Abstract
Mitochondria are key players in the generation and regulation of cellular bioenergetics, producing the majority of adenosine triphosphate molecules by the oxidative phosphorylation system (OXPHOS). Linked to numerous signaling pathways and cellular functions, mitochondria, and OXPHOS in particular, are involved in neuronal development, connectivity, plasticity, and differentiation. Impairments in a variety of mitochondrial functions have been described in different general and psychiatric disorders, including schizophrenia (SCZ), a severe, chronic, debilitating illness that heavily affects the lives of patients and their families. This article reviews findings emphasizing the role of OXPHOS in the pathophysiology of SCZ. Evidence accumulated during the past few decades from imaging, transcriptomic, proteomic, and metabolomic studies points at OXPHOS deficit involvement in SCZ. Abnormalities have been reported in high-energy phosphates generated by the OXPHOS, in the activity of its complexes and gene expression, primarily of complex I (CoI). In addition, cellular signaling such as cAMP/protein kinase A (PKA) and Ca+2, neuronal development, connectivity, and plasticity have been linked to OXPHOS function and are reported to be impaired in SCZ. Finally, CoI has been shown as a site of interaction for both dopamine (DA) and antipsychotic drugs, further substantiating its role in the pathology of SCZ. Understanding the role of mitochondria and the OXPHOS in particular may encourage new insights into the pathophysiology and etiology of this debilitating disorder.
Keywords: mitochondria, oxidative phosphorylation system, complex I, Schizophrenia, cAMP/PKA and Ca+2 signaling, neurodevelopment and plasticity, dopamine
Abstract
Les mitochondries sont un acteur clé dans la génération et la régulation de la bioénergétique cellulaire, produisant la majorité des molécules ATP par le système de phosphorylation oxydative (OXPHOS). Liées à de nombreuses voies de signalisation et fonctions cellulaires, les mitochondries, et OXPHOS en particulier, sont impliqués dans le développement neuronal, la connectivité, la plasticité et la différenciation. Des déficiences d’une variété de fonctions mitochondriales ont été décrites dans différents troubles généraux et psychiatriques, dont la schizophrénie (SCZ), une maladie grave, chronique et débilitante qui affecte lourdement la vie des patients et de leur famille. Cet article passe en revue les résultats mettant l’accent sur le rôle d’OXPHOS dans la pathophysiologie de la SCZ. Les données probantes accumulées au cours des récentes décennies dans des études d’imagerie, transcriptomiques, protéomiques et métabolomiques dénoncent la participation du déficit d’OXPHOS à la SCZ. Des anomalies ont été signalées dans les phosphates à haute énergie produits par le système OXPHOS, dans l’activité de ses complexes et de son expression génétique, principalement du complexe I (CoI). En outre, la signalisation cellulaire, comme cAMP/PKA et Ca+2, le développement neuronal, la connectivité et la plasticité ont été liés à la fonction OXPHOS et sont déclarés déficients dans la SCZ. Finalement, CoI s’est avéré être un site d’interaction pour la dopamine (DA) et les antipsychotiques, ce qui étaye encore son rôle dans la pathologie de la SCZ. Comprendre le rôle des mitochondries et d’OXPHOS en particulier peut susciter de nouvelles idées pour la pathophysiologie et l’étiologie de ce trouble débilitant.
Brain development, neuronal plasticity, and synapse connectivity are highly complex processes, related to various brain functions such as learning, memory, emotions, cognition, and sensorimotor function. These core processes facilitate the brain’s constant interaction with and adaption to the environment and are highly dependent on continuous oxygen supply.1,2 The latter is evident by high energetic demands and oxygen uptake of the brain, roughly 20% of total body consumption while only about 2% of body’s weight.3 Energy in the form of adenosine triphosphate (ATP) is generated mainly in mitochondria by the oxidative phosphorylation (OXPHOS) process, in which electrons produced by the citric acid cycle are transferred down the mitochondrial respiratory complexes. Mitochondria and the OXPHOS are not only responsible for production of high-energy phosphates, such as ATP and phosphocreatine, but are also involved in a variety of cellular processes, including calcium homeostasis, cAMP/protein kinase A (PKA) signaling, inflammation, reactive oxygen species (ROS) production, and apoptosis.4–9 Therefore, it is not surprising that multifaceted OXPHOS dysfunctions, originating from both genetic (maternal, or mendelian inheritance) and environmental influences, have been reported in many diseases and disorders, including neuropsychiatric disorders such as Alzheimer and Parkinson diseases, schizophrenia (SCZ), and bipolar disorder (BD).2,10–12 SCZ is a severe mental disorder, heavily affecting the lives of those afflicted and their families. The disorder is characterized by various abnormal cognitive, affective, and motor behavioural features, associated with a variety of impairments in occupational and social functioning. No single symptom is pathognomonic of SCZ; consequently, the disorder is noted for its great heterogeneity across individuals and for its variability within individuals over time.13 One consistent pathological finding implicated in SCZ is abnormal brain energy metabolism in specific neuronal circuits. Mitochondria, key players in brain bioenergetics, portray various deficits in SCZ. This review focuses on malfunctioning of the OXPHOS, source of ATP production, and main driving force of various mitochondrial-related cellular functions.
Brain Energy Metabolism in SCZ
Brain activity and function depend profoundly on ATP supply, with energetic demands varying significantly according to neuron type and brain activity levels.6,14,15 Cellular buffering of brain ATP is mainly regulated by ATPase and creatine kinase reactions.14 Creatine is phosphorylated by creatine kinase and converted into phosphocreatine (PCr), a high-energy phosphate, capable of donating a phosphate group to adenosine diphosphate (ADP), forming ATP, and vice versa.6 In SCZ patients, most imaging studies using positron emission tomography (PET), functional magnetic resonance imaging (fMRI), magnetic resonance spectroscopy (MRS), phosphorous magnetic resonance spectroscopy (31P-MRS), and single-photon emission tomography (SPECT) reveal altered metabolism, as expressed by changes in glucose, PCr, and ATP in different brain regions, including the prefrontal cortex (PFC), left temporal lobe, and frontal lobe.16–22 Interestingly, the severity of negative symptoms and neuropsychological performance correlate with ATP and PCr levels.23 In BD, which also portrays psychotic symptoms and shares genetic risk with SCZ, 31P-MRS studies did not find significant alterations in ATP levels.24,25 However, similar alterations have been reported for both disorders in other brain bioenergetic indicators such as elevated lactate and decreased intracellular pH (pHi) levels, as well as abnormalities in PCr and creatine kinase.24–30 It has been suggested that this shift from aerobic respiration to anaerobic glycolysis increases the risk for metabolic syndromes in these patients.31,32 Such maladaptations to the energetic demands of the central nervous system (CNS) point to a dysfunction of brain mitochondrial OXPHOS. Producing about 90% of ATP molecules generated in the brain, the OXPHOS is responsible for powering cell signaling and neuronal activity processes, such as pre- and postsynaptic action potentials, neurotransmitter release, and postsynaptic currents.14,33 Thus, damage to the OXPHOS can have detrimental effects on the CNS energetic balance that may lead to various neuronal dysfunctions.
