Abstract
Carbapenemase-producing organisms have spread worldwide, and infections with these bacteria cause significant morbidity. Horizontal transfer of plasmids carrying genes that encode carbapenemases plays an important role in the spread of multidrug-resistant Gram-negative bacteria. Here we investigate parameters regulating conjugation using an Escherichia coli laboratory strain that lacks plasmids or restriction enzyme modification systems as a recipient and also using patient isolates as donors and recipients. Because conjugation is tightly regulated, we performed a systematic analysis of the transfer of Klebsiella pneumoniae carbapenemase (blaKPC)-encoding plasmids into multiple strains under different environmental conditions to investigate critical variables. We used four blaKPC-carrying plasmids isolated from patient strains obtained from two hospitals: pKpQIL and pKPC-47e from the National Institutes of Health, and pKPC_UVA01 and pKPC_UVA02 from the University of Virginia. Plasmid transfer frequency differed substantially between different donor and recipient pairs, and the frequency was influenced by plasmid content, temperature, and substrate, in addition to donor and recipient strain. pKPC-47e was attenuated in conjugation efficiency across all conditions tested. Despite its presence in multiple clinical species, pKPC_UVA01 had lower conjugation efficiencies than pKpQIL into recipient strains. The conjugation frequency of these plasmids into K. pneumoniae and E. coli patient isolates ranged widely without a clear correlation with clinical epidemiological data. Our results highlight the importance of each variable examined in these controlled experiments. The in vitro models did not reliably predict plasmid mobilization observed in a patient population, indicating that further studies are needed to understand the most important variables affecting horizontal transfer in vivo.
INTRODUCTION
Carbapenemase-producing organisms (CPO) have spread worldwide, and the Centers for Disease Control and Prevention have categorized these organisms as an “urgent” threat (1) with high mortality rates ranging from 30% to more than 70% among infected patients (2–4). These carbapenemases include Klebsiella pneumoniae carbapenemase (KPC, encoded by blaKPC), NDM-1, IMP, VIM, and OXA family enzymes (5). Bacteria producing these enzymes are frequently resistant to nearly all antibiotics, because they harbor additional resistance genes carried on plasmids. blaKPC is becoming prevalent in parts of the United States, Europe, Asia, and South America (6–8).
The global spread of blaKPC has been due in part to vertical transmission through a relatively small set of successful bacterial host lineages (9, 10) such as K. pneumoniae sequence type 258 (ST258), which has disseminated globally (11, 12). Although the determinants behind the dramatic success of ST258 are unknown (13–15), genes transmitted in parallel with plasmids carrying blaKPC genes may contribute to advantages either in pathogenicity or fitness (14–17) possibly through unique capsule antigens or type IV secretion systems (11, 14, 15, 18).
In addition to vertical transfer, horizontal gene transfer (HGT) of DNA can contribute to the spread of antibiotic resistance. Conjugative plasmids carry genes that enable gene transfer to a heterologous recipient cell (19). Also, the resistance gene blaKPC is often located on the mobile transposon Tn4401 found on diverse plasmids (20). The blaKPC-containing plasmid backbone pKpQIL (IncFII replicon) examined here is endemic in Israel (8) and has been identified in outbreaks in the United States. IncN replicon plasmids carrying carbapenemase genes, also examined here, have been identified worldwide with increasing frequency (6, 21). Recently, it was suggested in a study analyzing clinical isolates that blaKPC-IncN/ST-15 plasmids may have more efficient intra- and interspecies conjugation than the pKpQIL/FIIK2 plasmids; however, this has not been proven, and the microbiology factors influencing this propensity to conjugate require further exploration (22).
The blaKPC gene has been identified in at least 15 species in the Enterobacteriaceae family (21, 23–25) as well as several species not in the Enterobacteriaceae family (23, 24). One might speculate that the gastrointestinal (GI) environment within a patient, given its high bacterial densities, could potentially provide an ideal environment for gene transfer (26). However, despite this high potential for spread, there are relatively few reports of blaKPC interspecies transfer within patients (22, 27–29). Plasmid transfer between bacteria within the hospital or community environment adds an additional layer of complexity (21). To better understand the clinical cases and outbreaks for which HGT appears to be frequent, compared to those for which HGT appears to be rare, we analyzed several conditions in vitro to approximate different settings, with a goal to fill in the knowledge gap surrounding the potential influences on conjugation.
In this work, we performed in vitro experiments to investigate factors that influence conjugation of four plasmids. The epidemic 114-kb pKpQIL plasmid was isolated during an outbreak at the National Institutes of Health Clinical Center (NIH) (4, 21). pKPC-47e, a 50-kb plasmid with an IncN replicon, was isolated from patients and the environment at NIH (21). At the University of Virginia Health System (UVA), an outbreak of carbapenemase-producing Enterobacteriaceae was partially attributed to an unprecedentedly high rate of proposed HGT of pKPC_UVA01, a nontypeable 43-kb plasmid, with transfer to at least 10 different species from seven genera (2, 30, 31). pKPC_UVA02, a 113-kb nontypeable plasmid, was also isolated during the outbreak and from subsequent surveillance cultures, providing evidence of possible HGT; however, it was identified in fewer species than pKPC_UVA01 (2, 30, 31). We analyzed these plasmids using in vitro and in silico methods along with hospital epidemiologic data to investigate factors that regulate their transfer.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids are listed in Table 1. Bacteria were grown at 37°C in tryptic soy broth (TSB) (Thermo Fisher Scientific, Waltham, MA), on blood agar (BA) (Remel, Lenexa, KS), or on tryptic soy agar (TSA) (Thermo Fisher Scientific) plates. The plates were supplemented with tetracycline (30 μg/ml), kanamycin (50 μg/ml), gentamicin (50 μg/ml), meropenem (0.5 μg/ml for E. coli XL-1 and 4 μg/ml meropenem for all other Enterobacteriaceae), where indicated.
TABLE 1.
