Abstract
Objectives
The World Health Organization called for the elimination of maternal-to-child transmission (MTCT) of HIV and syphilis, a harmonized approach for the improvement of health outcomes for mothers and children. Testing early in pregnancy, treating seropositive pregnant women, and preventing syphilis re-infection can prevent MTCT of HIV and syphilis. We assessed the health and economic outcomes of a dual testing strategy in a simulated cohort of 100,000 antenatal care patients in Malawi.
Methods
We compared four screening algorithms: (1) HIV rapid test only, (2) dual HIV and syphilis rapid tests, (3) single rapid tests for HIV and syphilis, and (4) HIV rapid and syphilis laboratory tests. We calculated the expected number of adverse pregnancy outcomes, the expected costs, and the expected newborn disability adjusted life years (DALYs) for each screening algorithm. The estimated costs and DALYs for each screening algorithm were assessed from a societal perspective using Markov progression models. Additionally, we conducted a Monte Carlo multi-way sensitivity analysis, allowing for ranges of inputs.
Results
Our cohort decision model predicted the lowest number of adverse pregnancy outcomes in the dual HIV and syphilis rapid test strategy. Additionally, from the societal perspective, the costs of prevention and care using a dual HIV and syphilis rapid testing strategy was both the least costly ($226.92 per pregnancy) and resulted in the fewest DALYs (116,639) per 100,000 pregnancies. In the Monte Carlo simulation the dual HIV and syphilis algorithm was always cost saving and almost always reduced disability adjusted life years (DALYs) compared to HIV testing alone.
Conclusion
The results of the cost-effectiveness analysis showed that a dual HIV and syphilis test was cost saving compared to all other screening strategies. Adding dual rapid testing to the existing prevention of mother-to-child HIV transmission programs in Malawi and similar countries is likely to be advantageous.
Keywords: HIV, syphilis, dual testing, cost-effectiveness, adverse pregnancy outcomes, screening, elimination of mother-to-child transmission of HIV and syphilis, EMTCT
MESH Keywords: HIV, Syphilis, Point of Care Technology, diagnosis, Cost-Effectiveness Analysis, Antenatal Screening, Prenatal Diagnosis
Introduction
In 2008, the global burden of active syphilis in pregnant women was estimated at 1.36 million women.1 Africa had the highest proportion of women with sero-positive syphilis tests during antenatal care, at 2.13% compared to all other regions.1 Without screening and treatment, maternal syphilis can lead to serious adverse pregnancy outcomes, including stillbirth, prematurity, low birth weight, neonatal mortality, and infant syphilis infection.2–5 Maternal treatment, which consists of a single intramuscular injection of benzathine penicillin,6 greatly reduces the risks of adverse pregnancy outcomes.7,8 However, syphilis screening has been inconsistent, primarily because of challenges associated with laboratory testing and until recently, low prioritization by local health systems and global governing bodies4,9.
In contrast, maternal HIV infection, which can also be transmitted to the infant, is screened for in a significantly larger proportion of pregnant women. Antenatal HIV testing and treatment has received tremendous support from donors and governments,4 leading to strengthened health systems and increased rates of case identification through point-of-care testing. Although antenatal HIV screening has been very successful, without syphilis screening babies continue to die from congenital syphilis.10 Implementation of syphilis point-of-care testing in resource limited settings has been more recent.11–17 The World Health Organization is calling for a harmonized approach to the elimination of HIV and syphilis.5 The similarity of interventions needed to prevent adverse pregnancy outcomes due to HIV and syphilis suggests that an integrated approach to the elimination of maternal-to-child transmission of HIV and syphilis is feasible. Dual elimination would address Millennium Development Goals 4, 5, and 6 by improving maternal and child health outcomes and reducing the spread of HIV infection.18
During antenatal care, screening for HIV and syphilis is the first step toward treatment and prevention of transmission. While rapid point-of-care HIV tests are consistently used in sub-Saharan Africa, screening options for syphilis vary considerably; there are currently options for rapid point-of-care tests, laboratory-based tests, and new dual point-of care tests that combine HIV and syphilis testing into a single rapid test.19 While the performance of those tests has been previously reported,19–23 to our knowledge, the cost-effectiveness of different HIV and syphilis screening programs including the use of dual HIV and syphilis tests, is not available. We undertook a cost-effectiveness analysis of various HIV and syphilis screening algorithms in order to provide funders and policy-makers with additional information into the costs and benefits of each option.
