Abstract
Background
Understanding the seminal complications leading to death after pediatric cardiac surgery may provide opportunities to reduce mortality. We analyzed all mortalities at two pediatric cardiac surgical programs and developed a method to identify the seminal complications and modes of death.
Methods
Trained nurses abstracted all cases of in-hospital mortality meeting inclusion criteria from each site over 5 years (2008–2012). Complication definitions were consistent with those of a multi-center clinical registry. An adjudication committee assigned a seminal complication in each case (the complication initiating the cascade of events leading to death). Seminal complications were grouped into categories to designate “mode of death”. The epidemiology of seminal complications and mode of death was described.
Results
In 191 subjects, low cardiac output syndrome (LCOS, 71% of all subjects), cardiac arrest (52%), and arrhythmia (48%) were the most common complications. The committee assigned LCOS (30%), failure to separate from bypass (16%), and cardiac arrest (12%) most frequently as seminal complications. Seminal complications occurred a median 2 hours (interquartile range, [IQR] 0–35 hours) post-operatively. Patients experienced a median 7 (IQR, 3–12) additional complications prior to death at median 15 days (IQR, 4–46). Systemic circulatory failure was the most common mode of death (51%), followed by inadequate pulmonary blood flow (13%) and cardiac arrest (12%).
Conclusions
Seminal complications occurred early post-operatively and systemic circulatory failure was the most common mode of death. Our classification system is likely scalable for subsequent multi-center analysis to understand cause-specific mortality variation across hospitals and drive quality improvement.
Keywords: Congenital Heart Disease, Cardiac Surgery, Complications, Postoperative Care, Outcomes
Despite advances in operative technique and perioperative care, surgical mortality for children undergoing complex congenital cardiac surgery remains high, with rates approaching 25% for more complex procedures like the Norwood operation [1, 2]. Furthermore, significant variation in postoperative mortality exists across centers, suggesting opportunities for continued improvement [3, 4].
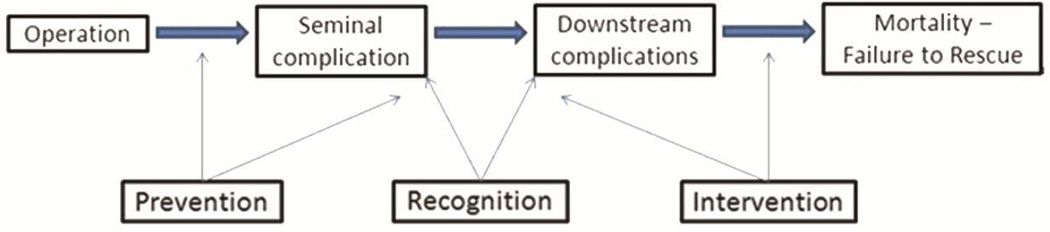
For adults undergoing cardiac surgery, postoperative mortality has declined significantly [5], and this improvement resulted in part from research elucidating high-leverage postoperative complications and the drivers of differences in mortality between low- and high-performing surgeons [6, 7]. Methods to identify the seminal complication or “mode of death” – the first complication that begins a cascade of downstream events ultimately leading to death (Figure 1) – served as the foundation for successful quality improvement initiatives to improve mortality rates. In contrast, the epidemiology of seminal complications leading to death after pediatric cardiac surgery is not well established. Further, the existing literature [8] has not been translated into actionable quality improvement. Determining the modes of death, cause-specific mortality rates, and variation in these rates across centers could inform efforts to reduce preventable deaths for children undergoing cardiac surgery.
Figure 1.
Conceptual model of postoperative complications leading to mortality
In this context, we performed a detailed analysis to characterize post-operative complications leading to mortality at two high-volume pediatric cardiac surgical centers. We aimed to identify the seminal complication that ultimately led to mortality using standardized definitions from a multi-institutional clinical registry - the Pediatric Cardiac Critical Care Consortium (PC4) [9] - in order to facilitate future research efforts intended to characterize causes of perioperative mortality, describe variation between centers, and highlight targets for quality improvement.
