Abstract
Antibody-mediated rejection has emerged as the leading cause of late graft loss in kidney transplant recipients, and inhibition of donor-specific antibody production should lead to improved transplant outcomes. The fusion protein CTLA4-Ig blocks T-cell activation and consequently inhibits T-dependent B cell antibody production, and the current paradigm is that CTLA4-Ig is effective on naïve T cells and less so on activated or memory T cells. In this study, we used a mouse model of allo-sensitization to investigate the efficacy of continuous CTLA4-Ig treatment, initiated 7 or 14 days post-sensitization, at inhibiting ongoing allo-specific B cell responses. Delayed treatment with CTLA4-Ig collapsed the allo-specific germinal center (GC) B cell response and inhibited alloantibody production. Using adoptively transferred TCR-transgenic T cells and a novel approach to track endogenous graft-specific T cells, we demonstrate that delayed CTLA4-Ig minimally inhibited graft-specific CD4+ and T follicular helper (Tfh) responses. Remarkably, delaying CTLA4-Ig until day 6 post-transplantation in a fully-mismatched heart transplant model inhibited alloantibody production and prevented acute rejection, while transferred hyperimmune sera reversed the effects delayed CTLA4-Ig. Collectively, our studies revealed the unexpected efficacy of CTLA4-Ig at inhibiting ongoing B cell responses even when the graft-specific T cell response has been robustly established.
Introduction
Successful solid organ transplantation is one of the major medical developments of the past century. Despite improved prevention and reversal of acute rejection through the use of immunosuppressive drugs[1-5], chronic rejection of allografts remains a major problem and the 10-year allograft survival rate for kidney grafts in the US is only 34-46%[6]. Donor-specific alloantibodies (DSA) play an important role in the development of chronic rejection, and patients who develop DSA exhibit a higher rate of graft failure five years post-transplantation than patients who do not[7-9]. Furthermore, T cell-mediated rejection (TCMR) with DSA or C4d deposition has a worse prognosis than pure TCMR [10, 11], suggesting that therapies that can control DSA production during acute rejection may be able to extend the survival of allografts in the clinic.
Current attempts to control chronic alloantibody-mediated rejection have relied on drugs such as calcineurin inhibitors and anti-proliferative agents that prevent T cell activation and expansion, and indirectly, the activation of B cells and production of T-dependent alloantibodies[1-3, 12]. In the case of presensitized recipients where memory B cells and plasma cells contribute to the production of DSA post-transplantation, B cell-directed therapies are being tested, including the use of rituximab, bortezomib, IVIG and plasmapheresis[13-17]. However, such approaches appear to be only partially or transiently effective[18, 19].
Belatacept, a high affinity CTLA4-Ig that blocks CD28-CD80/CD86 interactions, has been approved for the prevention of acute rejection in adult kidney transplant recipients[20, 21]. CTLA4-Ig is a fusion protein that inhibits the activation of naïve T-cells by preventing CD28 costimulation on T cells via binding to CD80 and CD86[22]. In addition, the binding of CTLA4-Ig to CD80 and CD86 has been reported to induce reverse signaling and the production of indoleamine 2,3-dioxygenase (IDO), which catalyses the degradation of tryptophan and creates a local inhibitory environment for T cells[23, 24]. This reverse signaling also induces in antigen presenting cells the nuclear translocation of the transcription factor Foxo3[25], which inhibits the production of IL-6 and tumor necrosis factor-alpha while increasing the secretion of suppressive cytokines such as IL-10[26]. Thus, the inhibition of B cell responses by CTLA4-Ig is presumed to be due to the inhibition of T cell activation, thereby denying B cells from receiving T cell help.
In this study we investigated the ability of the clinically approved human CTLA4-Ig, abatacept, to halt ongoing B cell responses in mice[27]. We build on our previous demonstration that delayed treatment with CTLA4-Ig, starting from seven days post-sensitization when B cell germinal center (GC) responses had been fully established, was able to halt the production of alloantibodies[28]. However, the mechanisms by which CTLA4-Ig shut down an established antigen-specific B-cell response had not been determined. We report here that delayed CTLA4-Ig is remarkably effective at reversing established GC B cell allospecific responses and resolving ongoing acute rejection.
Materials and Methods
Mice
Female C57BL/6 (B6, H-2b), BALB/c (B/c, H-2d) and TCRαβ−/− C57BL/6 mice, age 8–9 weeks, were purchased from The Jackson (Bar Harbor, ME) or Harlan Laboratories (Madison, WI). TCR75 mice [29] were obtained from Dr. R. P. Bucy (University of Birmingham, AL). 2W-OVA transgenic C57BL/6 mice [30] were bred with BALB/c mice to obtain 2W-OVA F1 mice.
Adoptive transfer of T cells
CD45.1+ CD44lo Vβ8.3+ CD4+ T cells were purified with CD4+ T cell negative selection beads (Miltenyi Biotec, Bergisch Gladbach, Germany) from total spleen and lymph node cells of TCR75 mice. CD4+ purity exceeded 95%, and CD44loVB8.3+ purity exceeded 80%. We back-calculated CD45.1+ CD44lo Vβ8.3+ CD4+ T cell yields of 500 cells and adoptively transferred them into C57BL/6 recipients 1 day prior to sensitization with BALB/c splenocytes.
Sensitization
BALB/c or 2W-OVA F1 mice were sacrificed and their spleens were harvested, processed into a single cell suspension, and resuspended at a concentration of 100×106 cells/mL. 5×106 cells were injected subcutaneously near each of 4 limb joints. For generating hyperimmune serum, the mice were challenged again on day 28, sacrificed on day 35, serum harvested from 10 mice and pooled.
Transplantation
Heterotopic heart transplantations were performed as previously described [31] by grafting BALB/c hearts from 6-8 week old donors onto the aorta and inferior vena cava in the peritoneal cavity of 8-12 week old C57BL/6 recipients. 500 μg of CTLA4-Ig (abatacept; Bristol-Myers Squibb) per mouse, intraperitoneally, starting on day −2, 0 and 2 or on days 6, 8, 10, and then twice per week until the end of the experiment.
