Abstract
The potential role antigens play in growth stimulation or in clonal selection of follicular lymphomas is unknown. To study this issue, we sequenced the immunoglobulin heavy chain variable region genes expressed by a follicular lymphoma from multiple biopsy specimens and also cloned and sequenced the corresponding germ-line variable gene from this patient. Comparison to the germ-line gene revealed numerous nucleotide substitutions in all of the lymphoma variable gene sequences. Some of the substitutions may have occurred in the nonmalignant precursor B cell that gave rise to this lymphoma because they were shared among all of the variable genes, but many of the mutations accumulated as the malignant clone expanded. The mutations were distributed in such a way that strongly suggested the majority of tumor cells had been positively selected through their antigen receptor. This was especially evident for the mutations that developed late in the clonal evolution of this lymphoma. These findings indicate that antigen stimulation may be involved in the growth of follicular lymphoma tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 6 A resolution. Nature. 1985 Jan 10;313(5998):156–158. doi: 10.1038/313156a0. [DOI] [PubMed] [Google Scholar]
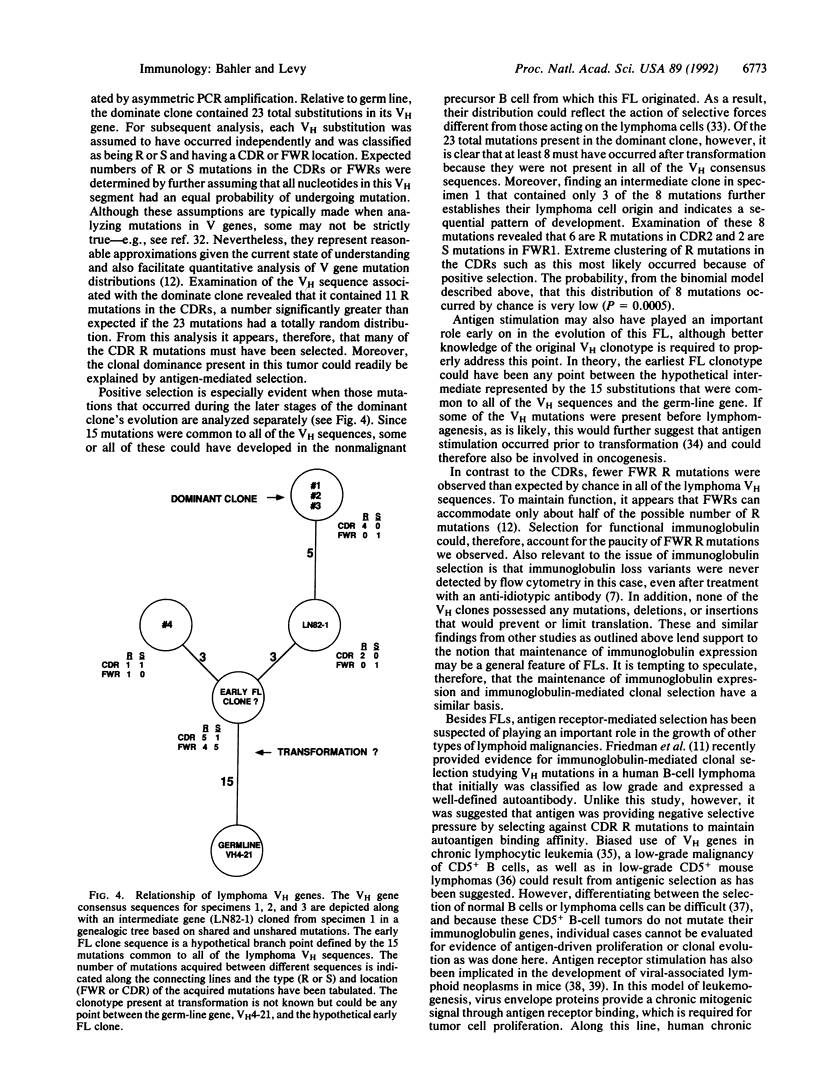
- Bahler D. W., Campbell M. J., Hart S., Miller R. A., Levy S., Levy R. Ig VH gene expression among human follicular lymphomas. Blood. 1991 Sep 15;78(6):1561–1568. [PubMed] [Google Scholar]
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Taylor L., Pollard J. W., Steele E. J. Distribution of mutations around rearranged heavy-chain antibody variable-region genes. Mol Cell Biol. 1990 Oct;10(10):5187–5196. doi: 10.1128/mcb.10.10.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. J., Zelenetz A. D., Levy S., Levy R. Use of family specific leader region primers for PCR amplification of the human heavy chain variable region gene repertoire. Mol Immunol. 1992 Feb;29(2):193–203. doi: 10.1016/0161-5890(92)90100-c. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Berry J., Flaherty D., Dunnick W. Somatic evolution of diversity among anti-phosphocholine antibodies induced with Proteus morganii. J Immunol. 1987 May 1;138(9):3060–3068. [PubMed] [Google Scholar]
- Cleary M. L., Galili N., Trela M., Levy R., Sklar J. Single cell origin of bigenotypic and biphenotypic B cell proliferations in human follicular lymphomas. J Exp Med. 1988 Feb 1;167(2):582–597. doi: 10.1084/jem.167.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Meeker T. C., Levy S., Lee E., Trela M., Sklar J., Levy R. Clustering of extensive somatic mutations in the variable region of an immunoglobulin heavy chain gene from a human B cell lymphoma. Cell. 1986 Jan 17;44(1):97–106. doi: 10.1016/0092-8674(86)90488-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero G., Hart S., Lim A., Borche L., Levy R., Miller R. A. Autoantibody activity of immunoglobulins isolated from B-cell follicular lymphomas. Blood. 1991 Aug 1;78(3):581–585. [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Morimoto C., McIntyre B. W., Rhynhart K., Lee N., Nadler L. M. Follicular non-Hodgkin's lymphoma cell adhesion to normal germinal centers and neoplastic follicles involves very late antigen-4 and vascular cell adhesion molecule-1. Blood. 1992 Jan 1;79(1):206–212. [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Friedman D. F., Cho E. A., Goldman J., Carmack C. E., Besa E. C., Hardy R. R., Silberstein L. E. The role of clonal selection in the pathogenesis of an autoreactive human B cell lymphoma. J Exp Med. 1991 Sep 1;174(3):525–537. doi: 10.1084/jem.174.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H., King J. L. Evolutionary nucleotide replacements in DNA. Nature. 1979 Oct 18;281(5732):605–606. doi: 10.1038/281605a0. [DOI] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stable expression and somatic hypermutation of antibody V regions in B-cell developmental pathways. Annu Rev Immunol. 1989;7:537–559. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- Kon S., Levy S., Levy R. Retention of an idiotypic determinant in a human B-cell lymphoma undergoing immunoglobulin variable-region mutation. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5053–5057. doi: 10.1073/pnas.84.14.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebecque S. G., Gearhart P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990 Dec 1;172(6):1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Matsuda F., Kinashi T., Kodaira M., Honjo T. A novel family of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1987 Jun 20;195(4):761–768. doi: 10.1016/0022-2836(87)90482-7. [DOI] [PubMed] [Google Scholar]
