Abstract
The contribution of the peripheral nervous system to opiate-induced hyperalgesia (OIH) is not well understood. Here, we determined the changes in excitability of primary sensory neurons after sustained morphine administration for 7 days. Changes in expression of glutamate receptors and glutamate transporters after morphine administration were ascertained in dorsal root ganglions (DRGs). Patch clamp recordings from intact DRGs (ex-vivo preparation) of morphine-treated rats showed increased excitability of small diameter (≤ 30 μm) neurons with respect to rheobase and membrane threshold, whereas the excitability of large diameter (> 30 μm) neurons remained unchanged. Small diameter neurons also displayed increased responses to glutamate, which were mediated mainly by GluN2B containing NMDA receptors (NMDARs), and to a lesser degree by the neuronal excitatory amino acid transporter 3 /excitatory amino acid carrier 1 (EAAT3/EAAC1). Co-administration in vivo of the GluN2B selective antagonist Ro 25-6981 with morphine for 7 days prevented the appearance of OIH and increased morphine-induced analgesia. Administration of morphine for 7 days led to an increased expression of GluN2B and EAAT3/EAAC1, but not of the AMPA, kainate or Group I metabotropic glutamate receptors, or of the vesicular glutamate transporter 2 (VGLUT2). These results suggest that peripheral glutamatergic neurotransmission contributes to OIH and that GluN2B subunit of NMDARs in the periphery may be a target for therapy.
1. Introduction
Opiates are the most prescribed drugs for controlling moderate to severe pain. The first few doses produce a robust analgesia, but this analgesic effect is usually not sustained as the nervous system adapts to the opiate, a phenomenon referred to as tolerance [26; 72]. Another consequence of this adaptation is the development of opiate-induced hyperalgesia (OIH), which is a paradoxical increase in pain despite dose escalation [11; 45; 65]. Many clinical studies have documented the existence of OIH [2; 8; 26; 30]. Studies on animal models of pain also suggest that opiates increase pain hypersensitivity and development of acute opioid tolerance [7; 16; 24; 27; 43; 52].
While much of the attention has focused on the involvement of the central nervous system (CNS) [78; 86], there is growing evidence that the peripheral nervous system contributes to OIH, because of documented increased firing of primary nociceptive neurons or their fibers after morphine administration [23; 31; 60; 76]. After morphine administration primary afferent terminals in the spinal cord display increased activity, greater release of glutamate, and larger N-methyl-D-aspartate receptor (NMDAR) mediated responses [90]. Finally, dissociated small diameter dorsal root ganglion (DRG) neurons pre-exposed to morphine, in vivo or in vitro, exhibit an increased excitability [60; 76].
NMDARs were previously identified as potential targets for prevention of OIH [45] after it was found that NMDAR antagonists co-administered with opiates blocked the appearance of hyperalgesia. The anti-hyperalgesic effect of these antagonists was largely believed to occur through central NMDARs [1; 46; 49]. Peripheral NMDARs, however, might also have been involved and could constitute better therapeutic targets because of the fewer side effects of NMDAR antagonists that do not cross the blood-brain-barrier [57]. For this reason, we chose to study OIH associated changes in the DRG since in this peripheral location opiate and glutamate receptors are abundant and participate in nociceptive transmission [21; 22; 36; 42; 44]. We investigated changes in glutamate receptors and transporters expressed on the neurons of the lumbar DRGs of rats that were administered morphine for 7 days and showed clear signs of OIH. The results indicate that increased nociception induced by continuous morphine administration is in part dependent on peripheral GluN2B expressing NMDARs and neuronal excitatory amino acid transporter 3 /excitatory amino acid carrier 1 (EAAT3/EAAC1).
2. Material and methods
2.1 Animals
Male Sprague-Dawley rats (220–250 g) were housed on a 12-hour light–dark cycle and given food and water ad libitum. For electrophysiological recordings, 10-16 animals/group were used. For Western blot analysis, 7 animals per group were used. For behavior analysis, 10 animals were used in control group, 8 animals were used for morphine group, and 6 animals were used for morphine and Ro 25-6981 group.
2.2 Ethics
The UCSF Institutional Animal Care and Use Committee approved the protocols used in this study, and the experiments were carried out in accordance with NIH regulations on animal use and care (Publication 85–23, Revised 1996)as well as according to recommendations of the International Association for the Study of Pain [91].
2.3 Drug administration and behavioral test
Drugs were systemically administered for 1 week using a mini-osmotic pump (2ML1, Alzet Co, USA). Briefly, after the rats were anesthetized with isoflurane, an incision was made on the nape, and the pump was inserted subcutaneously. The incision was then closed with 3-0 Vicryl sutures. Each pump contained either 2ml of sterile saline, morphine (15 mg/ml, West Ward, Illinois), saline + Ro 25-6981 (5 mg/kg), or morphine + Ro 25-6981 (5 mg/kg) [84]. The pump delivered the solution at a constant rate of 10 μl/h and lasted for 1 week. Continuous morphine administration for 7 days was previously shown to reliably induce OIH [60; 75]. Before implantation, the pumps were weighed. After rats were euthanized the pumps were recovered and weighed again to estimate the remaining fluid in the pump reservoir. All pumps were found to have approximately 0.3 ml of solution remaining when they were removed; indicating all the rats had the full infusion.
An investigator blind to the treatment groups performed behavior studies on rats. Heat pain threshold was measured daily by the Hargreaves plantar test between 9 to 11 AM. The animals were placed into the test area 60 min prior to testing. Then the withdrawal thresholds from both hind paws were measured 3 times, with a 5 min interval between each measure. The mean value was used as the thermal nociceptive threshold. A cutoff of 20 sec was used to prevent potential skin damage. The animals were first tested for 3 days in order to get a stable baseline; then on the 3rd day immediately after behavioral testing, they were implanted with a mini-osmotic pump. Animals were tested daily for 7 days following the pump implantation.
2.4 Patch clamp recordings
Intact lumbar DRGs were prepared as previously described [22]. Briefly, after inducing anesthesia with sodium pentobarbital (50 mg/kg, i.p.), a laminectomy was performed, then L4 and L5 DRGs were removed and placed into artificial cerebral spinal fluid (aCSF) bubbled with carbogen. The aCSF contained: 124 mM NaCl, 2.5 mM KCl, 1.2 mM NaH2PO4, 1.0 mM MgCl2, 2.0 mM CaCl2, 25 mM NaHCO3 and 10 mM glucose. The connective tissue surrounding the DRG was carefully removed under a dissecting microscope, and the ganglion was transferred to a recording chamber through which aCSF was perfused at a constant rate of 2-3 ml/min. A fine mesh anchor (SHD-22L, Harvard, USA) was used to stabilize the DRG during recordings. Five unit/ml of Liberase® (Roche) was applied locally via a pipette with a 5 μm diameter tip. After 15-20 minutes, the digested epineurium residue on a small area of the DRG surface was cleaned to expose the neurons.
DRG neurons were visualized with a 40X water-immersion objective using a microscope (FN-600; Nikon, Japan) equipped with infrared differential interference contrast optics. The image was captured with an infrared-sensitive CCD (IR-1000, Dage MTI, USA) and displayed on a black-white video monitor. The diameters of individual neurons were determined with a micrometer scale on the screen. Neurons ≤ 30 μm in diameter were categorized as small and those > 30 μm in diameter were categorized as large. Currents were recorded with an Axon 200B amplifier (Molecular Devices, USA) connected to a Digidata interface (Digidata 1322A, Molecular Devices, USA) and low-pass filtered at 5 kHz, digitized, and stored using pCLAMP 10.2 (Molecular Devices, USA). Patch pipettes were pulled from borosilicate glass capillary tubing (BF150-86-10, Sutter, USA) with a P97 pipette puller (Sutter, USA). The resistance of the pipette was 4-5 MΩ when filled with recording solution, which contained: 140 mM KCl, 2 mM MgCl2, 10 mM HEPES, 2 mM Mg-ATP, 0.5 mM Na2GTP, pH 7.4. The recordings were acquired in whole cell mode, and the access resistance was 10-20 MΩ with continuous monitoring. Data were discarded if the access resistance changed more than 15% during an experiment. For measuring the rheobase, a series of currents were injected to the neuron, starting at -0.1 nA with increments of 0.05 nA until the first action potential was generated. For measuring the membrane threshold, a 500 ms depolarizing ramp (2000 pA/s) was administered. For all currents induced by agonists except NMDA, the neurons were clamped at -70 mV. For NMDA recordings, the neurons were clamped at +40 mV, and Mg2+-free aCSF was used to remove the magnesium block. Also 10 μM of glycine was co-applied with NMDA to fully activate NMDA receptors (NMDARs).
