ABSTRACT
Sulfolobus islandicus serves as a model for studying archaeal biology as well as linking novel biology to evolutionary ecology using functional population genomics. In the present study, we developed a new counterselectable genetic marker in S. islandicus to expand the genetic toolbox for this species. We show that resistance to the purine analog 6-methylpurine (6-MP) in S. islandicus M.16.4 is due to the inactivation of a putative adenine phosphoribosyltransferase encoded by M164_0158 (apt). The application of the apt gene as a novel counterselectable marker was first illustrated by constructing an unmarked α-amylase deletion mutant. Furthermore, the 6-MP counterselection feature was employed in a forward (loss-of-function) mutation assay to reveal the profile of spontaneous mutations in S. islandicus M.16.4 at the apt locus. Moreover, the general conservation of apt genes in the crenarchaea suggests that the same strategy can be broadly applied to other crenarchaeal model organisms. These results demonstrate that the apt locus represents a new tool for genetic manipulation and sequence analysis of the hyperthermophilic crenarchaeon S. islandicus.
IMPORTANCE Currently, the pyrEF/5-fluoroorotic acid (5-FOA) counterselection system remains the sole counterselection marker in crenarchaeal genetics. Since most Sulfolobus mutants constructed by the research community were derived from genetic hosts lacking the pyrEF genes, the pyrEF/5-FOA system is no longer available for use in forward mutation assays. Demonstration of the apt/6-MP counterselection system for the Sulfolobus model renders it possible to again study the mutation profiles in mutants that have already been constructed by the use of strains with a pyrEF-deficient background. Furthermore, additional counterselectable markers will allow us to conduct more sophisticated genetic studies, i.e., investigate mechanisms of chromosomal DNA transfer and quantify recombination frequencies among S. islandicus strains.
INTRODUCTION
Diverse Sulfolobus islandicus strains belonging to the hyperthermophilic crenarchaea thrive in geographically isolated populations in hot springs around the world (1). These organisms provide an excellent system for studying microbial evolutionary ecology (2) and may be used as a genetic model system for studying novel molecular mechanisms in the TACK (Thaumarchaeota, Aigarchaeota, Crenarchaeota, and Korarchaeota) lineage of the archaeal domain, which has been hypothesized by some to be the most recent common ancestor of the eukaryotes on the tree of life (3). To date, the genomes of 20 diverse S. islandicus strains have been sequenced (2, 4–6). Versatile genetic tools have been developed for a few representative strains of S. islandicus (6–8), including two efficient plasmid shuttle vectors (9, 10), a set of new selectable markers (8, 11, 12), and conventional and novel methods of genetic manipulation (13), as well as clustered regularly interspaced short palindromic repeat-Cas-mediated genome editing protocols (14). Nevertheless, the pyrEF/5-fluoroorotic acid (5-FOA) counterselection system, which was first employed for forward mutation assays in Sulfolobus acidocaldarius (15), remains the sole counterselection marker in crenarchaeal genetics. Since most Sulfolobus mutants constructed by the research community were derived from the genetic hosts lacking the pyrE and pyrF genes (13, 16), a new genetic marker suitable for counterselection and forward mutation assays is of great importance for the genetic study of genome integrity and DNA damage repair in the Crenarchaeota. Furthermore, the availability of additional counterselectable markers would enable more sophisticated genetic studies, such as chromosomal DNA transfer and recombination among S. islandicus strains (17) and analyses of mutational frequency at various locations in the chromosome (18).
In addition to pyrEF/5-FOA, other counterselectable markers have been reported for bacteria and eukaryotes, especially genes that code for phosphoribosyltransferases (PRTases) and that are involved in the pyrimidine and purine salvage pathways (19–22). The counterselection is based on the fact that the active PRTases convert pyrimidine or purine analogs into toxic metabolites that kill the host cells, whereas mutant cells survive cell killing due to the lack of enzyme activity (23–26). A few archaeal PRTases have been characterized, but most of them are from methanogens or haloarchaea, which are members of the Euryarchaeota (27–31). These studies show that archaeal hpt (coding for hypoxanthine phosphoribosyltransferase) mutants exhibit resistance to purine analogs, such as 8-aza-2,6-diaminopurine (8-ADP), 8-azahypoxanthine (8-AHP), and 6-methylpurine (6-MP) (32, 33). This observation has facilitated the development of unmarked gene deletions based on hpt genes in different euryarchaea, including Methanosarcina acetivorans, Methanococcus maripaludis, and Methanosarcina mazei (34–36). More recently, the hpt gene (also named the xgprt gene) was used as a marker for developing genetic tools for use in the anaerobic hyperthermophiles Thermococcus kodakarensis and Pyrococcus furiosus (37, 38). In contrast to several observations of purine salvage pathways in euryarchaea, the purine salvage pathway in crenarchaea is poorly understood, although annotations of some key enzymes, such as purine PRTases, in the genomes of most crenarchaea have been made. Recently, the adenine and hypoxanthine-guanine-xanthine phosphoribosyltransferase of Sulfolobus solfataricus P2, encoded by Sso2342 and Sso2424, respectively, have been biochemically characterized (39); however, their use in genetic manipulations has not been demonstrated.
Here we tested the susceptibility of several Sulfolobus species, including S. islandicus M.16.4, a genetic model isolated from an acidic terrestrial hot springs in Kamchatka, Russia (4), to a set of purine analogs. 6-Methylpurine-resistant (6-MPr) mutants of this archaeon were obtained, and characterization of their genetic determinant of resistance revealed that it resulted from the loss function of an adenine phosphoribosyltransferase gene (the apt, or M164_0158, gene). Then, a new counterselectable method of genetic manipulation based on the apt gene was developed and employed to delete an α-amylase-encoding gene (amyA, M164_1052) in S. islandicus. Furthermore, the apt gene was used in a forward mutation assay to investigate the spectrum of spontaneous mutations at the apt locus in S. islandicus. Together, the findings of this study demonstrate that apt/6-MP functions as an efficient counterselection marker system in S. islandicus.
MATERIALS AND METHODS
Strains, media, and growth conditions.
All Sulfolobus strains (Table 1) were grown aerobically in standard DT medium at 75 to 78°C and pH 3.5 without shaking, as described previously (12). Plate medium was solidified with 1.6% (wt/vol) Phytagel or Gelrite agent (Sigma-Aldrich, USA). For the cultivation of a triple mutant derived from S. islandicus M.16.4, S. islandicus RJW004 (ΔargD ΔpyrEF ΔlacS) (12), 20 μg/ml uracil and 20 μg/ml agmatine were added to the DT medium (designated DTUA medium). For the growth of S. islandicus strains with mutations in the apt gene, the liquid medium was supplemented with 0.5 mM GMP disodium salt hydrate (Sigma-Aldrich, USA) or 0.5 mM AMP disodium salt (Sigma-Aldrich, USA). The purine analogs 6-MP, 6-thioguanine, 8-azaguanine, 2,6-diaminopurine, 2-aminopurine, 2-amino-6-methylmercaptopurine, and 6-methylaminopurine (Sigma-Aldrich, USA) were added from sterile stocks at concentrations ranging from 1 μM to 3 mM. In particular, 80 μM 6-MP was used to isolate spontaneous 6-MPr colonies from wild-type Sulfolobus strains and 150 to 300 μM 6-MP was used for counterselection procedures when the Δapt and ΔamyA deletion mutants were constructed.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| S. islandicus M.16.4 | Wild type | 4 |
| S. islandicus REY15A | Wild type | 5 |
| S. solfataricus P1 | Wild type | DSMZ |
| S. solfataricus P2 | Wild type | DSMZ |
| S. acidocaldarius | Wild type | DSMZ |
| S. islandicus RJW004 | S. islandicus M.16.4 with argD, pyrEF, and lacS deletions | 12 |
| S. islandicus RJW009 | S. islandicus RJW004 with an in-frame apt deletion | This study |
| S. islandicus RJW010 | S. islandicus RJW009 with an in-frame amyA deletion | This study |
| S. islandicus pMID-apt-T | RJW004 double-crossover transformants generated with pMID-apt via downstream insertion | This study |
| S. islandicus pMID-amyA-T | RJW009 double-crossover transformants generated with pMID-amyA via upstream insertion | This study |
| S. islandicus pC-apt-T | Δapt ΔargD ΔpyrEF ΔlacS::Sso-argD-Sso-apt, apt complementation strain | This study |
| Plasmids | ||
| pRJW8 | pUC19 carrying the lacS, pyrEF, and argD expression cassettes from S. solfataricus P2 | 12 |
| pRJW9 | pUC19 carrying the lacS, apt, and argD expression cassettes from S. solfataricus P2 | This study |
| pMID-apt | pRJW8 carrying the Up-arm and Dn-arm of apt and a partial apt gene (Tg-arm), apt-knockout plasmid | This study |
| pMID-amyA | pRJW9 carrying the Up-arm and Dn-arm of apt and a partial amyA gene (Tg-arm), amyA-knockout plasmid | This study |
| pC-SsoargD | pUC19 carrying the Up-arm and Dn-arm of lacS and the Sso-argD marker cassette | 12 |
| pC-Ssoapt | NheI/NcoI-cut pC-SsoargD into which the apt expression cassette from S. solfataricus P2 was inserted, apt complementation plasmid | This study |
Screening and sequencing of spontaneous Sulfolobus apt mutants.
