Abstract
Daily treatment with cannabinoids results in tolerance to many, but not all, of their behavioral and physiologic effects. The present studies investigated the effects of 7-day exposure to 10 mg/kg daily of Δ9-tetrahydrocannabinol (THC) on the diuretic and antinociceptive effects of THC and the synthetic cannabinoid AM4054. Comparison studies determined diuretic responses to the κ-opioid agonist U50,488 and furosemide. After determination of control dose-response functions, mice received 10 mg/kg daily of THC for 7 days, and dose-response functions were re-determined 24 hours, 7 days, or 14 days later. THC and AM4054 had biphasic diuretic effects under control conditions with maximum effects of 30 and 35 ml/kg of urine, respectively. In contrast, antinociceptive effects of both drugs increased monotonically with dose to >90% of maximal possible effect. Treatment with THC produced 9- and 7-fold rightward shifts of the diuresis and antinociception dose-response curves for THC and, respectively, 7- and 3-fold rightward shifts in the AM4054 dose-response functions. U50,488 and furosemide increased urine output to >35 ml/kg under control conditions. The effects of U50,488 were attenuated after 7-day treatment with THC, whereas the effects of furosemide were unaltered. Diuretic effects of THC and AM4054 recovered to near-baseline levels within 14 days after stopping daily THC injections, whereas tolerance to the antinociceptive effects persisted longer than 14 days. The tolerance induced by 7-day treatment with THC was accompanied by a 55% decrease in the Bmax value for cannabinoid receptors (CB1). These data indicate that repeated exposure to THC produces similar rightward shifts in the ascending and descending limbs of cannabinoid diuresis dose-effect curves and to antinociceptive effects while resulting in a flattening of the U50,488 diuresis dose-effect function.
Introduction
Marijuana and other cannabis products historically have been illegal in most countries, although their regulated medicinal or recreational use is becoming more prevalent (UNODC, 2014). The use of cannabis products (hereafter referred to by their principal psychoactive constituent, Δ9-tetrahydrocannabinol, or THC) is often characterized by repeated or long-term intake, whether as recreational drugs or for their reported medicinal effects in managing chronic conditions, including pain, anxiety, nausea, and anorexia (Pacher et al., 2006). Consequently, a full understanding of THC’s pharmacology necessitates the evaluation of how its effects are altered by repeated or chronic administration.
Repeated exposure to THC or other cannabinoid agonists in vivo has been shown to produce both downregulation and desensitization of CB1 cannabinoid receptors, leading to decreases in downstream signaling processes (Breivogel et al., 1999; Sim-Selley and Martin, 2002; Sim-Selley et al., 2006). These changes in CB1 receptor signaling are consistent with observations in earlier behavioral studies that demonstrated tolerance to the effects of THC or other CB1 receptor agonists in the four “tetrad” effects originally used to characterize cannabinoid drugs (Martin et al., 1991; Fan et al., 1994); however, the development of tolerance is not always uniform, nor is it necessarily evident across all behavioral endpoints. For example, mice treated daily with the CB1 agonists JWH-018 or JWH-073 demonstrated a complete loss of hypothermic responses after the second daily injection, whereas locomotor suppression induced by these drugs continued throughout the period of daily injections (Tai et al., 2015). Moreover, the magnitude of cannabinoid tolerance and cross-tolerance can vary with either the drug given chronically or the drug tested acutely (Fan et al., 1994). In one example of selective cross-tolerance, 32 mg/kg daily of THC eliminated the hypothermic effects of both THC and CP 55,940 and produced a rightward shift of the THC dose-response curve for rate-decreasing effects without altering the response-suppressing effects of CP 55,940 on operant behavior (Singh et al., 2011). Conventional receptor theory has been invoked to attribute such differences in the expression of cross-tolerance among drugs to documented differences in their efficacy. From this perspective, partial agonists that require greater receptor occupancy to produce their effects, like THC, are more likely to induce and show tolerance than are putative full agonists, such as CP55, 940 and JWH-018, which should have greater receptor reserves (Hruba et al., 2012). Thus, although considerable tolerance may develop to the behavioral effects of cannabinoids, its magnitude and time course may differ according to the drugs and endpoints under study.
We recently reported that cannabinoid-induced diuresis is a CB1-receptor mediated effect in rodents and, additionally, that these diuretic actions in mice are biphasic (Chopda et al., 2013; Paronis et al., 2013). Diuretic effects of cannabinoids were first reported anecdotally, and subsequently confirmed, in early studies of cannabis in humans (Parker and Wrigley, 1947; Ames, 1958). Yet diuresis in human subjects is not frequently mentioned in existing literature, suggesting that despite the widespread use of cannabis products, most people either do not notice or do not complain about THC-related diuresis. Given that marijuana exposure in humans often continues over years or even decades (e.g., Budney et al., 2003; Copersino et al., 2006), it may be that tolerance develops to the diuretic effects of marijuana and other cannabinoids. With this in mind, the present studies were conducted to evaluate changes in the diuretic effects of two cannabinoids, the CB1 partial agonist THC and the CB1 putative full agonist AM4054, consequent to a 7-day dosing regimen with 10 mg/kg of THC. In particular, given the curvilinear nature of the cannabinoid diuresis function in mice, we were interested in determining whether both the ascending and descending limbs of the function are susceptible to tolerance. For comparison, the diuretic effects of the κ-opioid agonist U50,488 and the loop diuretic furosemide also were determined in mice exposed to the same course of daily THC administration. Additional experiments were conducted to provide information on the effects of 10 mg/kg daily THC administration on a more conventional cannabinoid-induced effect, antinociception, as well as on cannabinoid CB1 receptor binding in mouse cerebellum.
Methods
Subjects.
Male CD-1 mice (Charles River Laboratories, Wilmington, MA) weighing 20–25 g at the start of the study were housed four/cage in a climate controlled vivarium with food and water available ad libitum. Mice were acclimatized to the study procedures twice before testing; each mouse was used in only one type of assay. All experiments were performed during the light portion of the light/dark cycle. All studies were approved by the Northeastern University Animal Care and Use Committee in accordance with guidelines established by the National Research Council.