Plasmids and bacterial strains used in this study
| Plasmid or bacterial strain | Description or relevant characteristica | Purpose in study | Source and/or reference |
|---|---|---|---|
| Plasmids | |||
| pKpQIL | pKpQIL-6e6 from strain KPNIH14 | Donor plasmid | NIH Clinical Center (4) |
| pAAC154-a50 | From KPNIH14 | Extra plasmid in donor | NIH Clinical Center (4) |
| pKPN-498 | From KPNIH14 | Extra plasmid in donor | NIH Clinical Center (4) |
| pKPC-47e | pKPC-47e from strain ECNIH3 | Donor plasmid | NIH Clinical Center (21) |
| pKpQ-GFP | pKpQIL-6e6 tagged with GFP | Donor plasmid | This study |
| p47e-GFP | pKPC-47e tagged with GFP | Donor plasmid | This study |
| pKPC_UVA01 | pKPC_UVA01 from Kpn1016 | Donor plasmid | UVA Medical Center (2) |
| pKPC_UVA02 | pKPC_UVA02 from strain Kox1015 | Donor plasmid | UVA Medical Center (2) |
| pKPC_CAV1193 | pKPC_CAV1193 from strain CAV1193 | Donor plasmid | UVA Medical Center (34) |
| pCAV1193-3741 | From CAV1193 | Extra plasmid in donor | UVA Medical Center (34) |
| pCAV1193-78 | From CAV1193 | Extra plasmid in donor | UVA Medical Center (34) |
| pCAV1193-166 | From CAV1193; confers Gentr | Extra plasmid in donor | UVA Medical Center (34) |
| pCAV1193-258 | From CAV1193 | Extra plasmid in donor | UVA Medical Center (34) |
| pKPN-065 | From Kpn223 | Extra plasmid in recipient | This study |
| pKPN-7c3 | From Kpn555 | Extra plasmid in recipient | This study |
| pKPN-d90 | From Kpn555 | Extra plasmid in recipient | This study |
| Bacterial strains | |||
| KPNIH1760 | K. pneumoniae patient isolate | Donor | 4; this study |
| ECNIH3 | E. cloacae patient isolate | Intermediate | 21 |
| ECONIH1 | E. coli patient isolate | Donor | 21 |
| ECONIH2 | E. coli patient isolate | Donor | This study |
| KPNIH39 | K. pneumoniae patient isolate | Donor | This study |
| Kpn1760CURE | KPNIH1760 cured of pKpQIL-6e6 | Intermediate | 32 |
| Kpn1760CT_pKpQIL | KPNIH1760 cured and transformed with pKpQIL-6e6 | Donor | 32 |
| Kpn1760CT_pKpQGFP | KPNIH1760 cured and transformed with pKpQ-GFP | Donor | This study |
| Kpn1760CT_pKPC47e | KPNIH1760 cured and transformed with pKPC-47e | Donor | This study |
| Kpn1760CT_p47eGFP | KPNIH1760 cured and transformed with p47e-GFP | Donor | This study |
| Kpn1760CT_pUVA01 | KPNIH1760 and transformed with pKPC_UVA01 | Donor | This study |
| Kpn1760CT_pUVA02 | KPNIH1760 cured and transformed with pKPC_UVA02 | Donor | This study |
| ECONIH2CURE | ECONIH2 cured of blaKPC plasmid | Recipient | This study |
| XL-1 | E. coli XL-1 Blue | Recipient | Stratagene |
| Kpn223 | K. pneumoniae thigh wound isolate, Sept. 2013 | Recipient | NIH Clinical Center |
| Kpn555 | K. pneumoniae urine isolate, Feb. 2013 | Recipient | NIH Clinical Center |
| Kpn808 | K. pneumoniae urine isolate, March 2014 | Recipient | NIH Clinical Center |
| Kpn731 | K. pneumoniae sputum isolate, March 2013 | Recipient | NIH Clinical Center |
| Kpn889 | K. pneumoniae urine isolate, Feb. 2014 | Recipient | NIH Clinical Center |
| Kpn164 | K. pneumoniae skin lesion isolate, Aug. 2013 | Recipient | NIH Clinical Center |
| Kpn110 | K. pneumoniae urine isolate, Aug. 2013 | Recipient | NIH Clinical Center |
| Kpn331 | K. pneumoniae urine isolate, Sept. 2013 | Recipient | NIH Clinical Center |
| Eco385 | E. coli blood isolate, Aug. 2013 | Recipient | NIH Clinical Center |
| Eco889 | E. coli urine isolate, Feb. 2014 | Recipient | NIH Clinical Center |
| Eco046 | E. coli urine isolate, Oct. 2013 | Recipient | NIH Clinical Center |
| Eco067 | E. coli sputum isolate, Oct. 2013 | Recipient | NIH Clinical Center |
| Eco207 | E. coli tracheal aspirate isolate, Feb. 2013 | Recipient | NIH Clinical Center |
| Eco452 | E. coli urine isolate, Nov. 2013 | Recipient | NIH Clinical Center |
| Eco556 | E. coli urine isolate, Feb. 2013 | Recipient | NIH Clinical Center |
| Eco603 | E. coli blood isolate, Oct. 2013 | Recipient | NIH Clinical Center |
| CAV1193 | K. pneumoniae pKPC_CAV1193; Meror Gentr | Donor | UVA Medical Center (34) |
| C1193_KPCCURE | Meros Gentr; cured of pKPC_CAV1193 | Recipient | This study |
| C1193_GentCURE | Meror Gents; cured of Gentr plasmids | Intermediate | This study |
| C1193_CURE | Meros Gents; cured of pKPC_CAV1193 Gentr plasmids | Intermediate | This study |
| C1193CT_pKpQGFP | Meror Gents; transformed with pKpQ-GFP | Donor | This study |
| CAV1205 | K. pneumoniae pKPC_UVA02 | Donor | UVA Medical Center (31) |
| CAV1237 | K. pneumoniae pKPC_UVA01 | Donor | UVA Medical Center (31) |
| Kpn1016 | K. pneumoniae pKPC_UVA01 | Donor | UVA Medical Center (2) |
| Kox1015 | K. oxytoca pKPC_UVA02 | Donor | UVA Medical Center (2) |
Gentr and Gents, gentamicin resistance and sensitivity, respectively; Meror and Meros, meropenem resistance and sensitivity, respectively. The isolation date (month and year) of some isolates are given. The months were abbreviated as follows: Feb., February; Aug., August; Sept., September; Oct., October; Nov. November.
Plasmid elimination experiments.
Plasmid elimination experiments were performed by serial passage at elevated temperature as described previously (32).
Construction of GFP-tagged plasmids and strains.
Plasmid DNA was extracted and purified from K. pneumoniae KPNIH1760 with a QIAfilter Midi-Prep kit (Qiagen, Valencia, CA). To fluorescently tag pKpQIL, a GFP:kanamycin (GFP stands for green fluorescent protein) cassette was cloned into aadA of pKpQIL, encoding a truncated form of AadA (33). Primers were designed to add 40 nucleotides of aadA sequence (AadAGFP For [For stands for forward] [AGG CAG GCT TAT CTT GGA CAA GAA GAT CGC TTG GCC TCG CGC GCA ACG CAA TTA ATG TGA] and AadAGFP Rev [Rev stands for reverse] [TGA TTG GGA GAG TGG CGG AAA CGA ACA CCA ACA TAT GCA GCA GAA AGC CAC GTT GTG TCT CAA]) to a GFP:kanamycin construct under the control of a lac promoter. The resulting construct aadA:GFPkan:aadA was cloned into a TOPO TA cloning vector (Invitrogen), amplified by PCR, and purified by QIAquick PCR purification kit (Qiagen). An E. coli strain (NM1100) that expresses lambda proteins (kindly provided by Susan Gottesman) was transformed with pKpQIL and plated on CRE medium (RambaChrom KPC; Gibson Biosciences, Lexington, KY). Lambda red recombination procedure was performed with NM1100(pKpQIL) and transformed with aadA:GFPkan:aadA. Colonies were isolated on kanamycin selective media and confirmed to express GFP by fluorescence microscopy. Plasmid DNA (pKpQ-GFP) was sequenced for confirmation. The same procedure was repeated using fluorescently tagged pKPC-47e isolated from Enterobacter cloacae ECNIH3 to generate p47e-GFP by insertion of GFP:kanamycin into the truncated form of aadA on pKPC-47e (AadAGFPIncN For [TCG TAA ACT GTA ATG CAA GTA GCG TAT GCG CTC ACG CAA CGC GCA ACG CAA TTA ATG TGA] and AadAGFPIncN Rev [TTG TAG GTC AAT GCT TTA AAA AGT AGC TGC TCC CCT TTC GAA AGC CAC GTT GTG TCT CAA]). Strain KPNIH1760 was cured of the plasmid pKpQIL to generate Kpn1760CURE, followed by transformation with pKpQIL or pKpQ-GFP to generate Kpn1760CT_pKpQIL (CT stands for cured and transformed) or Kpn1760CT_pKpQGFP, respectively. PCR and susceptibility testing were performed on isolates to confirm transformation. PCR was used to confirm retention of the two other plasmids (pAAC154-a50 and pKPN-498) carried by strain KPNIH1760 (21) (data not shown). The two isogenic strains exhibited growth rates similar to KPNIH1760 (data not shown). There was no significant difference in the conjugation rates of KPNIH1760, Kpn1760CT_pKpQIL, and Kpn1760CT_pKpQGFP to two Enterobacteriaceae recipients (see Table S1 in the supplemental material). Similar results were seen with Kpn1760CT_pKPC47e and Kpn1760CT_p47eGFP (data not shown). All subsequent conjugation studies utilized the GFP-tagged isolate Kpn1760CT_pKpQGFP or Kpn1760CT_p47eGFP.
Plasmids pKPC_UVA01 and pKPC_UVA02 were originally derived from strains Kpn1016 and Kox1015 (2). These plasmids were electroporated into strain Kpn1760CURE to generate Kpn1760CT_pUVA01 and Kpn1760CT_pUVA02, which were confirmed by sequence analysis and susceptibility testing.
