Supplemental Digital Content is available in the text
Keywords: enhanced recovery, fast track, hepatectomy, liver resection, multimodal approach, systematic review and meta-analysis
Abstract
Background:
FT program (FT) is a multimodal approach used to enhance postoperative rehabilitation and accelerate recovery. It was 1st described in open heart surgery, then modified and applied successfully in colorectal surgery. FT program was described in liver resection for the 1st time in 2008. Although the program has become widely accepted, it has not yet been considered the standard of care in liver surgery.
Objectives:
we performed this systematic review and meta-analysis to evaluate the impact of using the FT program compared to the traditional care (TC), on the main clinical and surgical outcomes for patients who underwent elective liver resection.
Methods:
PubMed/Medline, Scopus, and Cochran databases were searched to identify eligible articles that compared FT with TC in elective liver resection to be included in this study. Subgroup meta-analysis between laparoscopic and open surgical approaches to liver resection was also conducted. Quality assessment was performed for all the included studies. Odds ratios (ORs) and mean differences (MDs) were considered as a summary measure of evaluating the association in this meta-analysis for dichotomous and continuous data, respectively. A 95% confidence interval (CI) was reported for both measures. I2 was used to assess the heterogeneity across studies.
Results:
From 2008 to 2015, 3 randomized controlled trials (RCTs) and 5 cohort studies were identified, including 394 and 416 patients in the FT and TC groups, respectively. The length of hospital stay (LoS) was markedly shortened in both the open and laparoscopic approaches within the FT program (P < 0.00001). The reduced LoS was accompanied by accelerated functional recovery (P = 0.0008) and decreased hospital costs, with no increase in readmission, morbidity, or mortality rates. Moreover, significant results were found within the FT group such as reduced operative time (P = 0.03), lower intensive care unit admission rate (P < 0.00001), early bowel opening (P ≤ 0.00001), and rapid normal diet restoration (P ≤ 0.00001).
Conclusion:
FT program is safe, feasible, and can be applied successfully in liver resection. Future RCTs on controversial issues such as multimodal analgesia and adherence rate are needed. Specific FT guidelines should be developed for liver resection.
1. Introduction
Liver resection is now the preferred treatment for a variety of benign and malignant liver diseases. Major abdominal surgical procedures such as liver resections cause a considerable surgical stress reaction and possible disturbance of metabolic functions. In addition, liver resection has its specific complications, such as postoperative hemorrhage, biliary leakage, and even liver failure.[1]
Fast track (FT) program, recently referred to as the enhanced recovery after surgery program, is considered one of the modern protocols used to reduce morbidity and accelerate convalescence. FT combines various approaches that are used in the care of patients who undergo elective operations. These approaches include preoperative information and education of the patients, avoiding bowel preparation and prolonged fasting, using short-acting anesthesia, keeping normal body temperature, choosing minimally invasive techniques, optimizing perioperative fluid therapy, avoiding insertion of nasogastric tubes and peritoneal drains, pain control, and aggressive postoperative rehabilitation, including early ambulation and enteral nutrition combined with nutritional supplements. The core principle of the FT program lies in the combination of these approaches, aiming for reducing the surgical stress response and organ dysfunction, thereby markedly shortening the time required for a full recovery.[2]
Before the introduction of the FT program, no institutions had a written or agreed perioperative care pathway. There were no specific multimodal measures to avoid prolonged perioperative fasting, nasogastric decompression, excessive use of intravenous fluids, prophylactic abdominal drains, and postoperative immobilization. The postoperative traditional care (TC) emphasized prolonged rest for both the patient and the gastrointestinal tract.[3]
In 1990, Krohn et al[4] described the “accelerated recovery” program, which was successfully used to achieve better outcomes in patients who underwent open heart surgery. This program was later termed “fast track” by Engelman et al[5] in 1994. Kehlet[6] modified the program in 1997 and developed a multimodal approach to be applied to different types of interventions. Subsequently, it was applied successfully to colonic surgery in 1999.[7] The program has become widely accepted, and several components of the program are increasingly being implemented in modern operative care worldwide. Despite this, FT program has not yet been accepted as the standard of care in many fields, including liver surgery. van Dam et al[3] described the use of the FT program in liver resection for the 1st time in 2008.
To be implemented, the FT program requires cooperative teamwork from surgeons, anesthesiologists, physical therapists, nurses, and radiologists.[8]
We performed this systematic review and meta-analysis to evaluate the impact of using the FT program compared to TC, on the main clinical and surgical outcomes for patients who underwent elective liver resection.
2. Methods
This meta-analysis was conducted according to the preferred reporting items for systematic review and meta-analysis statement.[9] Ethical approval was not necessary because this study was a systematic review and meta-analysis. Therefore, our data were based on published studies only.
2.1. Protocol registration
The protocol for this systematic review and meta-analysis was registered into the PROSPERO database (www.crd.york.ac.uk/prospero) with registration number CRD42015020830.
2.2. Eligibility criteria
Only studies that clearly compared the FT program to TC in patients who underwent elective liver resection were included in the systematic review and, if applicable, included in the meta-analysis. If more than 1 study was reported by the same institute, only the most recent or higher level study was included.
Unpublished studies, abstracts, letters, editorials, reviews without original data, and case reports were excluded. Studies that lacked a control group, compared the FT program with a non-TC pathway or compared the FT program in both arms were also excluded.
2.3. Search strategy
PubMed/MEDLINE, Scopus, and Cochrane databases were searched to identify all published full-text articles on the use of the FT program in liver resection. Searches were limited to English-language studies only.
The following combination of keywords were used: (“Fast Track” OR “Fast-Track” OR “Enhanced Recovery” OR “ERAS” OR “Rapid Recovery” OR “Early Recovery” OR “Early Discharge” OR “Rapid Discharge” OR “Multimodal Approach” OR “Multi-Modal Approach” OR “Multimodal Program” OR “Multi-Modal Program” OR “Multimodal Protocol” OR “Multi-Modal Protocol” OR “Multimodal Measures” OR “Multi-Modal Measures”) AND (“Liver” OR “Hepatic” OR “Hepatectomy” OR “Hepatectomies” OR “Hepatobiliary” OR “Hepato-Biliary”). For all databases, the last search was completed on May 25, 2015.
2.4. Study selection
Two authors (EAA, RM) independently screened the titles and abstracts of the primary studies that were identified in the electronic search. Then, the bibliographies of relevant articles were manually reviewed to identify additional trials. Duplicate studies were excluded. Discrepancies between the 2 reviewers were resolved by a 3rd expert reviewer (MV).
2.5. Data extraction and outcomes of interest
The following parameters were extracted from each study by 2 authors (EAA, DN) independently: name of the first author and his/her affiliation, year of publication, study design, the number of patients in each arm, patient characteristics, and study quality.
