ABSTRACT
Members of the Fungi convert nitrate (NO3−) and nitrite (NO2−) to gaseous nitrous oxide (N2O) (denitrification), but the fungal contributions to N loss from soil remain uncertain. Cultivation-based methodologies that include antibiotics to selectively assess fungal activities have limitations, and complementary molecular approaches to assign denitrification potential to fungi are desirable. Microcosms established with soils from two representative U.S. Midwest agricultural regions produced N2O from added NO3− or NO2− in the presence of antibiotics to inhibit bacteria. Cultivation efforts yielded 214 fungal isolates belonging to at least 15 distinct morphological groups, 151 of which produced N2O from NO2−. Novel PCR primers targeting the p450nor gene, which encodes the nitric oxide (NO) reductase responsible for N2O production in fungi, yielded 26 novel p450nor amplicons from DNA of 37 isolates and 23 amplicons from environmental DNA obtained from two agricultural soils. The sequences shared 54 to 98% amino acid identity with reference P450nor sequences within the phylum Ascomycota and expand the known fungal P450nor sequence diversity. p450nor was detected in all fungal isolates that produced N2O from NO2−, whereas nirK (encoding the NO-forming NO2− reductase) was amplified in only 13 to 74% of the N2O-forming isolates using two separate nirK primer sets. Collectively, our findings demonstrate the value of p450nor-targeted PCR to complement existing approaches to assess the fungal contributions to denitrification and N2O formation.
IMPORTANCE A comprehensive understanding of the microbiota controlling soil N loss and greenhouse gas (N2O) emissions is crucial for sustainable agricultural practices and addressing climate change concerns. We report the design and application of a novel PCR primer set targeting fungal p450nor, a biomarker for fungal N2O production, and demonstrate the utility of the new approach to assess fungal denitrification potential in fungal isolates and agricultural soils. These new PCR primers may find application in a variety of biomes to assess the fungal contributions to N loss and N2O emissions.
INTRODUCTION
Denitrification is a key process responsible for loss of fixed nitrogen (N) in soils and sediments and is mediated by both abiotic and microbial processes (1, 2). Of the microorganisms involved in denitrification, members of the Bacteria are well studied and considered key contributors; however, some saprotrophic fungi also conserve energy from the reduction of nitrate (NO3−) or nitrite (NO2−) to nitrous oxide (N2O), the main end product of fungal denitrification (3–5). Fungi have been implicated in N turnover for over 30 years (1, 3, 6), but the ecological importance of fungal contributions to denitrification remain uncertain. Potential roles of fungi in soil N loss and greenhouse gas (i.e., N2O) emission are underexplored, and monitoring tools (e.g., quantitative or endpoint PCR) to specifically address the presence and abundance of denitrifying fungi are largely lacking. To date, the majority of the known denitrifying fungal isolates reside in the phylum Ascomycota (7–9). Although some reports suggest that fungi within the Basidiomycota and Zygomycota are denitrifiers (8–10), only a few isolates are available, and it remains to be confirmed if members of other phyla harbor nar, nap, nir, and nor gene clusters implicated in steps of the denitrification pathway. Previous investigations of fungal denitrification have relied on laboratory cultivation and substrate-induced respiration inhibition (SIRIN) tests (3, 7, 10). The SIRIN technique applies NO3− or NO2− in concert with broad-spectrum antibiotics (e.g., streptomycin) or fungicides (e.g., cycloheximide) to partition the bacterial and fungal contributions to N2O or dinitrogen (N2) formation. These efforts suggested that fungi play a large but unrecognized role in N turnover and N2O flux (9, 11–13). Although the SIRIN methodology has provided valuable insight into the fungal contribution to denitrification, the application of SIRIN to soils can be problematic as incomplete inhibition can bias the observations (14–16). Hence, alternative approaches (e.g., PCR) that selectively target fungal genes involved in denitrification are desirable to advance understanding of the fungal diversity contributing to N cycling in environments such as soils, aquifers, and deep-ocean sediments, where denitrifying fungi have been cultivated (7, 13, 17, 18).
Denitrifying fungi possess a unique protein of the CYP55 family of P450 cytochromes, cytochrome P450 nitric oxide reductase (P450nor). P450nor lacks mono-oxygenase activity and a conserved N-terminal membrane anchor domain found in other eukaryotic P450 cytochromes (19). It directly binds the electron donor NAD(P)H but otherwise is structurally conserved and shares similarity with other cytochrome P450 proteins (19). P450nor is responsible for the reduction of nitric oxide to N2O, the dominant end product, although N2 formation has been observed (5, 7, 20). Denitrifying bacteria and archaea are also capable of reducing nitric oxide to N2O but possess a distinct gene locus (norB) that encodes two isozymes, cNor and qNor, each relying on a distinct electron donor (c-type cytochrome or ubiquinol pool, respectively) (21, 22). Furthermore, only bacterial and archaeal denitrifiers possess the nosZ gene, which encodes the enzyme responsible for reduction of the greenhouse gas N2O to inert N2. Thus, the p450nor gene could serve as a diagnostic marker for fungal denitrification potential, but the lack of molecular tools targeting p450nor currently limits efforts to assess the contribution of fungi to N cycling. In the present study, a translated alignment of available p450nor sequences identified key conserved amino acid residues within P450nor and enabled the design of p450nor-specific degenerate primers. We demonstrated the broad utility of these new primers by performing PCR amplification of p450nor genes from fungal isolates and environmental DNA obtained from two agricultural soils with distinct physicochemical properties. Metagenomic data sets derived from the same agricultural soils did not reveal the presence of fungal denitrification genes within either soil. This finding emphasizes the value of cultivation-based efforts and the p450nor-targeted PCR assays for assessments of fungal contributions to denitrification in complex environmental systems such as soils.
MATERIALS AND METHODS
Medium preparation.
A modified Czapek liquid medium containing nitrate (2 mM) or nitrite (1 mM) (23) was utilized for fungal isolation and cultivation unless otherwise noted. Briefly, ultrapure water (≥18.0 MΩ · cm) (Milli-Q system; Millipore, Billerica, MA) was combined with (in g liter−1) KCl (0.5), K2SO4 (0.35), magnesium glycerophosphate (0.5), and FeCl2 (0.01), and the pH was adjusted to 6.8 by adding sodium hydroxide (0.5 M) before the medium was autoclaved.
Fungal enrichment and isolation.
