Abstract
Vitamin C (Ascorbic Acid), the antiscorbutic vitamin, cannot be synthesized by humans and other primates, and has to be obtained from diet. Ascorbic acid is an electron donor and acts as a cofactor for fifteen mammalian enzymes. Two sodium-dependent transporters are specific for ascorbic acid, and its oxidation product dehydroascorbic acid is transported by glucose transporters. Ascorbic acid is differentially accumulated by most tissues and body fluids. Plasma and tissue vitamin C concentrations are dependent on amount consumed, bioavailability, renal excretion, and utilization. To be biologically meaningful or to be clinically relevant, in vitro and in vivo studies of vitamin C actions have to take into account physiologic concentrations of the vitamin. In this paper, we review vitamin C physiology; the many phenomena involving vitamin C where new knowledge has accrued or where understanding remains limited; raise questions about the vitamin that remain to be answered; and explore lines of investigations that are likely to be fruitful.
Keywords: Vitamin C, Dehydroascorbic Acid, Vitamin C Transport, Scurvy, Dose Concentration Relationship, Recommended Dietary Allowance
Introduction
Vitamin C (Ascorbic acid, abbreviated as AA; the terms vitamin C and ascorbic acid are used interchangeably) is synthesized by all plants and most animals (Smirnoff et al., 2001). It is a vitamin for humans because the gene for gulonolactone oxidase, the terminal enzyme in the AA synthesis pathway has undergone mutations that make it non-functional (Linster & Van Schaftingen, 2007). Animals that have lost the ability to synthesize ascorbic acid do not have a phylogenetic relationship with each other. These animals include non-human primates, guinea pigs, capybara and some birds and fish (Chaudhuri & Chatterjee, 1969, Chatterjee, 1973, Cueto et al., 2000). Deficiency of ascorbic acid produces the fatal disease scurvy, which can be cured only by the administration of vitamin C.
In this paper, we review general aspects of vitamin C biology as it pertains to human health. Those aspects of vitamin C that have been extensively reviewed elsewhere are only briefly discussed here. These include the importance of proper sample processing and reliability of assays to ensure that vitamin C measurements are accurate and precise (Levine et al., 1999b); the role of vitamin C as an antioxidant (Padayatty et al., 2003); and the chemistry and biology of pharmacologic (high dose) intravenously administered vitamin C (Levine et al., 2011) (Parrow et al., 2013). A large number of epidemiological and intervention studies have examined the effects of vitamin C consumption and/or supplementation on physiological parameters, biomarkers and clinical end points. In general these studies found either no effect attributable to vitamin C intake or reported ambiguous results. Although these results are not reviewed here in great detail, we emphasize the importance of dose concentration relationships in designing and interpreting studies. Getting concentrations and amounts “just right”, as Goldilocks has described in clear fashion, is discussed throughout. The attached figures and figure legends are largely self-contained, and repetition of figure material in the manuscript is minimized.
History of scurvy and the discovery of vitamin C
Although scurvy has been known since ancient times (Clemeston, 1989), it became a notable cause of large scale deaths in the last five hundred years. Scurvy afflicted large land armies and cities in Northern Europe, especially in winter and during siege warfare. In the age of exploration, scurvy became the principle factor limiting sea voyage, often killing many sailors after two to three months at sea. In perhaps the first controlled clinical trial, James Lind showed that scurvy could be cured by citrus fruits (Lind, 1953b). However, this simple remedy was not used widely for several decades. The finding did not fit in with the extant scientific knowledge, and existing theories of the causation of disease had no concept of nutritional deficiency. Ascorbic acid was first isolated by Albert Szent-Gyorgyi in 1928 and shown to be the antiscorbutic factor by Szent-Gyorgyi and King in 1932 (Svirbely & Szent-Gyorgyi, 1932, King & Waugh, 1932).
Scurvy
The earliest symptom of scurvy is subtle, and was described by James Lind in his treatise on the scurvy (1753) as lassitude (Lind, 1953b). It was a predictable affliction of sailors who developed the disease after a month or two at sea. In its early stage, sailors lost initiative and the will to work, but could work normally when compelled to do so. Frank scurvy is now rare but in full form presents with striking signs and symptoms. These include hypochondriasis and depression; perifollicular hyperkeratosis with coiled hairs; swollen and friable gingivae; anemia, petechial hemorrhage, erythema, and purpura; arthralgia and/or joint effusions; breakdown of old wounds; bleeding into the skin, subcutaneous tissues, muscles, joints and subperiosteal hemorrhages; fever; shortness of breath; infections; and confusion (Hodges et al., 1971, Hood et al., 1970). Subjects can present with only some symptoms or signs, and in these cases diagnosis is often missed initially (Bernardino et al., 2012). The clinical picture is confusing in the presence of multiple vitamin deficiencies (Blanchard et al., 2014) or when atypical symptoms such as dyspnea are predominant (Kupari & Rapola, 2012). Untreated, the condition is fatal.
There is not a definitive low vitamin C plasma concentration at which scurvy develops. Studies using radiolabeled vitamin C predict that body stores in healthy humans are about 1500 mg. Scurvy is thought to occur when this falls below 300 mg (Hodges et al., 1971), with plasma vitamin C concentrations <10 µM. However, these experiments were done at a time when vitamin C measurements used colorimetric assays that detected not only vitamin C but other unknown interfering substances. Overestimation of vitamin C was more marked when the concentrations of vitamin C measured were low (Baker et al., 1969, Baker et al., 1971, Hodges et al., 1971). Modern vitamin C assays are much more accurate and precise. These generally use high performance liquid chromatography (HPLC) to separate vitamin C from other substances and flow-by electrochemistry (amperometry), flow-through electrochemistry (coulometry), ultraviolet (UV), or fluorescence to detect and measure vitamin C. Recently, mass spectrometry is another tool to detect vitamin C, coupled to liquid chromatography (called LC-MS)(Leveque et al., 2000, Szultka et al., 2014, Gentili et al., 2008). At present, the most thoroughly characterized method is HPLC with electrochemical detection (Levine et al., 1999b). Thus, because of inaccurate assays, the measurements of plasma vitamin C concentrations in scurvy may have shown values that were two to three fold higher than the true value. In depletion-repletion studies which utilized a modern HPLC electrochemical assay, healthy young men and women attained plasma vitamin C concentrations of 8 µM without developing scurvy (Levine et al., 1996b, Levine et al., 2001b). However, many subjects experienced lassitude, and it was considered unsafe to further deplete them of vitamin C. Therefore, scurvy remains a clinical diagnosis, confirmed by low plasma vitamin C concentrations, but as yet without a definite diagnostic concentration. Values below 10µM are perhaps not far from incipient scurvy, but physical signs may appear only at much lower values, perhaps as low as 3-5µM. In epidemiological studies, plasma vitamin C concentrations less than 11.4 µM (0.2 mg/dl) are deemed to indicate deficiency (Jacob et al., 1987, Schleicher et al., 2009).
Chemistry and metabolism of vitamin C
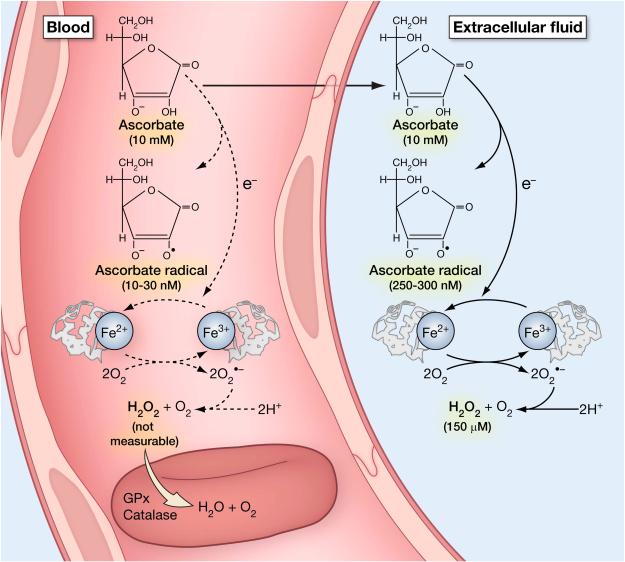
Chemically, vitamin C is an electron donor, or reducing agent, and electrons from ascorbate account for all of its known physiological effects. Vitamin C chemistry is detailed in figure 1. Because electrons from vitamin C can reduce oxidized species, or oxidants, vitamin C is often termed an antioxidant, but this terminology is misleading. Electrons from ascorbate can reduce metals such as copper and iron, leading to formation of superoxide and hydrogen peroxide, and subsequent generation of reactive oxidant species. Thus, under some circumstances ascorbate, through its action as a reducing agent, will generate oxidants. This chemistry occurs in vivo when pharmacologic ascorbate concentrations, in the millimolar range, are achieved in the plasma and in the extra cellular fluids, and can also occur with physiologic concentration of ascorbate in cell culture media when metals are present (Parrow et al., 2013).