Diuresis Procedures.
Mice were injected s.c. with vehicle or drug solutions using a constant volume of 1 ml/100 g. Immediately after injection, and without abdominal manipulation, they were placed on an elevated grid floor and isolated under a plastic cup (10 cm × 5 cm; d × h). Weigh boats placed underneath each mouse collected voided urine, and these were weighed every 2 hours over a total period of 6 hours. All mice were tested with saline before any drug or vehicle exposure. After this initial test, 120 mice were divided into four groups used to examine effects of THC or its vehicle, AM4054, U50,488, or furosemide. All mice were tested once before and 1 or 2 times after daily THC exposure with the same drug, with the exception that mice in the furosemide group were tested with one of the other drugs at 7 or 14 days after THC exposure. Dose selection for individual mice varied and the effects of each dose were determined in 6 to 10 animals, except where noted.
Antinociception Procedures.
Antinociception was determined using a warm water tail-withdrawal assay. A water bath maintained water temperature at 52.0 ± 0.5°C. Each mouse was gently hand held, and the distal 2–3 cm of the tail was immersed in the water; latency to tail withdrawal was measured using a stopwatch, and a cutoff time of 8 seconds was established to avoid tissue damage. Baseline latencies were determined twice on each test day with a 10-minute interval between determinations. Mice were divided into three groups of eight animals. One group of animals was used to evaluate the time course of the antinociceptive effects of AM4054; these mice were tested once per week, with all doses of AM4054 given in a randomized order. In the remaining two groups of animals, complete dose-response curves for either THC or AM4054 were generated in each mouse using cumulative dosing procedures similar to those described previously (Paronis and Woods, 1997). Briefly, 60 minutes after an injection, tail-withdrawal latencies were determined, and mice were then injected with the next dose, such that the total cumulative dose was increased by 0.25 or 0.5 log units. This procedure was repeated until the tail-withdrawal latency reached the cutoff or no longer increased with subsequent increases in drug dose. Dose-response curves were determined in all animals before THC treatment and at 24 hours, 7 days, and 14 days after seven daily THC injections of 10 mg/kg.
Binding Procedures.
Two groups of mice (n = 6) were injected daily with vehicle or 10 mg/kg of THC for 7 days. At 24 hours after the last injection, mice were sacrificed using cervical dislocation; whole brain was then collected and frozen at −80°C until further analysis. On the day of the binding assay and using methods previously described (Gatley et al., 1998; Gifford et al., 1999), the cerebellum from each brain was isolated, weighed, and separately homogenized in TME (100 mM Tris, 5 mM MgCl2, 1 mM EDTA) buffer containing 3% bovine serum albumin to obtain a 10 mg/ml homogenate. 3 ml of homogenate was transferred to another tube, to which 1.5 μCi [125I]AM281 was added. Binding assays were completed by incubating 100 μl of radiolabeled homogenate with 100 μl of TME buffer or 1 pM–10 μM AM251 and 800 μl homogenizing buffer for 90 minutes at room temperature on a shaker, followed by centrifugation (14,000 rpm) for 12 minutes at 4°C. The supernatant was removed, and pellets were blotted dry. Radioactivity was measured with a γ-counter; specific activity and total radioactivity were determined using 100 μl of homogenate plus [125I]AM281. All samples were run in duplicate.
Drugs and Dosing Procedures.
Δ9-THC was obtained from the National Institute on Drug Abuse (Rockville, MD); U50,488 [trans-(+/−)3,4-dichloro-N-methyl-N-(2-[1-pyrrolidinyl]-cyclohexyl)-benzeneacetamide methane sulfate] and furosemide were purchased from Sigma-Aldrich (St. Louis, MO). AM4054 [9β-(hydroxymethyl)-3-(1-adamantyl)-hexahydrocannabinol] was synthesized at the Center for Drug Discovery, Northeastern University. U50,488 was dissolved in saline. Furosemide was dissolved in 1% 1N NaOH and sterile water. THC and AM4054 were initially dissolved in ethanol, to which an equal volume of emulphor-620 (Rhodia, Cranbury, NJ) was added and then further diluted with saline to achieve a 1:1:18 mixture; this 1:1:18 solution, without drug, is referred to as vehicle. Drug doses are expressed in terms of the weight of free base, and injections were delivered s.c. in volumes of 1 ml/100g body weight. All drug injections were given between 8 and 10 AM.
Data Analysis.
Urine volumes were normalized to volume per body weight (ml/kg). Data obtained after saline injection in individual animals were grouped according to the drug tested and averaged to obtain group means (S.E.M.) and 99% confidence interval (CI). Minimum effective doses (MED) were defined as those found 0.5 ml/kg outside the 99% CI of the mean saline values, calculated using interpolation of two data points found above and within or below the 99% CI. Tail-withdrawal latencies are expressed as a percentage of maximum possible effect (%MPE ± S.E.M.), calculated using the following formula: %MPE = [(test latency − baseline latency)/(8 – baseline latency)] × 100. Antinociception ED50 values (with 95% CI) were calculated from grouped data using linear regression of data points ≥10% MPE. Potency ratios were calculated by dividing the baseline ED50 value by the ED50 value after daily THC exposure. Binding data were normalized to the protein content of the brain homogenate. Specific binding was determined by subtracting nonspecific binding from total binding, and Bmax values (±S.E.M.) were determined using Scatchard analysis (GraphPad Prism v.5.02, GraphPad Software, La Jolla, CA). All drug data were plotted and analyzed using log-transformed values of doses. Data were analyzed using paired t tests, one-way, or two-way analysis of variance (ANOVA), followed by Dunnett’s or Bonferroni’s multiple comparison tests; significance for all tests was set at P < 0.05.
Results
Cannabinoid Diuresis.