Mitochondrial OXPHOS
The OXPHOS consists of 5 protein complexes and 2 electron carriers embedded in the inner mitochondrial membrane.34,35 High-energy phosphate production is achieved by coupling electron transfer to proton translocation across the inner membrane, resulting in an electrochemical gradient, which generates a motive force driving ATP synthesis by the fifth complex, ATP synthase (complex V [CoV]). During respiration, electrons are first transferred from the citric acid cycle products, NADH and succinate, through complexes I (CoI) and II (CoII), respectively, to ubiquinone. They then pass through complex III (CoIII) and cytochrome c, terminating at complex IV (CoIV). In this process, CoIV reduces O2 to H2O.35 The interaction between OXPHOS complexes and their organization is still unclear. According to the “fluid-state model,” these complexes diffuse freely across the membrane, transferring electrons by randomly colliding. In contrast, the “solid-state model” supports the formation of stable supercomplex assemblies, or respirasomes, composed of several complexes functioning together.36,37 The assembly process of the OXPHOS complexes is tremendously intricate and is composed of multiple stages in which many structural, catalytic, and assembly proteins participate. In addition, proteins encoded by nuclear DNA (nDNA, ∼70 proteins), synthesized in the cytosol and imported into the mitochondria, have to assemble with proteins encoded by mitochondrial DNA (mtDNA, 13 proteins).38 Deficiencies in OXPHOS complexes formation and function are associated with either mtDNA or nDNA mutations and can lead to various defects, including synapse damage, axon degradation, ROS production, apoptosis, and cell death.4,39–41
Functional and Genetic Alterations of the OXPHOS in SCZ
Many patients diagnosed with mitochondrial diseases with a variety of OXPHOS deficiencies, CoI in particular, portray psychotic-like symptoms.12 On the other hand, OXPHOS dysfunction has been widely reported in disorders with psychotic features, such as SCZ and BD.42–45 Taken together, these findings suggest a role for mitochondrial dysfunction in the pathophysiology of psychosis. Dysfunctions of the OXPHOS have been observed at the level of high-energy phosphate production as well as enzymatic activity and subunit expression of OXPHOS complexes. Genetic studies implicate OXPHOS complexes, particularly CoI, in both disorders.23,27,44,46–50
Functional impairments of OXPHOS complexes I, II, and IV have long been described in various brain areas of SCZ patients.47–49,51 In BD, however, only 1 study has reported a decrease in CoI activity in the PFC, with no change in SCZ patients.52 We have reported aberrant OXPHOS activity in platelets of SCZ patients, with CoI showing the most consistent impairments. Our studies show that in blood cells, the enzymatic activity of CoI, but not that of complexes I to III and CoIV, exhibits disease state–dependent alterations. Thus, increased CoI activity positively correlates with positive symptomology and active psychosis, while decreased activity is associated with the residual state.51 Transcriptomic, proteomic, and metabolomic studies in different brain areas, mostly the PFC, have demonstrated specific robust changes (both increases and decreases) in gene expression and protein level associated with mitochondrial function in SCZ, among them OXPHOS genes and proteins. For example, the expression levels of several CoI subunits, such as NDUFV1, NDUFV2, and NDUFS1, which comprise one functional subunit that includes CoI’s substrate binding site, were significantly altered in the PFC, striatum, hippocampus, and parieto-occipital cortex as well as in somatic cells.27,53–58 Interestingly, 1 study reported a positive correlation between the extent of change in blood cells and positive symptomology in SCZ first-episode patients.50 In BD, transcriptomic abnormalities of mitochondrial genes were also reported mainly in subunits of complexes I, IV, and V.59,60 However, both disorders differ with respect to the extent and characteristics of mitochondrial impairments, energy metabolism, and ROS production. Overall, there is an inconsistency in the literature regarding alterations in OXPHOS gene expression. These discrepancies may be explained in part by our findings that the change in gene expression exhibits a disease-specific neuroanatomical pattern44 and/or is cell type specific.56 Moreover, varying results may be due to disease subtype and ethnicity of the tested population, as has been shown for NDUFV2 protein expression in lymphoblastoid cells of Japanese and European Caucasian BD-I and BD-II patients.58,61 We have previously suggested that change in expression of at least some of the mitochondrial genes in SCZ can be attributed to abnormal expression of the transcription factor Sp1, which controls many nDNA encoded mitochondrial genes, including NDUFV2 and NDUFV1.62 Indeed, a highly significant positive correlation and a parallel pattern of change in transcripts of Sp1 and CoI subunits in various brain areas and blood cells have been observed.62
A growing number of studies have found susceptibility loci and associated risk genes, including those of the OXPHOS in SCZ, even though genome-wide association studies (GWAS) have failed to consistently replicate genetic risk factors among these genes.63–65 One of these NDUFV2 has been reported to be a high-risk gene for SCZ.66 Single-nucleotide polymorphisms (SNPs) in its promoter, introns, and 3′-UTR regions, have been associated with both SCZ and BD.67,68
A small but growing number of studies report mtDNA SNPs as risk factors in SCZ,69,70 possibly due to somatic rather than inherited mutations.29 SNPs in ND1, ND4, and ND5 mtDNA encoded subunits of CoI have been repeatedly reported in SCZ.71,72 In line with the latter, a significant comorbidity was observed between SCZ and mitochondrial disease.12,73 Taken together, the data obtained from functional, expression, and genetic evidence point to a role for OXPHOS complexes, particularly CoI, in SCZ. In this context, it is noteworthy that CoI has been shown to be the rate-limiting enzyme for O2 consumption in nerve terminals, as a 10% inhibition of CoI is sufficient for major alterations in O2 consumption.74
The OXPHOS and cAMP/PKA Signaling
Driving cellular respiration, on one hand, and mitochondrial apoptosis cascade, on the other, the OXPHOS is linked to different, tightly regulated cell signaling pathways, with cyclic AMP (cAMP) and its effector PKA being the most studied OXPHOS-related cellular signaling pathway.8,75 The significance of this pathway in cell growth, survival, neuro-protection, axon regeneration, and ROS production is well established.76–78 However, relatively little is known about the mechanisms of action of mitochondrial cAMP and PKA.8,75 In mitochondria, it has been shown that this pathway plays a role in fission/fusion process via phosphorylation and inactivation of the fission protein dynamin-related protein 1 (Drp1), thereby promoting mitochondrial elongation and facilitating fusion, which is important for neuronal survival.79,80 Identification of cAMP and soluble adenylyl cyclase (sAC) inside the mitochondria indicates the local production of mitochondrial cAMP.8,81 PKA, the downstream target of cAMP, has also been shown to be located inside the mitochondria at different compartments (outer membrane, intermembrane space, and the matrix), despite not having a mitochondrial targeting sequence.8 In line with these findings, cAMP/PKA signaling has been shown to regulate transcription and phosphorylation of OXPHOS subunits from both nuclear and mitochondrial origin.8,75,82 cAMP/PKA-dependent phosphorylation of nuclear CoI encoded subunits has been reported for NDUFB11, NDUFA1, NDUFS4 NDUFA7, NDUFA10, NDUFC2, and GRIM19.83–87 Interestingly, 1 study has shown that phosphorylation of NDUFB11 results in a reduction of CoI activity.88 In line with the latter, inhibition of sAC, which product cAMP was shown to prevent degradation of CoI subunits NDUFS4, NDUFS2, and NDUFA9, reduced CoI activity.89 A tissue-specific activity of the cAMP/PKA pathway was also shown to regulate CoIV activity by phosphorylation of various subunits.90,91 This interaction is further exhibited by the involvement of targets downstream to cAMP/PKA, such as cAMP-responsive element-binding protein (CREB) and peroxisome proliferator–activated receptor coactivator 1α (PGC-1α), in the transcription of mitochondrial genes from both mitochondrial and nuclear origin.82,92,93 CREB, in addition to its functions in the nucleus, is imported into the mitochondria via the translocase of the outer membrane (TOM complex). In the mitochondria, CREB was shown to regulate the expression of mtDNA encoded genes, ND1, ND6, and COXIII in the periphery and ND2, ND4 and ND5 in brain.94 In brain, CREB induced changes in the mtDNA encoded genes affecting CoI activity and CoI-dependent respiration.94 PGC-1α activates transcription factors regulating the transcription of OXPHOS subunits by Sp1 and nuclear respiratory factors (NRF1 and NRF2).95,96 cAMP/PKA signaling pathway also affects cellular calcium homeostasis, which is discussed in the following section.