Strain CAV1193 harbors five plasmids: the pKPC_UVA01 slightly modified derivative pKPC_CAV1193 (34) that contains blaKPC, two plasmids containing gentamicin resistance genes, and two additional plasmids. To generate a blaKPC plasmid-free strain of CAV1193 (C1193_KPCCURE), gentamicin selection was maintained during plasmid elimination experiments. CAV1193 was cured of its plasmids that carry genes that encode gentamicin resistance to generate the gentamicin-sensitive strain C1193_GentCURE. Finally, C1193_KPCCURE was passaged at elevated temperature to generate the carbapenem- and gentamicin-sensitive strain C1193_CURE. Plasmid loss and/or retention were confirmed by PCR and susceptibility testing (see Table S2 in the supplemental material). pKpQ-GFP was transformed into the double cured strain, generating C1193CURE_pKpQGFP.
Conjugation experiments.
Conjugation experiments were performed with modifications from a previously described procedure (35). Briefly, Enterobacteriaceae donor and recipient cultures were grown overnight with agitation at 37°C in TSB. Saturated cultures were subcultured into fresh TSB and incubated with agitation for approximately 2 h, until cultures reached an absorbance (600 nm) of 0.4 to 0.6. For broth culture matings, donor and recipient cultures were mixed at a 1:10 ratio in fresh TSB and incubated at 37°C without agitation for 20 h. Experiments were conducted using time points from 1 h to 20 h for some combinations (see additional Materials and Methods in the supplemental material). The endpoint of 20 h was selected given the lack of conjugation for many combinations at shorter time points. The culture was diluted in phosphate-buffered saline (PBS) and used for enumeration of transconjugants on TSA plates containing either tetracycline or gentamicin and meropenem. Recipients were enumerated on TSA plates containing tetracycline or gentamicin only. For filter matings, donor and recipient cultures were mixed at a 1:10 ratio and then spotted onto 0.22-μm nitrocellulose filters placed on BA plates for 20 h. Transconjugants were confirmed by PCR and/or fluorescence microscopy. To confirm expression and function of the pKpQ-GFP-encoded resistance elements following conjugation, susceptibility profiles of recipients and transconjugants were compared. Recipient strains Kpn223 and Kpn731 were susceptible to doripenem, meropenem, imipenem, and ertapenem prior to conjugation (Table S3). Kpn223 and Kpn731 pKpQ-GFP transconjugants were resistant to doripenem, ertapenem, imipenem, and meropenem, as well as to several other antibiotics in accordance with known resistance mechanisms encoded by pKpQIL (33). Conjugation frequency is given as the number of CFU of transconjugants/number of CFU of recipients. When no transconjugants were detected, the limit of detection was set at 9 CFU/ml, and used to calculate the conjugation frequency, which was denoted using open circles. Mann-Whitney tests were performed using Graphpad Prism (San Diego, CA), where a P value of <0.05 was considered significant.
IncFII and IncN typing by multiplex PCR.
Bacterial strains used as conjugation recipients were screened for the presence of the IncN blaKPC plasmid by multiplex PCR (36) and IncFII blaKPC plasmid by PCR with FIIK FW/RV (forward/reverse) primers (37).
Genomic DNA sequencing and analysis.
Strains KPNIH1760, ECNIH3, CAV1193, CAV1205, Kpn1016, and Kox1015 have been previously sequenced (4, 21, 31, 34). Kpn223, Kpn555, and Eco889 were sequenced as part of this study. Genomic DNA was prepared for each isolate using the Promega Maxwell 16 nucleic acid purification system with the tissue DNA purification kit. DNA was further purified using the Zymo Genomic DNA Clean and Concentrator-10 kit. Libraries were constructed using the SMRTbell template kit, version 1.0. The DNA was size selected for the range 7 to 50 kb using a BluePippin system with a 0.75% gel cassette. Sequencing was performed on the PacBio RSII instrument using P5 polymerase binding and C3 sequencing kits with magnetic bead loading and 180-min acquisition. Genome assemblies were performed using HGAP and Quiver as part of SMRTAnalysis version 2.3.
Nucleotide sequence accession numbers.
The whole-genome sequencing data for strains Kpn223, Kpn555, and Eco889 can be retrieved at NCBI BioProject accession no. PRJNA279655, PRJNA279656, and PRJNA279654, respectively.
RESULTS
The temperature and substrate affected pKpQ-GFP conjugation.
The conjugation frequency of pKpQ-GFP from strain Kpn1760CT_pKpQGFP to the E. coli cloning strain XL-1 Blue (XL-1) is 10−3 at 37°C in broth culture (Fig. 1). This rate is 10 times greater (P < 0.05) than the conjugation rate at 25°C. To determine whether mating substrate contributes to conjugation efficiency, we compared conjugation of pKpQ-GFP in broth to nitrocellulose filters to mimic a biofilm environment. The conjugation rate of pKpQ-GFP from Kpn1760CT_pKpQGFP to XL-1 was ∼1 log unit higher in broth culture compared to filter matings at 37°C (P < 0.05 [Fig. 1]). These data indicate that conjugation of pKpQ-GFP varied significantly based on environmental factors.
FIG 1.
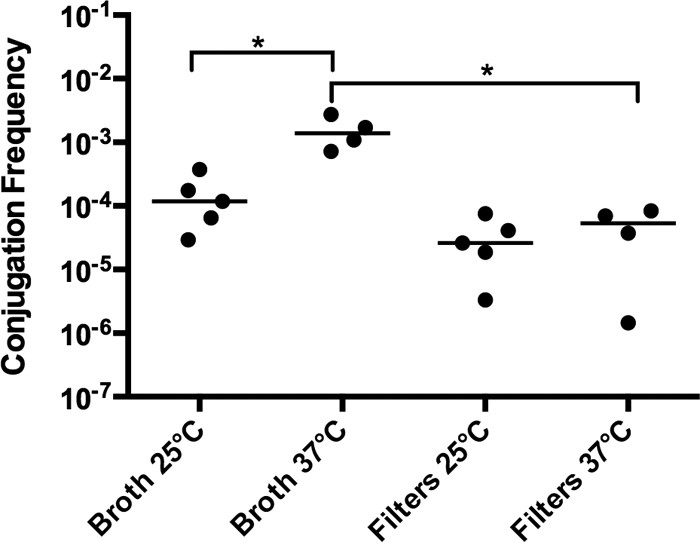
Effects of temperature and substrate on pKpQ-GFP conjugation frequency. The donor is Kpn1760CT_pKpQGFP, and the recipient is E. coli XL-1. Conjugation assays performed for 20 h in either broth culture or on filters placed on blood agar plates at 25°C or 37°C. The conjugation frequency is given by the number of CFU of transconjugants/number of CFU of recipients. Each symbol represents the value for an individual (n ≥ 4). Each bar indicates the median value for a group. Values that are statistically significantly different (P ≤ 0.05) are indicated by a bar and asterisk.
The recipient strain influenced pKpQ-GFP conjugation efficiency.
Many studies of conjugation use modified laboratory strains of E. coli as recipients; however, the applicability of the data are unclear (21). Therefore, we selected multiple clinical strains of K. pneumoniae as recipients (Table 1 and Fig. 2A). Transfer from strain Kpn1760CT_pKpQGFP was detected in six of eight K. pneumoniae patient isolate recipients, indicating that some K. pneumoniae strains are refractory to pKpQ-GFP conjugation under these experimental conditions (Fig. 2A). Of the recipients capable of receiving pKpQ-GFP, we observed a range of plasmid transfer frequencies from low (10−7) to high (10−2).
FIG 2.