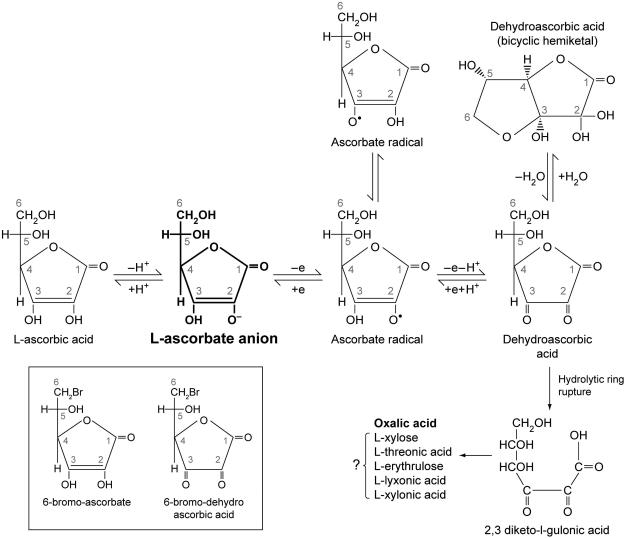
Figure 1. Ascorbic acid metabolism.
Ascorbic acid metabolism and halogenated analogues of ascorbic acid. Vitamin C (ascorbic acid) under physiological conditions is >99% in the form of ascorbate anion (shown in bold)(Beuttner & Schafer, 2004). It can sequentially donate two electrons from the double bond between carbons two and three. Loss of the first electron (oxidation) produces the free radical ascorbate radical (semidehydroascorbic acid). Some reactive free radicals produced by biological processes can be harmful because of their highly reactive nature. These can be reduced by ascorbic acid but in the process ascorbic acid is itself is converted (oxidized) into ascorbate radical. Ascorbate radical has a half-life of 10−3 seconds to several minutes, depending on the presence of oxygen and metals. Under physiologic conditions, ascorbate radical is comparatively unreactive compared to other free radicals. Ascorbate radical can be reduced back to vitamin C. Alternately, it can lose a second electron (oxidized) to form dehydroascorbic acid. Thus, because vitamin C loses electrons, it acts as an antioxidant or free radical scavenger (Buettner, 1993).
Dehydroascorbic acid is also unstable with a half-life of several minutes (Lewin, 1976). While dehydroascorbic acid exists in different forms, the dominant form in vivo is likely to be a hydrated hemiketal (Lewin, 1976, Corpe et al., 2005). Dehydroascorbic acid undergoes hydrolysis, with irreversible ring rupture to form 2, 3–diketogulonic acid, whose metabolic products include oxalate, threonate, and possibly xylose, xylonic acid, and lynxonic acid (Lewin, 1976). Oxalic acid is the clinically significant metabolic product in humans (bold). In some animals, products of vitamin C catabolism may enter the pentose phosphate pathway or other pathways of carbohydrate metabolism (Banhegyi & Loewus, 2004). Dehydroascorbic acid may be reduced back sequentially to ascorbate radical and ascorbic acid by glutathione or directly to ascorbic acid by enzyme dependent mechanisms (Linster & Van Schaftingen, 2007). Under suitable conditions (millimolar concentrations of ascorbic acid, presence of metal ions), hydrogen peroxide may be formed. Analogues of vitamin C have been synthesized by replacing the OH group at carbon 6 with bromine or iodine. These halogenated analogues of vitamin C (shown in box) can be oxidized to bromo dehydroascorbic acid and iodo dehydroascorbic acid respectively. However, oxidized halogen ascorbate analogues cannot form a cyclized hydrated hemiketal (Fig. 2). Note that many of the reactions above have been shown only in vitro or in animals and not under physiological conditions in vivo or in humans. From (Washko et al., 1992). Modified and reproduced with permission of Analytical Biochemistry.
Ascorbate loses electrons sequentially. When one electron is lost, the first product is ascorbate radical. Most radical species have short lives less than one millisecond. Ascorbate radical is different, in that the half live can be in many seconds, or even minutes, depending on absence of oxygen and electron acceptors, especially iron (Buettner, 1993). For example, under some conditions ascorbate radical can be measured in blood and extracellular fluid samples (Chen et al., 2007). When a second electron is lost, a more stable species formed, in comparison to ascorbate free radical. The formed species is dehydroascorbic acid, which exists in hydrated and anhydrous forms. As discussed below, dehydroascorbic acid has affinity for facilitated glucose transporters and is transported by a number of them (Corpe et al., 2013) (Rumsey et al., 1997) (Rumsey et al., 2000a). Both dehydroascorbic acid and ascorbate radical are reversibly reduced to ascorbate. Dehydroascorbic acid half-life is only minutes, due to hydrolytic ring rupture. Once the ring structure is lost, the product 2,3 diketogulonic acid cannot reform its precursors dehydroascorbic acid, ascorbate radical, and ascorbate.
Known and postulated actions of vitamin C
Enzymology and vitamin C
As just described, all known and postulated actions of vitamin C Table 1 are accounted for by a single chemical property: that AA is an electron donor and thus a reducing agent. The most well characterized actions are those as an enzyme cofactor, including those in which it is an actual cosubstrate. Ascorbate acts as an electron donor for fifteen mammalian and three fungal enzymes (Englard & Seifter, 1986, Levine, 1986). These include two monooxygenases (Dopamine β-Hydroxylase, Peptidylglycine α-Amidating Monooxygenase), twelve dioxygenases (6 Prolyl 4-Hydroxylases; Prolyl 3-Hydroxylase; Lysyl Hydroxylase; Asparaginyl hydroxylase; Trimethyllysine Hydroxylase; γ-Butyrobetaine Hydroxylase; and 4-Hydroxyphenylpyruvate Dioxygenase) and one amine oxidase. Although details of these enzyme reactions and the role of ascorbate are described elsewhere (Levine et al., 2006) a brief summary follows.
Table 1.
Putative enzymatic and non-enzymatic effects of vitamin C in mammals and fungi are listed in the table. Three mammalian enzymes (prolyl 4-hydroxylase, prolyl 3-hydroxylase and lysyl hydroxylase) take part in collagen biosynthesis. Prolyl 4-hydroxylase has six isoenzymes, three of which hydroxylate proline residues in procollagen, and another three hydroxylate proline residues in Hypoxia-Inducible Factor (HIF). HIF regulates several hundred genes and its activity is modulated by hydroxylation. Factor Inhibiting HIF (FIH) in turn modulates HIF by hydroxylation of asparagine residues in HIF (Dann et al., 2002, Lando et al., 2002). FIH also hydroxylates asparagine, aspartate and histidine residues in ankyrin repeat domains (Yang et al., 2011a, Yang et al., 2011b). Enzymatic functions of vitamin C have been demonstrated in vitro but generally not in vivo. In clinical experiments, iron absorption is increased by vitamin C but its effect on hemoglobin concentration is uncertain. Postulated antioxidant and pro oxidant roles of vitamin C have been experimentally demonstrated but are unproven in humans. Vitamin C is a reducing agent and may exert beneficial effects as a water soluble antioxidant. Some antioxidant functions have been demonstrated in vitro but have not been proven in humans. Under certain conditions vitamin C may be a pro-oxidant, especially when it is present in pharmacologic concentrations. Note that all actions of ascorbate are based on its ability to donate electrons. Where electrons go determines anti-oxidant or pro-oxidant consequences. Modified from Padayatty SJ, Daruwala R, Wang Y, et al. Vitamin C: Molecular Actions to Optimum Intake. In: Cadenas E, Packer L, eds. Handbook of Antioxidants. 2nd ed. New York: Marcel Dekker, Inc.; 2002:117-145. With permission of Marcel Dekker Inc., New York.