Flow Cytometry
Splenocytes only or a mixture of splenocytes and lymphocytes from axial, brachial, and inguinal lymph nodes were stained for analysis, controlling for the same number of cells per sample tube (typically 107 cells). Samples were stained for flow cytometry using AquaFluor LiveDead solution to exclude dead cells. A dump channel was used to exclude unwanted cells, consisting of antibodies for DX5, CD11c, F4/80, Gr-1, Ter119, and either CD19, CD4, or CD8, depending on the stain. Additional antibodies for CD90.2, CD4, CD8, CD44, CD62L, VB8.3, CD45.1, CXCR5, FR4, PD-1, Foxp3, IFNγ, B220, CD138, IgD, Fas, and GL7 were used to stain T and B cells. B cells were initially stained for 30 minutes on ice with H-2Kd-PE and H-2Kd-APC tetramers bearing the irrelevant malarial peptide (SYIPSAEKI). 2W(EAWGALANWAVDSA):I-Ab tetramer (NIH) and OVA (SIINFEKL):H-2Kb-PE pentamer (Proimmune) incubation was performed at room temperature for 30 minutes prior to additional antibodies.
Donor Specific Antibody Assay
Fresh BALB/c splenocytes were harvested and their red blood cells were lysed and then 1×106 cells were stained with 1μL of serum from sensitized, transplanted, or naïve recipients. After two washes, cells were then stained with anti-B220, anti-IgM, and anti-IgG fluorescent antibodies. B220+ B cells were excluded due to their expression of IgM+, IgG+ and FcγR.
Stimulation for Cytokine Staining
Splenocyte stimulators were from TCRαβ−/− C57BL/6 mice and B/6XB/c F1 mice were treated with red blood cell lysing buffer (Sigma, St. Louis, MO). F1 splenocytes were depleted of T cells by pre-incubation with anti-CD90 and 2 consecutive sessions with rabbit complement at 37°C. 60×106 TCRαβ−/− or T cell-depleted splenocytes were then incubated overnight with 5 μg/mL LPS. The following day, 1×106 responder cells were incubated with 5×105 stimulators (200μL per well) in a 37°C incubator. 18h later, 1 μg of monensin was added and incubation continued an additional 6h. Cells were then collected for intracellular staining, which was performed in an ice water bath.
Histology
Hearts were cut longitudinally, fixed in 10% formalin for 24 hours, and embedded in paraffin. Sections were then cut and stained by Hematoxylin and Eosin or anti-C4d antibodies followed by anti-rat HRP. Slides were then scanned using the CRI Pannoramic Whole Slide Scanner (Perkin Elmer, Melville, NY).
Statistics
Statistical analysis was performed using GraphPad Prism (La Jolla, CA). Graft survival curves significance was assessed using a Mantel-Cox log rank test. Statistically significance differences between alloantibody curves over time were assessed using a two-way ANOVA test; between percentages of cell types by unpaired two-tailed t tests; and between total numbers of cell types by two-tailed Mann-Whitney ranked tests.
Results
Delayed treatment with CTLA4-Ig halted ongoing alloantibody production by collapsing the B cell germinal center response
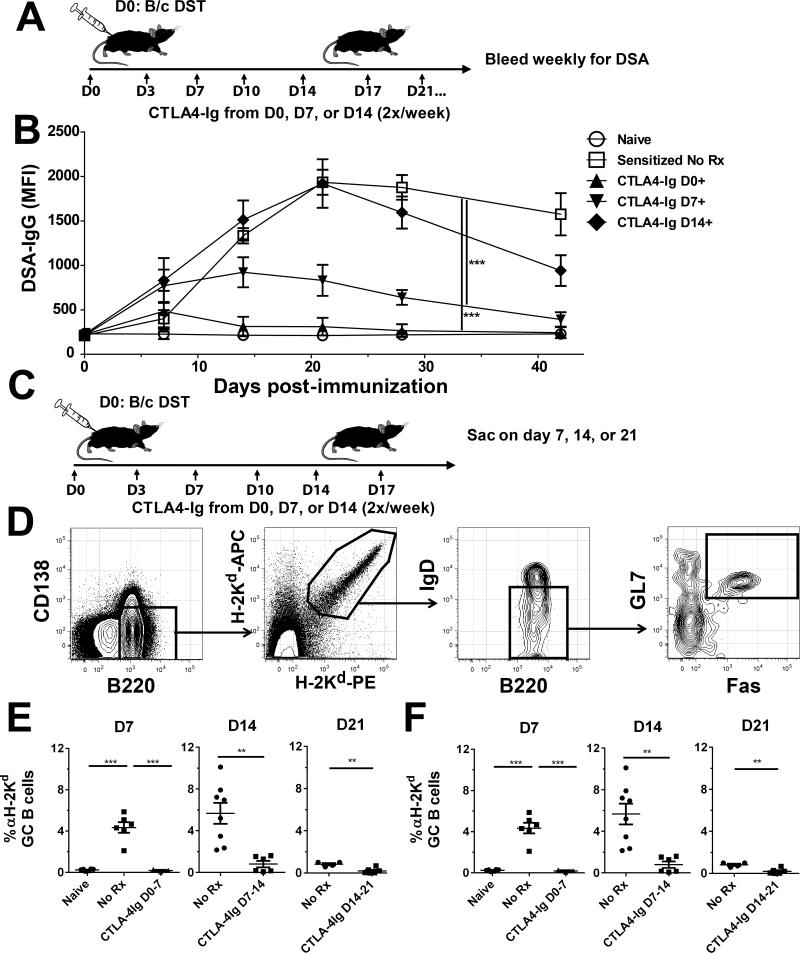
We have previously shown that alloantibody production triggered by allosensitization via donor splenocyte sensitization could be halted by delayed costimulatory blockade treatment that was started on day 7 post-sensitization, and that both the experimental agent anti-CD154 and clinically approved CTLA4-Ig, were equally efficacious[28]. However, it was unclear whether costimulatory blockade with CTLA4-Ig could be effective if treatment were to begin later than 7 days post-sensitization. We therefore sensitized C57BL/6 mice with 20×106 BALB/c splenocytes by subcutaneous injection and administered CTLA4-Ig i.p., biweekly, starting from day 0, 7, or 14 post-sensitization (Fig 1A). As previously reported, treatment beginning on day 0 nearly completely prevented allo-IgG production, while treatment from day 7 prevented further increase in alloantibody titers and promoted a downward trend towards baseline. In contrast, CTLA4-Ig treatment beginning on day 14 did not prevent the rise in circulating alloantibody titers as compared to untreated controls (p=0.92 by 2-way ANOVA) (Fig 1B). These results indicate that alloantibody production could be aborted by CTLA4-Ig only when treatment was initiated by day 7 post-sensitization.
Figure 1. CTLA4-Ig treatment delayed until day 14 post-immunization collapsed established germinal center B cell responses.