Triplicate soil cores were collected in November 2012 from agricultural sites in Havana, IL (latitude 40.296, longitude −89.994), and Urbana, IL (latitude 40.075, longitude −88.242), using a soil corer with a 30-cm depth range. The soil cores were placed in coolers and transported to the University of Illinois, Urbana-Champaign, for processing. Soil aliquots (5 to 10 g) from the 0- to 5-, 5- to 20-, and 20- to 30-cm depth ranges were shipped overnight on ice to the University of Tennessee. Soil samples were pooled and homogenized using an aseptic technique, and 5 g of soil was added to 30 ml of oxic Czapek medium (pH 6.8) in 60-ml glass serum bottles fitted with black butyl rubber stoppers and incubated at room temperature. Microcosms (n = 1 per treatment) were amended with either 2 mM sodium nitrate (NO3−) or 1 mM sodium nitrite (NO2−) as the electron acceptor and either sodium acetate, sodium formate, sodium pyruvate (3 mM each), or an autoclaved 1.5% (wt/vol) milled corn and soybean plant suspension as an exogenous substrate. All the serum bottles were amended with 30 μg ml−1 chloramphenicol (Fisher Scientific, USA) and 100 μg ml−1 streptomycin sulfate (Fisher Scientific, USA) from ethanolic or aqueous stocks, respectively, to inhibit bacterial growth. After 1 month of monitoring N2O production, the soil microcosms were shaken vigorously by hand, and 0.5 ml of homogenized suspension was transferred, using a sterile 1-ml plastic syringe fitted with a 19-gauge needle (Becton Dickinson, Franklin Lakes, NJ), to fresh anoxic medium containing the same carbon and nitrogen sources as the parent microcosms. The transfer cultures (n = 2 per treatment) received 50 μg ml−1 each of kanamycin sulfate (Fisher Scientific, USA) and ampicillin sodium salt (Fisher Scientific, USA) from sterile aqueous stocks to inhibit bacteria that might have possessed natural resistance to the antibiotics added to the initial enrichments. The cultures were then amended with 0.6 ml of filter-sterilized air (2% [vol/vol] headspace volume), and the NO3−, NO2−, and N2O concentrations were monitored. Following a 2-week incubation period, 2 ml of culture samples was transferred to 25 ml liquid (45°C) agar medium containing the same substrates as the parent cultures. Each tube was gently mixed, and its contents were poured into individual sterile plastic plates. The plates were incubated in the dark at room temperature and visually inspected each day for fungal growth. After filamentous colonies were observed, approximately 1-cm2 agar sections containing fungal mycelia were transferred to fresh agar plates. Subsequent transfers (at least three) were of 1-cm2 agar sections of the fungal colony margins to quarter-strength R2A agar (Fisher Scientific, USA) plates containing 100 μg ml−1 streptomycin sulfate. Contamination was monitored by microscopic observation and avoided by routine transfer of agar sections containing hyphae of consistent appearance to fresh R2A agar plates containing streptomycin or ampicillin and kanamycin. Aspergillus terreus strain NRRL 255 and Aspergillus flavus strain NRRL 3357 were obtained from the USDA Agricultural Research Service culture collection (the NRRL Collection). Both fungi were grown on quarter-strength R2A agar (Becton Dickinson, Franklin Lakes, NJ) plates amended with 50 μg ml−1 streptomycin for tissue collection and DNA extraction (see below).
Analytical procedures.
NO3− and NO2− were measured by ion chromatography with a Dionex 3000 instrument (Dionex, Sunnyvale, CA) as described previously (24, 25). N2O was monitored via 1-ml headspace injections into a MicroGC 3000A (Agilent Technologies, Palo Alto, CA) equipped with a polystyrene-divinylbenzene PlotQ column and a thermal conductivity detector. All nitrogen species were quantified using a dilution series of standards prepared in medium. The instrument limits of detection for NO2−, NO3−, and N2O were 7, 4, and 3 μmol, as determined by preparing replicate standards (n = 20) and following established procedures (26).
Denitrification activity of fungal isolates.
Fungal isolates were tested for denitrifying potential by transferring individual 1-cm2 agar sections to 1.5-ml plastic microcentrifuge tubes containing 1 ml of sterile water, followed by brief manual homogenization with sterile steel forceps. Then, 250 μl of the water-agar-mycelium mixture was inoculated into 26-ml Balch tubes containing 15 ml of oxic Czapek medium amended with 5 mM sodium acetate, 2 mM NO2−, and 100 μg ml−1 streptomycin. The headspace of each Balch tube was monitored over a 12-day period for N2O production.
DNA extraction.
Fungal biomass (∼0.3 g [wet weight]) was placed in a 2-ml screw-cap plastic tube containing approximately 200 mg of 0.5-mm silica and zirconium beads (Fisher Scientific, USA), and DNA was extracted following an established procedure (27) (see the supplemental material for details). Soil DNA extraction from soil samples (0.5 g of homogenized soil from 0- to 30-cm depth from Havana and Urbana and a separate 0- to 5-cm-depth Havana sample) was performed using the FastDNA spin kit for soil (MP Biomedicals, Santa Ana, CA) following the manufacturer's protocol. The soil DNA concentration and purity were analyzed with a NanoDrop 2000 spectrophotometer (Thermo Scientific) and a Qubit 2.0 fluorometer (Life Technologies, Carlsbad, CA) using the dsDNA BR Assay kit (Life Technologies) following the manufacturer's recommendations.
Primer design.
To design primers targeting p450nor gene sequences, primary literature resources and the National Center for Biotechnology Information (NCBI) database were queried to identify available p450nor gene and protein sequences (28). Multiple-sequence alignment was used to identify conserved residues, followed by selection of additional sequences by querying public sequence databases (GenBank and UniProt) using BLAST (29). Overall, 38 p450nor reference sequences were obtained and aligned using the program T-Coffee (30), followed by manual inspection. PAL2NAL (31) was used to generate a codon-aware nucleotide alignment, and conserved sites were selected for forward and reverse primer binding (Table 1; see the supplemental material for additional details about primer site selection). The p450nor sequences of A. terreus strain NIH2624 and A. flavus strain NRRL 3357 were among the 38 sequences used for primer design (see Data Set S1 in the supplemental material), and p450nor sequences derived from A. terreus strain NRRL 255 and A. flavus strain NRRL 3357 served as positive controls in all p450nor-targeted PCR assays (32, 33). Genomic DNA from the bacterium Dehalogenimonas lykanthroporepellens strain BLDC-9 (ATCC BAA-1523) was used as a negative control in PCR assays.
TABLE 1.
Characteristics of the p450nor gene-targeted primers designed and used in this study
| Primera | Primer sequenceb (5′→3′) | Melting temp range (°C)c | GC (%)c | Fold degeneracyd |
|---|---|---|---|---|
| p450nor394F | SCIACITTYGTIGAYATGGA | 45.6–55.9 | 35–60 | 8 (256) |
| p450nor809R | ATCATGTTIACBAIIGTIGCIT | 45.5–56.7 | 27–55 | 3 (3,072) |
| p450nor1008R | GMSGCRATKATNCCYTC | 42.2–54.3 | 41–71 | 128 |
The numeric positions of the primers are based on the A. terreus strain NIH2624 p450nor gene sequence.
S = G or C; Y = C or T; B = C, G, or T; M = A or C; R = A or G; K = G or T; N = A, G, C, or T; I = inosine.
Ranges were calculated using OligoCalc (70).
The numbers in parentheses indicate fold degeneracy if the IUPAC symbol “N” had been used instead of an inosine nucleoside.
PCR conditions.
PCR assays using the primer pair p450nor394F and p450nor809R (Table 1) consisted of 9.125 μl DNase-free water (Life Technologies, Carlsbad, CA), 2.5 μl of 10 mM magnesium chloride solution (Applied Biosystems, CA, USA), 2.5 μl of Ex Taq 10× buffer (Mg2+ free) (Clontech Laboratories, Inc., Mountain View, CA), 2.5 μl of 2.5 mM each deoxynucleoside triphosphate (dNTP) (Clontech), 0.5 μl each 100 μM primers, 6.25 μl of 200-ng/μl bovine serum albumin (BSA) solution (Fisher Scientific, USA), 0.125 μl of 5-U/μl TaKaRa Ex Taq DNA Polymerase (Clontech), and 1 μl DNA template (approximately 10 to 100 ng/μl) in a total reaction volume of 25 μl.
PCR conditions for p450nor amplification from soil DNA samples required a seminested approach for consistent results, with initial PCR using primers p450nor394F and p450nor1008R, followed by a second round of PCR using primers p450nor394F and p450nor809R. The initial PCR mixture contained the same ingredients described above, except that 0.8-μl volumes (each) of 25 μM primers p450nor394F and p450nor1008R were utilized in the initial amplification reaction. Then, 1 μl of the amplicon solution was used as the template DNA in a second round of PCR with primers p450nor394F and p450nor809R employing PCR conditions identical to those for fungal genomic DNA amplification.