All glutamate receptor and transporter agonists were purchased from Sigma (Sigma, USA); while antagonists like APV, CNQX, DL-threo-β-benzyloxyaspartate (TBOA)and Ro 25-6981 were purchased from Tocris (Tocris, USA). Drugs were dissolved in ultra-pure deionized water as stock solutions. All stock solutions were diluted to the desired concentration with aCSF immediately before use. All agonists were applied with focal pressure ejection via a pipette controlled by a Picrospitzer II (puff at 1-2 psi, General Valve, USA). The pipette was located approximately 50 μm from the recorded neuron so that the drugs reached all parts of the neuron. Changes in currents that were greater than 20% of baseline were determined to be inward currents induced by the agonists. All the inhibitors or antagonists were bath applied to reach the maximal effect. To confirm the contribution of GluN2B to NMDA currents, the potent GluN2B selective inhibitor Ro 25-6981 (0.3 μM) was perfused for 10 min to block GluN2B/NMDARs mediated currents. This dose has been used and verified previously for its inhibitory effect [84; 89].
2.5 Western blots
DRGs from L1-6 (from 7 animals) were homogenized in lysis buffer (30 mM Tris HCl, 1 mM EGTA, 0.1 mM Na3VO4, 10 mM Na4P2O7, 10 mM NaF, pH 7.4) containing complete protease inhibitor mini EDTA-free (Cat #: 1835170, Roche Diagnostics) and phosphatase inhibitor cocktail (Cat #: P0044, Sigma-Aldrich, St-Louis, USA). Tissue homogenates were centrifuged at 23,000 g for 15 minutes at 4°C. Then the supernatant was acquired and prepared for BCA to determine the protein concentration. For Western blot analysis, 25 μg of protein was separated on a 10% SDS-PAGE and transferred onto PVDF membranes as we described previously [29]. Membranes were blocked in LI-COR Odyssey Blocking Buffer (LI-COR Biosciences) for 1 hour at room temperature and incubated overnight at 4°C with primary antibodies: mouse monoclonal anti-β-actin (1:10,000, cat #: A2228, Sigma-Aldrich), rabbit polyclonal anti-NR2A/B (1:400, Cat #: AB1548, Chemicon, USA), rabbit polyclonal anti-GluN2B (1:1000, Cat #: 06-600, Millipore, USA), rabbit polyclonal anti-NR2C (1:400, Cat #: AGC-018, Alomone Labs, Israel), goat polyclonal anti-NR2D (1:500, Cat #: sc-1471, Santa Cruz, USA), mouse monoclonal anti-vGLUT2 (1:200, Cat #: 75-002, UC Davis/NIH NeuroMab Facility, USA), rabbit polyclonal anti-excitatory Amino Acid Transporter 3 (1:400, Cat #: AGC-023, Alomone Labs, Israel). After washing with PBST for 3 times (10 min each) the membranes were incubated for 1 hour at room temperature with fluorescent secondary antibodies (1:15,000, IRDye 680RD polyclonal donkey goat anti-mouse IgG Cat #: 926-68072, or IRDye 800CW polyclonal goat anti-rabbit IgG Cat #: 926-32211, LI-COR Biosciences, USA), washed with PBS for 3 times, scanned, and bands were quantified with the LI-COR Odyssey Infrared Imaging System. All the proteins were normalized to β-actin; the expression levels in saline animals were set as 100% and expression of the protein was shown as the percentage of saline group.
2.6 Statistical analysis
All results were presented as the mean ± SEM. For testing the blocking effect of receptor antagonist, the responses induced by each agonist were set as 100%, and the currents after antagonist application were expressed as the percentage of previous response. For animal behavior analysis, all the latencies were normalized to the baseline for each animal individually. One way repeated measure of ANOVA was used, followed by LSD test for further comparison. For comparison of currents and protein expression between saline and morphine groups, the statistical significance was determined using the Student's t-test. For comparing the responsive percentage, chi square test was used. A p level ≤ 0.05 was determined as statistically significant.
3. Results
3.1 Sustained morphine administration induced hyper-excitability of small diameter DRG neurons
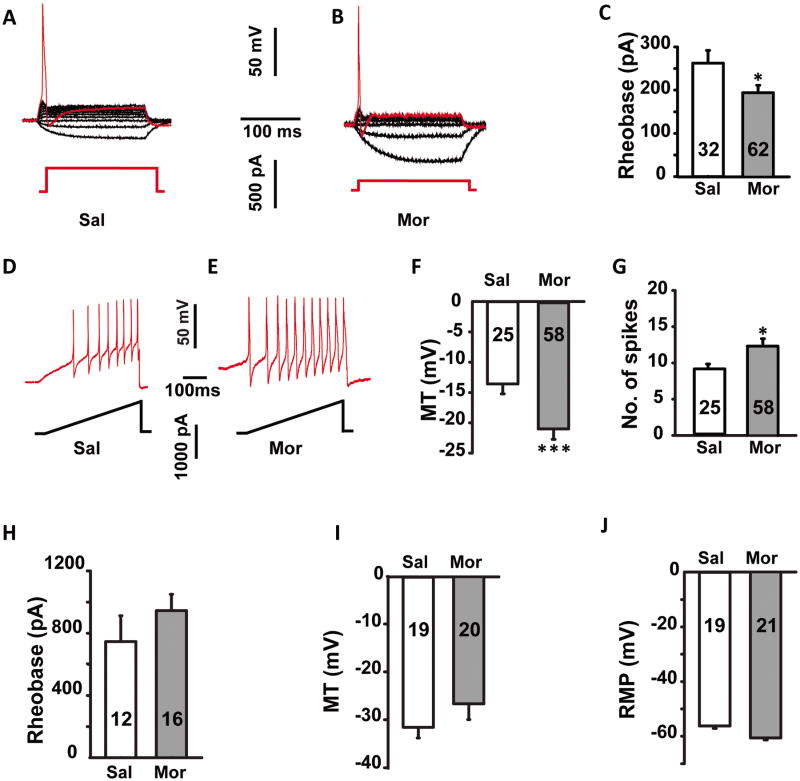
After one week of morphine or saline treatment, intact DRGs were collected and recorded. Small diameter neurons (≤ 30 μm) were first studied given that they are associated with nociceptive transmission [4]. Neurons from the saline group displayed an average rheobase of 260 ± 29.6 pA (n = 32, Fig. 1A). Morphine treatment significantly decreased the average rheobase to 190 ± 21.1 pA (n = 62, p ≤ 0.05; Fig.1B and C). Membrane threshold (MT) also decreased after morphine treatment from -14.7 ± 3.2 mV (n = 25) to -21.1 ± 3.8 mV (n = 58, p ≤ 0.001; Fig. 1D-F). The spike numbers elicited by the current ramp were also compared between the two groups. During a 500 ms ramp stimulus, the average number of spikes for neurons in the morphine group was 12.2 ± 1.6 (n = 58), compared with 7.8 ± 0.9 (n = 25) for the saline group (p ≤ 0.05, Fig. 1G). This data shows that following one week of morphine administration, small diameter neurons displayed a robust increase in excitability.
Fig. 1.
Sustained morphine administration for 1 week induced the hyper-excitability of the small diameter neurons (≤ 30 μm) in DRGs, but had no significant effects on larger neurons (> 30 μm). (A, B) Representative recording traces used to measure the rheobase of small neurons from saline and morphine treated rats. (C) Histogram comparing the rheobase in saline (Sal) and morphine (Mor) treated rats. Statistical analysis showed that the rheobase was significant lower in morphine compared to saline treated rats. (D, E) Representative traces used to measure the membrane threshold (MT) in small neurons from saline and morphine treated rats. Membrane threshold (F) was significant lower and the number of spikes (G) was greater for small neurons in morphine compared to saline treated rats. There was no significant difference in rheobase (H), membrane threshold (I) and resting membrane potential (J) in large neurons between saline and morphine treated rats. MT, membrane threshold; RMP, resting membrane potential. * p ≤ 0.05, ***, p ≤ 0.001. Integers in each column represent sample numbers.
In order to ascertain whether the excitatory effect of sustained morphine also involved larger DRG neurons, recordings were made from large diameter (> 30 μm) neurons. No significant differences in rheobase, MT, and resting membrane potentials (RMP) between the saline and morphine group (Fig. 1H-J) were seen in larger diameter neurons. Thus, sustained morphine administration selectively altered neuronal excitability of small diameter (≤ 30 μm) DRG neurons.