All relevant texts, tables, and figures were reviewed for data extraction; whenever further information was required, the corresponding author of the paper was contacted by e-mail.
To evaluate the impact of the FT program in liver resection compared to the TC, the following outcomes were studied: operative time, blood loss, the need for blood transfusion, and conversion rate in the laparoscopic resection. In addition, postoperative events were studied such as length of hospital stay (LoS), functional recovery, intensive care unit (ICU) admission, 1st bowel opening, restoration of oral fluid and normal diet, morbidity and mortality rates, pain score, C-reactive protein (CRP) level, hospital cost, readmission rate, and quality of life (QoL). The primary outcomes of this analysis were postoperative LoS and functional recovery, whereas the others were considered secondary outcomes.
Hospital stay was defined as the interval from the day of surgery to the day of actual discharge from the hospital. Functional recovery was determined by the number of days to reach a certain criteria of recovery, which included good pain control with oral analgesia, tolerance of solid food, normal body temperature, independent mobilization, normal or decreasing the serum bilirubin level, and willingness of the patients to be discharged. Readmission was defined as any hospital readmission within 30 days after discharge.
Operative time was defined as the interval from the incision to the suturing of the skin. The 1st bowel opening was determined by the 1st passage from the bowel of either stool or flatus while pain score was evaluated by using a visual analog scale (VAS) ranging from 0 to 10. Postoperative mortality was defined as the occurrence of death during hospitalization or within 30 days after surgery, while postoperative morbidity included the complication rate from the time of surgery to 90 days after discharge.
For assessment of the QoL, European quality of life-five dimensions model (EQ-5D)[10] was used. Repeated EQ-5D measurements were obtained in the postoperative period, and differences between groups in health-related QoL were calculated by using the area under the curve method. Alternatively, QoL could be assessed by completing the general comfort questionnaire (GCQ) based on the Kolcaba comfort line[11] which is determined on hospital discharge.
2.6. Qualitative analysis of each study
The quality of the retrospective studies were assessed according to the Newcastle–Ottawa Scale that scores patient selection, comparability between the 2 study groups, and assessment of outcomes. Studies that achieved ≥7 points were defined as “good” quality. The quality of the randomized controlled trials (RCTs) was assessed according to the Cochrane Collaboration tool for assessing risk of bias.[12] This tool analyzes the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. For each entry based on the risk of bias assessment guidelines, we made a judgment (low risk of bias, high risk of bias, or uncertain). All disagreements were resolved by discussion until a consensus agreement was achieved.
2.7. Statistical analysis
The meta-analysis was performed by using Review Manager (“RevMan” [Computer program], Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). Odds ratios (ORs) with Mantel–Haenszel method were used as a summary measure of efficacy for dichotomous data while mean differences (MDs) with inverse variance method were applied for continuous variables. A 95% confidence interval (CI) was reported for both measures. If the study provided medians and ranges instead of means and standard deviations, the means and standard deviations were imputed, as described by Hozo et al.[13] The fixed-effect model was used when no heterogeneity was detected among studies, while the random-effects model was preferred when variance existed. Statistical heterogeneity was evaluated by using the I2 statistic. I2 values of 0% to 25%, 25% to 50%, and >50% were considered indicative of homogeneity, moderate heterogeneity, and high heterogeneity, respectively. Forest plots were constructed and all data were considered statistically significant for P ≤ 0.05.
In this meta-analysis, we pooled the results from all the included studies in regards to the primary and secondary outcomes. In addition, we meta-analyzed the RCTs separately for each outcome; the results were only discussed if a difference was found compared to the pooled meta-analysis.
2.8. Risk of bias across studies
Assessment of the risk of publication bias across series for all outcome measures will be conducted according to the guidelines established by the Cochrane Handbook for Systematic Reviews.[14]
2.9. Additional analyses
Due to the variability of surgical approaches to liver resection (by either laparoscopy or open surgery), we will include a meta-analysis of subgroups for outcomes affected by this technical difference, such as operative outcomes, postoperative LoS, and morbidity and mortality rates.
3. Results
3.1. Eligible studies
The preferred reporting items for systematic review and meta-analysis flow diagram of the systematic literature search are shown in Fig. 1. The literature search included all articles published up to May 25, 2015, which yielded 1237 articles. After removing duplicates, the titles and abstracts of 839 articles were reviewed. Of these, 830 articles were excluded for the following reasons: 810 were not related to the FT program in liver resection, 10 were review articles,[1,15–23] 8 did not include a TC control group,[24–31] 1 was a commentary,[32] and 1 was only a protocol description.[33]
Figure 1.
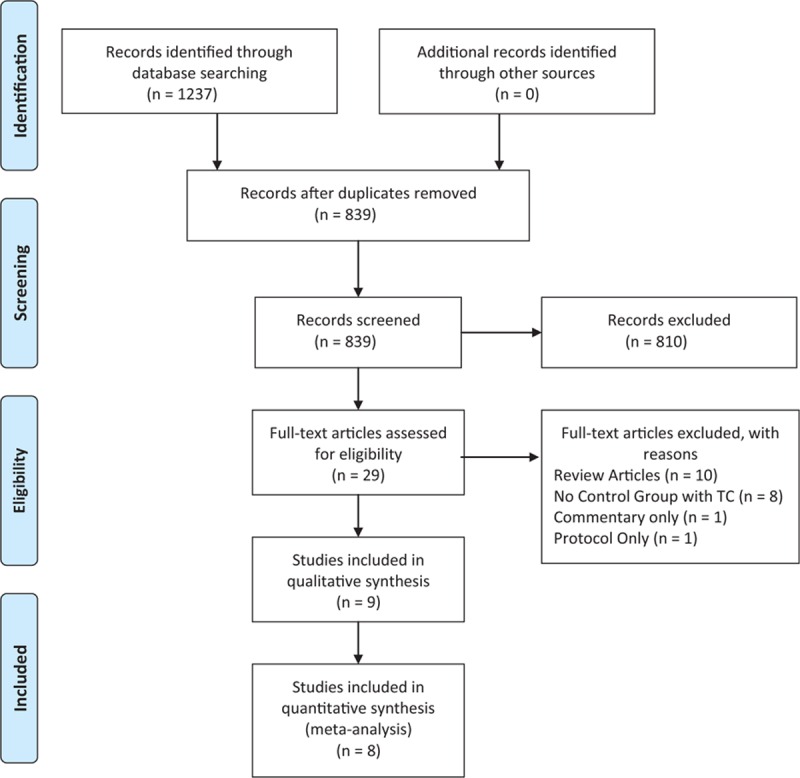
Preferred reporting items for systematic review and meta-analysis (PRISMA) flow diagram.