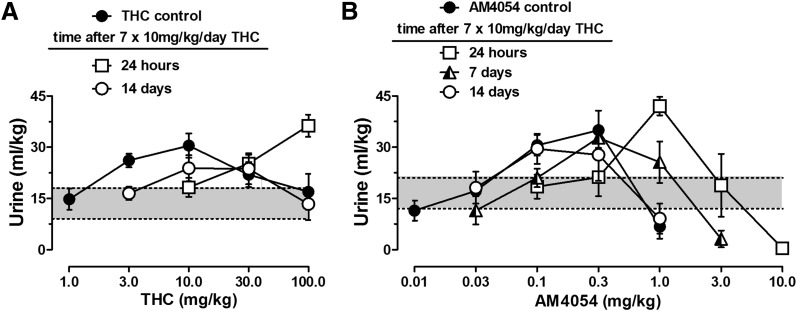
Saline injection resulted in an average of 16.3 ± 0.9 ml/kg of voided urine over 6 hours for all mice, and no significant differences were found among different groups of animals [F(3,16) = 1.754, not significant]. Injection of the cannabinoid vehicle had effects similar to those of saline, and six daily injections of vehicle did not alter diuretic responses measured on alternate days of treatment [F(2,14) = 0.566, not significant] (Table 1). Under control conditions, THC and AM4054 had curvilinear dose-response curves, dose dependently increasing and then decreasing urine output. For THC, doses of 1.0–10.0 mg/kg formed an ascending limb of the curve, and doses of 10–100 mg/kg formed a declining limb of the function. Similarly, dose-related diuresis occurred after 0.01–0.3 mg/kg of AM4054, and 1.0 mg/kg resulted in urine output below saline levels (Fig. 1).
TABLE 1.
Urine output (in ml/kg, mean ± S.E.M.) of mice injected daily with 10 mg/kg THC for 7 days (THC group, n = 8) or mice injected daily with vehicle for 6 days and on day 7 with 10 mg/kg THC (vehicle group, n = 8)
| Day | THC Group | Vehicle Group |
|---|---|---|
| 1 | 32.6 ± 4.0 | 15.7 ± 2.9 |
| 3 | 25.7 ± 4.8 | 12.5 ± 3.1 |
| 5 | 18.1 ± 3.1* | 14.6 ± 3.2 |
| 7 | 14.3 ± 2.8** | 31.1 ± 5.2## |
P < 0.05; **P < 0.01 compared with THC group, day 1; ##P < 0.01 compared with THC group, day 7.
Fig. 1.
Cannabinoid diuresis over 6 hours. (A) Effects of THC before (closed symbols) and 24 hours or 14 days after 7 days of THC administration. (B) Effects of AM4054 before (closed symbols) and 24 hours, 7 days, or 14 days after 7-day THC administration. Each point represents the mean of 6–10 mice (except n = 3 after 10 mg/kg AM4054), and vertical bars indicate ±S.E.M. The shaded area in between dotted lines marks the 99% CI for urine volume after saline injection. Ordinates: urine output over 6 hours in ml/kg of body weight. Abscissae: drug dose in mg/kg.
The diuretic effects of daily THC injection, 10 mg/kg, were measured on alternate days in a group of eight mice. This dose of THC initially produced a mean urine output of 32.6 ml/kg, and these effects decreased gradually with daily THC administration (Table 1). After the seventh daily injection of THC, only 14.3 ml/kg of urine was voided, similar to the effects of saline injection and significantly different from the effects observed after the first THC injection of 10 mg/kg [F(3,21) = 5.618, P < 0.01]. By comparison, in mice that had received repeated vehicle injections, the response to a single 10 mg/kg THC injection on day 7 was similar to the effects on day 1, and significantly different from those on day 7, of the THC-treated mice [F(2,21) = 6.047, P < 0.01] (Table 1).
As shown in Fig. 1A, a THC dose-response curve generated 24 hours after the last (i.e., seventh) daily injection of THC revealed a rightward shift in the ascending limb of the function, reflected in a 10-fold increase in the peak dose of THC, as well as in the lowest effective dose (Table 2). The descending limb of the THC dose response curve also appeared to shift rightward; however, the effects of THC doses higher than 100 mg/kg could not be determined because the vehicle required for high THC concentrations had measurable antidiuretic effects (unpublished observations). A two-way ANOVA on the effects of 10–100 mg/kg of THC revealed a significant interaction between dose and time [F(4,71) = 5.475; P < 0.01], and post hoc analysis indicated differences in urine volume of THC-treated mice after 10 and 100 mg/kg of THC compared with control conditions. No differences were found in the diuretic responses to 30 mg/kg THC, although notably this dose was found on the descending limb of the function under control conditions and on the ascending limb of the function after daily THC treatment. At 14 days after ending daily THC injections, the ascending limb of the THC dose-response curve remained to the right of the control dose-response curve (Fig. 1A). Although maximum urine volumes obtained at 24 hours or 14 days after daily THC treatment were marginally higher or lower, respectively, than control values, these difference were not significant [F(2,25) = 2.521].
TABLE 2.
Maximum urine outputs (mean ± S.E.M.) and minimum effective doses (MED)a calculated for ascending and descending limbs of diuresis dose-response curves
| Maximum Output (ml/kg) | MED (mg/kg) | ||
|---|---|---|---|
| Ascending Limb | Descending Limb | ||
| THC control | 30.5 ± 3.6 | 2.2 | NDb |
| 24 h post-THC | 36.3 ± 3.2 | 19.9 | ND |
| 14 days post-THC | 23.8 ± 3.8 | 8.0 | ND |
| AM4054 control | 35.0 ± 5.7 | 0.05 | 0.54 |
| 24 h post-THC | 42.0 ± 2.7 | 0.33 | 2.73 |
| 7 days post-THC | 32.7 ± 2.9 | 0.11 | 1.22 |
| 14 days post-THC | 29.2 ± 3.6 | 0.05 | 0.46 |
| U50,488 control | 37.0 ± 2.3 | 1.6 | ND |
| 24 h post-THC | 26.4 ± 2.0* | 8.4 | ND |
| 7 days post-THC | 25.1 ± 3.0** | 15.3 | ND |
| 14 days post-THC | 30.3 ± 2.9 | 2.7 | ND |
ND, not determined.
Minimum effective doses are defined as the lowest dose producing a 0.5-ml/kg increase or decrease outside of the 99% CI of saline and were calculated by interpolation of two points.