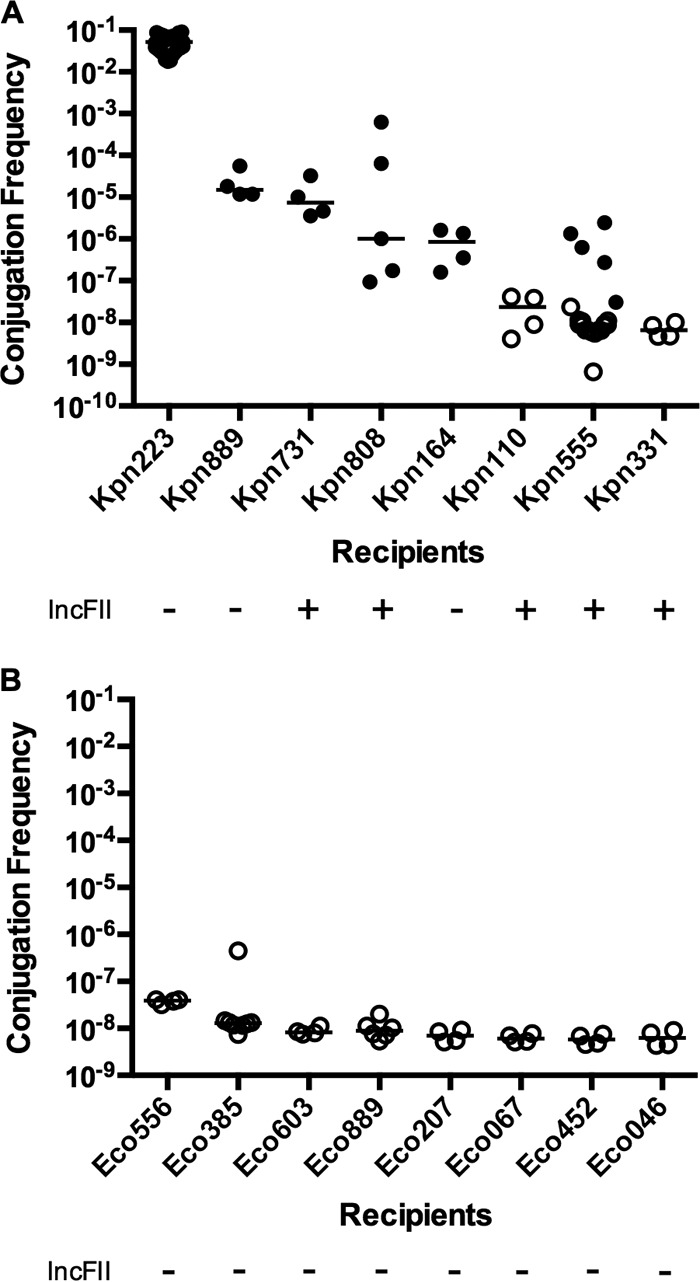
Effects of recipient strain and species on pKpQ-GFP conjugation frequency in broth. The donor is strain Kpn1760CT_pKpQGFP, and the recipients are K. pneumoniae (A) or E. coli (B) isolates from patients. Broth culture conjugations were performed for 20 h. The conjugation frequency is given by the number of CFU of transconjugants/number of CFU of recipients. Replicon typing was performed as described in Materials and Methods. Conjugation frequencies are indicated by solid circles. Open circles indicate that no transconjugants were observed at the limit of detection. Each symbol represents the value for an individual (n ≥ 4). Each bar indicates the median value for a group.
Plasmid incompatibility groups alone do not explain differences in conjugation efficiency.
This variation in transfer efficiencies could be due in part to plasmid incompatibility, namely, the presence of native plasmids that prevent the entry or maintenance of plasmids sharing the same replicon type (38). We analyzed by PCR whether K. pneumoniae recipients contained IncFII replicon plasmids. The most efficient recipient, Kpn223, lacked an IncFII plasmid, while the most refractory recipients (Kp110 and Kp331) contained at least one IncFII plasmid. However, Kpn731 and Kpn808 recipients contained at least one IncFII plasmid but still supported pKpQ-GFP conjugation. Kpn164 lacked an IncFII plasmid but only rarely produced pKpQ-GFP transconjugants (Fig. 2A). Furthermore, Kpn110, Kpn331, and Kpn555 were cured of their IncFII plasmids with minimal impact on conjugation efficiency (see Fig. S1 in the supplemental material).
E. coli was not an efficient recipient of pKpQ-GFP compared to K. pneumoniae.
Given that pKpQIL has been found in E. coli strains (39), we next tested eight patient E. coli clinical isolates as recipients to investigate species-specific factors in recipients. Strikingly, none of the eight E. coli patient isolates yielded a detectable number of transconjugants using strain Kpn1760CT_pKpQGFP as a donor (Fig. 2B). This indicated that with Kpn1760CT_pKpQGFP as a donor, clinical E. coli strains were more refractory recipients than E. coli XL-1 and multiple clinical K. pneumoniae strains. None of the E. coli recipients contained an IncFII plasmid (Fig. 2B). Given the inability of our E. coli recipients to accept plasmids from K. pneumoniae, we tested E. coli donors to determine whether intraspecies transfer would be more efficient. We used as donors the bacterial strain ECONIH1 containing the plasmid pKPC-47e (21), and the K. pneumoniae (KPNIH39) and E. coli (ECONIH2) strains containing pKpQIL plasmids. We used two of the previously tested E. coli strains and a plasmid-cured strain of ECONIH2 as recipients. We observed no difference in conjugation to E. coli recipients from either E. coli or K. pneumoniae donors (see Fig. S2 in the supplemental material).
Substrate, but not temperature, affected p47e-GFP conjugation.
pKPC-47e differs from pKpQIL in replicon type, size, and accessory genes (21). Conjugation was tested using strain Kpn1760CT_p47eGFP in broth culture and filter mating conditions using XL-1 as a recipient (Fig. 3). In this set of experiments, conjugation of p47e-GFP was observed only in the filter condition, in contrast to the result from pKpQ-GFP, which showed efficient conjugation in both broth and filters (Fig. 1).
FIG 3.
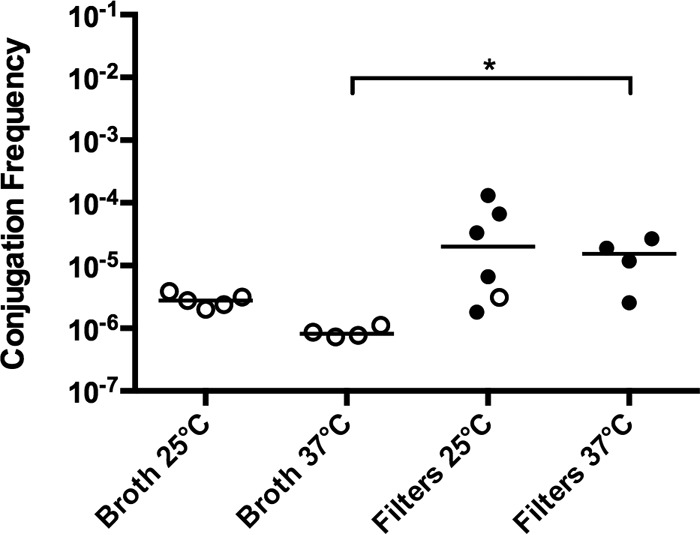
Effects of temperature and substratum on p47e-GFP conjugation frequency. The donor is Kpn1760CT_p47eGFP, and the recipient is E. coli XL-1. Conjugations were performed for 20 h in either broth culture or on filters placed on blood agar plates at 25°C or 37°C. The conjugation frequency is given by the number of CFU of transconjugants/number of CFU of recipients. Each symbol represents the value for an individual (n ≥ 4). Each bar indicates the median value for a group. Symbols: solid circles, conjugation frequency; open circles, no transconjugants observed at the limit of detection; *, P ≤ 0.05.
Both recipient strain and species influenced p47e-GFP conjugation.
IncN family plasmids typically have a broad host range (40), in contrast to IncFII plasmids such as pKpQIL. To test the species specificity of p47e-GFP, we analyzed conjugation of p47e-GFP to eight patient K. pneumoniae recipients and eight E. coli recipients (Fig. 4). Filter mating conjugation with strain Kpn1760CT_p47eGFP as the donor reproducibly produced transconjugants in only two of eight K. pneumoniae recipients, and the transfer rate was low (10−7 to 10−8) (Fig. 4A). Transconjugants were not detected for any of the eight E. coli patient strain recipients (Fig. 4B). Broth mating gave similar results (see Fig. S3 in the supplemental material).
FIG 4.