Nine comparative studies published between 2008 and 2015 matched our inclusion and exclusion criteria and were selected for inclusion in our systematic review and meta-analysis.[3,8,34–40] The abstract for one of the articles was available but the full-text could not be found in any database, despite after 6 weeks of dedicated searching. The corresponding author of the article was contacted through e-mail but we did not receive a response.[37] Therefore, a final total of 8 studies was included in the quantitative synthesis.
3.2. Study characteristics
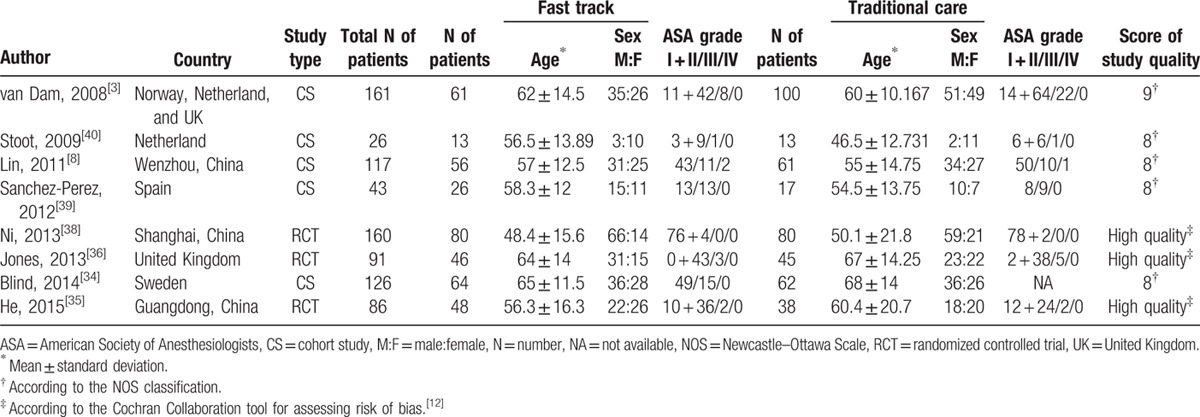
The characteristics of these studies are summarized in Table 1. The 8 studies included 810 patients: 394 in the FT group and 416 in the TC group. The sample size of these studies ranged from 26 to 161 patients. The patient characteristics, including sex, age, and American society of anesthesiologists score, were collected from all of the included studies.
Table 1.
Characteristics of the included studies.
The indications and extent of liver resection are shown in Table, Supplemental digital Content 1. A detailed FT program, including the most frequently described elements in each study and the respective adherence rate (if reported), is summarized in Table 2.
Table 2.
Fast track program in liver resection with adherence rate in each study (if reported).

The final selected articles included 3 RCTs[35,36,38] and 5 cohort studies.[3,8,34,39,40] Three studies were conducted by laparoscopy[35,39,40] and 5 by open surgery.[3,8,34,36,38]
3.3. Quality assessment
All included RCTs are of high quality as summarized in Fig. 2. One cohort study was scored 9,[3] while the remaining 4 cohort studies were scored 8.[8,34,39,40] Consequently, the quality of these studies was considered good, according to the Newcastle–Ottawa Scale.
Figure 2.
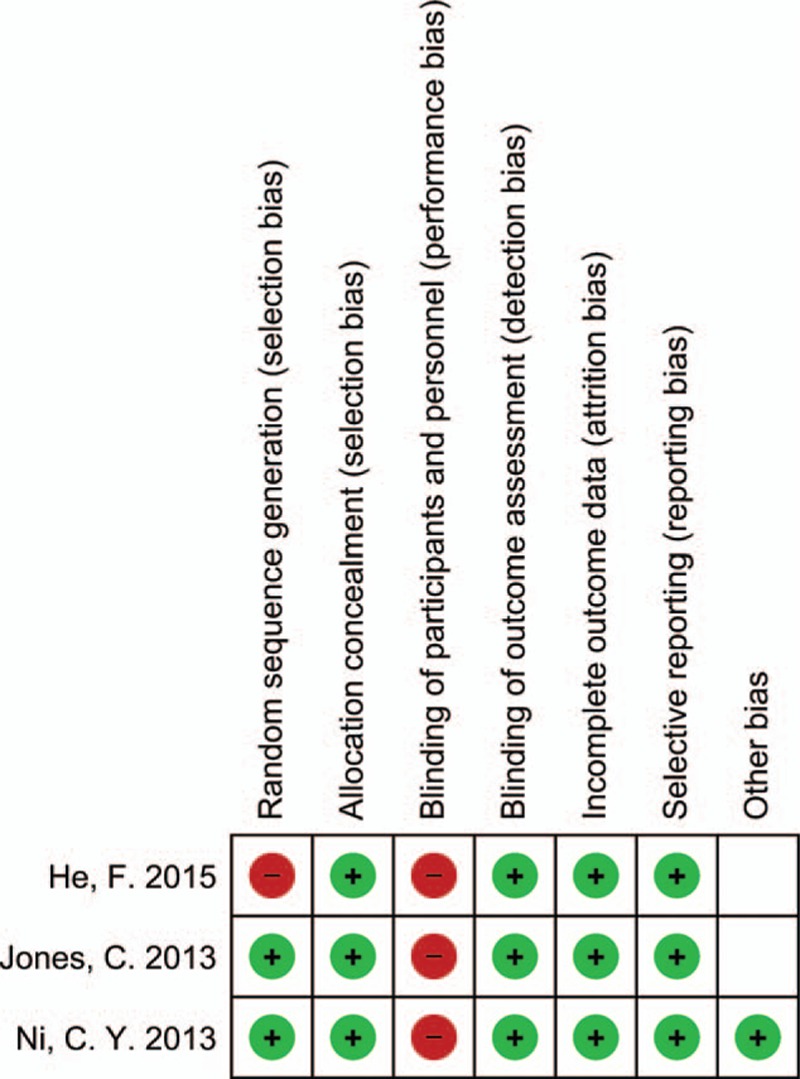
Risk of bias summary in randomized controlled trials (RCTs). The symbol of (−) indicates that there is a high risk of bias, of (+) indicates a low risk of bias and the blank field indicates uncertainty.
3.4. Meta-analysis of the primary outcomes
3.4.1. Length of hospital stay (LoS)
Hospital stay was reported in 5 studies of open liver resection,[3,8,34,36,38] and a highly statistically significant result between the FT and TC groups was found, in favor of the FT group (MD [CI 95%] = −2.41 [−3.69, −1.13]; P = 0.0002), with marked heterogeneity (I2 = 71%). LoS was also reported in all studies of laparoscopic liver resection,[35,39,40] and the findings demonstrated a significant result in favor of the FT group (MD [CI 95%] = −3.20 [−5.17, −1.22]; P = 0.001), with marked heterogeneity (I2 = 70%). Overall, these results demonstrated a highly statistically significant difference between the FT and TC groups, in favor of the FT group (MD [CI 95%] = −2.74 [−3.60, −1.87]; P < 0.00001). However, marked heterogeneity between these studies was observed; I2 = 73% (Fig. 3A).