Material and Methods
Setting
This analysis was performed at The Children’s Hospital of Philadelphia and the University of Michigan C.S. Mott Children’s Hospital. Both centers participate in the Pediatric Cardiac Critical Care Consortium (PC4), a quality improvement collaborative for children with critical cardiovascular disease maintaining a clinical registry on all surgical patients admitted to the cardiac intensive care unit (CICU) pre- and post-operatively. We undertook this study to inform a subsequent prospective investigation of variation in mode of death across PC4 centers.
Case selection
We used surgical registry data collected for the Society of Thoracic Surgeon’s (STS) Congenital Heart Surgery Database to identify every in-hospital mortality after cardiothoracic surgery between 1/1/2008 and 12/31/2012 at both hospitals. We excluded cases from analysis according to the following criteria: 1) patient died in the operating room, 2) postoperative care not provided in the CICU, 3) procedure other than cardiovascular surgery or thoracic surgery with cardiopulmonary bypass, 4) primary procedure was PDA ligation in neonate <2.5kg, or 5) the patient’s only surgical procedure was placement of temporary pacemaker wires for congenital heart block and fetal hydrops. Both Institutional Review Boards provided approval for the study without the need for written informed consent prior to data collection.
Data collection
Our methodology largely mirrors the seminal work of O’Connor [6], and methods explored by Ma [8] in a congenital cardiac population. In each of these analyses patient charts were abstracted and from these data expert clinicians assigned a mode of death. Mode of death was the complication or condition that represented the primary reason for the patient’s death.
Chart abstraction forms were created and included pre-operative characteristics, comorbidities, anatomy, all major invasive procedures, and intraoperative variables from the index cardiothoracic operation. We defined prematurity in neonates as gestational age at birth <39 weeks [10]. We classified surgical complexity using the Society of Thoracic Surgeons- European Association for Cardiothoracic Surgery (STAT) mortality categories [11]. Complications were defined according to the PC4 data dictionary, which will be the data source for follow-up investigation. The definition of almost every shared field matches that of the STS database. The investigators defined low cardiac output syndrome and complications not currently collected by either registry in the study manual of operations.
Trained cardiac surgical research nurses with clinical experience in caring for postoperative pediatric cardiac surgical patients reviewed each chart including physician notes, laboratory data, flowsheets, and imaging reports. The abstractors recorded every defined postoperative complication and the date and time the complication occurred. Complications could be recorded multiple times. Investigators held regular meetings with the teams to review definitions, discuss coding difficulties, and ensure consistency between sites.
Identifying the seminal complication
After determining the sequence of complications the abstractor was asked to code the seminal complication as described by O’Connor et al [6]. The seminal complication is that which starts the cascade of clinical events that ultimately lead to a patient’s demise, and frequently is not the terminal condition immediately preceding death. For example, a patient undergoing arterial switch operation may fail to separate from cardiopulmonary bypass necessitating mechanical circulatory support. While that patient may eventually be weaned, if he/she subsequently develops sepsis and multiorgan failure, the initial complication of failure to separate from bypass would be considered the seminal complication. The conceptual framework for this approach suggests that downstream complications are likely the result of those occurring earlier. In the previous example, the patient who requires extracorporeal membrane oxygenation (ECMO) assumes greater risk for infection given the dependence on invasive devices, tissue damage, inflammation, delay in feeding, and multiple interventions. Sepsis and multiorgan failure likely would not have occurred if the patient separated easily from bypass and did not require ECMO.
In most cases, the seminal complication was one of the first to occur after the initial index cardiothoracic surgery. For patients undergoing a second planned operation after the index procedure during the same hospitalization (e.g. superior cavopulmonary anastomosis after stage 1 palliation for functional single ventricle), we only considered complications occurring after the second procedure as possible seminal complications. We believed patients who survived a first operation to undergo an elective planned operation with an intervening period of clinical stability were most likely to die as the result of an event occurring after the most recent surgery. For patients having unplanned surgical re-intervention we assessed complications after the index surgery.
