Abstract
The eukaryotic genome is packaged in the three-dimensional nuclear space by forming loops, domains, and compartments in a hierarchical manner. However, when duplicated genomes prepare for segregation, mitotic cells eliminate topologically associating domains and abandon the compartmentalized structure. Alongside chromatin architecture reorganization during the transition from interphase to mitosis, cells halt most DNA-templated processes such as transcription and repair. The intrinsically condensed chromatin serves as a sophisticated signaling module subjected to selective relaxation for programmed genomic activities. To understand the elaborate genome–epigenome interplay during cell cycle progression, the steady three-dimensional genome requires a time scale to form a dynamic four-dimensional and a more comprehensive portrait. In this review, we will dissect the functions of critical chromatin architectural components in constructing and maintaining an orderly packaged chromatin environment. We will also highlight the importance of the spatially and temporally conscious orchestration of chromatin remodeling to ensure high-fidelity genetic transmission.
Keywords: Chromatin architecture, Genome stability, Epigenome, Cell cycle, Cancer
Introduction
DNA constitutes the basis of our existence. Fittingly, it is meticulously packaged into chromatin, via an association of DNA with histones and other architectural proteins. Chromatin serves as a platform for genetic transmission and epigenetic regulation. Proper organization of chromatin is critical to efficient execution of all DNA-templated processes including DNA replication, transcription, recombination, and repair [1, 2]. The chromatin structure is constantly undergoing a cycle of condensation and decondensation to accommodate diverse cellular purposes. In recent years, innovations of chromosome conformation capture (3C) technology and high-resolution microscopy has effectively illuminated genome organization, resulting in an unprecedented expansion of the details of chromatin topology and gene regulation [3–5]. These findings significantly strengthen our understanding of three-dimensional (3D) chromatin organization within the nucleus as well as its effects on gene activity.
Chromatin loops, topologically associating domains, and higher-order compartments emerge as a well-accepted organizing principle of the genome [6]. However, this hierarchical principle of spatial organization may not apply to highly condensed mitotic chromosomes [7]. The structure of mitotic chromosomes has been fascinating yet baffling to cell biologists until recently—several models have been proposed based on studies using 3C methods, chromosome painting/banding, and imaging-assisted high-throughput approaches [7–9]. In contrast to interphase chromatin that comprises a hierarchy of higher-order structures such as domains and compartments, mitotic chromosomes exhibit a much simpler organization pattern containing only chromatin loops, the primary folding units. More significantly, this homologous loop-compression organization is a general principle applicable to any gene locus, common to all chromosomes, and consistent among cell types [7]. Consistent with simplified chromatin organization, mitotic cells shut down most transcription [10] and inactivate DNA repair [11] to prepare for the challenging task of segregating genetic information.
While it resides in a 3D nuclear space, the genome is also surrounded by a dynamic chromatin environment, which constantly undergoes condensation and decondensation. A fourth dimension of time is therefore required to describe and understand genome behavior, notably the duplication-segregation cycle that maintains its identity and integrity. Four-dimensional (4D) orchestration of chromatin remodeling events ensures spatially and temporally coordinated transitions between chromatin compaction and decompaction. This review is aimed at summarizing evidence that faithful transmission of the genome demands a dynamic coordination of the 4D chromatin environment. We will integrate current knowledge of eukaryotic genome–epigenome interaction in both interphase and mitosis into an overview of chromatin architecture and function. Stemming from these principles, we will examine the relationship between genomic instability and cancer, which may potentially evolve form the deregulation of chromatin condensation.
Architectural and functional components of chromatin
Chromosome biology has been revitalized since the advent of chromosome conformation capture (3C) technology, a DNA capture method designed to detect the frequency of interaction between genomic loci in their native 3D state [12]. Studies using this technology revealed a highly conserved functional unit of the genome called topologically associating domain (TAD) [13, 14]. In interphase cells, TADs can be further segregated into active or inactive compartments [15, 16]. These multiple levels of hierarchical genome organization are associated with distinct chromatin signatures and are controlled by a host of factors, including histones, non-histone architectural proteins, chromatin scaffold proteins, as well as chromatin remodelers. Via these diverse components, chromatin is governed by a plethora of pathways that interact to accommodate fundamental machineries, which process genetic information via DNA replication/segregation, transcription, recombination, and repair.
Histones and the histone code
Histones are a critical and the most abundant protein component of chromatin. Together with DNA, histones form a repeating structural unit of chromatin called the nucleosome, which merely comprises the first level of chromatin packaging [17]. Histones are basic globular proteins whose globular domains form the nucleosomal core whereas unstructured tails jut out of the nucleosome [18]. Based on X-ray crystallography data, the nucleosome core consists of the 8-histone octamer in which a H3-H4 tetramers complexes with two H2A-H2B dimers [19, 20]. Linker histone, H1, completes the chromatosome by guarding the nucleosome opening and linker DNA. In addition to serving the structural center of chromatin, histones are extensively modified—these modifications alter histone interaction with associated proteins and ultimately regulate the dynamics and accessibility of chromatin [17]. As specific histone post-translational modification patterns or combinations correlate with distinct chromatin states, the concept of a “histone code” evolves to suggest that histone modifications resemble a molecular bar code that directs protein recruitment and chromatin structural alteration [18, 21]. As such, the covalent modifications are added or removed by enzymes (“writers” and “erasers”), and can be recognized by chromatin-binding proteins (“readers”). While the histone tails serve as a major repository of post-translational modifications that either disrupt chromatin contacts or recruit non-histone proteins to chromatin [22], the globular domains can also be modified to affect the intranucleosomal histone–DNA interaction [23].
A host of post-translational modifications exist, among which methylation and acetylation are particularly common in the formation of chromatin domains [24–26]. Histone can be methylated at different levels, predominantly on two basic residues, lysine (K) [27] and arginine (R) [28]. Histone methylation is reversible and can be linked to both gene activation and repression, depending on the target site. Most commonly, trimethylation of H3K4, H3K36, or H3K79 characterizes open euchromatin, whereas trimethylation of H3K9, H3K27, or H4K20 marks condensed heterochromatin [17, 24, 29].
Acetylation of histones affects many different biophysical properties of nucleosomes. Lysine acetylation of the core histone tails inhibits self-association, likely encoding an open chromatin conformation [30]. Acetylation also neutralizes the positive charge of histones and reduces the interaction between acetylated histones and DNA, exerting a strong impact on chromatin unfolding [31, 32]. Histone acetyltransferases (HATs) and their functional antagonists, histone deacetylases (HDACs), execute reversible histone acetylation and deacetylation. A diverse and complex range of HATs and HDACs constitute an important epigenetic regulatory mechanism that underlies chromatin remodeling. For example, acetylation on lysines is written by HATs (“writers”) and removed by HDACs (“erasers”), whereas non-histone proteins such as bromodomain-containing proteins can recognize these modifications and act as “readers” [33]. Lysine 16 on the N-terminal tail of histone H4 (H4K16) is one of several commonly examined sites in regards to chromatin condensation. Chemical ligation analysis demonstrated that acetylated H4K16 inhibits crosslinking mechanisms for nucleosome formation and chromatin compaction, leading to chromatin decondensation and transcription activation [17, 34, 35].
Histone variants represent an additional layer of complexity to the histone code. They are isoforms of the canonical histones that create specialized nucleosomes. Unlike traditional histones, these minor members of the histone gene family are expressed at lower levels independent of chromosome replication [36, 37]. In addition, histone variants differ temporally in their entrance into the nucleosome during the cell cycle [38, 39]. Eleven variants comprise the linker histone H1 gene family, which exhibit cell type and tissue-specific expression patterns; their expression alters during differentiation, development and cancer progression [40, 41]. Among core histones, variants largely occur on histones H3 and H2A, while histones H4 and H2B are generally invariant [42]. Histone H2A has the greatest spectrum of variants, whereas chromatin incorporation of different H2A variants functionally diversifies nucleosomes by altering nucleosome stability and dynamics [43–46]. Among these variants, H2A.X is a universal variant localized to chromosome fragile sites [47] and has been indicated as a recruiter of repair proteins in response to DNA damage [48–50]. Human histone H3 has six different variants, among which CENP-A (CenH3, centromeric H3) is the most well known [51]. CENP-A plays a critical role to chromosome segregation via centromere association [42, 52]. It replaces H3 at centromeres and is crucial to dictating centromeric positioning on chromosomes. The timing of CENP-A incorporation is critical as unscheduled CENP-A insertion disrupts kinetochore assembly and function [53].