Figure 3.
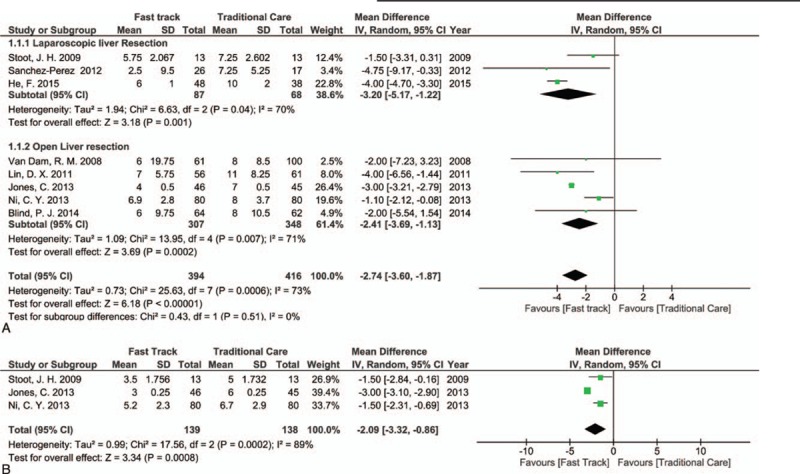
Forest plot of comparison: fast track versus traditional care, outcome: (A) length of hospital stay; (B) functional recovery.
3.4.2. Functional recovery
Three studies, including 2 RCTs and 1 cohort study, reported data on functional recovery[36,38,40] and showed statistically significant results in favor of the FT group (MD [CI 95%] = −2.09 [−3.32, −0.86]; P = .0008, I2 = 89%, Fig. 3B).
3.5. Meta-analysis of the secondary outcomes
3.5.1. Operative time (minute)
In regards to open liver resection, 4 studies reported the data for the operative time[3,8,34,38] and the difference between the FT and TC groups was significant, in favor of the FT group (MD [CI 95%] = −27.56 [−55.01, −0.10]; P = 0.05), with marked heterogeneity (I2 = 88%). On the other hand, 3 studies of laparoscopic liver resection reported the data for the operative time[35,39,40] and showed that the difference between the FT and TC groups was not statistically significant (MD [CI 95%] = −13.67 [−45.74, 18.40]; P = 0.40), with I2 = 66%. Overall, the differences in outcome were significant statistically in favor of the FT group (MD [CI 95%] = −20.61 [−39.08, −2.15]; P = 0.03), with marked heterogeneity between studies; I2 = 81% (Fig. 4A).
Figure 4.

Forest plot of comparison: fast track versus traditional care, outcome: (A) operative time; (B) intensive care unit (ICU) admission rate.
3.5.2. Blood loss (mL)
Studies that reported the intraoperative blood loss during open[3,8,34,36,38] or laparoscopic[35,40] hepatectomy did not show a statistically significant difference between the FT and TC groups (MD [CI 95%] = −74.67 [−152.50, 3.17]; P = .06, I2 = 68%, see Figure, Supplemental Digital Content 2).
3.5.3. Need for blood transfusion
Three studies of open liver resection[8,36,38] and 3 studies of laparoscopic liver resection[35,39,40] reported the need for blood transfusion data and showed not statistically significant results (OR [CI 95%] = 1.03 [0.67, 1.60]; P = .89, I2 = 1%, see Figure, Supplemental Digital Content 3).
3.5.4. Conversion rate
The conversion rate from laparoscopic into open liver resection was reported in all studies of laparoscopic liver resection[35,39,40] and showed no statistically significant differences between both groups (OR [CI 95%] = 0.99 [0.34, 2.87]; P = .099, I2 = 0%, see Figure, Supplemental Digital Content 4).
3.5.5. ICU admission and stay
Three studies reported the data regarding ICU admission and stay;[8,38,39] 2 reported the percentages of ICU admission of patients,[8,39] and there were fewer admitted FT group patients with high statistical difference than TC group patients, (OR [CI 95%] = 0.05 [0.02, 0.10]; P < 0.00001, I2 = 0%, Fig. 4B). The 3rd study[38] described the length of postoperative ICU stay, which showed a trend toward decreased length of ICU stay in the FT group, but this was not statistically significant (1.2 ± 0.2 vs 1.3 ± 0.6 days; P = 0.08).
3.5.6. First bowel opening
Two RCTs described this outcome[35,38] and showed that differences in the results between the FT and TC groups were statistically significant, where there was earlier bowel activity in the FT group (MD [CI 95%] = −0.98 [−1.26, −0.70]; P < 0.00001, I2 = 0%, Fig. 5A).
Figure 5.
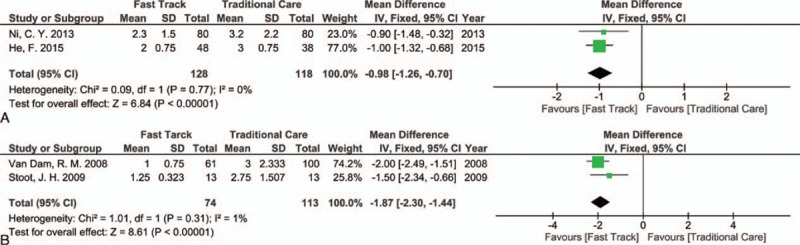
Forest plot of comparison: fast track versus traditional care, outcome: (A) first bowel opening; (B) restoration of normal diet.
Jones et al[36] reported that bowel sounds recurred sooner (P < 0.001) and flatus passed earlier (P = 0.008) in the FT group, but these data were not included in the meta-analysis because there were, only the P values.
3.5.7. Restoration of oral fluid and normal diet
Stoot et al[40] reported that in nearly all patients in both the FT and TC groups, oral fluid intake was resumed within the 1st 24 hours after surgery (1 ± 0.577 vs 2 ± 1.826, respectively), and there was no statistically significant difference between the 2 groups (P = 0.86). Jones et al[36] reported that patients in the FT group resumed oral intake earlier after surgery than those in the TC group (median 115 vs 330 minutes; P < 0.001, respectively) and drank more fluids in the 1st 24 hours (1375 vs 810 mL; P < 0.001, respectively). However, these data had no range, so it was not possible to include them in our meta-analysis. van Dam et al[3] also described that fluid intake was resumed 4 hours after surgery in 56 patients (92%) in the FT group.