A, Diagram of experimental protocol. C57BL/6 mice were given 20×106 BALB/c splenocytes injected subcutaneously at each limb at day 0, followed by CTLA4-Ig given twice weekly starting on day 0, 7, or 14. B, Donor-specific IgG antibodies (DSA-IgG). Serum samples were incubated with BALB/c splenocytes, then stained with rabbit anti-mouse IgG and anti-B220 to exclude B cells. Data from 2 independent experiments (N=8/group) are presented as the mean fluorescence intensity (MFI) on non-B cells. C, Diagram of treatment method for GC analysis. C57BL/6 mice sensitized with BALB/c splenocytes on day 0, were treated with CTLA4-Ig from day 0, 7, or 14 and sacrificed after 7 days of treatment. D, Example gating strategy for GC B cells, showing gating on B220+ B cells, H-2Kd-binding, IgDlo activated B cells, and Fas+GL7+ GC B cells. E&F, Quantification of GC H-2Kd-binding B cells. Pooled spleens and axial, brachial, and inguinal LNs were harvested on day 7 post-treatment in mice given CTLA4-Ig at day 0, 7, or 14 post-sensitization along with untreated controls. The percentage of activated IgDlo Fas+GL7+ GC B cells among the H-2Kd-binding population and their total number of are shown. Data pooled from 3 independent experiments (N=5-8/group) are presented; and statistically significant differences are indicated (*p<0.05, **p<0.01, ***p<0.005).
We next investigated why treatment with CTLA4-Ig from day 14 post-sensitization failed to prevent or reverse alloantibody production. Since we had previously reported that CTLA4-Ig rapidly collapsed donor-specific GC B cell (B220+IgDloFas+GL7+), we tested the effect of CTLA4-Ig on alloreactive GC B cells when treatment was delayed till day 14 post-sensitization. BALB/c splenocyte-sensitized mice were treated with CTLA4-Ig beginning on days 0, 7, or 14 post-sensitization and sacrificed after 7 days of treatment (Fig 1C). We utilized a previously validated dual-fluorochrome, single MHC Class I tetramer method to identify allo-MHC-reactive (H-2Kd-reactive) B cells (Fig 1D). Surprisingly, treatment with CTLA4-Ig significantly reduced both the percentage and number of H-2Kd-reactive B220+IgDloFas+GL7+ GC B cells regardless of when CTLA4-Ig treatment was initiated (Fig 1 E-F). However, we noted that in the untreated group, the percentage and total number of H-2Kd-reactive GC B cells peaked on day 7-14 and were dramatically reduced by day 21 post-sensitization, with only 1% of B cells existing as GC B cells by day 21, as compared to approximately 5% at either on day 7 or 14 (p<0.01) (Fig 1E-F). We inferred from these observations that while CTLA4-Ig was capable of collapsing B cell GC responses regardless of the time of intervention, the majority of alloreactive GC B cells fully differentiated into antibody secreting cells (ASC) by day 14.
Delayed CTLA4-Ig does not prevent the accumulation of adoptively transferred, alloreactive TCR75 T cells
The surprising effectiveness of CTLA4-Ig at collapsing ongoing GC responses raised the possibility that it was inhibiting T follicular helper (Tfh) cell generation, which would then deny GC B cells the necessary survival and expansion signals. To test this possibility, we used a TCR-transgenic CD4+ T cell population, TCR75, which is specific for the H-2Kd54-68 peptide presented by I-Ab[29]. The use of TCR75 cells provides the additional benefit of tracking a T cell population that is capable of engaging in cognate interactions with the H-2Kd-reactive B cells tracked in Figure 1.
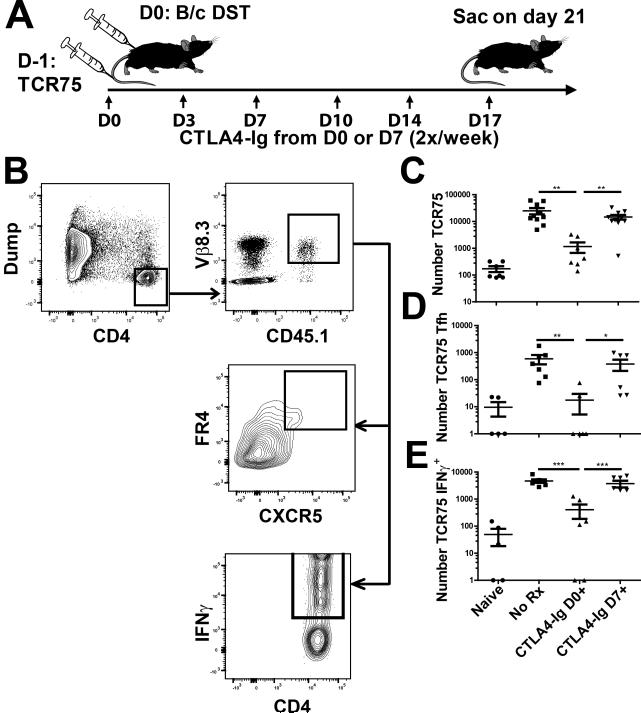
To keep their numbers similar to endogenous antigen-specific T cell populations, we seeded each C57BL/6 mouse with just 500 TCR75 cells via tail vein injection 1 day prior to sensitization. Mice were then sensitized with BALB/c splenocytes, treated with CTLA4-Ig starting on either on day 0 or 7 post-sensitization and sacrificed on day 21, a time point that allowed 2 weeks of CTLA4-Ig treatment when it was started on day 7 and when the TCR75 cells were still readily detectable (Fig 2A and B). We analyzed cells pooled from the spleen and lymph nodes by flow cytometry, gating on the CD45.1+ TCR75 population and assessing cell numbers, Tfh differentiation, and IFNγ production after stimulation with allogeneic APCs (Fig 2B). Accordingly, there was an increase in total TCR75 cells, as well as those with a Tfh (FR4+CXCR5+) or a Th1 (IFNγ+) phenotype in mice sensitized with BALB/C splenocytes (Fig 2C-E). While PD-1 and ICOS have typically been used to identify Tfh cells, Iyer et al. [32] reported that they are downregulated after activation, whereas the folate receptor, FR4, was maintained long-term. We confirmed that similar percentages of CXCR5+ cells expressed FR4 and PD-1 on day 10-14 post-sensitization (Figure S1). CTLA4-Ig treatment from day 0 largely prevented the expansion of TCR75 cells; in contrast, treatment beginning from day 7 had no significant effect on the TCR75 cells, with essentially the same number of TCR75 cells recovered on day 21 as in the untreated control group (Fig 2C). Likewise, the number of TCR75 cells that had differentiated into a Tfh phenotype was only reduced relative to untreated controls when CTLA4-Ig treatment began on day 0, but not on day 7 (Fig 2D). Finally, the number of IFNγ+ TCR75 cells, detected by ex vivo stimulation with T cell-depleted F1 splenocytes, was also not reduced by delayed CTLA4-Ig treatment as compared with animals not receiving CTLA4-Ig (Fig 2E). These observations suggest that the collapse of GC B cells was not due to an inhibition of TCR75 expansion, differentiation or persistence.