| Cofactor for enzymes | |
| Enzyme | Function of enzyme |
| Mammalian enzymes | |
| Dopamine β-monooxygenase
Peptidyl-glycine α-amidating monooxygenase |
Norepinephrine biosynthesis (Levine et al., 1991)
Amidation of peptide hormones (Prigge et al., 1999) |
| Prolyl 4-hydroxylase Three Collagen isoenzymes Prolyl 3-hydroxylase Lysyl hydroxylase |
Collagen hydroxylation (Prockop & Kivirikko, 1995) |
| Prolyl 4-hydroxylase Three Hypoxia-inducible Factor (HIF) isoenzymes |
Hypoxia-inducible Factor (HIF) hydroxylation
(Myllyharju, 2008) |
| Asparaginyl hydroxylase or FIH-1 (Factor
Inhibiting HIF) |
Regulation of HIF (Dann et al., 2002, Lando et al., 2002) |
| Trimethyllysine hydroxylase
γ-Butyrobetaine hydroxylase |
Carnitine biosynthesis (Rebouche, 1991a) |
| 4-hydroxyphenylpyruvate dioxygenase | Tyrosine metabolism (Lindblad et al., 1970) |
| Flavin adenine dinucleotide-dependent
amine oxidase (lysine-specific demethylase 1) |
Histone demethylation (Tsukada & Zhang, 2006) |
| Fungal enzymes | |
| Deoxyuridine 1'-hydroxylase Thymine 7-hydroxylase Pyridine deoxyribonucleoside 2' hydroxylase |
Reutilization pathways for pyrimidines or the
deoxyribose moiety of deoxynucleosides (Wondrack et al., 1978) (Stubbe, 1985) |
| Reducing agent | |
| Site | Action |
| Small intestine | Promote iron absorption (Hallberg et al., 1987) |
| Antioxidant | |
| Site | Action |
| Cells | Regulate gene expression and mRNA translation,
prevent oxidant damage to DNA and intracellular proteins (Hitomi & Tsukagoshi, 1996, Toth et al., 1995, Padayatty et al., 2003, Qiao & May, 2011, Sram et al., 2012). |
| Plasma | Increase endothelium dependent vasodilatation,
reduce extracellular oxidants from neutrophils, reduce low density lipoprotein oxidation, quench aqueous peroxyl radicals and lipid peroxidation products (Polidori et al., 2004, Richards et al., 2015, Ceriello et al., 2013, Traber & Stevens, 2011). |
| Stomach | Prevent formation of N-nitroso compounds
(Helser et al., 1992,
Aditi & Graham, 2012). |
| Pro oxidant | |
| Target | Effect |
| DNA | DNA damage (Podmore et al., 1998) |
| Lipid hydroperoxidase | Decomposition of lipid peroxidase leading to
DNA damage (Lee et al., 2001) |
| Downstream targets of hydrogen
peroxide |
Damage to cancer cells (Chen et al., 2007) (Chen et al., 2008) (Parrow et al., 2013) |
Dopamine β-Hydroxylase, found in neurosecretory vesicles and in adrenal chromaffin granules (Levine et al., 1941b) is necessary for the synthesis of norepinephrine in the nervous system and in the adrenal glands. It requires molecular oxygen and ascorbate (Levine et al., 1941b), and the reaction consumes ascorbate (Fleming & Kent, 1991, Stewart & Klinman, 1988). Peptidylglycine α-Amidating Monooxygenase, found in secretory vesicles is required to amidate many peptide hormones (Eipper & Mains, 1991, Glembotski, 1986) (Prigge et al., 2000) (Kumar et al., 2015) to make them biologically active. These include many hypothalamic and gastrointestinal hormones. The enzyme requires molecular oxygen, copper and ascorbate, and consumes ascorbate (Prigge et al., 2000). However, for amidation, other electron donors can replace ascorbate in vitro (Prigge et al., 2000). Trimethyllysine Hydroxylase and γ-Butyrobetaine Hydroxylases are required for carnitine synthesis from the essential amino acids lysine and methionine. The enzyme requires iron, α-ketoglutarate and a reductant, of which ascorbate is the most optimal, at least in vitro (Dunn et al., 1984). Because carnitine is obtained from diet and also synthesized in the body, the relative importance of these two sources, and the role of ascorbate in its synthesis are difficult to characterize in vivo (Thoma & Henderson, 1984, Englard & Seifter, 1986) (Rebouche, 1991a, Rebouche, 1991b). 4-Hydroxyphenylpyruvate Dioxygenase is required for the catabolism of tyrosine (Lindblad et al., 1970). Ascorbate deficiency leads to impaired tyrosine catabolism and increased plasma concentrations of tyrosine (Englard & Seifter, 1986, Levine et al., 1941a).
For enzymes in which ascorbate is involved in prolyl or lysyl hydroxylation, ascorbate acts as a cofactor rather than a cosubstrate. As a cofactor, the number of product molecules generated is in great excess in comparison to number of molecules of ascorbate utilized. Note that prolyl 4 hydroxylase has three isoenzymes involved in collagen hydroxylation and another three in the hydroxylation of HIF -1 (Pekkala et al., 2003) (Myllyharju, 2008).
Collagen Hydroxylation
Common symptoms of scurvy include wound dehiscence, poor wound healing and loosening of teeth, all pointing to defects in connective tissue (Lind, 1953a) (Crandon et al., 1940, Hirschmann & Raugi, 1999). Collagen provides connective tissue with structural strength. Vitamin C catalyzes enzymatic (Peterkofsky, 1991) post-translational modification of procollagen to produce and secrete adequate amounts of structurally normal collagen by collagen producing cells (Kivirikko & Myllyla, 1985) (Prockop & Kivirikko, 1995). Precollagen, synthesized in the endoplasmic reticulum, consists of amino acid repeats rich in proline. Specific prolyl and lysyl residues are hydroxylated, proline is converted to either 3 hydroxyproline or 4 hydroxyproline, and lysine is converted to hydroxylysine. The reactions catalyzed by prolyl 3-hydroxylase, prolyl 4-hydroxylase, and lysyl hydroxylase (Peterkofsky, 1991) (Prockop & Kivirikko, 1995) (Pekkala et al., 2003) require vitamin C as a cofactor. Hydroxylation aids in the formation of the stable triple helical structure of collagen, which is transported to the Golgi apparatus and eventually secreted by secretory granules. In the absence of hydroxylation, secretion of procollagen decreases (Peterkofsky, 1991) and it probably undergoes faster degradation. However, some hydroxylation can occur even in the absence of vitamin C (Parsons et al., 2006). Secreted procollagen is enzymatically cleaved to form tropocollagen that spontaneously forms collagen fibrils in the extracellular space. These fibrils form intermolecular collagen cross links, giving collagen its structural strength. Independent of its effects on hydroxylation, ascorbate may stimulate collagen synthesis (Geesin et al., 1988) (Sullivan et al., 1994). Collagen synthesis may be decreased in scorbutic animals (Peterkofsky, 1991, Kipp et al., 1996, Tsuchiya & Bates, 2003). Reduced collagen cross links may be a marker of vitamin C deficiency in the guinea pig (Tsuchiya & Bates, 2003) but this may not be specific to vitamin C deficiency. Though many features of human scurvy appear to be due to weakening of connective tissue, it has not been shown that these lesions are due to defective collagen synthesis.