Chromosome scaffold proteins
Earlier elegant experiments demonstrated that higher-order chromosome structures persist even after histone depletion [54, 55], suggesting that non-histone proteins constitute a chromosome scaffolding structure. The chromosome scaffold was found to consist mainly of two major proteins with high molecular weights, the 170-kDa Sc1 and 135-kDa Sc2 [56]. These two abundant components were later identified as topoisomerase II [57, 58] and the structural maintenance of chromosomes 2 (SMC2) [59].
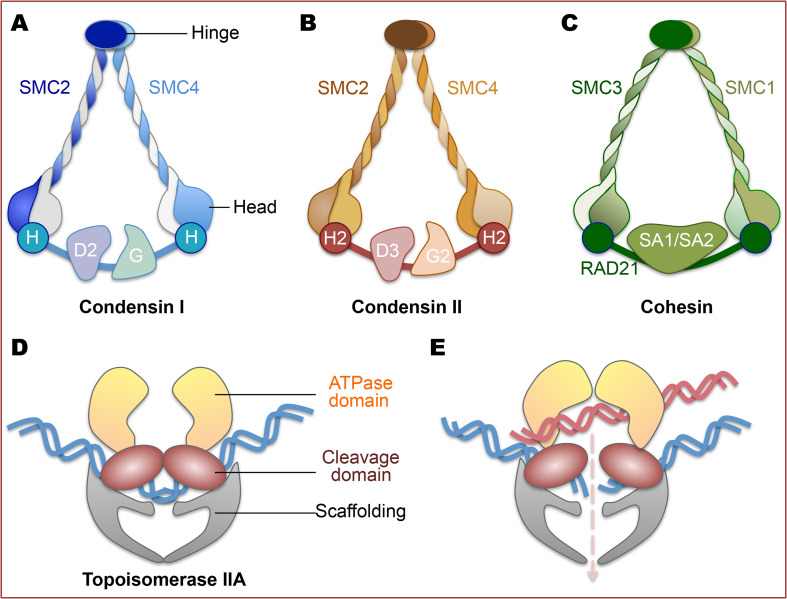
SMC2 is a member of the SMC ATPase family of multi-subunit protein complexes named condensins [60]. In eukaryotes, six SMC proteins form three heterodimers; Smc1–Smc3 (the cohesin complex) [61–63], Smc2–Smc4 (condensins) [60, 64], and the Smc5–Smc6 dimer [65, 66]. Cohesins and condensins are the central regulators of two major chromosome structure organization events required for faithful chromosome segregation: sister chromatid cohesion and chromosome condensation [67]. In addition to the control of mitotic chromosome segregation, cohesins and condensins also play important roles in interphase chromatin organization, transcription, and DNA repair [68–74]. Eukaryotes have two types of condensin complexes, known as condensins I and II, that share the same pair of subunits, SMC2 and SMC4 [75]. During interphase, condensin I is localized in the cytoplasm, whereas condensin II is found in the nucleus—this localization is conserved among many eukaryotic species [76, 77]. Condensin I governs lateral and looping chromatin compaction while condensin II controls axial chromatin compaction [78]. The eukaryotic pentameric condensin complex is composed of a heterodimer of SMC proteins (SMC2 and SMC4), a kleisin protein and two HEAT-repeat proteins (Fig. 1a, b). SMC2 and SMC4 form long anti-parallel coiled coils flanked by an ATPase head domain and a hinge domain that links two SMC subunits. The head domains interact with the kleisin subunit to form a closed ring [79]. This ring may entrap two strands of DNA from the same chromosome, resulting in chromosome condensation. Cohesin complexes have similar shape and subunit composition as condensins, while its ring structure locks the sister DNAs at the nascent replication fork (Fig. 1c). Cohesin complexes are removed at the anaphase onset, triggering chromosome segregation [80].
Fig. 1.
Architecture of chromosome scaffold proteins. a–c SMC complexes including condensin I, II, and cohesion. The core of each SMC complex is formed by two SMC proteins; each SMC protein contains an ATPase head domain, a hinge domain and a coiled coil that links the two. In addition to SMC proteins, each condensin complex contains a kleisin protein (H stands for GAP-H in condensin I whereas H2 stands for CAP-H2 in condensin II) and two HEAT-repeat proteins (CAP-D2 and CAP-G in condensin I whereas CAP-D3 and CAP-G2 in condensin II). Cohesin is composed of SMC1 and SMC3 heterodimer, as well as a kleisin subunit (Rad21 or Scc1) that binds to HEAT-repeat subunits called SA1/SA2 or Scc3. d, e Type II topoisomerases use ATPase activity to cleave both strands of a duplex DNA (blue) and pass another duplex DNA (red) through the transient break
Topoisomerase II is a type II topoisomerase that catalyzes the ATP-dependent DNA topological transition to promote chromosome disentanglement [81–89]. Strand synthesis during DNA replication produces intertwined daughter duplexes (termed precatenanes). These duplexes may progress to mitosis as tangled DNAs that result in defects in chromosome segregation. Topoisomerase II plays a key role in decatenating these topological linkages and controls the DNA decatenation checkpoint [89–92]. Topoisomerase II uses the energy released by ATP hydrolysis to introduce a double-strand break into one DNA helix to pass a second helix through the break site and to reseal the initial break (Fig. 1d, e). There are two vertebrate isoforms of topoisomerase II (α and β). While topoisomerase IIα is essential for chromosome condensation, replication, and segregation, type IIβ topoisomerase tends to function in DNA repair, transcription, and development [93]. In addition to its decatenating activity, topoisomerase II also plays an essential role in chromatin compaction by cooperating with the SMC proteins. SMCs and topoisomerase II co-localize as part of the protein network that stabilizes long-range contacts between chromosomal segments [94].
Chromatin non-histone architectural proteins
Besides histones and chromatin scaffold proteins, other non-histone architectural proteins also assist in regulating global chromatin organization, differential nucleosome packaging, and local chromatin architecture for the fidelity of chromosome duplication and segregation. Important non-histone chromatin architectural proteins include heterochromatin protein 1 (HP1) and the zinc finger factor, CCCTC-binding factor (CTCF).
HP1 proteins are central players in heterochromatin structure and function. The first HP1 gene was identified in Drosophila melanogaster [95] and Swi6 is the ortholog in Schizosaccharomyces pombe. There are three HP1 paralogs in mammals (HP1α, HP1β and HP1γ) that are characterized by two distinct functional domains. The hydrophobic chromodomain (CD) recognizes di- and tri-methylated H3K9 whereas the chromoshadow domain (CSD) forms a dimerization interface for ligand recruitment. Histone methyltransferase SUV39H1 trimethylates the lysine residue 9 of histone H3; this recruits HP1 and establishes constitutive heterochromatin at pericentromeric and telomeric sites [96, 97]. Interestingly, the CD and CSD can coordinate to induce an oligomerization process, leading to heterochromatin spreading [98–100]. In S. pombe, Swi6 acts as a transcriptional repressor and its repressive function is vital for sister chromatid cohesion, telomere maintenance and DNA repair [101]. Independently of H3K9 interaction, HP1 can actively participate in repair of DNA double strand breaks (DSBs) [102].
CCCTC-binding factor (CTCF) was initially characterized as a transcription factor [103, 104] and has been gradually recognized as an organizer of higher-order chromatin structure that facilitates the establishment of genome topology [105]. CTCF is conserved in most bilaterian phyla but is absent in yeast, Caenorhabditis elegans and plants [106]. ChIP (chromatin immunoprecipitation) and Hi-C (genome-wide 3C) analysis suggested that this zinc-finger protein functions as an insulator throughout the genome, partitioning it into various TADs and sub-TADs [107, 108]. Among CTCF-associated proteins, cohesin is essential for the formation or stabilization of long-range chromatin loops from CTCF binding sites [109]. Both CTCF and cohesin are enriched at the borders of TADs, playing a critical role in domain demarcation. There are two models that describe anchoring of the cohesin ring structure at chromatin loops. A single cohesin ring can wrap around two DNA fibers to tether sister chromatids (“embrace” model), or alternatively, two cohesin rings interact and each ring encircles one DNA fiber (“handcuff” model, Fig. 2). Despite the concurrent occupancy on chromatin, cohesin and CTCF may play different roles in genome organization and gene regulation. Cohesin mainly anchor chromatin loops within chromatin domains, whereas CTCF maintains the domain boundaries [110].