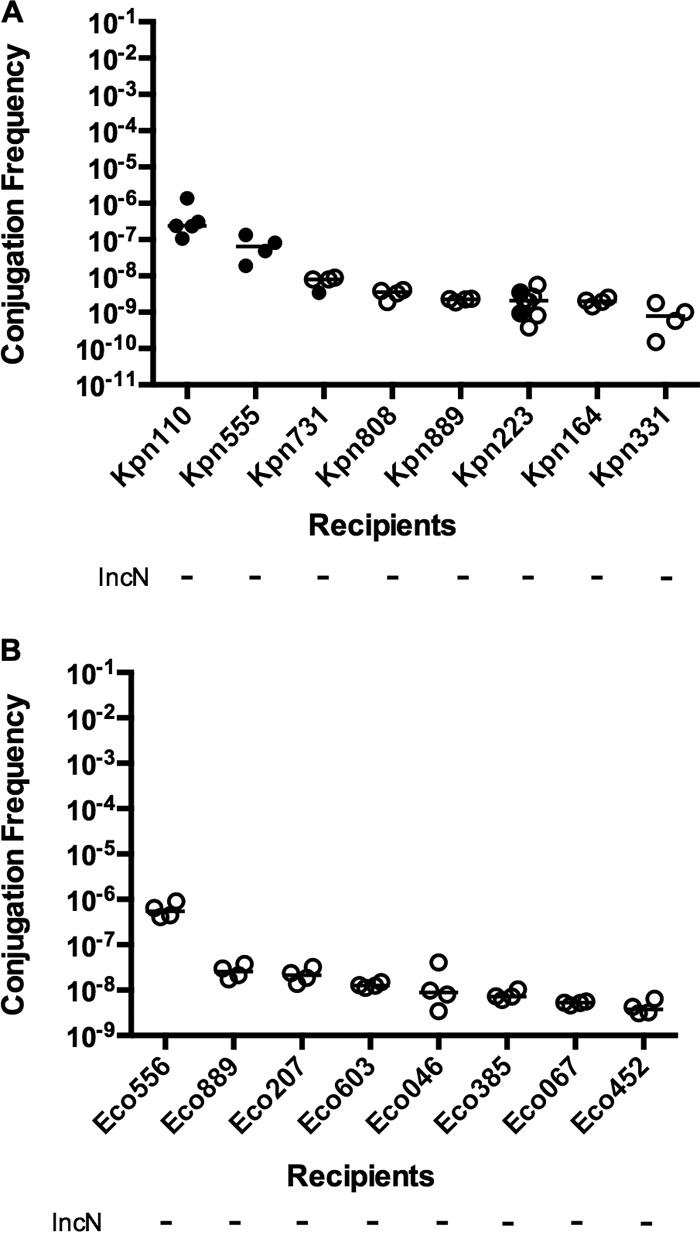
Effects of recipient strain and species on p47e-GFP conjugation frequency on filters. The donor is strain Kpn1760CT_p47eGFP, and recipients are K. pneumoniae (A) or E. coli (B) isolates from patients. Filter matings were performed for 20 h. The conjugation frequency is given by the number of CFU of transconjugants/number of CFU of recipients. Replicon typing was performed as described in Materials and Methods. Symbols: solid circles, conjugation frequency; open circles, no transconjugants observed at the limit of detection.
Donor and recipient strains contributed to the efficiency of UVA outbreak plasmid transfer in vitro.
We next investigated whether pKPC_UVA01 would demonstrate a high conjugation efficiency in vitro, as this “promiscuous” plasmid was observed in at least 10 species at UVA (2, 30). We performed conjugation assays with strains Kpn1760CT_pUVA01 and Kpn1760CT_pUVA02 to the Kpn223 recipient in broth culture at 37°C (Fig. 5). We detected a lower conjugation efficiency of pKPC_UVA01 conjugation from strain Kpn1760CT_pUVA01 to strain Kpn223 compared to pKpQ-GFP. Conversely, the efficiency of pKPC_UVA02 transfer in the same donor background was high into Kpn223.
FIG 5.
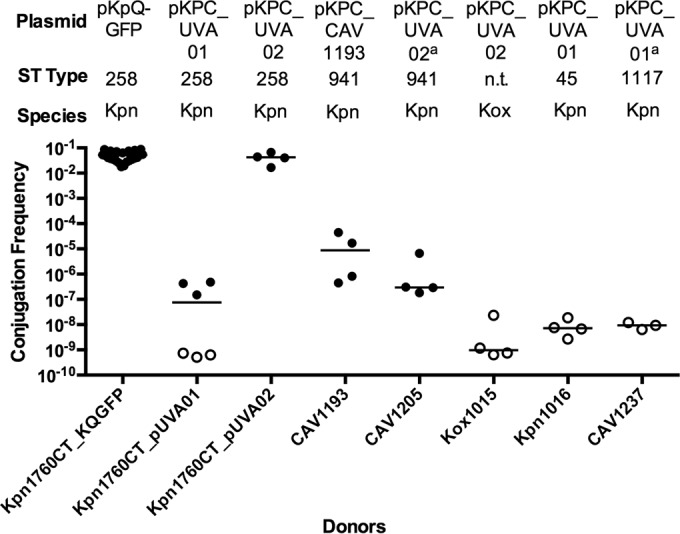
Conjugation ability of Enterobacteriaceae isolates containing pKPC_UVA01, pKPC_CAV1193, or pKPC_UVA02 to Kpn223. Donors are indicated on the x axis, and the recipient is Kpn223. Broth culture conjugations were performed for 20 h. The conjugation frequency is given by the number of CFU of transconjugants/number of CFU of recipients. The plasmid, sequence type, and species are given above the graph. The species are K. pneumoniae (Kpn) and K. oxytoca (Kox). Each symbol represents the value for an individual (n ≥ 3). Each bar indicates the median value for a group. Symbols: solid circles, conjugation frequency; open circles, no transconjugants observed at the limit of detection. The superscript a for two plasmids indicates that the plasmid was determined by whole-genome sequencing on the Illumina HiSeq platform and long-range PCR (31). n.t., not tested.
We next tested whether the donor background strain influenced conjugation by comparison of Kpn1760CT_pUVA01 or Kpn1760CT_pUVA02 to several unmodified patient donor strains. Original parent strains Kpn1016 and Kox1015 carrying pKPC_UVA01 and pKPC_UVA02, respectively, did not yield transconjugants, in contrast to Kpn1760CT_pUVA01 and Kpn1760CT_pUVA02 (Fig. 5), indicating donor influences on the process. Distinct differences in conjugation efficiency were observed between XL-1, Kpn555, and Eco385 as recipients (see Fig. S4 in the supplemental material), indicating additional recipient-level regulatory factors. We next asked whether the number of plasmids acquired by the original isolate CAV1193 was a consequence of enhanced recipient ability, as such hypothesized recipients would possibly contribute to the spread of resistant plasmids in the hospital. We generated a panel of CAV1193 isogenic strains (see Table S2 and Fig. S5 in the supplemental material), which contain the pKPC_UVA01-like plasmid pKPC_CAV1193 (34) to obtain a set of donors that are carbapenem resistant due to a blaKPC plasmid and gentamicin susceptible and a recipient that is carbapenem susceptible and gentamicin resistant. “Self-transfer” of pKPC_CAV1193 from the gentamicin-sensitive C1193_GentCURE into the plasmid-cured recipient C1193_KPCCURE was not detected (Fig. 6). The pKpQ-GFP plasmid transferred more easily into the cured CAV1193 strain than the original blaKPC-carrying plasmid of the clinical isolate. The conjugation efficiency of pKPC_UVA01 from strain Kpn1016 was also low. These findings suggest that for our experimental conditions, CAV1193 may not be an inherently efficient recipient, although the effect of plasmids lost during curing on conjugation efficiency is unknown.
FIG 6.