3.2 Sustained morphine administration increased responses to glutamate in small, but not large diameter DRG neurons
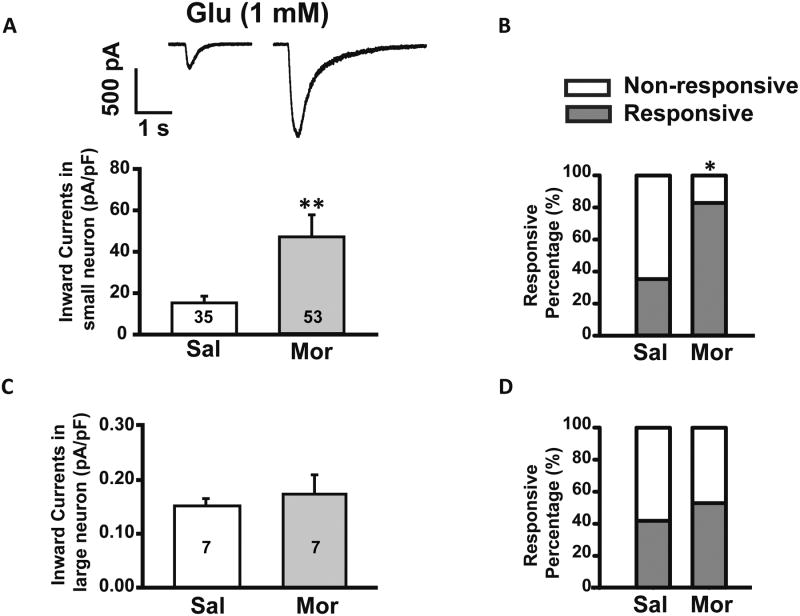
Recent studies indicate that glutamatergic transmission occurs in sensory ganglions and that intraganglionic glutamate receptors are involved in certain forms of pain such as neuropathic pain [22; 42; 44]. We therefore investigated glutamate receptor responses in primary sensory neurons of rats after OIH was induced by sustained morphine administration for 7 days. DRGs from the morphine-treated group demonstrated an increase in the amplitudes of inward currents induced by glutamate, with average amplitude of 47.2 ± 10.6 pA/pF compared to average amplitude of 15.3 ± 3.3 pA/pF from saline-treated DRGs (p ≤ 0.01, n = 35, Fig. 2A). In DRGs from saline-treated rats, puff application of 1 mM glutamate induced inward currents in 37.1% of small diameter neurons, whereas in DRGs from morphine-treated rats, glutamate induced inward currents in 84.2% of the small diameter neurons (p ≤ 0.05, Chi square test, Fig.2B).
Fig. 2.
Small diameter DRG neurons (≤ 30 μm) showed an increased response to glutamate in the morphine (Mor) group compared to saline (Sal). (A) Upper: Representative traces induced by glutamate (1 mM) puff application on small diameter neurons from saline (left) and morphine (right) treated rats. Lower: Histogram comparing the currents in saline and morphine treated rats. Statistical analysis showed that glutamate induced larger inward currents in neurons from morphine treated rats. (B) More neurons were responsive to glutamate in morphine treated rats compared with saline. (C) No differences were discerned in the responses of large diameter neurons (> 30 μm) to glutamate application between morphine and saline treated rats. Of note, compared with small diameter DRG neurons large neurons exhibited much smaller glutamate induced currents. (D) No difference was discerned in responsive percentage of large neurons to glutamate between morphine and saline treated rats. *, p ≤ 0.05, **, p ≤ 0.01. Integers in each column represent sample numbers.
We then verified whether sustained morphine administration affected glutamate-induced currents in larger diameter neurons (> 30 μm). In larger neurons from DRGs (n=7 from 4 DRGs) of the saline-treated group, 1 mM glutamate induced minimal inward currents, with an average current density of 0.15 ± 0.01 pA/pF. In large DRG neurons from the morphine-treated group, glutamate-induced inward currents similarly showed an average density of 0.17 ± 0.03 pA/pF (p > 0.05, 7 neurons from 3 DRGs, Fig.2C). Responsive percentages to glutamate in large neurons were comparable between saline- and morphine-treated rats (Fig. 2D). Therefore, sustained morphine administration did not significantly impact glutamate-induced currents in large diameter neurons.
To exclude the possibility that increased response to glutamate was due to opiate withdrawal, another batch of recordings were made in small diameter neurons after adding morphine (10μM) to the aCSF bathing the DRGs (8 small diameter neurons from 4 DRGs in the morphine treated group). With the presence of morphine in aCSF, the amplitudes of glutamate-induced currents were not different from those recorded when morphine was omitted from the bathing solution (data not shown).
3.3 Sustained morphine administration upregulated GluN2B expressing NMDARs
Glutamate-induced currents in DRGs are mediated by several subtypes of glutamate receptors that include the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), NMDA, kainate (KA), and group I metabotropic glutamate receptors (group I mGluRs). The glutamate transporters can also generate glutamate-induced currents [40; 77].
To determine which specific glutamate subunit/subtype were involved in mediating changes in currents after morphine treatments, specific glutamate agonists and antagonists were used in patch clamp recordings.
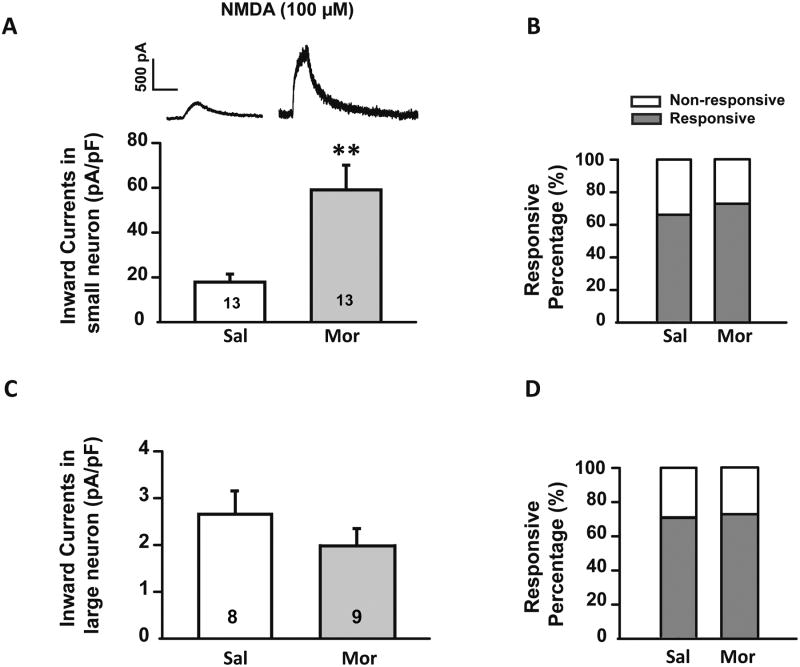
In the morphine group the average amplitude of NMDA induced currents was greater than in saline controls (59.1 ± 11.0 pA/pF vs. 17.9 ± 3.6 pA/pF, n = 13 for each group, p ≤ 0.01, Fig. 3A), suggesting that the expression of NMDARs is increased after morphine treatment. The percentage of small neurons responding to NMDA, however, was similar in the morphine and saline treated groups, 72.4% and 65.1% respectively (p >0.05, chi square test, Fig. 3B).
Fig. 3.
Sustained morphine administration induced an up-regulation of NMDARs. (A) Upper: representative current traces induced by NMDA (100 μM) puff application on small diameter neurons (≤ 30 μm) from saline (left) and morphine (right) treated rats. Lower: Histogram comparing the currents in saline (Sal) and morphine (Mor) treated rats. Statistical analysis showed that NMDA induced larger inward currents in neurons from morphine treated compared with saline treated rats. (B) No significant changes in the percentage of small diameter neurons responsive to NMDA were detected between saline and morphine treated rats. (C) No significant differences were discerned in the responses of large diameter neurons (> 30 μm) to NMDA application between morphine and saline treated rats. Of note, compared with small diameter DRG neurons, large neurons exhibited much smaller NMDA induced currents. (D) No difference was discerned in percentage of large neurons responsive to NMDA between morphine and saline treated rats. *, p ≤ 0.05, **, p ≤ 0.01. Integers in each column represent sample numbers.
Given that NMDARs are expressed by DRG neurons of all sizes [54], we also analyzed NMDARs responses in larger diameter (> 30 μm) neurons. We found that in comparison to small diameter neurons, the amplitude of NMDA (100 μM) induced inward currents in larger neurons was much less and did not differ between saline and morphine treated animals. In the saline group, the average NMDA current density was 2.59 ± 0.56 pA/pF, whereas in the morphine group the current density was 1.89 ± 0.19 pA/pF (p = 0.256, Fig. 3C). Similarly to what was observed in small neurons, the numbers of NMDA-responsive large diameter neurons did not increase after morphine treatment (Fig. 3D).