Fig. 2.
Distinct chromatin organizations in interphase and mitotic cells and the two-step compaction process during the transition from interphase to mitosis. A hierarchical organization of chromatin loops, topologically associating domains and higher-order compartments is shown in the interphase cell (lower left corner). When the cell prepares for division, linear compaction results in the formation of 80–120 kb consecutive loops. Further axial and lateral compression ultimately gives rise to the rod-shaped mitotic chromosome. CTCF (red barrel) and cohesion (green ring) cooperate to form and stabilize chromatin domains. Two models of cohesion anchoring chromatin loops are shown in the box. In the mitotic cell, the different colored rings (match Fig. 1) represent condensin I (blue), condensin II (brown), and cohesin (green)
In addition to the above-listed chromatin components, chromatin remodelers are an important group of ATP-hydrolysis-dependent enzyme complexes that shape the genome by altering nucleosome positioning relative to the DNA sequence, as detailed in many comprehensive reviews [111–114].
Genome stability and epigenome dynamics from linear looping to 4D remodeling
The means by which integrity of eukaryotic genomes is maintained in the context of continuous chromatin reorganization remains mysterious. From 3C to circularized chromosome conformation Capture (4C), carbon-copy chromosome conformation capture (5C) and genome-wide chromosome conformation capture (Hi-C) methods, recent research has illuminated genome organization in three dimensions in the interphase nucleus [115]. The revelation of long-distance chromatin looping and TADs offers sophisticated interpretation of cellular function and prediction of cell fate. On top of this hierarchical 3D genome organization, appending a fourth dimension of time is inevitable due to the dynamic nature of chromatin. It is of interest to note that chromatin organization changes dramatically when duplicated genomes prepare for cell division [7, 116]. How do cells maintain genomic integrity and cope with the challenge of chromatin restructuring during the transition between interphase and mitosis? Here we focus on three fundamental genome maintenance processes—interphase-restricted DNA replication, DNA repair and mitosis-specific chromosome segregation—to highlight the importance of spatial and temporal coordination of chromatin components.
Interphase chromatin architecture for efficient genome duplication and repair
Perpetuation of life requires both faithful genome duplication and efficient DNA repair, both operating in a hierarchically organized and interphase-restricted chromatin environment. DNA replication requires transient chromatin disruption ahead of the DNA polymerase complex; thus, this process is generally associated with open and accessible chromatin. Replication is initiated at transcriptionally permissive TADs, and is completed in transcriptionally repressive TADs [117, 118]. Chromatin remodeling complexes facilitate the sliding or removal of nucleosomes from replication forks. Nucleosomes upstream of replication forks are first disrupted and histones are then displaced by histone chaperones; following this process, new histones are deposited on the new strands via de novo assembly [119].
The origin of DNA replication must be selected and activated within the local chromatin environment. Origin recognition complexes (ORCs) congregate at nucleosome-depleted regions and are associated with cohesin loading [120]. ORCs bind to DNA, creating pre-replication complexes (PreRCs); these PreRCs load MCM helicase-complexes on to DNA, licensing DNA for replication [121]. The PreRC is then inhibited until the following G1 phase [121]. Specific and reproducible genome locations have been identified as eukaryotic replication origins and ORC binding sits [122], suggesting that eukaryotes determine ORC binding based on the local chromatin environment. Activating histone modifications such as histone acetylation are associated with DNA replication in early S phase, whereas a local perturbation of histone acetylation shifts the time of replication and impairs the recruitment of replication proteins [123]. These data indicate that local changes in histone modification are capable of fine-tuning DNA replication activity.
Intrinsic chromatin condensation is subjected to selective unwinding, allowing accessibility not only to replication factors, but also to the DNA repair machinery during damage. Essentially, repair mechanisms loosen the nucleosome for accessibility of histones and repair factors [124, 125]. DSBs are the most severe type of DNA damage, as insufficient correction can lead to chromosomal loss [126]. Studies have observed the absence of nucleosomes at DSBs [127]. Nucleosome packaging and condensed chromatin architecture surrounding DSBs can spatially hinder loading of repair proteins [119]. Khurana et al. demonstrated that DSB repair involves a “biphasic” chromatin organization. Chromatin is first relaxed via PARP remodelers at DSBs; afterwards, it is compacted again via the recruitment of a histone H2 variant and an H3K9 methyltransferase, both repressive in nature and facilitative of condensation-dependent BRCA1 recruitment at DSBs for homologous recombination [128]. In support of this notion, fast and transient HP1α recruitment to DNA damage sites is essential for homology-directed repair by facilitating the loading of important repair proteins such as 53BP1 and Rad51 [129].
Emerging evidence suggests a critical role of non-proteolytic ubiquitination of adjacent chromatin areas to generate binding sites for DNA repair factors. This requires the sequential action of two E3 ubiquitin ligases RNF8 and RNF168, as well as an E3 ubiquitin-conjugating enzyme UBC13 which specifically generates K63-linked ubiquitin chains [130–136]. Interestingly, a recent study demonstrates that H1-type linker histones, but not core histones, represent major chromatin-associated targets of RNF8-mediated ubiquitination at DSB sites [137]. These findings reveal ubiquitination of linker histones as novel histone marks for recognition by factors involved in DNA repair. In summary, chromatin remodeling via the histone code is critical in mediating DNA damage repair.
Mitotic loop compression and faithful chromosome segregation
The most fascinating and challenging outcome of the cell cycle is the equal delivery of intact genetic material to the daughter cells, which is accompanied by chromosome condensation and segregation. Unlike interphase chromatin, mitotic chromatin has considerably fewer modified sites, less accessibility, and an overall more compact structure [138]. As chromatin is condensed, chromatin-binding proteins and transcription factors are discharged [117]. Mitotic chromatin condensation is marked by H4K20me and limitation of H4 acetylation, which recruit condensin II to mediate early compaction. Subsequently, phosphorylation of histone H3 by aurora B kinase serves to recruit the condensin I complex, bind repressive proteins, while expelling other chromatin-bound factors [117, 139]. Consequently, DNA is packaged for high-fidelity inheritance by daughter strands and low topological hindrance during chromosome segregation [117, 140].
Although mitotic chromosomes have the most distinct morphology in a cell, it was not until recently that the structure of mitotic chromosomes and underlying packaging mechanisms were illuminated. 5C and Hi-C technologies first distinguished the homologous folding state of mitotic chromosomes from the highly compartmentalized and cell type-specific interphase chromatin organization [7]. The authors found that mitotic chromosomes lose TADs and chromatin compartments, the basic organization characteristics of interphase chromatin. Polymer simulation suggested that mitotic chromosomes are organized into arrays of consecutive 80- to 120-kb loops that may form in early prophase. These findings led to a two-step compaction model of mitotic chromosomes—chromatin is first compacted into an array of consecutive loops, and then followed by a series of compression, giving rise to the rod-shaped mitotic chromosome (Fig. 2). Further investigation is required to characterize molecular and epigenetic activities that drive the domain disassembly and loop compaction process during the transition from interphase to mitosis.
Several other models of mitotic chromosomes have also evolved. A thin-plate model based on banded karyotype and cytogenetic data suggests that mitotic chromatin forms many stacked layers of planar perpendicular to the chromosome axis [8]. Using electron microscopy-assisted nucleosome interaction capture (EMANIC) cross-linking experiments, a more recent study proposed a modified model in which zigzag nucleosome chains are packaged into hierarchical loops [9]. This model highlighted the role of linker histone unloading in promoting lateral association between the nucleosome-chain loops that results in hierarchical folding of proximal loops at various angular orientations.
Although mitosis only comprises about 5 % of the time within a cell cycle, there are distinct mitosis sub-phases (prophase, prometaphase, metaphase, anaphase, and telophase) with distinct morphologies. A recent imaging study uncovered a discontinuous process of chromosome compaction and expansion during the mitotic progression from prophase to metaphase [141]. Chromosome architectural proteins including cohesins, condensins, and topoisomerase II, play critical roles in driving the morphological changes during chromosome compaction and expansion. These findings extend the steady-state 3D models of mitotic chromosome compaction to provide a 4D dynamic picture in which the transition among distinct mitotic sub-phases is driven by chromatin architectural alterations.