Abnormalities in the cAMP/PKA signaling pathway have been reported in SCZ. For example, postmortem analysis of SCZ patients revealed an asymmetry of cAMP binding to PKA in temporal cortices.97 An alteration in PKA activity has been reported in the dorsolateral prefrontal cortex (DLPFC) but not in the anterior cingulate (ACC) in SCZ. No changes were observed in levels of PKA catalytic subunits.98 A more significant change in the cAMP/PKA signaling pathway was observed in BD, with a widespread decrease in cAMP binding to PKA in brain99 and abnormal PKA level and activity.100,101 In platelets of both SCZ and BD patients, abnormal levels of the catalytic subunits of PKA were reported.102,103 Alterations in CREB expression and protein levels were also reported to vary according to brain region, in a disease-specific manner, with a decrease in the cingulate gyrus (CG) and in the DLPFC in BD and a decrease in the CG with no change in the DLPFC in SCZ.104 Finally, a number of phosphodiesterases (PDEs) have been shown to inactivate cAMP by hydrolysis.105–108 PDE4 has been shown to interact with the protein disrupted in schizophrenia 1 (DISC1) in a cAMP/PKA-dependent manner and inactivate cAMP.109 Both proteins have been localized to the mitochondria,110 suggesting that variations in both can affect mitochondrial cAMP catabolism, with elevated cellular cAMP in SCZ leading to dissociation of PDE4B from DISC1 and increased PDE4B activity.109 All 3 proteins have been implicated in SCZ. For example, alterations in PDE4A and PDE10 protein levels have been observed in different brain regions of SCZ patients, and SNPs were identified in several PDEs and in DISC1.66,111–113 These findings attribute an additional role for cAMP/PKA signaling pathways in the interaction between mitochondria and the cell, regulating mitochondrial activity according to cell demands and vice versa.
The OXPHOS and Calcium Homeostasis
Calcium, one of the cells’ most common second messengers, is involved in many essential cellular functions, including gene transcription, signaling pathways, cell proliferation and regulation of neuronal functions, synaptic plasticity, learning, memory, and cognition. Mitochondria play an important role in Ca+2 buffering and signaling, while Ca+2 regulates mitochondrial localization, movement within the neuron, and their degradation.114–117 N-methyl-d-aspartate (NMDA) receptors (NMDARs) are responsible for the main flux of Ca+2 into CNS cells.30 Cellular Ca+2 is mainly stored within the endoplasmic reticulum (ER), where its concentration is several orders of magnitude higher than in the cytoplasm.118,119 ER and the mitochondria connect and interact via the mitochondria-associated ER membrane, allowing release of Ca+2 at maximal proximity.120,121 The main mechanism of ER calcium release is through inositol-1,4,5-trisphosphate (IP3), which activates the IP3 receptor (IP3 R) on the ER membrane, leading to Ca+2 release into the cytosol.122,123 The cAMP/PKA pathway plays an important role in Ca+ signaling, with PKA modulating both IP3 R capacity124 and NMDAR permeability for extracellular Ca+2.30 Cytosolic Ca+2 enters the mitochondria via the Ca+2 uniporter, due to the membrane potential driving force generated by the OXPHOS.123,125 Intramitochondrial Ca+2 () affects OXPHOS function through different mechanisms. For example, modulates and stimulates sAC activity and thereby the PKA signaling cascade.126 In addition, stimulates the phosphorylation of CREB-dependent protein kinases, initiating transcription of CRE-regulated genes.127,128 has been suggested to facilitate activation, regulation, and proper function of OXPHOS genes by activating key dehydrogenases of citric acid, including pyruvate dehydrogenase, NAD+-isocitrate dehydrogenase, and oxoglutarate dehydrogenase.123,129,130 A direct effect of on regulation of ATP production has been suggested via the activation of complex V and an increase of electron flow through CoIII.131,132 A reciprocal interaction between OXPHOS and has been suggested as CoI, CoII, and CoIV deficiencies are associated with abnormalities in Ca+2 signaling.133–135 The possible involvement of Ca+2 signaling in SCZ had been suggested as early as 1979 based on correlations between SCZ psychotic symptoms and increased cerebrospinal fluid Ca+2 levels.136 More recently, an increase in Ca+2 levels has been reported in platelets of SCZ and BD patients.137,138 Genetic studies also support the involvement of Ca+2 in both disorders, with polymorphisms found in calcium channels,139–141 NMDAR, and their related genes.142–144 Altered levels of specific subunits of the NMDA complex have been demonstrated in SCZ postmortem brains.145,146 Notably, administration of NMDAR antagonists (e.g., ketamine and phencyclidine) can produce SCZ-like symptoms.147–149 Additional pathways related to neuronal Ca+2 signaling are impaired in SCZ, for example, IP3, GSK-3, and ryanodine receptor signaling pathways.149,150 While Ca2+ signaling alterations in psychiatric disorders have still not been directly related to OXPHOS abnormalities, the regulatory effects of Ca2+ signaling on the OXPHOS suggest a possible link between the two.
The OXPHOS in Neuronal Development and Plasticity
Adaptation of the nervous system to the ever changing environment by neurogenesis and active modulation of synaptic connections between neurons is a high-energy demanding process, termed synaptic plasticity, a concept initially proposed by Donald Hebb in 1949.151 Defects in neuronal connectivity, synaptic modeling, and neuronal signaling have been suggested to be part of the underling pathophysiological mechanisms of SCZ.43,152–156 Mitochondria, localized in dendrites and axons, participate in essential processes related to plasticity, including morphological changes such as development of new synapses and remodeling of mature ones, Ca+2 signaling, generation of action potential, synaptic transmission, and ion homeostasis.157–159 Attached to vesicles, they are transported along microtubules to synaptic terminals, enabling these high-energy demanding processes.160,161 Removal of mitochondria from nerve endings can lead to abnormal synaptic transmission.162,163 Interestingly, DISC1, implicated in SCZ, affects mitochondria localization and microtubule transport.164
In cells, mitochondria routinely fuse, divide (fission), branch, and change their size in a dynamic manner. This process, termed mitochondrial network dynamics, enables proper mitochondrial function, including inheritance and maintenance of mtDNA, regulation of metabolic energy, mitochondrial trafficking, and maintenance of a healthy mitochondrial population.159,165–167 Mutations related to this process have been previously linked to neurodegenerative diseases such as Parkinson and Huntington diseases, and more recently, impairments in mitochondrial network dynamics have been reported by us in SCZ and by others in BD-derived cells.168–173 The extent to which the OXPHOS affects neuronal branching and plasticity is still an open question. However, in CoI mutagenized Caenorhabditis elegans, an increased number of dendrites and their branching in sensory neurons were observed.174 In humans, we have demonstrated impairments in differentiation and maturation into dopaminergic and glutamatergic neurons of SCZ-derived induced pluripotent stem cells (iPSCs), alongside a reduction in CoI-driven respiration. In addition, dissipation in mitochondrial membrane potential, impaired mitochondrial network structure and connectivity, and abnormal expression levels of NDUFV1, NDUFV2, and NDUFS1 were reported.170 Mammalian embryonic stem cell (ESCs), which originate from the blastocyst inner cell mass, are naturally exposed to hypoxic conditions,175 with mitochondria showing immature morphology at this stage.176–178 Not surprising, the main source of energy at this stage comes mainly from glycolysis and not oxidative phosphorylation. Only later during differentiation, O2 levels rise and an increase in mitochondria number is accompanied by a shift towards oxidative phosphorylation respiration.177,179 Indeed, it was shown that cellular differentiation of ESCs and iPSCs depends on OXPHOS and is hampered by the inhibition of CoI or CoIII.180–182 In line with these findings, we have shown that abnormalities in mitochondrial function are associated with a failure of SCZ-derived iPSCs to differentiate into dopaminergic and glutamatergic neurons.170 These findings imply a key role for mitochondria and their OXPHOS in synaptic plasticity and differentiation into neurons.
