Abstract
Autism and schizophrenia share multiple phenotypic and genotypic markers, and there is ongoing debate regarding the relationship of these two disorders. To examine whether cortical dynamics are similar across these disorders, we directly compared fMRI responses to visual, somatosensory and auditory stimuli in adults with autism (N=15), with schizophrenia (N=15), and matched controls (N=15). All participants completed a one-back letter detection task presented at fixation (to control attention) while task-irrelevant sensory stimulation was delivered to the different modalities. We focused specifically on the response amplitudes and the variability in sensory fMRI responses of the two groups, given the evidence of greater trial-to-trial variability in adults with autism. Both autism and schizophrenia individuals showed weaker signal-to-noise ratios (SNR) in sensory-evoked responses compared to controls (d>0.42), but for different reasons. For the autism group, the fMRI response amplitudes were indistinguishable from controls but were more variable trial-to-trial (d=0.47). For the schizophrenia group, response amplitudes were smaller compared to autism (d=0.44) and control groups (d=0.74), but were not significantly more variable (d<0.29). These differential group profiles suggest (1) that greater trial-to-trial variability in cortical responses may be specific to autism and is not a defining characteristic of schizophrenia, and (2) that blunted response amplitudes may be characteristic of schizophrenia. The relationship between the amplitude and the variability of cortical activity might serve as a specific signature differentiating these neurodevelopmental disorders. Identifying the neural basis of these responses and their relationship to the underlying genetic bases may substantially enlighten the understanding of both disorders.
Keywords: schizophrenia, autism, fMRI, variability, sensory perception
1.0 Introduction
Autism and schizophrenia share similar phenotypes including impairments in social, cognitive, and sensory behavior (Eack et al., 2013; Sugranyes et al., 2011; King & Lord, 2011; Cheung et al., 2010; Couture et al., 2010). Whereas autism manifests in childhood, the first psychotic break for schizophrenia occurs between late adolescence and young adulthood. The DSM-II included autism under the umbrella of schizophrenia, although later editions separated the two diagnoses (for a review, see Parnas & Bovet, 1991). Despite the segregation, the overlap between the disorders is quite apparent: in one study, half the individuals with autism met the criteria for schizophrenia (Konstantareas et al., 2001; Ghaziuddin, Tsai & Ghaziuddin, 1992), and in another, the neurocognitive and social-cognitive performance across a large neuropsychological battery was nearly identical between autism and schizophrenia (Eack et al., 2013).
Closer scrutiny of the biology of autism and schizophrenia reveals many similarities, including in genetics (Burbach & van der Zwaag, 2009; Leblond et al., 2012; Peykov et al., 2015; Sebat et al., 2007; Malhotra et al., 2011; Sullivan et al., 2012). One review investigating ‘at risk’ genotypes in autism and schizophrenia, Crespi et al. (2010) found that the two conditions may be genetically diametric or dose-dependent: certain CNV replications in autism were deleted in schizophrenia and vice versa. There are also similarities in brain function. Relative to controls, individuals with either disorder showed under-activation in prefrontal cortex (autism: Baron-Cohen et al., 1999; Happé et al., 1996; schizophrenia: Callicott et al., 2000; Russell et al., 2000; Schneider et al., 1998) and in fusiform gyrus (autism: Hall, Szechtman & Nahmias, 2003; Pierce et al., 2001; Schultz, Romanski & Tsatsanis, 2000; schizophrenia: Quintana et al., 2003; Streit et al., 2001).
Despite abnormal sensory behavior being a key commonality, there are differential cortical dynamics of sensory responses. The majority of sensory fMRI studies in schizophrenia have reported weaker activation (i.e. weaker signal-to-noise ratios, SNR) in sensory cortices (Silverstein et al., 2009; Gaebler et al., 2015; Kircher et al., 2004; Woodruff et al., 1997). Autism individuals show either greater (Green et al., 2015; Kaiser et al., 2015; Takarae et al., 2–14; Green et al., 2013) or weaker fMRI activation compared to healthy controls (Dinstein et al., 2012; Haigh et al., 2014; Cascio et al., 2012). Very few studies have compared the two groups directly under identical conditions. Doing so is critical to reach definitive conclusions about transdiagnostic similarities between the groups.
We have shown perturbations in neural processing in autism in response to sensory stimuli (Dinstein et al., 2012; Haigh et al., 2014). Relative to matched controls, autism individuals evinced greater trial-to-trial variability in fMRI responses, despite responses being indistinguishable in amplitude, resulting in weaker SNRs. Greater variability has been reported in the amplitude and latency of P1 ERP responses to visual stimuli (Milne, 2011). There are similar reports in schizophrenia (Jordanov et al., 2011; Müller et al., 1986), which could potentially contribute to smaller average responses (Iyer, Boutros & Zouridakis, 2011). Greater trial-to-trial variability may be the result of an imbalance between neural excitation and inhibition, which is associated with autism (Jamain et al., 2002; Markram, Rinaldi & Masrkram, 2007; Vattikuti & Chow, 2010; Rubenstein & Merzenich, 2003; Sigurdsson, in press; Uhlhaas, 2013; Lisman, 2012), and with schizophrenia (Baron-Cohen et al., 2009; Gomot et al., 2002; Simmons et al., 2009). One hypothesis is that there is excess excitation due to either increased glutamatergic activity, or reduced GABAergic signaling. The neural variability may be correlated across time and clusters of neurons, thereby affecting the fMRI signal. Variability in sensory responses could impact more complex information processing: if the individual is unable to gain reliable information about their surroundings, then this might make complex environments like social situations confusing and potentially over-whelming, leading to social withdrawal (Dinstein, Heeger & Behrmann, 2015).
Greater trial-to-trial variability offers a potential signature of the cortical response in autism and the key question is whether greater variability in sensory-evoked activity is specific to autism, or is apparent in schizophrenia as well. If the latter, this would offer a transdiagnostic endophenotype related to the sensory abnormalities seen in autism and schizophrenia, and may relate to their shared genetic markers. Differences in response variability across the two groups would alternatively indicate that the overt manifestation of the underlying neurobiology may differ or be differentially modulated by environmental or other genetic factors.