Chromatin condensation during the interphase-metaphase transition
Although we have gained essential insights into the broad principle of eukaryotic genome organization, i.e., the hierarchical loop-domain-compartment organization in interphase cells, our knowledge about the dynamics of in vivo chromatin folding/unfolding is still limited. In particular, what drives the transition between TAD-mediated interphase chromatin structuring and TAD-null mitotic chromosome compaction remains enigmatic. Here we summarize evidence for general mechanisms of chromatin condensation mediated by either the histone code or chromosome scaffold proteins SMC condesins and topoisomerase II. It is likely that both mechanisms jointly mediate chromatin condensation during the interphase–metaphase transition.
A recent study successfully reconstituted mitotic chromosomes in vitro using six purified factors, core histones, three histone chaperones, topoisomerase II, and condensin I [142]. This finding emphasizes the potent role of condensins and topoisomerase II in the formation of the mitotic chromosome architecture. As described in the earlier section, condensins are the major non-histone component of the chromosome scaffold that possesses a broad spectrum of function in regulating chromatin structure and dynamics [143]. Condensins facilitate the initial mitotic chromatin condensation event that forms 80- to 120-kb linear chromatin loops [7, 144]. Moreover, condensin can recognize and bind monomethylated H4K20, inducing compaction, while dimethylated H4K20 disrupts condensin association to DNA, and consequently leads to decondensation [145].
In addition to condensins, the histone code plays a critical role in driving chromatin condensation. Above all histone modifications, the phosphorylation status of histone H3 serine 10 (H3S10) and the acetylation status of histone H4 appear to be the most directive in chromatin compaction [146, 147]. In yeast, phosphorylation of histone H3 recruits the lysine deacetylase Hst2p to deacetylate H4K16 during early mitosis, resulting in chromatin condensation [146]. Aside from H3S10 phosphorylation, the methylation and acetylation of nearby residues, lysines 9 and 14 can affect heterochromatin formation [148]. In interphase cells, acetylation of histone H4K16 prevents its interaction with the acidic patch of neighboring nucleosome [34]. In addition to H3 and H4 modifications, the H2A acid patch is also crucial for chromatin condensation [149]. A crosstalk may exist between the condensin pathway and the histone pathway in regulating chromatin condensation. For example, in embryonic stem cells, phosphorylated H3S10 marks condensin-dependent chromatin domains [150]. Phosphorylation of H3S10 may also enhance the flexibility of the chromatin fiber, allowing the recruitment of non-histone proteins including condensin or topoisomerase II [151]. While it seems that the histone code and condensin-mediated complexes play paralleled and coordinative roles in chromatin condensation to facilitate the transition from interphase to metaphase, further studies are necessary to elucidate the stepwise temporal progression of mitotic chromosome compaction.
Chromatin deregulation, genomic instability, and cancer
Given the complex regulatory network of chromatin dynamics and the importance of chromatin organization in genome maintenance, even subtle epigenome alterations may cause aberrant genome behaviors. Recent research has uncovered new chromatin players and novel mechanisms of chromatin regulation, which helps understand the genome–epigenome interplay. For example, the tumor suppressor PTEN has been found to promote chromatin condensation by interacting with histones and other chromatin architecture proteins [152, 153]. PTEN is among the most frequently mutated genes in human cancer. The non-canonical function of PTEN in regulating chromatin structure can serve to interrogate how normally packaged chromatin protects the genome and how aberrant chromatin architecture may cause cancer.
PTEN as an emerging regulator of chromatin condensation
PTEN is a tumor suppressor and its best-known function is the antagonism of the PI3K/AKT signaling pathway as a lipid phosphatase [154]. However, this is not the sole mechanism for PTEN function in tumor suppression. Accumulating evidence indicates that the lack of PTEN leads to structural and numerical chromosome aberrations [155–158], suggesting that PTEN is essential for faithful genomic transmission. In particular, nuclear PTEN facilitates DNA repair by acting on chromatin to modulate gene transcription [156]. A plethora of microarray data also revealed that PTEN depletion or overexpression causes global gene transcription profile alterations [159–164]. The mechanism for these earlier observations has been uncovered only recently, which points to the direct involvement of PTEN in chromatin dynamics. PTEN interacts with histone H1 to maintain chromatin condensation and heterochromatin structure [152, 153]. Loss of PTEN hinders H1-mediated loading of HP1α. Consequently, this promotes chromatin decondensation and heterochromatin disruption. Aside from the impedance of chromatin loading of histone H1 and HP1α, PTEN null cells also exhibit an increase in acetylation of histone H4K16, a post-translational modification associated with relaxed chromatin and disrupted nucleosome interaction [34, 165]. Moreover, PTEN physically interacts with the chromosome architecture protein topoisomerase II to facilitate the resolution of chromatin precatenanes and tangled chromosomes [166]. In the absence of PTEN, DNA decatenation defects give rise to mitotic aberrations manifested by anaphase bridges. In the presence of erroneous decatenation machinery, defective spindle architecture during mitosis induces chromosome missegregation, leading to further impairment of genomic inheritance. We have observed that loss of PTEN causes aberrant mitotic spindle geometry, massive chromosome misalignment and mitotic catastrophe. As a result of chromatin and chromosome architecture deregulation, PTEN mutations and functional deficiency have been associated with defective DNA replication, chromosome missegregation, massive chromosome aberrations and a variety of tumors [156, 167–170]. Therefore, the role of PTEN in regulating chromatin organization directly contributes to genomic stability and tumor suppression.
Chromatin and chromosome condensation: the verdict
All DNA-templated processes including transcription, replication, and repair are associated with chromatin opening and accessibility. In this scenario, an overall state of chromatin condensation appears to hinder accessibility by blocking chromatin loading of cognate machineries. In fact, an intrinsically condensed chromatin conformation maintained by both constitutive and facultative architectural factors is of fundamental importance in the maintenance of normal transcription profiles and genome stability. For example, the linker histone H1 is a constitutive architectural protein that maintains higher-order chromatin structure. A recent study demonstrated that reduced expression or chromatin association of H1 impairs the recruitment of DNA repair factors to damage-flanking chromatin [137]. PTEN uses its C-terminal region to bind H1, to maintain heterochromatin structure and to suppress the transcription machinery [152], whereas the deletion of this region causes oncogene activation, centromere instability, and tumor development [170].
Although chromatin and chromosome condensation are a fundamental facet of cellular function at various cell cycle stages, condensation must not only be maintained to an appropriate degree but also carefully coordinated both spatially and temporally to accommodate specific genome activities. Aberrant chromatin remodeling often results in chromosome abnormality, leading to genomic instability. For example, loss of PTEN induces chromatin decondensation and hyperacetylation of H4K16 [152], which prevents chromatin loading of essential replication factors, leading to intra-S checkpoint defects and premature progression into mitosis [167]. Interestingly, a recent report suggested that enforced recruitment of a histone-modification factor causes unscheduled DNA repair in mitosis, which can result in sister telomere fusion and aneuploidy [171]. Comparably, incomplete genome duplication due to replication stress often results in unscheduled DNA replication in mitosis, which impairs the mitotic chromosome condensation process, giving rise to chromosome gaps and breaks [172]. A nuclease-mediated pathway was found to promote DNA synthesis at these chromosome fragile sites and as a result, chromosome missegregation and non-disjunction was reduced. However, this might represent merely a transient consequence within the same cell cycle whereas the long-term outcome may be unfavorable in terms of genomic stability. Indeed, the unscheduled mitotic DNA synthesis was found more frequently in aneuploidy cancer cells that exhibit intrinsically high levels of chromosomal instability [172]. These data suggest that the inappropriate timing of DNA replication impairs mitotic chromosome compaction, resulting in aneuploidy. Therefore, a temporal and spatial orchestration of chromatin condensation is fundamentally necessary to prevent genomic instability.