In the unique situation where a patient was cannulated to ECMO in the operating room, this was always selected as the seminal complication and was termed “failure to separate from bypass.” We did not attempt to determine the specific etiology for failure to separate. All other events leading to mechanical circulatory support were preceded by another complication, and that complication was selected as the seminal complication.
After assignment of the seminal complication by the abstractors, the investigators convened an adjudication meeting to confirm or change the seminal complication designation. Three pediatric cardiac surgeons, two cardiologists, and one cardiac intensivist met in-person to review the data abstraction forms and perform a limited secondary review of the medical record. The adjudication committee rendered its decision on the seminal complication independently, and agreed unanimously on all cases.
Classifying mode of death
Following the methodology of O’Connor [6], seminal complications were grouped into categories based on consensus among the clinician investigators (see AppendixTable 1): systemic circulatory failure, inadequate pulmonary blood flow, cardiac arrest, postoperative hemorrhage, respiratory failure, arrhythmia, and other. These categories represent the mode of death.
Analysis
Standard descriptive statistics were used to characterize the overall cohort and frequencies of complications. Differences in seminal complication designation between the nurse abstractors and clinical adjudication committee are presented, and an overall percentage agreement calculated. Since systemic circulatory failure was the most common mode of death seen in just over half the patients, we performed univariate comparisons of patient and operative variables between those with systemic circulatory failure and those with other modes; Chi-square and fisher’s exact tests for categorical variables, and Wilcoxon rank-sum test for continuous variables. All analyses were performed using SAS Version 9.4 (SAS Institute Inc, Cary, NC), with statistical significance at a p-value less than 0.05.
Results
Across both institutions there were 205 in-hospital mortalities during the study period. Of these, 14 were excluded based on the defined criteria: 4 patients died in the operating room, 5 patients did not receive care in the CICU post-operatively, 3 patients had only placement of temporary pacing wires for congenital heart block and hydrops fetalis, and two patients did not have a qualifying surgery.
Characteristics of the 191 patients included for analysis are shown in Table 1. Neonates and infants made up 87% of the total cohort. Pre-operative shock, mechanical circulatory support, and cardiac arrest occurred infrequently. Most patients (n=136, 71%) were classified in STAT categories 4 or 5, or had unassignable primary procedures.
Table 1.
Overall cohort characteristics
| Characteristic | N(%), Med (IQR) |
|---|---|
| Patient/Pre-operative | |
| Age, days | 13 (5–129) |
| Neonate (0–30 days) | 110 (57%) |
| Infant (1 mo – 1 year) | 57 (30%) |
| Child (1 – 18 years) | 24 (13%) |
| Weight for age z-score | |
| < −2 | 81 (42%) |
| −2 to +2 | 108 (57%) |
| >2 | 2 (1%) |
| Hospital | |
| Philadelphia | 95 (50%) |
| Michigan | 96 (50%) |
| Non-cardiac abnormalities | |
| Genetic abnormality | 18 (9%) |
| Extracardiac anomaly | 35 (18%) |
| Syndrome | 39 (20%) |
| Prematurity (<39 weeks gestational age) if neonate | 71/110 (65%) |
| 37–39 weeks | 40 (36%) |
| <37 weeks | 31 (28%) |
| Pre-operative risk factors | |
| Cardiac arrest | 4 (2%) |
| Pre-op MCS | 2 (1%) |
| Shock at surgery | 1 (1%) |
| Shock, resolved | 10 (5%) |
| Mechanical ventilation | 51 (27%) |
| Previous cardiothoracic surgery | 38 (20%) |
| Pre-operative non-surgical intervention during hospitalization | 38 (20%) |
| Operative | |
| STAT category (or score) | |
| 1 | 4 (2%) |
| 2 | 23 (12%) |