2.0 Methods and Materials
2.1 Participants
Ten males and five females (mean age 26, range 19–34 years) with schizophrenia or schizoaffective disorder (diagnosed using the Structured Clinical Interview for DSM-IV (First et al., 2005) and the Brief Psychiatric Rating Scale (BPRS) (Lukoff, Nuechterlein & Ventura, 1986) by an expert diagnostician) participated in a 90-minute study and were paid $75 for their time (see Table 1 for demographics). Fourteen of the individuals with schizophrenia were taking antipsychotics (average chlorpromazine equivalent was 255 mg, SD 306 mg) (see Supplementary Materials for more information on medication use).
Table 1.
Demographic and medication information for the individuals with schizophrenia. BPRS=Brief Psychiatric Rating Scale; CPZ=chlorpromazine equivalents.
| Participant | Gender | Age (years) | BPRS Score | Medication CPZ (mg/day) |
Full-Scale IQ |
|---|---|---|---|---|---|
| 1 | F | 24 | 28 | 93.3 | 96 |
| 2 | M | 33 | 47 | 75.0 | 94 |
| 3 | M | 34 | 30 | 200.0 | 95 |
| 4 | F | 31 | 28 | 33.3 | 96 |
| 5 | M | 23 | 32 | 266.7 | 100 |
| 6 | M | 24 | 36 | 0.0 | 102 |
| 7 | M | 19 | 23 | 100.0 | 117 |
| 8 | M | 25 | 29 | 50.0 | 102 |
| 9 | F | 30 | 33 | 507.1 | 112 |
| 10 | M | 25 | 33 | 968.1 | 97 |
| 11 | M | 22 | 28 | 33.3 | 129 |
| 12 | F | 19 | 33 | 783.3 | 101 |
| 13 | M | 28 | 29 | 0.0 | 113 |
| 14 | F | 24 | 44 | 100.0 | 89 |
| 15 | M | 26 | 18 | 100.0 | 109 |
Data from twelve male and three female age-matched individuals with autism (mean age 26, range 19–36 years), and eleven male and four female typical controls (mean age 27, range 20–40 years) were included in this study, and were previously reported (Dinstein et al., 2012; Haigh et al., 2014). Participants were chosen according to closest match to the schizophrenia group on age. All of the individuals with autism were Caucasian and met the DSM-IV criteria for autism based on the Autism Diagnostic Observation Schedule (ADOS-G) (Lord et al., 2000) and Autism Diagnostic Interview (ADI) (Le Couteur et al., 1989; Lord, Rutter & Le Couteur, 1994). These assessments were carried out at the Center For Excellence in Autism Research, at the University of Pittsburgh (see Table 2 for demographics) and confirmed by expert opinion (NJM). One individual with autism was taking antipsychotic medication, and six were taking antidepressants (see Supplementary Materials).
Table 2.
Demographic and clinical information for the individuals with autism. ADOS=Autism Diagnostic Observation Schedule; ADI=Autism Diagnostic Interview.
| Participant | Gender | Age (years) |
ADOS social |
ADOS communication |
ADOS stereotypical |
ADI social |
ADI communication |
ADI stereotypical |
Full scale IQ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 19 | 7 | 5 | 3 | 27 | 20 | 6 | 107 |
| 2 | M | 33 | 5 | 3 | 3 | 26 | 18 | 12 | 131 |
| 3 | M | 36 | 8 | 2 | 1 | 20 | 11 | 3 | 125 |
| 4 | F | 31 | 10 | 6 | 3 | 15 | 9 | 6 | 121 |
| 5 | M | 22 | 13 | 6 | 1 | 23 | 13 | 4 | 88 |
| 6 | M | 22 | 6 | 5 | 6 | 19 | 11 | 4 | 127 |
| 7 | M | 21 | 9 | 5 | 1 | 22 | 15 | 5 | 108 |
| 8 | M | 27 | 6 | 2 | 3 | 20 | 16 | 7 | 104 |
| 9 | F | 31 | 7 | 2 | 4 | 10 | 8 | 6 | 123 |
| 10 | M | 21 | 8 | 4 | 2 | 21 | 17 | 6 | 123 |
| 11 | M | 36 | 8 | 2 | 1 | 20 | 11 | 3 | 129 |
| 12 | M | 19 | 7 | 3 | 3 | 22 | 15 | 5 | 96 |
| 13 | M | 30 | 10 | 6 | 2 | 23 | 17 | 6 | 128 |
| 14 | M | 22 | 11 | 5 | 3 | 20 | 15 | 3 | 107 |
| 15 | M | 29 | 6 | 3 | 1 | 15 | 12 | 2 | 116 |
All autism and schizophrenia participants had an IQ above 88, had normal or corrected-to-normal vision, and gave their written consent to take part in the study. The Institutional Review Boards at Carnegie Mellon University (CMU) and the University of Pittsburgh approved the experimental procedures, which were in compliance with the safety guidelines for MRI research, and the individuals with autism consented to the use of their data in this study.