Methods
Procedures (Screening algorithms & Model structure)
We conducted a cost-effectiveness analysis of four HIV and syphilis screening algorithms that are currently used in antenatal care: (1) HIV rapid test only, (2) dual HIV and syphilis rapid test, (3) single rapid tests for HIV and syphilis, and (4) HIV rapid and syphilis laboratory-based tests. We assumed the laboratory tests were a combination of rapid plasma reagin and Treponema pallidum particle agglutination assay.24 Using a hypothetical cohort of 100,000 antenatal patients, we calculated the expected number of adverse pregnancy outcomes, the expected costs, and the expected newborn disability adjusted life years (DALYs) for each screening algorithm.25,26 We used disability weights of zero for losses due to stillbirth and miscarriages. The estimated costs and DALYs for each screening algorithm were assessed from a societal perspective using Markov progression models. The analytic horizon was the life expectancy of the child. Schematics of the Markov model can be seen in the web appendix [Figure w1].
Input parameters
We estimated the number of expected adverse pregnancy outcomes (fetal death or stillbirth, neonatal death, prematurity or low birth weight, congenital syphilis infection and HIV mother-to-child transmission) through a decision tree. Each pregnancy was assumed to be singleton. The tree incorporated the epidemiology of HIV and syphilis, the reported uptake for testing, test sensitivity, the likelihood of treatment versus loss-to-follow-up and the anticipated pregnancy outcomes given the woman’s disease and treatment status. All model inputs were determined from the published literature, unless otherwise stated (Table 1). When available, we used data from Malawi for the setting of the analysis. Malawi was chosen as a real example of a low-income economy in sub-Saharan Africa with endemic HIV and syphilis and a response similar to other countries in the region. Malawi has approximately 638,900 births annually, so our hypothetical cohort of 100,000 women presenting for antenatal care represented approximately one sixth of the country’s annual births.27 For the primary analysis we used an HIV prevalence at antenatal care of 10.6% with 24.8% of HIV-infected women presenting with AIDS.28,29 Syphilis prevalence among HIV uninfected women was 1.1% and among HIV infected women was 2.2%.28,29 For the single rapid syphilis test, results on sensitivity and specificity from 3 published studies were averaged and the range from the three was used for sensitivity analyses. 30-w2 Ranges used for sensitivity analyses were otherwise assumed using a 50% spread around the base case estimate or the 95% confidence interval from the literature. Each method for range estimation is displayed in Table 1. Variables that have strong evidence for inputs from the literature or the Malawi government reports were not ranged. Additionally, because the prevalence of adverse pregnancy outcomes in some women (e.g. those with untreated syphilis and HIV infection) totaled 100%, these outcomes were kept stable in the model without a range.
Table 1.
· Description, point values, range and sources for all variables used in a cost-effectiveness model of different algorithms of HIV and syphilis testing in pregnancy·
| Variable | Base-case value | Range | Sources |
|---|---|---|---|
| Maternal characteristics | |||
| Median age at first birth | 20 | w18 | |
|
| |||
| Disease prevalence | |||
| HIV Prevalence among pregnant women | 10·6% | 5·3%–15·9%† | 28 |
| AIDS prevalence among HIV infected pregnant women | 24·82% | Assumption | |
| Syphilis prevalence in HIV uninfected pregnant women | 1·09% | 0·54%–1·63%† | 28 |
| Syphilis prevalence in HIV infected pregnant women | 2·17% | 1·09%–3·26%† | 28 |
| History of syphilis infection (Adequately treated previous infections) in HIV uninfected women | 5% | 2·5%–7·5%† | Assumption |
| History of syphilis infection (Adequately treated previous infections) in HIV infected women | 10% | 5%–15%† | Assumption |
|
| |||
| Disease progression | |||
| Average progression time HIV to AIDS in treated child | 10 years | Assumption | |
| Average progression time AIDS to death in treated child | 5 years | Assumption | |
| Average progression time HIV to AIDS in untreated child | 1 year | Assumption | |
| Average progression time AIDS to death in treated child | 1 year | Assumption | |
|
| |||
| Test performance | |||