Descending limb of function was not determined.
P < 0.05; **P < 0.01 compared with U50,488 control.
Both limbs of the AM4054 dose-response curve were shifted to the right after daily THC treatment (Fig. 1B). The lowest effective dose calculated for AM4054-induced diuresis was 0.05 mg/kg under control conditions, and the lowest dose that produced less diuresis than saline was 0.54 mg/kg. At 24 hours after the last daily injection of THC, both MED values for AM4054 were increased by 5- to 7-fold (Tables 2 and 3). A two-way ANOVA on the effects of 0.1–1.0 mg/kg of AM4054 revealed a significant interaction between dose and time [F(4,44) = 10.19; P < 0.01], and post hoc analysis indicated differences in urine volume of THC-treated mice after 1.0 mg/kg AM4054 compared with control conditions. There was partial recovery in the position of both limbs of the AM4054 diuresis dose-response curves 7 days after the last daily THC injection, as the MED values were 2-fold higher than those obtained under control conditions. There was a full recovery to control effects of AM4054 by 14 days after ending daily THC injections (Fig. 1B). The maximum urine outputs produced by AM4054 administration were not statistically different from control values [F(3,19) = 1.639] (Table 2).
TABLE 3.
Control ED50 values and potency ratiosa at different times after stopping daily THC injections.
| Potency Ratio |
|||||
|---|---|---|---|---|---|
| MED | 24 h | 7 day | 14 day | ||
| THC | Diuresis-ascending | 2.2 mg/kgb | 9.2 | ND | 3.7 |
| Antinociception | 13.2 mg/kg | 7.1 | ND | 4.6 | |
| AM4054 | Diuresis-ascending | 0.05 mg/kgb | 7.0 | 2.3 | 1.0 |
| Diuresis-descending | 0.54 mg/kgb | 5.5 | 2.5 | 1.0 | |
| Antinociception | 0.3 mg/kg | 2.9 | 3.2 | 3.1 | |
ND, not determined.
Calculated by dividing the post-THC treatment MED or ED50 values by the baseline MED or ED50 values.
Taken from Table 2; included here for clarity.
Noncannabinoid Diuresis.
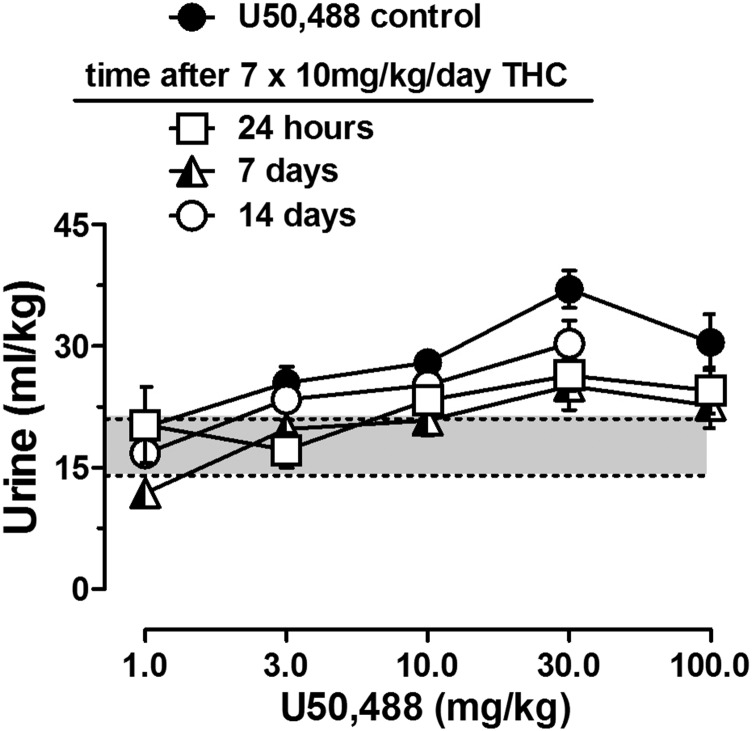
The ability of THC treatment to alter other diuretic responses were evaluated in mice tested with saline, furosemide, or U50,488. Daily THC exposure did not alter diuretic responses to saline or to furosemide (Table 4). In contrast, the diuretic effects of U50,488 were diminished in THC-treated mice at 24 hours and 7 days after ending THC injections (Fig. 2; Table 2). The MED values for U50,488 were higher in THC-treated mice relative to control values; however, changes in the U50,488 dose-response function were different from the effects seen in the THC and AM4054 dose-response curves. In contrast to the effects obtained with cannabinoid agonists, the peak diuretic effects of U50,488 were decreased as a function of THC treatment; yet the dose producing peak effects, 30 mg/kg, was unchanged [F(3,44) = 5.1; P < 0.01]. Thus, daily THC injection of 10 mg/kg resulted in a flattening of the U50,488 dose-response curve that was evident for more than 7 days after the last injection of THC (Fig. 2). As noted for both AM4054 and THC, by 14 days after ending the daily THC injections, there was substantial recovery of the diuretic effects of U50,488.
TABLE 4.
Urine output (in ml/kg, mean ± S.E.M.) after saline or furosemide before and 24 hours after 7-day treatment with 10 mg/kg THC (n = 6/group)
| Saline |
Furosemide |
||
|---|---|---|---|
| 1 ml/100g | 10 mg/kg | 30 mg/kg | |
| Control | 14.6 ± 3.6 | 36.4 ± 3.1 | 41.5 ± 2.4 |
| 24 h after THC | 18.5 ± 2.6 | 34.3 ± 3.1 | 39.4 ± 2.7 |
Fig. 2.
Diuretic effects of U50,488 before (closed symbols) and 24 hours, 7 days, or 14 days after 7-day THC administration (n = 6–10); other details as in Fig. 1.
Antinociception.