Mid-log-phase Sulfolobus cells were spun down for 10 min at 10,000 rpm and then resuspended in DT medium with a normalized optical density at 600 nm (OD600) of 0.5. An aliquot of 400 μl of cells was plated undiluted via overlay on selective medium containing 80 μM 6-MP. Single 6-MPr colonies were picked and resuspended in 400 μl DT medium. Two microliters of cell culture was used as the DNA template for PCR amplification according to a procedure described previously (12). The apt gene, together with its putative promoter and terminator regions, from different Sulfolobus strains was PCR amplified using the primers Sispt-3-seq-F/R, whose sequences are shown in Table 2. The resulting PCR products were treated with ExoSAP-IT for the PCR product cleanup kit (Affymetrix, USA) and then sent to the University of Illinois at Urbana-Champaign Core DNA Sequencing Facility for sequencing. In particular, to extensively study the spontaneous mutation spectrum of the apt gene in S. islandicus M.16.4, 215 6-MPr isolates in total from 24 independent cell cultures were examined, whereas 10 6-MPr isolates of each of the other Sulfolobus strains were screened.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| Sispt-1-seq-F | GCTGTAGAGAAGGCTAAGAGAGA |
| Sispt-1-seq-R | CCAGTTGTAGTGTCAATAAGCG |
| Sispt-2-seq-F | GATTACCGAGCATTCATATTTAA |
| Sispt-2-seq-R | AGAGAGAGATGGCATGAAAGTTA |
| Sispt-3-seq-F | TACCCGGATCATATAACCCAG |
| Sispt-3-seq-R | AAGGTTTTTGTGGTTGGTGAT |
| Sacapt-seq-F | CCTATTATTCCTATTTTTGCTTT |
| Sacapt-seq-R | TTGAAAAGTTGAGCCAAGAAG |
| Ssoapt-seq-F | GTATAGTCCAGATCCGCCAA |
| Ssoapt-seq-R | GAAATTAGCACAAGATGCAGAA |
| Stoapt-seq-F | TTAAATTGCCTAAGATACCTGTT |
| Stoapt-seq-R | AAAGACATTTGGTGGAGCTAT |
| apt-Up-F | TCGCGTCGACGACCTCCTCTGCTGTAACTGG |
| apt-Up-R | ACTAGCTAGCGGGCAAAATTAACTCCCTAA |
| apt-Dn-F | ACTAGCTAGCATAATCTAATTAATATAGCCTATACCTTA |
| apt-Dn-R | ACGCGGATCCTGGTCTTATAATTGTGGAGAGG |
| apt-Tg-F | ACATGCATGCTGAAAGTAGTCACATGGGATGA |
| apt-Tg-R | GAAACGGCCGTTAGATTATTTTTCTTCTTTTCATTTC |
| apt-flankP-F | GTAACCTTTGAATTAACGCATA |
| apt-flankP-R | ATAGAACTGACAAGGAGTTTCA |
| apt-intP-F | GATAGCAAGAGGAGGTTTAGTTC |
| apt-intP-R | TGAGATTGGTGGGTCATTTAT |
| lacS-flankP-F | TACGGGAAGTAACACGGAGC |
| lacS-flankP-R | TACGGGAAGTAACACGGAGC |
| Sso-apt-F | CATGCCATGGTCATTTTGCTCAATCATAAGATG |
| Sso-apt-R | TGATGCTAGCTTTGTTGTTGGTGATGAAGTG |
| Sso-argD/apt-F | ACTAACGCGTTACTTTCTTACTGCT |
| Sso-argD/apt-R | GAAACGGCCGTTTGTTGTTGGTGATGAAGTG |
| argD-F | CATGCCATGGATTCTCCAATATATGGGGTTT |
| lacS-R | AAAACGCGTCCTAGTGTTGCAAGGCAGAT |
| amyA-Up-F | CGCGGATCC TTCAGTAGTGTTTGGAGGATATG |
| amyA-Up-R | CGGGGTACCTTTAGGGAGATTAACCATTGAT |
| amyA-Dn-For | CGGGGTACCGTAATGAAGAGAGGTCACATTTAG |
| amyA-Dn-Rev | AAGCGTCGACACCTAATCGCATTTTTAGTCC |
| amyA-Tg-F | GAAACGGCCGATGATAAAAGCCTCATGTTTAT |
| amyA-Tg-R | ACATGCATGCAGTGTGTAGCCATACCCAAG |
| amyA-flankP-F | AAATCTATATCCGTATTCATCACC |
| amyA-flankP-R | TTATCGGGACAATCCTAGTGT |
Added restriction sites are underlined.
Construction of gene deletion and complementation vectors.
Plasmid pRJW8 harboring a hybrid marker cassette, argD-pyrEF-lacS (12), was used to construct the apt deletion plasmid via the recently established marker insertion and unmarked target gene deletion (MID) method (12, 40). The regions upstream (the upstream arm [Up-arm], 773 bp) and downstream (the downstream arm [Dn-arm], 703 bp) of the apt gene were amplified from S. islandicus M.16.4 genomic DNA using the primer sets apt-Up-F/R and apt-Dn-F/R, respectively (Table 2). SalI/NheI and NheI/BamHI sites were introduced into the products obtained by PCR with primer sets apt-Up-F/R and apt-Dn-F/R, respectively. The reverse primer apt-Up-R started at position +6 relative to the apt TTG start codon, and the forward primer apt-Dn-F started 9 bp 5′ to the apt TAA stop codon. A triple ligation with Up-arm, Dn-arm, and the cloning vector pRJW8 resulted in plasmid pKapt-UD, which contained the in-frame Δapt allele. Afterwards, a partial targeted apt gene (Tg-arm; 617 bp) was amplified using the primer pair apt-Tg-F/R. apt-Tg-F started at position +16 relative to the apt start codon and the added SphI site, whereas apt-Tg-R started at the end of the apt stop codon and contained an EagI site. SphI/EagI-digested Tg-arm was cloned into pKapt-UD at the corresponding sites, yielding the apt deletion plasmid pMID-apt.
To construct the apt complementation plasmid pC-Ssoapt, the wild-type copy of apt from S. solfataricus P2 (Sso-apt), constituting the open reading frame of Sso2342 as well as its native promoter and terminator region (824 bp in total), was PCR amplified with primer pair Sso-apt-F/R (Table 2), with NcoI and NheI sites being introduced at the 5′ and 3′ ends, respectively. Then, the resulting PCR fragment was digested with NcoI/NheI and inserted at the corresponding sites of a nonreplicative plasmid, pC-SsoargD, harboring the S. solfataricus argD (Sso-argD) expression cassette flanked by the upstream and downstream regions of lacS in S. islandicus M.16.4 (12).