| Syphilis test sensitivity | |||
| Dual HIV and syphilis test | 0·89 | 0·84–0·94 | 20 |
| Syphilis rapid | 0·82 | 0·63–0·97 | 30 w1 w2 |
| Syphilis laboratory-based | 1 | 24 | |
| Syphilis test specificity | |||
| Dual HIV and syphilis test | 0·99 | 0·97–1.00 | 20 |
| Syphilis rapid | 0·96 | 0·92–0·99 | 30 w1 w2 |
| Syphilis Laboratory-based | 1 | w16 | |
| Syphilis test specificity in those with previously treated syphilis infection | |||
| Dual HIV and syphilis test | 0·91 | Assumption | |
| Syphilis rapid | 0·91 | w1 | |
| Syphilis laboratory-based | 1 | ||
| HIV Test sensitivity | |||
| Dual HIV and syphilis test | 0·99 | 0·95–1·00 | 20 |
| HIV rapid | 1 | w10 | |
| HIV Test specificity | |||
| Dual HIV and Syphilis test | 0·99 | 0·997–·999 | 20 |
| HIV rapid | 0·96 | 0·85–1 | w10 |
|
| |||
| Loss to Follow-up | |||
| Syphilis loss to follow up (for laboratory tests) | 20% | w19 | |
| HIV loss to follow-up | 23·9% | w20 | |
|
| |||
| HIV and Syphilis Treatment | |||
| Infants born to women who test positive for HIV and receive treatment who receive Nevirapine | 77·19% | Assumption | |
| Proportion of children known to be HIV-exposed who were enrolled in ART | 67% | 28 | |
| Probability of syphilis treatment success for the mother | 98% | w16 | |
|
| |||
| Pregnancy outcomes | |||
| Syphilis Uninfected mothers | |||
| Stillbirth/early fetal death | 4·6% | 3·0%–7·1% | 3 |
| Neonatal death | 3% | 2·1%–4·3% | 3 |
| Prematurity or low birth weight | 6·3% | 3·5%–11·0% | 3 |
| MTCT of HIV in HIV treated mothers (in utero + intrapartum/postnatal, 12 month) | 4·99% | w21 | |
| MTCT of HIV in HIV untreated mothers (in utero + intrapartum/postnatal, 12 month) | 24.17% | w22 | |
| Syphilis infected mothers (syphilis untreated) | |||
| Congenital syphilis | 15·5% | 7·5%–29·0% | 3 |
| Prematurity or low birth weight | 12·1% | 3·9%–31·8% | 3 |
| Neonatal death | 12·3% | 9·3%–16·2% | 3 |
| Stillbirth/early fetal death | 25·6% | 18·5%–34·2% | 3 |
| Additional effects in HIV co-infected mothers (HIV treated) | |||
| MTCT of HIV in HIV treated mothers (in utero + intrapartum/postnatal) | 9·05% | 29 | |
| Prematurity or low birth weight | 2·74% | 3 | |
| Additional effects in HIV co-infected mothers (HIV untreated) | |||
| MTCT of HIV | 34·5% | 29 | |
| Prematurity or low birth weight & MTCT of HIV | 9·36% | 3,29 | |
|
| |||
| Costs (2012 USD) | |||
| Labor Costs | |||
| Pre-test counseling (both HIV & syphilis) | $0·44 | w5 Φ | |
| Sample collection (single test) | $0·27 | w5 Φ | |
| Preparing and inoculating test (single test) | $0·37 | 24 | |
| Reading and recording results (single test) | $0·63 | 24 | |
| Post-test counseling, syphilis positive | $0·62 | w5 Φ | |
| Post-test counseling, syphilis negative | $0·18 | w5 Φ | |
| Post-test counseling, HIV positive | $1·24 | w5 Φ | |
| Post-test counseling, HIV negative | $0·18 | w5 Φ | |
| Patient cost | |||
| Travel cost | $1·49 | Assumption | |
| Testing time cost (dual test) | $0·48 | 24 | |
| Testing time cost (single tests) | $0·71 | Assumption | |
| Test Cost | |||
| Single syphilis rapid test | $0·55 | WHO Catalog | |
| Single HIV rapid test | $0·81 | UNICEF agreement | |
| Laboratory-based syphilis tests | $2·53 | w13 | |
| Dual HIV and syphilis test | $1·30 | $1·20–$2·60 | Assumption |
| Early infant diagnosis | $32·50 | w23 | |
| Treatment for syphilis (2·4 MU benzathine penicillin) | $2·38 | w16 | |
| Pregnancy outcome cost | |||
| Healthy | $72·59 | 36·30–108·89† | w16 |
| Congenital syphilis | $804·54 | 402·27–1206·81† | w16 |
| Premature | $1508·84 | 754·42–2263·26† | w16 |
| Neonatal death | $3577·58 | 1788·79–5366·37† | w16 |
| Stillbirth | $72·59 | 36·30–108·89† | w16 |
| MTCT HIV | $1358·18 | 679·09–2037·27† | w24 |
| Nevirapine for 12 months for infant | $16·60 | 8·30–24·90† | |
|
| |||
| Disability weights | |||
| AIDS (no treatment) | 0·545 | 25 | |
| HIV | 0·053 | 25 | |
| Death | 1 | - | |
| Congenital syphilis (3 years) | 0·315 | 26 | |
| Low birth weight (1 year) | 0·106 | 26 | |
| Neonatal death | 1 | - | |
| Stillbirth | 0 | - | |
| Miscarriage | 0 | - | |
|
| |||
| Test Uptake | |||
| Syphilis rapid test | 0·08 | 0·04–0·12† | w9 |
| Syphilis laboratory test | 0·08 | 0·04–0·12† | w9 |
| HIV rapid test | 0·83 | 0·415–0·95** | w9 |
| Dual HIV and syphilis test | 0·83 | 0·415–0·95** | Assumption |
| Early infant diagnosis | 0·9 | w23 | |
|
| |||
| Other | |||
| Life expectancy of newborn | 50 years | w25 | |
| Discount rate | 3% | w26 | |
