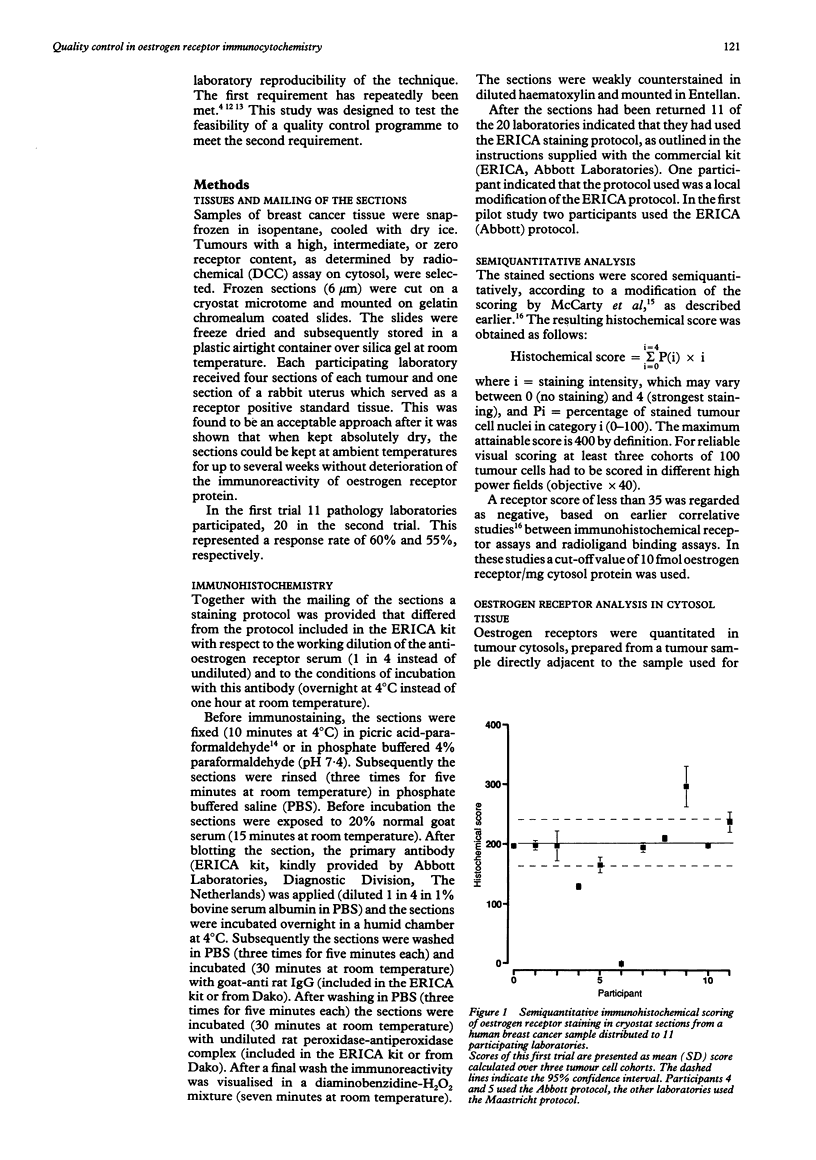
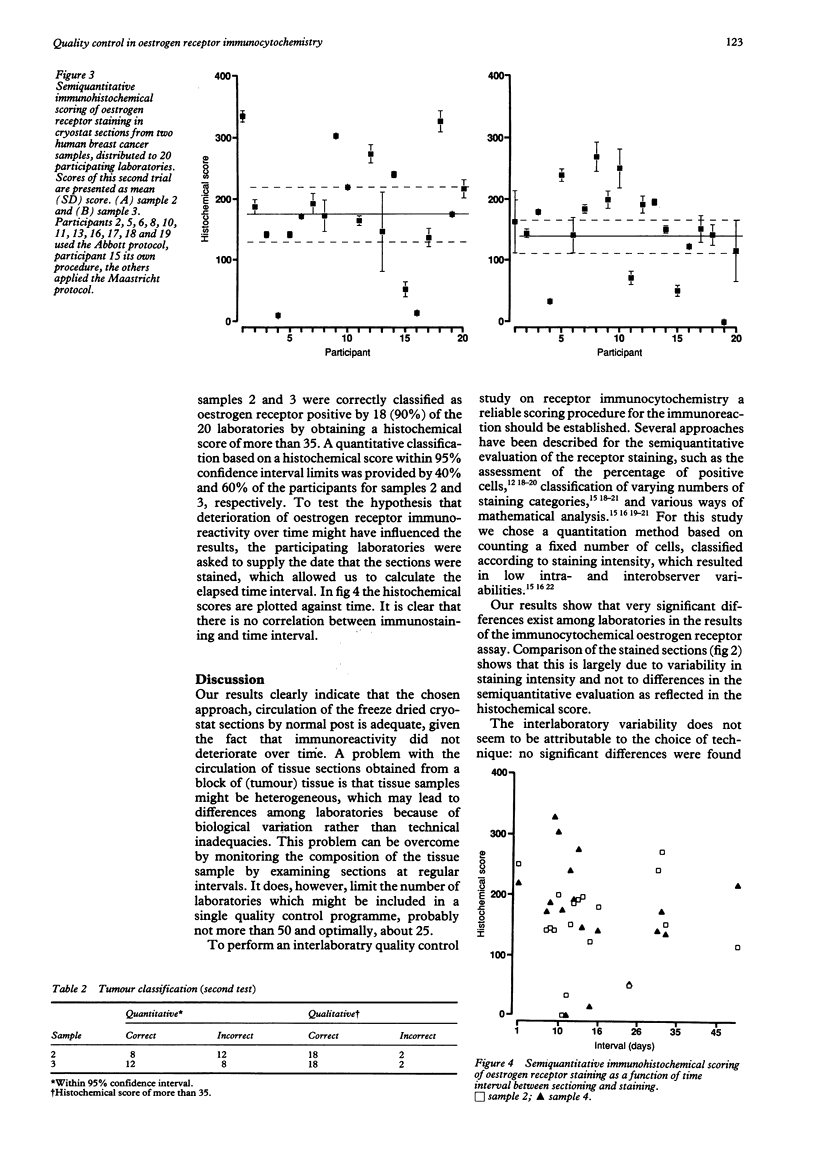
AIMS: To evaluate the feasibility of an interlaboratory quality control programme in immunohistochemistry. METHODS: Several pathology laboratories were asked to carry out immunohistochemical oestrogen receptor staining on a set of freeze dried cryostat sections of breast cancer tissue. The sections and protocols for staining and semi-quantitative scoring were mailed to the participating laboratories in two trials. The oestrogen receptor content of the breast cancer samples was determined by radioligand binding assay on the tumour cytosol. RESULTS: In the first trial 11 laboratories (response rate 60%) participated. Eight (73%) of the participants scored within a 95% confidence interval and all but one correctly classified the tumour as receptor positive. In the second trial all 20 participating laboratories (response rate 55%) correctly scored one tumour sample as negative and 18 of them (90% of respondents) correctly classified the two other tumour samples as receptor positive. In a quantitative evaluation a histochemical score within 95% confidence interval limits was provided by eight (40%) and 12 (60%) of the participants. CONCLUSIONS: Semiquantitative scoring of immunocytochemical staining is valuable for performing correlative inter-laboratory studies, although this scoring protocol may not be required for diagnosis or prognosis. Significant inter-laboratory variability exists, leading to qualitatively correct receptor classification in 100% of receptor negative and 80% of receptor positive cases, and quantitative agreement in only about half of the cases. The perceived variability is not caused by systematic differences in the choice of the immunocytochemical technique, or the mailing of freeze dried sections. Quality control programmes should be included in the standard procedures of each diagnostic immunohistochemistry laboratory.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J., Bentzen S. M., Poulsen H. S. Relationship between radioligand binding assay, immunoenzyme assay and immunohistochemical assay for estrogen receptors in human breast cancer and association with tumor differentiation. Eur J Cancer Clin Oncol. 1988 Mar;24(3):377–384. doi: 10.1016/s0277-5379(98)90006-2. [DOI] [PubMed] [Google Scholar]