Dopamine and Antipsychotic Drugs Interact with the OXPHOS
Dopamine (DA) has been suggested to play a pivotal role in the pathophysiology of a number of mental disorders, particularly in SCZ.183–186 The DA hypothesis in SCZ originally stemmed from the ability of antipsychotic drugs to inhibit DA receptors, primarily D2 receptors, and that of psychostimulants to activate DA transmission.187 Even though the mechanisms of action of antipsychotic drugs are not entirely clear, a reduction in energy-demanding processes induced by these substances in the frontal lobes and basal ganglia of medicated SCZ patients has been reported.23,188 Concomitantly, in vitro exposure of rat pancreas cells to clozapine resulted in reduced levels of glucose oxidation and ATP production.189 Similarly, inhibition of ATP-related responses was demonstrated following exposure of PC12 cells to haloperidol.190 We and others have shown that both typical and atypical antipsychotic drugs inhibit CoI activity and CoI-driven respiration in isolated mitochondria and in intact neuronal cells.191–194 In mice, acute and chronic haloperidol administration specifically inhibited CoI in extrapyramidal brain regions, the extent of inhibition correlating with D2 abundancy.195 In human and rat brain specimens, a drug- and dose-dependent in vitro inhibition of CoI activity was observed with haloperidol > chlorpromazine > risperidone > zotepine > clozapine.169,194,196 Interestingly, we have shown that CoI activity is increased in peripheral blood cells of medicated and nonmedicated SCZ patients at the acute stage, while decreased in chronically medicated SCZ patients at the residual state.169 Similar to antipsychotics, DA also affects mitochondrial functions.197 In neuronal cell cultures, L-3,4-dihydroxyphenylalanine (L-DOPA) and DA reduced striatum CoI activity and ATP production.198–201 We have shown that these effects on mitochondria are due to the ability of DA to be taken up by the mitochondria and elicit a dose-dependent inhibition of CoI activity but not that of complexes II, IV, and V.201 Although DA and antipsychotics both inhibit CoI activity, they interact with the complex at different sites, DA with the hydrophilic matrix penetrating arm and antipsychotics with the hydrophobic inner membrane embedded arm of the complex.169 The clinical efficiency of antipsychotic drugs has been attributed to their antagonism of the D2 receptor, while in the mitochondria, these drugs mimic DA action on CoI. This drug-mitochondria interaction may be one mechanism involved in the side effects exhibited by the drugs. Alternatively, DA and antipsychotic drugs may both interact independently with the mitochondria, participating in a compensatory mechanism aimed to overcome mitochondrial dysfunction.
Conclusion
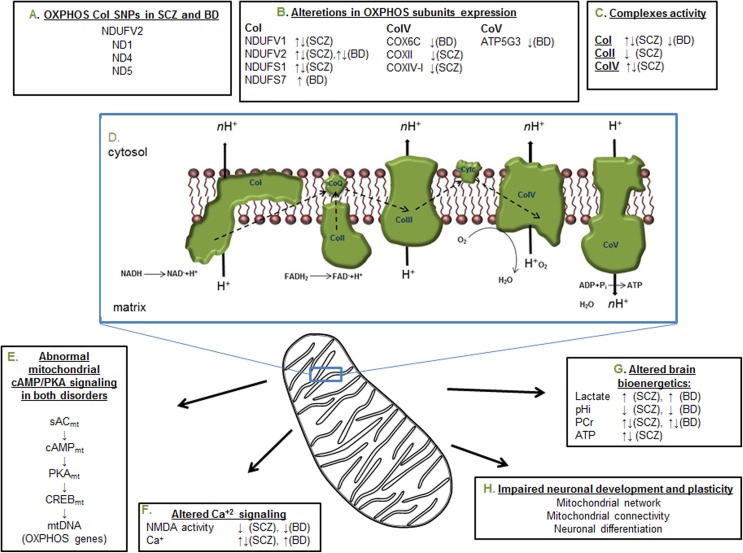
Neuronal energetic demands render them heavily dependent on the mitochondria, particularly on the OXPHOS. Apart from ATP production, the OXPHOS is a major player in many cellular processes, including calcium buffering, cell signaling, ROS production, and apoptosis. In mental disorders, mitochondrial deficits are significant yet of a relatively limited magnitude. Therefore, a deviation from normal functioning rather than lack of functioning and cell death is expected, specifically in cells highly dependent on energy supply for their activity. Indeed, in mental disorders, mild alterations in brain development, synaptic plasticity, and neuronal network connectivity have been observed. Such changes, however, can affect brain functioning, which may ultimately manifest in distorted cognitive and emotional behaviours, characteristic of mental disorders. As expected, many studies have found defects in various components of the OXPHOS protein apparatus (Figure 1). The fact that the OXPHOS interacts with antipsychotic drugs (typical and atypical) and with dopamine suggests mitochondria in general and CoI in particular, as an additional pathological factor in SCZ, which may serve as a novel target for future treatment strategies.
Figure 1.
Summary of the most reproducible deficiencies in the oxidative phosphorylation system (OXPHOS) and its related cellular signaling in schizophrenia (SCZ) and bipolar disorder (BD). (A) The most frequent single-nucleotide polymorphisms (SNPs) reported in nuclear and mitochondrial DNA (nDNA and mtDNA, respectively) encoded subunits of complex I. (B) Increase and decrease in the expression of various subunits of the OXPHOS complexes. (C) Reduced and enhanced enzymatic activity of 3 complexes of the OXPHOS. (D) The respiratory chain complexes, electron transfer, and adenosine triphosphate (ATP) production. (E) The mitochondrial cAMP/protein kinase A (PKA) signaling pathway, which affects the expression of mtDNA encoded subunits of the OXPHOS complexes. (F) Altered glutamate NMDA receptor transmission and intracellular Ca2+ concentration and signaling. (G) Alterations in mitochondrial originated high-energy phosphates, lactate, and pH, indicating impaired energy production in cell or tissue. (H) Disease-related neurodevelopmental consequences of the alterations presented in A to G. Arrows indicate the direction of alteration. PCr, phosphocreatine.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. [DOI] [PubMed] [Google Scholar]
- 2. Ben-Shachar D. Mitochondrial complex I as a possible novel peripheral biomarker for schizophrenia In: Ritsner MS. Ed. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes. Vol III Springer: Netherlands: p. 71–83. [Google Scholar]
- 3. Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. [DOI] [PubMed] [Google Scholar]
- 4. Ricci JE, Muñoz-Pinedo C, Fitzgerald P, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. [DOI] [PubMed] [Google Scholar]
- 5. Seth RB, Sun L, Ea CK, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kB and IRF3. Cell. 2005;122:669–682. [DOI] [PubMed] [Google Scholar]
- 6. Du F, Zhu X-H, Zhang Y, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105:6409–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Acin-Perez R, Salazar E, Kamenetsky M, et al. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valsecchi F, Ramos-Espiritu LS, Buck J, et al. cAMP and mitochondria. Physiology (Bethesda). 2013;28:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hüttemann M, Helling S, Sanderson TH, et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta Bioenerg. 2012;1817:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. [DOI] [PubMed] [Google Scholar]
- 11. Schon EA, Manfredi G. Neuronal degeneration and mitochondrial dysfunction. J Clin Invest. 2003;111:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fattal O, Budur K, Vaughan AJ, et al. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47:1–7. [DOI] [PubMed] [Google Scholar]
- 13. McGlashan TH, Fenton WS. The positive-negative distinction in schizophrenia: review of natural history validators. Arch. Gen. Psychiatry. 1992;49:63–72. [DOI] [PubMed] [Google Scholar]
- 14. Erecinska M, Silver I. ATP and brain function. J Cereb Blood Flow Metab. 1989;9(1):2–19. [DOI] [PubMed] [Google Scholar]
- 15. Moujahid A, D’Anjou A, Graña M. Energy demands of diverse spiking cells from the neocortex, hippocampus, and thalamus. Front Comput Neurosci. 2014;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gur RE, Resnick SM, Alavi A, et al. Regional brain function in schizophrenia. Arch Gen Psychiatry. 1987;44:119–125. [DOI] [PubMed] [Google Scholar]
- 17. Pettegrew JW, Keshavan MS, Panchalingam K, et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics: a pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 1991;48:563–568. [DOI] [PubMed] [Google Scholar]