Impaired chromatin condensation and cancer
The discovery of the epigenome sheds light on the etiology for cancer independent of genetic mutations, which integrates and processes the environmental signals, influencing the transcription and transmission of the genome [173]. Numerous recent studies explicated the mechanism of specific epigenetic modifications and remodelers upon the development and progression of cancer, leading this field into an unprecedented depth of understanding. Moreover, emerging research has revealed epigenomic profiles of various diseases including cancer; thus, the functional and mechanistic relationship between chromatin compaction and the fidelity of genome and epigenome inheritance is critical to the illumination of cancer evolution mechanisms. Here we highlight the perspective of deregulated chromatin architecture in neoplasms, using the frequently mutated PTEN gene as an example.
As a powerful and broad-spectrum tumor suppressor, PTEN physically interacts with histone H1 through its C-terminal domain and guards the condensed state of chromatin. Loss of PTEN induces H4K16 acetylation level, which results in the relaxation of chromatin and alteration of gene expression profiles including oncogene upregulation [152]. Specific deletion of the PTEN C-terminal domain results in multiple forms of genomic instability and leads to development of various cancers and premalignant lesions [170]. Similarly, in acute myelogenous leukemia cells, the enlarged and finely distributed nuclear chromatin indicates a decondensed chromatin state, which arises from enhanced leukemia-related gene expression [174].
Cancer evolves by overriding fundamental genomic transmission machineries such as DNA replication, repair, and segregation. Recently, we and others have found that PTEN, which sustains chromosome condensation and heterochromatin structure [152, 153], mediates fork progression and recovery during replication via recruitment of replication factor 1, Rad51 recombinase, and MCM2 helicase [167–169]. These studies highlight the essential role of PTEN in maintaining chromatin condensation and ensuring efficient recruitment of vital replication factors for genome duplication. Moreover, PTEN has also been reported to act on chromatin to regulate the repair of DNA double-strand breakage [156, 175]. Furthermore, our most recent study uncovered the essential roles of PTEN in controlling of DNA decatenation [166]. Interestingly, loss of PTEN induces chromatin decondensation and hyperacetylation of H4K16 [152], which prevents chromatin loading of essential replication factors, leading to intra-S checkpoint defects and premature progression into mitosis [167]. These findings collectively illustrate how PTEN guards fundamental and successive cell cycle events including DNA replication, decatenation, and repair in the context of chromatin condensation to ensure genome and epigenome stability.
During malignant transformation, cells acquire epigenetic alterations including abnormal DNA methylation, aberrant histone modification, and impaired heterochromatin structure [176]. Abnormal DNA methylation was the first epigenetic change described in human cancers, which is associated with mutations in DNA methyltransferases as well as a global loss of H4 modifications [177, 178]. Knockout of the H3K9 methyltransferases Suv39h1/2 causes heterochromatin instability, aneuploidy, and B cell lymphoma [179]—this highlights the importance of heterochromatin in protection against genomic instability. Advances in genome-wide sequencing technology have uncovered diverse mutations of genes encoding chromatin remodeling enzymes as well as histones themselves [180]. For example, mutations of histone H3, H3K27M and H3G34R/V, have been identified as driver mutations in pediatric glioma [181, 182]. A better understanding of the etiology of cancer opens a world of potential treatments, and of the mechanism in which external environmental factors cooperate with internal genome/epigenome instability in tumorigenesis is a new area worthy further exploration.
Conclusions and perspectives
The organization of the eukaryotic genome in both interphase and mitosis, particularly in steady states, has been extensively explored and progressively clarified. However, the relationship between chromatin reorganization and the concurrently ongoing DNA replication and chromosome segregation remains murky. Understanding the 4D interaction between the genome and the epigenome, in both high resolution and real time, demands further investigation of chromatin topology and dynamics, as well as the functional coordination with genetic inheritance.
This review largely discussed the impact of chromatin structure and epigenetic state on the genome and genetic transmission. However, this demonstrates solely one side of the coin—in fact, the genome–epigenome interplay is bidirectional and may very well be cyclic. Thus, cancer may evolve as a consequence of aberrant reprogramming and reshaping processes that are mutual and can be triggered by either a gene mutation or a spatiotemporal error of chromatin dynamics. This relationship is attested by a PTEN mutation-induced cascade of chromatin decondensation, chromosome instability, and tumorigenesis. As 3C and imaging techniques for real-time study of chromosome conformation and dynamics become more feasible, a live picture will better demonstrate the intricate interplay between chromatin architecture and DNA behavior. Further application of these advanced technologies to the samples of cancer patients and preclinical tumor models will provide invaluable information to elucidate how impairment of the genome–epigenome crosstalk leads to cancer evolution. As it becomes more evident that genome instability and epigenome deregulation are both central hallmarks of cancer, better therapeutic strategies can be devised to target genetic or epigenetic alterations that trigger the signaling cascade leading to tumorigenesis.
Acknowledgments
Work in the authors’ laboratory is supported by NIH Grant R01GM100478.
References
- 1.Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 2.Schneider R, Grosschedl R. Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev. 2007;21:3027–3043. doi: 10.1101/gad.1604607. [DOI] [PubMed] [Google Scholar]
- 3.Phillips-Cremins JE, Sauria ME, Sanyal A, et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281–1295. doi: 10.1016/j.cell.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shachar S, Voss TC, Pegoraro G, et al. Identification of gene positioning factors using high-throughput imaging mapping. Cell. 2015;162:911–923. doi: 10.1016/j.cell.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowen JM, Fan ZP, Hnisz D, et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell. 2014;159:374–387. doi: 10.1016/j.cell.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibcus JH, Dekker J. The hierarchy of the 3D genome. Mol Cell. 2013;49:773–782. doi: 10.1016/j.molcel.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naumova N, Imakaev M, Fudenberg G, et al. Organization of the mitotic chromosome. Science. 2013;342:948–953. doi: 10.1126/science.1236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daban JR. Stacked thin layers of metaphase chromatin explain the geometry of chromosome rearrangements and banding. Sci Rep. 2015;5:14891. doi: 10.1038/srep14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigoryev SA, Bascom G, Buckwalter JM, et al. Hierarchical looping of zigzag nucleosome chains in metaphase chromosomes. Proc Natl Acad Sci USA. 2016;113:1238–1243. doi: 10.1073/pnas.1518280113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Balbas MA, Dey A, Rabindran SK, et al. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 1995;83:29–38. doi: 10.1016/0092-8674(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 11.Heijink AM, Krajewska M, van Vugt MA. The DNA damage response during mitosis. Mutat Res. 2013;750:45–55. doi: 10.1016/j.mrfmmm.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Dekker J, Rippe K, Dekker M, et al. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 13.de Laat W, Dekker J. 3C-based technologies to study the shape of the genome. Methods. 2012;58:189–191. doi: 10.1016/j.ymeth.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon JR, Selvaraj S, Yue F, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman-Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao SS, Huntley MH, Durand NC, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 19.Davey CA, Sargent DF, Luger K, et al. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 Å resolution. J Mol Biol. 2002;319:1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
- 20.Luger K, Mader AW, Richmond RK, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Fischle W, Cheung W, et al. Beyond the double helix: writing and reading the histone code. Novartis Found Symp. 2004;259:3–17. doi: 10.1002/0470862637.ch2. [DOI] [PubMed] [Google Scholar]
- 22.Hansen JC, Tse C, Wolffe AP. Structure and function of the core histone N-termini: more than meets the eye. Biochemistry. 1998;37:17637–17641. doi: 10.1021/bi982409v. [DOI] [PubMed] [Google Scholar]
- 23.Tropberger P, Schneider R. Scratching the (lateral) surface of chromatin regulation by histone modifications. Nat Struct Mol Biol. 2013;20:657–661. doi: 10.1038/nsmb.2581. [DOI] [PubMed] [Google Scholar]