Figure 2. Adoptively transferred CD4+ alloreactive TCR75 T cell responses are not controlled by delayed CTLA4-Ig treatment.
A, Diagram of experimental protocol. C57BL/6 mice were given 500 TCR75 CD4+ T cells 1d prior to sensitization with BALB/c splenocytes, followed by CTLA4-Ig 2x/week starting from day 0 or 7. Controls were unsensitized (Naïve) or sensitized but untreated (No Rx) C57BL/6 mice. B, Example gating strategy for TCR75 T cells. Gating for TCR75 was based on CD4+ CD45.1 and Vβ8.3 expression, and TCR75+Tfh cells were identified as FR4+CXCR5hi. Gating for TCR75+ IFNγ+ cells is also illustrated. Analysis was performed on the TCR75+ cells concurrently transferred and harvested from all four groups. C, The total number of TCR75+ T cells and D, of TCR75+ Tfh cells from combined spleen and axial, brachial, and inguinal LNs was calculated for each group described in 2A. Cell counts of 0 were arbitrarily set to 1 to fit on a logarithmic scale. E, IFNγ production was evaluated by incubating responder cells with previously LPS-stimulated and T cell-depleted C57BL/6 syngeneic or F1 allogeneic stimulator cells. Total numbers of TCR75+ IFNγ+ cells were determined after subtracting syngeneic cell stimulation from allogeneic cell stimulation. Data pooled from 3 independent experiments (N=5=8/group) are presented; and statistically significant differences are indicated (*p<0.05, **p<0.01, ***p<0.005).
Delayed CTLA4-Ig treatment does not alter the expansion or differentiation of endogenous antigen-specific T cells
Several studies now suggest that high-affinity monoclonal TCR-transgenic T cells can expand and differentiate more effectively than endogenous antigen-specific cell populations, and their presence could drive other cell populations to behave differently as well [33, 34]. To make sure that our observations were not aberrantly skewed by the TCR-transgenic T cell system, we utilized a novel approach to track endogenous antigen-specific T cell in C57BL/6 hosts immunized with splenocytes from donor mice that expressed the 2W-OVA transgene under the control of the actin promoter[30]. The 2W-OVA transgene comprises the gene for chicken ovalbumin and the gene for the 2W peptide (EAWGALANWAVDSA) inserted between the sequences for OVA and the Db transmembrane domain, and is regulated by the chicken ß-actin promoter to allow ubiquitous expression. When 20×106 2W-OVA F1 splenocytes were injected subcutaneously into C57BL/6 mice, an expansion of 2W:I-Ab (2W) tetramer-binding CD4+ T cells and of SIINFEKL:H-2Kb (OVA) pentamer-binding CD8+ T cells was observed (Fig 3).
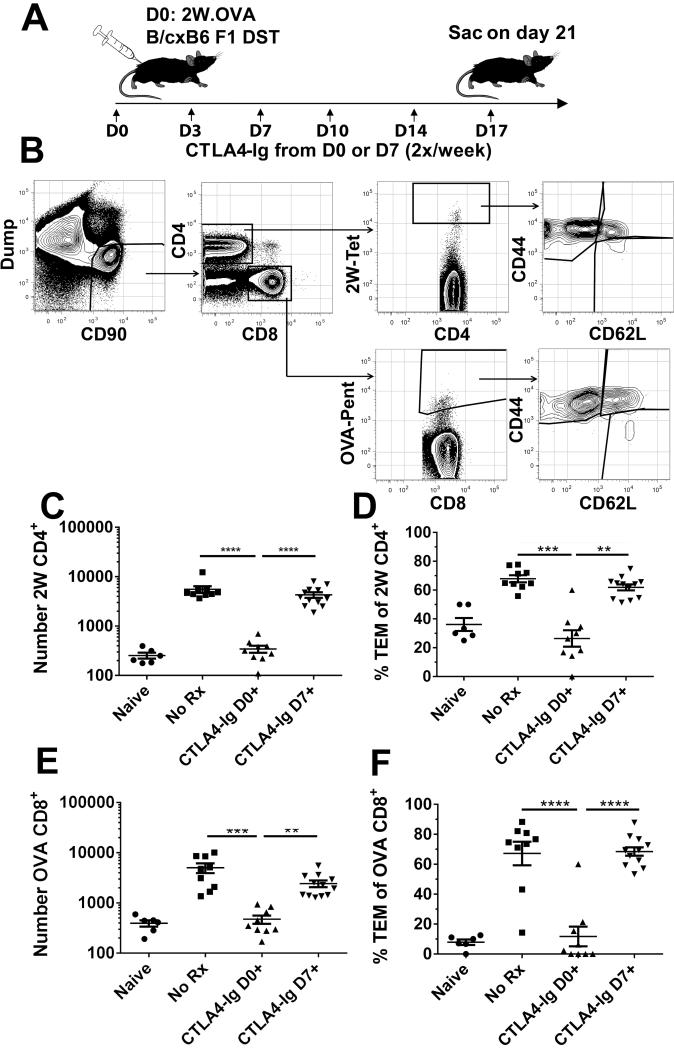
Figure 3. Endogenous antigen-specific CD4+ and CD8+T cell expansion is not reversed by delayed CTLA4-Ig treatment.
A, Diagram of experimental protocol. C57BL/6 mice were sensitized with 20×106 splenocytes from 2W-OVA B/6×B/c F1 mice. Recipients were treated biweekly with 250μg CTLA4-Ig 2x/week starting at either day 0 or 7; sensitized and untreated (No Rx), or naïve mice served as controls. B, Example gating strategy of sensitized C57BL/6 mouse. Combined spleens and axial, brachial, and inguinal LNs were stained and gated on Dump−CD90+ T cells, and separated into CD4+ and CD8+ gates. CD4+ and CD8+ T cells were examined for I-Ab:2W-binding (2W) or H-2Kb:OVA-binding (OVA), and for CD44 and CD62L expression. C&D, The total number of 2W CD4+ T cells, and CD44hiCD62Llo effectors among 2W cells is shown. E&F, The total number of OVA CD8+ T cells, and CD44hiCD62Llo effectors among OVA cells is shown. Data from 4 independent experiments (N=6-10/group) are presented; and statistically significant differences are indicated (**p<0.01, ***p<0.005, ****p<0.001).