| 3 | 28 (15%) |
| 4 | 83 (43%) |
| 5 | 45 (24%) |
| Unassignable | 8 (4%) |
| Primary procedure (groups) | |
| Hypoplastic left heart and related | 44 (23%) |
| Palliative procedures | 27 (14%) |
| Right heart lesions | 20 (10%) |
| Thoracic arteries/veins | 17 (9%) |
| Pulmonary venous anomalies | 14 (7%) |
| Septal defects | 14 (7%) |
| Transposition of the great arteries | 12 (6%) |
| Other single ventricle | 11 (6%) |
| Left heart lesions | 9 (5%) |
| Ventricular assist device | 9 (5%) |
| Truncus arteriosus | 4 (2%) |
| Double outlet right ventricle | 3 (2%) |
| Conduit operations | 2 (1%) |
| Electrophysiological | 2 (1%) |
| Pericardial disease | 1 (1%) |
| Thoracic and mediastinal disease | 1 (1%) |
| Miscellaneous procedures | 1 (1%) |
| Primary procedure | |
| Single ventricle, with arch involvement | 43 (23%) |
| Single ventricle, no arch involvement | 34 (18%) |
| Two ventricle, with arch involvement | 14 (7%) |
| Two ventricle, no arch involvement | 100 (52%) |
| Cardiopulmonary bypass time, minutes | 106 (73–158) |
MCS, mechanical circulatory support; STAT, Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery
Epidemiology of all postoperative complications
Table 2 shows the percentage of patients who experienced each complication at least once postoperatively. Low cardiac output syndrome (LCOS), cardiac arrest, arrhythmia, need for mechanical circulatory support, and chronic respiratory failure were the most common complications. Also shown in Table 2 is the epidemiology of the initial complication for each patient. LCOS was the most common initial complication, followed by failure to separate from bypass and arrhythmia.
Table 2.
Frequency of complications
| Complication | Ever among all patients, N (%) |
As initial complication, N (%) |
|---|---|---|
| Cardiac and operative | ||
| Low cardiac output syndrome | 135 (71%) | 73 (38%) |
| Need for MCS | 88 (46%) | 30 (16%) |
| Cardiac arrest | 99 (52%) | 12 (6%) |
| Unplanned cardiac re-intervention | 62 (32%) | 2 (1%) |
| Pulmonary hypertension | 18 (9%) | 9 (5%) |
| Hypoxemia | 53 (28%) | 11 (6%) |
| Pericardial effusion | 9 (5%) | 0 |
| Arrhythmia | 92 (48%) | 25 (13%) |
| CHB requiring PPM | 3 (2%) | 0 |
| Hemorrhagic/Thrombotic | ||
| Bleeding requiring transfusion | 40 (21%) | 7 (4%) |
| Bleeding requiring operation | 27 (14%) | 4 (2%) |
| Thrombosis | 28 (15%) | 0 |
| Shunt thrombosis | 5 (3%) | 0 |
| Respiratory | ||
| Acute respiratory failure | 41 (21%) | 3 (2%) |
| Chronic respiratory failure | 83 (43%) | 1 (1%) |
| Chylothorax | 28 (15%) | 1 (1%) |
| Pleural effusion requiring drainage | 47 (25%) | 1 (1%) |
| Prolonged pleural drainage | 18 (9%) | 0 |
| Vocal cord paralysis | 4 (2%) | 0 |
| Diaphragm paralysis | 1 (1%) | 0 |
| Neurologic | ||
| Seizure | 42 (22%) | 0 |
| Stroke | 16 (8%) | 0 |
| Hypoxic-ischemic encephalopathy | 37 (19%) | 0 |
| Renal | ||
| Acute kidney injury | 51 (27%) | 2 (1%) |
| Acute renal failure requiring dialysis | 43 (23%) | 0 |
| Other | ||
| Necrotizing enterocolitis | 17 (9%) | 1 (1%) |
| Hospital acquired infection | 74 (39%) | 0 |
| Hepatic failure | 28 (9%) | 0 |
| Other | 122 (64%) | 9 (5%) |
CHB, Complete heart block; MCS, mechanical circulatory support; PPM, permanent pacemaker
Epidemiology of seminal complications
Table 3 illustrates the frequency of seminal complications designated by the clinical adjudication committee and compared to the nurse abstractors. The seminal complication (assigned by the committee) was most commonly LCOS, failure to separate from bypass, or cardiac arrest. These were also the three most common chosen by the nurse abstractors, but there were differences on individual cases. The clinical adjudication committee and the nurse abstractors agreed in 66% of cases; agreement includes both complication type and timing (i.e. if a patient had multiple episodes of LCOS, both the committee and abstractor chose the same episode).