An α-amylase-knockout plasmid, pMID-amyA, was also constructed by the MID method (40), with slight modifications. The argD-apt cassette was amplified from pC-Ssoapt with the primer sets Sso-argD/apt-F and Sso-argD/apt-R (Table 2) and replaced the argD-pyrEF cassette in pRJW8, generating pRJW9. Similarly, three homologous arms, namely, the amyA Up-arm, amyA Dn-arm, and amyA Tg-arm, were sequentially inserted into pRJW9, thereby generating the amyA deletion plasmid pMID-amyA.
Construction of an S. islandicus mutant with unmarked in-frame apt and amyA deletions.
S. islandicus RJW004 (ΔargD ΔpyrEF ΔlacS) (12) was used as the host strain to construct the Δapt mutant (RJW009). The linearized knockout plasmid pMID-apt was introduced into RJW004 by an electroporation-mediated transformation procedure that was described earlier (41) and modified as described in reference 12. The transformants with the hybrid marker cassette argD-pyrEF-lacS integrated into the RJW004 chromosome were screened on selective plates without agmatine. The sequence of one candidate strain (pMID-apt-T) was further confirmed by PCR using the primers apt-flankP-F/R, which were specific for sequences located outside the apt flanking region (Table 2). To obtain unmarked apt deletion strains, colonies in a 6-methylpurine-resistant culture were first enriched in liquid medium containing uracil (20 μg/ml), agmatine (1 mg/ml), GMP (0.5 mM), and 6-MP (150 to 300 μM) and then plated on solid medium. Single colonies were screened by colony PCR using two distinct primer sets, apt-flankP-F/R and apt-intP-F/R, which annealed outside the flanking regions and internal sequences of apt, respectively (Table 2). The sequences of the resulting PCR fragments amplified by primer set apt-flankP-F/R were further confirmed by sequencing of the apt locus.
The ΔamyA mutant (RJW010) was constructed by using RJW009 (RJW004 Δapt) as a parent strain. Again, the transformant (pMID-amyA-T) with a hybrid maker cassette argD-apt-lacS integrated into the RJW009 chromosome was selected without agmatine, and successful integration was confirmed by PCR using the proper primer sets shown in Table 2. Cell cultures positive for pMID-amyA-T were then directly subjected to counterselection in DT medium containing uracil (20 μg/ml), agmatine (1 mg/ml), GMP (0.5 mM), and 6-MP (150 to 300 μM). The in-frame deletion of amyA was confirmed by PCR and sequencing analysis at the amyA locus with appropriate primers (Table 2).
Complementation of the Δapt deletion mutant.
Complementation of the Δapt strain was achieved upon electroporation with BamHI-linearized pC-Ssoapt. The transformants with the Sso-argD and Sso-apt cassette inserted at the ΔlacS locus via double-crossover homologous recombination were screened by selection for agmatine prototrophs. Colonies prototrophic for agmatine were examined with the primer sets lacS-flankP-F/R and apt-flankP-F/R (Table 2), whose sequences are specific for regions located outside the lacS and apt flanking regions, respectively, and one positive candidate was colony purified three times for further study.
Phylogenetic analysis of apt homologs.
Sequences to be analyzed were retrieved from the NCBI microbial protein database (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi). The sequences of S. islandicus M164_0158 (Apt) and M164_0233 (Gpt) were aligned with the selected sequences using the MUSCLE program (v3.7), which was configured to achieve the highest accuracy. Phylogenetic analysis was performed on the Phylogeny.fr platform (www.phylogeny.fr) (42). After alignment, ambiguous regions (i.e., regions containing gaps and/or poorly aligned regions) were removed by use of the Gblocks program (v0.91b), which was performed using the following parameters: the minimum length of a block after gap cleaning was 10, no gap positions were allowed in the final alignment, all segments with contiguous nonconserved positions of greater than 8 were rejected, and the minimum proportion of sequences for a flank position was 85%. The phylogenetic tree was reconstructed using the maximum likelihood method implemented in the PhyML program (v3.0 aLRT). The default substitution model was selected by assuming an estimated proportion of invariant sites of 0.021 and 4 gamma-distributed rate categories to account for rate heterogeneity across sites. The gamma shape parameter was estimated directly from the data (gamma = 1.044). The statistical robustness and reliability of the branching order within each phylogenetic tree were confirmed with a bootstrap analysis using 1,000 replicates. The tree was represented by use of the TreeDyn program (v198.3).
α-Amylase plating assay.
The α-amylase activities of RJW010 ΔamyA and its parent strain, RJW009, were detected by a method described previously (43, 44). Briefly, cell cultures were washed twice and resuspended in Brock's medium (45), and then 8 μl cells (with a normalized OD600 of 0.5) was spotted on Brock's medium plates supplemented with 0.2% starch (Sigma-Aldrich, USA) as a carbon source and 5 mM l-glutamic acid (Sigma-Aldrich, USA) as an alternative carbon and energy source (43). After 11 days of incubation at 78°C, the plates were flooded with 5 ml Gram's iodine solution (Sigma-Aldrich, USA) to visualize the halos generated by starch hydrolysis.
RESULTS
S. islandicus M.16.4 is highly sensitive to the purine analog 6-MP.
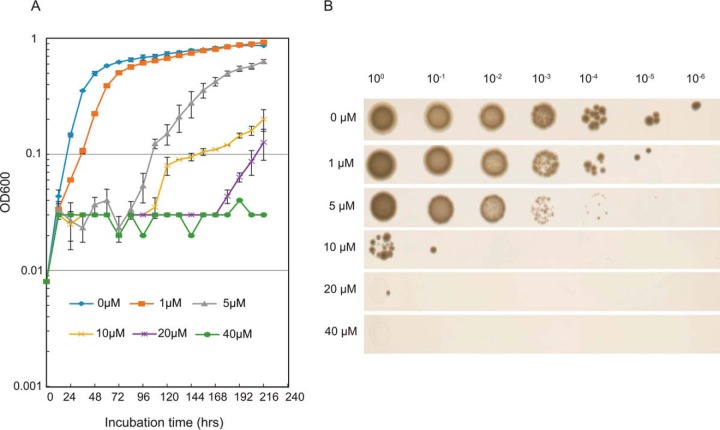
First, we examined the growth of wild-type strain S. islandicus M.16.4 in the presence of a few purine analogs (Table 3). We found that 6-methylpurine (6-MP), 8-azaguanine, and 6-thioguanine apparently inhibited cell growth in liquid medium, with 6-MP showing the lowest MIC (Table 3). The influence of this drug on cell growth was studied in more detail. As shown in Fig. 1A, the culture exhibited slightly retarded growth in the presence of 1 μM 6-MP but could reach approximately the same optical density as cultures growing in the absence of 6-MP. Furthermore, concentrations of 6-MP above 5 μM significantly affected the growth of S. islandicus M.16.4, and the cell growth in liquid medium was completely inhibited by 40 μM 6-MP.
TABLE 3.
MICs of various purine analogs for wild-type strain S. islandicus M.16.4a
| Purine analog | MIC (mM) |
|---|---|
| 6-Methylpurine | 0.04 |
| 6-Thioguanine | 0.5 |
| 8-Azaguanine | 0.1 |
| 2,6-Diaminopurine | 3.0 |
| 2-Aminopurine | >3.0 |
| 2-Amino-6-methylmercaptopurine | 3.0 |
| 6-Methylaminopurine | >3.0 |
Sulfolobus cell cultures were inoculated into 45 ml DT liquid medium containing various concentrations of purine analogs. The initial OD600 of the cells was normalized to 0.008 by calculation. Cells were incubated at 75 to 78°C without shaking, and the growth was monitored by measuring the cell density after 10 days.
FIG 1.
Growth-inhibiting effect of various concentrations of 6-methylpurine on wild-type S. islandicus M.16.4. (A) Growth curves of S. islandicus M.16.4 in DT liquid medium containing 0, 1, 5, 10, 20, and 40 μM 6-methylpurine. The initial OD600 of the different cell cultures was normalized to 0.008, cells were grown without shaking, and cell growth was monitored every 12 h. Error bars represent standard deviations from three independent experiments. (B) Spotting assay with S. islandicus M.16.4 on a plate with medium containing various concentrations of 6-methylpurine. Eight-microliter cell dilutions were dropped onto the appropriate plates, and the growth was checked after 9 days of incubation at 78°C.