- 18. Buchsbaum MS, Hazlett EA. Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull. 1998;24:343–364. [DOI] [PubMed] [Google Scholar]
- 19. Volz HP, Rossger G, Riehemann S, et al. Increase of phosphodiesters during neuroleptic treatment of schizophrenics: a longitudinal 31P-magnetic resonance spectroscopic study. Biol Psychiatry. 1999;45:1221–1225. [DOI] [PubMed] [Google Scholar]
- 20. Shenton ME, Dickey CC, Frumin M, et al. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gangadhar BN, Jayakumar PN, Subbakrishna DK, et al. Basal ganglia high-energy phosphate metabolism in neuroleptic-naive patients with schizophrenia: a 31-phosphorus magnetic resonance spectroscopic study. Am J Psychiatry. 2004;161:1304–1306. [DOI] [PubMed] [Google Scholar]
- 22. Du F, Cooper AJ, Thida T, et al. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 2014;71:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volz HP, Rzanny R, Rößger G, et al. Decreased energy demanding processes in the frontal lobes of schizophrenics due to neuroleptics? A 31P-magneto-resonance spectroscopic study. Psychiatry Res Neuroimaging. 1997;76:123–129. [DOI] [PubMed] [Google Scholar]
- 24. Kato T, Takahashi S, Shioiri T, et al. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J Affect Disord. 1993;27:53–59. [DOI] [PubMed] [Google Scholar]
- 25. Hamakawa H, Murashita J, Yamada N, et al. Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci. 2004;58:82–88. [DOI] [PubMed] [Google Scholar]
- 26. Dager SR, Friedman SD, Parow A, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. [DOI] [PubMed] [Google Scholar]
- 27. Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14:241–253. [DOI] [PubMed] [Google Scholar]
- 28. MacDonald ML, Naydenov A, Chu M, et al. Decrease in creatine kinase messenger RNA expression in the hippocampus and dorsolateral prefrontal cortex in bipolar disorder. Bipolar Disord. 2006;8:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skeberdis VA, Chevaleyre V, Lau CG, et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat. Neurosci. 2006;9:501–510. [DOI] [PubMed] [Google Scholar]
- 31. Newcomer JW, Craft S, Fucetola R, et al. Glucose-induced increase in memory performance in patients with schizophrenia. Schizophr Bull. 1999;25:321–335. [DOI] [PubMed] [Google Scholar]
- 32. Regenold WT, Phatak P, Marano CM, et al. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol Psychiatry. 2009;65:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall C, Klein-Flügge M, Howarth C, et al. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32:8940–8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuno-yagi A, Hatefi Y. Studies on the mechanism of oxidative phosphorylation. J Biol Chem. 1985;280:14424–14427. [PubMed] [Google Scholar]
- 35. Papa S, Martino PL, Capitanio G, et al. The oxidative phosphorylation system in mammalian mitochondria. Adv Exp Med Biol. 2012;942:3–37. [DOI] [PubMed] [Google Scholar]
- 36. Lenaz G, Genova ML. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am J Physiol Cell Physiol. 2007;292:C1221–C1239. [DOI] [PubMed] [Google Scholar]
- 37. Lapuente-Brun E, Moreno-Loshuertos R, Acín-Pérez R, et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. [DOI] [PubMed] [Google Scholar]
- 38. Walker JE, Carroll J, Altman MC, et al. Mass spectrometric characterization of the thirteen subunits of bovine respiratory complexes that are encoded in mitochondrial DNA. Methods Enzymol. 2009;456:111–131. [DOI] [PubMed] [Google Scholar]
- 39. Fukui H, Moraes CT. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci. 2008;31:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Breuer ME, Koopman WJ, Koene S, et al. The role of mitochondrial OXPHOS dysfunction in the development of neurologic diseases. Neurobiol Dis. 2013;51:27–34. [DOI] [PubMed] [Google Scholar]
- 41. Papa S, De Rasmo D. Complex I deficiencies in neurological disorders. Trends Mol Med. 2013;19:61–69. [DOI] [PubMed] [Google Scholar]
- 42. Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2:180–190. [DOI] [PubMed] [Google Scholar]
- 43. Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int Rev Neurobiol. 2004;59:273–296. [DOI] [PubMed] [Google Scholar]
- 44. Ben-Shachar D, Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One. 2008;3:e3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rezin GT, Amboni G, Zugno AI, et al. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34:1021–1029. [DOI] [PubMed] [Google Scholar]
- 46. Kato T, Takahashi S, Shioiri T, et al. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1992;26:223–230. [DOI] [PubMed] [Google Scholar]
- 47. Cavelier L, Jazin EE, Eriksson I, et al. Decreased cytochrome-c oxidase activity and lack of age-related accumulation of mitochondrial DNA deletions in the brains of schizophrenics. Genomics. 1995;29:217–224. [DOI] [PubMed] [Google Scholar]
- 48. Prince J, Blennow K, Gottfries CG, et al. Mitochondrial function is differentially altered in the basal ganglia of chronic schizophrenics. Neuropsychopharmacology. 1999;21:372–379. [DOI] [PubMed] [Google Scholar]
- 49. Maurer I, Zierz S, Möller H-J. Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophr Res. 2001;48:125–136. [DOI] [PubMed] [Google Scholar]
- 50. Akarsu S, Torun D, Bolu A, et al. Mitochondrial complex I and III gene mRNA levels in schizophrenia, and their relationship with clinical features. J Mol Psychiatry. 2014;2:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ben-Shachar D, Zuk R, Gazawi H, et al. Increased mitochondrial complex I activity in platelets of schizophrenic patients. Int J Neuropsychopharmacol. 1999;2:245–253. [DOI] [PubMed] [Google Scholar]
- 52. Andreazza AC, Shao L, Wang J-F, et al. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. [DOI] [PubMed] [Google Scholar]
- 53. Dror N, Klein E, Karry R, et al. State-dependent alterations in mitochondrial complex I activity in platelets: a potential peripheral marker for schizophrenia. Mol Psychiatry. 2002;7:995–1001. [DOI] [PubMed] [Google Scholar]
- 54. Karry R, Klein E, Ben-Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol Psychiatry. 2004;55:676–684. [DOI] [PubMed] [Google Scholar]
- 55. Konradi C, Eaton M, MacDonald ML, et al. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. [DOI] [PubMed] [Google Scholar]
- 56. Altar CA, Jurata LW, Charles V, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. [DOI] [PubMed] [Google Scholar]
- 57. Mehler-Wex C, Duvigneau JC, Hartl RT, et al. Increased mRNA levels of the mitochondrial complex I 75-kDa subunit: a potential peripheral marker of early onset schizophrenia? Eur Child Adolesc Psychiatry. 2006;15:504–507. [DOI] [PubMed] [Google Scholar]
- 58. Washizuka S, Iwamoto K, Kakiuchi C, et al. Expression of mitochondrial complex I subunit gene NDUFV2 in the lymphoblastoid cells derived from patients with bipolar disorder and schizophrenia. Neurosci Res. 2009;63:199–204. [DOI] [PubMed] [Google Scholar]
- 59. Gawryluk JW, Wang J-F, Andreazza AC, et al. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. [DOI] [PubMed] [Google Scholar]
- 60. Nakatani N, Hattori E, Ohnishi T, et al. Genome-wide expression analysis detects eight genes with robust alterations specific to bipolar I disorder: relevance to neuronal network perturbation. Hum Mol Genet. 2006;15:1949–1962. [DOI] [PubMed] [Google Scholar]
- 61. Washizuka S, Kakiuchi C, Mori K, et al. Expression of mitochondria-related genes in lymphoblastoid cells from patients with bipolar disorder. Bipolar Disord. 2005;7(2):146–152. [DOI] [PubMed] [Google Scholar]