NOTE: Women are assumed to have unknown HIV and syphilis status at time of testing·
ranges are calculated using a 50% reduction for the lower bound and an assumption for the top
ranges calculated using a 50% spread
lower end of range is another common way to determine the disability weight of co-infection: highest disability weight of the two infections
Labor costs were calculated using salaries published by the WHO (w5) and assumed times for length of each procedure
Malawi, along with other sub-Saharan African countries, is currently implementing Option B+ for the prevention of mother-to-child transmission of HIV. Under Option B+, HIV-infected mothers start combination antiretroviral therapy from 14 weeks of gestation and continue for life, regardless of CD4 t-cell count.w3 Our model included option B+ for HIV treatment and the WHO-recommended syphilis treatment consisting of a single intramuscular dose of benzathine penicillin 2.4 MU.w4
Costs for individual test materials and supplies were determined from the literature, negotiated agreements between suppliers and UNICEF or WHO, or direct communication with suppliers. Costs incurred by the health system for time for each test were calculated using WHO published health worker salaries for the regionw5 and when available, published times needed for each procedure.24 Patient costs for clinic attendance and testing were included.24 Test procurement and distribution costs of tests were not included. Treatment costs for both syphilis and HIV infection in mothers at time of screening and lifetime for the child were included. Those costs included health system and patient cost at time of testing, and the health system throughout treatment. All costs were converted to 2012 dollars using the World Bank GDP deflator.w6 Costs and DALYs were discounted annually by 3%.w7
We assumed that pregnancy outcomes in women with effectively treated syphilis would have the same rates of pregnancy outcomes as women without syphilis infection. The model also assumed that if syphilis relapse were to occur, it will occur during the first year.w8 We used an uptake of HIV testing of 83% and 8% for syphilis based on reported test coverage in the country in July through September 2013.w9 We assumed an uptake of the dual test equal to that of the HIV rapid test. For the testing algorithms that looked at two separate tests for HIV and syphilis, the model assumed that if a woman were tested for syphilis, she was also tested for HIV. We assumed that there was no loss-to-follow-up among children with congenital syphilis whose mothers were tested and received treatment for syphilis, but for whom the treatment failed.
Sensitivity analyses
We conducted several sensitivity analyses. We conducted one-way sensitivity analyses to determine the impact that key inputs had on the cost and effectiveness estimates, as shown in Table 1. Additionally, we conducted three Monte Carlo multi-way sensitivity analyses, allowing all the variables with ranges in Table 1 to vary, assuming uniform distributions. We conducted Monte Carlo simulations to sample randomly from those distributions for 1000 model iterations and calculated the incremental cost and effectiveness compared to each other test algorithm.
This analysis was non-human subject research that did not require institutional review board oversight. All analyses were conducted using TreeAge Pro Software 2015 (Williamstown, MA, USA).
Results
Health and Cost Outcomes
Our cohort decision model for 100,000 pregnant women attending antenatal care in Malawi predicted a total of 15,820 adverse pregnancy outcomes in the HIV rapid test only strategy, 15,779 adverse pregnancy outcomes in the HIV rapid and laboratory-based syphilis testing strategy, 15,778 adverse pregnancy outcomes in the single rapid test for HIV and syphilis strategy, and 15,370 adverse pregnancy outcomes in the dual HIV and syphilis rapid test strategy [Web Appendix Table]. Given the base-case parameters, the strategy using the dual HIV and syphilis rapid test was both the least costly ($214.79 per pregnancy) and resulted in the fewest DALYs (108,693) per 100,000 pregnancies. The results of the cost-effectiveness analysis showed that a dual HIV and syphilis test was cost saving compared to all other screening strategies; all other screening strategies that included testing were strictly dominated, indicating they were both more costly and less effective [Table 2].
Table 2.