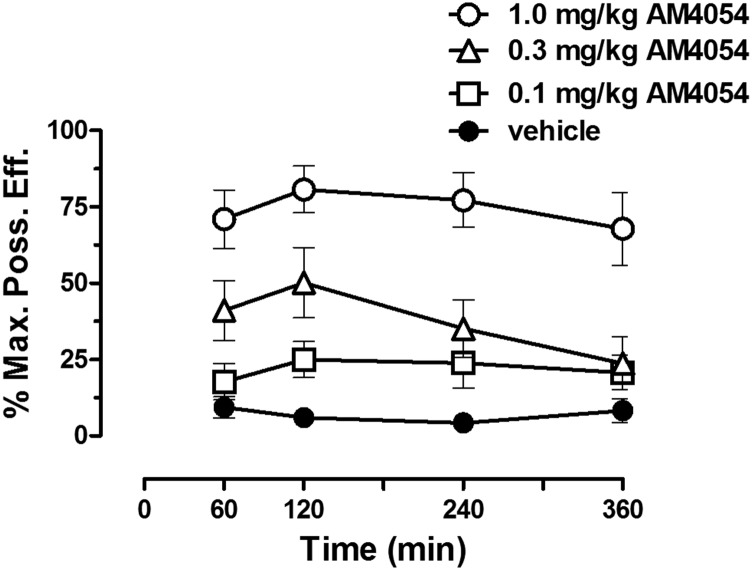
Initial studies evaluated the time course of the antinociceptive effects of AM4054 to determine a suitable interinjection interval for subsequent studies using cumulative dosing with this drug. Averaged baseline tail-withdrawal latencies did not vary significantly across conditions [F(3,28) = 1.782] and ranged from 1.8 ± 0.2 to 2.2 ± 0.1 seconds. All doses of AM4054 tested reached near peak antinociceptive effects within 1 hour of injection, and these effects were sustained for at least 4 hours (Fig. 3); based on these results, a 1-hour interjection interval was used for all cumulative dosing studies.
Fig. 3.
Time course of the antinociceptive effects of AM4054. Ordinate: antinociceptive response, expressed as a percentage of maximum possible effect (%MPE). Abscissae: time (in minutes) since injection of AM4054.
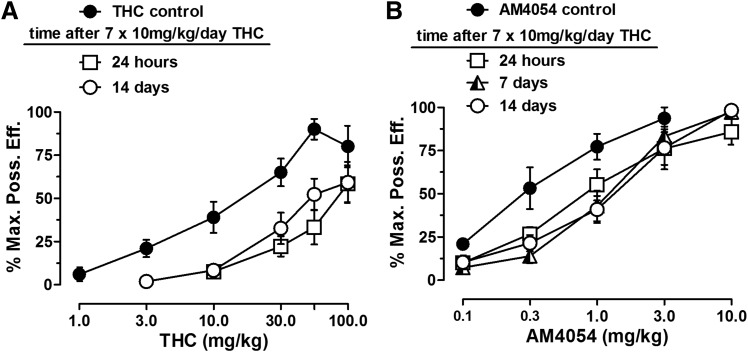
Averaged baseline tail-withdrawal latencies for mice tested with THC and AM4054 were 1.9 ± 0.1 and 2.5 ± 0.1 seconds, respectively. Both cannabinoid drugs produced dose-related increases in antinociception under control conditions, with ED50 values (and 95% CI) of 13.2 (7.6, 22.9) and 0.3 (0.2, 0.5) mg/kg, respectively. The initial antinociceptive effects of 10 mg/kg THC were moderate, resulting in 38.7% ± 10.1% MPE in individual animals. These moderate effects were eliminated during daily treatment with 10 mg/kg THC; after the third daily THC injection, the average antinociceptive effect was 14% ± 4% MPE. The daily injection of 10 mg/kg THC produced a rightward shift of the full THC dose-response curve, yielding an ED50 value of 94.2 mg/kg (Fig. 4). There was little recovery toward baseline up to 14 days after ending the daily THC injections, and the potency ratio compared with control conditions was greater than 4-fold (Table 2).
Fig. 4.
Antinociceptive effects of AM4054 and THC. (A) Effects of THC before and after 7-day THC administration (n = 8). (B) Effects of AM4054 before and after 7-day THC administration (n = 8). Ordinates: antinociceptive response, expressed as a percentage of maximum possible effect (%MPE). Abscissae: cumulative drug dose in mg/kg of body weight.
Daily THC injections also produced a smaller, but significant, rightward shift of the dose-response curve for the antinociceptive effects of AM4054, and the mean ED50 value increased approximately 3-fold, to 1.0 (0.7, 1.4) mg/kg. As observed with THC, there was no recovery of the AM4054 dose-response curve to its basal position, and the potency ratio was still 3-fold at 14 days after ending the daily THC injections.
Binding.
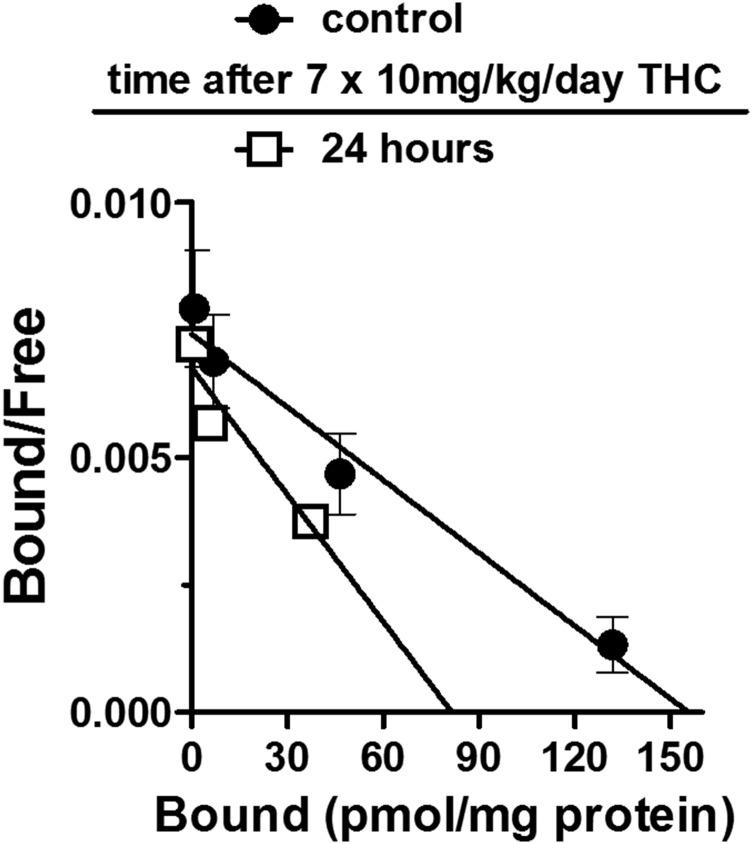
CB1 receptor levels in cerebellum were compared in mice that received 7-day treatment with either vehicle or 10 mg/kg THC and were then sacrificed 24 hours after the last injection (Fig. 5). Mouse cerebellum has previously been shown to have a high density of CB1 receptors sites (Herkenham et al., 1991) that are sensitive to downregulation (McKinney et al., 2008). The Bmax for CB1 receptors in vehicle-mice was 170 ± 30 pmol/mg. The Bmax value in mice that received daily THC was 75 ± 9 pmol/mg, significantly lower than Bmax values obtained from vehicle treated mice [F(2,6) = 29.77, P < 0.01.].