2.2 Experimental design
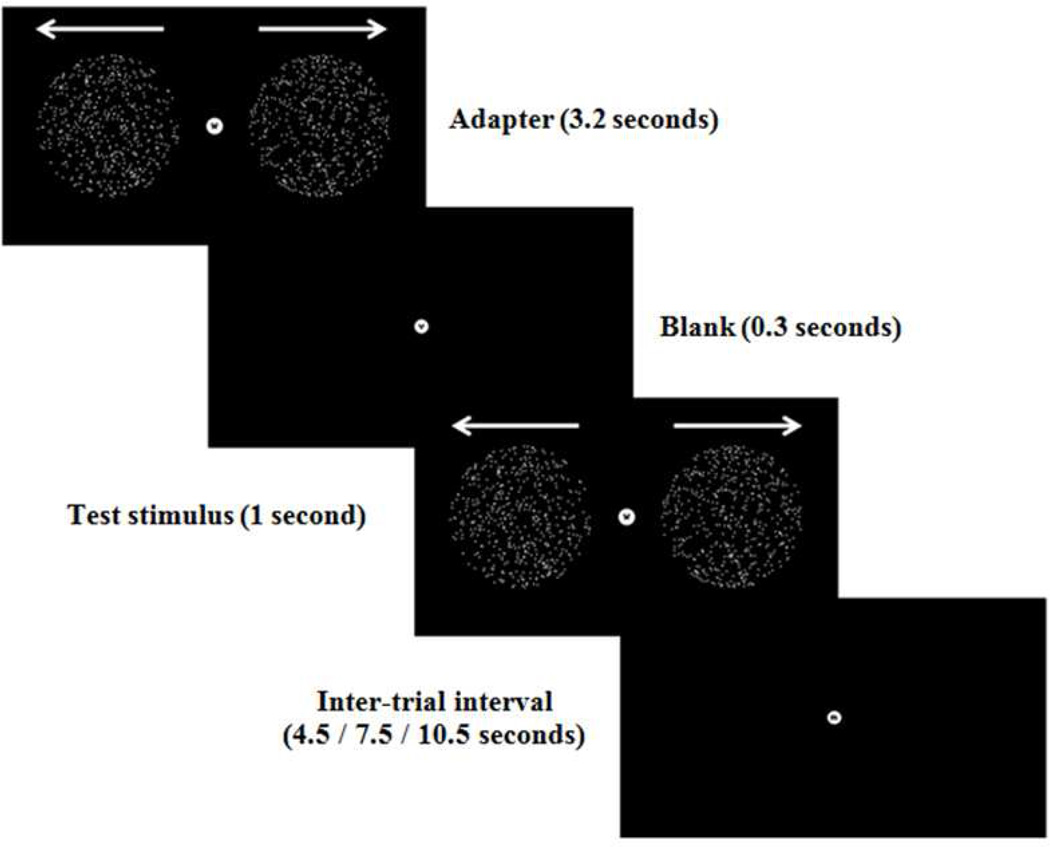
The design of the experiment was identical to that described previously (Dinstein et al., 2012; Haigh et al., 2014). Participants took part in a single fMRI session in which neural responses to visual, auditory and somatosensory stimuli were measured in separate runs following an event-related design (see Figure 1 for example of visual display and the timing of a single trial). Participants were presented with 72 trials for each of three sensory modalities over two runs, which were blocked and the blocks were randomly interleaved across modality. For each modality, the trial began with an adapter followed by a test stimulus. Adapters were either 2 circular apertures containing 500 white dots each (visual), 11 air puffs directed to the back of the left hand (somatosensory), or eleven pure tone beeps (auditory). The test stimuli were either identical to the adapters (the adapted condition); different from the adapter (the unadapted condition) in motion direction (visual), body location (location on left hand, somatosensory), or tone frequency (auditory); or no test was presented (the no-test condition).
Figure 1.
An example trial from the visual experiment. The adapter was shown for 3.2 s followed by a blank screen for 0.3 s, and the test stimulus for 1 s. The inter-trial intervals between trials were 4.5, 7.5 or 10.5 s in duration (in a randomized order). Auditory and somatosensory experiments had an identical structure.
During the sensory stimulation, participants were asked to complete a one-back task which was orthogonal (and irrelevant) to the sensory stimuli. This ensured that any sensory differences between groups were not a function of differential attention. Participants were instructed to attend to a sequence of letters and identify immediate repetitions. The letters, shown in lower case, were presented at fixation throughout each block of trials, one at a time and changed every 500ms. Participants used their right index finger to indicate when a repetition was noted. Participants had 1 s to respond and received feedback (correct response – fixation green; incorrect response – fixation red). Misses were not indicated.
2.3 Data Acquisition
All data were collected on the same 3T Siemens MRI scanner at CMU. Six functional (two per sensory modality) and one anatomical scan were acquired per participant. The scanner was equipped with a Siemens 12 channel birdcage head coil, which was used for RF transmit and receive. Functional images were acquired with a T2*-sensitive echo planar imaging pulse sequence (repetition time=1,500 ms, echo time=30 ms, flip angle=75°, 24 slices, 3×3×3 mm voxels, field of view= 192 mm). Anatomical volumes were acquired with a T1-weighted 3D–MPRAGE pulse sequence (1×1×1 mm).
2.4 Data Analysis
fMRI data were preprocessed using Brain Voyager, in-house software written in Matlab (Mathworks, Natick, MA) and the NeuroElf toolbox (http://neuroelf.net/, JW). Preprocessing included 3D motion correction, temporal high-pass filtering with a cutoff frequency of 6 cycles per scan, spatial smoothing using a Gaussian kernel with 8 mm width at half height, alignment with the anatomical volume using trilinear interpolation, and transformation to the Talairach coordinate system (Talairach & Tournoux, 1988). Scans containing head movements in excess of 2 mm (approximately 7% of scan volumes) were excluded from data analysis. Voxel intensity was corrected for the residual motion, by regressing the head motion on the fMRI responses and then using the residuals to calculate the adjusted fMRI responses. There was no significant difference in the amount of head motion between the autism, schizophrenia and control groups (see Supplementary Materials, Figure S1).
Individual regions of interest (ROIs) were created by identifying the 200 most significant voxels within the relevant sensory area of the cortex bilaterally for each participant. This ensured that ROI size was equivalent across participants and across modalities (see Figure S2 for activation maps, which appeared to be similar across groups), and was consistent with previous studies (Dinstein et al., 2012; Haigh et al., 2014). The response from each hemisphere was analysed. The first functional scan from each sensory modality was used to define these bilateral ROIs, unless the scan was removed from analysis due to excess motion artefact (see head motion section) in which case the remaining scan was used to define the ROIs. Responses from both runs were analysed.