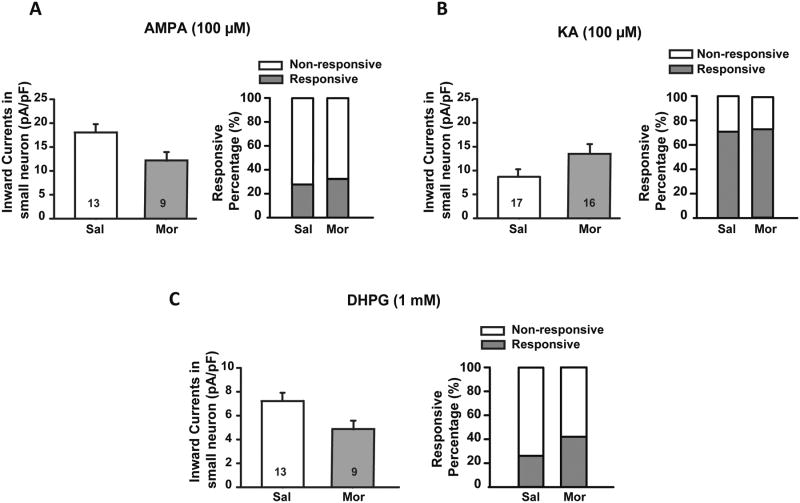
In contrast to NMDA, application of 100 μM AMPA or KA on small diameter neurons did not produce any significant difference in average current amplitude between saline and morphine groups (Fig. 4A and B). Similarly to NMDA, the percentage of responsive neurons remained unchanged (Fig. 4A and B). Finally, puff application of 1 mM DHPG, a specific agonist for group I mGluRs, did not induce significant changes in the average amplitude or responsive percentage between two groups (Fig. 4C). Overall, no significant changes were detected for non-NMDA glutamate receptors between the saline- and morphine-treated groups.
Fig. 4.
Histograms showing the effect of AMPA, KA, and DHPG puff application on small diameter neurons (≤ 30 μm) in saline (Sal) vs. morphine (Mor) treated rats. Sustained morphine administration had no effect on AMPA (100 μM, A), KA (100 μM, B) or DHPG (1 mM, C) induced currents in small diameter DRG neurons (≤ 30 μm) or on the percentage of responsive neurons compared with saline treated rats. Integers in each column represent sample numbers.
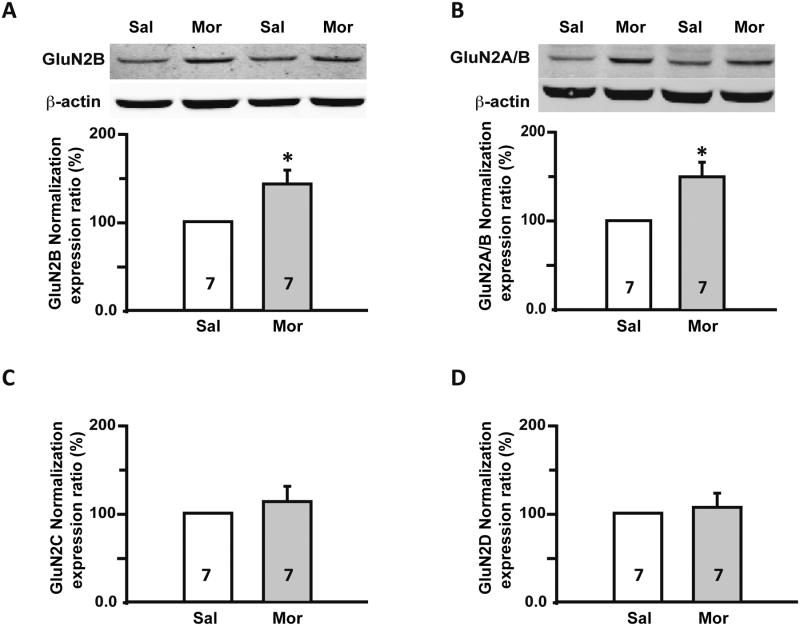
Previous studies have determined that the GluN2B subunit of the NMDAR is expressed mainly by small and medium diameter DRG neurons [50], and that GluN2B is involved in nociception and OIH [85; 90]. We performed Western blot to examine changes in GluN2B expression after morphine treatment. The housekeeping protein β-actin served as an internal control. The expression of the GluN2B subunit in DRGs from morphine-treated rats increased by 42.5 ± 15.6% compared to the saline group (p ≤ 0.05, n = 7, Fig. 5A). To evaluate the contribution of GluN2A subunit, we used an antibody that recognizes the GluN2A/2B subunits. Western blot analysis showed that relative expression ratio of GluN2A/B in morphine group was increased by 49.2 ± 16.1% (n = 7, p ≤ 0.05, Fig. 5B) but was not different from the increase shown with the GluN2B selective antibody, leading us to conclude that GluN2A did not increase.
Fig. 5.
Sustained morphine administration specifically up-regulated GluN2B, but not other NMDAR subunits. (A) Western blots: representative blots for GluN2B NMDAR subunits in DRGs from saline (Sal) and morphine (Mor) treated rats. Histogram: Statistical analysis showed that there was up-regulation of GluN2B subunits in the DRGs from morphine treated rats. (B) Western blots: representative blots for GluN2A/B NMDARs subunits in DRGs from saline and morphine treated rats. Histogram: Statistical analysis showed that there was up-regulation of GluN2A/B subunits in DRGs from morphine treated rats. Western blot analysis did not reveal any significant difference in GluN2C (C) or GluN2D (D) subunits expression between saline and morphine treated rats. Sal, Saline treated group; Mor, Morphine treated group. *, p ≤ 0.05; **, p ≤ 0.01. Integers in each column represent sample numbers.
Next, we determined if expression of GluN2C and GluN2D NMDAR subunits was altered in the DRGs after morphine treatment. The expression of GluN2C (n = 7/group, Fig. 5C) and GluN2D was not significantly different after morphine treatment (p > 0.05, n = 7/group, Fig. 5D). Based on Western blot analysis from the DRGs, the GluN2B subunit emerged as the only NMDA subunit with a significant change in expression after sustained morphine administration.
3.4 The GluN2B selective antagonist Ro 25-6981 prevented the increased response to NMDA and the hyperalgesia following sustained morphine administration
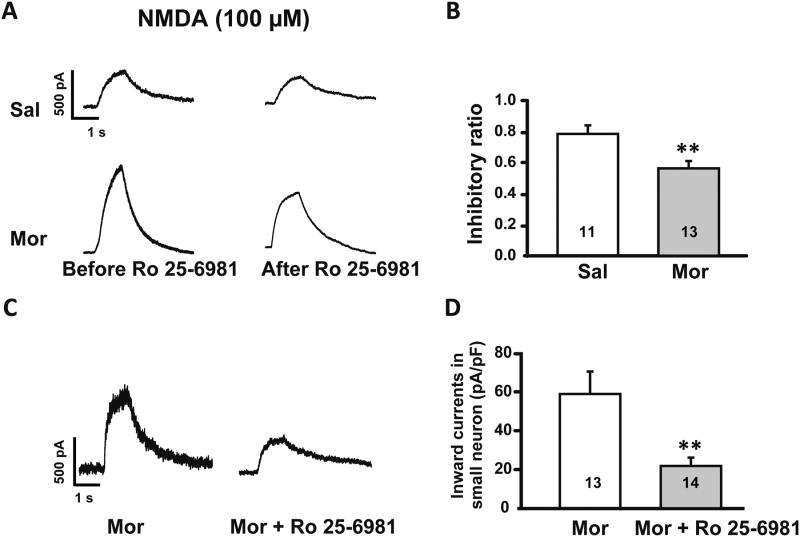
In order to determine if the increase in the GluN2B subunit of the NMDAR contributed to increased neuronal response to glutamate, we used the selective GluN2B antagonist Ro 25-6981 during patch clamp recording on intact DRGs. We first established that run down effects or desensitization did not occur from repeated NMDA applications. The results showed that NMDA-induced currents were only reduced to 98.7 ± 2.5% (p > 0.05) when NMDA was reapplied 10 min after the first application (data not shown). Then we found that in DRGs from control rats, bath application of Ro 25-6981 inhibited NMDA currents to 78.8 ± 4.1% of the original response. While in DRGs from morphine-treated rats, Ro 25-6981 inhibited the NMDA-induced currents to 56.6 ± 4.0%, which was a significantly greater inhibition (p ≤ 0.01, n = 13/group, Fig. 6A and B).
Fig. 6.
Both in vitro and in vivo application of the GluN2B selective antagonist Ro 25-6981 inhibited NMDA induced currents more prominently in morphine (Mor) treated rats than in controls (Sal). (A) Upper: representative currents induced by NMDA application before and after bath application of Ro 25-6981 in small diameter DRG neurons (≤ 30 μm) from saline treated rats. Lower: Currents recorded in small DRG neurons from morphine treated rats. (B) Histogram showing the inhibitory effect of Ro 25-6981 in morphine vs. saline treated rats (** p ≤ 0.01). (C) Left: representative trace shows NMDA induced current in a small DRG neuron from a morphine treated rat. Right: NMDA induced current in a small DRG neuron from a morphine + Ro 25-6981 treated rat. (D) Application of Ro 25-6981 blocked the enhancement of NMDA induced currents in small DRG neurons caused by morphine administration (**, p ≤ 0.01). Integers in each column represent sample numbers.
In subsequent experiments, we tested the effects of in vivo administration of Ro 25-6981 on neuronal responses to application of NMDA. We co-administered morphine (15 mg/ml) + Ro 25-6981 (5 mg/kg) (n = 6) via a sub-cutaneous pump delivering 10 μl/h for one week. Control rats were treated with morphine (15 mg/ml; n = 8).Patch clamp recordings of small diameter neurons on intact DRGs showed that co-administration of Ro 25-6981 with morphine blocked the morphine associated increase in NMDA currents to 21.7 ± 3.2 pA/pF vs. 59.1 ± 11.0 pA/pF in the morphine treated group (p ≤ 0.01, Fig. 6C and D).