Amplification of fungal nirK genes from isolate DNA were performed as previously described by using primers nirKfF/nirKfR and fnirK2F/fnirK1R (34, 35) with the following modifications. The durations of the annealing and elongation steps were increased by 15 s (34) or decreased by 15 s (35), respectively, to allow simultaneous amplification of similar-size amplicons (∼450 to 500 bp). A total of 35 amplification cycles were performed for both the nirKfF/nirKfR and the fnirK2F/fnirK1R primer sets.
Amplification of the fungal internal transcribed spacers 1 and 2 was performed using primers ITS4 (36) and EF3RCNL (37). Amplification and sequencing of the nuclear small-subunit ribosomal (18S rRNA) gene from fungal isolates utilized 0.5 μl each of 10 μM primers PNS1, NS1, NS3, NS4, NS7, NS8, SR6, and NS19b (36, 38, 39) and the PCR conditions described above for the p450nor394F and p450nor1008R primers.
All DNA amplifications were carried out on a Veriti thermal cycler (Life Technologies, Carlsbad, CA) at 95°C for 2 min and 10 s, followed by 35 cycles each of 95°C for 30 s, 47°C for 45 s, and 72°C for 1 min (1.5 min for 18S rRNA genes), with a final extension step at 72°C for 7 min. For amplification of p450nor from soil DNA templates, as well as amplification of isolate ITS regions or isolate 18S rRNA genes, the annealing temperature was increased to 50, 53, or 55°C, respectively. Annealing temperatures for amplifications with nirK-targeted primer pairs nirKfF/nirKfR and fnirK2F/fnirK1R were 54 and 50°C, respectively (34, 35). For visualization of amplicons, 5 μl of the PCR assay mixture was combined with 1 μl of a loading dye solution (25 mg each bromophenol blue and xylene cyanol and 4 g sucrose dissolved in 10 ml of ultrapure water) and loaded onto a 1% (wt/vol) agarose gel, and electrophoresis was conducted at 90 V for 1 h at an initial amperage of 200 mA.
Cloning and sequencing.
Cloning of the ITS region and p450nor amplicons from DNA obtained from isolates and agricultural soil was performed using the TOPO TA cloning kit (Life Technologies, Carlsbad, CA) following the manufacturer's protocols. Of the 60 amplicons generated from soil DNA, 23 were selected for sequencing based on restriction digest analysis (HaeIII digestion using NEBuffer 4 solution [New England BioLabs, Ipswitch, MA]). Briefly, PCR amplicons (5 μl) were incubated at 37°C for 12 h in a 12-μl reaction volume (5.3 μl PCR grade water, 1.2 μl 10× NEBuffer 4 solution, 0.5 μl HaeIII), and fragment patterns were analyzed via gel electrophoresis. The cloned amplicons were Sanger sequenced using an ABI Big-Dye v3.1 cycle-sequencing mixture on an ABI 3130 analyzer (Applied Biosystems) at the University of Tennessee Molecular Biology Resource Facility. Electropherograms of sequenced amplicons were manually inspected using Geneious software (Biomatters Ltd., Auckland, New Zealand) following automatic trimming with a 0.03 error probability limit, and overlapping regions were assembled de novo after vector sequence removal using the UniVec database within Geneious.
Fungal taxonomy.
The script FungalITSPipeline.pl (40) was used to extract ITS1 and ITS2 regions from the cloned ITS sequences of the fungal isolates and to determine taxonomic affiliations within the International Nucleotide Sequence Database Collaboration (INSDC) (41). The 18S rRNA gene sequences were annotated using the SILVA database and the SINA alignment tool (42, 43).
Metagenomic analysis.
Metagenomic reads previously generated from Havana (SRR1152189) and Urbana (SRR1153387) soils (44) were retrieved from NCBI's Sequence Read Archive and unpacked with the SRA Toolkit (45). SolexaQA++ (46) was used to trim reads with a 0.01 error probability limit (Phred score, 20), followed by discarding reads shorter than 50 nucleotides. This procedure resulted in totals (including paired and single reads) of 236,437,515 and 291,298,696 high-quality sequences from Havana and Urbana soils, respectively. Fungal presence in the metagenomes was established by nucleotide alignment (BLASTn) against a custom ITS database provided by the FHiTINGS ITS analysis software, followed by analysis of the results with the same software (47). BLASTn alignment of the metagenomic reads against the cloned ITS sequences from the enriched fungal isolates was performed to uncover the presence/absence and relative abundances of isolated fungi within the metagenomes. A similar analysis was performed with cloned p450nor sequences, except a translated nucleotide alignment (BLASTx) was used.
P450nor sequence analyses.
The 38 reference P450nor sequences were combined with newly obtained p450nor sequences from isolates and soil DNA. Following the removal of identical sequences, all the sequences were aligned with the UniRef90 database with BLASTp (29, 48). Significant alignments (≥70% alignment length and ≥30% sequence identity) with eukaryotic sequences were retained for each query sequence, and hits with ≥90% identity to P450nor query sequences or uncertain NCBI taxonomy were removed. MAFFT v7.130b (49) was used with default parameters to initially align all the sequences, followed by manual editing with Jalview and SEA VIEW to retain the region amplified by the primer set p450nor394F and p450nor809R (50–52). The resulting alignment consisted of 108 taxa with an average length of 153 (minimum, 128; maximum, 158) amino acids. The alignment was analyzed with ProtTest v3.4 (53) to select an optimized evolutionary model for the aligned sequences prior to tree building. Phylogenetic reconstruction was performed using the FastTree 2 v2.1.7 SSE3 software package (54). Approximate maximum-likelihood tree reconstruction with FastTree 2 was performed using the Whelan and Goldman (55) amino acid substitution model with gamma-distributed rate heterogeneity (discrete distribution with 4 rate categories) and the subtree-pruning-regrafting algorithm. Support for internal nodes was assessed using 5,000 replications of the Shimodaira-Hasegawa test performed within FastTree 2. An additional alignment was generated using MAFFT that contained full-length sequences from a subset (n = 16) of all the sequences used in phylogenetic reconstruction. This alignment was submitted to the ESPript 3.0 Web server (http://espript.ibcp.fr; 56) to visualize key conserved residues in a diversity of P450nor sequences.
Nucleotide sequence accession numbers.
The p450nor, ITS, and 18S rRNA gene sequences are available under GenBank accession numbers KM889490 to KM889526 and KT714145 to KT714154, KM889527 to KM889556, and KT714155 to KT714191, respectively.
RESULTS
Fungal isolation.
Fungal cultivation techniques applied to Havana and Urbana soil-derived transfer cultures produced 214 fungal isolates. Growth experiments in medium containing NO2− demonstrated that about two-thirds of the isolates (151 out of 214) produced N2O within a 12-day incubation period (data not shown). Based on the gross morphology of mature fungal colonies, the 214 isolates from both the Havana and Urbana soils were divided into 15 groups, and 30 distinct isolates (including at least one representative isolate from each morphological group) were selected for further analysis. Since each morphological grouping may not align with the underlying isolate genotype, ITS region analysis was performed, which revealed 10 phylogenetically distinct groups, four of which were composed of singleton ITS sequences (see Data Set S2 in the supplemental material). Five additional isolates were later selected for p450nor analysis based on morphology consistent with that of N2O-producing isolates in previously assigned groupings, but only 18S rRNA gene identification was performed on these isolates (see Data Set S2 in the supplemental material).
Fungal-denitrifier morphology and taxonomy.