An epoch of the fMRI time series, for each voxel in the ROI, was then extracted from adapter-onset to 12 s (8 time-points) after adapter-onset. Response amplitudes were calculated, separately for each trial, by averaging the responses at time-points 4 and 5, which corresponded to the peak of the haemodynamic response. Response standard deviations (SD) were calculated by averaging the response across time-points 3–6 (to capture the peak of the fMRI response, whilst attaining a more accurate measure of response variability), separately for each trial, and then computing the SD across trials. SNRs were calculated by dividing the response amplitudes by the response variances. We also performed complementary randomization tests to assess differences between groups without assuming normal distributions, and an additional regression analysis using a general linear model to utilize more of the data rather than just the peak of the fMRI response (see Supplementary Materials). The responses from the no-test condition (12 no-test presentations per scan) were used for the main analysis. The results were similar (see Supplementary Materials).
Effect sizes were calculated for group differences in fMRI response amplitude, SDs and SNR, using the following formulae:
Formula 1. Calculations for Cohen’s d effect size for each group comparison. N=number of observations; SD=standard deviation; G1=group 1, G2=group 2.
3.0 Results
Mixed analyses of variance were conducted separately for the fMRI response amplitudes, the SD in fMRI responses and the SNR, and separately for each pairwise group comparison. Sensory modality served as the within-subjects variable (responses from visual, somatosensory and auditory ROIs) and group served as the between-subjects variable (autism, control and schizophrenia).
For all analyses, there was a significant main effect of sensory modality, due to the smaller fMRI responses, smaller variability, and weaker SNRs in the somatosensory modality. Significant interactions between modality and group are highlighted and are of key interest.
3.1 fMRI analyses
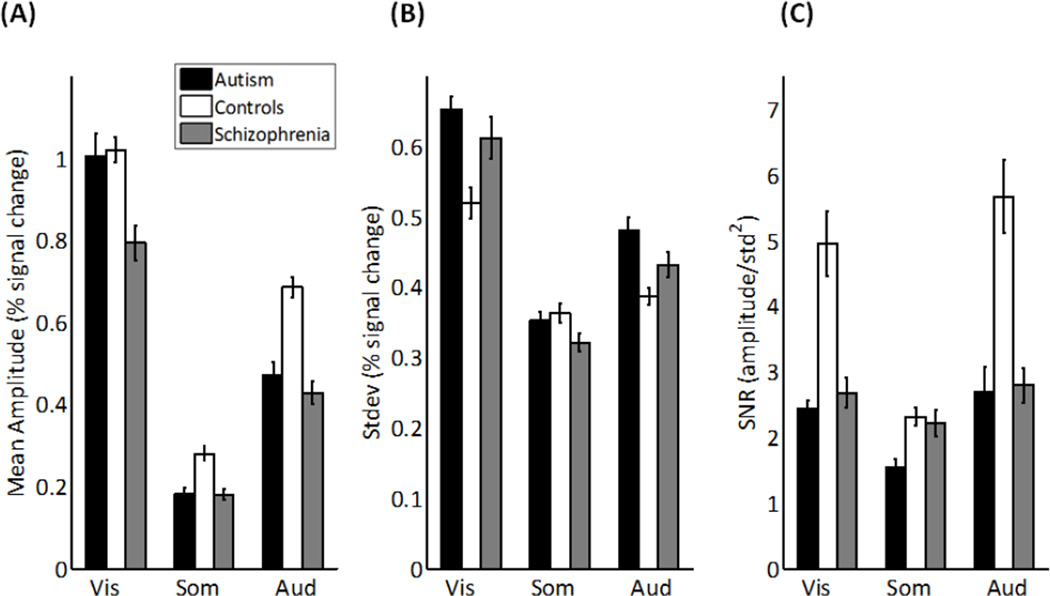
Individuals with autism produced statistically indistinguishable fMRI response amplitudes compared to controls (Figure 2A; F(1,21)=0.60, p=.446; d=0.17). However, individuals with schizophrenia produced smaller fMRI amplitudes compared to controls (F(1,23)=11.68, p=.002; d=0.74), and marginally smaller amplitudes compared to autism (F(1,22)=4.01, p=.058; d=0.44).
Figure 2.
The fMRI responses for autism, schizophrenia and control group for the visual, somatosensory and auditory stimuli. A) Mean response amplitudes. B) Standard deviations of the responses. C) Signal-to-noise ratios. Error bars represent one standard error.
The SD of the fMRI response were greater in autism compared to controls (Figure 2B; F(1,21)=4.58, p=.044; d=0.47), but there was no significant difference between autism and schizophrenia on SD (F(1,22)=1.64, p=.213; d=0.17), or between controls and schizophrenia (F(1,23)=0.59, p=.451; d=0.28).
There was no significant difference between schizophrenia and autism in SNR (Figure 2C; F(1,22)=.04, p=.842; d=0.04). Both autism and schizophrenia exhibited smaller SNRs than controls (autism versus controls: F(1,21)=4.20, p=.053; d=0.45; schizophrenia versus controls: F(1,23)=4.35, p=.048; d=0.45). The smaller SNR in autism was due to the greater variability in fMRI responses, whereas, the smaller SNR in schizophrenia was due to the smaller response amplitudes.
Analysis of the responses in the adapted and unadapted trials yielded qualitatively similar results (Supplementary Materials).
Because the individuals with schizophrenia showed consistently smaller fMRI response amplitudes compared to controls, any differences in SD in fMRI responses between groups might be difficult to interpret. In particular, if the variance increases with the mean fMRI response (a Poisson distribution), then any difference in SD might be a direct consequence of the difference in response amplitudes. To circumvent this potential confound, fMRI response amplitudes were equated across groups by selecting individuals from the groups who were closely matched on overall amplitude (N=10 in each group). Individuals with autism still exhibited greater SD in fMRI responses than controls (F(1,18)=8.05, p=.011), but there was still no significant difference between autism and schizophrenia on SD in fMRI responses (F(1,18)=0.76, p=.394), or schizophrenia and control groups (F(1,18)=2.74, p=.115; see Supplementary Materials for further analyses).