Summary results from the cohort decision model comparing the expected effects (Disability-Adjusted Life-Years (DALYs)) of the pregnancy and total costs (2012 U.S. Dollars) for all 4 antenatal HIV and syphilis testing algorithms in the Malawi setting of a theoretical cohort of 100,000 pregnant women receiving antenatal care using the base-case value model inputs.
| Total Costs | Incremental Cost | DALYs Lost | Incremental DALYs Lost | |
|---|---|---|---|---|
| Dual HIV and syphilis test | $21,479,390 | - | 108,693 | - |
| Single rapid tests for HIV & syphilis | $21,864,363 | $384,972 | 110,691 | 1,998 |
| HIV rapid test screening only | $21,875,298 | $395,908 | 110,875 | 2,182 |
| HIV rapid test & laboratory-based test for syphilis | $21,893,483 | $414,092 | 110,697 | 2,004 |
Monte Carlo Simulations (Multi-way Sensitivity Analysis)
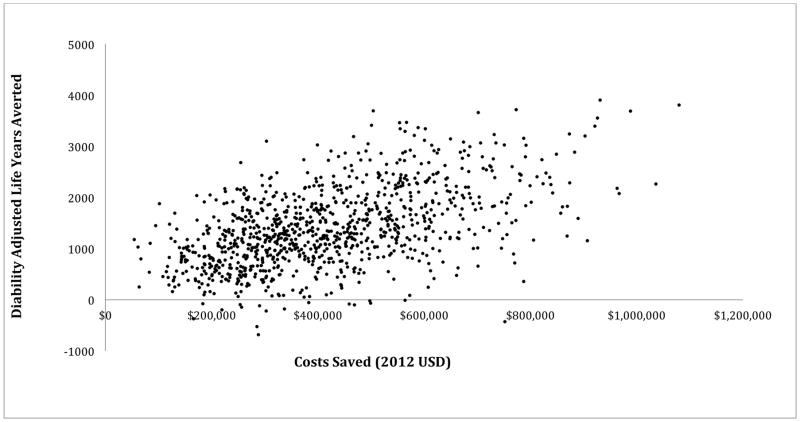
The Monte Carlo simulation [Figure 1] showed that the dual HIV and syphilis test remained the most cost-effective algorithm for nearly all iterations and was cost saving for all iterations and had the highest number of disability adjusted life years averted for all but a few iterations compared to the HIV rapid test only algorithm.
Figure 1.
Distribution of costs saved versus effectiveness from Monte Carlo simulation when using the dual HIV and syphilis test algorithm compared to the rapid HIV test only algorithm. Each dot is representative of an iteration of the model run (n=1000). The dual HIV and syphilis algorithm was always cost saving and almost always reduced disability adjusted life years (DALYs) compared to HIV testing alone.
One-way Sensitivity Analyses
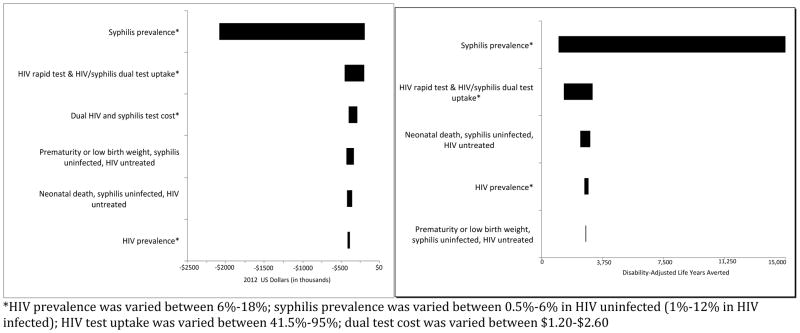
Figures 2a and 2b reflect the impact of altering model parameter values from lower to upper end ranges [Table 1] on incremental cost and effectiveness of HIV rapid test only versus dual HIV and syphilis testing. Parameters that had a large effect on incremental cost included HIV and syphilis prevalence, risk of prematurity or low birth weight among syphilis uninfected and HIV-positive untreated mothers, and probability of neonatal death in syphilis uninfected and HIV-positive untreated mothers. Variables that influenced the relative effectiveness were HIV prevalence among pregnant women, uptake of dual HIV and syphilis testing, syphilis prevalence in HIV uninfected women, and stillbirth or early fetal death among syphilis uninfected HIV-positive untreated mothers. The higher the HIV and syphilis prevalence, the more money that was saved and the more that were DALYs averted using a dual HIV and syphilis test rather than an HIV rapid test only. In each one-way sensitivity analysis dual HIV and syphilis testing remained relatively less expensive and more effective compared to HIV rapid testing alone. We found that the cost of the dual test needed to be greater than $6.04 in order for the dual testing algorithm to no longer be most dominating. We conducted an additional sensitivity analysis in which we removed the patient time and labor costs from the model and found that the dual HIV and syphilis test algorithm remained the most cost-effective.
Figure 2.
Figure 2a. Tornado diagram of sensitivity analysis: incremental cost of HIV rapid test only versus dual HIV and syphilis test. This tornado diagram is a graphical representation of how the relative cost is impacted by varying model parameters from lower to upper ranges.