- 24.Bartova E, Krejci J, Harnicarova A, et al. Histone modifications and nuclear architecture: a review. J Histochem Cytochem. 2008;56:711–721. doi: 10.1369/jhc.2008.951251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JK, Howe LJ. Histone acetylation: truth of consequences? Biochem Cell Biol. 2009;87:139–150. doi: 10.1139/O08-112. [DOI] [PubMed] [Google Scholar]
- 27.Murray K. The occurrence of epsilon-N-methyl lysine in histones. Biochemistry. 1964;3:10–15. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- 28.Byvoet P, Shepherd GR, Hardin JM, et al. The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch Biochem Biophys. 1972;148:558–567. doi: 10.1016/0003-9861(72)90174-9. [DOI] [PubMed] [Google Scholar]
- 29.Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–R551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Hayes JJ. Acetylation mimics within individual core histone tail domains indicate distinct roles in regulating the stability of higher-order chromatin structure. Mol Cell Biol. 2008;28:227–236. doi: 10.1128/MCB.01245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliva R, Bazett-Jones DP, Locklear L, et al. Histone hyperacetylation can induce unfolding of the nucleosome core particle. Nucleic Acids Res. 1990;18:2739–2747. doi: 10.1093/nar/18.9.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 34.Shogren-Knaak M, Ishii H, Sun JM, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 35.Akhtar A, Becker PB. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell. 2000;5:367–375. doi: 10.1016/S1097-2765(00)80431-1. [DOI] [PubMed] [Google Scholar]
- 36.Osley MA. The regulation of histone synthesis in the cell cycle. Annu Rev Biochem. 1991;60:827–861. doi: 10.1146/annurev.bi.60.070191.004143. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell. 2002;9:1191–1200. doi: 10.1016/S1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad K, Henikoff S. Histone H3 variants specify modes of chromatin assembly. Proc Natl Acad Sci USA. 2002;99(Suppl 4):16477–16484. doi: 10.1073/pnas.172403699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulard M, Bouvet P, Kundu TK, et al. Histone variant nucleosomes: structure, function and implication in disease. Subcell Biochem. 2007;41:71–89. [PubMed] [Google Scholar]
- 40.Happel N, Doenecke D. Histone H1 and its isoforms: contribution to chromatin structure and function. Gene. 2009;431:1–12. doi: 10.1016/j.gene.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Terme JM, Sese B, Millan-Arino L, et al. Histone H1 variants are differentially expressed and incorporated into chromatin during differentiation and reprogramming to pluripotency. J Biol Chem. 2011;286:35347–35357. doi: 10.1074/jbc.M111.281923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamakaka RT, Biggins S. Histone variants: deviants? Genes Dev. 2005;19:295–310. doi: 10.1101/gad.1272805. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Capetillo O, Lee A, Nussenzweig M, et al. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Redon C, Pilch D, Rogakou E, et al. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/S0959-437X(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 45.Ausio J, Abbott DW. The many tales of a tail: carboxyl-terminal tail heterogeneity specializes histone H2A variants for defined chromatin function. Biochemistry. 2002;41:5945–5949. doi: 10.1021/bi020059d. [DOI] [PubMed] [Google Scholar]
- 46.Millar CB. Organizing the genome with H2A histone variants. Biochem J. 2013;449:567–579. doi: 10.1042/BJ20121646. [DOI] [PubMed] [Google Scholar]
- 47.Szilard RK, Jacques PE, Laramee L, et al. Systematic identification of fragile sites via genome-wide location analysis of gamma-H2AX. Nat Struct Mol Biol. 2010;17:299–305. doi: 10.1038/nsmb.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo J, Kim SC, Lee HS, et al. Genome-wide profiles of H2AX and gamma-H2AX differentiate endogenous and exogenous DNA damage hotspots in human cells. Nucleic Acids Res. 2012;40:5965–5974. doi: 10.1093/nar/gks287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celeste A, Petersen S, Romanienko PJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celeste A, Difilippantonio S, Difilippantonio MJ, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–383. doi: 10.1016/S0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filipescu D, Muller S, Almouzni G. Histone H3 variants and their chaperones during development and disease: contributing to epigenetic control. Annu Rev Cell Dev Biol. 2014;30:615–646. doi: 10.1146/annurev-cellbio-100913-013311. [DOI] [PubMed] [Google Scholar]
- 52.Valdivia MM, Hamdouch K, Ortiz M, et al. CENPA a genomic marker for centromere activity and human diseases. Curr Genomics. 2009;10:326–335. doi: 10.2174/138920209788920985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKinley KL, Cheeseman IM. Polo-like kinase 1 licenses CENP-A deposition at centromeres. Cell. 2014;158:397–411. doi: 10.1016/j.cell.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adolph KW, Cheng SM, Laemmli UK. Role of nonhistone proteins in metaphase chromosome structure. Cell. 1977;12:805–816. doi: 10.1016/0092-8674(77)90279-3. [DOI] [PubMed] [Google Scholar]
- 55.Paulson JR, Laemmli UK. The structure of histone-depleted metaphase chromosomes. Cell. 1977;12:817–828. doi: 10.1016/0092-8674(77)90280-X. [DOI] [PubMed] [Google Scholar]
- 56.Lewis CD, Laemmli UK. Higher order metaphase chromosome structure: evidence for metalloprotein interactions. Cell. 1982;29:171–181. doi: 10.1016/0092-8674(82)90101-5. [DOI] [PubMed] [Google Scholar]
- 57.Earnshaw WC, Halligan B, Cooke CA, et al. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985;100:1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gasser SM, Laroche T, Falquet J, et al. Metaphase chromosome structure. Involvement of topoisomerase II. J Mol Biol. 1986;188:613–629. doi: 10.1016/S0022-2836(86)80010-9. [DOI] [PubMed] [Google Scholar]
- 59.Saitoh N, Goldberg IG, Wood ER, et al. ScII: an abundant chromosome scaffold protein is a member of a family of putative ATPases with an unusual predicted tertiary structure. J Cell Biol. 1994;127:303–318. doi: 10.1083/jcb.127.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–521. doi: 10.1016/S0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- 61.Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/S0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 62.Guacci V, Koshland D, Strunnikov A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae . Cell. 1997;91:47–57. doi: 10.1016/S0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barbero JL. Cohesins: chromatin architects in chromosome segregation, control of gene expression and much more. Cell Mol Life Sci. 2009;66:2025–2035. doi: 10.1007/s00018-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirano T. Condensins: organizing and segregating the genome. Curr Biol. 2005;15:R265–R275. doi: 10.1016/j.cub.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 65.Potts PR. The Yin and Yang of the MMS21-SMC5/6 SUMO ligase complex in homologous recombination. DNA Repair (Amst) 2009;8:499–506. doi: 10.1016/j.dnarep.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 66.De Piccoli G, Torres-Rosell J, Aragon L. The unnamed complex: what do we know about Smc5–Smc6? Chromosome Res. 2009;17:251–263. doi: 10.1007/s10577-008-9016-8. [DOI] [PubMed] [Google Scholar]
- 67.Ball AR, Jr, Yokomori K. The structural maintenance of chromosomes (SMC) family of proteins in mammals. Chromosome Res. 2001;9:85–96. doi: 10.1023/A:1009287518015. [DOI] [PubMed] [Google Scholar]
- 68.Sjogren C, Nasmyth K. Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae . Curr Biol. 2001;11:991–995. doi: 10.1016/S0960-9822(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 69.Strom L, Lindroos HB, Shirahige K, et al. Postreplicative recruitment of cohesin to double-strand breaks is required for DNA repair. Mol Cell. 2004;16:1003–1015. doi: 10.1016/j.molcel.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 70.Degner SC, Verma-Gaur J, Wong TP, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci USA. 2011;108:9566–9571. doi: 10.1073/pnas.1019391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan J, Enge M, Whitington T, et al. Transcription factor binding in human cells occurs in dense clusters formed around cohesin anchor sites. Cell. 2013;154:801–813. doi: 10.1016/j.cell.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 72.Heale JT, Ball AR, Jr, Schmiesing JA, et al. Condensin I interacts with the PARP-1-XRCC1 complex and functions in DNA single-strand break repair. Mol Cell. 2006;21:837–848. doi: 10.1016/j.molcel.2006.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallace HA, Bosco G. Condensins and 3D organization of the interphase nucleus. Curr Genet Med Rep. 2013;1:219–229. doi: 10.1007/s40142-013-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piazza I, Haering CH, Rutkowska A. Condensin: crafting the chromosome landscape. Chromosoma. 2013;122:175–190. doi: 10.1007/s00412-013-0405-1. [DOI] [PubMed] [Google Scholar]