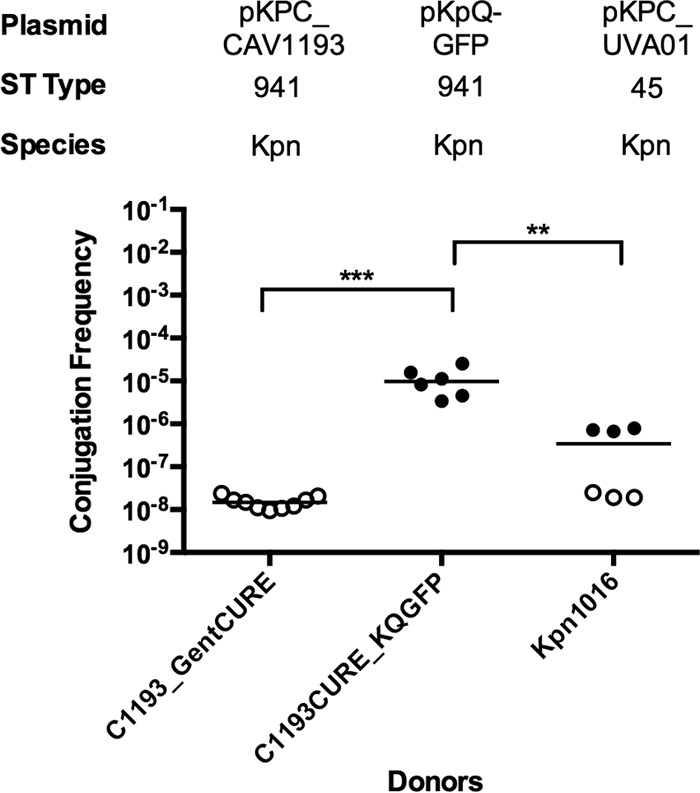
Effect of isogenic donor-recipient strains on conjugation. The recipient is strain C1193_KPCCURE, and donors are indicated on the x axis. Broth culture conjugations were performed for 20 h. The plasmid, sequence type, and species (K. pneumoniae [Kpn]) are given above the graph. The conjugation frequency is given by the number of CFU of transconjugants/number of CFU of recipients. Each symbol represents the value for an individual (n ≥ 6). Each bar indicates the median value for a group. Symbols: solid circles, conjugation frequency; open circles, no transconjugants observed at the limit of detection. Values that are statistically significantly different are indicated by bars and asterisks as follows: **, P < 0.005; ***, P < 0.0005.
Sequence analysis of plasmids highlights complexity of genetics that regulate conjugation.
Genomics is a powerful tool that has been utilized to track the origin of outbreaks (4) and infer plasmid evolution and host range (40). Thus, we sought to determine whether there were “signatures” that could predict conjugation efficiency. We analyzed plasmids from donor and recipient strains using Illumina MiSeq and single-molecule, real-time (SMRT) sequencing. We also included a few recently described plasmids from other studies that examined plasmids carrying carbapenemase genes, their conjugation ability, and HGT (29, 41). Using a candidate gene approach, we selected a set of 77 genes that are potentially associated with plasmid transfer, replication, or maintenance based on current literature, realizing that this is not an exhaustive list. We examined the plasmids for the presence of sequences matching our selected gene set. The presence/absence of predicted proteins was plotted as a heatmap, along with percent protein identity to show the variation in protein sequence and the limitations of annotation methods when separated from direct studies of protein function (see Fig. S6 in the supplemental material).
Plasmids from Kpn223 and Kpn555 recipients were analyzed due to strikingly different conjugation frequencies. Genes that mediate surface exclusion (traS and traT) were present in Kpn555 plasmids, but not predicted to be present in the Kpn223 plasmid. There was also a difference in the number of plasmids detected in the two recipients. Kpn555 contained two unique plasmids with tra genes, while Kpn223 carried one plasmid that appears to lack conjugation genes. Uncovering all of the significance differences between the strains would require an additional evaluation of the potential chromosomal regulatory factors and further in vitro experiments.
We next compared the sequences of donor carbapenemase gene-containing plasmids. Interestingly, the “promiscuous” plasmid pKPC_UVA01 carried many fewer transfer genes from our list based on protein comparison than most of the plasmids, including plasmids recently described in other studies (see Fig. S6 in the supplemental material). Chen et al. (41) describe the conjugative resistance plasmid pBK30683, and also pBK30661, which had lost many of the tra genes, providing evidence for the spread of resistance through both clonal spread and horizontal transfer. Dortet et al. (29) also describe a conjugative resistance plasmid, pOXA-48, and a related plasmid with a deletion in the tra operon. The set of proteins in pOXA-48, a plasmid reported to show very efficient conjugation in both intraspecies and interspecies experiments (22), has a set of predicted transfer genes that differ from those of pKPC_UVA01 and from plasmids shown in the heatmap. Our findings highlight the striking differences in presumed conjugation genes between this small set of plasmids and illustrate that it would be a challenge to use sequence analysis of the genes to fully explain the in vitro differences in plasmid transfer efficiency.
DISCUSSION
Nosocomial outbreaks of multidrug-resistant bacteria are of great concern due to their increasing incidence and associated mortality rate. Both vertical and horizontal transmissions contribute to these outbreaks (22, 30). HGT via conjugation is influenced by environmental and bacterial factors, but the precise role of each remains unknown. We used in vitro and in silico approaches to characterize variables affecting conjugal transfer of clinically isolated blaKPC-containing plasmids. Commonly, blaKPC plasmid transfer studies conflate the effect of donor strain and plasmid. We uncoupled the donor bacterial strain from the plasmid by generating a unique set of reagents through plasmid elimination and electroporation. We also compared our conjugation data with the observed plasmid molecular epidemiology.
IncN family plasmids accounted for four of the eight environmental plasmids isolated in a recent study (21), and our results may help explain the predominance of this plasmid in the environment, as p47e-GFP transfer was more efficient on filters than in broth. The filter method is a simplified simulation of an environmental biofilm, and a true biofilm may demonstrate even greater differences in conjugation efficiencies. Our data are consistent with previous work demonstrating that IncN plasmids had greater conjugation efficiencies on a solid substrate than in a liquid substrate (42). It is interesting to note the proposed HGT of the IncN/ST-15 plasmids in patients examined in the Adler et al. study (22). The HGT described in that study was proposed to be within the patient, not the environment; therefore, more-detailed conjugation studies of these plasmids would be valuable. It would also be of interest to directly examine IncN/ST-15 and IncN p47e-GFP under the same experimental conditions to compare conjugation efficiencies.
To our knowledge, this is the first analysis to investigate the role of the recipient strain on blaKPC-containing plasmid transfer by testing several clinical isolate recipients. A similar study examining blaNDM-1 plasmids conjugated into multiple patient isolates yielded species-specific variations in conjugation frequencies (43). Species-level differences may be coordinated by shufflon genes (44), while strain-level differences may be due to outer membrane proteins (19, 45) or exclusion factors (46). The first report of plasmid-borne colistin resistance is a recent example illustrating the importance of testing a range of recipients for plasmid transfer studies (47). Liu et al. (47) showed conjugation of the colistin-resistant plasmid to the E. coli K-12 strain EC600, but no transconjugants were obtained from clinical isolate recipients, including K. pneumoniae, E. coli, and Pseudomonas aeruginosa. Our data highlight the manipulation required for E. coli cloning strains to become permissive plasmid recipients, as E. coli XL-1 received pKpQ-GFP with much higher efficiency than the tested clinical E. coli isolates. Less efficient conjugation into E. coli and subsequent instability of the plasmids has been noted in the past (48). Given the recovery of blaKPC-containing plasmids in clinical E. coli strains in recent studies (22, 49), this issue deserves further examination to understand the critical factors regulating HGT in E. coli.
Our experiments targeted the possible origins of interspecies spread at UVA (30). As just one possible reason to explain the spread of resistance in the UVA Medical Center, we hypothesized that strain CAV1193 could be an inherently efficient recipient given that it had accepted and retained multiple plasmids. However, we did not detect transconjugants after mating a modified CAV1193 donor to a plasmid-cured CAV1193 recipient, and other donors to this recipient resulted in low to modest conjugation efficiency. We do not know the actual donor for the presumed HGT during the outbreak, and we cannot know how closely our contrived plasmid-cured CAV1193 matched the original recipient; furthermore, CAV1193 may have originally acquired pKPC_CAV1193 through a different mechanism of HGT. Other modes of HGT may be involved, as the role of transposons (20) and integrative and conjugative elements in blaKPC transfer were not addressed by our experiments. Direct DNA import may also play an underappreciated role in blaKPC transfer (50). Another limitation to note is that although our calculation of efficiency accounts for differences in recipient CFU, the analysis is limited by lack of growth rate data of donors, transconjugants, and recipients that may have some effect on the conjugation ratio. Our experimental protocol is commonly used for conjugation analyses, and this limitation is true for similar studies.