Fig. 5.
Linear Scatchard transformations of the binding of [125I]AM281 to cerebellum in mice sacrificed after 7-day vehicle administration (closed circles) or in mice sacrificed 24 hours after 7-day THC administration (open squares).
Discussion
The results of these experiments demonstrate that the diuretic effects of cannabinoid agonists are subject to tolerance. Moreover, for THC and AM4054, roughly equivalent tolerance developed to both diuretic and antinociceptive effects, providing further evidence that similar receptor mechanisms may underlie these two different cannabinoid effects (Chopda et al., 2013). These findings notwithstanding, there was a slight difference in the magnitude of tolerance to the two cannabinoid agonists and, additionally, the dose-response curve of AM4054 showed faster recovery than did the dose-response curve of THC. These observations are in keeping with the designation of THC as a partial CB1 agonist and AM4054 as a putative full agonist (Paronis et al., 2012; Desai et al., 2013). An unexpected finding of the present study was that chronic administration of 10 mg/kg daily THC also resulted in attenuation of the diuretic effects of the κ-opioid agonist U50,488. The effect of chronic administration of THC on the diuretic response to U50,488 suggests that prolonged exposure to cannabinoid agonists has effects that extend beyond the cannabinoid system and, more specifically, to the κ-opioid system.
Unlike other CB1 receptor-mediated effects, the dose-response curve for cannabinoid diuresis in mice consistently displays a biphasic function. An earlier study revealed that the increasing and decreasing effects of AM4054 on diuresis are both antagonized by rimonabant, although slightly greater antagonism was evident in the ascending limb of the function (Chopda et al., 2013). In contrast, the descending limb of the function was more sensitive to antagonism by AM6545, a CB1 antagonist with poor CNS penetration, suggesting involvement of peripheral mechanisms in the decreases in urine output (Tam et al., 2010; Chopda et al., 2013). Thus, it was of particular interest to determine whether both limbs of the diuresis dose-effect curves for cannabinoids are similarly subject to tolerance. In THC-treated mice, the entire AM4054 dose-response curve showed a rightward displacement, and changes in the position of the THC dose response curve appeared qualitatively similar to those seen with AM4054. Moreover, the magnitude of the rightward shifts of the THC and AM4054 dose-response curves for diuresis approximate those observed for the antinociceptive effects of both drugs. These data, coupled with the 50% decrease in binding sites, are consistent with results of previous findings on cannabinoid tolerance in mice and extend them to include diuretic effects (Fan et al., 1994; McKinney et al., 2008; Singh et al., 2011; Nguyen et al., 2012). Of note, earlier studies typically used doses of THC ranging from 20 to 60 mg/kg daily to investigate the development of tolerance, with dosing regimens lasting from 2 to 13 days (Fan et al., 1994; Bass and Martin, 2000; McKinney et al., 2008). The present studies demonstrate that a 1-week exposure to a lower daily dosage of THC suffices to reveal substantial tolerance to cannabinoid effects, comparable to that reported using higher daily dosages.
The baseline positions of the ascending and descending portions of the AM4054 dose-response curve were recovered by 14 days after ending daily THC treatment, whereas the ascending limb of the THC dose response function remained to the right of the control positon, as did the curves for antinociceptive effects of both agonists. Slow or incomplete recovery of full cannabinoid effects in THC-treated mice has been reported elsewhere, and recovery time varies with the effect measured. For example, Bass and Martin (2000) found full recovery of the locomotor activity–suppressant effects of THC within 7 days after cessation of 20 mg/kg daily THC, whereas the dose-response function for antinociceptive effects was recovered fully only after 12 days. Similarly, McMahon and colleagues reported an incomplete recovery for hypothermic and operant response rate-decreasing effects of CP55,940 and THC more than 10 days after stopping daily THC treatment with 32 mg/kg (Singh et al., 2011). In another study, a single intracerebroventricular administration of WIN 55,212 produced tolerance to the antinociceptive effects of the drug that lasted more than 14 days (Garzón et al., 2009). The variable, yet overall slow, return of dose-response curves to their baseline position after repeated exposure to THC may depend on the prolonged, regionally dependent, recovery rate of CB1 receptor levels or, alternatively, to altered coupling to second messenger systems (Sim-Selley et al., 2006; Garzón et al., 2008).