The majority of the representative fungal isolates were assigned to the genera Fusarium (teleomorph Giberella; 19 in total) or Trichoderma (5 in total) based on ITS and 18S rRNA gene sequence analysis. Microscopic observations of mature fungal colonies corroborated these results and revealed characteristic features of both the Fusarium (e.g., banana-shaped septate ascospores) and Trichoderma (e.g., presence of green conidia aggregated into fascicles) isolates (see Fig. S1 in the supplemental material) (57). Taxonomic prediction based on 18S rRNA gene sequence classification was successful for all but two fungal isolates. ITS sequence analysis identified these two isolates as putative Trichoderma sp. and Fusarium sp. Morphotyping (e.g., appearance of aerial hyphae with a white, feathery appearance on agar plates) and 18S rRNA gene sequence analysis assigned two additional isolates to the order Mortierellales. Other fungi isolated in this study included members of the genera Verticillium (1 isolate), Humicola (2 isolates), Chaetomium (3 isolates), Podospora (1 isolate), Lecythophora (1 isolate), and Guehomyces (1 isolate) (see Data Set S3 in the supplemental material), all of which belong to fungal lineages known to harbor denitrifying representatives (33).
p450nor and nirK PCR amplification.
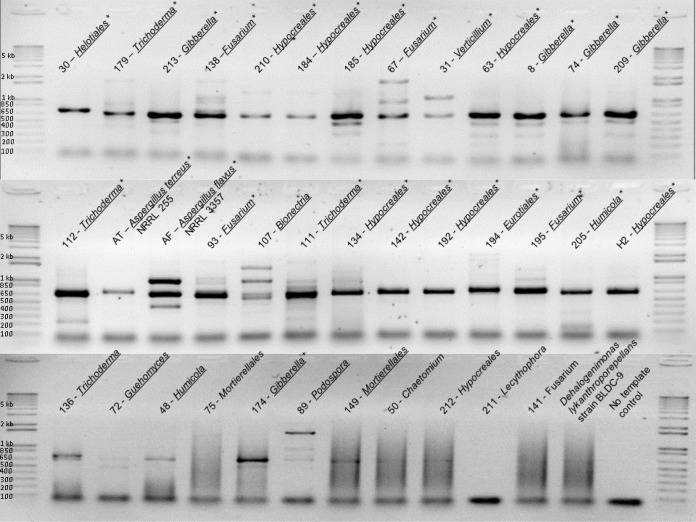
Application of primers p450nor394F and p450nor809R to isolate genomic DNA consistently resulted in a DNA band between 650 and 850 bp in size, and sequencing confirmed the amplification of p450nor gene fragments in 23 out of 23 (100%) N2O-producing fungal isolates (Fig. 1; see Data Set S2 in the supplemental material). Several nonspecific amplicons 100 to 200 bp below the predicted p450nor amplicon size of 536 to 890 bp were consistently observed (see Data Set S4 in the supplemental material), requiring gel extraction and cloning of the amplicons prior to sequencing. Sequence similarity among nontarget amplicons indicated that they originated from a putative ATPase gene and a noncoding genomic region. Nine isolates displayed a p450nor amplicon, but N2O was not detected in liquid culture under the conditions tested (Table 2). nirK amplification was detected in 17 out of 23 (74%) N2O-producing fungal isolates using primer set nirKfF/nirKfR. Amplification of both a p450nor and a nirK gene was observed in 20 out of 37 (54%) isolates. There were two instances in which nirK was amplified but p450nor could not be detected (isolate identifiers [ID] 50 and 141), and in 12 cases, p450nor was amplified but nirK was not detected (Table 2). Using primer set fnirK2F/fnirK1R, nirK was detected in only 3 out of 23 (13%) N2O-producing fungal isolates (Table 2).
FIG 1.
PCR amplification of p450nor genes from 37 fungal isolates. The names of isolates with positive p450nor bands between 650 and 850 bp are underlined. Fungal isolates whose p450nor genes have been sequenced are marked with asterisks. All the isolates have unique identifiers (shown before the taxonomic names) and possess their lowest taxonomic designation (e.g., genus) using the 18S rRNA gene classification from the SILVA database. A. terreus strain NRRL 255 and A. flavus strain NRRL 3357 were used as positive controls for p450nor amplification. Genomic DNA from the bacterium Dehalogenimonas lykanthroporepellens strain BLDC-9 was used as a negative control.
TABLE 2.
Select characteristics and gene contents of fungal isolates related to their capacity to perform denitrification
| Isolate ID | Isolate sourcea | Taxonomic affiliationb | N2O productionc | Targeted PCR |
||
|---|---|---|---|---|---|---|
| p450nord | nirK primer set 1e | nirK primer set 2f | ||||
| 30 | H | Helotiales | + | + | + | − |
| 179 | U | Trichoderma | + | + | + | − |
| 213 | U | Gibberella | + | + | + | − |
| 138 | H | Fusarium | + | + | + | + |
| 210 | U | Hypocreales | + | + | − | − |
| 184 | U | Hypocreales | + | + | − | − |
| 185 | U | Hypocreales | + | + | + | + |
| 67 | U | Fusarium | + | + | + | − |
| 31 | U | Verticillium | + | + | + | − |
| 63 | H | Hypocreales | + | + | + | − |
| 8 | H | Gibberella | + | + | + | − |
| 74 | H | Gibberella | + | + | + | − |
| 209 | U | Gibberella | + | + | + | − |
| 112 | H | Trichoderma | − | + | − | − |
| 93 | H | Fusarium | + | + | + | + |
| 107 | H | Bionectria | + | + | − | − |
| 111 | U | Trichoderma | + | + | + | − |
| 134 | H | Hypocreales | − | + | − | − |
| 142 | H | Hypocreales | − | + | + | − |
| 192 | H | Hypocreales | − | + | + | − |
| 194 | H | Eurotiales | + | + | + | − |
| 195 | U | Fusarium | − | + | − | − |
| 205 | H | Humicola | − | + | − | + |
| H2 | H | Hypocreales | + | + | − | − |
| 136 | H | Trichoderma | − | + | + | − |
| 72 | U | Guehomyces | + | + | − | − |
| 48 | U | Humicola | − | + | − | − |
| 75 | U | Mortierellales | − | − | − | − |
| 174 | U | Gibberella | + | + | + | − |
| 89 | H | Podospora | − | + | − | − |
| 149 | H | Mortierellales | + | + | + | − |
| 50 | U | Chaetomium | − | − | + | + |
| 212 | H | Hypocreales | − | − | − | − |
| 211 | H | Lecythophora | − | − | − | − |
| 141 | H | Fusarium | − | − | + | − |
| ATg | USDA | Aspergillus | + | + | − | − |
| AFh | USDA | Aspergillus | + | + | + | − |
H and U, Havana and Urbana soils, respectively; USDA, Agricultural Research Service culture collection.
Lowest taxonomic rank based on 18S rRNA gene classification (see Materials and Methods for more information). In some cases, the ITS sequence was used for classification when 18S rRNA gene classification was unsuccessful.
+, detected; −, not detected. N2O production was determined in defined mineral salts medium using nitrite as an electron acceptor (see Materials and Methods).
+, detected; −, not detected. p450nor was classified as detected when a visible band was confirmed between 536 and 890 bp by electrophoresis on a 1% (wt/vol) agarose gel (see Materials and Methods).
+, detected; −, not detected. nirK amplification was with primer set nirKfF/nirKfR (34). nirK positive detection was indicated by a visible band at 480 bp by electrophoresis on a 1% (wt/vol) agarose gel (see Materials and Methods).
+, detected; −, not detected. nirK amplification was with primer set fnirK2F/fnirK1R (35). nirK was classified as detected when a visible band was confirmed at 468 bp by electrophoresis on a 1% (wt/vol) agarose gel (see Materials and Methods).