HIF-1 Hydroxylation
In addition to its role in the hydroxylation of collagen, vitamin C may also play a role in the hydroxylation of specific proline residues in Hypoxia Inducible Factor -1 alpha (HIF-1α) by a separate set of prolyl 4 hydroxylases. These enzymes contain non haeme iron, and require ascorbate, molecular oxygen, and 2-oxoglutarate (Bruick & McKnight, 2001) (Myllyharju, 2003) (Pekkala et al., 2003) (Knowles et al., 2003) (Dengler et al., 2014). HIF-1 is a transcription factor that is a key to oxygen sensing in multicellular animals (Taabazuing et al., 2014) (Semenza, 2014). It consists of an oxygen regulated α subunit and a constitutively expressed β subunit (Dengler et al., 2014). Under normal conditions, including normal oxygen tension, HIF-1α is hydroxylated at specific proline and asparagine residues. Hydroxylated HIF -1 α is targeted for degradation by proteasomes (Dengler et al., 2014). Under hypoxic conditions, whether due to reduced atmospheric oxygen or due to local ischemia, hydroxylation is inhibited and HIF-1 α is stabilized. Metal induced ascorbate depletion in cultured cells also inhibits hydroxylation and stabilizes HIF-1 α (Kaczmarek et al., 2007) though metals may also have other effects on this system. Because unhydroxylated HIF-1α is stable, it accumulates long enough to translocate into the nucleus. In the nucleus, HIF-1α forms dimers with HIF-1 β, and the dimer binds to DNA to transcribe target genes (Dengler et al., 2014). In perfused lungs, HIF-1 α was induced by hypoxia in one hour but was degraded under normoxic conditions in minutes (Yu et al., 1998). HIF-1 activates gene transcription by binding to parts of the DNA within the Hypoxia Response Element, a cis acting regulatory element. HIF-1 may regulate several hundred genes, but regulation is limited to a smaller number of specific genes for each cell type (Semenza, 2011) (Taabazuing et al., 2014). HIF-1 plays an important role in normal physiology (Semenza, 2011). As reviewed elsewhere, HIF-1 has a role in the control of erythropoiesis (Franke et al., 2013); in lung disease (Shimoda & Semenza, 2011); in heart disease (Semenza, 2014); in diabetes (Catrina, 2014, Ichiki & Sunagawa, 2014); and in cancer (Semenza, 2013) (Borsi et al., 2015) . All of these conditions have varying degrees of oxygen deprivation at the tissue level. Ascorbate has demonstrable effects on HIF-1 in vitro. Ascorbate inhibited HIF -1 activity in cell culture and prevented gene transcription specific to HIF-1 stimulation (Vissers et al., 2007, Kuiper et al., 2014). Increased systolic pulmonary artery pressure induced by hypoxia in (ascorbate and iron replete) healthy subjects was unaffected by intravenously administered vitamin C but was reduced by iron infusion (Talbot et al., 2014). Whether these effects were mediated by HIF-1 is not known though HIFs are thought to be mediators of hypoxia induced pulmonary hypertension (Shimoda & Laurie, 2014).
HIF-1 in turn is negatively regulated by Factor Inhibiting HIF (FIH). FIH is a hydroxylase that also requires ascorbate (Flashman et al., 2010). FIH appears necessary for hydroxylation of asparagine and/or aspartate residues on HIF 1, and also on the structural protein ankyrin. Hydroxylation in vitro was dependent on ascorbate or ascorbate analogs that are electron donors (Yang et al., 2011b). It is not known if other intracellular electron donors will suffice, ie. glutathione. Manipulation of HIF-1 and the factors involved in its enzymatic pathways may present attractive therapeutic targets (Myllyharju, 2008). The concentration -dependent role of ascorbate in vivo in human physiology and disease states that may potentially act via HIF-1 are not yet known.
Enzymology in vivo and in situ
The role of vitamin C as a cofactor or cosubstrate for many enzymes has not been well studied in vivo. Neither is it certain that only vitamin C can fulfill these roles in vivo. It is possible that other reducing agents can replace ascorbate, to varying extents. Some signs and symptoms of scurvy appear to be due to specific defects in enzyme actions where vitamin C is known to play a role. Lassitude and signs of autonomic dysfunction might be due to impaired norepinephrine biosynthesis. Breakdown of wounds, bleeding gingivae, loosening of teeth and other apparent defects in connective tissue might be due to impaired hydroxylation reactions in collagen, producing either smaller amounts of functional collagen, or weaker collagen or both. Impaired amidation of peptide hormones (Eipper et al., 1992) may produce widespread hormone defects, but such specific defects have not been experimentally shown to be the cause of human or animal scurvy.
Most enzyme reactions where vitamin C acts as a cofactor require relatively low concentrations of the vitamin in comparison to the normal in vivo concentration. It is possible that as consequence of vitamin C transporter activity, many tissues contain significant amounts of vitamin C even in the face of deficiency, though such measurements in humans are not available. Further, only limited data exist regarding intracellular distribution of vitamin C. In scurvy, the cell as a whole may contain significant amounts of the vitamin but it may not be available at the requisite concentrations at specific enzyme reaction sites within the cell, ie. within specific subcellular organelles. One example of how ascorbate concentrations can affect enzymatic reactions is for norepinephrine biosynthesis. The concentration of ascorbate is approximately 12-15 mM in adrenal medullary chromaffin granules, the site of norepinephrine biosynthesis. The enzyme that produces norepinephrine from dopamine is dopamine beta-monoxygenase. The Km of this enzyme for ascorbate is < 1 mM, meaning that this enzyme works at close to maximal rate unless severe ascorbate lack occurs (Dhariwal et al., 1989) (Dhariwal et al., 1991a). It is unknown whether adrenal vitamin C is so depleted in scurvy that concentrations in adrenal medullary secretory granules are less than 1 mM.
It is also possible that intracellular vitamin C may play additional roles that are different from that as cofactors for enzymes. For example, ascorbate may act as an intracellular chemical reducing agent (antioxidant). While some of these reactions have been demonstrated in vitro, it is unknown whether they have clinical relevance. Glutathione is another intracellular reducing substance, often present at millimolar concentrations (Montero et al., 2013, Wu et al., 2004). It is not known whether there are specific intracellular non-enzymatic chemical reactions that utilize ascorbate as an electron donor, but not glutathione.
Ascorbic acid and dehydroascorbic acid transport
Vitamin C is transported by specific transporters termed Sodium-dependent Vitamin C Transporter (SVCT) 1 and 2 (Tsukaguchi et al., 1999) (Daruwala et al., 1999, Wang et al., 1999, Wang et al., 2000). The distribution of these transporters in animals and humans has been determined by studying mRNA expression of the two transport proteins. Although antibodies are available, their reliability has not been consistent. Functional studies have clarified the role of these transporters in certain tissues using cell and animal models. SVCT1 and 2 belong to a family of nucleobase transporters that are highly conserved through evolution.
SVCT1 is the absorptive vitamin C transporter and occurs in the intestine, in renal tubules and in the liver (Fig 2). SVCT1 knockout mice, in which the transporter is not expressed, lose large amounts of vitamin C in the urine compared to wild type mice. Urinary loss occurs because vitamin C that is filtered at the glomerulus is not reabsorbed, because SVCT1 is absent (Corpe et al., 2010). However, SVCT1 knockout mice absorb both vitamin C itself as well as a structural analog, 6-bromo ascorbate, from the intestinal lumen. As discussed in detail below, oxidized 6-bromo ascorbate is not transported by glucose transporters (GLUTs), and there are no SVCT1 transporters in SVCT1 knockout mice. Therefore, these mice transport AA and 6-bromo ascorbate from the intestinal lumen either via SVCT2 or through an unknown transporter.
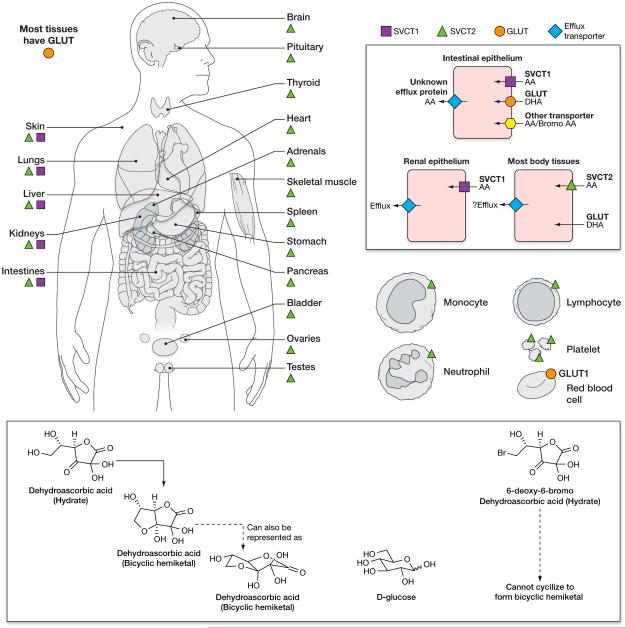
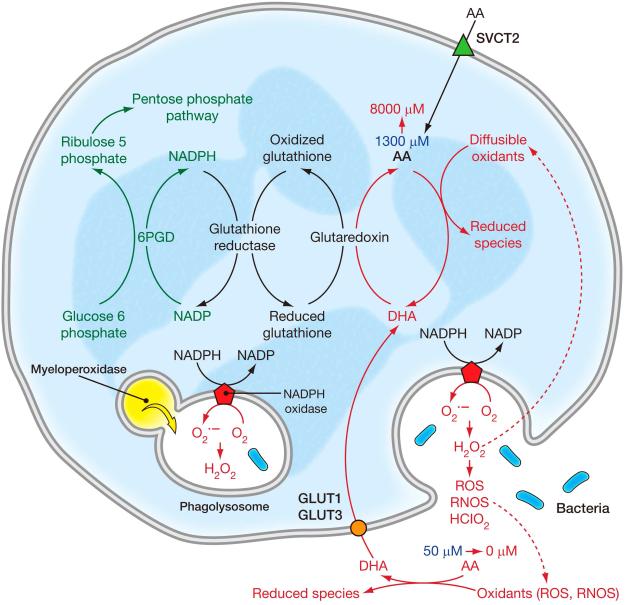
Figure 2. Vitamin C transporters.