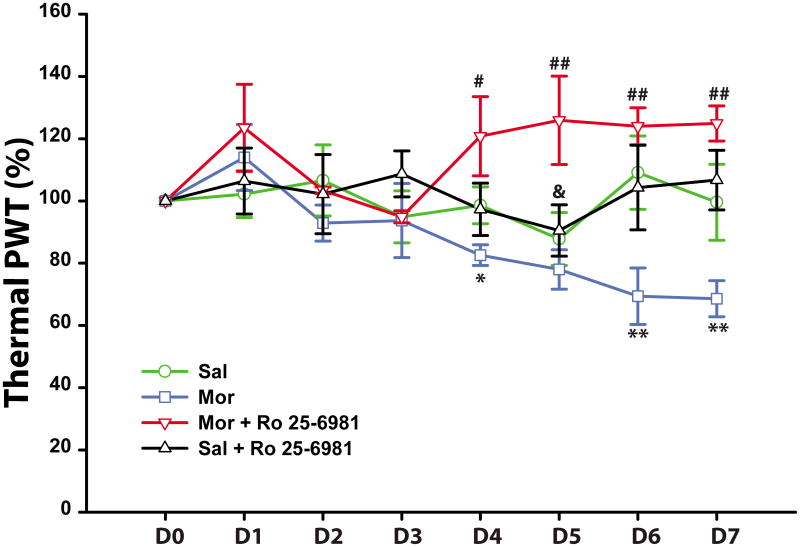
Next, we monitored the response to a thermal nociceptive stimulus in rats that were administered with the following solutions via a subcutaneous mini-osmotic pump: saline (n = 10), morphine (n = 8), morphine + Ro 25-6981 (n = 6), or saline + Ro 25-6981 (n = 6). Baseline withdrawal latencies to heat before pump implantation were similar for all rats in all treatment groups (6.6 ± 0.7, 6.3 ± 0.6, 7.9 ± 0.4, and 7.1 ± 0.6 s for saline, morphine, morphine + Ro 25-6981 and saline + Ro 25-6981 respectively).Mini-osmotic pumps were then implanted and withdrawal latencies continued to be measured daily. Four days after morphine treatment, the withdrawal latencies became shorter (82.1 ± 3.2% of their baseline, p < 0.05), a sign of OIH. In rats that were given morphine + Ro 25-6981, there was no thermal hyperalgesia and withdrawal latencies increased to 121.5 ± 13.2% of their baseline (Fig. 7, p < 0.05). From day 5 onwards, in the morphine group the withdrawal latencies decreased further relative to their baseline (78.5 ± 3.9% to 68.6 ± 5.8%, p < 0.05). While the morphine + Ro 25-6981 group continued to show sustained anti-nociception (126.4 ± 14.7% to 124.9 ± 5.7% of baseline, p < 0.01).In the saline alone and saline + Ro 25-6981 groups, the withdrawal latencies remained unchanged throughout the 7 day period (p > 0.05, compared with baseline). Intergroup comparisons show a separation of the four groups at day 4 (Fig. 7). The morphine treated animals developed decreased tolerance to nociceptive heat, whereas the morphine + Ro 25-6981 treated animals became less sensitive to the same stimulus.
Fig. 7.
Thermal withdrawal threshold measured in vivo with the Hargreaves heat plantar test. Starting 4 days after the pump implantation, morphine treated rats showed thermal hyperalgesia. Co-administration of morphine and Ro 25-6981 (Mor + Ro 25-6981) blocked the hyperalgesic effect and enhanced the antinociceptive effect of morphine. * p ≤ 0.05; ** p ≤ 0.01, morphine vs. saline or saline + Ro 25-6981; #, p ≤ 0.05; ##, p ≤ 0.01, morphine + Ro 25-6981 vs. morphine. &, p< 0.05, morphine + Ro 25-6981 vs. saline or saline + Ro 25-6981. N = 10 for saline group, n = 8 for morphine group and n = 6 for Morphine + Ro 25-6981 or saline + Ro 25-6981. In the axis the time post-pump implantation are indicated from day 1 (D1) to day 7 (D7). D0 is day of the pump implantation and the paw withdrawal threshold (PWT) on that day (100%) represents the baseline paw withdrawal value.
3.5 Sustained morphine administration upregulated EAAT3/sEAAC1 in DRGs
Glutamate currents generated in DRG neurons are mainly mediated by AMPA receptors, NMDA receptors, KA receptors and group I mGlu receptors [22; 42]. Accordingly we used selective antagonist CNQX (10 μM), APV (50 μM), and DL-AP3 (60 μM) to block all glutamate receptor-mediated currents. We reasoned that glutamate transporters would mediate the residual currents. In agreement with this notion, application of TBOA (100 μM), a specific glutamate transporter blocker, blocked 90.1% of the remaining currents.
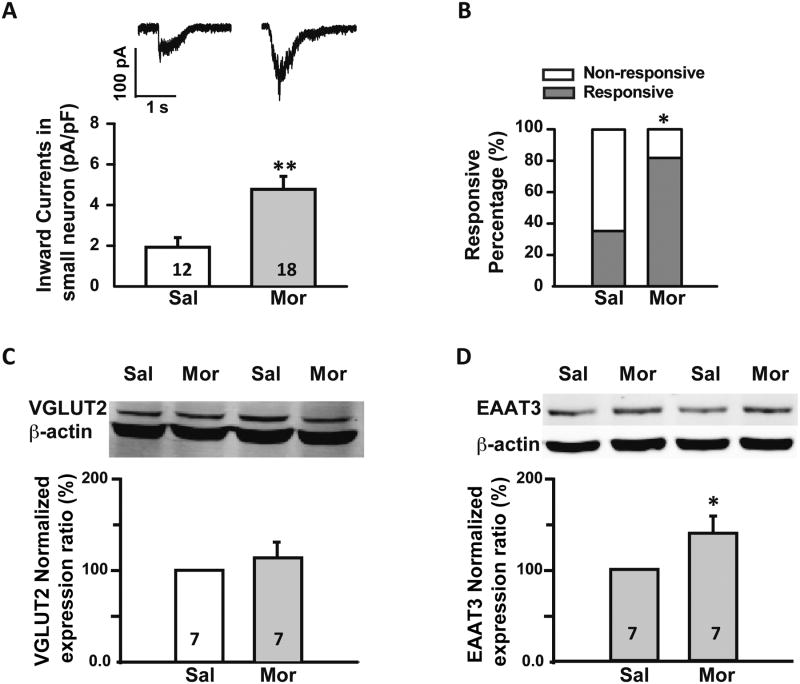
Even after blocking the glutamate receptors the amplitude of glutamate-mediated inward currents was still greater in the morphine compared with saline treated group. The average amplitude of these currents in the saline group was 1.9 ± 0.5pA/pF (n = 12 neurons), whereas in the morphine group the amplitude was 4.8 ± 0.6pA/pF (n= 18 neurons, p ≤ 0.01, Fig. 8A).
Fig. 8.
Sustained morphine administration induced an up-regulation of the glutamate transporter EAAT3/EAAC1 but not of VGLUT2. (A) Patch clamp data. Upper: representative current traces induced by glutamate (1 mM) puff application in the presence of APV (50 μM), CNQX (10 μM) and DL-AP3 (60 μM) on small diameter neurons (≤ 30 μm) from saline (left) and morphine (right) treated rats. Lower: histogram comparing glutamate transporter currents in saline (Sal) and morphine (Mor) treated rats. Statistical analysis showed that there was larger glutamate transporter mediated inward currents in neurons from morphine compared to saline treated rats. (B) Patch clamp data. In addition, when transporter currents were isolated, more neurons were responsive to glutamate in morphine treated compare to saline treated rats. (C) Western blots. Upper: representative blots for VGLUT2 in DRGs from saline (Sal) and morphine (Mor) treated rats. Lower: histogram comparing VGLUT2 expression in saline (Sal) and morphine (Mor) treated rats. Statistical analysis showed that there was no change in expression of VGLUT2 in the DRGs from morphine compared to saline treated rats. (D) Western blots. Upper: representative blots for EAAT3/EAAC1 in DRGs from saline (Sal) and morphine (Mor) treated rats. Lower: histogram comparing EAAT3/EAAC1 expression in saline (Sal) and morphine (Mor) treated rats. Statistical analysis showed that there was increased expression of EAAT3/EAAC1 in DRGs from morphine compared to saline treated rats. *, p ≤ 0.05; **, p ≤ 0.01. Integers in each column represent sample numbers.