The bacterial strains Kpn223 and Kpn555 were strikingly different as recipients, prompting a comparison of the strains. The number of plasmids and type of replicon differed between strains Kpn223 and Kpn555, but the role of the number of recipient plasmids on conjugation efficiency is inconclusive (51–53). Kpn223 seems to carry only a plasmid with limited presumptive transfer genes and unclear conjugal ability, which may explain, in part, its high-efficiency recipient ability. Plasmid content data on other recipient strains were not determined. Finally, plasmid incompatibility is an important factor in plasmid inheritance based on previously published studies, but it is known not to be absolute (38, 54). pKpQ-GFP could transfer into certain IncFII-positive strains, and our experiments with strains cured of the IncFII plasmid clearly reveal that incompatibility groups are not the dominant determinant of conjugation efficiency in these donor/recipient combinations. Future refinement of incompatibility designations may be important.
Investigators in the past have tried to predict plasmid promiscuity based on genomic signatures, such as tracking specific trinucleotide frequencies (40). We wondered if there might be a pattern of genes that correlated with promiscuity, allowing us to discern important differences between pKPC_UVA01 and the other plasmids. As might be expected given the vast literature on conjugation, our sequence analysis of these plasmids did not reveal a definitive gene set that could easily predict conjugation efficiency. The analysis does highlight the significant differences seen in plasmid conjugation content; however, the sample size was quite small, so future studies are needed to investigate specific genes and gene redundancy further. Our findings are consistent with a study that did not identify recipient genes required specifically for conjugation (55). When comparing the plasmids in strain Kpn223 versus those in Kpn555, we see the surface exclusion genes, traT and traS (56), on Kpn555 plasmids. Sequence analysis revealed a wide range of transfer and maintenance genes among different donor plasmids. Our limited data suggest that it would be a challenge to predict the potential of plasmid spread in vivo based on the transfer-related genes carried on blaKPC plasmids. Recent studies examine the spread of resistance as HGT or via a clonal transmission and describe modifications to the tra operon in plasmids, which highlights the increasing role of sequence and conjugation analyses in investigation of these clinically significant plasmids (29, 41).
Decades ago, investigators were studying factors relevant to plasmid transfer in the human gut (51), and we are still asking similar questions. When considering horizontal plasmid transfer in a patient, we know that the microbiome of the patient does not match the characteristics of a laboratory cloning strain. What parameters of donor, recipient, plasmid, and surrounding milieu are most critical for promoting or blocking conjugation and how important is regulation of conjugation compared to subsequent selective pressure?
To extend our understanding, animal models will be useful to investigate the role the microbiome plays in conjugation. In our study, only a limited number of donor/recipient combinations were tested, and a dense community of GI organisms, including uncultured bacterial donors, would allow a much higher number of transfer combinations. The recent clinical observation that the specific parent strain as well as the plasmid type may influence conjugation rate suggests that there is much to learn about HGT regulation (22). Elucidating these fundamental mechanisms of transfer may unlock new strategies for treatment and facilitate a broader understanding of the interchangeable components of bacterial genomes. The findings in the UVA Medical Center prompted a hypothesis about the ease of HGT of specific plasmids (2, 30), consistent with other publications in the field; however, our investigation of these clinically relevant plasmids clearly demonstrates the complexity of HGT regulation, as those plasmids did not easily conjugate in the experiments performed, and highlights the limits of our collective knowledge.
Supplementary Material
ACKNOWLEDGMENTS
We thank Susan Gottesman and Nadim Majdalani for critical reagents and advice.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The contents are the sole responsibility of the authors.
We declare no competing financial interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00014-16.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2:e00204-11. doi: 10.1128/mBio.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez F, Van Duin D. 2013. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med 80:225–233. doi: 10.3949/ccjm.80a.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, NISC Comparative Sequencing Program Group, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viau R, Frank KM, Jacobs MR, Wilson B, Kaye K, Donskey CJ, Perez F, Endimiani A, Bonomo RA. 2016. Intestinal carriage of carbapenemase-producing organisms: current status of surveillance methods. Clin Microbiol Rev 29:1–27. doi: 10.1128/CMR.00108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade LN, Curiao T, Ferreira JC, Longo JM, Climaco EC, Martinez R, Bellissimo-Rodrigues F, Basile-Filho A, Evaristo MA, Del Peloso PF, Ribeiro VB, Barth AL, Paula MC, Baquero F, Canton R, Darini AL, Coque TM. 2011. Dissemination of blaKPC-2 by the spread of Klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among Enterobacteriaceae species in Brazil. Antimicrob Agents Chemother 55:3579–3583. doi: 10.1128/AAC.01783-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu SK, Wu TL, Chuang YC, Lin JC, Fung CP, Lu PL, Wang JT, Wang LS, Siu LK, Yeh KM. 2013. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PLoS One 8:e69428. doi: 10.1371/journal.pone.0069428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leavitt A, Chmelnitsky I, Ofek I, Carmeli Y, Navon-Venezia S. 2010. Plasmid pKpQIL encoding KPC-3 and TEM-1 confers carbapenem resistance in an extremely drug-resistant epidemic Klebsiella pneumoniae strain. J Antimicrob Chemother 65:243–248. doi: 10.1093/jac/dkp417. [DOI] [PubMed] [Google Scholar]
- 9.Adler A, Miller-Roll T, Assous MV, Geffen Y, Paikin S, Schwartz D, Weiner-Well Y, Hussein K, Cohen R, Carmeli Y. 2015. A multicenter study of the clonal structure and resistance mechanism of KPC-producing Escherichia coli isolates in Israel. Clin Microbiol Infect 21:230–235. doi: 10.1016/j.cmi.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y, Brolund A, Giske CG. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 53:3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, Bonomo RA, Musser JM, Kreiswirth BN. 2014. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U S A 111:4988–4993. doi: 10.1073/pnas.1321364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 14.Bowers JR, Kitchel B, Driebe EM, MacCannell DR, Roe C, Lemmer D, de Man T, Rasheed JK, Engelthaler DM, Keim P, Limbago BM. 2015. Genomic analysis of the emergence and rapid global dissemination of the clonal group 258 Klebsiella pneumoniae pandemic. PLoS One 10:e0133727. doi: 10.1371/journal.pone.0133727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathers AJ, Peirano G, Pitout JD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chmelnitsky I, Shklyar M, Hermesh O, Navon-Venezia S, Edgar R, Carmeli Y. 2013. Unique genes identified in the epidemic extremely drug-resistant KPC-producing Klebsiella pneumoniae sequence type 258. J Antimicrob Chemother 68:74–83. doi: 10.1093/jac/dks370. [DOI] [PubMed] [Google Scholar]