Maximum urine volumes were marginally higher in mice tested with either THC or AM4054 at 24 hours after ending daily THC treatment than under control conditions. This stands in marked contrast to the nearly complete attenuation of U50,488-induced diuresis measured at the same time point. This was the most unexpected result of our studies, both because the tolerance to U50,488 represents a cross-tolerance across different drug classes and also because the expression of the effect: a downward- rather than rightward-shift in the dose-response curve - was different and in opposition to the changes seen in the effects of cannabinoids. THC and U50,488 both result in a free-water diuresis, suggesting that perhaps cannabinoids, like κ-opioid agonists, produce their diuretic effects centrally by suppressing vasopressin release (Sofia et al., 1977; Leander et al., 1985, 1987; Chopda et al., 2013). This raises the possibility that repeated THC exposure may alter circulating levels of vasopressin; however, in contrast to decreased effects of U50,488 and cannabinoid agonists, diuretic responses to furosemide or 10 ml/kg of saline were unchanged in THC-treated mice. Together, these results indicate that the cross-tolerance induced by daily THC exposure does not extend to peripherally acting loop diuretics and that fluid handling, per se, was not affected by the daily THC injections, suggesting that there is some specificity to the effects induced by daily THC, although the mechanisms through which THC exposure reduces U50,488-induced diuresis are not clear.
Interactions between cannabinoids and κ-opioids have been explored previously, based on their common signaling transduction mechanisms or their often similar behavioral effects (Hampson et al., 2000; Walentiny et al., 2010; Maguire et al., 2014). For example, the κ-opioid antagonist nor-binaltorphimine will block the antinociceptive effects of THC, whereas coadministration of cannabinoid and κ-opioid agonists has additive antinociceptive effects (Smith et al., 1994; Maguire and France, 2016). Early reports of a bidirectional antinociceptive cross-tolerance between THC and κ-opioid agonists led to subsequent studies demonstrating the involvement of the endogenous κ-opioid dynorphin in the antinociceptive effects of THC (Smith et al., 1994; Pugh et al., 1996). Dynorphin and κ-opioid receptor activation also has been implicated in reducing the reinforcing effects of cannabinoid agonists in mice, although blockade or removal of κ-opioid receptors does not alter other “tetrad” effects of THC (Ghozland et al., 2002; Mendizábal et al., 2006). It is conceivable that dynorphin also mediates the diuretic effects of cannabinoids, although preliminary studies from our laboratory found no effects of pretreatment with the opioid antagonist naltrexone on THC or AM4054-induced diuresis.
Other investigators have pursued more direct interactions between cannabinoids and κ-opioid receptors, demonstrating that CB1 and κ-opioid receptors colocalize, and potentially dimerize, in cultured cells and also that the CB1 antagonist rimonabant is able to antagonize the effects of the κ-opioid agonist U69,593 on [35S]GTPγS binding in KOR-transfected human embryonic kidney (HEK)293 cells (Rios et al., 2006; Walentiny et al., 2010). Further, mice treated for 10 days with the cannabinoid antagonist AM281 showed increases in the expression of both prodynorphin and κ-opioid receptor genes (Rios et al., 2006; Saez-Cassanelli et al., 2007). These latter studies open the possibility that repeated exposure to a cannabinoid agonist, such as THC, may induce a downregulation within the κ-opioid system, resulting in decreased responsiveness to κ-opioid agonists. Alternatively, administration of THC may indirectly decrease κ-opioid receptor availability. Stimulated vasopressin release has been associated with a near doubling of κ-opioid receptors in the plasma membrane of hypothalamic neurosecretory cells (Shuster et al., 1999). If cannabinoid agonists produce diuresis through inhibition of vasopressin release, this may be associated with decreased trafficking of κ-opioid receptors and the resultant downward shift in the U50,488 dose-response curve seen in the present studies. Although intriguing, this suggestion must remain speculative without further investigation, and notwithstanding similarities in the acute effects of these drugs, it remains difficult to explain the loss of U50,488-mediated diuresis in THC-treated mice, especially in comparison with the overall rightward shifts of the cannabinoid dose-response curves.
In summary, the present results confirm and extend earlier observations of cannabinoid tolerance by demonstrating that a relatively low dose of 10 mg/kg daily THC is adequate to produce antinociceptive tolerance that endures for at least 14 days. Using the same dosing regimen, we further show that roughly equivalent tolerance is produced to the diuretic effects of cannabinoids. Moreover, despite the curvilinear nature of the diuresis dose-effect curves, rightward shifts were obtained in both the ascending and descending limbs of the functions, suggesting the cannabinoid tolerance is receptor-mediated and surmountable; however, daily exposure to THC also decreased the effects of a κ-opioid agonist, demonstrating that THC-induced tolerance extends beyond the cannabinoid system, specifically to the κ-opioid receptor system. The attenuation of U50,488-mediated diuresis was different in appearance, as the dose-response curve was shifted downward, not rightward, like the cannabinoid dose-response curves, and yet the duration of the tolerance was similar to that expressed by THC and AM4054. Cannabinoid CB1 receptors are frequently cited as being among the most abundant G-protein– coupled receptors, widely distributed within the central nervous system; thus, it is not unreasonable to anticipate that acutely administered cannabinoid agonists interact with ligands of other receptor systems (Herkenham et al., 1991; Gifford et al., 1999; Sim-Selley et al., 2006). Our findings suggest that, in addition to acute effects, repeated exposure to cannabinoid agonists also may profoundly alter the effects of drugs that produce their effects through other neurotransmitter systems.
Acknowledgments
The authors thank Joseph B. Anderson for excellent technical assistance and Jack Bergman and Roger D. Spealman for comments on a previous version of the manuscript.
Abbreviations
- AM4054
9β-(Hydroxymethyl)-3-(1-adamantyl)-hexahydrocannabinol
- ANOVA
analysis of variance
- CB
cannabinoid
- CI
confidence interval
- MED
minimum effective dose
- %MPE
percent of maximum possible effect
- THC, U50,488
trans-(-)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide
Authorship Contributions
Participated in research design: Chopda, Gatley, Paronis.
Conducted experiments: Chopda, Parge.
Contributed new reagents: Thakur, Makriyannis.
Performed data analysis: Chopda, Parge, Paronis.
Wrote or contributed to the writing of the manuscript: Chopda, Paronis.
Footnotes
This work was supported by Northeastern University Institutional funds and the National Institutes of Health (Grant DA035411).
Portions of this work were previously presented in G.R.C.’s doctoral dissertation defense: Cannabinoid-mediated diuresis in mice. Doctoral dissertation, Northeastern University, Boston, Massachusetts, and as Sonti S, Chopda GR, Paronis, CA, Gatley SJ. (2013). The effects of acute and chronic administration of delta-9-tetrahydrocannabinol (Δ9-THC) on receptor downregulation in mice at the 23rd Annual Symposium of the International Cannabinoid Research Society, Vancouver, BC, Canada.