Distribution of vitamin C transporters in human tissues. Sodium Vitamin C Transporters (SVCTs) are mainly responsible for vitamin C transport into cells in humans and other mammals. SVCT1 is primarily expressed in absorptive tissues, including the intestinal epithelium and the proximal convoluted tubules and the descending part of the loop of Henle in the kidney. SVCT 1 is also expressed in liver. SVCT2 is expressed in most body tissues, as are Glucose Transporters (GLUTs). SVCT 1 and 2 transport ascorbic acid but not dehydroascorbic acid into cells. GLUTs 1, 2, 3, 4 and 8 (but not other GLUTS) (Corpe et al., 2013, Rumsey et al., 2000a, Rumsey et al., 1998, Burzle & Hediger, 2012), transport dehydroascorbic acid but not ascorbic acid into cells. Some GLUTs have a higher affinity for DHA than for glucose. The transporter responsible for exporting ascorbic acid from cells into the extracellular fluid or plasma has not been identified. The only ascorbate containing cell known to lack an SVCT is the mature red blood cell. The red blood cell obtains its ascorbate by transporting dehydroascorbic acid and immediately reducing it internally. Dehydroascorbic acid is transported into human red blood cells by GLUT1, and into mouse red blood cells by GLUT 3 and/or 4 (Tu et al., 2015). D-Glucose closely resembles the ring form (hydrated hemiketal form) of dehydroascorbic acid, which is likely to account for its transport by some GLUTs. When bromo ascorbic acid is oxidized to form bromo dehydroascorbic acid, this compound is not transported by GLUTs. SVCT distribution was inferred by the presence of specific mRNA for SVCT1 and 2, and in some cases by antibodies. Figure based on data from: (Tsukaguchi et al., 1999, Savini et al., 2008) (Daruwala et al., 1999, Wang et al., 1999, Wang et al., 2000).
Another means to study vitamin C transport mechanisms was creation of SVCT2 knockout mice. SVCT2 is widely distributed and based on mRNA expression is the predominant tissue transporter for vitamin C. SVCT2 knockout mice die at birth, and fetal tissues show very low vitamin C concentrations in all tissues measured (Sotiriou et al., 2002).
As noted above, the ascorbate oxidation product dehydroascorbic acid (DHA) exists in several different forms and is transported by GLUTs (Corpe et al., 2013) (Rumsey et al., 1997) (Rumsey et al., 2000a, Vera et al., 1993). Several GLUTS have a higher affinity for DHA than for glucose. The hydrated form of DHA forms bicyclic hemiketal structures, some of which resemble glucose in three dimensions (Fig 2) (Corpe et al., 2005). As soon as DHA is transported, it undergoes immediate intracellular reduction to ascorbate. The process of extracellular oxidation to DHA, DHA transport, and immediate intracellular reduction is termed ascorbate recycling (Washko et al., 1993). Based on data from in vitro cell models, DHA has been proposed to serve either as an alternate path or dominant path for ascorbate accumulation (Vera et al., 1993) (Nualart et al., 2003) (Agus et al., 1997).
In vivo, our understanding of the roles of DHA and GLUTs in vitamin C economy in the intact organism is unclear, especially for humans. Available data indicate that it is unlikely that DHA transport is the major pathway for ascorbate transport for most tissues. This is because the SVCT2 knockout mouse dies at birth and has virtually no ascorbate in all tissues measured other than the liver, where ascorbate is synthesized (Sotiriou et al., 2002). If DHA transport via GLUTs could serve as a salvage pathway, ascorbate should be present in at least some tissues. Mice are different than humans, and it remains possible that humans have DHA pathways that mice lack. Also, not every tissue was measured for ascorbate in the SVCT2 knockout mouse, and it is possible that some tissues do utilize the ascorbate recycling pathway. A recent example comes from experiments in red blood cells from both mice and humans (Tu et al., 2015). Red blood cells are one cell type that exclusively utilizes dehydroascorbic acid as the transported substrate. Once transported inside red blood cells, dehydroascorbic acid is immediately reduced to vitamin C.
In the physiological state, the amount of DHA in plasma is estimated as <1-2% of that of ascorbate. Liquid chromatography mass spectrometry assays in the near future may be able to address our current inability to measure DHA directly in plasma. These assays will have to account for DHA instability and hydrolysis in plasma as a biological fluid, and will either have to correct for hydrolysis or provide sample stability. At present there are no direct means to measure DHA accurately. Current techniques are to measure ascorbate, and then to re-measure ascorbate after the sample is reduced. The value before reduction is subtracted from the value after reduction. Accuracy is reduced because a large number is usually subtracted from a large number, and the resulting difference may not be distinguishable from zero. Under conditions where ascorbate oxidation occurs locally, it is possible that DHA concentrations are higher than the estimated 1-2% plasma values. Local DHA concentrations may be higher when cells produce reactive oxidant species, such as that by activated neutrophils and monocytes. In addition, when ascorbate is given pharmacologically, it is possible that DHA concentrations rise proportionately. Vitamin C analogs with halogen substitutions at the 6th carbon position may be useful in the future in dissecting vitamin C transport mechanisms, especially the contribution of DHA uptake to overall ascorbate content in cells. The halogen analogs are transported by SVCTs, but when oxidized, are not transported by GLUTs. Substitution of the hydroxyl group with halogen prevents the oxidized halogen DHA species from forming a three dimensional configuration, as a bicyclic hemiketal, that is similar to glucose (Corpe et al., 2005)(Fig 2).
Interest remains in characterizing the role of DHA transport in ascorbate economy because of DHA transport by GLUTs, and the possibility of dysregulated DHA transport with hyperglycemia, as in diabetes. Nearly 40 years ago, a general hypothesis was linked vitamin C to diabetes via DHA (Mann & Newton, 1975). Unfortunately, until recently specific explanations were lacking that tied diabetes pathophysiology to DHA transport or aberrant DHA transport (Will & Byers, 1996) (Chen et al., 2006). Promising new data from mouse and human red blood cells indicate that DHA is the only transported species into these cells, and hyperglycemia inhibited transport in vitro and in vivo (Tu et al., 2015). Red blood cells from diabetic subjects had lower vitamin C and were more rigid, with decreased structural protein beta-spectrin. Thus, red blood cells with low vitamin C concentrations in diabetes could contribute to or even cause microvascular hypoxia that is the hallmark of diabetic vascular disease (Tu et al., 2015, May, 2015). Because glucose transporters differ in mouse and human red blood cells, clinical research in humans without and with diabetes will be essential in future studies.
To summarize: SVCT 1 and 2 transport vitamin C into cells. In addition, vitamin C accumulation into cells under some circumstances may occur by other mechanisms: ascorbate recycling, or direct transport of DHA. As indicated above, ascorbate recycling means that extracellular ascorbate is oxidized to dehydroascorbic acid, which is transported by facilitative glucose transporters and then effectively trapped by intracellular reduction to ascorbate.