The randomization test and the regression analysis showed similar results to the trial-triggered analyses, except that individuals with schizophrenia did not exhibit significant differences in response amplitudes compared to controls (F(1,15)=0.10, p=.761) or individuals with autism (F(1,17)<.01, p=.993; Supplementary Materials, Figure S4). We discuss the apparent inconsistency in the outcome of these two analyses below.
There was no significant correlation dosage between antipsychotic medication and fMRI responses in the schizophrenia group, and no significant effect of antidepressants on responses in the autism group (see Supplementary Materials for details). There were also no significant correlations between IQ and amplitude, SD or SNR for the autism or the schizophrenia group (p>.05).
3.2 Behavioral Responses
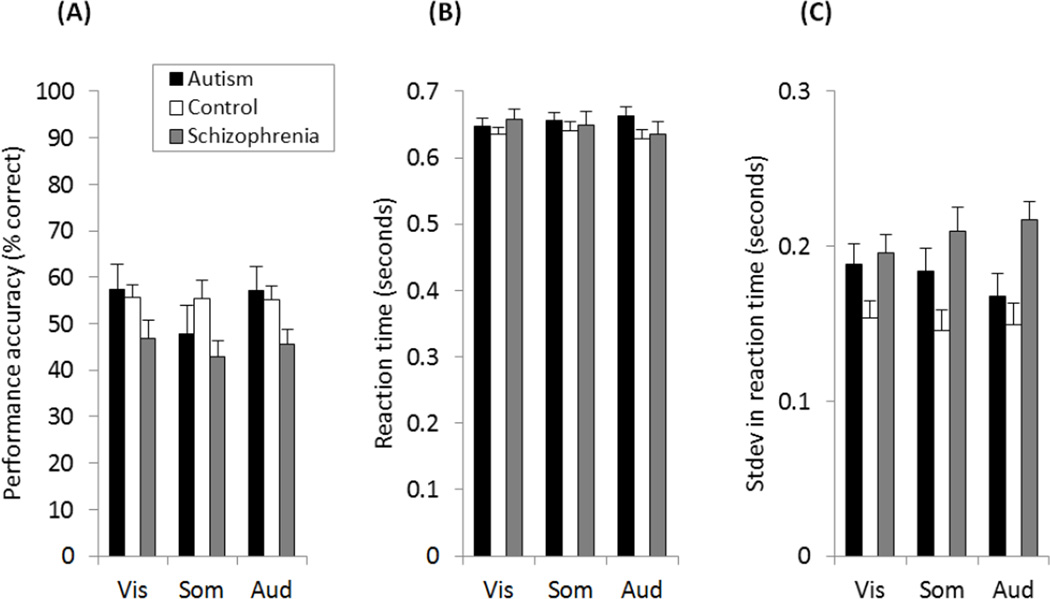
One possible explanation for the group differences in fMRI responses is that certain groups might have been more attentive/variable over time. If the former, then we would expect to see poorer response accuracy and/or slower reaction times (RT) in behavioral responses. If the latter, then we would expect to see more variable RT. We measured performance accuracy and RT on the letter repetition detection task at fixation, as a proxy for attention (Figure 3).
Figure 3.
Behavioral performance from the autism, schizophrenia and control group. (A) Accuracy, the percent of letter repeats that were correctly identified. (B) Reaction time. (C) Standard deviation in reaction times. Error bars show one standard error.
There were no significant differences in accuracy (% correct), or in mean RT to the repeated letter between autism, schizophrenia and control groups (see Supplementary Materials for statistical comparisons). Individuals with schizophrenia, however, exhibited significantly greater trial-to-trial variability in RT compared to controls (F(1,23)=9.52, p=.005), but not compared to autism (F(1,24)=2.14, p=.156), and there was no significant difference between autism and controls (F(1,23)=2.69, p=.114). There were also no significant correlations between SDs in RT and fMRI responses that were consistent across the sensory modalities (see Supplementary Materials).
The greater trial-to-trial variability in RT in the schizophrenia group might suggest that their attentional state may have been more variable. But there was no evidence for greater variability in the fMRI responses from the schizophrenia group. Hence, these findings do not indicate that the differences in fMRI responses between groups were due to differences in attention or performance per se.
4.0 Discussion
This investigation was designed to characterize sensory fMRI responses in autism and schizophrenia, which is critical given questions about their common pathophysiology. Compared to controls, both autism and schizophrenia produced weaker SNRs (somatosensory responses were weaker in amplitude across the board, potentially yielding a floor effect for somatosensory SNR). For autism, weaker SNR arose from greater trial-to-trial variability in fMRI responses (in particular, for visual and auditory responses) while the amplitude was indistinguishable from controls. For schizophrenia, weaker SNR arose from smaller fMRI amplitudes, while trial-to-trial variability was indistinguishable from autism and control groups. These results held across a number of analytic approaches, and could not be attributed to differences in behavioral responses, motion artifacts during scanning, nor to medication. Together, these findings provide differential signatures of cortical activation in autism versus schizophrenia.
One potential concern about this study is the small sample size (15 participants per group), which may result in the analyses being under-powered or the findings difficult to replicate. First, the greater trial-to-trial variability in autism, originally reported by Dinstein et al (2012), was subsequently replicated (Haigh et al., 2014), and the current study shows a medium effect size (Cohen, 1988). Second, the effect size for differences in trial-to-trial variability between schizophrenia and autism or control groups was small, so it is unlikely that increasing the sample size would yield different findings. A power analysis of the largest group effect size that was not significant (d=0.28) would require at least 200 participants in each group to have 90% power in the results (Faul et al., 2007; Faul et al., 2009). Third, a number of analyses were conducted, including non-parametric randomization tests, to confirm that the findings were not an artefact of the analysis. Therefore, it is unlikely that these results were confounded by the small sample size. Finally, we note that the reduction in response amplitude in the schizophrenic participants was only evident in the trial-triggered analyses and not in the regression analysis. While this inconsistency suggests that the finding of a reduction in response amplitude in schizophrenia ought to be treated with caution, many existing studies have demonstrated such a result in sensory cortices spanning MRI and EEG/ERP methodologies (Silverstein et al., 2009; Gaebler et al., 2015; Kircher et al., 2004; Woodruff et al., 1997; Umbricht & Krljes, 2005; Salisbury et al., 2009). The robust evidence of hypo-activation in schizophrenia confirms that our observation of reduced amplitude in schizophrenia in this study is likely to be valid.