- 62. Ben-Shachar D, Karry R. Sp1 expression is disrupted in schizophrenia; a possible mechanism for the abnormal expression of mitochondrial complex I genes, NDUFV1 and NDUFV2. PLoS One. 2007;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alaerts M, Del-Favero J. Searching genetic risk factors for schizophrenia and bipolar disorder: learn from the past and back to the future. Hum Mutat. 2009;30:1139–1152. [DOI] [PubMed] [Google Scholar]
- 65. Wang KS, Liu XF, Aragam N. A genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorder. Schizophr Res. 2010;124:192–199. [DOI] [PubMed] [Google Scholar]
- 66. Ayalew M, Le-Niculescu H, Levey DF, et al. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry. 2012;17(9):887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Washizuka S, Kametani M, Sasaki T, et al. Association of mitochondrial complex I subunit gene NDUFV2 at 18p11 with schizophrenia in the Japanese population. Am J Med Genet Neuropsychiatr Genet. 2006;141B:301–304. [DOI] [PubMed] [Google Scholar]
- 68. Xu C, Li PP, Kennedy JL, et al. Further support for association of the mitochondrial complex I subunit gene NDUFV2 with bipolar disorder. Bipolar Disord. 2008;10:105–110. [DOI] [PubMed] [Google Scholar]
- 69. Amar S, Shamir A, Ovadia O, et al. Mitochondrial DNA HV lineage increases the susceptibility to schizophrenia among Israeli Arabs. Schizophr Res. 2007;94:354–358. [DOI] [PubMed] [Google Scholar]
- 70. Verge B, Alonso Y, Valero J, et al. Mitochondrial DNA (mtDNA) and schizophrenia. Eur Psychiatry. 2011;26:45–56. [DOI] [PubMed] [Google Scholar]
- 71. Marchbanks RM, Ryan M, Day INM, et al. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res. 2003;65:33–38. [DOI] [PubMed] [Google Scholar]
- 72. Martorell L, Segués T, Folch G, et al. New variants in the mitochondrial genomes of schizophrenic patients. Eur J Hum Genet. 2006;14:520–528. [DOI] [PubMed] [Google Scholar]
- 73. Manji H, Kato T, Di Prospero NA, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13(5):293–307. [DOI] [PubMed] [Google Scholar]
- 74. Telford JE, Kilbride SM, Davey GP. Complex I is rate-limiting for oxygen consumption in the nerve terminal. J Biol Chem. 2009;284:9109–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hüttemann M, Lee I, Samavati L, et al. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta Mol Cell Res. 2007;1773:1701–1720. [DOI] [PubMed] [Google Scholar]
- 76. Gottesman MM, Fleischmann RD. The role of cAMP in regulating tumour cell growth. Cancer Surv. 1986;5:291–308. [PubMed] [Google Scholar]
- 77. Teng FYH, Tang BL. Axonal regeneration in adult CNS neurons: signaling molecules and pathways. J Neurochem. 2006;96:1501–1508. [DOI] [PubMed] [Google Scholar]
- 78. Lau BYB, Fogerson SM, Walsh RB, et al. Cyclic AMP promotes axon regeneration, lesion repair and neuronal survival in lampreys after spinal cord injury. Exp Neurol. 2013;250:31–42. [DOI] [PubMed] [Google Scholar]
- 79. Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Merrill R, Dagda RK, Dickey AS, et al. Mechanism of neuroprotective mitochondrial remodeling by pka/akap1. PLoS Biol. 2011;9:e10000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bundey RA, Insel PA. Discrete intracellular signaling domains of soluble adenylyl cyclase: camps of cAMP? Sci STKE. 2004;2004:pe19. [DOI] [PubMed] [Google Scholar]
- 82. Papa S, Rasmo D, De Technikova-Dobrova Z, et al. Respiratory chain complex I, a main regulatory target of the cAMP/PKA pathway is defective in different human diseases. FEBS Lett. 2012;586:568–577. [DOI] [PubMed] [Google Scholar]
- 83. Chen R, Fearnley IM, Peak-Chew SY, et al. The phosphorylation of subunits of complex i from bovine heart mitochondria. J Biol Chem. 2004;279:26036–26045. [DOI] [PubMed] [Google Scholar]
- 84. Schilling B, Aggeler R, Schulenberg B, et al. Mass spectrometric identification of a novel phosphorylation site in subunit NDUFA10 of bovine mitochondrial complex I. FEBS Lett. 2005;579:2485–2490. [DOI] [PubMed] [Google Scholar]
- 85. Palmisano G, Sardanelli AM, Signorile A, et al. The phosphorylation pattern of bovine heart complex I subunits. Proteomics. 2007;7:1575–1583. [DOI] [PubMed] [Google Scholar]
- 86. Papa S, Scacco S, De Rasmo D, et al. CAMP-dependent protein kinase regulates post-translational processing and expression of complex I subunits in mammalian cells. Biochim Biophys Acta Bioenerg. 2010;1797:649–658. [DOI] [PubMed] [Google Scholar]
- 87. Yadava N, Potluri P, Scheffler IE. Investigations of the potential effects of phosphorylation of the MWFE and ESSS subunits on complex I activity and assembly. Int J Biochem Cell Biol. 2008;40:447–460. [DOI] [PubMed] [Google Scholar]
- 88. Scacco S, Vergari R, Scarpulla RC, et al. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum- starved mouse fibroblast cultures. J Biol Chem. 2000;275:17578–17582. [DOI] [PubMed] [Google Scholar]
- 89. De Rasmo D, Signorile A, Santeramo A, et al. Intramitochondrial adenylyl cyclase controls the turnover of nuclear-encoded subunits and activity of mammalian complex I of the respiratory chain. Biochim Biophys Acta Mol Cell Res. 2015;1853:183–191. [DOI] [PubMed] [Google Scholar]
- 90. Steenaart NE, Shore GC. Mitochondrial cytochrome c oxidase subunit IV is phosphorylated by an endogenous kinase. FEBS Lett. 1997;415:294–298. [DOI] [PubMed] [Google Scholar]
- 91. Fang JK, Prabu SK, Sepuri NB, et al. Site specific phosphorylation of cytochrome c oxidase subunits I, IVi1 and Vb in rabbit hearts subjected to ischemia/reperfusion. FEBS Lett. 2007;581:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Conkright MD, Canettieri G, Screaton R, et al. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. [DOI] [PubMed] [Google Scholar]
- 93. Wu Z, Huang X, Feng Y, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103:14379–14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee J, Kim C-H, Simon DK, et al. Mitochondrial cyclic AMP response element-binding protein (CREB) mediates mitochondrial gene expression and neuronal survival. J Biol Chem. 2005;280:40398–40401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. [DOI] [PubMed] [Google Scholar]
- 96. Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci. 2008;1147:321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nishino N, Kitamura N, Hashimoto T, et al. Increase in [3 H]cAMP binding sites and decrease in Gi alpha and Go alpha immunoreactivities in left temporal cortices from patients with schizophrenia. Brain Res. 1993;615:41–49. [DOI] [PubMed] [Google Scholar]
- 98. Funk AJ, McCullumsmith RE, Haroutunian V, et al. Abnormal activity of the MAPK- and cAMP-associated signaling pathways in frontal cortical areas in postmortem brain in schizophrenia. Neuropsychopharmacology. 2012;37:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rahman S, Li PP, Young LT, et al. Reduced [3 H]cyclic AMP binding in postmortem brain from subjects with bipolar affective disorder. J Neurochem. 1997;68:297–304. [DOI] [PubMed] [Google Scholar]
- 100. Fields A, Li PP, Kish SJ, et al. Increased cyclic AMP-dependent protein kinase activity in postmortem brain from patients with bipolar affective disorder. J Neurochem. 1999;73:1704–1710. [DOI] [PubMed] [Google Scholar]
- 101. Chang A, Li P, Warsh J. Altered cAMP-dependent protein kinase subunit immunolabeling in post-mortem brain from patients with bipolar affective disorder. J Neurochem. 2003;84:781–791. [DOI] [PubMed] [Google Scholar]
- 102. Tardito D, Tura GB, Bocchio L, et al. Abnormal levels of cAMP-dependent protein kinase regulatory subunits in platelets from schizophrenic patients. Neuropsychopharmacology. 2000;23:216–219. [DOI] [PubMed] [Google Scholar]
- 103. Perez J, Tardito D, Mori S, et al. Abnormalities of cyclic adenosine monophosphate signaling in platelets from untreated patients with bipolar disorder. Arch Gen Psychiatry. 1999;56:248–253. [DOI] [PubMed] [Google Scholar]