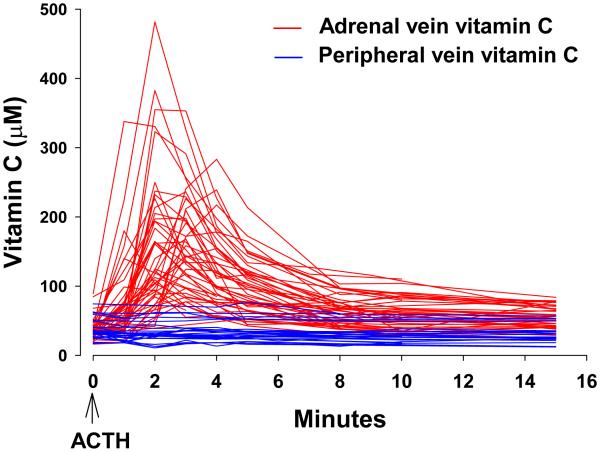
Regardless of how transport occurs, either via SVCTs or via ascorbate recycling, vitamin C transported into some cells must exit from them, as in intestine and kidney. Also, other tissues secrete vitamin C. These include liver, the site of vitamin C synthesis in animals able to synthesize the vitamin; adrenal; reproductive organs; and stomach. Mechanisms of exit, or efflux, are not known. Because vitamin C is an anion at physiologic pH, it cannot simply diffuse across the cell membrane to extracellular fluid. Efflux transporters for the vitamin have not been identified (Eck et al., 2013). For intestinal epithelium and proximal renal tubular cells, vitamin C is absorbed by luminal transporters such as SVCT1. Once inside intestinal epithelia and renal tubular cells, the vitamin must exit, presumably by efflux transporters on the basolateral surface, to enter plasma or extracellular fluid. Vitamin C is secreted into the gastric juice, cerebrospinal fluid, and aqueous humor, all of which have higher concentrations than those in plasma (Fig 3). Vitamin C is also secreted by the adrenal gland in humans in response to ACTH. Vitamin C secretion from the adrenals in humans is rapid, within minutes. The amount secreted is sufficient to increase local plasma vitamin C concentrations several fold in the adrenal vein (see section: Human Adrenals secrete vitamin C in response to ACTH), although insufficient to increase systemic concentrations (Padayatty et al., 2007). Animal data indicate that testes and ovaries might also secrete vitamin C in response to hormone signaling (Koba et al., 1971) (Musicki et al., 1996). For rapid hormone dependent secretion of vitamin C to occur, a transport mechanism is likely to be responsible, but is as yet unidentified. Transport kinetics properties of SVCT1 and 2 are ideal for transporting the vitamin into cells, but not for its secretion into plasma or extra cellular fluids from cells (Eck et al., 2013).
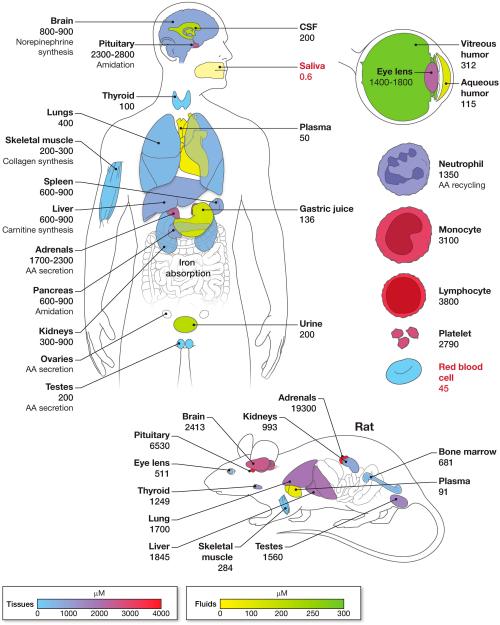
Figure 3. Vitamin C concentrations in human and rat tissues and fluids.
Concentration of vitamin C in body tissues and body fluids are shown in µM. Ascorbic acid concentrations are much higher in tissues than in plasma. Tissues and body fluids are shown in separate concentration dependent color schemes. Organs that are uncolored (white) indicate that data on vitamin C concentrations are unavailable. For clarity, some organs shown are slightly displaced from their correct anatomical positions. Vitamin C concentrations shown are derived from published reports. Values for white blood cells and urinary vitamin C concentrations were obtained from depletion repletion studies in young healthy women, at the study phase when they were at steady state for vitamin C intake of 100 mg per day (Levine et al., 2001b), which is close to the recommended dietary allowance for vitamin C. Note that the red blood cell is the only cell with internal concentrations of vitamin C that are below those in plasma (values less than plasma are shown in red). Of body fluids, only saliva has a vitamin C concentration lower than that of plasma. Putative functions of vitamin C as a cofactor for enzymes are indicated, as are selected clinical or experimental observations of phenomenon involving vitamin C. Values shown should be considered approximate as they were obtained from tissues collected under non uniform conditions including clinical and post mortem samples. Some vitamin C concentrations shown are the mean values of results from two or more studies. Further, the dietary intake or plasma concentrations of vitamin C were not known in many cases and the methods used in sample collection and storage, and for vitamin C assays, varied widely. Values may also vary with age and possibly in disease states. Data obtained from (Hornig, 1975, Voigt et al., 2002)(Dostalova, 1982, Koliakos et al., 2002, Vobecky et al., 1982, Evans et al., 1982, Omaye et al., 1987, Berger et al., 1996, Taylor et al., 1997, Rathbone et al., 1989, Sobala et al., 1989, Levine et al., 2001b, Jacob et al., 1987). Reported salivary vitamin C concentrations varied widely. Salivary vitamin C concentration shown (median 0.6 µM, range 0.2-19 µM) in nonsmokers was measured by HPLC. Corresponding median plasma vitamin C concentration was 53 µM (range 16 µM-89 µM)(Schock et al., 2004). Note that tissue vitamin C concentrations shown for the rat are much higher than for humans. Explanations are that fresh tissue samples can be obtained from animals (while many human tissue samples were obtained post mortem), or that there are true species differences. Compared to other tissues, AA concentrations in the rat pituitary and adrenal glands are higher, and therefore they are shown in arbitrary colors (and not according to the concentration dependent color scale) (Hornig, 1975). Vitamin C concentration data for mice are similar to that of the rat (Sotiriou et al., 2002, Corpe et al., 2010).
The transporter SVCT1 is responsible for vitamin C re-absorption in the proximal convoluted tubule of the kidney. SVCT1 knockout mice are unable to reabsorb vitamin C in the urine and have high perinatal mortality. The high perinatal mortality can be reversed almost completely by supplementing the mother with vitamin C (Corpe et al., 2010). In these knockout mice, low vitamin C concentrations that do no apparent harm to adult mice are deleterious to the fetus in the peripartum period. Clinical studies to test whether vitamin C could improve outcomes in high risk pregnancies have been performed in patients with histories of pre-eclampsia. Effects were inconsistent, in large part because vitamin C intake was enough to produce tissue saturation even in the control groups, as discussed below. Women were not enrolled based on low or high vitamin C concentrations, in part because measurements were not performed. It remains unknown whether vitamin C could improve pregnancy outcomes in patients with low concentrations of the vitamin (Rumbold et al., 2015, Rossi & Mullin, 2011).
Several Single Nucleotide Polymorphisms (SNPs) have been described in SVCTs. SNPs can be in the protein coding (exonic SNP) region of the gene or in other regions including introns (intronic SNP), promoter or intergenic regions. Some exonic SNPs may change the amino acid sequence of the protein product (nonsynonymous SNP, missense SNP). For other exonic SNPs, the variation in nucleotide sequence does not result in a change in the amino acid sequence of the protein (synonymous SNP). Nonsynonymous SNPs are more likely to change SVCT protein function, which may then be reflected in altered vitamin C physiology. However even SNPs that do not change amino acid sequence of the protein product (intronic SNPs, synonymous SNPs) may produce a functional change in vivo, because the difference in the DNA sequence may have many effects, including influencing promoter activity or messenger RNA stability (Shastry, 2009) .
Several SNPs have been found in the human SVCT1 gene that, by reducing vitamin C renal reabsorption, could recapitulate low vitamin C concentrations (Corpe et al., 2010) (Timpson et al., 2010). The relevant polymorphisms are more common in populations from sub-Saharan Africa (Eck et al., 2004) (Erichsen et al., 2006) than those from other parts of the world (Timpson et al., 2010) and may simply reflect the greater genetic diversity found in Africa (Jorde et al., 2000, Witherspoon et al., 2007, Tishkoff & Williams, 2002, Tishkoff & Verrelli, 2003, Reed & Tishkoff, 2006). Alternately, the relatively easy availability of vitamin C rich food may have reduced the danger from impaired vitamin C transport by SVCT1 and allowed such SNPs to persist in this population. Other, as yet unknown selective pressures may also account for these SNPs. Another possibility is that such SNPs are rarer in the non-African populations because these populations may have been subject to deprivations, especially that of vitamin C containing food, in their migration out of Africa. In the presence of low vitamin C intake, it is necessary to conserve vitamin C, and SNPs that result in vitamin C loss would be rapidly selected out.