- Levy N. S., Malipiero U. V., Lebecque S. G., Gearhart P. J. Early onset of somatic mutation in immunoglobulin VH genes during the primary immune response. J Exp Med. 1989 Jun 1;169(6):2007–2019. doi: 10.1084/jem.169.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R., Levy S., Cleary M. L., Carroll W., Kon S., Bird J., Sklar J. Somatic mutation in human B-cell tumors. Immunol Rev. 1987 Apr;96:43–58. doi: 10.1111/j.1600-065x.1987.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Levy S., Mendel E., Kon S., Avnur Z., Levy R. Mutational hot spots in Ig V region genes of human follicular lymphomas. J Exp Med. 1988 Aug 1;168(2):475–489. doi: 10.1084/jem.168.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J., Johnson G. D., Gordon J., MacLennan I. C. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992 Jan;13(1):17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Mason D. Y., Johnson G. D., Abbot S., Gregory C. D., Hardie D. L., Gordon J., MacLennan I. C. Germinal center cells express bcl-2 protein after activation by signals which prevent their entry into apoptosis. Eur J Immunol. 1991 Aug;21(8):1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- Logtenberg T., Schutte M. E., Inghirami G., Berman J. E., Gmelig-Meyling F. H., Insel R. A., Knowles D. M., Alt F. W. Immunoglobulin VH gene expression in human B cell lines and tumors: biased VH gene expression in chronic lymphocytic leukemia. Int Immunol. 1989;1(4):362–366. doi: 10.1093/intimm/1.4.362. [DOI] [PubMed] [Google Scholar]
- Mann D. L., DeSantis P., Mark G., Pfeifer A., Newman M., Gibbs N., Popovic M., Sarngadharan M. G., Gallo R. C., Clark J. HTLV-I--associated B-cell CLL: indirect role for retrovirus in leukemogenesis. Science. 1987 May 29;236(4805):1103–1106. doi: 10.1126/science.2883731. [DOI] [PubMed] [Google Scholar]
- McGrath M. S., Tamura G., Weissman I. L. Receptor mediated leukemogenesis: murine leukemia virus interacts with BCL1 lymphoma cell surface IgM. J Mol Cell Immunol. 1987;3(4):227–242. [PubMed] [Google Scholar]
- McGrath M. S., Weissman I. L. AKR leukemogenesis: identification and biological significance of thymic lymphoma receptors for AKR retroviruses. Cell. 1979 May;17(1):65–75. doi: 10.1016/0092-8674(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Meeker T., Lowder J., Cleary M. L., Stewart S., Warnke R., Sklar J., Levy R. Emergence of idiotype variants during treatment of B-cell lymphoma with anti-idiotype antibodies. N Engl J Med. 1985 Jun 27;312(26):1658–1665. doi: 10.1056/NEJM198506273122602. [DOI] [PubMed] [Google Scholar]
- Pascual V., Victor K., Lelsz D., Spellerberg M. B., Hamblin T. J., Thompson K. M., Randen I., Natvig J., Capra J. D., Stevenson F. K. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4-21 gene segment is responsible for the major cross-reactive idiotype. J Immunol. 1991 Jun 15;146(12):4385–4391. [PubMed] [Google Scholar]
- Pennell C. A., Arnold L. W., Haughton G., Clarke S. H. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J Immunol. 1988 Oct 15;141(8):2788–2796. [PubMed] [Google Scholar]
- Pennell C. A., Mercolino T. J., Grdina T. A., Arnold L. W., Haughton G., Clarke S. H. Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur J Immunol. 1989 Jul;19(7):1289–1295. doi: 10.1002/eji.1830190721. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Zelenetz A. D., Campbell M. J., Bahler D. W., Takahashi S., Oren R., Esserman L., Umetsu D. T., Kwak L. W., Maloney D. G., Brown S. Follicular lymphoma: a model of lymphoid tumor progression in man. Ann Oncol. 1991 Feb;2 (Suppl 2):115–122. doi: 10.1007/978-1-4899-7305-4_18. [DOI] [PubMed] [Google Scholar]
- van Es J. H., Gmelig Meyling F. H., van de Akker W. R., Aanstoot H., Derksen R. H., Logtenberg T. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. J Exp Med. 1991 Feb 1;173(2):461–470. doi: 10.1084/jem.173.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]