Table 3.
Seminal complications
| Complication | N (%) seminal complication by adjudication commmittee |
N (%) seminal complication by abstractor |
|---|---|---|
| Cardiac and operative | ||
| Low cardiac output syndrome | 57 (30%) | 78 (41%) |
| Failure to separate from bypass | 31 (16%) | 30 (16%) |
| Cardiac arrest | 22 (12%) | 18 (9%) |
| Unplanned cardiac re-intervention | 7 (4%) | 4 (2%) |
| Pulmonary hypertension | 12 (6%) | 7 (4%) |
| Hypoxemia | 13 (7%) | 14 (7%) |
| Arrhythmia | 8 (4%) | 18 (9%) |
| Hemorrhagic/Thrombotic | ||
| Bleeding requiring transfusion | 4 (2%) | 4 (2%) |
| Bleeding requiring operation | 6 (3%) | 1 (1%) |
| Thrombosis | 1 (1%) | 3 (2%) |
| Shunt thrombosis | 1 (1%) | 0 |
| Respiratory | ||
| Acute respiratory failure | 3 (2%) | 5 (3%) |
| Chronic respiratory failure | 9 (5%) | 1 (1%) |
| Chylothorax | 3 (2%) | 2 (1%) |
| Diaphragm paralysis | 1 (1%) | 0 |
| Neurologic | ||
| Stroke | 1 (1%) | 0 |
| Hypoxic-ischemic encephalopathy | 0 | 1 (1%) |
| Renal | ||
| Acute kidney injury | 1 (1%) | 1 (1%) |
| Other | ||
| Necrotizing enterocolitis | 1 (1%) | 1 (1%) |
| Hospital acquired infection | 1 (1%) | 1 (1%) |
| Hepatic failure | 0 | 1 (1%) |
| Other | 9 (5%) | 1 (1%) |
The seminal complication was the initial postoperative complication in only 66% of cases. Seminal complications occurred at median 2 hours (interquartile range [IQR], 0–35 hours) after admission to the CICU. In 36% of cases the seminal complication was present at CICU arrival, and in 73% the seminal complication occurred within the first 24 hours. The median time from seminal complication to death was 15 days (IQR, 4 – 46 days), and patients experienced a median of 7 (IQR, 3–12) additional complications prior to death.
Mode of death
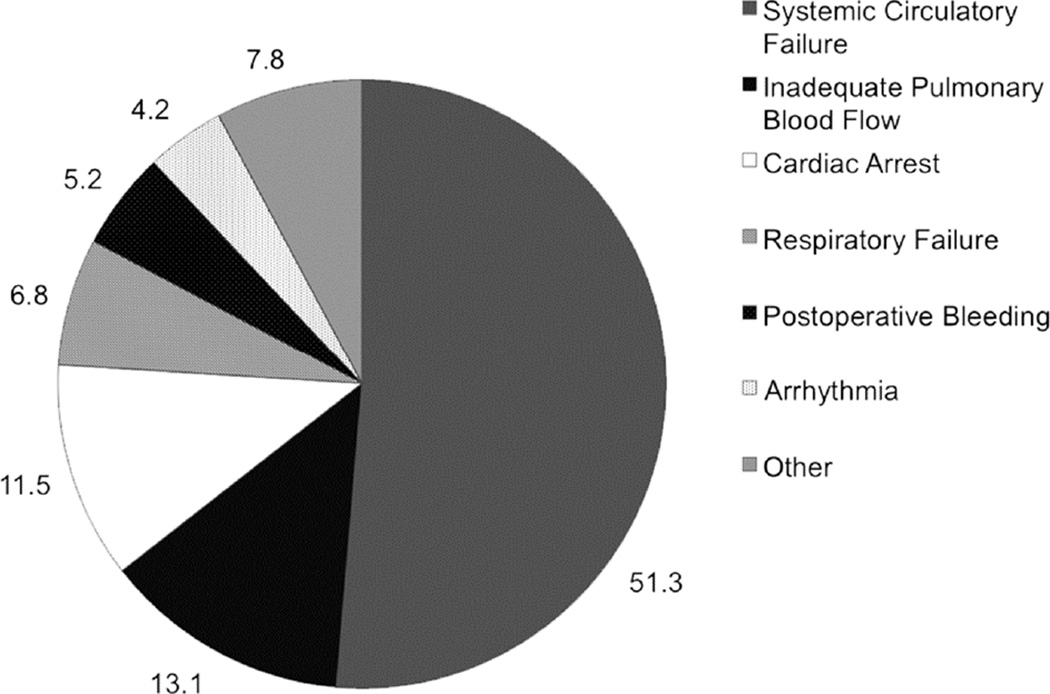
Individual complications were grouped into categories defining mode of death as described above. As shown in Figure 2, systemic circulatory failure was the most common mode of death, followed by inadequate pulmonary blood flow and cardiac arrest. Table 4 shows the comparison of patients with systemic circulatory failure to those in all other mode of death categories. Patients with systemic circulatory failure had longer median total bypass time (130 vs. 91 minutes, p<0.0001) and were less likely to have undergone a pre-operative non-surgical intervention (14% vs. 26%, p=0.046) than patients in other mode of death categories. Age, preoperative risk factors, non-cardiac anomalies, and surgical complexity were similar between the groups.
Figure 2.
Distribution of modes of death (%, N=191)
Table 4.