In addition to the common SVCT1 SNPs whose effect on vitamin C transport have been studied in vitro (see below) (Corpe et al., 2010), many uncommon or rare SVCT1 SNPs have been identified. With increasing use of DNA sequencing in research, it has become apparent that these SNPs are numerous with several hundred identified in publically available databases (ENSEMBL, 2015a, NCBI, 2015a). Because there are so many different SVCT1 SNPs (though each individual SNP is uncommon), the uncommon or rare SVCT1 SNPs cumulatively may account for a greater proportion of SVCT1 SNPs in the general population than that accounted for by the well-studied common SVCT1 SNPs. The population distribution of these uncommon SNPs is not known and it is possible that the preponderance of SVCT1 SNPs described in African populations may reflect incomplete knowledge or ascertainment bias (Michels et al., 2013).
The effect of SVCT1 exon SNPs on vitamin C transport has been studied in vitro using the Xenopus laevis oocyte expression system. When either the common type of SVCT1 or SVCT1 containing SNPs frequently found in humans was expressed in oocytes, oocytes that expressed SVCT1 transporters harboring SNPs were found to have reduced ability to accumulate vitamin C from the surrounding media (Corpe et al., 2010). Data from these in vitro vitamin C transport studies were used to estimate plasma vitamin C concentrations in humans using a mathematical model based on data from normal subjects (Graumlich et al., 1997, Padayatty et al., 2004). The calculations showed that SVCT1 SNPs significantly reduced steady state fasting vitamin C concentration at intakes in the range of 30 to 2500 mg/day (Corpe et al., 2010). Population studies have shown reduced plasma vitamin C concentration in subjects with specific SVCT1 SNPs, though these reductions were less than that predicted by the pharmacokinetic modeling described above (Michels et al., 2013) (Timpson et al., 2010) (Cahill & El-Sohemy, 2009). In people with low vitamin C intake, such polymorphisms in SVCT1 and SVCT2 were associated with low vitamin C concentrations in the lens and aqueous humor (Senthilkumari et al., 2014). A negative association was seen between two SVCT2 SNPs (one intronic and the other exonic) and colorectal adenoma, but colorectal adenomas were not associated with the common SVCT1 SNPs (Erichsen et al., 2008). In another study, a synonymous SVCT1 SNP conferred an increased risk of Crohn’s disease (Amir Shaghaghi et al., 2014). In these association studies, there were no vitamin C measurements reported for plasma, tissue or urine. Therefore it is not possible to attribute the findings to alterations in vitamin C physiology (Michels et al., 2013). A metaanalysis of five studies with a total of more than 18000 patients found no relationship between SVCT1 SNPs and metabolic parameters that contribute to the risk of cardiovascular disease (Wade et al., 2015). Currently, the clinical and physiological significance of intronic and exonic SVCT polymorphisms are unknown. The role of genetic polymorphisms on vitamin C physiology is reviewed elsewhere (Michels et al., 2013, Shaghaghi et al., 2016).
SVCT2 is found throughout the body and transports vitamin C into tissues. As noted above, SVCT2 knockout mice die immediately after birth; and fetal organs have undetectable or very low vitamin C concentrations in comparison to heterozygous and wildtype mice (Sotiriou et al., 2002). In vitamin C synthesizing species such as mice, the developing embryo does not synthesize vitamin C in the early part of fetal development. Placental transport of vitamin C from the mother to the fetus is essential for fetal survival, and such transport is mediated by SVCT2 in placenta. Placental vitamin C transport maintains higher vitamin C concentration on the fetal side of the placenta than on the maternal side. Therefore, vitamin C concentrations are higher in the umbilical cord blood than in the maternal blood.
That SVCT2 knockout mice do not survive is the best available in vivo evidence that vitamin C acquisition via the uptake of DHA by glucose transporters is not physiologically dominant nor is it adequate to stave off fatal scurvy at the tissue level. However, because ascorbate was not measured in every tissue from SVCT2 knockout mice, it was still possible that the DHA pathway was utilized by one or more tissues or cell types in vivo. Thus, DHA is the exclusive pathway utilized by mouse and human red blood cells (Tu et al., 2015). Red blood cell ascorbate was not measured in SVCT2 knockout mice because an assay for RBC was not available at that time (Li et al., 2012).
The SVCT2 gene harbors many more SNPs (more than 2000) than the SVCT1 gene but they are either intronic or synonymous SNPs (a few nonsynonymous SNPs have been recently identified in public databases) (ENSEMBL, 2015b, NCBI, 2015b). It may be that SVCT2 is less tolerant of changes in its amino acid sequence reflecting its critical importance in transporting vitamin C into a wide variety of cells (Michels et al., 2013) (shown by lethality at birth in SVCT2 knockout mice) (Sotiriou et al., 2002).
Absorption and tissue distribution of vitamin C
Vitamin C is absorbed from the small intestine in humans, achieving peak plasma vitamin C concentrations approximately 120-180 minutes after ingestion. Although SVCT 1 is believed to be the candidate transporter, other SVCTs may exist. Alternatively, some ascorbate may be oxidized in intestine, transported as DHA by GLUT2, and then immediately reduced (Corpe et al., 2013). Of note, intestinal transport of DHA by GLUTs, with subsequent reduction to ascorbate in the enterocyte or mesenteric system, does not account for the experimental findings that there is absorption of an ascorbic acid analog in mice that do not have SVCT1. When oxidized to its dehydro form, this analog cannot form a bicyclic hemiketal structure (Fig 2), and is not transported by GLUTs, even though the analog is absorbed in SVCT knockout mice.
After absorption, because vitamin C is water soluble, it is distributed from blood throughout the extracellular space. Tissues accumulate vitamin C (Fig 3) against a concentration gradient, most likely via SVCT2 as discussed above. Tissue concentrations are dependent upon plasma and extracellular fluid vitamin C concentrations, which in turn are dependent on dietary intake of vitamin C. Tissue concentrations of vitamin C, which are frequently millimolars (fig 3), are far in excess of what is required for its actions as a coenzyme. Millimolar concentrations of intracellular vitamin C may simply serve as a reservoir of the vitamin, or may have other unknown functions.
It should be noted that for many human tissues, measurements of accurate vitamin C concentrations in health and in disease states are not known. The major portion of vitamin C in humans is in the liver, brain, and skeletal muscle. Although skeletal muscle vitamin C concentrations are not high when compared to other cell types (Fig 3), skeletal muscle constitutes 31-38% of body mass (Janssen et al., 2000). Skeletal muscle vitamin C concentrations appear to be in equilibrium with dietary intake of the vitamin (Carr et al., 2013). Estimates of tissue vitamin C in humans (Fig 3) are based on data compiled from multiple sources, often using post mortem samples and inaccurate AA essays. When human body fluids or tissues are not promptly and properly processed, vitamin C may be lost by oxidation and/or sample handling practices, and assay results may be artificially low. To calculate body stores of vitamin C that result from differing vitamin C intake, it is possible in theory to match tissue / cell concentrations to plasma concentrations. Unfortunately, only limited matched values are available, for monocytes, lymphocytes, neutrophils, mixed mononuclear cells, skeletal muscle, and red blood cells (Levine et al., 1996b) (Levine et al., 2001b) (Tu et al., 2015, Carr et al., 2013). These data are insufficient to calculate total body stores of vitamin C in relation to doses. Prior to use of modern HPLC assays, radiolabelled ascorbate administered to humans was used to estimate body stores (Baker et al., 1969, Kallner et al., 1979, Baker et al., 1971). Limitations of these data are that the radiolabel was assumed to remain as ascorbate in humans, but no mass measurements were performed to confirm the assumption.
