Abstract
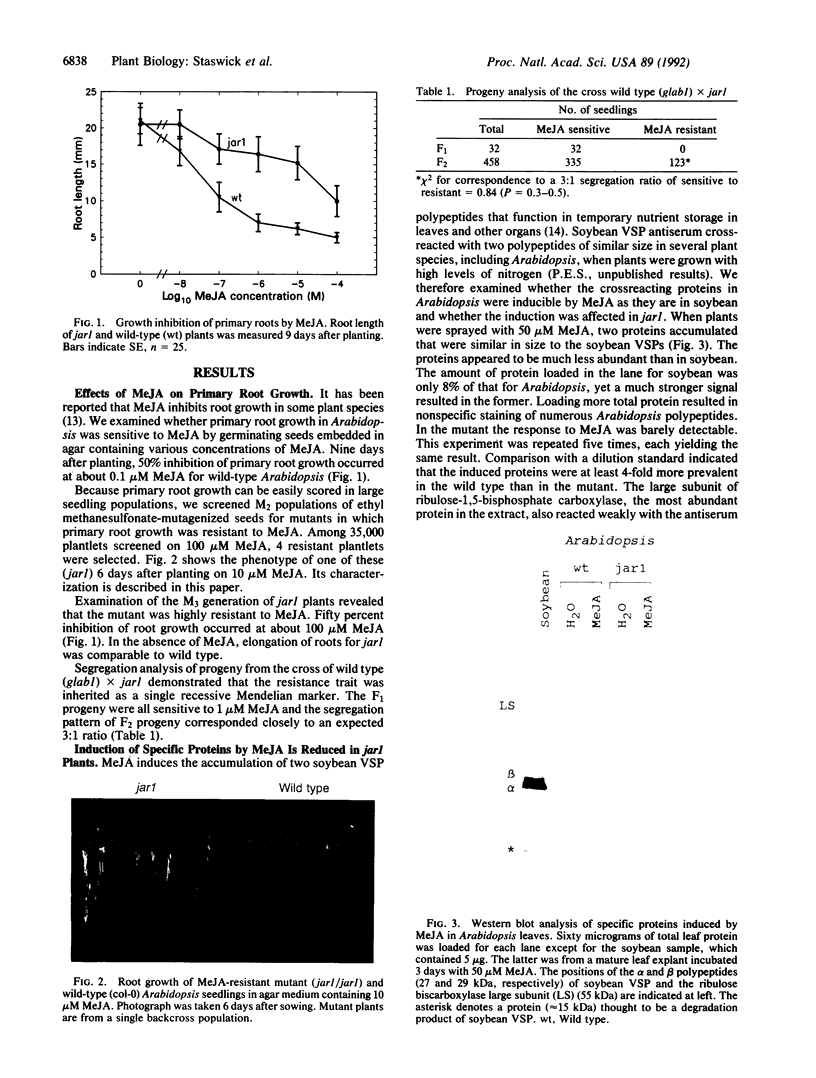
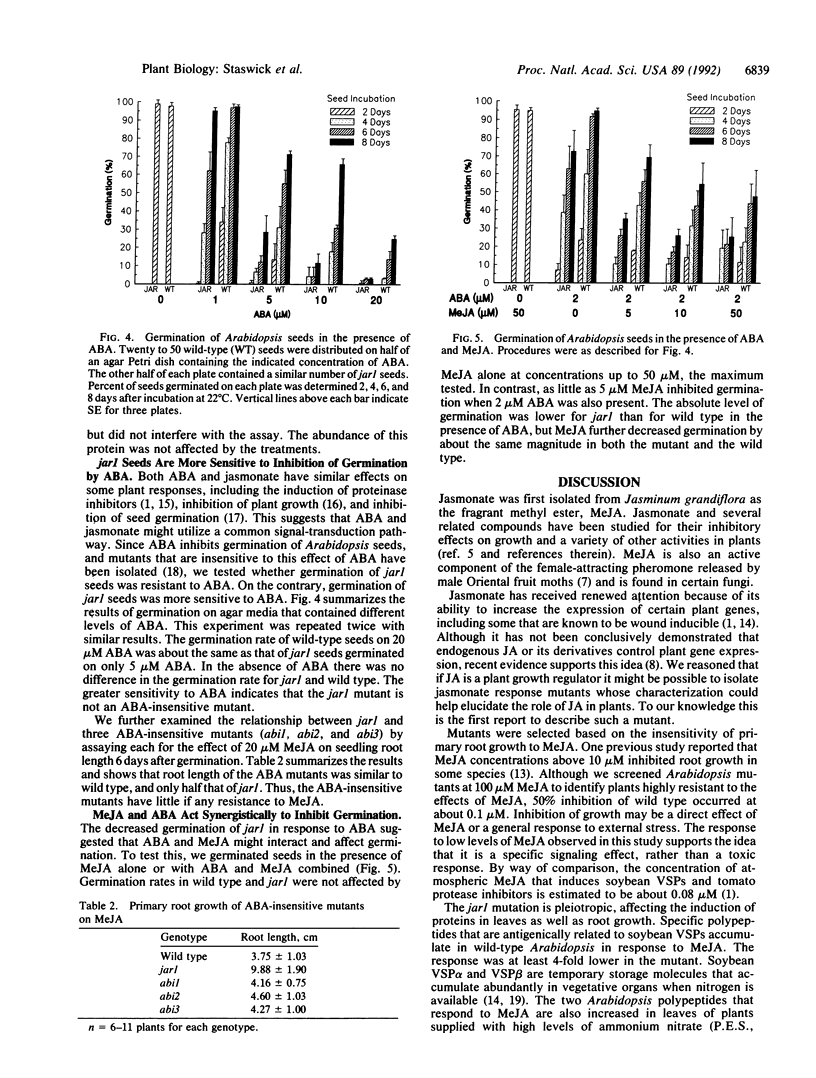
Jasmonic acid and its methyl ester, methyl jasmonate (MeJA), are plant signaling molecules that affect plant growth and gene expression. Primary root growth of wild-type Arabidopsis thaliana seedlings was inhibited 50% when seedlings were grown on agar medium containing 0.1 M MeJA. An ethyl methanesulfonate mutant (jar1) with decreased sensitivity to MeJA inhibition of root elongation was isolated and characterized. Genetic data indicated the trait was recessive and controlled by a single Mendelian factor. MeJA-induced polypeptides were detected in Arabidopsis leaves by antiserum to a MeJA-inducible vegetative storage protein from soybean. The induction of these proteins by MeJA in the mutant was at least 4-fold less in jar1 compared to wild type. In contrast, seeds of jar1 plants were more sensitive than wild type to inhibition of germination by abscisic acid. These results suggest that the defect in jar1 affects a general jasmonate response pathway, which may regulate multiple genes in different plant organs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. C., Nishida R., Roelofs W. L. Close-range attraction of female oriental fruit moths to herbal scent of male hairpencils. Science. 1981 Dec 18;214(4527):1359–1361. doi: 10.1126/science.214.4527.1359. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Octadecanoid Precursors of Jasmonic Acid Activate the Synthesis of Wound-Inducible Proteinase Inhibitors. Plant Cell. 1992 Feb;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. R., Somerville C. R. Three Classes of Abscisic Acid (ABA)-Insensitive Mutations of Arabidopsis Define Genes that Control Overlapping Subsets of ABA Responses. Plant Physiol. 