Characteristics of patients with circulatory failure vs. others
| Characteristic | Patients with circulatory failure (N=98) |
Patients with other mode of death (N=93) |
p‡ |
|---|---|---|---|
| Patient/Pre-operative | |||
| Age | 0.41 | ||
| Neonate (0–30 days) | 60 (61%) | 50 (54%) | |
| Infant (1 month – 1 year) | 25 (26%) | 32 (34%) | |
| Child (1 – 18 years) | 13 (1413%) | 11 (12%) | |
| Weight for age z-score | 0.07 | ||
| < −2 | 36 (37%) | 56 (60%) | |
| −2 to +2 | 60 (61%) | 47 (51%) | |
| >2 | 2 (2%) | 0 | |
| Hospital | 0.03 | ||
| Philadelphia | 41 (42%) | 54 (58%) | |
| Michigan | 57 (58%) | 39 (42%) | |
| Non-cardiac abnormalities | |||
| Genetic abnormality | 8 (8%) | 10 (11%) | 0.54 |
| Extracardiac anomaly | 15 (15%) | 20 (22%) | 0.27 |
| Syndrome | 18 (18%) | 21 (23%) | 0.45 |
| Prematurity (<39 wks) if neonate | 37/60 (62%) | 34/50 (68%) | 0.40 |
| Pre-operative risk factors | |||
| Cardiac arrest | 1 (1%) | 3 (3%) | 0.36 |
| Pre-operative MCS | 0 | 2 (2%) | 0.24 |
| Shock at surgery | 0 | 1 (1%) | 0.49 |
| Shock, resolved | 5 (5%) | 5 (5%) | 1.00 |
| Mechanical ventilation | 25(25%) | 26 (28%) | 0.70 |
| Previous cardiothoracic surgery | 18 (18%) | 20 (22%) | 0.56 |
| Pre-operative non-surgical intervention during hospitalization | 14 (14%) | 24 (26%) | 0.046 |
| Operative | |||
| STAT category | 0.70 | ||
| 1–3 | 27 (28%) | 28 (30%) | |
| 4–5 and unassigned | 71 (72%) | 65 (70%) | |
| Primary procedure (groups) | 0.35 | ||
| Single ventricle, arch | 26 (27%) | 17 (18%) | |
| Single ventricle, no arch | 14 (14%) | 20 (21%) | |
| Two ventricle, arch | 6 (6%) | 8 (9%) | |
| Two ventricle, no arch | 52 (53%) | 48 (52%) | |
| Cardiopulmonary bypass time, minutes | 130 (91–196) | 91 (71–119) | <0.0001 |
Chi-square/fisher’s exact for categorical; Wilcoxon rank-sum for continuous
MCS, mechanical circulatory support; STAT, Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery
Comment
We performed a detailed analysis characterizing the epidemiology of postoperative complications from all in-hospital mortalities at two large pediatric cardiac surgical centers over five years. In this analysis, the seminal complication usually occurred within 24 hours after postoperative admission to the CICU, and patients experienced several downstream complications thereafter prior to death. Systemic circulatory failure was the most common mode of death, occurring in approximately half of the overall cohort. Important case mix factors such as age, comorbidities, and surgical complexity were similarly distributed among those with systemic circulatory failure and all other modes of death.