To examine the effect of 6-MP on the growth of S. islandicus M.16.4 on plates, an exponentially growing culture was used to prepare a series of dilutions of cell suspensions, which were then spotted on DT medium plates containing different concentrations of 6-MP and incubated for 9 days. As shown in Fig. 1B, 6-MP at a concentration of 40 μM completely inhibited cell growth (Fig. 1B), consistent with the results obtained with liquid medium. We tested 6 different Sulfolobus species for their 6-MP tolerance in liquid medium and found that the MICs fell into the range of 20 to 100 μM. Interestingly, S. acidocaldarius showed a much higher level of resistance to 6-MP, with the MIC for S. acidocaldarius being more than 400 μM (see Table S1 in the supplemental material).
6-MPr results from the loss of function of M164_0158.
To reveal the genetic determinant of the 6-MP resistance in S. islandicus M.16.4, 10 6-MPr colonies were picked up from a DT medium plate containing 80 μM 6-MP, and then the putative promoter and coding regions of each annotated PRTase gene, i.e., M164_1910 (Sispt-1), M164_0233 (Sispt-2), and M164_0158 (Sispt-3), were screened by PCR. Sequencing of the PCR products revealed that 6-MPr mutations were exclusively located in the Sispt-3 gene, either on its promoter or in the coding region. A phylogenetic analysis showed that M164_0158 is a homolog of SSO2342 (see Fig. S1 in the supplemental material), a biochemically well-characterized adenine PRTase (APRTase) in Sulfolobus solfataricus P2 (39), indicating that M164_0158 (named apt here) codes for a putative APRTase in S. islandicus M.16.4.
Deletion of M164_0158 (apt) confers resistance to 6-MP, but AMP or GMP is required to maintain normal cell growth.
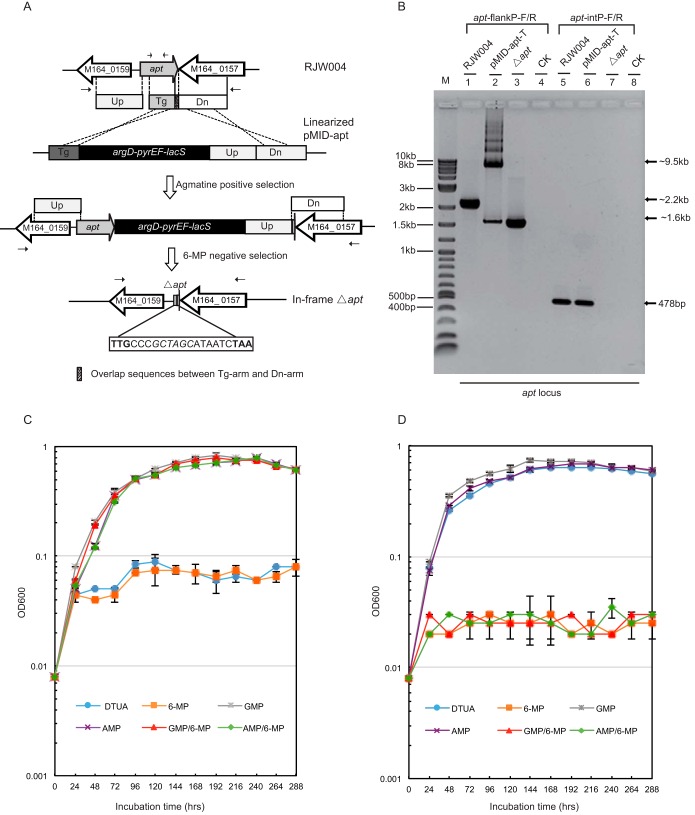
To verify that the apt gene could be the sole target of 6-MP, we sought to create an apt deletion mutant following the recently established MID methodology (12). Transformants of strain RJW004 (ΔargD ΔpyrEF ΔlacS) that had acquired the marker cassette via double homologous recombination were selected by growth on a plate with medium lacking agmatine (Fig. 2A), and the transformation/recombination efficiency was estimated to be 10 to 50 CFU/μg DNA. The sequence of a representative transformant, designated strain pMID-apt-T, was then confirmed by PCR analysis (Fig. 2B). An ∼2.2-kb fragment was obtained with wild-type DNA (RJW004), whereas two fragments with expected sizes of ∼9.5 kb and ∼1.6 kb were amplified with DNA from the transformant. These corresponded to the recombination allele and the apt deletion allele, respectively, as illustrated in Fig. 2B. To obtain the apt deletion mutants, 6-MPr cells were propagated by cultivating the pMID-apt-T recombinant in 6-MP-containing liquid medium, and then the cells were plated to isolate single 6-MPr colonies (see Fig. S2 in the supplemental material). The 6-MPr colonies were generated by either spontaneous apt mutation or excision of the marker cassette of pMID-apt-T recombinants, which were distinguishable by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining. Figure 2B shows the results of PCR analysis of a representative apt deletion mutant with two different primer sets, apt-flankP-F/R and apt-intP-F/R. PCRs with both primer sets revealed that the 6-MPr strain carried the designed allele with the apt deletion (Fig. 2B). This was further confirmed by sequencing of the 1.6-kb PCR products amplified from the 6-MPr strain with primers apt-flankP-F/R.
FIG 2.
Construction and phenotype characterization of the Δapt mutant. (A) Schematic of the apt deletion construct in the background of RJW004 (ΔargD ΔpyrEF ΔlacS). Arrows, positions of PCR primer sets. (B) PCR analysis of the apt locus in host strain RJW004, transformant pMID-apt-T, and the Δapt mutant using two different primer sets (apt-flankP-F/R and apt-intP-F/R). The expected sizes of the PCR products are indicated. Lane M, 2-log DNA ladder (New England Biolabs [NEB]); lanes CK (lanes 4 and 8), no DNA template was added when PCR amplifications were performed. (C) Growth profiles of the Δapt mutant (RJW009) in DTUA liquid medium with or without 6-MP, GMP, AMP, GMP and 6-MP, or AMP and 6-MP. These growth experiments were conducted with each cell culture at an initial OD600 of 0.008 and 78°C without shaking. Cell growth was monitored very 24 h for 12 days. Error bars represent standard deviations from three independent experiments. (D) Growth profiles of host strain RJW004 in DTUA liquid medium with or without 6-MP, GMP, AMP, GMP and 6-MP, or AMP and 6-MP.
The growth profiles of the Δapt strain, named RJW009 (Fig. 2C), and its parent strain, RJW004 (Fig. 2D), were documented by cultivating them in different types of media. It was found that the Δapt strain was resistant to a high concentration of 6-MP (0.15 mM, 3.75-fold the MIC); however, AMP or GMP was required to maintain its normal cell growth (Fig. 2C). In contrast, the growth of wild-type strain RJW004 was completely inhibited by 6-MP within 12 days of incubation, even in the presence of AMP or GMP (Fig. 2D).