Furthermore, a greater percentage of neurons exhibited glutamate transporter currents following sustained morphine administration. In the saline group 38.0% of neurons were responsive, whereas in the morphine group, 83.9% of neurons were responsive (p ≤ 0.05; Fig. 8B). These data suggest that glutamate transporters in small diameter DRG neurons underlie some of the increases in inward currents and the responsive percentage change after sustained morphine administration.
In large diameter DRG neurons, glutamate transporter-mediated currents were much smaller than those seen in small diameter neurons. The average current density was 0.06 ± 0.01 pA/pF in the large diameter neurons from the saline treated group. Morphine treatment did not change the average transporter current density, which remained at 0.07 ± 0.01 pA/pF (p > 0.05 from 6 large neurons/group).
Of the eight glutamate transporters, only three are consistently expressed in small diameter DRG neurons. These include the vesicular glutamate transporter 2 (VGLUT2), the vesicular glutamate transporter 3 (VGLUT3), and EAAT3/EAAC1 [51; 64; 70]. Since both VGLUT2 and EAAT3/EAAC1 expression is associated with nociceptive transmission [20; 63], we used Western blot to assess for possible change in protein levels. Sustained morphine administration did not significantly change the expression of VGLUT2, which showed a relative expression ratio of 112.4 ± 17.1%, comparable to saline group (p > 0.05, Fig. 8C). For EAAT3/EAAC1, the relative expression in morphine group was increased to 136.5 ± 16.3% of the saline group (p ≤ 0.05, Fig. 8D). Taken together, this data suggests that increases in glutamate induced currents in small diameter neurons after sustained morphine treatment, is in part due to increased expression of the EAAT3/EAAC1.
4. Discussion
The main finding of our study is that sustained morphine administration leads to increased excitability of small diameter, but not of larger (> 30 μm) DRG neurons. The increased neuronal excitability is accompanied by greater responses to glutamate that are mediated in part by the NMDARs, and the neuronal glutamate transporter EAAT3/EAAC1. The NMDAR GluN2B subunit is upregulated following morphine administration and appears to be critically involved in OIH.
4.1 Morphine and small diameter DRG neurons
Clinically, repeated opiates administration leads to both tolerance and hyperalgesia [33; 45]. Tolerance is a common phenomenon associated with repeated opiate administration, where the medication becomes less effective over time and increasingly larger doses are required to relieve pain [33]. OIH is a paradoxical increase in pain sensitivity with morphine administration [3]. In contrast to tolerance, increasing the dose of opiates worsens OIH [45]. In our study, thermal nociceptive threshold, in otherwise normal animals, were progressively decreased even in presence of constant level of morphine, suggesting that this phenomenon is due to OIH (Fig. 7).
Our data suggests that morphine induces OIH by a direct action on small diameter primary sensory neurons, many of which are nociceptive. Large diameter neurons that convey innocuous sensations, failed to display a change in excitability or glutamate-induced responses. This is consistent with clinical observations that OIH is an increase in nociceptive sensations whereas other senses are unchanged [32; 88]. Opiate receptors, especially the mu-opiate receptor (MOR), are critically involved in OIH [17; 66; 86] and MOR-deficient mice are not hyperalgesic after opiate treatment [46]. We suggest that MORs on small diameter DRG neurons are instrumental in initiating OIH. This is supported by previous reports that in adult DRGs most MORs are expressed by small diameter neurons [5; 80]. Also MOR agonists impact the function of small, but not large diameter neurons. For instance calcium transport is altered [68], release of excitatory amino acids is increased [18; 76], and peripheral C and A-delta sensory fibers become more excitable [31].
Opiate receptor independent mechanisms may also be involved in OIH [67]. The Toll-like receptor 4 (TLR4) has been implicated in OIH [34]. In DRGs TLR4 is expressed by neurons rather than by glial cells [12; 74]. Binding of morphine or possibly its metabolite morphine-3-glucuronide (M3G) to TLR4 activates a cascade of events leading to greater neuronal excitability through an increased activity of sodium channels [12; 58; 60], transient receptor potential channels [15; 60; 61], and inflammatory mediators [12; 81]. Some of these events are likely contributors to the altered function of primary sensory neurons observed here.
4.2 Role of GluN2B expressing NMDARs in OIH
Most of the reported data on morphine-induced changes in GluN2B expressing NMDARs and pain comes from studies in the CNS [38; 41; 47; 48; 85; 87; 90]. In OIH increased expression and phosphorylation of GluN2B containing NMDARs contributes to increased neural activity and pain behavior. Our data suggest that in the periphery, GluN2B expressing NMDARs also contribute to OIH and in that location they are potential targets for developing new therapeutics.
NMDARs comprise four subunits, including two constant GluN1 subunits and two GluN2 and/or GluN3 subunits [79]. An increase in GluN2B is relevant to inflammatory, neuropathic or incisional pain [9; 37; 39; 84; 85]. Our western blot analysis of the DRGs revealed an upregulation of GluN2B following morphine administration, but not of other subunits (Fig. 4). Since protein lysates from whole DRG include small and large neurons, as well as satellite glial cells, all of which express NMDARs [13; 42; 54], we used patch clamp recording to confirm neuron-specific upregulation. Patch clamp of DRG neurons confirmed that the GluN2B containing NMDARs were increased after morphine treatment, whereas AMPA, KA and mGluR1 receptor responses remained unchanged. This selective increase in GluN2B might be unique to opiate therapy as in other conditions such as nerve injury all glutamate receptors increase in the DRG [22].
Similarly to our observations for the DRGs, Zhao and colleagues found morphine induced increase in GluN2B expressing NMDAR current in primary afferent terminals in the spinal cord [90]. Also, blocking NMDARs in with the non-selective antagonist AP5 given intrathecally increases the analgesic effect of morphine [90]. We extended these findings by showing that simultaneous administration of morphine and the GluN2B antagonist Ro 25-6981 reverses OIH and enhances morphine analgesia.
Morphine-induced enhanced responses to glutamate probably results from greater surface expression of NMDARs and increased ion conductance through the channel portion of this receptor. In accordance, morphine promotes phosphorylation of NMDAR subunits and greater expression of GluN2B containing NMDARs at the membrane [19; 25; 28; 73; 90]. MOR and NMDAR interactions at the neuronal membrane would be key in these morphine-induced changes [19]. Morphine binding to MOR leads to both an enhanced activity of NMDAR through PKC mediated phosphorylation of GluN1 and GluN2 (presumably contributing to OIH) and to a decreased activity of MOR (presumably contributing to opiate tolerance) [19]. Subunit diversity, in addition to phosphorylation, is another key determinant of NMDAR properties. The increased expression of the GluN2B subunit seen here is associated with greater conductance to Ca++ [55].
The function of NMDARs is modulated by polyamine [6; 56; 82; 83], which are organic compounds containing two or more primary amino groups. At physiological pH, polyamine can positively modulate NMDAR function by inhibiting GluN1-GluN2B channel function [56; 71]. Decreasing the polyamine levels in the diet can ameliorate hyperalgesic status by decreasing glutamate levels in the spinal cord [14] and potentiate the analgesic effect of morphine [59]. Using models of hyperalgesia (inflammatory, post-incision, neuropathic, OIH, and non-nociceptive environmental stress), Rivat and colleagues showed that a polyamine-deficient diet prevented phosphorylation on tyrosine residues of the GluN2B subunit and inhibited the appearance of hyperalgesia [59]. Our observation here that the GluN2B selective antagonist Ro 25-6981 prevented the development of OIH is similar to the findings with a polyamine-deficient diet [59]. While Rivat and colleagues used spinal cord tissue to measure levels of GluN2B phosphorylation, it might be possible that a polyamine-deficient diet counters OIH as well via altered tyrosine phosphorylation of the GluN2B subunits in DRG. Taken together, these data suggest that the modulation of GluN2B subunit is key in regulating OIH.
4.3 The role of glutamate transporters in OIH
Sustained morphine administration leads to a down-regulation of glutamate transporters, especially the glial transporters GLAST and GLT1, resulting in elevation of extracellular glutamate [53; 69]. In the DRG this additional glutamate is associated with a NMDAR mediated increased excitability of sensory neurons [44]. At variance with previous reports, we observed that sustained morphine lead to an increase expression of neuronal EAAC1/EAAT3 together with a rise in glutamate induced transporter currents. These currents are generated by the co-transport of one negatively charged glutamate with 3 Na+ and 1H+ ions in the cell in exchange for one K+ out of the cell resulting in a net entry of two positive charges across the membrane [40]. Normally, EAAC1/EAAT3 accounts for only 20% of the glutamate reuptake in the striatum [62]. This percentage is likely to increase with morphine-induced inhibition of GLAST and GLT1 leading to an increment of transient depolarizing currents associated with EAAC1/EAAT3 activity. The impact of these altered transporter currents on primary sensory neuron excitability has not been reported and will need to be studied further to determine how it could contribute to nociception.