We used this experimental model to test the effect of CTLA4-Ig treatment, beginning on either day 0 or 7 post-sensitization, and sacrificing the recipients on day 21 for flow cytometric analysis (Figure 3A,B). As shown in Figure 3C-F, treatment with CTLA4-Ig from the day of immunization completely inhibited endogenous 2W- and OVA-specific T cell responses, preventing their expansion and differentiation into effector memory TEM cells (CD44+CD62L−). In contrast, delayed CTLA4-Ig did not affect the increase in total number of 2W-reactive CD4+ (Fig 3C) or OVA-reactive CD8+ (Fig 3E) T cells and minimally affected their differentiation into CD44+CD62L− effectors (Fig 3D-F).
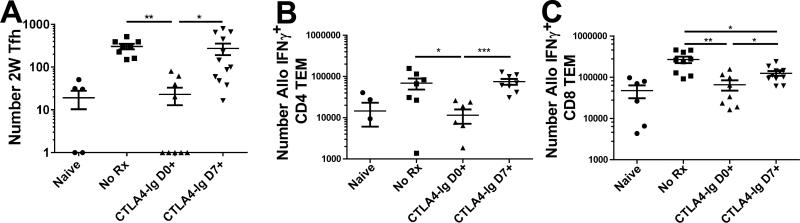
We also investigated the differentiation of endogenous donor-reactive T cells into Tfh or IFNγ-producing cells (Fig 4). Similarly to seeded TCR75 cells, delayed CTLA4-Ig treatment did not decrease the number of 2W-reactive CD4+ Tfh cells as compared to untreated controls, confirming that the collapse of GC B cells was not due to an inhibition of Tfh differentiation per se (Fig 4A). Furthermore, delayed CTLA4-Ig therapy did not prevent the accumulation of allospecific CD4+ IFNγ-producing cells, detected by the ex vivo stimulation with T cell-depleted F1 splenocytes. Delayed CTLA4-Ig was able to induce a modest 35% decrease in the number of alloreactive CD8+ T cells producing IFNγ compared to the untreated controls (Fig 4C). Overall, the effect of delayed CTLA4-Ig treatment on the endogenous donor-reactive CD4+ T cells was minimal, and the robust inhibition of alloreactive GC B cell responses by delayed CTLA4-Ig is likely to be due to inhibiting Tfh interaction with GC B cells.
Figure 4. Endogenous antigen-specific CD4+ Tfh and IFNγ-producing responses are not controlled by delayed CTLA4-Ig.
C57BL/6 mice were as described for Figure 3. A, The total number of I-Ab:2W-binding (2W) Tfh cells from pooled spleens and axial, brachial, and inguinal LNs. B&C, Total numbers of allospecific IFNγ+CD4+ or IFNγ+CD8+ T cells were determined as described in Figure 2. Cell counts of 0 were arbitrarily set to 1 to fit on a logarithmic scale, and data pooled from 4 independent experiments (N=6-9/group) are presented; and statistically significant differences are indicated (*p<0.05, **p<0.01, ***p<0.005).
Delayed CTLA4-Ig therapy reverses allospecific B cell responses and rescues heart allografts from acute rejection
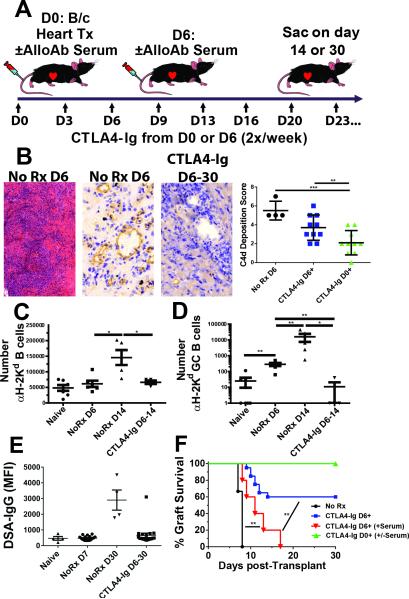
We next investigated whether the effects of delayed CTLA4-Ig on B cell responses can be replicated in a model of heterotopic heart transplantation. To this end, C57BL/6 mice were transplanted with BALB/c hearts, a model in which heart allografts are typically rejected 7-8 days post-transplantation. CTLA4-Ig treatment was initiated either on the day of transplant or 6 days post-transplant (Figure 5A), when there was already significant C4d deposition (Fig 5B, left and middle panels). The total number of H-2Kd-reactive B cells was not significantly increased on day 6 post-transplantation, but significantly increased by 3-fold between days 6 and 14 post-transplantation (Fig 5C). This increase was completely inhibited by the administration of CTLA4-Ig starting on day 6 post-transplantation. In contrast, a 10-fold increase in the total number of H-2Kd-reactive B cells with the GC phenotype were observed by day 6 post-transplantation, and a 1000-fold increase was observed on day 14 post-transplantation (Fig 5D). The administration of CTLA4-Ig starting on day 6 post-transplantation completely reduced the H-2Kd-reactive GC B cell response to levels observed in naïve mice. Consistent with this early control of alloreactive B cell responses, mice receiving delayed CTLA4-Ig exhibited excellent long-term control over alloantibody production (Fig 5E). Of the recipients treated delayed CTLA4-Ig, 8 of 12 had palpable heartbeat at day 30 post-transplantation (Fig 5F). Immunohistochemistry of the surviving allografts on day 30 post-transplantation revealed a modest but not statistically significant, reduction in C4d deposition compared to grafts at day 6 post-transplantation (Fig 5B, right panel). Notably, the adoptively transferred hyperimmune serum was able to precipitate acute allograft rejection in 5/5 mice treated with delayed CTLA4-Ig. In contrast, mice that received serum with CTLA4-Ig on day 0 did not reject their grafts (Fig 5F).
Figure 5. Delayed CTLA4-Ig rescued allogeneic heterotopic heart transplants but did not reverse established damage.