The finding of a differential signature across the two conditions suggests that a consideration of both the variability and the amplitude of sensory fMRI responses might be useful in differentiating the sensory cortical dynamics characteristic of autism and schizophrenia. The reduction in response amplitude in schizophrenic participants has been demonstrated in many studies, during visual (Silverstein et al., 2009) and auditory processing (Gaebler et al., 2015; Kircher et al., 2004), particularly in those with auditory hallucinations (Woodruff et al., 1997), and correlates with reduced performance at sensory tasks (Holcomb et al., 2000; Volz et al., 2001; Kim et al., 2011). This hypo-responsiveness has been linked to dendritic toxicity (shorter and fewer dendritic spines, especially in auditory cortex) (Sweet et al., 2008), and abnormalities in PING (Pyramidal Interneuron Network Gamma) circuits (Gonzalez-Burgos & Lewis, 2008; Lewis et al., 2012; Gonzalez-Burgos, Fish & Lewis, 2011).
The greater variability may reflect the noise in the sensory systems. Approaches to noise reduction include the use of oxytocin (Owen et al., 2013); oxytocin is lower in autism (Modahl et al., 1998; Wu et al., 2005), and oxytocin-related treatments for autism are on the rise (Kuehn, 2011; Modi & Young, 2012; Gordon et al., 2013). As autism is a neurodevelopmental disorder, the greater variability may affect sensory input throughout development. Human sensory systems learn by detecting statistical regularities in the environment; unreliable sensory signals would make learning more difficult (perhaps leading to the repetitious behavior in autism), and make sensory environments unpredictable, leading to withdrawal from social situations.
In conclusion, both autism and schizophrenia evinced weaker SNRs in sensory fMRI responses compared to controls. However, the profile of the weaker SNRs appeared to differ between the two groups of individuals: autism was associated with greater trial-to-trial variability, whereas schizophrenia was associated with smaller response amplitudes. These dissociations might help differentiate between the two groups and aid in the elucidation of the neural mechanisms underlying each condition. Furthermore, differences in the neurobiological profile and cortical dynamics might offer potential targets for differential interventions.
Supplementary Material
Acknowledgments
The authors thank Ryan Egan for helping with participant recruitment and fMRI testing and the staff at the Center for Excellence in Autism Research at the University of Pittsburgh for recruitment and assessment of participants. The authors have no conflict of interest to declare.
Funding
This work was supported by a grant from the Simons Foundation Autism Research Initiative (177638) to DH and MB, and a National Institutes of Health/National Institute of Child Health and Human Development grant (HD055748) to NJM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Contributions
Sarah M Haigh – helped design and run the study, analyzed and interpreted the data, and wrote the manuscript
Akshat Gupta – recruited the participants, coordinated all involved with the scanning, and helped run the study
Scott M Barb – recruited the individuals with schizophrenia, helped run the study, and helped with manuscript preparation
Summer A F Glass – recruited the individuals with schizophrenia, provided demographic and symptom information, and helped with manuscript preparation
Nancy J Minshew – recruited the individuals with autism, clinically assessed the participants, helped with manuscript preparation and provided comments on the final version
Ilan Dinstein – helped with data analysis, helped with manuscript preparation and provided comments on the final version
David J Heeger – helped with the design of the study, data analysis, manuscript preparation and provided final comments on the final version
Shaun M Eack – recruited the individuals with autism, clinically assessed the participants, helped with manuscript preparation and provided comments on the final version
Marlene Behrmann – helped with the design of the study, data analysis, manuscript preparation and provided final comments on the final version
All authors reviewed the manuscript before submission
References
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SCR. Social intelligence in the normal and autistic brain: an fMRI study. Eur. J. Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Burbach JPH, van der Zwaag B. Contact in the genetics of autism and schizophrenia. Trends Neurosci. 2009;32:69–72. doi: 10.1016/j.tins.2008.11.002. doi: http://dx.doi.org/10.1016/j.tins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological Dysfunction of the Dorsolateral Prefrontal Cortex in Schizophrenia Revisited. Cereb. Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Moana-Filho EJ, Guest S, Nebel MB, Weisner J, Baranek GT, Essick GK. Perceptual and Neural Response to Affective Tactile Texture Stimulation in Adults with Autism Spectrum Disorders. Autism Res. 2012;5:231–244. doi: 10.1002/aur.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q, Sham P, Chua S, McAlonan G. Autistic Disorders and Schizophrenia: Related or Remote? An Anatomical Likelihood Estimation. PLoS One. 2010;5:e12233. doi: 10.1371/journal.pone.0012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. New Jersey: Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol. Med. 2010;40:569–579. doi: 10.1017/S003329170999078X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi B, Stead P, Elliot M. Comparative genomics of autism and schizophrenia. Proc. Natl. Acad. Sci. 2010;107:1736–1741. doi: 10.1073/pnas.0906080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. Unreliable Evoked Responses in Autism. Neuron. 2012;75:981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein, Heeger DJ, Behrmann M. Neural variability: friend or foe? Trends Cogn Sci. 2015;19:322–328. doi: 10.1016/j.tics.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Eack SM, Bahorik AL, McKnight SAF, Hogarty SS, Greenwald DP, Newhill CE, Phillips ML, Keshavan MS, Minshew NJ. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr. Res. 2013;148:24–28. doi: 10.1016/j.schres.2013.05.013. doi: http://dx.doi.org/10.1016/j.schres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York: New York State Psychiatric Institute; 2005. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. [Google Scholar]