5. Conclusions
The finding that the peripheral nervous system is involved in OIH opens new avenues to prevent this and other unwanted effects of opiates such as tolerance, which could be improved by blocking peripheral NMDARs [10]. Brain non-penetrant NMDAR antagonists would avoid many of the unwanted CNS side effects of the current drugs such as ketamine. Given that peripheral opiate receptors contribute substantially to opiate analgesia [35], the combination of a peripherally acting opiate agonist with a NMDAR antagonists might offer better and more durable analgesia.
Acknowledgments
We would like to thank Dr. Peter Ohara for his critical suggestions and thoughtful discussions. We thank Dr. Burcu Hasdemir and Dr. Min Liao for their help with DRG lysate preparation and western blot analysis. The Painless Research Foundation as well as NIH grants R01 NS080921-01 and R21 NS079897-01A1 supported the experiments.
Footnotes
Conflict of interest statement: The authors report no conflict of interest.
References
- 1.Ahmadi S, Golbaghi H, Azizbeigi R, Esmailzadeh N. N-methyl-D-aspartate receptors involved in morphine-induced hyperalgesia in sensitized mice. Eur J Pharmacol. 2014;737:85–90. doi: 10.1016/j.ejphar.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 2.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106(1-2):49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349(20):1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beland B, Fitzgerald M. Mu- and delta-opioid receptors are downregulated in the largest diameter primary sensory neurons during postnatal development in rats. Pain. 2001;90(1-2):143–150. doi: 10.1016/s0304-3959(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 6.Bernardi M, Bertolini A, Szczawinska K, Genedani S. Blockade of the polyamine site of NMDA receptors produces antinociception and enhances the effect of morphine, in mice. Eur J Pharmacol. 1996;298(1):51–55. doi: 10.1016/0014-2999(95)00778-4. [DOI] [PubMed] [Google Scholar]
- 7.Celerier E, Gonzalez JR, Maldonado R, Cabanero D, Puig MM. Opioid-induced hyperalgesia in a murine model of postoperative pain: role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology. 2006;104(3):546–555. doi: 10.1097/00000542-200603000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7(1):43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.D'Mello R, Marchand F, Pezet S, McMahon SB, Dickenson AH. Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain. Mol Ther. 2011;19(10):1780–1792. doi: 10.1038/mt.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danysz W, Kozela E, Parsons CG, Sladek M, Bauer T, Popik P. Peripherally acting NMDA receptor/glycineB site receptor antagonists inhibit morphine tolerance. Neuropharmacology. 2005;48(3):360–371. doi: 10.1016/j.neuropharm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 11.David A, Edwards LC. The Evidence for Opioid-Induced Hyperalgesia Today. Austin Journal of Anesthesia and Analgesia. 2014;2(4) [Google Scholar]
- 12.Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, Yin H, Khanna R, White FA. Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. J Neuroinflammation. 2012;9:200. doi: 10.1186/1742-2094-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari LF, Lotufo CM, Araldi D, Rodrigues MA, Macedo LP, Ferreira SH, Parada CA. Inflammatory sensitization of nociceptors depends on activation of NMDA receptors in DRG satellite cells. Proc Natl Acad Sci U S A. 2014;111(51):18363–18368. doi: 10.1073/pnas.1420601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrier J, Bayet-Robert M, Pereira B, Daulhac L, Eschalier A, Pezet D, Moulinoux JP, Balayssac D. A polyamine-deficient diet prevents oxaliplatin-induced acute cold and mechanical hypersensitivity in rats. PLoS One. 2013;8(10):e77828. doi: 10.1371/journal.pone.0077828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forster AB, Reeh PW, Messlinger K, Fischer MJ. High concentrations of morphine sensitize and activate mouse dorsal root ganglia via TRPV1 and TRPA1 receptors. Mol Pain. 2009;5:17. doi: 10.1186/1744-8069-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francesco F, Tuan T, Theresa-Alexandra MM, Sophie L, Thomas DG, Louis-Etienne L, Annie C, Nicolas D, Wenbo Z, Antoine GG, Daniela M, Simon B, Karen V, Jean-Martin B, Catherine MC, Michael WS, Yves De K. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl- homeostasis. Nature Neuroscience. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardell LR, King T, Ossipov MH, Rice KC, Lai J, Vanderah TW, Porreca F. Opioid receptor-mediated hyperalgesia and antinociceptive tolerance induced by sustained opiate delivery. Neurosci Lett. 2006;396(1):44–49. doi: 10.1016/j.neulet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(15):6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garzon J, Herrero-Labrador R, Rodriguez-Munoz M, Shah R, Vicente-Sanchez A, Wagner CR, Sanchez-Blazquez P. HINT1 protein: a new therapeutic target to enhance opioid antinociception and block mechanical allodynia. Neuropharmacology. 2015;89:412–423. doi: 10.1016/j.neuropharm.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Gegelashvili G, Bjerrum OJ. High-affinity glutamate transporters in chronic pain: an emerging therapeutic target. J Neurochem. 2014;131(6):712–730. doi: 10.1111/jnc.12957. [DOI] [PubMed] [Google Scholar]
- 21.Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26(3):953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong K, Kung LH, Magni G, Bhargava A, Jasmin L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One. 2014;9(4):e95491. doi: 10.1371/journal.pone.0095491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gozariu M, Bouhassira D, Willer J, Le Bars D. Temporal summation and a C-fibre reflex in the rat: effects of morphine on facilitatory and inhibitory mechanisms. Eur J Pharmacol. 2000;394(1):75–84. doi: 10.1016/s0014-2999(00)00114-x. [DOI] [PubMed] [Google Scholar]
- 24.Gracious R, Ross ARG, Dewey William L, Akbarali Hamid I. Opioid-induced hypernociception is associated with hyperexcitability and altered tetrodotoxin-resistant Na+ channel function of dorsal root ganglia. AJP: Cell Physiology. 2011;302(8) doi: 10.1152/ajpcell.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain. 2009;5:76. doi: 10.1186/1744-8069-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, Fletcher D, Chauvin M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93(2):409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Guirimand F, Chauvin M, Willer JC, Le Bars D. Effects of intravenous morphine and buprenorphine on a C-fiber reflex in the rat. J Pharmacol Exp Ther. 1995;273(2):830–841. [PubMed] [Google Scholar]
- 28.Guo RX, Zhang M, Liu W, Zhao CM, Cui Y, Wang CH, Feng JQ, Chen PX. NMDA receptors are involved in upstream of the spinal JNK activation in morphine antinociceptive tolerance. Neurosci Lett. 2009;467(2):95–99. doi: 10.1016/j.neulet.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Hasdemir B, Mahajan S, Bunnett NW, Liao M, Bhargava A. Endothelin-converting enzyme-1 actions determine differential trafficking and signaling of corticotropin-releasing factor receptor 1 at high agonist concentrations. Mol Endocrinol. 2012;26(4):681–695. doi: 10.1210/me.2011-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hina N, Fletcher D, Poindessous-Jazat F, Martinez V. Hyperalgesia induced by low-dose opioid treatment before orthopaedic surgery: An observational case-control study. Eur J Anaesthesiol. 2014 doi: 10.1097/EJA.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 31.Hogan D, Baker AL, Moron JA, Carlton SM. Systemic morphine treatment induces changes in firing patterns and responses of nociceptive afferent fibers in mouse glabrous skin. Pain. 2013;154(11):2297–2309. doi: 10.1016/j.pain.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooten WM, Lamer TJ, Twyner C. Opioid-Induced Hyperalgesia in Community-Dwelling Adults with Chronic Pain. Pain. 2015 doi: 10.1097/j.pain.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24(1):83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jagla C, Martus P, Stein C. Peripheral opioid receptor blockade increases postoperative morphine demands--a randomized, double-blind, placebo-controlled trial. Pain. 2014;155(10):2056–2062. doi: 10.1016/j.pain.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15(12):8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang M, Zhang W, Ma Z, Gu X. Antinociception and prevention of hyperalgesia by intrathecal administration of Ro 25-6981, a highly selective antagonist of the 2B subunit of N-methyl-D-aspartate receptor. Pharmacol Biochem Behav. 2013;112:56–63. doi: 10.1016/j.pbb.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Jiang YQ, Andrade A, Lipscombe D. Spinal morphine but not ziconotide or gabapentin analgesia is affected by alternative splicing of voltage-gated calcium channel CaV2.2 pre-mRNA. Mol Pain. 2013;9:67. doi: 10.1186/1744-8069-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Cho HY, Ahn YJ, Kim J, Yoon YW. Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain. 2012;153(5):1022–1029. doi: 10.1016/j.pain.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Kirischuk S, Kettenmann H, Verkhratsky A. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch. 2007;454(2):245–252. doi: 10.1007/s00424-007-0207-5. [DOI] [PubMed] [Google Scholar]
- 41.Ko SW, Wu LJ, Shum F, Quan J, Zhuo M. Cingulate NMDA NR2B receptors contribute to morphine-induced analgesic tolerance. Mol Brain. 2008;1:2. doi: 10.1186/1756-6606-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kung LH, Gong K, Adedoyin M, Ng J, Bhargava A, Ohara PT, Jasmin L. Evidence for glutamate as a neuroglial transmitter within sensory ganglia. PLoS One. 2013;8(7):e68312. doi: 10.1371/journal.pone.0068312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg. 2002;94(5):1263–1269. doi: 10.1097/00000539-200205000-00040. table of contents. [DOI] [PubMed] [Google Scholar]