AT, Aspergillus terreus strain NRRL 255.
AF, Aspergillus flavus strain NRRL 335.
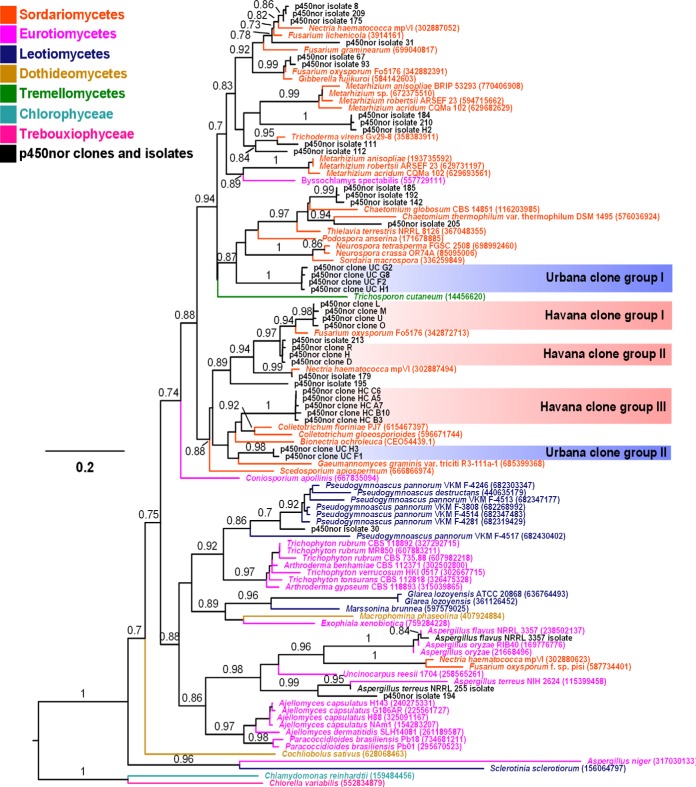
The nested-PCR amplification of soil DNA resulted in the recovery of a total of 23 environmental p450nor clone sequences. Pairwise nucleotide and amino acid identities of the 23 environmental p450nor sequences ranged from 65.8 to 100% and 63.8 to 100%, respectively. Phylogenetic analysis of the environmental p450nor sequences revealed the presence of five distinct soil clades (e.g., Urbana clone groups I and II and Havana clone groups I, II, and III) (Fig. 2). The clone groups from both soils were affiliated with sequences of representative Sordariomycetes denitrifiers (e.g., Fusarium and Trichoderma) and p450nor sequences derived from N2O-producing fungal isolates recovered from these soils (isolate ID 213 and 179) (Fig. 2). Overall, the environmental clone sequences displayed 59 to 100% amino acid identity with P450nor sequences from fungal isolates derived from the same agricultural soils. The alignment of the amplified region of the p450nor genes (approximately 160 amino acids) and P450nor tree reconstruction supported the monophyly of the cloned p450nor sequences with previously reported p450nor sequences from confirmed fungal denitrifiers (Fig. 2). The p450nor sequences recovered from isolate DNA and soil DNA clustered with p450nor sequences from members of the Leotiomycetes (one sequence), Eurotiomycetes (one sequence), and Sordariomycetes (47 sequences), three diverse fungal lineages within the phylum Ascomycota that are known to harbor denitrifying representatives (33).
FIG 2.
Rooted maximum-likelihood tree of fungal P450nor and related P450 amino acid sequences. Translated p450nor sequences amplified from isolate and clone sequences generated in this study are in black letters. Values along branches represent support (out of 5,000 resamples) for the tree topology at the nearest node, determined using the Shimodaira-Hasegawa test implemented in FastTree2. Only values ≥0.70 are reported. The scale bar indicates amino acid substitutions per site. GenInfo Identifier (GI) numbers of proteins from the NCBI database are in parentheses. HC, Havana clone; UC, Urbana clone. Clones with single letters were generated from a distinct Havana soil sample compared to the HC clones. Note that for Bionectria ochroleuca, an EMBL protein accession number is provided in parentheses, since a GI number was not available.
Soil denitrification activity.
Previous investigations have demonstrated that the majority of denitrifying fungal isolates lack the capacity to reduce NO3−. Therefore, replicate microcosms received NO3− or NO2− to assess denitrification activity. N2O production and visible fungal biomass (i.e., hyphae) were observed in all microcosms (16 total) that received antibiotics to inhibit bacterial growth (see Fig. S3 in the supplemental material); however, N2O formation in Havana soil microcosms amended with NO3− was low (see Fig. S3, top left, in the supplemental material). Havana transfer cultures amended with NO2− mirrored patterns observed in the NO2−-amended soil microcosms, but N2O formation was variable in the transfer cultures that received NO3− (see Fig. S4 in the supplemental material). N2O concentrations declined in Urbana microcosms after 2 weeks of incubation, whereas N2O production continued in Havana microcosms (see Fig. S3 in the supplemental material). A decline in the NO3− concentration in Urbana transfer cultures was observed, but concomitant formation of N2O did not occur (see Fig. S4 in the supplemental material).
Metagenomic analyses.
BLAST analysis of the Havana and Urbana metagenomic sequences identified 5,213 and 3,373 unique reads out of 236 and 291 million reads, respectively, that were alignable with fungal ITS database sequences. Fewer than 200 reads from each metagenome aligned with ITS sequences amplified from the representative fungal isolates obtained from the same soil samples. No metagenomic reads aligned with amplified and sequenced p450nor (this study) or previously reported p450nor sequences.
DISCUSSION
A new means to assess fungal denitrification potential.
The p450nor sequences identified in this study formed a monophyletic group with reference p450nor sequences and demonstrated the specificity of the degenerate primers for p450nor genes in isolate and soil DNA (Fig. 2). New sequences sharing 54 to 98% amino acid identity to reference p450nor sequences were recovered, suggesting the primers allow the recovery of novel sequence diversity within the predicted 536- to 890-bp sequence region spanned by the conserved primer binding sites (see Data Set S4 in the supplemental material). Phylogenetic analysis of the p450nor environmental clones permitted the identification of distinct groups (Havana clone groups I, II, and III and Urbana clone group I) (Fig. 2). These data suggest that geochemical differences between Havana and Urbana soils could be driving fungal-denitrifier diversity, but additional efforts are needed to determine to what extent geochemical conditions determine fungal p450nor diversity.
Recent investigations into fungal denitrification have relied on PCR primers targeting the fungal nirK gene to demonstrate the NO2−-reducing potential of the fungal community (34, 58). Although a significant advance in our ability to target fungal denitrifiers, nirK primer sets targeting the NO2−-to-NO step do not directly reveal the N2O-producing potential of the fungal community. The application of the fungal nirK gene-targeted primer set fnirK2F/fnirK1R (35) yielded only five positive results, whereas primer set nirKfF/nirKfR (34) revealed that many (17 out of 23) of the N2O-producing fungal isolates possessed nirK. Apparently, the latter primer set targets a greater diversity of fungal nirK genes but still failed to amplify nirK genes from 26% of the NO2−-reducing and N2O-producing fungal isolates. Interestingly, in two nondenitrifying fungal isolates, nirK was amplified but p450nor was not detected. On the other hand, in 12 isolates, p450nor amplification occurred but nirK was not detected (Table 2). Of these 12 organisms, five were shown to reduce NO2− to N2O. These observations suggest that the characterization of fungal nirK sequence space is incomplete, and additional sequence information from denitrifying fungal isolates is desirable. To date, p450nor is the sole gene known to encode the N2O-producing phenotype in fungi (3) and thus represents an ideal target for assessing N2O formation potential. Due to substantial heterogeneity in available p450nor sequences, primer design is challenging (33). Consequently, the design of PCR primers that amplify all p450nor sequences while excluding all other sequences of the p450 gene family will probably not be possible. Still, the new p450nor primers presented here represent a major advance for assessing fungal N2O production potential.