- Gaebler AJ, Mathiak K, Koten JW, König AA, Koush Y, Weyer D, Depner C, Matentzoglu S, Edgar JC, Willmes K, Zvyagintsev M. Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia. Brain. 2015 doi: 10.1093/brain/awv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziuddin M, Tsai L, Ghaziuddin N. Comorbidity of autistic disorder in children and adolescents. Eur. Child Adolesc. Psychiatry. 1992;1:209–213. doi: 10.1007/BF02094180. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard M-H, Adrien J-L, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: Electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA Neurons and the Mechanisms of Network Oscillations: Implications for Understanding Cortical Dysfunction in Schizophrenia. Schizophr. Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, Zagoory-Sharon O, Leckman JF, Feldman R, Pelphrey KA. Oxytocin enhances brain function in children with autism. Proc. Natl. Acad. Sci. 2013;110:20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Hernandez L, Tottenham N, Krasileva K, SY B, Dapretto M. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015;72:778–786. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, Tottenham N, Dapretto M, Bookheimer SY. Overreactive Brain Responses to Sensory Stimuli in Youth With Autism Spectrum Disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:1158–1172. doi: 10.1016/j.jaac.2013.08.004. doi: http://dx.doi.org/10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh SM, Heeger DJ, Dinstein I, Minshew N, Behrmann M. Cortical Variability in the Sensory-Evoked Response in Autism. J. Autism Dev. Disord. 2014;45:1176–1190. doi: 10.1007/s10803-014-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GB, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. Am. J. Psychiatry. 2003;160:1439–1441. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- Happé F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R, Frith C. “Theory of mind” in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996:8. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Weiler M, Dannals RF, Tamminga CA. Brain Activation Patterns in Schizophrenic and Comparison Volunteers During a Matched-Performance Auditory Recognition Task. Am. J. Psychiatry. 2000;157:1634–1645. doi: 10.1176/appi.ajp.157.10.1634. [DOI] [PubMed] [Google Scholar]
- Iyer D, Boutros NN, Zouridakis G. Aberrant auditory evoked responses in schizophrenia: evidence from single-trial analysis. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2011;2011:4406–4409. doi: 10.1109/IEMBS.2011.6091093. [DOI] [PubMed] [Google Scholar]
- Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, Gillberg C, Leboyer M, Bourgeron T null. Linkage and association of the glutamate receptor 6 gene with autism. Mol. Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Mei W, Zhang J, Jing J, Wu Q, Zhuo Y, Xiao Z. Abnormal auditory sensory gating-out in first-episode and never-medicated paranoid schizophrenia patients: an fMRI study. Exp. Brain Res. 2013;229:139–147. doi: 10.1007/s00221-013-3600-7. [DOI] [PubMed] [Google Scholar]
- Jordanov T, Popov T, Weisz N, Elbert T, Paul-Jordanov I, Rockstroh B. Reduced mismatch negativity and increased variability of brain activity in schizophrenia. Clin. Neurophysiol. 2011;122:2365–2374. doi: 10.1016/j.clinph.2011.05.002. doi: http://dx.doi.org/10.1016/j.clinph.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Yang DY-J, Voos AC, Bennett RH, Gordon I, Pretzsch C, Beam D, Keifer C, Eilbott J, McGlone F, Pelphrey KA. Brain Mechanisms for Processing Affective (and Nonaffective) Touch Are Atypical in Autism. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedy SK, Rosen C, Khine T, Rajarethinam R, Janicak PG, Sweeney JA. An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Res. Neuroimaging. 2009;172:16–23. doi: 10.1016/j.pscychresns.2008.06.003. doi: http://dx.doi.org/10.1016/j.pscychresns.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park S, Blake R. Perception of Biological Motion in Schizophrenia and Healthy Individuals: A Behavioral and fMRI Study. PLoS One. 2011;6:e19971. doi: 10.1371/journal.pone.0019971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BH, Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011;1380:34–41. doi: 10.1016/j.brainres.2010.11.031. doi: http://dx.doi.org/10.1016/j.brainres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Rapp A, Grodd W, Buchkremer G, Weiskopf N, Lutzenberger W, Ackermann H, Mathiak K. Mismatch Negativity Responses in Schizophrenia: A Combined fMRI and Whole-Head MEG Study. Am. J. Psychiatry. 2004;161:294–304. doi: 10.1176/appi.ajp.161.2.294. [DOI] [PubMed] [Google Scholar]
- Konstantareas MMM, Hewitt T. Autistic Disorder and Schizophrenia: Diagnostic Overlaps. J. Autism Dev. Disord. 2001;31:19–28. doi: 10.1023/a:1005605528309. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA. 2011;305:659–661. doi: 10.1001/jama.2011.117. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. J. Autism Dev. Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, Konyukh M, Chaste P, Ey E, Rastam M, Anckarsäter H, Nygren G, Gillberg IC, Melke J, Toro R, Regnault B, Fauchereau F, Mercati O, Lemière N, Skuse D, Poot M, Holt R, Monaco AP, Järvelä I, Kantojärvi K, Vanhala R, Curran S, Collier DA, Bolton P, Chiocchetti A, Klauck SM, Poustka F, Freitag CM, Waltes R, Kopp M, Duketis E, Bacchelli E, Minopoli F, Ruta L, Battaglia A, Mazzone L, Maestrini E, Sequeira AF, Oliveira B, Vicente A, Oliveira G, Pinto D, Scherer SW, Zelenika D, Delepine M, Lathrop M, Bonneau D, Guinchat V, Devillard F, Assouline B, Mouren M-C, Leboyer M, Gillberg C, Boeckers TM, Bourgeron T. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012;8:e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Salisbury DF, et al. SME. Fusiform gyrus volume reduction in first-episode schizophrenia: A magnetic resonance imaging study. Arch. Gen. Psychiatry. 2002;59:775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. doi: http://dx.doi.org/10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr. Opin. Neurobiol. 2012;22:537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the expanded brief psychiatric rating scale. Schizophr Bull. 1986;12:594–602. [Google Scholar]
- Malhotra D, McCarthy S, Michaelson JJ, Vacic V, Burdick KE, Yoon S, Cichon S, Corvin A, Gary S, Gershon ES, Gill M, Karayiorgou M, Kelsoe JR, Krastoshevsky O, Krause V, Leibenluft E, Levy DL, Makarov V, Bhandari A, Malhotra AK, McMahon FJ, Nöthen MM, Potash JB, Rietschel M, Schulze TG, Sebat J. High Frequencies of De Novo CNVs in Bipolar Disorder and Schizophrenia. Neuron. 2011;72:951–963. doi: 10.1016/j.neuron.2011.11.007. doi: http://dx.doi.org/10.1016/j.neuron.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Rinaldi T, Markram K. The intense world syndrome--an alternative hypothesis for autism. Front. Neurosci. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: A postmortem study. Am. J. Psychiatry. 2000;157:40–47. doi: 10.1176/ajp.157.1.40. [DOI] [PubMed] [Google Scholar]