In regards to the restoration of a normal diet, 2 studies reported these data[3,40] and showed a highly significant difference between the FT and TC groups, in favor of the FT group (MD [CI 95%] = −1.87 [−2.30, −1.44]; P < 0.00001, I2 = 1%, Fig. 5B).
3.5.8. Morbidity and mortality rates
All of the selected studies reported these outcomes.[3,8,34–36,38–40] No statistically significant differences in morbidity were found between the FT and TC groups in open or laparoscopic liver resection (OR [CI 95%] = 0.90 [0.66, 1.21]; P = 0.48, I2 = 16%, Fig. 6A). When this meta-analysis included only RCTs,[35,36,38] a significant decrease in morbidity rate was observed in the FT group (OR [CI 95%] = 0.54 [0.33, 0.89]; P = 0.02, I2 = 0%, Fig. 6B). No mortality was registered in the FT and TC groups after laparoscopic liver resection, and results were not significantly different between the 2 groups after open liver resection (OR [CI 95%] = 0.68 [0.14, 3.34]; P = 0.64, I2 = 0%, see Figure, Supplemental Digital Content 5).
Figure 6.
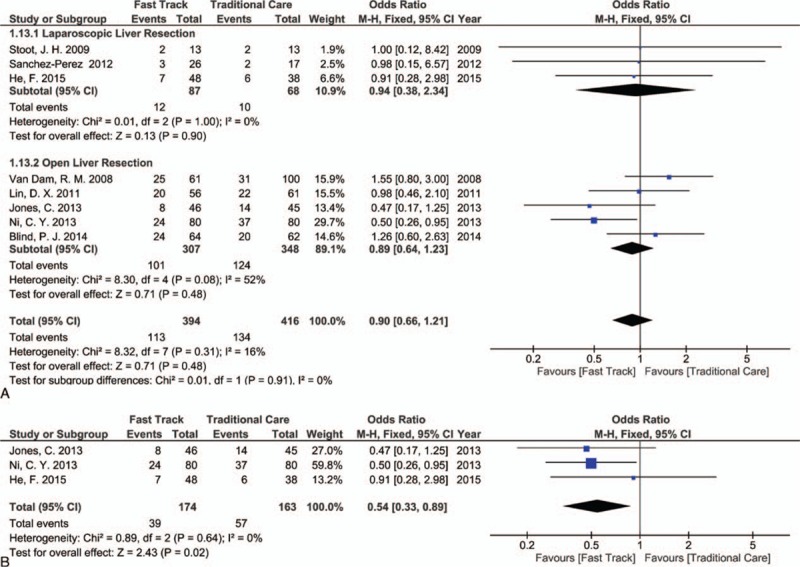
Forest plot of comparison: fast track versus traditional care, outcome: (A) morbidity rate (all studies); (B) morbidity rate (randomized controlled trials [RCTs] only).
3.5.9. Pain score
One RCT reported data for the pain score,[36] and it showed that there were no differences between the FT and TC groups except on the 2nd postoperative day (POD), where VAS was significantly lower in the FT than the TC group (2.5 ± 1.4 vs 3.3 ± 2.0, respectively; P = 0.04).
3.5.10. C-reactive protein level (CRP)
One RCT[38] reported the serum CRP level on POD 1, 3, and 5; its level in the FT group was significantly lower than the TC group (all P < 0.05).
3.5.11. Hospital cost
Two studies reported data for hospital costs; He et al[35] showed that the average hospital costs were 9470 ± 1540 Renminbi (Chinese yuan) (RMB) between the TC group and only 7742 ± 1200 RMB in the FT group (P = 0.03). Lin et al[8] reported that the median of charges (without range) during hospital stay was 21,004 RMB for the FT group, which is significantly less than the 26,626 RMB for the TC group (P < 0.05). Sanchez-Perez et al[39] reported that the use of the FT program reduced hospital costs by about 30%; the study did not include any other cumulative data that could be used in our meta-analysis.
3.5.12. Readmission rate
Seven studies reported the readmission rate[3,8,34–36,39,40] and showed that there were no statistically significant results between the FT and TC groups (OR [CI 95%] = 1.17 [0.61, 2.23]; P = 0.64, I2 = 0%, see Figure, Supplemental Digital Content 6).
3.5.13. Quality of life
The 3 RCTs reported this outcome; 2 of them[35,36] reported it according to EQ-5D and described significant changes in QoL scores for the FT group over time. In both the FT and TC groups, the QoL measures after surgery showed an initial decrease from baseline. Over the 1st month after surgery, QoL improved considerably in the FT group. The median area under the curve was higher in the FT group than the TC group, as reported by He et al (36.9 vs 35.2; P = 0.04, respectively)[35] and Jones et al (37.2 vs 35.6, P = 0.002, respectively).[36]
The 3rd trial[38] described QoL according to GCQ and found that the mean of its measures in the FT group was higher than the TC group (101.2 ± 13.0 vs 93.4 ± 21.4, respectively; P < 0.01), which is statistically significant in favor of the FT group.
3.6. Risk of bias across studies
According to the Cochran handbook for systematic reviews, the test for publication bias is unreliable when less than 10 studies are included in a meta-analysis. Therefore, funnel plots were not constructed in this study.