The variable presence/absence of introns within p450nor genes can lead to amplicon size variations and create additional difficulties in identifying p450nor amplicons. To assist in identifying a range of expected amplicon sizes, we analyzed the intron structure in p450nor sequences from 47 p450nor-containing fungal genomes (see Fig. S5 and Data Set S4 in the supplemental material). Overall, predicted p450nor amplicons contained between one and four introns and varied in size between 536 and 890 bp. Although introns or other genetic variations may prevent p450nor amplification, their presence within a primer binding region was observed in only a single case (Glarea lozoyensis strain ATCC 20868) (see Fig. S5 in the supplemental material). The observed intron in G. lozoyensis is more likely due to low transcript coverage at the p450nor locus, since the forward primer site (present in exons of all other fungi examined) was detected within the predicted p450nor gene sequence. Primer sequence degeneracy contributes to nonspecific amplification, and thus, cloning and sequencing were required to confirm p450nor amplification. A general limitation of degenerate PCR primers is diminished binding efficiency leading to uneven amplification of target sequences (Table 1). In spite of these obstacles, all the sequenced amplicons fell in the expected 536- to 890-bp size range and were identified as p450nor. Importantly, primers p450nor394F/p450nor809R amplified p450nor from all N2O-producing isolates, indicating the utility of this approach to target a broad diversity of fungal denitrifiers. In nine instances, p450nor was detected but N2O was not produced by fungal isolates in liquid culture. Amplicon sequencing ruled out false positives, suggesting that the chosen cultivation conditions were not conducive to N2O production in these isolates.
The seminested-PCR methodology provides additional confidence in assaying for the presence/absence of p450nor in soils where fungi may not be abundant at the time of sampling or fungal DNA is underrepresented in soil DNA extracts. Application of the seminested-PCR approach to a dilution series of genomic DNAs extracted from A. terreus strain NRRL 255, A. flavus strain NRRL 3357, and Fusarium graminearum indicated a 10- to 100-fold increase in sensitivity over the direct (i.e., nonnested) approach. Experiments with dilutions of genomic DNA suggested that the seminested-PCR approach yielded positive results when at least 3,000 to 24,000 p450nor gene copies were present per assay (data not shown). Thus, the nonnested-PCR approach may yield false-negative results (inability to detect p450nor when present), and failure to detect p450nor amplicons in isolate or environmental DNA samples should be interpreted accordingly.
Diversity of the denitrifying fungi.
It is widely accepted that the majority of soil bacteria cannot be cultivated by conventional methods (59). Although this issue may not be as extensive for fungi, only approximately 2 to 7% of the estimated 1.5 to 5.1 million fungal species have been cultivated (60, 61). Furthermore, only a few hundred of the 100,000 documented fungal species have been tested for the ability to produce N2O from N oxyanions. Our knowledge of fungi capable of denitrification is constantly expanding, and fungal representatives spanning eight orders and three phyla have now been documented to produce N2O (33, 58), including a diversity of organisms examined here. Therefore, it is very likely that a far greater diversity of fungi contributing to this process exists in nature than is currently acknowledged.
The enrichment and isolation techniques utilized in the present study resulted in the cultivation of fungal taxa largely representative of currently classified fungal denitrifiers. For example, several members of the genera Fusarium and Trichoderma, both known to harbor species capable of N2O production, were cultured (8, 17, 18). Also identified were two Mortierella isolates (Mortierellomycotina, formally Zygomycota), a genus that includes members known to produce N2O (33, 58). Only one of the two Mortierella isolates produced N2O in liquid culture after 12 days of incubation, indicating that not all members of the genus may share this phenotype. Several other fungal isolates possessed ITS regions highly similar (>99% similarity) to those of known denitrifiers, but not all produced N2O under the cultivation conditions tested (see Data Set S3 in the supplemental material). Similarly, a recent study demonstrated that only 50 of 113 species in the order Hypocreales, many belonging to the same genus (i.e., Fusarium), tested positive for N2O production, suggesting the denitrifying phenotype varies even among closely related fungi (58).
N2O formation in soil microcosms and enrichments.
The Havana and Urbana site microcosms amended with NO2− consistently produced N2O, but N2O formation from NO3− was limited to Urbana soil microcosms. Possible explanations for this observation include a lack of fungal denitrifiers possessing nitrate reductase (nar and nap) genes, inhibition of fungal nitrate reductase activity by antibiotics intended to inhibit bacterial denitrifiers (62), and the rapid reduction of NO3− to ammonium in Havana soils (63). Of note, the N2O concentration declined or N2O was not detected in Urbana microcosms (see Fig. S1 and S2 in the supplemental material), likely because bacterial denitrifiers were not completely inhibited by the addition of antibiotics and consumed the available N2O. In support of this explanation, inoculation of solid medium with suspensions from Urbana soil microcosms resulted in the formation of colonies of presumably antibiotic-resistant bacteria. Microscopic observations corroborated bacterial growth on antibiotic-treated solid medium. Incomplete inhibition of all or specific microbial processes is a known weakness of SIRIN analyses and may explain the observed variation in N2O production between sites (13, 17, 64, 65). The observed variability in NO3− reduction is consistent with other studies describing a diversity of fungi that are incapable of reducing NO3− to N2O (7, 10). Interestingly, most sequenced genomes of fungal denitrifiers possess a putative napA gene encoding periplasmic nitrate reductase (3), but a detailed understanding of the variation in fungal NO3− reduction capacity has yet to be obtained. Overall, the observed production of N2O in microcosms established with Havana and Urbana soils is in line with the magnitude and variability observed in other studies, in which antibiotics were employed to probe the fungal contribution to N2O production (8, 13, 18).
Low sequence depth limits detection of fungal sequences in metagenomes.
p450nor was not detected in Havana and Urbana metagenomes, but fungal presence was established by the presence of fungal ITS sequences (∼5,200 and 3,400 reads, respectively) and alignment of >200 million metagenomic reads to the fungal-isolate ITS regions (∼200 reads in both metagenomes). The newly designed primer sets amplified p450nor genes using soil DNA extracted from the same soil samples used for metagenomics analysis, emphasizing their utility to screen for fungal denitrification potential. In most documented cases, a single p450nor gene copy was found per fungal genome, although exceptions have been noted (e.g., three p450nor gene copies were detected in Nectria haematococca [Fig. 2]). Not surprisingly, the detection of single-copy protein-encoding genes in metagenomics data sets is more challenging than detecting high-copy-number ribosomal operons (which includes the fungal ITS region) (66). Hence, current metagenomics approaches may not be optimal for detection of sequences from organisms that contribute small amounts of DNA (e.g., fungi) or are present in low abundance, and increased sequencing depth of environmental DNA samples is needed to uncover the functional potential of fungi in soils (67–69).
Conclusions.
A curated set of 38 p450nor sequences enabled the design of degenerate PCR primers, which were demonstrated to effectively uncover fungal N2O production potential in denitrifying isolates and soil DNA samples. Hence, the new p450nor-targeted primers complement existing primer sets to assess fungal NO2− reduction potential (34, 58). Given the potential biases associated with DNA extraction and direct-sequencing methodologies (e.g., metagenomics analysis), approaches that rely on the targeted amplification of low-abundance, process-specific genes are crucial for unraveling the functional potential of microorganisms in soils.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hawa Henderson, Jenny Onley, and Meng Bi for their assistance with fungal culture transfers; Yi Yang for assistance with calculations involving quantitative geochemical data; and Ashley M. Frank for manuscript revisions.
