Abstract
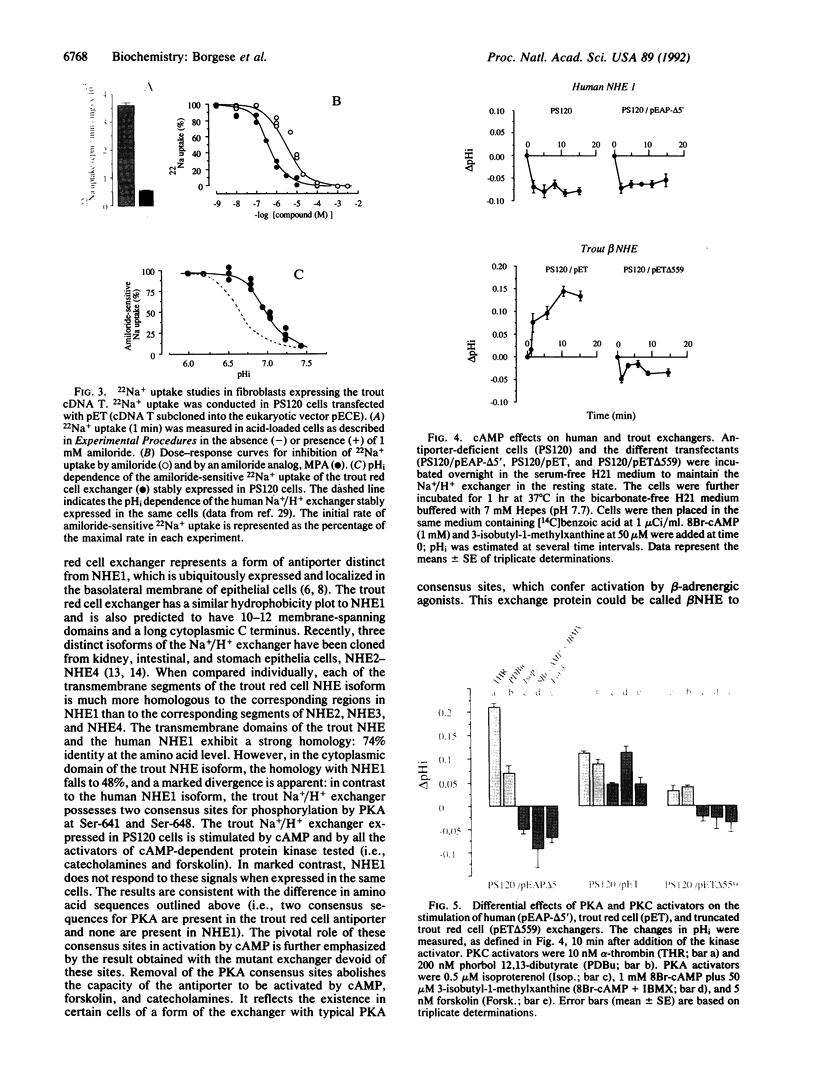
The ubiquitous plasma membrane Na+/H+ exchanger (termed NHE1) is activated by diverse hormonal signals, with the notable exception of hormones acting through cAMP as second messenger. Therefore, the Na+/H+ exchanger found in the nucleated trout red cell is of particular interest since it is activated by catecholamines, forskolin, and cAMP analogues. We report here that a cloned cDNA encoding the red cell exchanger restores functional Na+/H+ activity when transfected into Na+/H+ antiporter-deficient fibroblasts (i.e., it regulates intracellular pH in a Na-dependent and amiloride-sensitive manner). This red cell exchanger represents an additional form of Na+/H+ exchanger (termed beta NHE), which is characterized by a specific cytoplasmic domain involved in activation by the cAMP-dependent signaling pathway. After transfection in the same cellular context, beta NHE, but not NHE1, is activated by cAMP or by hormones that increase cAMP levels. Comparison of the amino acid sequences of exchangers shows that beta NHE, but not NHE1, contains two clustered consensus motifs for phosphorylation by a cAMP-dependent protein kinase (protein kinase A; PKA). A deletion mutant devoid of the C-terminal region of the cytoplasmic loop containing the two PKA sites restores Na+/H+ activity but is no longer activated by cAMP analogues or catecholamines. In red blood cells, the Na+/H+ exchanger is also activated by another pathway involving protein kinase C (PKC). Expression of beta NHE in fibroblasts shows that these two independent signaling pathways impinge on two distinct domains of the exchanger. The cytoplasmic segment containing PKA consensus sites, which is crucial for cAMP activation, is unnecessary for stimulation by PKC activators.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Barber D. L., McGuire M. E., Ganz M. B. Beta-adrenergic and somatostatin receptors regulate Na-H exchange independent of cAMP. J Biol Chem. 1989 Dec 15;264(35):21038–21042. [PubMed] [Google Scholar]
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Fievet B., Claireaux G., Thomas S., Motais R. Adaptive respiratory responses of trout to acute hypoxia. III. Ion movements and pH changes in the red blood cell. Respir Physiol. 1988 Oct;74(1):99–113. doi: 10.1016/0034-5687(88)90144-2. [DOI] [PubMed] [Google Scholar]
- Franchi A., Cragoe E., Jr, Pouysségur J. Isolation and properties of fibroblast mutants overexpressing an altered Na+/H+ antiporter. J Biol Chem. 1986 Nov 5;261(31):14614–14620. [PubMed] [Google Scholar]
- Garcia-Romeu F., Motais R., Borgese F. Desensitization by external Na of the cyclic AMP-dependent Na+/H+ antiporter in trout red blood cells. J Gen Physiol. 1988 Apr;91(4):529–548. doi: 10.1085/jgp.91.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Haggerty J. G., Agarwal N., Reilly R. F., Adelberg E. A., Slayman C. W. Pharmacologically different Na/H antiporters on the apical and basolateral surfaces of cultured porcine kidney cells (LLC-PK1). Proc Natl Acad Sci U S A. 1988 Sep;85(18):6797–6801. doi: 10.1073/pnas.85.18.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpel R., Olami Y., Taglicht D., Schuldiner S., Padan E. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10408–10414. [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Knickelbein R. G., Aronson P. S., Dobbins J. W. Membrane distribution of sodium-hydrogen and chloride-bicarbonate exchangers in crypt and villus cell membranes from rabbit ileum. J Clin Invest. 1988 Dec;82(6):2158–2163. doi: 10.1172/JCI113838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Mahé Y., Garcia-Romeu F., Motais R. Inhibition by amiloride of both adenylate cyclase activity and the Na+/H+ antiporter in fish erythrocytes. Eur J Pharmacol. 