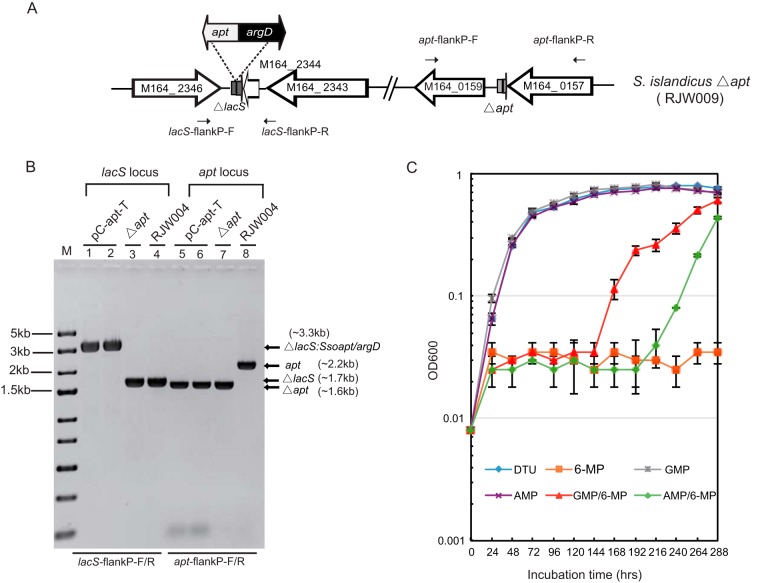
Finally, the apt deficiency was complemented by the insertion of the S. solfataricus apt gene (Sso2342) at the lacS locus of S. islandicus via double crossover, as illustrated in Fig. 3A. The sequences of the recombinants (strain pC-apt-T; Fig. 3B), in which Sso-apt and Sso-argD were integrated at the lacS locus, were confirmed by PCR analysis using primers with sequences specific for regions located outside the lacS and apt flanking regions, respectively (Fig. 3A and B). Growth analysis of one representative complemented Δapt strain (pC-apt-T) in liquid medium showed that its sensitivity to 6-MP was restored, and no GMP or AMP was required to maintain normal growth (Fig. 3C). Together, these results indicate that the apt gene is solely responsible for 6-MP sensitivity in this organism.
FIG 3.
Complementation of the S. islandicus Δapt mutant. (A) Integration of the apt expression cassette into the lacS gene deletion locus of S. islandicus Δapt via double crossover. The apt deletion strains were transformed with linearized pC-apt containing two homologous flanking regions of lacS, selectable markers for the agmatine prototroph (Sso-argD), and the apt expression cassette from S. solfataricus P2 (Sso-apt). The transformants were selected for agmatine prototrophs. (B) PCR analysis of the S. islandicus Δapt complemented strain. Primers with sequences specific for regions outside the 5′ and 3′ flanking regions of the lacS (lacS-flankP-F/R) or apt (apt-flankP-F/R) gene were designed. Lane M, DNA markers; lanes 1 to 4, the results of PCR with two individual pC-apt-T transformants, a Δapt mutant, and the host strain (RJW004), respectively, and primers lacS-flankP-F/R; lanes 5 to 8, the results of PCR with two individual pC-apt-T transformants, a Δapt mutant, and the host strain (RJW004), respectively, and primers apt-flankP-F/R. Arrows, the amplified fragments expected. (C) Growth profiles of representative complemented strain pC-apt-T in DT liquid medium with uracil (DTU) with or without 6-MP, GMP, AMP, GMP and 6-MP, or AMP and 6-MP.
Unmarked chromosomal gene deletion using the apt gene as a counterselection marker in S. islandicus.
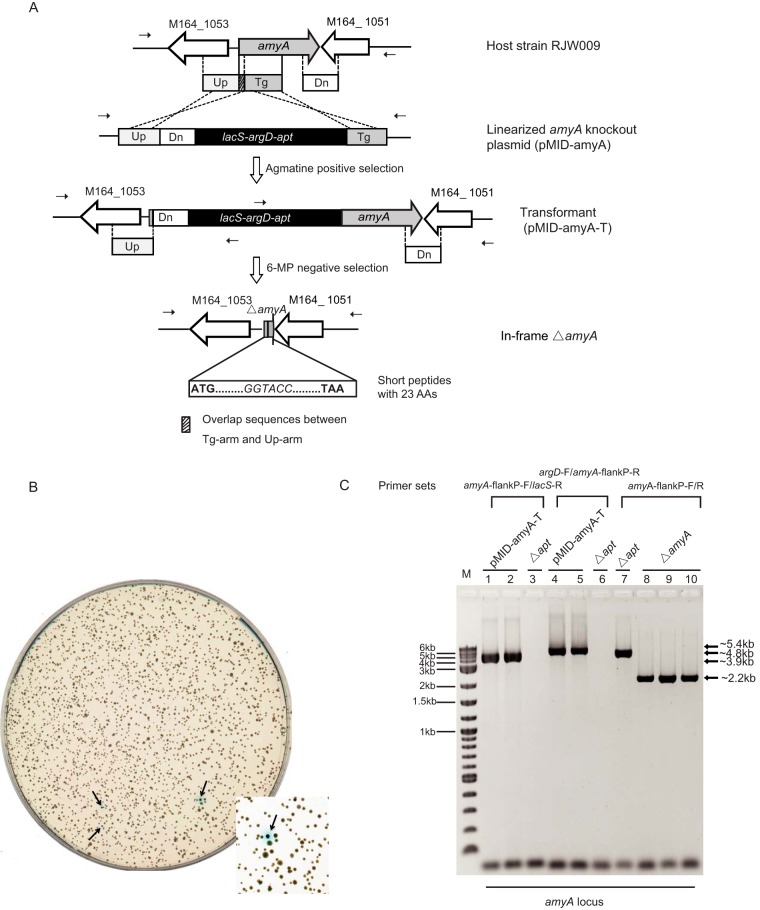
Next we studied the application of the apt/6-MP-based counterselection system to conduct unmarked chromosomal gene deletions in S. islandicus. A new integrative vector, pRJW9, containing a marker cassette consisting of the lacS, argD, and apt genes was made and employed to construct knockout plasmids. We chose to delete M164_1052, a putative α-amylase gene (amyA) coding for a protein whose amino acid sequence shared 92% and 61% identity with the amino acid sequences of the well-characterized α-amylases of S. solfataricus (SSO1172) and S. acidocaldarius (Saci_1162), respectively (43, 46, 47). The knockout plasmid was introduced into S. islandicus Δapt (i.e., strain RJW009 [ΔargD ΔpyrEF ΔlacS Δapt]) by electroporation, and transformants were selected by growth on a plate with medium lacking agmatine (Fig. 4A). PCR amplifications of two representative transformants (pMID-amyA-T-1 and pMID-amyA-T-2) confirmed that the knockout plasmid was integrated into the host chromosome via homologous recombination, as illustrated in Fig. 4A and C. We found that the 6-MPr colonies with unmarked amyA deletions (recombinants; Fig. 4B, white) could be effectively selected during a counterselection step with 0.15 mM 6-MP, 1 mM agmatine, and 0.5 mM GMP, whereas the frequency of spontaneous 6-MPr colonies (spontaneous mutants; Fig. 4B, blue) occurred at a ∼500-fold lower frequency. The amyA deletions from three randomly selected white colonies were confirmed by PCR with primers whose sequences bind to regions outside the amyA flanking regions (Fig. 4C, lanes 8 to 10).
FIG 4.
Construction of mutants (RJW010) with an unmarked amyA gene deletion based on the apt/6-MP counterselection system. (A) Schematic overview of the recombination strategy used to generate an unmarked mutant with an in-frame amyA gene deletion via 6-MP counterselection. S. islandicus RJW009 (ΔargD ΔpyrEF ΔlacS Δapt) was used as the host strain for genetic manipulation. Cells that had undergone a double-crossover homologous recombination were selected by agmatine prototrophy. Cells (strain pMID-amyA-T) that suffered from a second single crossover at two repeated homologous Up-arms generated mutants with an in-frame unmarked amyA gene deletion in the presence of 150 to 300 μM 6-MP. AAs, amino acids. (B) X-Gal staining of 6-MPr colonies on 6-MP-containing plates. (C) PCR verification of the amyA locus in the host strain RJW009 Δapt, pMID-amyA-T transformants, and the ΔamyA mutant (RJW010). The pMID-amyA-T sequence was verified with two primer sets, amyA-flankP-F/lacS-R and argD-F/amyA-flankP-R, and the sequences of the ΔamyA mutants were analyzed with primers whose sequences were specific for regions located outside the 5′ and 3′ flanking regions. The expected sizes of the PCR products are indicated. Lane M, 2-log DNA ladder (NEB).