4. Discussion
Major surgery is still associated with many undesirable consequences such as pain, cardiopulmonary, gastrointestinal, infectious, and thromboembolic complications associated with a prolonged convalescent period. The key pathogenic factor in postoperative morbidities, excluding failures of surgical and anesthetic techniques, is the surgical stress response and the subsequent increased demand for organ function, especially if it is associated with preoperative risk factors and comorbidities.[4,6]
FT program aims to reduce morbidity and accelerate convalescence through preoperative information and education of the patients, stress reduction, optimizing pain control, and aggressive postoperative rehabilitation, including early enteral nutrition and ambulation associated with nutritional supplements.[2,6]
Stress reduction can be obtained by avoiding preoperative bowel preparation and prolonged fasting; patients were allowed to eat and drink until 6 hours, with clear fluids permitted until 2 hours before surgery.[3,30] Using short-acting anesthesia and regional anesthesia and analgesia, avoiding hypothermia, and choosing minimally invasive techniques were essentials. In particular, some authors suggested that laparoscopic liver resection could improve postoperative recovery, decrease morbidity, and therefore, may enhance the results of FT program.[17,41,42] Goal-directed fluid therapy based on individual optimization of stroke volume has been demonstrated to improve outcomes in liver surgery through keeping lower central venous pressure (CVP) during the resection procedure and enhancing gastrointestinal and pulmonary function postoperatively.[36,43–45] Routine drainage of the peritoneal cavity and insertion of nasogastric tube are not recommended within the FT program.[3]
Optimal management of postoperative pain is a prerequisite for FT surgery and it should be a balanced multimodal analgesia.[3,6,8,36] This reduces postoperative nausea and vomiting and allows for early ambulation that is recommended 8 hours after surgery or maximum delayed to the 1st POD.[2] It also facilitates early enteral nutrition; patients within the FT program are encouraged to drink oral fluids 4 to 6 hours after surgery and eat normal food on the 1st POD.[3,34,35]
However, Anders[46] pointed out potential problems with the FT program and suggested that there were adverse effects on the quality of care. He also claimed that hospitals wanted to discharge patients more quickly despite their poorer health state, to decrease their expenses. Moreover, due to the complexity of the FT program and its multiple elements, many barriers to the implementation of the program have arisen, including institutional barriers such as lack of experienced medical and nursing staff. In addition, there were intervention-specific barriers due to limited or weak supporting evidence for several elements of the program. Individual barriers arose from perioperative teams who resisted the change of traditional methods, and poor collaboration between team members.[47,48] Therefore, TC still prevails in many centers as the preferred choice of many surgeons.[49]
This argument about the FT program resulted in a few published reports that compared the FT and TC programs, particularly in liver resection. The systematic search found 6 previous systematic reviews,[1,16,18–20,23] 2 of them with meta-analyses[16,20] and 3 discussed upper abdominal surgeries, including liver resections.[18,19,23] None of these reviews investigated the effect of a laparoscopic approach compared with the open one within the FT program in liver resection. Moreover, none of the publications assessed the QoL, pain score, and ICU admission rate after liver resection in regards to the FT program. In addition, insufficient data about functional recovery, hospital costs, and assessment of protocol adherence were reported. In our present study, we discussed 16 outcomes and aimed to properly assess the FT program compared to the TC program in liver resection.
A meta-analysis, as a quantitative method for therapeutic evaluation, may be used when controversy persists in order to clarify the results of different studies by developing supporting evidence and recommendations. It was possible to include 8 studies in this meta-analysis, which in total contained 810 patients; 3 studies were RCTs and 5 were cohort studies.
In regards to the postoperative outcomes in this meta-analysis, LoS, which is a primary outcome was markedly shortened in both open and laparoscopic liver resections within the FT program. LoS ranged from 2.5 to 7 days compared with the TC group, which ranged from 7.25 to 11 days (P < 0.00001). Moreover, functional recovery was accelerated by the program (P = 0.0008). Currently, functional recovery is considered to be more accurate than LoS in describing the actual time needed for complete recovery, as it is not affected by other social or psychological events.[1] Despite its importance, it was reported by only 3 of the included studies.[36,38,40]
The reduced LoS and accelerated functional recovery in the FT group are accompanied by a lower ICU admission rate (P < 0.00001) and markedly reduced hospital costs, with no increase in the readmission, morbidity, or mortality rates. When including only RCTs in the meta-analysis, a significant decrease in morbidity rate was noticed in the FT group (P = 0.02), in concordance with the results of 2 previous meta-analyses.[16,20] Decreased perioperative fasting periods, restricted fluid infusion, and hypothermia prevention could be the causes of these advantages in the FT group. Maintaining perioperative normal blood glucose level prevents the sense of thirst, hunger, and anxiety.[50] In addition, restricting over-hydration prevents delayed gastrointestinal function and reduces interstitial edema, lung compliance, and cardiac overload.[35] Therefore, amelioration of the overall surgical stress response was resulted, associated with a successive decrease in the complication rate and enhanced recovery.
A significant result in favor of the FT group in regards to earlier bowel opening (P < .00001) was observed. Oral fluid intake was resumed within the 1st 24 hours after surgery nearly in all patients of the FT group, but the results were not statistically significant between the FT and TC groups. In the FT program, normal diet was restored successfully on the 1st POD compared to the TC group (P < 0.00001). These results may be due to the restriction of perioperative over-hydration, early ambulation, and good pain control.
Only 1 study reported the serum level of CRP, which is used as a laboratory indicator of the surgical stress response. The study showed that levels of CRP were significantly lower in the FT group compared to the TC group (P < 0.05).[38] Also, a 1 study reported that there was no difference in pain score between the FT and TC groups, except on the 2nd POD where the VAS score was significantly lower in the FT group (P = 0.044).[36]
QoL measures are an important factor in the evaluation of these programs. After surgery, EQ-5D measures showed an initial decrease from baseline in the FT and TC groups. Over the 1st postoperative month, QoL considerably improved in the FT group.[35,36] Ni et al[38] assessed QoL by using a GCQ based on Kolcaba comfort line and found significantly better results in the FT group (P < 0.01).
In regards to the operative outcomes in this meta-analysis, surgical time was significantly reduced in patients that underwent open hepatectomy within the FT program compared to the TC group (P = 0.05). However, no significant difference was noticed between the 2 groups in patients that underwent laparoscopic liver resection. This effect in open hepatectomy with FT may be attributed to the differences in anesthetic agents administered, which may be associated with better hemodynamic stability and less use of vasopressors. This beneficial effect in laparoscopic hepatectomy is mitigated by the technical difficulty of the intervention, which is often time-consuming. Other operative parameters including intraoperative blood loss, blood transfusion, and conversion rate were not significantly different between the FT and TC groups.
Interestingly, it was noticed that the FT program did not add any significant advantage to patients who underwent laparoscopic liver resection; perhaps, the minimally invasive approach itself enhanced the postoperative recovery, and the introduction of the FT program in this subgroup of patients does not add any other beneficial effects.
As evidenced by a Cochrane meta-analysis,[51] simply using the FT program does not guarantee improved results unless there is stringent oversight of protocol adherence by all members of the team.[18] In the selected studies, there were confusing and insufficient data about the adherence to the FT program elements, and there was a high discrepancy between these elements. Only 2 out of 8 studies properly described the adherence rate to every single element of the program with a reported high compliance, except for the early removal of the urinary catheter (79% and 65% adherence rates, respectively).[35,36] In addition, most of these described elements were derived from the colorectal FT program, which may be not suitable for liver surgery.
Moreover, the available data about the anesthetic agents used were heterogeneous; some of these studies proposed the standard anesthetic protocol for both groups based on opioids,[35,36,38] but others used short-term anesthetic agents.[3,40] The majority of the included studies considered thoracic epidural as the regional anesthesia of choice in the FT program. On the other hand, some authors considered that epidural use is recommended in colorectal surgery, but its use is questionable in liver resection, as it may impair the postoperative recovery and it should be replaced by intrathecal morphine.[16,50,52,53] Furthermore, Hughes et al[16] reported that a small liver remnant after major resection may be a contraindication to the administration of paracetamol as it may induce liver damage. However, recent evidence recommended that paracetamol is safe in hepatic patients when its maximum dose is reduced to 2 to 3 g/day; nonsteroidal antiinflammatory drugs and opioids are better avoided.[54,55] As pain control is considered one of the most important elements in the FT program, further evaluation of analgesia with alternative methods in liver surgery is required to establish optimal results.