1985 Oct 22;116(3):199–206. doi: 10.1016/0014-2999(85)90154-2. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Moran A., Montrose M. H., Murer H. Regulation of Na+-H+ exchange in cultured opossum kidney cells by parathyroid hormone, atrial natriuretic peptide and cyclic nucleotides. Biochim Biophys Acta. 1988 Apr 2;969(1):48–56. doi: 10.1016/0167-4889(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Motais R., Garcia-Romeu F., Borgese F. The control of Na+/H+ exchange by molecular oxygen in trout erythrocytes. A possible role of hemoglobin as a transducer. J Gen Physiol. 1987 Aug;90(2):197–207. doi: 10.1085/jgp.90.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moule S. K., McGivan J. D. Epidermal growth factor and cyclic AMP stimulate Na+/H+ exchange in isolated rat hepatocytes. Eur J Biochem. 1990 Feb 14;187(3):677–682. doi: 10.1111/j.1432-1033.1990.tb15353.x. [DOI] [PubMed] [Google Scholar]
- Murer H., Hopfer U., Kinne R. Sodium/proton antiport in brush-border-membrane vesicles isolated from rat small intestine and kidney. Biochem J. 1976 Mar 15;154(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- Orlowski J., Kandasamy R. A., Shull G. E. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992 May 5;267(13):9331–9339. [PubMed] [Google Scholar]
- Palfrey H. C., Greengard P. Hormone-sensitive ion transport systems in erythrocytes as models for epithelial ion pathways. Ann N Y Acad Sci. 1981;372:291–308. doi: 10.1111/j.1749-6632.1981.tb15482.x. [DOI] [PubMed] [Google Scholar]
- Pollock A. S., Warnock D. G., Strewler G. J. Parathyroid hormone inhibition of Na+-H+ antiporter activity in a cultured renal cell line. Am J Physiol. 1986 Feb;250(2 Pt 2):F217–F225. doi: 10.1152/ajprenal.1986.250.2.F217. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Petersen K. U. Cyclic AMP inhibits Na+/H+ exchange at the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1985 Mar;85(3):409–429. doi: 10.1085/jgp.85.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood R. P., Emmer E., Wesolek J., McCullen J., Husain Z., Cohen M. E., Braithwaite R. S., Murer H., Sharp G. W., Donowitz M. Regulation of the rabbit ileal brush-border Na+/H+ exchanger by an ATP-requiring Ca++/calmodulin-mediated process. J Clin Invest. 1988 Sep;82(3):1091–1097. doi: 10.1172/JCI113665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Sardet C., Fafournoux P., Pouysségur J. Alpha-thrombin, epidermal growth factor, and okadaic acid activate the Na+/H+ exchanger, NHE-1, by phosphorylating a set of common sites. J Biol Chem. 1991 Oct 15;266(29):19166–19171. [PubMed] [Google Scholar]
- Sardet C., Franchi A., Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989 Jan 27;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Semrad C. E., Chang E. B. Calcium-mediated cyclic AMP inhibition of Na-H exchange in small intestine. Am J Physiol. 1987 Mar;252(3 Pt 1):C315–C322. doi: 10.1152/ajpcell.1987.252.3.C315. [DOI] [PubMed] [Google Scholar]
- Tse C. M., Brant S. R., Walker M. S., Pouyssegur J., Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem. 1992 May 5;267(13):9340–9346. [PubMed] [Google Scholar]
- Tse C. M., Ma A. I., Yang V. W., Watson A. J., Levine S., Montrose M. H., Potter J., Sardet C., Pouyssegur J., Donowitz M. Molecular cloning and expression of a cDNA encoding the rabbit ileal villus cell basolateral membrane Na+/H+ exchanger. EMBO J. 1991 Aug;10(8):1957–1967. doi: 10.1002/j.1460-2075.1991.tb07725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi S., Fafournoux P., Sardet C., Pouysségur J. The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls "H(+)-sensing". Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2424–2428. doi: 10.1073/pnas.89.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]