- 104. Ren X, Rizavi HS, Khan MA, et al. Alteration of cyclic-AMP response element binding protein in the postmortem brain of subjects with bipolar disorder and schizophrenia. J Affect Disord. 2014;152-154:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Beavo JA, Conti M, Heaslip RK. Multiple cyclic nucleotide phosphodiesterases. Mol Pharmacol. 1994;46(3):399–405. [PubMed] [Google Scholar]
- 106. Houslay MD, Milligan G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci. 1997;22:217–224. [DOI] [PubMed] [Google Scholar]
- 107. Loughney K, Snyder PB, Uher L, et al. Isolation and characterization of PDE10A, a novel human 3′, 5′-cyclic nucleotide phosphodiesterase. Gene. 1999;234:109–117. [DOI] [PubMed] [Google Scholar]
- 108. Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–179. [DOI] [PubMed] [Google Scholar]
- 109. Millar JK, Pickard BS, Mackie S, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. [DOI] [PubMed] [Google Scholar]
- 110. James R, Adams RR, Christie S, et al. Disrupted in Schizophrenia 1 (DISC1) is a multicompartmentalized protein that predominantly localizes to mitochondria. Mol Cell Neurosci. 2004;26:112–122. [DOI] [PubMed] [Google Scholar]
- 111. Fatemi SH, King DP, Reutiman TJ, et al. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr. Res. 2008;101:36–49. [DOI] [PubMed] [Google Scholar]
- 112. Hennah W, Thomson P, McQuillin A, et al. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. 2009;14:865–873. [DOI] [PubMed] [Google Scholar]
- 113. Siuciak JA. The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs. 2008;22:983–993. [DOI] [PubMed] [Google Scholar]
- 114. Rintoul GL, Filiano AJ, Brocard JB, et al. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mironov SL. Spontaneous and evoked neuronal activities regulate movements of single neuronal mitochondria. Synapse. 2006;59:403–411. [DOI] [PubMed] [Google Scholar]
- 116. MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20:102–112. [DOI] [PubMed] [Google Scholar]
- 117. Rintoul GL, Reynolds IJ. Mitochondrial trafficking and morphology in neuronal injury. Biochim Biophys Acta Mol Basis Dis. 2010;1802:143–150. [DOI] [PubMed] [Google Scholar]
- 118. Somlyo AP. Cell physiology: Cellular site of calcium regulation. Nature. 1984;309:516–517. [DOI] [PubMed] [Google Scholar]
- 119. Raturi A, Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim Biophys Acta Mol Cell Res. 2013;1833:213–224. [DOI] [PubMed] [Google Scholar]
- 120. Csordás G, Renken C, Várnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fujimoto M, Hayashi T. New insights into the role of mitochondria-associated endoplasmic reticulum membrane. Int Rev Cell Mol Biol. 2011;292:73–117. [DOI] [PubMed] [Google Scholar]
- 122. Rizzuto R, Brini M, Murgia M, et al. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. [DOI] [PubMed] [Google Scholar]
- 123. Benedicte O, Prete D, Mouni C. Mitochondrial calcium signalling: role in oxidative phosphorylation diseases. 2012. Available from: http://www.intechopen.com/books/bioenergetics/mitochondrial-calcium-signalling-role-in-oxidative-phosphorylation-diseases.
- 124. Desouza N, Reiken S, Ondrias K, et al. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002;277:39397–39400. [DOI] [PubMed] [Google Scholar]
- 125. De Stefani D, Raffaello A, Teardo E, et al. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci U S A. 2003;100:10676–10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Brindle P, Nakajima T, Montminy M. Multiple protein kinase A–regulated events are required for transcriptional induction by cAMP. Proc Natl Acad Sci U S A. 1995;92:10521–10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. [DOI] [PubMed] [Google Scholar]
- 129. McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. [DOI] [PubMed] [Google Scholar]
- 130. Gellerich FN, Gizatullina Z, Trumbeckaite S, et al. The regulation of OXPHOS by extramitochondrial calcium. Biochim Biophys Acta Bioenerg. 2010;1797:1018–1027. [DOI] [PubMed] [Google Scholar]
- 131. Murphy AN, Kelleher JK, Fiskum G. Submicromolar Ca2+ regulates phosphorylating respiration by normal rat liver and AS-30D hepatoma mitochondria by different mechanisms. J Biol Chem. 1990;265:10527–10534. [PubMed] [Google Scholar]
- 132. Territo PR, Mootha VK, French SA, et al. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–C435. [DOI] [PubMed] [Google Scholar]
- 133. Willems PHGM, Valsecchi F, Distelmaier F, et al. Mitochondrial Ca2+ homeostasis in human NADH: ubiquinone oxidoreductase deficiency. Cell Calcium. 2008;44:123–133. [DOI] [PubMed] [Google Scholar]
- 134. Willems PHGM, Smeitink JM, Koopman WJH. Mitochondrial dynamics in human NADH: ubiquinone oxidoreductase deficiency. Int J Biochem Cell Biol. 2009;41:1773–1782. [DOI] [PubMed] [Google Scholar]
- 135. Valsecchi F, Esseling JJ, Koopman WJH, et al. Calcium and ATP handling in human NADH: ubiquinone oxidoreductase deficiency. Biochim Biophys Acta Mol Basis Dis. 2009;1792:1130–1137. [DOI] [PubMed] [Google Scholar]
- 136. Jimerson DC, Post RM, Carman JS, et al. CSF calcium: clinical correlates in affective illness and schizophrenia. Biol Psychiatry. 1979;14:37–51. [PubMed] [Google Scholar]
- 137. Kusumi I, Koyama T, Yamashita I. Thrombin-induced platelet calcium mobilization is enhanced in bipolar disorders. Biol Psychiatry. 1992;32:731–734. [DOI] [PubMed] [Google Scholar]
- 138. Řípová D, Strunecká A, Němcová V, et al. Phospholipids and calcium alterations in platelets of schizophrenia patients. Physiol Res. 1997;46:59–68. [PubMed] [Google Scholar]
- 139. Ferreira MAR, O’Donovan MC, Meng Y, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sklar P, Smoller JW, Fan J, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Berger SM, Bartsch D. The role of L-type voltage-gated calcium channels Cav1.2 and Ca v1.3 in normal and pathological brain function. Cell Tissue Res. 2014;357:463–476. [DOI] [PubMed] [Google Scholar]
- 142. Sakurai K, Toru M, Yamakawa-Kobayashi K, et al. Mutation analysis of the N-methyl-D-aspartate receptor NR1 subunit gene (GRIN1) in schizophrenia. Neurosci Lett. 2000;296:168–170. [DOI] [PubMed] [Google Scholar]
- 143. Giegling I, Genius J, Benninghoff J, et al. Genetic findings in schizophrenia patients related to alterations in the intracellular Ca-homeostasis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1375–1380. [DOI] [PubMed] [Google Scholar]
- 144. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Akbarian S, Sucher NJ, Bradley D, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Woo T-UW, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. [DOI] [PubMed] [Google Scholar]
- 147. Snyder SH. Phencyclidine. Nature. 1980;285:355–356. [DOI] [PubMed] [Google Scholar]
- 148. Malhotra AK, Pinals DA, Adler CM, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. [DOI] [PubMed] [Google Scholar]
- 149. Berridge MJ. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion. 2013;7:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Berridge MJ. Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res. 2014;357:477–492. [DOI] [PubMed] [Google Scholar]
- 151. Hebb DO. The organization of behavior. New York: John Wiley; 1949. [Google Scholar]
- 152. Goldsmith SK, Joyce JN. Alterations in hippocampal moss fiber pathway in schizophrenia and Alzheimer’s disease. Biol Psychiatry. 1995;37:122–126. [DOI] [PubMed] [Google Scholar]
- 153. Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Eastwood SL, Burnet PWJ, Harrison PJ. Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience. 1995;66:309–319. [DOI] [PubMed] [Google Scholar]
- 155. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. [DOI] [PubMed] [Google Scholar]