The experience of the Northern New England Cardiovascular Disease Study Group suggests that understanding the epidemiology of postoperative complications and analyzing variation in mode of death across hospitals can be a cornerstone for effective initiatives to reduce operative mortality [7]. Their analysis of mode of death after coronary artey bypass grafting demonstrated that variation in low output heart failure accounted for 80% of the difference in surgeon mortality rates within the collaborative [6]. Armed with this information, clinicians and researchers were able to focus on the key drivers of death from low output heart failure, and discovered the practices of high performing surgeons and hospitals that positively impacted prevention, identification, and treatment of this seminal complication. After disseminating these practices throughout the region the group achieved significant and longlasting reductions in operative mortality [7, 12].
Based on this roadmap for successful quality improvement in cardiac surgical care, we sought to develop a methodology for analyzing the seminal complication and mode of death after pediatric cardiac surgery in a way that can be scaled up for analysis in a multi-institutional quality collaborative. We believe that understanding variation in mode of death across hospitals may provide actionable data to help elucidate the hospital structures and processes that underlie high quality pediatric cardiac surgical care.
Several important insights were gleaned from this study to inform future research efforts. Our findings suggest that we cannot simply assign the first complication chronologically as the seminal complication; one-third of the seminal complications adjudicated by the clinicians were not the initial complication. Secondly, this study illustrates the necessity of the adjudication committee review; the committee changed the seminal complication in 33% of cases based on a different interpretation of the clinical events. Similarly, in O’Connor’s study [6] the seminal complication could be assigned according to coding rules in only 40% of cases, while the clinical endpoints committees assigned mode of death in the remainder. Clinical adjudication is a logistical challenge for a multi-center study, but our analysis and previous work suggest that simpler methods may not lead to accurate classification of cases.
Our study represents a necessary initial step in a larger effort to characterize the epidemiology of postoperative mortality after pediatric cardiac surgery and plan interventions to reduce preventable deaths. However, our analysis remains limited in important ways, in part due to the heterogeneity of the patient population with regard to age and disease. We have characterized the mode of death using observable variables in the medical record captured in a clinical registry, but there are additional factors that might provide a deeper view into the etiology of perioperative mortality, and may be lesion and/or procedure specific. For example, systemic circulatory failure could be due to severe pre-operative myocardial dysfunction, poor myocardial preservation during bypass, residual cardiac lesions, or ineffective recognition or treatment of postoperative circulatory failure. Further study must identify the high-leverage modes of death driving the difference between low-and high-mortality hospitals. Ultimately, this will inform further study of specific etiologies of complications, and the clinical practices and hospital factors that lead to high quality care.
Our study is also limited similar to previous mode of death analyses by not including survivors. Determining the frequency of complications in all patients is necessary to understand whether low-mortality hospitals achieve these results through prevention or treatment of key complications. Recent literature suggests that a hospital’s response to complications may be of equal or greater importance than prevention. Failure-to-rescue (mortality rate among patients with a complication) explains much of the variation in overall perioperative mortality between hospitals across multiple surgical specialties, including pediatric cardiac surgery [4, 13]. Future analyses that demonstrate how high-performing hospitals achieve better rescue rates are necessary to create actionable data that can be disseminated to drive quality improvement. Since all events and therapies are time-stamped in the PC4 database, it will be possible in subsequent studies to delineate the frequency and sequence of all postoperative complications. This will facilitate calculation of cause-specific mortality and failure-to-rescue rates since the frequency of complications will be known in survivors and non-survivors. Analysis of cause-specific failure-to-rescue in a multi-institutional dataset would be unique and could provide valuable insight that accelerates improvements in perioperative care for pediatric cardiac surgical patients.