S.A.H. acknowledges additional support from the NSF SCALE-IT IGERT at the University of Tennessee and the DOE SCGSR Fellowship Program.
Funding Statement
This research was supported by the U.S. Department of Energy (DOE), Office of Biological and Environmental Research, Genomic Science Program (award DE-SC0006662).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00243-16.
REFERENCES
- 1.Zumft W. 1997. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield DE, Glazer AN, Falkowski PG. 2010. The evolution and future of Earth's nitrogen cycle. Science 330:192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- 3.Shoun H, Fushinobu S, Jiang L, Kim S-W, Wakagi T. 2012. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos Trans R Soc Lond B Biol Sci 367:1186–1194. doi: 10.1098/rstb.2011.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usuda K, Toritsuka N, Matsuo Y, Kim DH, Shoun H. 1995. Denitrification by the fungus Cylindrocarpon tonkinense: anaerobic cell growth and two isozyme forms of cytochrome P-450nor. Appl Environ Microbiol 61:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanimoto T, Hatano K, Kim D, Uchiyama H, Shoun H. 1992. Co-denitrification by the denitrifying system of the fungus Fusarium oxysporum. FEMS Microbiol Lett 93:177–180. doi: 10.1111/j.1574-6968.1992.tb05086.x. [DOI] [Google Scholar]
- 6.Shoun H, Sudo Y, Seto Y, Beppu T. 1983. Purification and properties of a cyotchrome P-450 of a fungus, Fusarium oxysporum. J Biochem 94:1219–1229. [DOI] [PubMed] [Google Scholar]
- 7.Shoun H. 1992. Denitrification by fungi. FEMS Microbiol Lett 94:277–281. doi: 10.1111/j.1574-6968.1992.tb05331.x. [DOI] [PubMed] [Google Scholar]
- 8.Mothapo NV, Chen H, Cubeta MA, Shi W. 2013. Nitrous oxide producing activity of diverse fungi from distinct agroecosystems. Soil Biol Biochem 66:94–101. doi: 10.1016/j.soilbio.2013.07.004. [DOI] [Google Scholar]
- 9.Wei W, Isobe K, Shiratori Y, Nishizawa T, Ohte N, Otsuka S, Senoo K. 2014. N2O emission from cropland field soil through fungal denitrification after surface applications of organic fertilizer. Soil Biol Biochem 69:157–167. doi: 10.1016/j.soilbio.2013.10.044. [DOI] [Google Scholar]
- 10.Tsuruta S, Takaya N, Zhang L, Shoun H, Kimura K, Hamamoto M, Nakase T. 1998. Denitrification by yeasts and occurrence of cytochrome P450nor in Trichosporon cutaneum. FEMS Microbiol Lett 168:105–110. doi: 10.1111/j.1574-6968.1998.tb13262.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma WK, Farrell RE, Siciliano SD. 2008. Soil formate regulates the fungal nitrous oxide emission pathway. Appl Environ Microbiol 74:6690–6696. doi: 10.1128/AEM.00797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoun H. 1992. Fungal denitrification and cytochrome P-450. J Agric Chem Soc Japan 66:154–157. [Google Scholar]
- 13.Laughlin RJ, Stevens RJ. 2002. Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Biogeochemistry 66:1540–1548. [Google Scholar]
- 14.Johnson CK, Vigil MF, Doxtader KG, Beard WE. 1996. Measuring bacterial and fungal substrate-induced respiration in dry soils. Soil Biol Biochem 28:427–432. doi: 10.1016/0038-0717(96)00004-1. [DOI] [Google Scholar]
- 15.Blagodatskaya E, Dannenmann M, Gasche R, Butterbach-Bahl K. 2010. Microclimate and forest management alter fungal-to-bacterial ratio and N2O-emission during rewetting in the forest floor and mineral soil of mountainous beech forests. Biogeochemistry 97:55–70. doi: 10.1007/s10533-009-9310-3. [DOI] [Google Scholar]
- 16.Yanai Y, Toyota K, Morishita T, Takakai F, Hatano R, Limin SH, Darung U, Dohong S. 2007. Fungal N2O production in an arable peat soil in Central Kalimantan, Indonesia. Soil Sci Plant Nutr 53:806–811. doi: 10.1111/j.1747-0765.2007.00201.x. [DOI] [Google Scholar]
- 17.Cathrine SJ, Raghukumar C. 2009. Anaerobic denitrification in fungi from the coastal marine sediments off Goa, India. Mycol Res 113:100–109. doi: 10.1016/j.mycres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Jasrotia P, Green SJ, Canion A, Overholt WA, Prakash O, Wafula D, Hubbard D, Watson DB, Schadt CW, Brooks SC, Kostka JE. 2014. Watershed-scale fungal community characterization along a pH gradient in a subsurface environment cocontaminated with uranium and nitrate. Appl Environ Microbiol 80:1810–1820. doi: 10.1128/AEM.03423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriks J, Oubrie A, Castresana J, Urbani A, Gemeinhardt S, Saraste M. 2000. Nitric oxide reductases in bacteria. Biochim Biophys Acta 1459:266–273. doi: 10.1016/S0005-2728(00)00161-4. [DOI] [PubMed] [Google Scholar]
- 20.Shoun H, Tanimoto T. 1991. Denitrification by the fungus Fusarium oxysporum and involvement of cytochrome P-450 in the respiratory nitrite reduction. J Biol Chem 266:11078–11082. [PubMed] [Google Scholar]
- 21.Philippot L. 2002. Denitrifying genes in bacterial and archaeal genomes. Biochim Biophys Acta 1577:355–376. doi: 10.1016/S0167-4781(02)00420-7. [DOI] [PubMed] [Google Scholar]
- 22.Jones CM, Stres B, Rosenquist M, Hallin S. 2008. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol Biol Evol 25:1955–1966. doi: 10.1093/molbev/msn146. [DOI] [PubMed] [Google Scholar]
- 23.Atlas RM. 2004. Handbook of microbiological media, 3rd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 24.Banihani Q, Sierra-Alvarez R, Field J. 2009. Nitrate and nitrite inhibition of methanogenesis during denitrification in granular biofilms and digested domestic sludges. Biodegradation 20:801–812. doi: 10.1007/s10532-009-9268-9. [DOI] [PubMed] [Google Scholar]
- 25.Yoon S, Sanford RA, Löffler FE. 2013. Shewanella spp. use acetate as an electron donor for denitrification but not ferric iron or fumarate reduction. Appl Environ Microbiol 79:2818–2822. doi: 10.1128/AEM.03872-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long GL, Winefordner JD. 1983. Limit of detection. A closer look at the IUPAC definition. Anal Chem 55:712A–724A. [Google Scholar]
- 27.Hughes KW, McGhee LL, Methven AS, Johnson JE, Petersen RH. 1999. Patterns of geographic speciation in the genus Flammulina based on sequences of the ribosomal ITS1-5.8S-ITS2 area. Mycologia 91:978–986. doi: 10.2307/3761628. [DOI] [Google Scholar]
- 28.Geer LY, Marchler-Bauer A, Geer RC, Han L, He J, He S, Liu C, Shi W, Bryant SH. 2010. The NCBI Biosystems database. Nucleic Acids Res 38:D492–D496. doi: 10.1093/nar/gkp858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 30.Notredame C, Higgins DG, Heringa J. 2000. T-coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 31.Suyama M, Torrents D, Bork P. 2006. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34:W609–W612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stief P, Fuchs-Ocklenburg S, Kamp A, Manohar C-S, Houbraken J, Boekhout T, de Beer D, Stoeck T. 2014. Dissimilatory nitrate reduction by Aspergillus terreus isolated from the seasonal oxygen minimum zone in the Arabian Sea. BMC Microbiol 14:35. doi: 10.1186/1471-2180-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mothapo N, Chen H, Cubeta MA, Grossman JM, Fuller F, Shi W. 2015. Phylogenetic, taxonomic and functional diversity of fungal denitrifiers and associated N2O production efficacy. Soil Biol Biochem 83:160–175. doi: 10.1016/j.soilbio.2015.02.001. [DOI] [Google Scholar]