1990 Nov;94(3):1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Grimes H. D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H., Müller M. J., Kutchan T. M., Zenk M. H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C., Britton J. H., Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990 Nov;2(11):1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Cortes H., Willmitzer L., Sanchez-Serrano J. J. Abscisic Acid Mediates Wound Induction but Not Developmental-Specific Expression of the Proteinase Inhibitor II Gene Family. Plant Cell. 1991 Sep;3(9):963–972. doi: 10.1105/tpc.3.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett F. B., Wilson A. K., Estelle M. The aux1 Mutation of Arabidopsis Confers Both Auxin and Ethylene Resistance. Plant Physiol. 1990 Nov;94(3):1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol. 1989 Jan;89(1):309–315. doi: 10.1104/pp.89.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E., Huang J. F., Rhee Y. Nitrogen and methyl jasmonate induction of soybean vegetative storage protein genes. Plant Physiol. 1991 May;96(1):130–136. doi: 10.1104/pp.96.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen R. W., van Rooijen G. J., Pearce D. W., Pharis R. P., Holbrook L. A., Moloney M. M. Effects of jasmonic Acid on embryo-specific processes in brassica and linum oilseeds. Plant Physiol. 1991 Feb;95(2):399–405. doi: 10.1104/pp.95.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V. M., Colwell G. Acid phosphatase-1 from nematode resistant tomato : isolation and characterization of its gene. Plant Physiol. 1991 Sep;97(1):139–146. doi: 10.1104/pp.97.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. K., Pickett F. B., Turner J. C., Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990 Jul;222(2-3):377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983 Sep;73(1):125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]