- 156. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mattson MP, Partin J. Evidence for mitochondrial control of neuronal polarity. J Neurosci Res. 1999;56:8–20. [DOI] [PubMed] [Google Scholar]
- 158. Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:351–361. [DOI] [PubMed] [Google Scholar]
- 159. Li Z, Okamoto KI, Hayashi Y, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. [DOI] [PubMed] [Google Scholar]
- 160. Nakata T, Terada S, Hirokawa N. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J Cell Biol. 1998;140:659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, et al. Assembling the presynaptic active zone: a characterization of an active zone precursor vesicle. Neuron. 2001;29:131–143. [DOI] [PubMed] [Google Scholar]
- 162. Stowers RS, Megeath LJ, Górska-Andrzejak J, et al. Axonal transport of mitochondria to synapses depends on Milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. [DOI] [PubMed] [Google Scholar]
- 163. Guo X, Macleod GT, Wellington A, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. [DOI] [PubMed] [Google Scholar]
- 164. Atkin TA, MacAskill AF, Brandon NJ, et al. Disrupted in Schizophrenia-1 regulates intracellular trafficking of mitochondria in neurons. Mol Psychiatry. 2011;16:122–124. [DOI] [PubMed] [Google Scholar]
- 165. Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. [DOI] [PubMed] [Google Scholar]
- 166. Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. [DOI] [PubMed] [Google Scholar]
- 167. Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta Bioenerg. 2012;1817:1833–1838. [DOI] [PubMed] [Google Scholar]
- 168. Cataldo AM, McPhie DL, Lange NT, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. 2010;177:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rosenfeld M, Brenner-Lavie H, Ari SGB, et al. Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69:980–988. [DOI] [PubMed] [Google Scholar]
- 170. Robicsek O, Karry R, Petit I, et al. Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle–derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry. 2013;18:1067–1076. [DOI] [PubMed] [Google Scholar]
- 171. Chen H, Chan DC. Mitochondrial dynamics-fusion, fission, movement, and mitophagy in neurodegenerative diseases. Hum Mol Genet. 2009;18:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Chang DTW, Rintoul GL, Pandipati S, et al. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. [DOI] [PubMed] [Google Scholar]
- 173. Cui M, Tang X, Christian WV, et al. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem. 2010;285:11740–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Gioran A, Nicotera P, Bano D. Impaired mitochondrial respiration promotes dendritic branching via the AMPK signaling pathway. Cell Death Dis. 2014;5:e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Houghton FD, Leese HJ. Metabolism and developmental competence of the preimplantation embryo. Eur J Obstet Gynecol Reprod Biol. 2004;115:92–96. [DOI] [PubMed] [Google Scholar]
- 177. St John JC, Ramalho-Santos J, Gray HL, et al. The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning Stem Cells. 2005;7:141–153. [DOI] [PubMed] [Google Scholar]
- 178. Oh SK, Kim HS, Ahn HJ, et al. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells. 2005;23:211–219. [DOI] [PubMed] [Google Scholar]
- 179. Ramalho-Santos J, Rodrigues AS. From oocytes and pluripotent stem cells to fully differentiated fates: (also) a mitochondrial odyssey. In: St. John JC. Ed. Mitochondrial DNA, Mitochondria, Disease and Stem Cells Totowa (NJ; ): Humana; 2013. p. 69–86. [Google Scholar]
- 180. Spitkovsky D, Sasse P, Kolossov E, et al. Activity of complex III of the mitochondrial electron transport chain is essential for early heart muscle cell differentiation. FASEB J. 2004;18:1300–1302. [DOI] [PubMed] [Google Scholar]
- 181. Chung S, Dzeja PP, Faustino RS, et al. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S60–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chen C-T, Shih Y-RV, Kuo TK, et al. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. [DOI] [PubMed] [Google Scholar]
- 183. Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. [DOI] [PubMed] [Google Scholar]
- 184. Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. [DOI] [PubMed] [Google Scholar]
- 185. Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kapur S, Kapur S, Remington G, et al. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–883. [DOI] [PubMed] [Google Scholar]
- 187. Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 2000;97:7673–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Jayakumar PN, Gangadhar BN, Venkatasubramanian G, et al. High energy phosphate abnormalities normalize after antipsychotic treatment in schizophrenia: a longitudinal 31P MRS study of basal ganglia. Psychiatry Res Neuroimaging. 2010;181:237–240. [DOI] [PubMed] [Google Scholar]
- 189. Sasaki N, Iwase M, Uchizono Y, et al. The atypical antipsychotic clozapine impairs insulin secretion by inhibiting glucose metabolism and distal steps in rat pancreatic islets. Diabetologia. 2006;49:2930–2938. [DOI] [PubMed] [Google Scholar]
- 190. Koizumi S, Ikeda M, Nakazawa K, et al. Inhibition by haloperidol of adenosine 5′-triphosphate-evoked responses in rat pheochromocytoma cells. Biochem Biophys Res Commun. 1995. May;210:624–630. [DOI] [PubMed] [Google Scholar]
- 191. Brenner-Lavie H, Klein E, Ben-Shachar D. Mitochondrial complex I as a novel target for intraneuronal DA: modulation of respiration in intact cells. Biochem Pharmacol. 2009;78:85–95. [DOI] [PubMed] [Google Scholar]
- 192. Burkhardt C, Kelly JP, Lim YH, et al. Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol. 1993;33:512–517. [DOI] [PubMed] [Google Scholar]
- 193. Whatley SA, Curti D, Das Gupta F, et al. Superoxide, neuroleptics and the ubiquinone and cytochrome b5 reductases in brain and lymphocytes from normals and schizophrenic patients. Mol Psychiatry. 1998;3:227–237. [DOI] [PubMed] [Google Scholar]
- 194. Casademont J, Garrabou G, Miró O, et al. Neuroleptic treatment effect on mitochondrial electron transport chain: peripheral blood mononuclear cells analysis in psychotic patients. J Clin Psychopharmacol. 2007;27:284–288. [DOI] [PubMed] [Google Scholar]
- 195. Balijepalli S, Kenchappa RS, Boyd MR, et al. Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: comparison with atypical antipsychotics. Neurochem Int. 2001;38:425–435. [DOI] [PubMed] [Google Scholar]
- 196. Maurer I, Möller H. Inhibition of complex I by neuroleptics in normal human brain cortex parallels the EP toxicity of neuroleptics. Mol Cell Biochem. 1997;174(1-2):255–259. [PubMed] [Google Scholar]
- 197. Ben-Shachar D. Mitochondrial dysfunction in schizophrenia: a possible linkage to dopamine. J Neurochem. 2002;83:1241–1251. [DOI] [PubMed] [Google Scholar]
- 198. Przedborski S, Jackson-Lewis V, Muthane U, et al. Chronic levodopa administration alters cerebral mitochondrial respiratory chain activity. Ann Neurol. 1993;34:715–723. [DOI] [PubMed] [Google Scholar]
- 199. Ben-Shachar D, Zuk R, Glinka Y. Dopamine neurotoxicity: inhibition of mitochondrial respiration. J Neurochem. 1995;64:718–723. [DOI] [PubMed] [Google Scholar]
- 200. Ben-Shachar D, Zuk R, Gazawi H, et al. Dopamine toxicity involves mitochondrial complex I inhibition: implications to dopamine-related neuropsychiatric disorders. Biochem Pharmacol. 2004;67:1965–1974. [DOI] [PubMed] [Google Scholar]
- 201. Brenner-Lavie H, Klein E, Zuk R, et al. Dopamine modulates mitochondrial function in viable SH-SY5Y cells possibly via its interaction with complex I: relevance to dopamine pathology in schizophrenia. Biochim Biophys Acta Bioenerg. 2008;1777:173–185. [DOI] [PubMed] [Google Scholar]