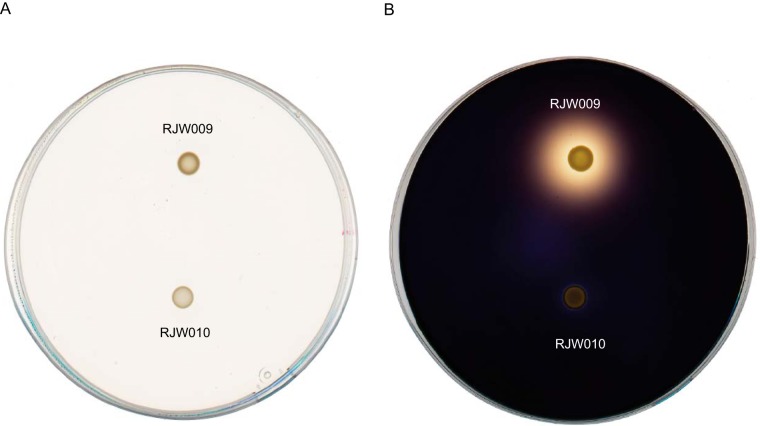
To reveal the phenotype of the deletion mutants, the amylase activities of one of the ΔamyA strains (RJW010) and its parent strain (RJW009 Δapt) were assayed on starch-containing plates, and this revealed a large halo surrounding the colony of the wild-type strain (Fig. 5B), which was absent from the colony of the ΔamyA mutant (Fig. 5B). Taken together, the successful construction of a strain with an unmarked amyA deletion indicated that apt/6-MP represents a counterselection system useful for genetic manipulation in S. islandicus.
FIG 5.
Starch hydrolysis test with the ΔamyA mutant (RJW010) and its parent strain, RJW009. Cells were grown on solid medium containing 0.2% starch and 5 mM glutamic acid for 11 days at 78°C. (A) No treatment; (B) treatment with Gram's iodine solution.
Application of the apt gene in a forward mutation assay.
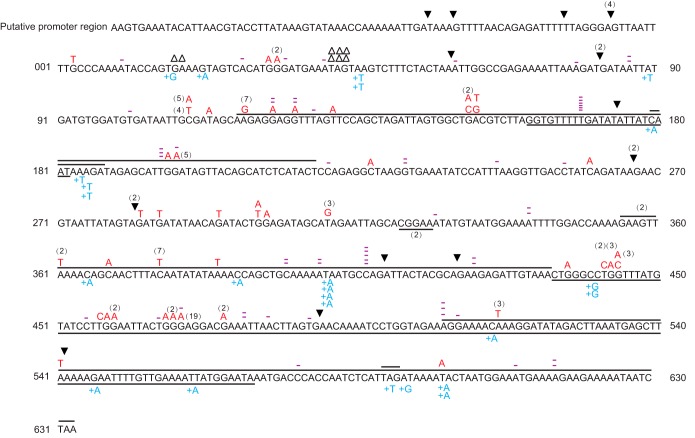
Subsequently, we tested the usefulness of the apt gene as a genetic marker to evaluate the spectrum of spontaneous mutation in S. islandicus M.16.4. This was done by selecting 215 independent 6-MPr colonies from 24 independent experiments and analyzing their apt mutant alleles. The apt coding sequence and its flanking regions, including 122 bp upstream of the start codon and 62 bp downstream of the stop codon, were amplified by PCR using primers Sispt-3-F/R (Table 2). The sequences of the resulting PCR fragments were determined by DNA sequencing and compared with the sequence of the wild-type gene. Mutations of different types were observed, including base substitutions (BPSs; 46.5%); small indels (<3 bp) causing frameshift mutations (38.6%); and large indels, including large deletions (1.4%) and large insertion mutations of tandem duplications and an insertion sequence (IS) element (ISC1205) (see Fig. S3 in the supplemental material)-based mutation (13.5%) (Table 4). The molecular characteristics of all these mutations, except for a large deletion occurring in the region from positions −40 to +455 of the gene, are illustrated graphically in Fig. 6. This indicated that apt constitutes another useful reporter gene suitable for use in forward mutation assays.
TABLE 4.
Summary of spontaneous mutations at the apt locus in wild-type strain S. islandicus M.16.4
| Mutation | No. of 6-MPr S. islandicus M.16.4 isolates (% of total) |
|---|---|
| BPSs | 100 (46.5) |
| Transitions | 71 (33.0) |
| G:C→A:T | 60 (27.9) |
| A:T→G:C | 11 (5.1) |
| Transversions | 29 (13.5) |
| G:C→T:A | 13 (6.0) |
| G:C→C:G | 2 (0.9) |
| A:T→C:G | 3 (1.4) |
| A:T→T:A | 11 (5.1) |
| Frameshifts | 83 (38.6) |
| −1 bp | 58 (27.0) |
| +1 bp | 24 (9.6) |
| −2 bp | 1 (0.5) |
| Insertions | 29 (13.5) |
| Tandem duplications | 10 (4.7) |
| IS-mediated insertions | 19 (8.8) |
| Deletions | 3 (1.4) |
| 3 bp | 2 (0.9) |
| 495 bp | 1 (0.5) |
| Total | 215 (100) |
FIG 6.
The spontaneous mutational spectra in S. islandicus M.16.4 at the apt locus. The coding region of the apt gene is labeled starting from base 1 (T) and going to base 633 (A). The intergenic region between apt (M164_0158) and M164_0159 is listed as base −83 (A) to base −1 (T). BPSs are denoted with a base in red above the sequence. Single-base-pair insertions or deletions are represented by a base with a plus sign in blue or a minus sign in purple, respectively. △△ and △△△, deletions of 2 and 3 bp, respectively; underlining or overlining, tandem duplications of the corresponding sequences; ▼, insertion sequence element (IS1205)-mediated insertions. A large deletion of the fragment of the apt gene from the A at position −40 to the C at position +455 is not shown. The numbers in parentheses indicate the number of independent isolates with the same mutation event.
DISCUSSION
In this study, we provide genetic evidence that M164_0158 encodes an adenine phosphoribosyltransferase (APRTase) in the hyperthermophilic crenarchaeon S. islandicus M.16.4 and a mutation in the apt gene confers 6-MP resistance by preventing lethal incorporation of this toxic purine analog. For this reason, the apt/6-MP system represents another counterselection marker, in addition to the pyrEF/5-FOA system. We show that this marker has two important applications in S. islandicus: (i) the apt/6-MP system serves as an efficient counterselection marker for constructing markerless gene deletions, and (ii) it is a useful reporter gene for studying the profile of spontaneous mutations.
Recently, Sso2342 (apt) and Sso2424 (gpt) of S. solfataricus P2 have been characterized biochemically, and they have been found to encode adenine and hypoxanthine-guanine-xanthine phosphoribosyltransferases, respectively (39). Here we show that S. islandicus apt is the genetic target of 6-MP selection. Since the amino acid sequence of the apt-encoded protein (M164_0158) shares 90% identity with the S. solfataricus SSO2342 amino acid sequence, this suggests that M164_0158 also encodes an APRTase. Furthermore, phylogenetic analyses of proteins that show sequence similarity to the characterized APRTase (SSO2342, Apt) and guanosine PRTase (GPRTase; SSO2424, Gpt) of S. solfataricus P2 (39) show that they fall into different clades (see Fig. S1B in the supplemental material). This indicates that the Apt and Gpt clades of proteins are well conserved in crenarchaea and perhaps the result of duplication at this locus before divergence within this clade. We also found that a few other Sulfolobus species, including S. acidocaldarius, Sulfolobus tokodaii, and S. islandicus REY15A, that carry an apt gene (i.e., Saci_0998, St0484, and SiRe_0139, respectively) and that are sensitive to 6-MP (see Table S1 in the supplemental material) and exhibit 6-MP counterselection. Together, this finding suggests that the APRTase is conserved in the genus Sulfolobus and probably in all crenarchaea whose genomes code for an apt homolog and they could be tested for application of the apt/6-MP counterselection system in genetic studies.