Figure 2b. Tornado diagram of sensitivity analysis: incremental effectiveness of HIV rapid test only versus dual HIV and syphilis test. This tornado diagram is a graphical representation of how the relative effectiveness is impacted by varying model parameters from lower to upper ranges.
Discussion
We used health services data and economic estimates to compare four testing approaches for preventing adverse pregnancy outcomes due to maternal HIV and syphilis infection. Our model of 100,000 pregnant women in Malawi found that using a dual HIV and syphilis rapid test algorithm in antenatal care would reduce the number of adverse outcomes of pregnancy. The dual HIV and syphilis rapid test algorithm was found to lead to lower overall costs and decreased newborn DALYs when compared to the other screening algorithms, given the base-case parameters, which were chosen to match the current epidemiologic state of HIV and syphilis in Malawi.
Using a Monte Carlo simulation comparing the dual HIV and syphilis rapid test algorithm to HIV rapid test only algorithm we were able to show that even when accounting for uncertainty of inputs the dual rapid HIV and syphilis test was always cost saving and almost always associated with fewer DALYs. We varied base case estimates for a number of the variables in the model and found that the dual HIV and syphilis rapid test algorithm dominated the HIV rapid test only algorithm.
Dual HIV and syphilis tests have performed very well in the laboratory, with sensitivies and specificities over 99% for both the HIV antibody and treponemal antibody detection.19,21,23 Additionally, a field evaluation of a dual HIV and syphilis test used in this cost-effectiveness analysis displayed excellent HIV antibody detection and very good treponemal antibody detection.20 Those performance results are similar to evaluations of single rapid tests.30 w1 w2 w10 Our analysis suggests that integrating the screening of syphilis into antenatal HIV prevention programs through dual rapid point-of-care testing would positively affect case finding and the prevention of maternal-to-child transmission of syphilis. A dual rapid point-of-care test also has potential to save costs and increase uptake by leveraging current procurement and testing systems that have already been strengthened in HIV testing programs. However, with the implementation of any changes in a program comes additional start-up costs, such as training, new contracts and product registration activities. Those costs were not included in our analysis, but would reflect one-time programmatic costs and would therefore likely have a minimal effect over time. Additionally, we assumed no loss to follow-up for rapid testing as women could be tested and treated at the same visit. In order to implement same visit testing and treatment programs, effective logistical coordination and consistent medication supply are needed.
Other cost-effectiveness studies have looked at HIV and syphilis integration of HIV and syphilis testing, however as far as we know, the current analysis was the first to include dual HIV and syphilis rapid tests.17 w11–w15 Owusu-Edusei and colleagues found that even in a low prevalence setting in China, integrating the screening of syphilis into HIV antenatal screening programs was considerably more cost-effective, with a cost-effectiveness ratio more than 15 times lower than screening for HIV alone.w13 Other studies using data from sub-Saharan Africa found that syphilis screening in antenatal care was cost saving.w14 w16 Additionally increased syphilis screening among HIV-infected men who have sex with men in North America was shown to be cost-effective.w17
Our analysis was subject to several limitations. Our analysis aimed to assess the health effects and costs in the infant by screening for syphilis and/or HIV in pregnancy. Therefore, we did not include health effects or costs after pregnancy for the mother. Consequently, the benefits of syphilis testing in antenatal care using a dual test may be underestimated because this intervention would have an effect on two individuals, the mother as well as the infant, at once. Additionally, we were not able to account for the costs of procurement and distribution of the tests. However, the most cost effective algorithm in the analysis was the dual HIV and syphilis rapid test, and because this algorithm requires procurement of a single test device as opposed to two or more test devices, it is likely that if procurement and distribution costs had been included, additional cost savings would have been identified. We did not account for adverse side effects or overtreatment rates of HIV and/or syphilis. Additionally, we assumed smooth implementation of dual test strategy with uptake at the same rate as the single HIV-test algorithm. While this is likely, given that it replaces a single test with another single test, the acceptability of the new test to both patients and healthcare providers will need to be evaluated. Dual tests have been shown good field performance in some settings, however, additional evaluations are required to understand how they will perform in specific settings.20 An additional limitation of this analysis is that our model was structured such that we assumed that each test’s sensitivity and specificity were independent. The strengths of our study were that we included the four most common test algorithms for HIV infection and syphilis screening, which allowed us to identify the most cost-effective algorithm of all four. Additionally, we conducted both 1-way and 2-way sensitivity analyses that allowed us to vary estimates to gain further insight on what factors had the largest impact on adverse pregnancy outcomes, progression of disease and cost.