- 44.Laursen JC, Cairns BE, Dong XD, Kumar U, Somvanshi RK, Arendt-Nielsen L, Gazerani P. Glutamate dysregulation in the trigeminal ganglion: a novel mechanism for peripheral sensitization of the craniofacial region. Neuroscience. 2014;256:23–35. doi: 10.1016/j.neuroscience.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–161. [PubMed] [Google Scholar]
- 46.Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res. 2001;86(1-2):56–62. doi: 10.1016/s0169-328x(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Liu Y, Zhang J, Zhang W, Sun YE, Gu X, Ma Z. Intrathecal administration of roscovitine prevents remifentanil-induced postoperative hyperalgesia and decreases the phosphorylation of N-methyl-D-aspartate receptor and metabotropic glutamate receptor 5 in spinal cord. Brain Res Bull. 2014;106:9–16. doi: 10.1016/j.brainresbull.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Liu XS, Hou Y, Yan TL, Guo YY, Han W, Guan FL, Chen T, Li T. Dopamine D3 receptor-regulated NR2B subunits of N-methyl-d-aspartate receptors in the nucleus accumbens involves in morphine-induced locomotor activity. CNS Neurosci Ther. 2014;20(9):823–829. doi: 10.1111/cns.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113(3):639–646. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- 50.Ma QP, Hargreaves RJ. Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience. 2000;101(3):699–707. doi: 10.1016/s0306-4522(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 51.Malet M, Vieytes CA, Lundgren KH, Seal RP, Tomasella E, Seroogy KB, Hokfelt T, Gebhart GF, Brumovsky PR. Transcript expression of vesicular glutamate transporters in lumbar dorsal root ganglia and the spinal cord of mice - effects of peripheral axotomy or hindpaw inflammation. Neuroscience. 2013;248:95–111. doi: 10.1016/j.neuroscience.2013.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100(3):213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 53.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22(18):8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446(4):325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- 55.Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157(8):1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mony L, Zhu S, Carvalho S, Paoletti P. Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J. 2011;30(15):3134–3146. doi: 10.1038/emboj.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, Leander JD. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014;23(2):243–254. doi: 10.1517/13543784.2014.852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol. 2011;186(11):6417–6426. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rivat C, Richebe P, Laboureyras E, Laulin JP, Havouis R, Noble F, Moulinoux JP, Simonnet G. Polyamine deficient diet to relieve pain hypersensitivity. Pain. 2008;137(1):125–137. doi: 10.1016/j.pain.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Ross GR, Gade AR, Dewey WL, Akbarali HI. Opioid-induced hypernociception is associated with hyperexcitability and altered tetrodotoxin-resistant Na+ channel function of dorsal root ganglia. Am J Physiol Cell Physiol. 2012;302(8):C1152–1161. doi: 10.1152/ajpcell.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowan MP, Szteyn K, Doyle AP, Gomez R, Henry MA, Jeske NA. beta-arrestin-2-biased agonism of delta opioid receptors sensitizes transient receptor potential vanilloid type 1 (TRPV1) in primary sensory neurons. Mol Pain. 2014;10:50. doi: 10.1186/1744-8069-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salvatore MF, Davis RW, Arnold JC, Chotibut T. Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at ser(19) Exp Neurol. 2012;234(2):428–436. doi: 10.1016/j.expneurol.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Scherrer G, Imamachi N, Cao YQQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137(6):1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462(7273):651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? Neuroreport. 2003;14(1):1–7. doi: 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- 66.Sufka KJ, Hughes RA, Giordano J. Effects of selective opiate antagonists on morphine-induced hyperalgesia in domestic fowl. Pharmacol Biochem Behav. 1991;38(1):49–54. doi: 10.1016/0091-3057(91)90588-s. [DOI] [PubMed] [Google Scholar]
- 67.Swartjes M, Mooren RA, Waxman AR, Arout C, van de Wetering K, den Hartigh J, Beijnen JH, Kest B, Dahan A. Morphine induces hyperalgesia without involvement of mu-opioid receptor or morphine-3-glucuronide. Mol Med. 2012;18:1320–1326. doi: 10.2119/molmed.2012.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taddese A, Nah SY, McCleskey EW. Selective opioid inhibition of small nociceptive neurons. Science. 1995;270(5240):1366–1369. doi: 10.1126/science.270.5240.1366. [DOI] [PubMed] [Google Scholar]
- 69.Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124(1-2):77–86. doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 70.Tao FLW, Zhang B, Yaster M, Rothstein JD, Johns RA, Tao YX. Evidence of neuronal excitatory amino acid carrier 1 expression in rat dorsal root ganglion neurons and their central terminals. Neuroscience. 2004;123(4):10451051. doi: 10.1016/j.neuroscience.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 71.Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268(5212):873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- 72.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251(4989):85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 73.Tsai RY, Chou KY, Shen CH, Chien CC, Tsai WY, Huang YN, Tao PL, Lin YS, Wong CS. Resveratrol regulates N-methyl-D-aspartate receptor expression and suppresses neuroinflammation in morphine-tolerant rats. Anesth Analg. 2012;115(4):944–952. doi: 10.1213/ANE.0b013e31825da0fb. [DOI] [PubMed] [Google Scholar]
- 74.Tse KH, Chow KB, Leung WK, Wong YH, Wise H. Primary sensory neurons regulate Toll-like receptor-4-dependent activity of glial cells in dorsal root ganglia. Neuroscience. 2014;279:10–22. doi: 10.1016/j.neuroscience.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 75.Tumati S, Roeske WR, Largent-Milnes T, Wang R, Vanderah TW, Varga EV. Sustained morphine-mediated pain sensitization and antinociceptive tolerance are blocked by intrathecal treatment with Raf-1-selective siRNA. Br J Pharmacol. 2010;161(1):51–64. doi: 10.1111/j.1476-5381.2010.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tumati S, Yamamura HI, Vanderah TW, Roeske WR, Varga EV. Sustained morphine treatment augments capsaicin-evoked calcitonin gene-related peptide release from primary sensory neurons in a protein kinase A- and Raf-1-dependent manner. J Pharmacol Exp Ther. 2009;330(3):810–817. doi: 10.1124/jpet.109.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev. 2013;93(4):1621–1657. doi: 10.1152/physrev.00007.2013. [DOI] [PubMed] [Google Scholar]
- 78.Vera-Portocarrero LP, Zhang ET, King T, Ossipov MH, Vanderah TW, Lai J, Porreca F. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2007;129(1-2):35–45. doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M, Vyklicky L. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–203. doi: 10.33549/physiolres.932678. [DOI] [PubMed] [Google Scholar]
- 80.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC. Coexpression of -and -opioid receptors in nociceptive sensory neurons. Proceedings of the National Academy of Sciences. 2010;107(29):13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109(16):6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams K, Dawson VL, Romano C, Dichter MA, Molinoff PB. Characterization of polyamines having agonist, antagonist, and inverse agonist effects at the polyamine recognition site of the NMDA receptor. Neuron. 1990;5(2):199–208. doi: 10.1016/0896-6273(90)90309-4. [DOI] [PubMed] [Google Scholar]
- 83.Williams K, Romano C, Dichter MA, Molinoff PB. Modulation of the NMDA receptor by polyamines. Life Sci. 1991;48(6):469–498. doi: 10.1016/0024-3205(91)90463-l. [DOI] [PubMed] [Google Scholar]
- 84.Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107–11116. doi: 10.1523/JNEUROSCI.1678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu LJ, Zhuo M. Targeting the NMDA receptor subunit NR2B for the treatment of neuropathic pain. Neurotherapeutics. 2009;6(4):693–702. doi: 10.1016/j.nurt.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu JTT, Zhao JYY, Zhao X, Ligons D, Tiwari V, Atianjoh FE, Lee CYY, Liang L, Zang W, Njoku D, Raja SN, Yaster M, Tao YXX. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. The Journal of clinical investigation. 2014;124(2):592–603. doi: 10.1172/JCI70236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan X, Yadav R, Gao M, Weng HR, R Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2013;62(7):1093–1109. doi: 10.1002/glia.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Ahmed S, Vo T, St Hilaire K, Houghton M, Cohen AS, Mao J, Chen L. Increased Pain Sensitivity in Chronic Pain Subjects on Opioid Therapy: A Cross-Sectional Study Using Quantitative Sensory Testing. Pain Med. 2014 doi: 10.1111/pme.12606. [DOI] [PubMed] [Google Scholar]
- 89.Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47(6):859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Zhao YLL, Chen SRR, Chen H, Pan HLL. Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-D-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. The Journal of biological chemistry. 2012;287(30):25073–25085. doi: 10.1074/jbc.M112.378737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]