Characterization of the growth of the Δapt strain showed that cell growth was significantly impaired in liquid medium with or without 6-MP during a period of 12 days of incubation (Fig. 3C); however, cell growth recovered after a prolonged cultivation (∼ 3 weeks; not shown in Fig. 3C). Interestingly, the addition of AMP or GMP was required to maintain robust growth (Fig. 3C). The reason that GMP can also support cell growth was probably due to the interconversion between GMP and AMP in purine metabolism, as is reported to occur in most microorganisms (48). The molecular mechanism for the requirement for AMP or GMP for the growth of apt-deficient strains of S. islandicus remains unknown, but a similar phenomenon was also described in the hyperthermophilic euryarchaeon P. furiosus, in which the growth of the Δhpt mutant was maintained well in the presence of 6 mM GMP (38). In contrast, in another hyperthermophilic euryarchaeon, T. kodakarensis, no retarded growth was observed in the medium by testing the hpt deletion strain (37) or 6-MPr strains with spontaneous mutations or indels in the hpt gene (T. J. Santangelo, personal communications). It was also noteworthy that the apt-deficient strain also showed relatively slower growth on Gelrite plates, which could be explained by the observation that single 6-MPr colonies appeared after approximately 14 days of incubation on the overlay plates to which AMP or GMP was not added.
The purine PRTase-encoding genes have been broadly applied in forward mutation assays for bacteria and eukaryotes, which were based on the inability of mutants to enzymatically convert a drug to a toxic metabolite (23–25). Recently, this counterselection system has been tested in T. kodakarensis, in which the hypoxanthine-guanine phosphoribosyltransferase-encoding gene hpt was used to compare the patterns of spontaneous mutations that occurred in wild-type and DNA polymerase B mutant strains (49). Here we have used the apt gene for the forward mutation assay in S. islandicus M.16.4. Another reporter gene that has been used in the forward mutation assay is the pyrEF/5-FOA system, with which mutation profiles have been obtained for S. acidocaldarius, S. solfataricus, and S. islandicus (50, 51). The profile of frameshifts, tandem duplications, and larger deletion and BPSs obtained in this work was generated from molecular analysis of the apt locus from over 200 6-MPr strains. We found that the mutation profile is comparable to that of T. kodakarensis, also assayed by 6-MP counterselection with an hpt gene (49). Interestingly, a 5-FOA-based forward mutation assay in S. acidocaldarius revealed that frameshift mutations are dominant in this organism (50). Furthermore, this and a few other studies have revealed that the transitions outnumbered transversions by more than 2-fold, and a very strong strand bias for G or C-to-A or T transitions was observed (49, 50, 52, 53). These should be further studied by generating mutants deficient in certain DNA repair mechanisms and investigating their effect on the profile of spontaneous mutations. Since most mutants are generated using pyrEF as a selection marker (e.g., see references 7 and 13), the pyrEF/5-FOA system for use in forward mutation assays is no longer available. The demonstration of an apt/6-MP counterselection system for the Sulfolobus model renders it possible again to study the mutation profile in mutants that have already been constructed in the background of pyrEF deficiency. Vice versa, when apt/6-MP is used for genetic manipulations, the mutation profile in the generated mutants can then be investigated using the pyrEF/5-FOA system. In conclusion, the apt marker developed here will further facilitate genetic studies in Sulfolobus organisms, which serve as the most broadly applicable genetic model in the Crenarchaeota.
Supplementary Material
ACKNOWLEDGMENT
We thank Isabelle Anna Zink from the Department of Ecogenomics and Systems Biology at the University of Vienna for providing helpful suggestions about the α-amylase plating assay.
Funding Statement
This work was supported by the National Aeronautics and Space Administration (NASA) through the NASA Astrobiology Institute under cooperative agreement no. NNA13AA91A, issued through the Science Mission Directorate.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00455-16.
REFERENCES
- 1.Whitaker RJ, Grogan DW, Taylor JW. 2003. Geographic barriers isolate endemic populations of hyperthermophilic archaea. Science 301:976–978. doi: 10.1126/science.1086909. [DOI] [PubMed] [Google Scholar]
- 2.Cadillo-Quiroz H, Didelot X, Held NL, Herrera A, Darling A, Reno ML, Krause DJ, Whitaker RJ. 2012. Patterns of gene flow define species of thermophilic Archaea. PLoS Biol 10:e1001265. doi: 10.1371/journal.pbio.1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guy L, Ettema TJG. 2011. The archaeal ‘TACK’ superphylum and the origin of eukaryotes. Trends Microbiol 19:580–587. doi: 10.1016/j.tim.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Reno ML, Held NL, Fields CJ, Burke PV, Whitaker RJ. 2009. Biogeography of the Sulfolobus islandicus pan-genome. Proc Natl Acad Sci U S A 106:8605–8610. doi: 10.1073/pnas.0808945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo L, Brugger K, Liu C, Shah SA, Zheng H, Zhu Y, Wang S, Lillestol RK, Chen L, Frank J, Prangishvili D, Paulin L, She Q, Huang L, Garrett RA. 2011. Genome analyses of Icelandic strains of Sulfolobus islandicus, model organisms for genetic and virus-host interaction studies. J Bacteriol 193:1672–1680. doi: 10.1128/JB.01487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaubert C, Danioux C, Oberto J, Cortez D, Bize A, Krupovic M, She Q, Forterre P, Prangishvili D, Sezonov G. 2013. Genomics and genetics of Sulfolobus islandicus LAL14/1, a model hyperthermophilic archaeon. Open Biol 3:130010. doi: 10.1098/rsob.130010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.She Q, Zhang C, Deng L, Peng N, Chen Z, Liang YX. 2009. Genetic analyses in the hyperthermophilic archaeon Sulfolobus islandicus. Biochem Soc Trans 37:92–96. doi: 10.1042/BST0370092. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Whitaker RJ. 2012. A broadly applicable gene knockout system for the thermoacidophilic archaeon Sulfolobus islandicus based on simvastatin selection. Microbiology 158:1513–1522. doi: 10.1099/mic.0.058289-0. [DOI] [PubMed] [Google Scholar]
- 9.Deng L, Zhu H, Chen Z, Liang YX, She Q. 2009. Unmarked gene deletion and host-vector system for the hyperthermophilic crenarchaeon Sulfolobus islandicus. Extremophiles 13:735–746. doi: 10.1007/s00792-009-0254-2. [DOI] [PubMed] [Google Scholar]
- 10.Berkner S, Grogan D, Albers SV, Lipps G. 2007. Small multicopy, non-integrative shuttle vectors based on the plasmid pRN1 for Sulfolobus acidocaldarius and Sulfolobus solfataricus, model organisms of the (cren-)archaea. Nucleic Acids Res 35:e88. doi: 10.1093/nar/gkm449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng T, Huang Q, Zhang C, Ni J, She Q, Shen Y. 2012. Development of a simvastatin selection marker for a hyperthermophilic acidophile, Sulfolobus islandicus. Appl Environ Microbiol 78:568–574. doi: 10.1128/AEM.06095-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Cooper TE, Krause DJ, Whitaker RJ. 2013. Augmenting the genetic toolbox for Sulfolobus islandicus with a stringent positive selectable marker for agmatine prototrophy. Appl Environ Microbiol 79:5539–5549. doi: 10.1128/AEM.01608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Tian B, Li S, Ao X, Dalgaard K, Gokce S, Liang Y, She Q. 2013. Genetic manipulation in Sulfolobus islandicus and functional analysis of DNA repair genes. Biochem Soc Trans 41:405–410. doi: 10.1042/BST20120285. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Pan S, Zhang Y, Ren M, Feng M, Peng N, Chen L, Liang YX, She Q. 2016. Harnessing type I and type III CRISPR-Cas systems for genome editing. Nucleic Acids Res 44:e34. doi: 10.1093/nar/gkv1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs KL, Grogan DW. 1997. Rates of spontaneous mutation in an archaeon from geothermal environments. J Bacteriol 179:3298–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner M, van Wolferen M, Wagner A, Lassak K, Meyer BH, Reimann J, Albers SV. 2012. Versatile genetic tool box for the crenarchaeote Sulfolobus acidocaldarius. Front Microbiol 3:214. doi: 10.3389/fmicb.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Krause DJ, Whitaker RJ. 2013. Sulfolobus islandicus: a model system for evolutionary genomics. Biochem Soc Trans 41:458–462. doi: 10.1042/BST20120338. [DOI] [PubMed] [Google Scholar]