- 75.Hirano T. At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- 76.Shintomi K, Hirano T. The relative ratio of condensin I to II determines chromosome shapes. Genes Dev. 2011;25:1464–1469. doi: 10.1101/gad.2060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee J, Ogushi S, Saitou M, et al. Condensins I and II are essential for construction of bivalent chromosomes in mouse oocytes. Mol Biol Cell. 2011;22:3465–3477. doi: 10.1091/mbc.E11-05-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Green LC, Kalitsis P, Chang TM, et al. Contrasting roles of condensin I and condensin II in mitotic chromosome formation. J Cell Sci. 2012;125:1591–1604. doi: 10.1242/jcs.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Onn I, Aono N, Hirano M, et al. Reconstitution and subunit geometry of human condensin complexes. EMBO J. 2007;26:1024–1034. doi: 10.1038/sj.emboj.7601562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haarhuis JH, Elbatsh AM, Rowland BD. Cohesin and its regulation: on the logic of X-shaped chromosomes. Dev Cell. 2014;31:7–18. doi: 10.1016/j.devcel.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Liu LF, Liu CC, Alberts BM. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980;19:697–707. doi: 10.1016/S0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- 82.Brown PO, Cozzarelli NR. A sign inversion mechanism for enzymatic supercoiling of DNA. Science. 1979;206:1081–1083. doi: 10.1126/science.227059. [DOI] [PubMed] [Google Scholar]
- 83.Gellert M, Mizuuchi K, O’Dea MH, et al. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goto T, Wang JC. Yeast DNA topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J Biol Chem. 1982;257:5866–5872. [PubMed] [Google Scholar]
- 85.Hsieh T, Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980;21:115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- 86.Roca J, Wang JC. The probabilities of supercoil removal and decatenation by yeast DNA topoisomerase II. Genes Cells. 1996;1:17–27. doi: 10.1046/j.1365-2443.1996.01001.x. [DOI] [PubMed] [Google Scholar]
- 87.Vologodskii AV, Zhang W, Rybenkov VV, et al. Mechanism of topology simplification by type II DNA topoisomerases. Proc Natl Acad Sci USA. 2001;98:3045–3049. doi: 10.1073/pnas.061029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charvin G, Bensimon D, Croquette V. Single-molecule study of DNA unlinking by eukaryotic and prokaryotic type-II topoisomerases. Proc Natl Acad Sci USA. 2003;100:9820–9825. doi: 10.1073/pnas.1631550100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baxter J, Sen N, Martinez VL, et al. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science. 2011;331:1328–1332. doi: 10.1126/science.1201538. [DOI] [PubMed] [Google Scholar]
- 90.Downes CS, Clarke DJ, Mullinger AM, et al. A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature. 1994;372:467–470. doi: 10.1038/372467a0. [DOI] [PubMed] [Google Scholar]
- 91.Luo K, Yuan J, Chen J, et al. Topoisomerase IIalpha controls the decatenation checkpoint. Nat Cell Biol. 2009;11:204–210. doi: 10.1038/ncb1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vos SM, Tretter EM, Schmidt BH, et al. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol. 2011;12:827–841. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maeshima K, Laemmli UK. A two-step scaffolding model for mitotic chromosome assembly. Dev Cell. 2003;4:467–480. doi: 10.1016/S1534-5807(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 95.James TC, Elgin SC. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol Cell Biol. 1986;6:3862–3872. doi: 10.1128/MCB.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krouwels IM, Wiesmeijer K, Abraham TE, et al. A glue for heterochromatin maintenance: stable SUV39H1 binding to heterochromatin is reinforced by the SET domain. J Cell Biol. 2005;170:537–549. doi: 10.1083/jcb.200502154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Cao M, O’Sullivan R, Peters AHFM, et al. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet. 2004;36:94–99. doi: 10.1038/ng1278. [DOI] [PubMed] [Google Scholar]
- 98.Canzio D, Chang EY, Shankar S, et al. Chromodomain-mediated oligomerization of HP1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol Cell. 2011;41:67–81. doi: 10.1016/j.molcel.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Canzio D, Liao M, Naber N, et al. A conformational switch in HP1 releases auto-inhibition to drive heterochromatin assembly. Nature. 2013;496:377–381. doi: 10.1038/nature12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teif VB, Kepper N, Yserentant K, et al. Affinity, stoichiometry and cooperativity of heterochromatin protein 1 (HP1) binding to nucleosomal arrays. J Phys Condens Matter. 2015;27:064110. doi: 10.1088/0953-8984/27/6/064110. [DOI] [PubMed] [Google Scholar]
- 101.Zeng W, Ball AR, Jr, Yokomori K. HP1: heterochromatin binding proteins working the genome. Epigenetics. 2010;5:287–292. doi: 10.4161/epi.5.4.11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luijsterburg MS, Dinant C, Lans H, et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J Cell Biol. 2009;185:577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baniahmad A, Steiner C, Kohne AC, et al. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–514. doi: 10.1016/0092-8674(90)90532-J. [DOI] [PubMed] [Google Scholar]
- 104.Lobanenkov VV, Nicolas RH, Adler VV, et al. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene. 1990;5:1743–1753. [PubMed] [Google Scholar]
- 105.Ong CT, Corces VG. CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet. 2014;15:234–246. doi: 10.1038/nrg3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heger P, Marin B, Bartkuhn M, et al. The chromatin insulator CTCF and the emergence of metazoan diversity. Proc Natl Acad Sci USA. 2012;109:17507–17512. doi: 10.1073/pnas.1111941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim TH, Abdullaev ZK, Smith AD, et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vietri Rudan M, Barrington C, Henderson S, et al. Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell Rep. 2015;10:1297–1309. doi: 10.1016/j.celrep.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hadjur S, Williams LM, Ryan NK, et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–413. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zuin J, Dixon JR, van der Reijden MI, et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci USA. 2014;111:996–1001. doi: 10.1073/pnas.1317788111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brien GL, Bracken AP. Transcriptomics: unravelling the biology of transcription factors and chromatin remodelers during development and differentiation. Semin Cell Dev Biol. 2009;20:835–841. doi: 10.1016/j.semcdb.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 112.Erdel F, Krug J, Langst G, et al. Targeting chromatin remodelers: signals and search mechanisms. Biochim Biophys Acta. 2011;1809:497–508. doi: 10.1016/j.bbagrm.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 113.Langst G, Manelyte L. Chromatin remodelers: from function to dysfunction. Genes (Basel) 2015;6:299–324. doi: 10.3390/genes6020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hauk G, Berger JM. The role of ATP-dependent machines in regulating genome topology. Curr Opin Struct Biol. 2016;36:85–96. doi: 10.1016/j.sbi.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dekker J, Marti-Renom MA, Mirny LA. Exploring the three-dimensional organization of genomes: interpreting chromatin interaction data. Nat Rev Genet. 2013;14:390–403. doi: 10.1038/nrg3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dekker J. Two ways to fold the genome during the cell cycle: insights obtained with chromosome conformation capture. Epigenetics Chromatin. 2014;7:25. doi: 10.1186/1756-8935-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma Y, Kanakousaki K, Buttitta L. How the cell cycle impacts chromatin architecture and influences cell fate. Front Genet. 2015;6:19. doi: 10.3389/fgene.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pope BD, Ryba T, Dileep V, et al. Topologically associating domains are stable units of replication-timing regulation. Nature. 2014;515:402–405. doi: 10.1038/nature13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 120.MacAlpine HK, Gordan R, Powell SK, et al. Drosophila ORC localizes to open chromatin and marks sites of cohesin complex loading. Genome Res. 2010;20:201–211. doi: 10.1101/gr.097873.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dorn ES, Cook JG. Nucleosomes in the neighborhood. Epigenetics. 2014;6:552–559. doi: 10.4161/epi.6.5.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karnani N, Taylor CM, Malhotra A, et al. Genomic study of replication initiation in human chromosomes reveals the influence of transcription regulation and chromatin structure on origin selection. Mol Biol Cell. 2010;21:393–404. doi: 10.1091/mbc.E09-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goren A, Tabib A, Hecht M, et al. DNA replication timing of the human beta-globin domain is controlled by histone modification at the origin. Genes Dev. 2008;22:1319–1324. doi: 10.1101/gad.468308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Y, Price BD. Chromatin dynamics and the repair of DNA double strand breaks. Cell Cycle. 2011;10:261–267. doi: 10.4161/cc.10.2.14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huyen Y, Zgheib O, Ditullio RA, Jr, et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature. 2004;432:406–411. doi: 10.1038/nature03114. [DOI] [PubMed] [Google Scholar]