- 34.Wei W, Isobe K, Shiratori Y, Nishizawa T, Ohte N, Ise Y, Otsuka S, Senoo K. 2015. Development of PCR primers targeting fungal nirK to study fungal denitrification in the environment. Soil Biol Biochem 81:282–286. doi: 10.1016/j.soilbio.2014.11.026. [DOI] [Google Scholar]
- 35.Long A, Song B, Fridey K, Silva A. 2015. Detection and diversity of copper containing nitrite reductase genes (nirK) in prokaryotic and fungal communities of agricultural soils. FEMS Microbiol Ecol 91:1–9. doi: 10.1093/femsec/fiu004. [DOI] [PubMed] [Google Scholar]
- 36.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 479 In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 37.Lord NS, Kaplan CW, Shank P, Kitts CL, Elrod SL. 2002. Assessment of fungal diversity using terminal restriction fragment (TRF) pattern analysis: comparison of 18S and ITS ribosomal regions. FEMS Microbiol Ecol 42:327–337. doi: 10.1111/j.1574-6941.2002.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 38.Hibbett DS. 1996. Phylogenetic evidence for horizontal transmission of group I introns in the nuclear ribosomal DNA of mushroom-forming fungi. Mol Biol Evol 13:903–917. doi: 10.1093/oxfordjournals.molbev.a025658. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell K, Cigelnik E, Benny GL. 1998. Phylogenetic relationships among the Harpellales and Kickxellales. Mycologia 90:624–639. doi: 10.2307/3761222. [DOI] [Google Scholar]
- 40.Nilsson RH, Bok G, Ryberg M, Kristiansson E, Hallenberg N. 2009. A software pipeline for processing and identification of fungal ITS sequences. Source Code Biol Med 4:1. doi: 10.1186/1751-0473-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y, Cochrane G, Karsch-Mizrachi I. 2013. The international nucleotide sequence database collaboration. Nucleic Acids Res 41:D21–D24. doi: 10.1093/nar/gks1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pruesse E, Peplies J, Glöckner FO. 2012. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orellana LH, Rodriguez-R LM, Higgins S, Chee-Sanford JC, Sanford RA, Ritalahti KM, Löffler FE, Konstantinidis KT. 2014. Detecting nitrous oxide reductase (nosZ) genes in soil metagenomes: method development and implications for the nitrogen cycle. mBio 5:e01193-14. doi: 10.1128/mBio.01193-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leinonen R, Sugawara H, Shumway M. 2011. The sequence read archive. Nucleic Acids Res 39:D19–D21. doi: 10.1093/nar/gkq1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox M, Peterson D, Biggs P. 2010. SolexaQA: at-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics 11:485. doi: 10.1186/1471-2105-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dannemiller KC, Reeves D, Bibby K, Yamamoto N, Peccia J. 2014. Fungal high-throughput taxonomic identification tool for use with next-generation sequencing (FHiTINGS). J Basic Microbiol 54:315–321. doi: 10.1002/jobm.201200507. [DOI] [PubMed] [Google Scholar]
- 48.Suzek BE, Huang H, McGarvey P, Mazumder R, Wu CH. 2007. UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics 23:1282–1288. doi: 10.1093/bioinformatics/btm098. [DOI] [PubMed] [Google Scholar]
- 49.Katoh K, Asimenos G, Toh H. 2009. Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 537:39–64. doi: 10.1007/978-1-59745-251-9_3. [DOI] [PubMed] [Google Scholar]
- 50.Clamp M, Cuff J, Searle SM, Barton GJ. 2004. The Jalview Java alignment editor. Bioinformatics 20:426–427. doi: 10.1093/bioinformatics/btg430. [DOI] [PubMed] [Google Scholar]
- 51.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galtier N, Gouy M, Gautier C. 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548. [DOI] [PubMed] [Google Scholar]
- 53.Darriba D, Taboada GL, Doallo R, Posada D. 2011. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2: approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whelan S, Goldman N. 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol 18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- 56.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gams W, Bisset J. 1998. Morphology and identification of Trichoderma, p 3–34. In Kubicek CP, Harman GE (ed), Trichoderma and Gliocladium, vol 1 Basic biology, taxonomy and genetics. Taylor & Francis, Bristol, PA. [Google Scholar]
- 58.Maeda K, Spor A, Edel-Hermann V, Heraud C, Breuil M-C, Bizouard F, Toyoda S, Yoshida N, Steinberg C, Philippot L. 2015. N2O production, a widespread trait in fungi. Sci Rep 5:9697. doi: 10.1038/srep09697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staley JT, Konopka A. 1985. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol 39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- 60.Blackwell M. 2011. The fungi: 1, 2, 3 … 5.1 million species? Am J Bot 98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 61.Hawksworth DL. 2012. Global species numbers of fungi: are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers Conserv 21:2425–2433. doi: 10.1007/s10531-012-0335-x. [DOI] [Google Scholar]
- 62.Brooks MH, Smith RL, Macalady DL. 1992. Inhibition of existing denitrification enzyme activity by chloramphenicol. Appl Environ Microbiol 58:1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takasaki K, Shoun H, Yamaguchi M, Takeo K, Nakamura A, Hoshino T, Takaya N. 2004. Fungal ammonia fermentation, a novel metabolic mechanism that couples the dissimilatory and assimilatory pathways of both nitrate and ethanol. Role of acetyl-CoA synthetase in anaerobic ATP synthesis. J Biol Chem 279:12414–12420. [DOI] [PubMed] [Google Scholar]
- 64.Chen H, Mothapo NV, Shi W. 2014. The significant contribution of fungi to soil N2O production across diverse ecosystems. Appl Soil Ecol 73:70–77. doi: 10.1016/j.apsoil.2013.08.011. [DOI] [Google Scholar]
- 65.Ma WK, Bedard-Haughn A, Siciliano SD, Farrell RE. 2008. Relationship between nitrifier and denitrifier community composition and abundance in predicting nitrous oxide emissions from ephemeral wetland soils. Soil Biol Biochem 40:1114–1123. doi: 10.1016/j.soilbio.2007.12.004. [DOI] [Google Scholar]
- 66.Moore D, Robson GD, Trinci APJ. 2011. 21st century guidebook to fungi, 1st ed Cambridge University Press, New York, NY. [Google Scholar]
- 67.Luo C, Rodriguez-R LM, Konstantinidis KT. 2013. A user's guide to quantitative and comparative analysis of metagenomic datasets, p 525–547. In DeLong EF. (ed), Microbial metagenomics, metatranscriptomics, and metaproteomics. Academic Press, Boston, MA. [DOI] [PubMed] [Google Scholar]
- 68.Deacon JW. 2005. Fungal biology, 4th ed Wiley-Blackwell, Hoboken, NJ. [Google Scholar]
- 69.Griffin DH. 1994. Fungal physiology, 2nd ed Wiley-Liss, New York, NY. [Google Scholar]
- 70.Kibbe WA. 2007. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35:W43–W46. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.