The results of the current analysis help provide important cost-effectiveness information about new dual rapid testing technology when compared with existing testing strategies. As dual point-of-care rapid testing programs are rolled out, actual costs and programmatic data, particularly testing uptake, should be evaluated. Further surveillance of syphilis infections, screening and adverse pregnancy outcomes may allow more accurate cost-effectiveness estimates. The dual HIV and syphilis rapid test algorithm was the most cost-effective strategy that we analyzed. Adding dual rapid testing to the existing prevention of mother-to-child HIV transmission programs in Malawi and similar countries is likely to be advantageous.
Supplementary Material
Expected adverse pregnancy outcomes resulting from the cohort decision model comparing each HIV and syphilis testing algorithm for a theoretical cohort of 100,000 pregnant women in Malawi
Key Messages.
Globally syphilis affects more pregnancies than HIV and millions of pregnant women are HIV and/or syphilis infected each year.
This article presents results from a cost-effectiveness analysis of various HIV and syphilis screening algorithms in order to provide funders and policy-makers with information into the costs and benefits of each option.
Use of a dual HIV and syphilis test was cost saving compared to all other screening strategies.
Adding dual rapid testing to the existing prevention of mother-to-child HIV transmission programs in Malawi and similar countries is likely to be advantageous.
Acknowledgments
Funding Statement: This analysis was funded in part by Standard Diagnostics through an unrestricted educational grant, through the UCLA Center for HIV Identification, Prevention, and Treatment under award number 5P30MH058107, and by the UCLA Center for AIDS Research under award number 5P30 AI028697. CCB acknowledges funding from NIDA T32 DA023356 and NIDA R01 DA037773-01A1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank those who helped review and provide feedback on this manuscript and the cost-effectiveness models: Thomas L. Gift and Kwame Owusu-Edusei of the U.S. Centers for Disease Control and Prevention and Fern Terris-Prestholt from the London School of Hygiene and Tropical Medicine.
Footnotes
Competing Interest: None declared.
Contributor statement: CCB performed the literature review, wrote the manuscript and provided support with model building and analysis. EL built the models, performed the analysis, supported literature review and provided review of the manuscript. LJA revised the models and performed sensitivity analyses. JDK conceived of the analysis and provided oversight.
Licence statement: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ and co-owners or contracting owning societies (where published by the BMJ on their behalf), and its Licensees to permit this article (if accepted) to be published in Sexually Transmitted Infections and any other BMJ products and to exploit all subsidiary rights, as set out in our licence.
References
- 1.Newman L, Kamb M, Hawkes S, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS medicine. 2013;10(2):e1001396. doi: 10.1371/journal.pmed.1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Santis M, De Luca C, Mappa I, et al. Syphilis Infection during pregnancy: fetal risks and clinical management. Infectious diseases in obstetrics and gynecology. 2012;2012:430585. doi: 10.1155/2012/430585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bulletin of the World Health Organization. 2013;91(3):217–26. doi: 10.2471/BLT.12.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klausner JD. The sound of silence: missing the opportunity to save lives at birth. Bulletin of the World Health Organization. 2013;91(3):158–58A. doi: 10.2471/BLT.13.118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Global guidance on criteria and processes for validation: Elimination of mother-to-child transmission of HIV and syphilis. Geneva, Switzerland: World Health Organization; 2014. Available at: http://www.who.int/reproductivehealth/publications/rtis/9789241505888/en/ [Google Scholar]
- 6.Workowski KA, Berman S, et al. Centers for Disease C. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 7.Alexander JM, Sheffield JS, Sanchez PJ, et al. Efficacy of treatment for syphilis in pregnancy. Obstetrics and gynecology. 1999;93(1):5–8. doi: 10.1016/s0029-7844(98)00338-x. [DOI] [PubMed] [Google Scholar]
- 8.Watson-Jones D, Gumodoka B, Weiss H, et al. Syphilis in pregnancy in Tanzania. II. The effectiveness of antenatal syphilis screening and single-dose benzathine penicillin treatment for the prevention of adverse pregnancy outcomes. The Journal of infectious diseases. 2002;186(7):948–57. doi: 10.1086/342951. [DOI] [PubMed] [Google Scholar]
- 9.Kamb ML, Newman LM, Riley PL, et al. A road map for the global elimination of congenital syphilis. Obstetrics and gynecology international. 2010 doi: 10.1155/2010/312798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peeling RW, Mabey D, Fitzgerald DW, et al. Avoiding HIV and dying of syphilis. Lancet. 2004;364(9445):1561–3. doi: 10.1016/S0140-6736(04)17327-3. [DOI] [PubMed] [Google Scholar]
- 11.Peeling RW, Holmes KK, Mabey D, et al. Rapid tests for sexually transmitted infections (STIs): the way forward. Sexually transmitted infections. 2006;82(Suppl 5):v1–6. doi: 10.1136/sti.2006.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. The Use of Rapid Syphilis Tests. 2006 Available at: http://whq.lib.doc.who.int/hq/2006/TDR_SDI_061_engpdf?ua=1.