A, Diagram of experimental protocol. C57BL/6 mice were given heterotopic BALB/c hearts, and given 250μg CTLA4-Ig 2x/week starting 6d post-transplantation until sacrifice, and compared with untreated controls. Hyperimmune sera (250 uL/mouse) was injected i.v. either at D0 or D6 post-transplantation, concurrently with the initiation of CTLA4-Ig treatment. B, Histology of acutely rejecting (AR) hearts at 6d post-transplantation (Left, H&E staining, 10x magnification. Center, C4d staining, 40x magnification) or at 30d post-transplant from animals treated with CTLA4-Ig from day 6-30 post-transplant (Right, C4d staining, 40x magnification). A quantification of C4d deposition of AR hearts (NoRx D6) on day 6 post-transplant, as well as CTLA4-Ig D6+ and CTLA4-Ig D0+ on day 30 post-transplantation, is shown (far right). C, Spleens and axial, brachial, and inguinal LN cells were pooled and the total number of H-2Kd-binding B cells/per mouse were determined on day 14 post-transplantation for naïve, day 6 or 14 post-heart transplant recipients with no treatment (No Rx), or treatment with CTLA4-Ig from day 6-14 post-transplantation. D, Total number of GC B cells among H-2Kd-specific B cells with a GC phenotype. (N=4-8 per group from 4 independent experiments). E, Circulating donor-specific IgG antibodies (DSA-IgG) levels in transplanted mice at day 6-7 or ≥30 post-transplantation ± CTLA4-Ig. F, Survival of allografts in untreated and delayed CTLA4-Ig-treatment (N=6-12 per group from 5 independent experiments). Some mice received 250 uL/mouse of pooled hyperimmune serum at time of CTLA4-Ig treatment (Day0+ (N=3) or D6+ N=5 from 2 independent experiments). Statistically significant differences are indicated (*p<0.05, **p<0.01, ***p<0.005).
Discussion
A number of risk factors have been identified for developing de novo DSA post-transplantation, including pre-transplant DSA, poor adherence to treatment or inadequate immunosuppression, and late acute antibody-mediated rejection[35, 36]. There is a general consensus in the clinical transplant community that current immunosuppressive agents do not effectively control antibody responses once they are established[37, 38], and that drugs with the ability to reverse established antibody responses would result in an improvement in the long-term outcomes of transplants in the clinic.
To develop a rational approach towards controlling established antibody responses, it is important to understand how ASCs are generated. Recent studies with model T-dependent antigens indicate that ASCs are generated in two distinct phases[39]. The early phase occurs independently of GC and is responsible for the production of antibodies from B cells that have undergone class switching and limited somatic hypermutation. In contrast, the later phase comprises ASC that have undergone extensive class switching and affinity maturation within the GC, and the antibodies they produce are likely to be of higher affinity. In allograft transplantation, effective inhibition of T cell responses results in virtually no alloantibody production[40] (Fig 5) suggesting that post-GC ASCs are the dominant source of alloantibodies, and inhibiting the GC reaction may be an effective way to control graft-specific B cell responses. Indeed, by tracking alloreactive B cells, we were able to demonstrate the ability of CTLA4-Ig administered even as late as day 14 post-immunization to collapse established GC responses and significantly reduce the total numbers of H-2Kd-specific B cells. DSA production was significantly inhibited by CTLA4-Ig administered from day 7 and less so when treatment was initiated from day 14 post-immunization. These latter observations suggest that post-GC ASCs had already been generated by day 14 and their production of alloantibodies was less resistant to CTLA4-Ig. Nevertheless, we observed a trend towards a more rapid decline the DSA at day 42, raising the possibility that CTLA4-Ig may inhibit antibody production by plasma cells (Fig 1B). Indeed, Lee and colleagues [41, 42] have reported that long-lived plasma cells resident in the bone marrow require CD28-B7 interactions for their survival. Thus, a more careful analysis of the impact of CTLA4-Ig on long-lived plasma cells is required, as there continues to be a strong clinical need to identify drugs that can reverse established DSA responses.
The ability of CTLA4-Ig, administered from the day of transplantation, to inhibit the development of germinal center B cell responses and alloantibody production has been explained by its ability to inhibit T cell activation [43]. In contrast, our observations that CTLA4-Ig treatment starting from day 7 or day 14 to collapse established GCs suggests that the maintenance of the GC is persistently dependent on CD28-B7 interactions between T cells and B cells. An alternative explanation is that CTLA4-Ig binds to B7 on dendritic cells, triggering their expression of IDO that inhibits the activation of conventional T cells and/or promotes the function of regulatory T cells[24, 44]. Indeed, there are reports that IDO expression can be induced in B cells; however IDO does not appear to have counter-regulatory effects in B cells and has even been reported to promote antibody-production[45-47]. Nevertheless, IDO produced by B cells may have immunomodulatory effects on T cells and induce regulatory T cells through a CTLA-4-TGFß/IDO pathway[48]. Thus, the profound effect of delayed CTLA4-Ig on the GC B cells could be explained by the inhibition of alloreactive T cell activation, a possibility we addressed by quantifying alloreactive CD4+ T cells.
We initially traced the fate of alloreactive T cells using the conventional approach of adoptively transferring alloreactive TCR-transgenic T cells. A 500 TCR75 dose was chosen because it was 2000-10000-fold lower than has been used in previous studies to trace the fate of alloreactive T cells[49-51], and close to the level of endogenous T cell populations. Even with this low dose of cells, we were able to track the cells and determine their fate post-sensitization. CTLA4-Ig administered from day 7 post-immunization was unable control the TCR75 CD4+ T cell response including their differentiation into Tfh cells. These observations suggest that the collapse of the GC B cell response was not due to the lack of Tfh cells. Additionally, we describe a novel and powerful approach for tracking the fate of endogenous alloreactive CD4+ and CD8+ T cells in C57BL/6 recipients sensitized with cells that express the model antigen 2W-OVA. By using MHC tetramers to track the CD4+ and CD8+ T cells that recognize the 2W and OVA antigens, respectively, we confirmed that the delayed administration of CTLA4-Ig to day 7 post-immunization was not able to reverse the already primed alloreactive CD4+ or CD8+ T cell response. These observations suggest that the ability of delayed CTLA4-Ig to inhibit and reverse established B cell responses is due to inhibition of B-cell:Tfh cell interactions leading to the death of B cells within the GC, and argue against a more global effect of CTLA4-Ig inhibiting T cell activation and differentiation via the generation of IDO and regulatory T cells.