The nonuse of abdominal drains following liver resection is also a matter of debate. Some trials revealed that routine abdominal drainage is unnecessary after elective hepatectomy.[56,57] On the other hand, other reports described many valuable diagnostic and therapeutic benefits of the drain, especially after major liver resection.[58,59] As the FT program discourages the use of abdominal drains, this element should be revised within the FT program in the context of liver surgery. Perhaps, a protocol for early versus late drain removal or even no drain placement, only in minor liver resection would be applicable.
Now, well-established FT program with guidelines was developed in different kinds of surgery, including colorectal/pelvic[60,61] and gynecologic/oncology surgeries,[62] and in pancreaticoduodenectomy,[63] cystectomy,[64] and gastrectomy[65] procedures. No guidelines for the implementation of the FT program in liver surgery were identified. As liver surgery is a special entity, we advise the development of well-determined FT program elements specifically for these types of operations to be used as guidelines. This allows for better evaluation and standardization of the FT program in the field of liver surgery.
Finally, this meta-analysis showed significant results in favor of the FT program in regards to LoS, functional recovery, operative time, ICU admission rate, 1st bowel opening, and restoration of a normal diet. No significant differences emerged between the FT program and TC when comparing blood loss, need for blood transfusion and conversion, readmission, and mortality rates. When considering RCTs only, significantly lower morbidity rates were observed in the FT program. None of the analyzed outcomes showed superior results in favor of the TC group.
This meta-analysis had several limitations: the majority of the included studies are retrospective in nature and there was a marked heterogeneity between the investigated outcomes. We used the random-effects model as appropriate, but this bias was impossible to overcome. Moreover, there is a marked insufficiency of available data in the literature in regards to the FT program in liver resection, and the comparison of several outcomes such as pain score with multimodal analgesia, hospital costs, the serum level of CRP, duration to 1st bowel opening, and restoration of oral fluid and normal diet. We recommend considering these outcomes in prospective randomized studies so that better judgments can be made for the FT program in liver resection.
5. Conclusion
LoS was markedly reduced and functional recovery was accelerated when the FT program was implemented, without increasing readmission, morbidity, or mortality rates. Based on our systematic review and meta-analysis, the FT program is safe, feasible, and can be applied successfully in liver resection. Future RCTs focusing on debated issues such as multimodal analgesia and adherence rate are needed. The development of specific FT guidelines in the field of liver resection is strongly recommended.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CRP = C-reactive protein, EQ-5D = European quality of life-five dimensions model, FT = fast track, GCQ = general comfort questionnaire, GDFT = goal-directed fluid therapy, ICU = intensive care unit, LoS = length of hospital stay, MD = mean difference, OR = odds ratio, POD = postoperative day, QoL = quality of life, RCT = randomized controlled trial, RMB = Renminbi (Chinese yuan), TC = traditional care, VAS = visual analog scale.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Coolsen MM, Wong-Lun-Hing EM, van Dam RM, et al. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB (Oxford) 2013; 15:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001; 322:473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dam RM, Hendry PO, Coolsen MM, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg 2008; 95:969–975. [DOI] [PubMed] [Google Scholar]
- 4.Krohn BG, Kay JH, Mendez MA, et al. Rapid sustained recovery after cardiac operations. J Thorac Cardiovasc Surg 1990; 100:194–197. [PubMed] [Google Scholar]
- 5.Engelman RM, Rousou JA, Flack JE, et al. Fast-track recovery of the coronary bypass patient. Ann Thorac Surg 1994; 58:1742–1746. [DOI] [PubMed] [Google Scholar]
- 6.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997; 78:606–617. [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg 1999; 86:227–230. [DOI] [PubMed] [Google Scholar]
- 8.Lin DX, Li X, Ye QW, et al. Implementation of a fast-track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys 2011; 61:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins CD, Jono RT, Stanton BA, et al. The measurement of health-related quality of life: major dimensions identified by factor analysis. Soc Sci Med 1990; 31:925–931. [DOI] [PubMed] [Google Scholar]
- 11.Kolcaba K, Wilson L. Comfort care: a framework for perianesthesia nursing. J Perianesth Nurs 2002; 17:102–111.quiz 111–103. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S. Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England; Hoboken, NJ: Wiley-Blackwell; 2008. [Google Scholar]
- 15.Page AJ, Ejaz A, Spolverato G, et al. Enhanced recovery after surgery protocols for open hepatectomy–physiology, immunomodulation, and implementation. J Gastrointest Surg 2015; 19:387–399. [DOI] [PubMed] [Google Scholar]
- 16.Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB (Oxford) 2014; 16:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravante G, Elmussareh M. Enhanced recovery for non-colorectal surgery. World J Gastroenterol 2012; 18:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall TC, Dennison AR, Bilku DK, et al. Enhanced recovery programmes in hepatobiliary and pancreatic surgery: a systematic review. Ann R Coll Surg Engl 2012; 94:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spelt L, Ansari D, Sturesson C, et al. Fast-track programmes for hepatopancreatic resections: where do we stand? HPB (Oxford) 2011; 13:833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei Q, Wang X, Tan S, et al. Fast-track programs versus traditional care in hepatectomy: a meta-analysis of randomized controlled trials. Dig Surg 2014; 31:392–399. [DOI] [PubMed] [Google Scholar]
- 21.Kapritsou M, Korkolis DP, Giannakopoulou M, et al. Fast-track recovery after major liver and pancreatic resection from the nursing point of view. Gastroenterol Nurs 2014; 37:228–233. [DOI] [PubMed] [Google Scholar]
- 22.Kehlet H. Multimodal approach to postoperative recovery. Curr Opin Crit Care 2009; 15:355–358. [DOI] [PubMed] [Google Scholar]
- 23.Olsen MF, Wennberg E. Fast-track concepts in major open upper abdominal and thoracoabdominal surgery: a review. World J Surg 2011; 35:2586–2593. [DOI] [PubMed] [Google Scholar]
- 24.Takamoto T, Hashimoto T, Inoue K, et al. Applicability of enhanced recovery program for advanced liver surgery. World J Surg 2014; 38:2676–2682. [DOI] [PubMed] [Google Scholar]