- 126.Ohsawa R, Seol J-H, Tyler JK. At the intersection of non-coding transcription, DNA repair, chromatin structure, and cellular senescence. Front Genet. 2013;4:136. doi: 10.3389/fgene.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tsukuda T, Fleming AB, Nickoloff JA, et al. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae . Nature. 2005;438:379–383. doi: 10.1038/nature04148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khurana S, Kruhlak MJ, Kim J, et al. A macrohistone variant links dynamic chromatin compaction to BRCA1-dependent genome maintenance. Cell Rep. 2014;8:1049–1062. doi: 10.1016/j.celrep.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baldeyron C, Soria G, Roche D, et al. HP1alpha recruitment to DNA damage by p150CAF-1 promotes homologous recombination repair. J Cell Biol. 2011;193:81–95. doi: 10.1083/jcb.201101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mailand N, Bekker-Jensen S, Faustrup H, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 131.Huen MS, Grant R, Manke I, et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kolas NK, Chapman JR, Nakada S, et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doil C, Mailand N, Bekker-Jensen S, et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell. 2009;136:435–446. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 134.Stewart GS, Panier S, Townsend K, et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell. 2009;136:420–434. doi: 10.1016/j.cell.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 135.Jackson SP, Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell. 2013;49:795–807. doi: 10.1016/j.molcel.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 136.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/S0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 137.Thorslund T, Ripplinger A, Hoffmann S, et al. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature. 2015;527:389–393. doi: 10.1038/nature15401. [DOI] [PubMed] [Google Scholar]
- 138.Khan WA, Rogan PK, Knoll JH. Localized, non-random differences in chromatin accessibility between homologous metaphase chromosomes. Mol Cytogenet. 2014;7:70. doi: 10.1186/s13039-014-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ono T, Losada A, Hirano M, et al. Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 2003;115:109–121. doi: 10.1016/S0092-8674(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 140.Koshland D, Strunnikov A. Mitotic chromosome condensation. Annu Rev Cell Dev Biol. 1996;12:305–333. doi: 10.1146/annurev.cellbio.12.1.305. [DOI] [PubMed] [Google Scholar]
- 141.Liang Z, Zickler D, Prentiss M, et al. Chromosomes progress to metaphase in multiple discrete steps via global compaction/expansion cycles. Cell. 2015;161:1124–1137. doi: 10.1016/j.cell.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shintomi K, Takahashi TS, Hirano T. Reconstitution of mitotic chromatids with a minimum set of purified factors. Nat Cell Biol. 2015;17:1014–1023. doi: 10.1038/ncb3187. [DOI] [PubMed] [Google Scholar]
- 143.Hirano T. Condensins: universal organizers of chromosomes with diverse functions. Genes Dev. 2012;26:1659–1678. doi: 10.1101/gad.194746.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alipour E, Marko JF. Self-organization of domain structures by DNA-loop-extruding enzymes. Nucleic Acids Res. 2012;40:11202–11212. doi: 10.1093/nar/gks925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu W, Tanasa B, Tyurina OV, et al. PHF8 mediates histone H4 lysine 20 demethylation events involved in cell cycle progression. Nature. 2010;466:508–512. doi: 10.1038/nature09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilkins BJ, Rall NA, Ostwal Y, et al. A cascade of histone modifications induces chromatin condensation in mitosis. Science. 2014;343:77–80. doi: 10.1126/science.1244508. [DOI] [PubMed] [Google Scholar]
- 147.Hendzel MJ, Wei Y, Mancini MA, et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 148.Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 149.Zhou J, Fan JY, Rangasamy D, et al. The nucleosome surface regulates chromatin compaction and couples it with transcriptional repression. Nat Struct Mol Biol. 2007;14:1070–1076. doi: 10.1038/nsmb1323. [DOI] [PubMed] [Google Scholar]
- 150.Fazzio TG, Panning B. Condensin complexes regulate mitotic progression and interphase chromatin structure in embryonic stem cells. J Cell Biol. 2010;188:491–503. doi: 10.1083/jcb.200908026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murnion ME, Adams RR, Callister DM, et al. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- 152.Chen ZH, Zhu M, Yang J, et al. PTEN interacts with histone H1 and controls chromatin condensation. Cell Rep. 2014;8:2003–2014. doi: 10.1016/j.celrep.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gong L, Govan JM, Evans EB, et al. Nuclear PTEN tumor-suppressor functions through maintaining heterochromatin structure. Cell Cycle. 2015 doi: 10.1080/15384101.2015.1044174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Puc J, Keniry M, Li HS, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 156.Shen WH, Balajee AS, Wang J, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 157.Maser RS, Choudhury B, Campbell PJ, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ehlers JP, Worley L, Onken MD, et al. Integrative genomic analysis of aneuploidy in uveal melanoma. Clin Cancer Res. 2008;14:115–122. doi: 10.1158/1078-0432.CCR-07-1825. [DOI] [PubMed] [Google Scholar]
- 159.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3 K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li G, Hu Y, Huo Y, et al. PTEN deletion leads to up-regulation of a secreted growth factor pleiotrophin. J Biol Chem. 2006;281:10663–10668. doi: 10.1074/jbc.M512509200. [DOI] [PubMed] [Google Scholar]
- 161.Mulholland DJ, Kobayashi N, Ruscetti M, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vivanco I, Palaskas N, Tran C, et al. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 163.Hong TM, Yang PC, Peck K, et al. Profiling the downstream genes of tumor suppressor PTEN in lung cancer cells by complementary DNA microarray. Am J Respir Cell Mol Biol. 2000;23:355–363. doi: 10.1165/ajrcmb.23.3.4002. [DOI] [PubMed] [Google Scholar]
- 164.Matsushima-Nishiu M, Unoki M, Ono K, et al. Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res. 2001;61:3741–3749. [PubMed] [Google Scholar]
- 165.Bodor DL, Mata JF, Sergeev M, et al. The quantitative architecture of centromeric chromatin. Elife. 2014;3:e02137. doi: 10.7554/eLife.02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kang X, Song C, Du X, et al. PTEN stabilizes TOP2A and regulates the DNA decatenation. Sci Rep. 2015;5:17873–17884. doi: 10.1038/srep17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.He J, Kang X, Yin Y, et al. PTEN regulates DNA replication progression and stalled fork recovery. Nat Commun. 2015;6:7620. doi: 10.1038/ncomms8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang G, Li Y, Wang P, et al. PTEN regulates RPA1 and protects DNA replication forks. Cell Res. 2015;25:1189–1204. doi: 10.1038/cr.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Feng J, Liang J, Li J, et al. PTEN controls the DNA replication process through MCM2 in response to replicative stress. Cell Rep. 2015;13:1295–1303. doi: 10.1016/j.celrep.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 170.Sun Z, Huang C, He J, et al. PTEN C-terminal deletion causes genomic instability and tumor development. Cell Rep. 2014;6:844–854. doi: 10.1016/j.celrep.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Orthwein A, Fradet-Turcotte A, Noordermeer SM, et al. Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science. 2014;344:189–193. doi: 10.1126/science.1248024. [DOI] [PubMed] [Google Scholar]
- 172.Minocherhomji S, Ying S, Bjerregaard VA, et al. Replication stress activates DNA repair synthesis in mitosis. Nature. 2015;528:286–290. doi: 10.1038/nature16139. [DOI] [PubMed] [Google Scholar]
- 173.Brookes E, Shi Y. Diverse epigenetic mechanisms of human disease. Annu Rev Genet. 2014;48:237–268. doi: 10.1146/annurev-genet-120213-092518. [DOI] [PubMed] [Google Scholar]
- 174.Trencsenyi G, Nagy G, Bako F, et al. Incomplete chromatin condensation in enlarged rat myelocytic leukemia cells. DNA Cell Biol. 2012;31:470–478. doi: 10.1089/dna.2011.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bassi C, Ho J, Srikumar T, et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341:395–399. doi: 10.1126/science.1236188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Morgan MA, Shilatifard A. Chromatin signatures of cancer. Genes Dev. 2015;29:238–249. doi: 10.1101/gad.255182.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 2005;45:629–656. doi: 10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- 178.Fraga MF, Ballestar E, Villar-Garea A, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 179.Peters AH, O’Carroll D, Scherthan H, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/S0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 180.Van Rechem C, Whetstine JR. Examining the impact of gene variants on histone lysine methylation. Biochim Biophys Acta. 2014;1839:1463–1476. doi: 10.1016/j.bbagrm.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 182.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]