We also demonstrated that delayed CTLA4-Ig treatment was able to treat acute rejection when administered as late as 6 days post-transplantation in a BALB/c into C57BL/7 heterotopic heart model. Treatment was started just 1-2 days before complete cessation of heartbeat to allow us to also test the efficacy of CTLA4-Ig at treating rejection. While this time point is likely to be too late to be clinically relevant, we reasoned that it serves as a robust model to demonstrate the ability of delayed CTLA4-Ig to rescue allografts from acute rejection. DSA levels were suppressed and cardiac allografts on day 30 post-transplant presented with lower C4d deposition compared to the day 6 allografts. Adoptively transferred hyperimmune sera into recipients treated with delayed CTLA4-Ig was able to induce acute rejection, but not when the sera was transferred into recipients treated with CTLA4-Ig from day of transplantation. Thus delayed CTLA4-Ig treatment was able to inhibit established donor-specific antibody response and halt established rejection. While a causal relationship between these two events is suggested by these observations, it has not been definitively proven and a possibility exists that the control of acute rejection may be due, either in part or entirely, to the inhibition of established T effector cell function by delayed CTLA4-Ig.
The ability of CTLA4-Ig to control established T cell mediated acute rejection appears to contradict the extensive literature that primed effector and memory T cells are resistant to blockade of the CD28-B7 or CD40-CD154 pathways[52-55]. However, the majority of those studies focused on tolerance induction that used a short treatment course of costimulation blockade, whereas in this study we continuously treated with CTLA4-Ig, which is more similar to clinical protocols[21, 56]. Indeed, our studies may provide insight into why CTLA4-Ig cannot establish tolerance in sensitized recipients, by demonstrating that it is ineffective at eliminating effector alloreactive T cells and suggesting that continued CTLA4-Ig is required to prevent these cells from reactivating to mediate graft rejection. The reported ability of CTLA4-Ig to inhibit memory CD4+ T cell IL-2 production and proliferation and partially inhibit IFNγ production [57], supports this possibility. Finally, the results that CTLA4-Ig can reverse established B cell responses and acute rejection provides a potential explanation for the clinical observation that despite higher early acute rejection rates in renal transplant patients on belatacept, the rate of developing de novo DSA was significantly lower and kidney function was superior compared to patients on cyclosporine A [58, 59].
In summary, our study utilizes novel approaches to track both endogenous graft-specific B and T cell responses to understand the behavior of these cells during sensitization and allograft rejection. This study demonstrates that when CTLA4-Ig treatment was delayed to day 6-7 post-sensitization or transplantation, a time when both T and B cell responses had been established, only the B cell response was reversed whereas the T cell response, by and large, developed comparably to untreated recipients. Finally, we show that delayed CTLA4-Ig treatment starting from day 6 post-transplantation, was able to halt established B cell responses and acute rejection. Collectively these observations underscore the potential utility of CTLA4-Ig for controlling established B cell responses and for treating acute rejection in the clinic.
Supplementary Material
Acknowledgments
We are grateful to Dr. Wink Baldwin, Cleveland Clinic, for his generous assistance in developing the C4d immunohistochemistry and Ms. Rachel C Young for her artistic contributions. This work was supported in part by grants (1R01AI110513, P01AI097113) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. JY received an American Heart Association postdoctoral fellowship award and was funded by a Respiratory Biology Training Grant (T32 HL07605). JSY received an American Heart Association post-doctoral fellowship award (15POST25700452) and was funded by a Respiratory Biology Training Grant (T32 HL07605). M.L.M. was funded by the Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), an HHMI Med-into-Grad Program training grant (56006772) and two AHA pre-doctoral fellowships (13PRE14550022 and 15PRE22180007). MHC Class I tetramers were provided by the NIH Tetramer Core Facility (contract HHSN272201300006C).
Abbreviations
- 2W
peptide, EAWGALANWAVDSA, or 2W:I-Ad tetramers
- APC
allophycocyanin
- ASC
antibody secreting cells
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- DSA
donor specific antibodies
- FR4
folate receptor 4
- GC
germinal center
- IDO
indoleamine 2,3-dioxygenase
- IFNγ
interferon-gamma
- IVIG
intravenous immunoglobulin
- OVA
ovalbumin or SIINFEKL:H-2Kb (OVA) pentamers
- TCR
T cell receptor
- TCMR
T cell-mediated rejection
- Tfh
T follicular cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 2.Hartono C, Muthukumar T, Suthanthiran M. Immunosuppressive drug therapy. Cold Spring Harb Perspect Med. 2013;3(9):a015487. doi: 10.1101/cshperspect.a015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aliabadi A, Cochrane AB, Zuckermann AO. Current strategies and future trends in immunosuppression after heart transplantation. Curr Opin Organ Transplant. 2012;17(5):540–5. doi: 10.1097/MOT.0b013e328358000c. [DOI] [PubMed] [Google Scholar]
- 4.Uslu A, Nart A. Treatment of first acute rejection episode: systematic review of level I evidence. Transplant Proc. 2011;43(3):841–6. doi: 10.1016/j.transproceed.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Kaltenborn A, Schrem H. Mycophenolate mofetil in liver transplantation: a review. Ann Transplant. 2013;18:685–96. doi: 10.12659/AOT.889299. [DOI] [PubMed] [Google Scholar]
- 6.Gondos A, et al. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation. 2013;95(2):267–74. doi: 10.1097/TP.0b013e3182708ea8. [DOI] [PubMed] [Google Scholar]
- 7.D'Orsogna L, et al. HLA donor-specific antibody detected by solid phase assay identifies high-risk transplantation pairs irrespective of CDC crossmatch results: case reports and literature review. Clin Transpl. 2006:497–501. [PubMed] [Google Scholar]
- 8.Tsapepas DS, et al. Preformed donor-specific antibodies and risk of antibody-mediated rejection in repeat renal transplantation. Transplantation. 2014;97(6):642–7. doi: 10.1097/01.TP.0000440954.14510.6a. [DOI] [PubMed] [Google Scholar]
- 9.Terasaki PI. A personal perspective: 100-year history of the humoral theory of transplantation. Transplantation. 2012;93(8):751–6. doi: 10.1097/TP.0b013e3182483713. [DOI] [PubMed] [Google Scholar]
- 10.Willicombe M, et al. Acute cellular rejection: impact of donor-specific antibodies and C4d. Transplantation. 2014;97(4):433–9. doi: 10.1097/01.TP.0000437431.97108.8f. [DOI] [PubMed] [Google Scholar]
- 11.Halloran PF, et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(7):1711–20. doi: 10.1681/ASN.2014060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn CJ, et al. Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral)1 in organ transplantation. Drugs. 2001;61(13):1957–2016. doi: 10.2165/00003495-200161130-00006. [DOI] [PubMed] [Google Scholar]
- 13.Campbell PM, et al. Pretransplant HLA antibodies are associated with reduced graft survival after clinical islet transplantation. Am J Transplant. 2007;7(5):1242–8. doi: 10.1111/j.1600-6143.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 14.Macklin PS, Morris PJ, Knight SR. A systematic review of the use of rituximab for desensitization in renal transplantation. Transplantation. 2014;98(8):794–805. doi: 10.1097/TP.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 15.Wahrmann M, et al. Effect of the proteasome inhibitor bortezomib on humoral immunity in two presensitized renal transplant candidates. Transplantation. 2010;89(11):1385–90. doi: 10.1097/TP.0b013e3181d9e1c0. [DOI] [PubMed] [Google Scholar]