- 25.Hughes M, Coolsen MM, Aahlin EK, et al. Attitudes of patients and care providers to enhanced recovery after surgery programs after major abdominal surgery. J Surg Res 2015; 193:102–110. [DOI] [PubMed] [Google Scholar]
- 26.MacKay G, O’Dwyer PJ. Early discharge following liver resection for colorectal metastases. Scott Med J 2008; 53:22–24. [DOI] [PubMed] [Google Scholar]
- 27.Schultz NA, Larsen PN, Klarskov B, et al. Evaluation of a fast-track programme for patients undergoing liver resection. Br J Surg 2013; 100:138–143. [DOI] [PubMed] [Google Scholar]
- 28.Wong-Lun-Hing EM, van Dam RM, Heijnen LA, et al. Is current perioperative practice in hepatic surgery based on enhanced recovery after surgery (ERAS) principles? World J Surg 2014; 38:1127–1140. [DOI] [PubMed] [Google Scholar]
- 29.Connor S, Cross A, Sakowska M, et al. Effects of introducing an enhanced recovery after surgery programme for patients undergoing open hepatic resection. HPB (Oxford) 2013; 15:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunne DF, Yip VS, Jones RP, et al. Enhanced recovery in the resection of colorectal liver metastases. J Surg Oncol 2014; 110:197–202. [DOI] [PubMed] [Google Scholar]
- 31.Hammond JS, Humphries S, Simson N, et al. Adherence to enhanced recovery after surgery protocols across a high-volume gastrointestinal surgical service. Dig Surg 2014; 31:117–122. [DOI] [PubMed] [Google Scholar]
- 32.Dejong CH, van Dam RM. Enhanced recovery programs in liver surgery. World J Surg 2014; 38:2683–2684. [DOI] [PubMed] [Google Scholar]
- 33.van Dam RM, Wong-Lun-Hing EM, van Breukelen GJ, et al. Open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery ERAS(R) programme (ORANGE II-trial): study protocol for a randomised controlled trial. Trials 2012; 13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blind PJ, Andersson B, Tingstedt B, et al. Fast-track program for liver resection–factors prolonging length of stay. Hepatogastroenterology 2014; 61:2340–2344. [PubMed] [Google Scholar]
- 35.He F, Lin X, Xie F, et al. The effect of enhanced recovery program for patients undergoing partial laparoscopic hepatectomy of liver cancer. Clin Transl Oncol 2015; 17:694–701. [DOI] [PubMed] [Google Scholar]
- 36.Jones C, Kelliher L, Dickinson M, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg 2013; 100:1015–1024. [DOI] [PubMed] [Google Scholar]
- 37.Lu H, Fan Y, Zhang F, et al. Fast-track surgery improves postoperative outcomes after hepatectomy. Hepatogastroenterology 2014; 61:168–172. [PubMed] [Google Scholar]
- 38.Ni CY, Yang Y, Chang YQ, et al. Fast-track surgery improves postoperative recovery in patients undergoing partial hepatectomy for primary liver cancer: a prospective randomized controlled trial. Eur J Surg Oncol 2013; 39:542–547. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Perez B, Aranda-Narvaez JM, Suarez-Munoz MA, et al. Fast-track program in laparoscopic liver surgery: theory or fact? World J Gastrointest Surg 2012; 4:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoot JH, van Dam RM, Busch OR, et al. The effect of a multimodal fast-track programme on outcomes in laparoscopic liver surgery: a multicentre pilot study. HPB (Oxford) 2009; 11:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou YM, Shao WY, Zhao YF, et al. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci 2011; 56:1937–1943. [DOI] [PubMed] [Google Scholar]
- 42.Abu Hilal M, Di Fabio F, Teng MJ, et al. Single-centre comparative study of laparoscopic versus open right hepatectomy. J Gastrointest Surg 2011; 15:818–823. [DOI] [PubMed] [Google Scholar]
- 43.Soni N. British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP): Cassandra's view. Anaesthesia 2009; 64:235–238. [DOI] [PubMed] [Google Scholar]
- 44.Kehlet H, Bundgaard-Nielsen M. Goal-directed perioperative fluid management: why, when, and how? Anesthesiology 2009; 110:453–455. [DOI] [PubMed] [Google Scholar]
- 45.Trinooson CD, Gold ME. Impact of goal-directed perioperative fluid management in high-risk surgical procedures: a literature review. AANA J 2013; 81:357–368. [PubMed] [Google Scholar]
- 46.Anders G. Health Against Wealth: HMOs and the Breakdown of Medical Trust. Boston: Houghton Mifflin; 1996. [Google Scholar]
- 47.Pearsall EA, Meghji Z, Pitzul KB, et al. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann Surg 2015; 261:92–96. [DOI] [PubMed] [Google Scholar]
- 48.Khoo CK, Vickery CJ, Forsyth N, et al. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 2007; 245:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gustafsson UO, Hausel J, Thorell A, et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011; 146:571–577. [DOI] [PubMed] [Google Scholar]
- 50.Koea JB, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg 2009; 2009:271986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011; 2:CD007635. [DOI] [PubMed] [Google Scholar]
- 52.Revie EJ, McKeown DW, Wilson JA, et al. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB (Oxford) 2012; 14:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes MJ, Ventham NT, McNally S, et al. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg 2014; 149:1224–1230. [DOI] [PubMed] [Google Scholar]
- 54.Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010; 85:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imani F, Motavaf M, Safari S, et al. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence-based recommendations. Hepat Mon 2014; 14:e23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun HC, Qin LX, Lu L, et al. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg 2006; 93:422–426. [DOI] [PubMed] [Google Scholar]
- 57.Gurusamy KS, Samraj K, Davidson BR. Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev 2007; 3:CD006232. [DOI] [PubMed] [Google Scholar]
- 58.Bekki Y, Yamashita Y, Itoh S, et al. Predictors of the effectiveness of prophylactic drains after hepatic resection. World J Surg 2015; 39:2543–2549. [DOI] [PubMed] [Google Scholar]
- 59.Jiao LR, Habib NA. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method (Br J Surg 2006; 93: 422–426). Br J Surg 2006; 93:1024–1025.author reply 1025. [DOI] [PubMed] [Google Scholar]
- 60.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 2012; 31:783–800. [DOI] [PubMed] [Google Scholar]
- 61.Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 2012; 31:801–816. [DOI] [PubMed] [Google Scholar]
- 62.Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations – Part I. Gynecol Oncol 2016; 140:313–322. [DOI] [PubMed] [Google Scholar]
- 63.Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. World J Surg 2013; 37:240–258. [DOI] [PubMed] [Google Scholar]
- 64.Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(R)) society recommendations. Clin Nutr 2013; 32:879–887. [DOI] [PubMed] [Google Scholar]
- 65.Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Br J Surg 2014; 101:1209–1229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