- De Goeij T. F., Bosman F. T., Berns E. M. Determination of steroid hormone-dependency of tumours utilizing tissue sections. Survey of histochemical techniques and their application in surgical pathology. J Pathol. 1986 Jul;149(3):163–172. doi: 10.1002/path.1711490302. [DOI] [PubMed] [Google Scholar]
- DeSombre E. R., Thorpe S. M., Rose C., Blough R. R., Andersen K. W., Rasmussen B. B., King W. J. Prognostic usefulness of estrogen receptor immunocytochemical assays for human breast cancer. Cancer Res. 1986 Aug;46(8 Suppl):4256s–4264s. [PubMed] [Google Scholar]
- Elias J. M., Gown A. M., Nakamura R. M., Wilbur D. C., Herman G. E., Jaffe E. S., Battifora H., Brigati D. J. Quality control in immunohistochemistry. Report of a workshop sponsored by the Biological Stain Commission. Am J Clin Pathol. 1989 Dec;92(6):836–843. doi: 10.1093/ajcp/92.6.836. [DOI] [PubMed] [Google Scholar]
- Giri D. D., Dangerfield V. J., Lonsdale R., Rogers K., Underwood J. C. Immunohistology of oestrogen receptor and D5 antigen in breast cancer: correlation with oestrogen receptor content of adjacent cryostat sections assayed by radioligand binding and enzyme immunoassay. J Clin Pathol. 1987 Jul;40(7):734–740. doi: 10.1136/jcp.40.7.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoie J., Stenwig A. E., Kullmann G., Lindegaard M. Distant metastases in papillary thyroid cancer. A review of 91 patients. Cancer. 1988 Jan 1;61(1):1–6. doi: 10.1002/1097-0142(19880101)61:1<1::aid-cncr2820610102>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Howat J. M., Harris M., Swindell R., Barnes D. M. The effect of oestrogen and progesterone receptors on recurrence and survival in patients with carcinoma of the breast. Br J Cancer. 1985 Feb;51(2):263–270. doi: 10.1038/bjc.1985.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C., Zava D. T., Eppenburger U., Kiser A., Sebek S., Dowdle E., Krozowski Z., Bennett R. C., Funder J., Holdaway I. M. Reliability of steroid hormone receptor assays: an international study. Eur J Cancer Clin Oncol. 1983 Mar;19(3):357–363. doi: 10.1016/0277-5379(83)90133-5. [DOI] [PubMed] [Google Scholar]