- 16.Robinson JA, et al. Plasmapheresis followed by intravenous immunoglobulin in presensitized patients awaiting thoracic organ transplantation. Ther Apher. 1997;1(2):147–51. doi: 10.1111/j.1744-9987.1997.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 17.Abe T, et al. Anti-huCD20 antibody therapy for antibody-mediated rejection of renal allografts in a mouse model. Am J Transplant. 2015;15(5):1192–204. doi: 10.1111/ajt.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Immenschuh S, et al. Indicators of treatment responsiveness to rituximab and plasmapheresis in antibody-mediated rejection after kidney transplantation. Transplantation. 2015;99(1):56–62. doi: 10.1097/TP.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 19.Bhimaraj A, Taylor DO. How to deal with presensitized candidates for heart transplantation? Curr Opin Organ Transplant. 2011;16(5):529–35. doi: 10.1097/MOT.0b013e32834a8c4d. [DOI] [PubMed] [Google Scholar]
- 20.Satyananda V, Shapiro R. Belatacept in kidney transplantation. Curr Opin Organ Transplant. 2014;19(6):573–7. doi: 10.1097/MOT.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 21.Vincenti F, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 22.Cutolo M, Nadler SG. Advances in CTLA-4-Ig-mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmun Rev. 2013;12(7):758–67. doi: 10.1016/j.autrev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Mellor AL, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16(10):1391–401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 24.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 25.Dejean AS, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10(5):504–13. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhoen M, et al. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176(10):6202–10. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 27.Senolt L, et al. Prospective new biological therapies for rheumatoid arthritis. Autoimmun Rev. 2009;9(2):102–7. doi: 10.1016/j.autrev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA4-Ig. Am J Transplant. 2013;13(9):2280–92. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honjo K, Xu X, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation. 2004;77(3):452–5. doi: 10.1097/01.TP.0000112937.12491.42. [DOI] [PubMed] [Google Scholar]
- 30.Moon JJ, et al. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self- peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci U S A. 2011;108(35):14602–7. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186(1):214–21. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyer SS, et al. Identification of novel markers for mouse CD4(+) T follicular helper cells. Eur J Immunol. 2013;43(12):3219–32. doi: 10.1002/eji.201343469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hataye J, et al. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312(5770):114–6. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 34.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26(6):827–41. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vo AA, et al. Factors Predicting Risk for Antibody-mediated Rejection and Graft Loss in Highly Human Leukocyte Antigen Sensitized Patients Transplanted After Desensitization. Transplantation. 2015;99(7):1423–30. doi: 10.1097/TP.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 36.Wiebe C, et al. Rates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients With De Novo Donor-Specific Antibody. Am J Transplant. 2015 doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 37.Jordan SC, Vo AA. Donor-specific antibodies in allograft recipients: etiology, impact and therapeutic approaches. Curr Opin Organ Transplant. 2014;19(6):591–7. doi: 10.1097/MOT.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery RA, et al. Humoral immunity and antibody-mediated rejection in solid organ transplantation. Semin Immunol. 2011;23(4):224–34. doi: 10.1016/j.smim.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Nutt SL, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160–71. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 40.Sucher R, et al. IDO and regulatory T cell support are critical for cytotoxic T lymphocyte-associated Ag-4 Ig-mediated long-term solid organ allograft survival. J Immunol. 2012;188(1):37–46. doi: 10.4049/jimmunol.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rozanski CH, et al. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med. 2011;208(7):1435–46. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozanski CH, et al. CD28 Promotes Plasma Cell Survival, Sustained Antibody Responses, and BLIMP-1 Upregulation through Its Distal PYAP Proline Motif. J Immunol. 2015;194(10):4717–28. doi: 10.4049/jimmunol.1402260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim EJ, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant. 2014;14(1):59–69. doi: 10.1111/ajt.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172(7):4100–10. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 45.Godin-Ethier J, et al. IDO expression by human B lymphocytes in response to T lymphocyte stimuli and TLR engagement is biologically inactive. Mol Immunol. 2011;49(1-2):253–9. doi: 10.1016/j.molimm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Merlo LM, et al. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J Immunol. 2014;192(5):2082–90. doi: 10.4049/jimmunol.1303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eleftheriadis T, et al. Inhibition of indoleamine 2,3-dioxygenase not only blocks autoreactive B cell activation, but it also reduces production of antibodies in general: comment on the article by Pigott and Mandik-Nayak. Arthritis Rheum. 2013;65(7):1951–2. doi: 10.1002/art.37946. [DOI] [PubMed] [Google Scholar]
- 48.Nouel A, et al. B-Cells induce regulatory T cells through TGF-beta/IDO production in A CTLA-4 dependent manner. J Autoimmun. 2015;59:53–60. doi: 10.1016/j.jaut.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Conlon TM, et al. Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol. 2012;188(6):2643–52. doi: 10.4049/jimmunol.1102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford ML, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204(2):299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinelli DF, et al. An anti-CD154 domain antibody prolongs graft survival and induces Foxp3(+) iTreg in the absence and presence of CTLA-4 Ig. Am J Transplant. 2013;13(11):3021–30. doi: 10.1111/ajt.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riella LV, Sayegh MH. T-cell co-stimulatory blockade in transplantation: two steps forward one step back! Expert Opin Biol Ther. 2013;13(11):1557–68. doi: 10.1517/14712598.2013.845661. [DOI] [PubMed] [Google Scholar]
- 53.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–9. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172(9):5456–66. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 55.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6(4):647–51. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 56.Vincenti F, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353(8):770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 57.Ndejembi MP, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177(11):7698–706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 58.Rostaing L, et al. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant. 2013;13(11):2875–83. doi: 10.1111/ajt.12460. [DOI] [PubMed] [Google Scholar]
- 59.Bray R, et al. Evaluation of donor-specific antibodies through 7 years with belatacept in BENEFIT and BENEFIT-EXT. 2015 American Transplant Congress. 2015 Abstract No. 458. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.