- 17.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae: a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Mathema B, Pitout JD, DeLeo FR, Kreiswirth BN. 2014. Epidemic Klebsiella pneumoniae ST258 is a hybrid strain. mBio 5:e01355-14. doi: 10.1128/mBio.01355-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas CM, Nielsen KM. 2005. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol 3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 20.Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. 2008. Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob Agents Chemother 52:1257–1263. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan S, Thomas PJ, Deming C, Park M, Lau AF, Dekker JP, Snitkin ES, Clark TA, Luong K, Song Y, Tsai YC, Boitano M, Dayal J, Brooks SY, Schmidt B, Young AC, Thomas JW, Bouffard GG, Blakesley RW, NISC Comparative Sequencing Program, Mullikin JC, Korlach J, Henderson DK, Frank KM, Palmore TN, Segre JA. 2014. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 6:254ra126. doi: 10.1126/scitranslmed.3009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler A, Khabra E, Paikin S, Carmeli Y. 12 April 2016. Dissemination of the blaKPC gene by clonal spread and horizontal gene transfer: comparative study of incidence and molecular mechanisms. J Antimicrob Chemother. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Arnold RS, Thom KA, Sharma S, Phillips M, Kristie Johnson J, Morgan DJ. 2011. Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South Med J 104:40–45. doi: 10.1097/SMJ.0b013e3181fd7d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picao RC, Cardoso JP, Campana EH, Nicoletti AG, Petrolini FV, Assis DM, Juliano L, Gales AC. 2013. The route of antimicrobial resistance from the hospital effluent to the environment: focus on the occurrence of KPC-producing Aeromonas spp. and Enterobacteriaceae in sewage. Diagn Microbiol Infect Dis 76:80–85. doi: 10.1016/j.diagmicrobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Tavares CP, Pereira PS, Marques Ede A, Faria C Jr, de Souza Mda P, de Almeida R, Alves Cde F, Asensi MD, Carvalho-Assef AP. 2015. Molecular epidemiology of KPC-2-producing Enterobacteriaceae (non-Klebsiella pneumoniae) isolated from Brazil. Diagn Microbiol Infect Dis 82:326–330. doi: 10.1016/j.diagmicrobio.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Aminov RI. 2011. Horizontal gene exchange in environmental microbiota. Front Microbiol 2:158. doi: 10.3389/fmicb.2011.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goren MG, Carmeli Y, Schwaber MJ, Chmelnitsky I, Schechner V, Navon-Venezia S. 2010. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg Infect Dis 16:1014–1017. doi: 10.3201/eid1606.091671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidjabat HE, Silveira FP, Potoski BA, Abu-Elmagd KM, Adams-Haduch JM, Paterson DL, Doi Y. 2009. Interspecies spread of Klebsiella pneumoniae carbapenemase gene in a single patient. Clin Infect Dis 49:1736–1738. doi: 10.1086/648077. [DOI] [PubMed] [Google Scholar]
- 29.Dortet L, Oueslati S, Jeannot K, Tande D, Naas T, Nordmann P. 2015. Genetic and biochemical characterization of OXA-405, an OXA-48-type extended-spectrum beta-lactamase without significant carbapenemase activity. Antimicrob Agents Chemother 59:3823–3828. doi: 10.1128/AAC.05058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheppard AE, Stoesser N, Wilson DJ, Sebra R, Kasarskis A, Anson LW, Giess A, Pankhurst LJ, Vaughan A, Grim CJ, Cox HL, Yeh AJ, Modernising Medical Microbiology Informatics Group, Sifri CD, Walker AS, Peto TE, Crook DW, Mathers AJ. 2016. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother 60:3767–3778. doi: 10.1128/AAC.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathers AJ, Stoesser N, Sheppard AE, Pankhurst L, Giess A, Yeh AJ, Didelot X, Turner SD, Sebra R, Kasarskis A, Peto T, Crook D, Sifri CD. 2015. Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae at a single institution: insights into endemicity from whole-genome sequencing. Antimicrob Agents Chemother 59:1656–1663. doi: 10.1128/AAC.04292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau AF, Wang H, Weingarten RA, Drake SK, Suffredini AF, Garfield MK, Chen Y, Gucek M, Youn JH, Stock F, Tso H, DeLeo J, Cimino JJ, Frank KM, Dekker JP. 2014. A rapid matrix-assisted laser desorption ionization-time of flight mass spectrometry-based method for single-plasmid tracking in an outbreak of carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 52:2804–2812. doi: 10.1128/JCM.00694-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2010. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob Agents Chemother 54:4493–4496. doi: 10.1128/AAC.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheppard AE, Stoesser N, Sebra R, Kasarskis A, Deikus G, Anson L, Walker AS, Peto TE, Crook DW, Mathers AJ. 2016. Complete genome sequence of KPC-producing Klebsiella pneumoniae strain CAV1193. Genome Announc 4(1):e01649-15. doi: 10.1128/genomeA.01649-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Tran JH, Jacoby GA, Zhang Y, Wang F, Hooper DC. 2003. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob Agents Chemother 47:2242–2248. doi: 10.1128/AAC.47.7.2242-2248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Fernandez A, Villa L, Moodley A, Hasman H, Miriagou V, Guardabassi L, Carattoli A. 2011. Multilocus sequence typing of IncN plasmids. J Antimicrob Chemother 66:1987–1991. doi: 10.1093/jac/dkr225. [DOI] [PubMed] [Google Scholar]
- 37.Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother 65:2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 38.Novick RP. 1987. Plasmid incompatibility. Microbiol Rev 51:381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hidalgo-Grass C, Warburg G, Temper V, Benenson S, Moses AE, Block C, Strahilevitz J. 2012. KPC-9, a novel carbapenemase from clinical specimens in Israel. Antimicrob Agents Chemother 56:6057–6059. doi: 10.1128/AAC.01156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki H, Yano H, Brown CJ, Top EM. 2010. Predicting plasmid promiscuity based on genomic signature. J Bacteriol 192:6045–6055. doi: 10.1128/JB.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Chavda KD, Melano RG, Hong T, Rojtman AD, Jacobs MR, Bonomo RA, Kreiswirth BN. 2014. Molecular survey of the dissemination of two blaKPC-harboring IncFIA plasmids in New Jersey and New York hospitals. Antimicrob Agents Chemother 58:2289–2294. doi: 10.1128/AAC.02749-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradley DE, Taylor DE, Cohen DR. 1980. Specification of surface mating systems among conjugative drug resistance plasmids in Escherichia coli K-12. J Bacteriol 143:1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potron A, Poirel L, Nordmann P. 2011. Plasmid-mediated transfer of the bla(NDM-1) gene in Gram-negative rods. FEMS Microbiol Lett 324:111–116. doi: 10.1111/j.1574-6968.2011.02392.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen YT, Lin JC, Fung CP, Lu PL, Chuang YC, Wu TL, Siu LK. 2014. KPC-2-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. J Antimicrob Chemother 69:628–631. doi: 10.1093/jac/dkt409. [DOI] [PubMed] [Google Scholar]
- 45.Arutyunov D, Frost LS. 2013. F conjugation: back to the beginning. Plasmid 70:18–32. doi: 10.1016/j.plasmid.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Garcillan-Barcia MP, de la Cruz F. 2008. Why is entry exclusion an essential feature of conjugative plasmids? Plasmid 60:1–18. doi: 10.1016/j.plasmid.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. [DOI] [PubMed] [Google Scholar]
- 48.Siu LK, Lin JC, Gomez E, Eng R, Chiang T. 2012. Virulence and plasmid transferability of KPC Klebsiella pneumoniae at the Veterans Affairs Healthcare System of New Jersey. Microb Drug Resist 18:380–384. doi: 10.1089/mdr.2011.0241. [DOI] [PubMed] [Google Scholar]
- 49.Chavda KD, Chen L, Jacobs MR, Bonomo RA, Kreiswirth BN. 25 April 2016. Molecular diversity and plasmid analysis of KPC-producing Escherichia coli. Antimicrob Agents Chemother. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burton B, Dubnau D. 2010. Membrane-associated DNA transport machines. Cold Spring Harb Perspect Biol 2:a000406. doi: 10.1101/cshperspect.a000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freter R, Freter RR, Brickner H. 1983. Experimental and mathematical models of Escherichia coli plasmid transfer in vitro and in vivo. Infect Immun 39:60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon DM. 1992. Rate of plasmid transfer among Escherichia coli strains isolated from natural populations. J Gen Microbiol 138:17–21. doi: 10.1099/00221287-138-1-17. [DOI] [PubMed] [Google Scholar]
- 53.Lereclus D, Menou G, Lecadet MM. 1983. Isolation of a DNA sequence related to several plasmids from Bacillus thuringiensis after a mating involving the Streptococcus faecalis plasmid pAM beta 1. Mol Gen Genet 191:307–313. doi: 10.1007/BF00334831. [DOI] [PubMed] [Google Scholar]
- 54.Datta N, Hedges RW. 1971. Compatibility groups among fi− R factors. Nature 234:222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- 55.Perez-Mendoza D, de la Cruz F. 2009. Escherichia coli genes affecting recipient ability in plasmid conjugation: are there any? BMC Genomics 10:71. doi: 10.1186/1471-2164-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Achtman M, Kennedy N, Skurray R. 1977. Cell–cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A 74:5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