References
- Ames F. (1958) A clinical and metabolic study of acute intoxication with Cannabis sativa and its role in the model psychoses. J Ment Sci 104:972–999. [DOI] [PubMed] [Google Scholar]
- Bass CE, Martin BR. (2000) Time course for the induction and maintenance of tolerance to Delta(9)-tetrahydrocannabinol in mice. Drug Alcohol Depend 60:113–119. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. (1999) Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem 73:2447–2459. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. (2003) The time course and significance of cannabis withdrawal. J Abnorm Psychol 112:393–402. [DOI] [PubMed] [Google Scholar]
- Chopda GR, Vemuri VK, Sharma R, Thakur GA, Makriyannis A, Paronis CA. (2013) Diuretic effects of cannabinoid agonists in mice. Eur J Pharmacol 721:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. (2006) Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict 15:8–14. [DOI] [PubMed] [Google Scholar]
- Desai RI, Thakur GA, Vemuri VK, Bajaj S, Makriyannis A, Bergman J. (2013) Analysis of tolerance and behavioral/physical dependence during chronic CB1 agonist treatment: effects of CB1 agonists, antagonists, and noncannabinoid drugs. J Pharmacol Exp Ther 344:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. (1994) Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther 271:1383–1390. [PubMed] [Google Scholar]
- Garzón J, de la Torre-Madrid E, Rodríguez-Muñoz M, Vicente-Sánchez A, Sánchez-Blázquez P. (2009) Gz mediates the long-lasting desensitization of brain CB1 receptors and is essential for cross-tolerance with morphine. Mol Pain 5:11. 10.1186/1744-8069-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Volkow ND, Pappas N, King P, Wong CT, Gifford AN, Pyatt B, Dewey SL, Makriyannis A. (1998) Imaging the brain marijuana receptor: development of a radioligand that binds to cannabinoid CB1 receptors in vivo. J Neurochem 70:417–423. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. (2002) Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci 22:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. (1999) Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther 288:478–483. [PubMed] [Google Scholar]
- Hampson RE, Mu J, Deadwyler SA. (2000) Cannabinoid and kappa opioid receptors reduce potassium K current via activation of G(s) proteins in cultured hippocampal neurons. J Neurophysiol 84:2356–2364. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. (2012) Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Δ⁹-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther 342:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander JD, Hart JC, Zerbe RL. (1987) Kappa agonist-induced diuresis: evidence for stereoselectivity, strain differences, independence of hydration variables and a result of decreased plasma vasopressin levels. J Pharmacol Exp Ther 242:33–39. [PubMed] [Google Scholar]
- Leander JD, Zerbe RL, Hart JC. (1985) Diuresis and suppression of vasopressin by kappa opioids: comparison with mu and delta opioids and clonidine. J Pharmacol Exp Ther 234:463–469. [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP. (2014) Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther 345:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav 40:471–478. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. (2008) Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 324:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizábal V, Zimmer A, Maldonado R. (2006) Involvement of kappa/dynorphin system in WIN 55,212-2 self-administration in mice. Neuropsychopharmacology 31:1957–1966. [DOI] [PubMed] [Google Scholar]
- Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ. (2012) β-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biol Psychiatry 71:714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CS, Wrigley F. (1947) Effects of cannabis. Lancet 2:223. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Nikas SP, Shukla VG, Makriyannis A. (2012) Δ(9)-Tetrahydrocannabinol acts as a partial agonist/antagonist in mice. Behav Pharmacol 23:802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronis CA, Thakur GA, Bajaj S, Nikas SP, Vemuri VK, Makriyannis A, Bergman J. (2013) Diuretic effects of cannabinoids. J Pharmacol Exp Ther 344:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronis CA, Woods JH. (1997) Clocinnamox dose-dependently antagonizes morphine-analgesia and [3H]DAMGO binding in rats. Eur J Pharmacol 337:27–34. [DOI] [PubMed] [Google Scholar]
- Pugh G, Jr, Smith PB, Dombrowski DS, Welch SP. (1996) The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther 279:608–616. [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. (2006) mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol 148:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Cassanelli JL, Fontanella GH, Delgado-García JM, Carrión AM. (2007) Functional blockage of the cannabinoid receptor type 1 evokes a kappa-opiate-dependent analgesia. J Neurochem 103:2629–2639. [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. (1999) Stimulus-dependent translocation of κ opioid receptors to the plasma membrane. J Neurosci 19:2658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Martin BR. (2002) Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther 303:36–44. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. (2006) Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol 70:986–996. [DOI] [PubMed] [Google Scholar]
- Singh H, Schulze DR, McMahon LR. (2011) Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharmacology (Berl) 215:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Welch SP, Martin BR. (1994) Interactions between delta 9-tetrahydrocannabinol and kappa opioids in mice. J Pharmacol Exp Ther 268:1381–1387. [PubMed] [Google Scholar]
- Sofia RD, Knobloch LC, Harakal JJ, Erikson DJ. (1977) Comparative diuretic activity of delta9-tetrahydrocannabinol, cannabidiol, cannabinol and hydrochlorothiazide in the rat. Arch Int Pharmacodyn Ther 225:77–87. [PubMed] [Google Scholar]
- Tai S, Hyatt WS, Gu C, Franks LN, Vasiljevik T, Brents LK, Prather PL, Fantegrossi WE. (2015) Repeated administration of phytocannabinoid Δ(9)-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol Res 102:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, et al. (2010) Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120:2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) (2014) World Drug Report, United Nations, http://www.unodc.org/wdr2014/.
- Walentiny DM, Vann RE, Warner JA, King LS, Seltzman HH, Navarro HA, Twine CE, Jr, Thomas BF, Gilliam AF, Gilmour BP, et al. (2010) Kappa opioid mediation of cannabinoid effects of the potent hallucinogen, salvinorin A, in rodents. Psychopharmacology (Berl) 210:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]