- Milne E. Increased intra-participant variability in children with autistic spectrum disorders: evidence from single-trial analysis of evoked EEG. Front. Psychol. 2011;2:51. doi: 10.3389/fpsyg.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modahl C, Green LA, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol. Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. doi: http://dx.doi.org/10.1016/S0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm. Behav. 2012;61:340–350. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HF, Achim A, Laur A, Buchbinder A. Topography and possible physiological significance of EEG amplitude variability in psychosis. Acta Psychiatr. Scand. 1986;73:665–675. doi: 10.1111/j.1600-0447.1986.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas J, Bovet P. Autism in schizophrenia revisited. Compr. Psychiatry. 1991;32:7–21. doi: 10.1016/0010-440x(91)90065-k. doi: http://dx.doi.org/10.1016/0010-440X(91)90065-K. [DOI] [PubMed] [Google Scholar]
- Peykov S, Berkel S, Schoen M, Weiss K, Degenhardt F, Strohmaier J, Weiss B, Proepper C, Schratt G, Nothen MM, Boeckers TM, Rietschel M, Rappold GA. Identification and functional characterization of rare SHANK2 variants in schizophrenia. Mol Psychiatry. 2015 doi: 10.1038/mp.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Müller R-A, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform `face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol. Psychiatry. 2003;53:1099–1112. doi: 10.1016/s0006-3223(02)01784-5. doi: http://dx.doi.org/10.1016/S0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TA, Rubia K, Bullmore ET, Soni W, Suckling J, Brammer MJ, Simmons A, Williams SCR, Sharma T. Exploring the social brain in schizophrenia: Left prefrontal underactivation during mental state attribution. Am. J. Psychiatry. 2000;157:2040–2042. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 Auditory Event-Related Potentials in First-Hospitalized and Chronic Schizophrenia. Schizophr. Bull. 2009 doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Müller-Gärtner HW. Differential amygdala activation in schizophrenia during sadness. Schizophr. Res. 1998;34:133–142. doi: 10.1016/s0920-9964(98)00085-1. doi: http://dx.doi.org/10.1016/S0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Romanski LM, Tsatsanis KD. Neurofunctional models of autistic disorder and Asperger syndrome: Clues from neuroimaging. In: Klin A, Volkmar FR, Sparrow SS, editors. Asperger Syndrome. New York, NY, US: Guilford Press; 2000. pp. 172–209. [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee Y-H, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimäki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King M-C, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong Association of De Novo Copy Number Mutations with Autism. Sci. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T. Neural circuit dysfunction in schizophrenia: Insights from animal models. Neuroscience. doi: 10.1016/j.neuroscience.2015.06.059. n.d. doi: http://dx.doi.org/10.1016/j.neuroscience.2015.06.059. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Berten S, Essex B, Kovacs I, Susmaras T, Little DM. A fMRI examination of visual integration in schizophrenia. J. Integr. Neurosci. 2009;08:175–202. doi: 10.1142/s0219635209002113. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. doi: http://dx.doi.org/10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Streit M, Ioannides A, Sinnemann T, Wölwer W, Dammers J, Zilles K, Gaebel W. Disturbed facial affect recognition in patients with schizophrenia associated with hypoactivity in distributed brain regions: a magnetoencephalographic study. Am. J. Psychiatry. 2001;158:1429–1436. doi: 10.1176/appi.ajp.158.9.1429. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism Spectrum Disorders and Schizophrenia: Meta-Analysis of the Neural Correlates of Social Cognition. PLoS One. 2011;6:e25322. doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Reichenberg AMC, et al. FAmily history of schizophrenia and bipolar disorder as risk factors for autism. Arch. Gen. Psychiatry. 2012;69:1099–1103. doi: 10.1001/archgenpsychiatry.2012.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced Dendritic Spine Density in Auditory Cortex of Subjects with Schizophrenia. Neuropsychopharmacology. 2008;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Luna B, Minshew NJ, Sweeney JA. Visual Motion Processing and Visual Sensorimotor Control in Autism. J. Int. Neuropsychol. Soc. 2014;20:113–122. doi: 10.1017/S1355617713001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 3-Dimensional proportional system: an approach to cerebral imaging. New York: Thieme; 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Toal F, Daly EM, Page L, Deeley Q, Hallahan B, Bloemen O, Cutter WJ, Brammer MJ, Curran S, Robertson D, Murphy C, Murphy KC, Murphy DGM. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol. Med. 2010;40:1171–1181. doi: 10.1017/S0033291709991541. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr. Opin. Neurobiol. 2013;23:283–290. doi: 10.1016/j.conb.2012.11.004. doi: http://dx.doi.org/10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr. Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. doi: http://dx.doi.org/10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Vattikuti S, Chow CC. A computational model for cerebral cortical dysfunction in autism spectrum disorders. Biol. Psychiatry. 2010;67:672–678. doi: 10.1016/j.biopsych.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz H-P, Nenadic I, Gaser C, Rammsayer T, Häger F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001:12. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Woodruff PWR, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SCR, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM. Auditory Hallucinations and the Temporal Cortical Response to Speech in Schizophrenia: A Functional Magnetic Resonance Imaging Study. Am. J. Psychiatry. 1997;154:1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive Association of the Oxytocin Receptor Gene (OXTR) with Autism in the Chinese Han Population. Biol. Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. doi: http://dx.doi.org/10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.