- 13.WHO. The Global Elimination of Congenital Syphilis: Rationale and Strategy for Action. 2007 Available at: www.who.int/reproductivehealth/publications/rtis/9789241595858/en/index.html.
- 14.Garcia PJ, Carcamo CP, Chiappe M, et al. Rapid Syphilis Tests as Catalysts for Health Systems Strengthening: A Case Study from Peru. PloS one. 2013;8(6):e66905. doi: 10.1371/journal.pone.0066905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang LG, Tucker JD, Liu FY, et al. Syphilis screening among 27,150 pregnant women in South Chinese rural areas using point-of-care tests. PloS one. 2013;8(8):e72149. doi: 10.1371/journal.pone.0072149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker JD, Hawkes SJ, Yin YP, et al. Scaling up syphilis testing in China: implementation beyond the clinic. Bulletin of the World Health Organization. 2010;88(6):452–7. doi: 10.2471/BLT.09.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickerman P, Peeling RW, Terris-Prestholt F, et al. Modelling the cost-effectiveness of introducing rapid syphilis tests into an antenatal syphilis screening programme in Mwanza, Tanzania. Sexually transmitted infections. 2006;82(Suppl 5):v38–43. doi: 10.1136/sti.2006.021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown NJ, Platt MP, Beattie RM. Women, children, and global public health: beyond the millennium development goals. BMJ. 2015;350:h1755. doi: 10.1136/bmj.h1755. [DOI] [PubMed] [Google Scholar]
- 19.Bristow CC, Adu-Sarkodie Y, Ondondo RO, et al. Multi-site laboratory evaluation of a dual HIV/syphilis point-of-care rapid test for simultaneous detection of HIV and syphilis. Open Forum Infectious Diseases. 2014;1(1):1–5. doi: 10.1093/ofid/ofu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristow CC, Leon SR, Huang E, et al. Field evaluation of a dual rapid diagnostic test for HIV infection and syphilis in Lima, Peru. Sexually Transmitted Infections. 2015 doi: 10.1136/sextrans-2015-052326. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristow CC, Leon SR, Ramos LB, et al. Laboratory Evaluation of a Dual Rapid Immunodiagnostic Test for HIV and Syphilis Infection. Journal of clinical microbiology. 2015;53(1):311–3. doi: 10.1128/JCM.02763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omoding D, Katawera V, Siedner M, et al. Evaluation of the SD Bioline HIV/Syphilis Duo assay at a rural health center in Southwestern Uganda. BMC research notes. 2014;7:746. doi: 10.1186/1756-0500-7-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphries RM, Woo JS, Chung JH, et al. Laboratory evaluation of three rapid diagnostic tests for dual detection of HIV and Treponema pallidum antibodies. Journal of Clinical Microbiology. 2014;52(12):4394–7. doi: 10.1128/JCM.02468-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blandford JM, Gift TL, Vasaikar S, et al. Cost-effectiveness of on-site antenatal screening to prevent congenital syphilis in rural eastern Cape Province, Republic of South Africa. Sexually transmitted diseases. 2007;34(7 Suppl):S61–6. doi: 10.1097/01.olq.0000258314.20752.5f. [DOI] [PubMed] [Google Scholar]
- 25.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez AD, Mathers CD, Ezzati M, et al. Measuring the Global Burden of Disease and Risk Factors. In: Lopez AD, Mathers CD, Ezzati M, et al., editors. Global Burden of Disease and Risk Factors. Washington (DC): 2006. [PubMed] [Google Scholar]
- 27.UNICEF. Malawi Statistics. 2012 Available at: http://www.unicef.org/infobycountry/malawi_statistics.html.
- 28.Malawi Government. 2012 GLOBAL AIDS RESPONSE PROGRESS REPORT: Malawi Country Report for 2010 and 2011. 2012. [Google Scholar]
- 29.Mwapasa V, Rogerson SJ, Kwiek JJ, et al. Maternal syphilis infection is associated with increased risk of mother-to-child transmission of HIV in Malawi. Aids. 2006;20(14):1869–77. doi: 10.1097/01.aids.0000244206.41500.27. [DOI] [PubMed] [Google Scholar]
- 30.Benzaken AS, Sabido M, Galban E, et al. Field performance of a rapid point-of-care diagnostic test for antenatal syphilis screening in the Amazon region, Brazil. International journal of STD & AIDS. 2011;22(1):15–8. doi: 10.1258/ijsa.2010.010145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expected adverse pregnancy outcomes resulting from the cohort decision model comparing each HIV and syphilis testing algorithm for a theoretical cohort of 100,000 pregnant women in Malawi