- King W. J., DeSombre E. R., Jensen E. V., Greene G. L. Comparison of immunocytochemical and steroid-binding assays for estrogen receptor in human breast tumors. Cancer Res. 1985 Jan;45(1):293–304. [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Koenders A., Thorpe S. M. Standardization of steroid receptor assays in human breast cancer--IV. Long-term within- and between-laboratory variation of estrogen and progesterone receptor assays. Eur J Cancer Clin Oncol. 1986 Aug;22(8):945–952. doi: 10.1016/0277-5379(86)90061-1. [DOI] [PubMed] [Google Scholar]
- McCarty K. S., Jr, Miller L. S., Cox E. B., Konrath J., McCarty K. S., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985 Aug;109(8):716–721. [PubMed] [Google Scholar]
- McCarty K. S., Jr, Reintgen D. S., Seigler H. F., McCarty K. S., Sr Cytochemistry of sex steroid receptors: a critique. Breast Cancer Res Treat. 1981;1(4):315–325. doi: 10.1007/BF01806747. [DOI] [PubMed] [Google Scholar]
- McGuire W. L. Prognostic factors in primary breast cancer. Cancer Surv. 1986;5(3):527–536. [PubMed] [Google Scholar]
- Remmele W., Hildebrand U., Hienz H. A., Klein P. J., Vierbuchen M., Behnken L. J., Heicke B., Scheidt E. Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A Pathol Anat Histopathol. 1986;409(2):127–147. doi: 10.1007/BF00708323. [DOI] [PubMed] [Google Scholar]
- Scheres H. M., De Goeij A. F., Rousch M. J., Hondius G. G., Willebrand D. D., Gijzen A. H., Bosman F. T. Quantification of oestrogen receptors in breast cancer: radiochemical assay on cytosols and cryostat sections compared with semiquantitative immunocytochemical analysis. J Clin Pathol. 1988 Jun;41(6):623–632. doi: 10.1136/jcp.41.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders M. P., De Goeij A. F., Koudstaal J., Rousch M. J., Scheres H. M., Stoot J. E., Bosman F. T. Is immunohistochemical analysis of oestrogen and progesterone receptors in endometrial carcinoma superior to the radioligand binding assay? J Pathol. 1990 Jun;161(2):129–135. doi: 10.1002/path.1711610207. [DOI] [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Underwood J. C. Oestrogen receptors in human breast cancer: review of histopathological correlations and critique of histochemical methods. Diagn Histopathol. 1983 Jan-Mar;6(1):1–22. [PubMed] [Google Scholar]