Finally, aspects of clinical decision-making - not captured in this study or any clinical registry – may profoundly impact patient outcome: was the correct surgery selected among the different options? Was the timing of the procedure optimal given the patient’s physiology and comorbidities? These questions and many others must be addressed through empirical analyses and collaborative learning approaches. These future iterations can be accomplished more effectively if the approach we present here is repeated across a multi-institutional dataset providing guidance for more refined investigation.
In conclusion, it remains unknown how mode of death after pediatric cardiac surgery varies across hospitals, and determining this variation could provide targets to improve clinical outcomes. We present a method for classifying the seminal complication and mode of death for children undergoing cardiac surgery that will be applied prospectively within a multi-institutional quality collaborative. Similar research efforts have proved invaluable in adult cardiac surgery quality improvement, and if replicated will perhaps lead to important advances in the pediatric realm as well.
Supplementary Material
Acknowledgments
This work is supported by NHLBI 1K08HL116639 (PI – Gaies) and internal grants from the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasquali SK, He X, Jacobs JP, et al. Measuring hospital performance in congenital heart surgery: administrative versus clinical registry data. Ann Thorac Surg. 2015;99(3):932–938. doi: 10.1016/j.athoracsur.2014.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs JP, O'Brien SM, Pasquali SK, et al. Variation in outcomes for benchmark operations: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2011;92(6):2184–2191. doi: 10.1016/j.athoracsur.2011.06.008. discussion 2191-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs JP, O'Brien SM, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 2-Clinical Application. Ann Thorac Surg. 2015 doi: 10.1016/j.athoracsur.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquali SK, He X, Jacobs JP, Jacobs ML, O'Brien SM, Gaynor JW. Evaluation of failure to rescue as a quality metric in pediatric heart surgery: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94(2):573–579. doi: 10.1016/j.athoracsur.2012.03.065. discussion 579-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor GT, Birkmeyer JD, Dacey LJ, et al. Results of a regional study of modes of death associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 1998;66(4):1323–1328. doi: 10.1016/s0003-4975(98)00762-0. [DOI] [PubMed] [Google Scholar]
- 7.Nugent WC. Building and supporting sustainable improvement in cardiac surgery: the Northern New England experience. Semin Cardiothorac Vasc Anesth. 2005;9(2):115–118. doi: 10.1177/108925320500900202. [DOI] [PubMed] [Google Scholar]
- 8.Ma M, Gauvreau K, Allan CK, Mayer JE, Jr, Jenkins KJ. Causes of death after congenital heart surgery. Ann Thorac Surg. 2007;83(4):1438–1445. doi: 10.1016/j.athoracsur.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 9.Gaies M, Cooper DS, Tabbutt S, et al. Collaborative quality improvement in the cardiac intensive care unit: development of the Paediatric Cardiac Critical Care Consortium (PC4) Cardiol Young. 2015;25(5):951–957. doi: 10.1017/S1047951114001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello JM, Pasquali SK, Jacobs JP, et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129(24):2511–2517. doi: 10.1161/CIRCULATIONAHA.113.005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JP, Jacobs ML, Maruszewski B, et al. Initial application in the EACTS and STS Congenital Heart Surgery Databases of an empirically derived methodology of complexity adjustment to evaluate surgical case mix and results. Eur J Cardiothorac Surg. 2012;42(5):775–779. doi: 10.1093/ejcts/ezs026. discussion 779-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connor GT, Plume SK, Morton JR, et al. Results of a regional prospective study to improve the in-hospital mortality associated with coronary artery bypass grafting. Jama. 1996;275:841–846. [PubMed] [Google Scholar]
- 13.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.