- 18.Krause DJ, Didelot X, Cadillo-Quiroz H, Whitaker RJ. 2014. Recombination shapes genome architecture in an organism from the archaeal domain. Genome Biol Evol 6:170–178. doi: 10.1093/gbe/evu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller KL, Bender KS, Wall JD. 2009. Development of a markerless genetic exchange system for Desulfovibrio vulgaris Hildenborough and its use in generating a strain with increased transformation efficiency. Appl Environ Microbiol 75:7682–7691. doi: 10.1128/AEM.01839-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graf N, Altenbuchner J. 2011. Development of a method for markerless gene deletion in Pseudomonas putida. Appl Environ Microbiol 77:5549–5552. doi: 10.1128/AEM.05055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song BF, Ju LZ, Li YJ, Tang LJ. 2014. Chromosomal insertions in the Lactobacillus casei upp gene that are useful for vaccine expression. Appl Environ Microbiol 80:3321–3326. doi: 10.1128/AEM.00175-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukagawa T, Hayward N, Yang J, Azzalin C, Griffin D, Stewart AF, Brown W. 1999. The chicken HPRT gene: a counter selectable marker for the DT40 cell line. Nucleic Acids Res 27:1966–1969. doi: 10.1093/nar/27.9.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skopek TR, Liber HL, Krolewski JJ, Thilly WG. 1978. Quantitative forward mutation assay in Salmonella typhimurium using 8-azaguanine resistance as a genetic marker. Proc Natl Acad Sci U S A 75:410–414. doi: 10.1073/pnas.75.1.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenwick RG Jr, Sawyer TH, Kruh GD, Astrin KH, Caskey CT. 1977. Forward and reverse mutations affecting the kinetics and apparent molecular weight of mammalian HGPRT. Cell 12:283–291. [DOI] [PubMed] [Google Scholar]
- 25.Albertini RJ. 2001. HPRT mutations in humans: biomarkers for mechanistic studies. Mutat Res 489:1–16. doi: 10.1016/S1383-5742(01)00064-3. [DOI] [PubMed] [Google Scholar]
- 26.Glaab WE, Mitchell LS, Miller JE, Vlasakova K, Skopek TR. 2005. 5-Fluorouracil forward mutation assay in Salmonella: determination of mutational target and spontaneous mutational spectra. Mutat Res 578:238–246. doi: 10.1016/j.mrfmmm.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Worrell VE, Nagle DP Jr. 1990. Genetic and physiological characterization of the purine salvage pathway in the archaebacterium Methanobacterium thermoautotrophicum Marburg. J Bacteriol 172:3328–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuer-Lauridsen B, Nygaard P. 1998. Purine salvage in two halophilic archaea: characterization of salvage pathways and isolation of mutants resistant to purine analogs. J Bacteriol 180:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen TL, Whitman WB. 1987. Incorporation of exogenous purines and pyrimidines by Methanococcus voltae and isolation of analog-resistant mutants. Appl Environ Microbiol 53:1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeMoll E, Auffenberg T. 1993. Purine metabolism in Methanococcus vannielii. J Bacteriol 175:5754–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeMoll E, Tsai L. 1986. Conversion of purines to xanthine by Methanococcus vannielii. Arch Biochem Biophys 250:440–445. doi: 10.1016/0003-9861(86)90747-2. [DOI] [PubMed] [Google Scholar]
- 32.Atomi H, Imanaka T, Fukui T. 2012. Overview of the genetic tools in the Archaea. Front Microbiol 3:337. doi: 10.3389/fmicb.2012.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farkas JA, Picking JW, Santangelo TJ. 2013. Genetic techniques for the archaea. Annu Rev Genet 47:539–561. doi: 10.1146/annurev-genet-111212-133225. [DOI] [PubMed] [Google Scholar]
- 34.Moore BC, Leigh JA. 2005. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J Bacteriol 187:972–979. doi: 10.1128/JB.187.3.972-979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchett MA, Zhang JK, Metcalf WW. 2004. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Appl Environ Microbiol 70:1425–1433. doi: 10.1128/AEM.70.3.1425-1433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehlers C, Jager D, Schmitz RA. 2011. Establishing a markerless genetic exchange system for Methanosarcina mazei strain Go1 for constructing chromosomal mutants of small RNA genes. Archaea 2011:439608. doi: 10.1155/2011/439608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santangelo TJ, Cubonova L, Reeve JN. 2010. Thermococcus kodakarensis genetics: TK1827-encoded beta-glycosidase, new positive-selection protocol, and targeted and repetitive deletion technology. Appl Environ Microbiol 76:1044–1052. doi: 10.1128/AEM.02497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreuzer M, Schmutzler K, Waege I, Thomm M, Hausner W. 2013. Genetic engineering of Pyrococcus furiosus to use chitin as a carbon source. BMC Biotechnol 13:9. doi: 10.1186/1472-6750-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen MR, Jensen KS, Rasmussen MS, Christoffersen S, Kadziola A, Jensen KF. 2014. Specificities and pH profiles of adenine and hypoxanthine-guanine-xanthine phosphoribosyltransferases (nucleotide synthases) of the thermoacidophile archaeon Sulfolobus solfataricus. Extremophiles 18:179–187. doi: 10.1007/s00792-013-0595-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Guo L, Deng L, Wu Y, Liang Y, Huang L, She Q. 2010. Revealing the essentiality of multiple archaeal pcna genes using a mutant propagation assay based on an improved knockout method. Microbiology 156:3386–3397. doi: 10.1099/mic.0.042523-0. [DOI] [PubMed] [Google Scholar]
- 41.Albers SV, Driessen AJ. 2008. Conditions for gene disruption by homologous recombination of exogenous DNA into the Sulfolobus solfataricus genome. Archaea 2:145–149. doi: 10.1155/2008/948014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dereeper A, Audic S, Claverie JM, Blanc G. 2010. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol Biol 10:8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worthington P, Hoang V, Perez-Pomares F, Blum P. 2003. Targeted disruption of the alpha-amylase gene in the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol 185:482–488. doi: 10.1128/JB.185.2.482-488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soo E, Rudrappa D, Blum P. 2015. Membrane association and catabolite repression of the Sulfolobus solfataricus α-amylase. Microorganisms 3:567–587. doi: 10.3390/microorganisms3030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brock TD, Brock KM, Belly RT, Weiss RL. 1972. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol 84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 46.Ellen AF, Albers SV, Driessen AJ. 2010. Comparative study of the extracellular proteome of Sulfolobus species reveals limited secretion. Extremophiles 14:87–98. doi: 10.1007/s00792-009-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi KH, Cha J. 2015. Membrane-bound amylopullulanase is essential for starch metabolism of Sulfolobus acidocaldarius DSM639. Extremophiles 19:909–920. doi: 10.1007/s00792-015-0766-x. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerman EF, Magasanik B. 1964. Utilization and interconversion of purine bases and ribonucleosides by Salmonella typhimurium. J Biol Chem 239:293–300. [PubMed] [Google Scholar]
- 49.Cubonova L, Richardson T, Burkhart BW, Kelman Z, Connolly BA, Reeve JN, Santangelo TJ. 2013. Archaeal DNA polymerase D but not DNA polymerase B is required for genome replication in Thermococcus kodakarensis. J Bacteriol 195:2322–2328. doi: 10.1128/JB.02037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grogan DW, Carver GT, Drake JW. 2001. Genetic fidelity under harsh conditions: analysis of spontaneous mutation in the thermoacidophilic archaeon Sulfolobus acidocaldarius. Proc Natl Acad Sci U S A 98:7928–7933. doi: 10.1073/pnas.141113098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackwan RR, Carver GT, Kissling GE, Drake JW, Grogan DW. 2008. The rate and character of spontaneous mutation in Thermus thermophilus. Genetics 180:17–25. doi: 10.1534/genetics.108.089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Redder P, Garrett RA. 2006. Mutations and rearrangements in the genome of Sulfolobus solfataricus P2. J Bacteriol 188:4198–4206. doi: 10.1128/JB.00061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindahl T, Nyberg B. 1974. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry 13:3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.