For animal vitamin C concentrations, rodent data are most abundant. Reported tissue concentrations of vitamin C in the rat are much higher than those in humans (Fig 3). This may be because fresh tissue is easily obtained under controlled conditions from rodents, thus avoiding oxidation and irreversible degradation of vitamin C. Note that plasma vitamin C was reported to be 90 µM in rat (Fig. 3), although more recent studies with modern assays have shown plasma concentrations of 40 – 70 µM in rat (Corpe et al., 2013) and mouse (Corpe et al., 2010). Higher plasma concentrations described in older reports on rodents may have been due to inaccurate spectrophotometric/colorimetric assays. These assays can over-estimate the amount of vitamin C when measured concentrations are at the low end of an assay range, as is the case for plasma samples. It is also possible that the higher reported measurements may have indicated a true increase in plasma vitamin C concentrations precipitated by acute stress. Because of interfering substances, spectrophotometric/colorimetric assays tend to be inaccurate when vitamin C concentrations are low, as in the micromolar range. Spectrophotometric/colorimetric assays are less affected by interfering substances (presumably because they are present in fixed amounts) when ascorbate concentrations are higher, especially in the mM range, as is the case with many tissues. It is also possible that there are species differences in plasma and tissue vitamin C concentrations, and in particular differences between vitamin C synthesizing (rat, mouse) and non-synthesizing (primate) species. Reliable vitamin C concentrations (i.e. derived from properly obtained and processed tissue samples and measured by HPLC assays) for many human tissues are as yet not available (especially in relation to simultaneously measured plasma vitamin concentrations), with exceptions noted above for circulating cells and skeletal muscle.
Red blood cells are the only body compartment other than saliva (Fig 3) that have vitamin C concentrations that are similar to or lower than that of plasma (Li et al., 2012) (Evans et al., 1982) (Jacob et al., 1987). Until recently RBC vitamin C was usually measured (Butler & Cushman, 1940, Barkhan & Howard, 1958, Kassan & Roe, 1940)}(Evans et al., 1982) (Jacob et al., 1987) using spectrophotometric/ colorimetric assays prone to artifact, such that vitamin C concentrations were either overestimated or indeterminate (Li et al., 2012, Mendiratta et al., 1998, Iheanacho et al., 1995, Okamura, 1980, Westerman et al., 2000, Lubschez, 1945, Iggo et al., 1956, Washko et al., 1992, Rumsey et al., 2000b). With advent of HPLC techniques to measure vitamin C in red blood cells, there is now promise of learning pathophysiology of vitamin C in disease, especially in diabetes (Tu et al., 2015).
In contrast to other tissues, RBCs obtain vitamin C via a dehydroascorbic acid pathway. Progenitor erythroid cells have SVCT2 transporters, but these are lost in the process of maturation, such that circulating RBCs do not have any known vitamin C transporter. Circulating RBCs have slightly lower vitamin C concentrations than plasma. RBCs obtain ascorbate via transport of trace concentrations of DHA, found in plasma, on glucose transporters followed by immediate intracellular reduction. RBCs can reduce DHA via the protein glutaredoxin, or by direct chemical reduction from the millimolar intracellular RBC concentrations of glutathione. RBCs are unlikely to be a simple storage reservoir for plasma vitamin C based on direct efflux from RBCs, although this hypothesis has been considered (Montel-Hagen et al., 2008), because ascorbate efflux from RBC is comparatively slow (May et al., 1996, Mendiratta et al., 1998, May et al., 2001). Because RBCs are more numerous than other blood cells, they do account for most of the vitamin C in circulating cells of whole blood (Barkhan & Howard, 1958, Li et al., 2012).
The function of ascorbate in RBCs is uncertain. Until recently (Li et al., 2012), function has been difficult to address because of inability to accurately measure ascorbate in RBCs. In addition to the postulated role of ascorbate efflux from RBCs, other evidence supports a function of ascorbate based transmembrane electron transfer across the RBC. The hypothesis is that ascorbate is an electron donor for transmembrane electron transfer from within the RBC to an acceptor externally, most likely ascorbate radical formed from ascorbate oxidation in plasma. Therefore, electrons from ascorbate within RBCs would help to maintain plasma ascorbate, but without direct exit of ascorbate itself from the RBC (May et al., 2000) (May et al., 2004). The hypothesis is consistent with observations about vitamin C stability in blood and plasma samples (Dhariwal et al., 1991b) (Levine et al., 1999b) (Kassan & Roe, 1940). Vitamin C in plasma samples prepared from human whole blood, stored for as long as 24 hours, is more stable than vitamin C in human plasma or serum samples that have been separated from RBCs and stored for the equivalent amount of time (Dhariwal et al., 1991b). Vitamin C may also have a separate role in maintaining the red blood cell structural protein ß-spectrin. In mice that cannot make vitamin C and that have low vitamin C concentrations, red blood cell ß-spectrin is decreased. ß-Spectrin returns to normal levels within days after deficient mice are re-supplemented (Tu et al., 2015). Other red blood cell structural proteins are not affected by variations in vitamin C concentrations. It is unknown why ascorbate is necessary for maintenance of ß-spectrin. It is possible that there are specific amino acids on ß-spectrin (i.e. arginine, asparagine) that are hydroxylated in an ascorbate-dependent fashion, analogous to ascorbate dependent hydroxylation of proline in collagen and HIF-1α.
Vitamin C physiology
Current knowledge of vitamin C physiology in humans is largely limited to vitamin C dose concentration relationships, bioavailability and renal excretion in healthy young subjects (Levine et al., 1996b, Levine et al., 2001b). Many investigations have documented depletion rates, absorption, plasma and cellular concentrations, and excretion of vitamin C in normal humans and in some disease states. (Vinson & Bose, 1988) (Mangels et al., 1993, Gregory, 1993) (Blanchard, 1991b, Blanchard et al., 1989, Blanchard, 1991a, Baker et al., 1971, Hodges et al., 1969). Results were variable, perhaps because of uncertain dietary control, imprecise vitamin C assays, or pharmacokinetics that was not performed at steady state (Piotrovskij et al., 1993).
Plasma vitamin C concentrations depend on dietary intake; vitamin C absorption by the gastrointestinal tract; distribution in body fluids and uptake by tissues; irreversible metabolism of vitamin C (utilization); and vitamin C excretion by the kidneys. All of these factors may be altered in disease, and may also vary depending on body composition, genetics, and perhaps other factors such as physical activity. However, the most important variable identified so far that determines plasma vitamin C concentration is dietary intake.
Many studies have detailed the relationship between dietary intake of vitamin C and the concentrations of vitamin C in plasma and in circulating cells. Low dietary intake and plasma concentrations of the vitamin are common, even in affluent countries. In a survey of 7277 non-institutionalized civilians in the US in 2003-2004 (Schleicher et al., 2009), mean plasma vitamin C concentrations in subjects more than 6 years of age were 48 µM in males, and 54.8 µM in females. However, 8.2% of males and 6% of females had plasma vitamin C concentrations < 11.4 µM, a concentration where frank scurvy could occur. Among men, 18% of smokers but only 5.3% of non-smokers had such low values. Among women, 15.3% of smokers and 4.2% of non-smokers had similarly low values. These values are to a large extent dependent on dietary intake and perhaps smoking, but some disease states may increase loss by oxidative degradation of the vitamin or through increased renal loss.
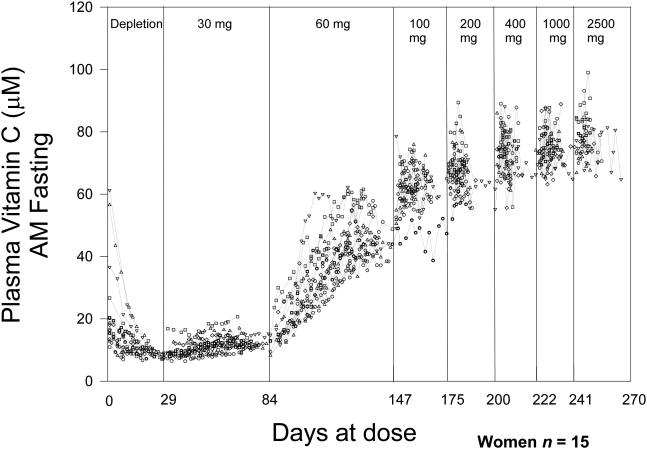
Absolute bioavailability of an oral dose of vitamin C can be determined when the subject is at steady state for a specific dose, as in depletion-repletion studies performed under carefully controlled conditions (Young, 1996, Graumlich et al., 1997) (Levine et al., 2001b)(Figs 4 A and 4B). Absolute bioavailability describes what percentage of an orally administered dose is absorbed when compared to an intravenous dose. For example, bioavailability of a 30 mg dose oral dose can be determined when the subject has consumed this dose (15 mg taken twice daily, total of 30 mg/ day) long enough for plasma and tissue concentrations of the vitamin to be at equilibrium for the dose. This equilibrium condition is termed steady-state. At the time of steady-state, plasma vitamin C concentrations after administration of a 30 mg oral and intravenous dose can be used to calculate absolute bioavailability of the 30 mg dose. Bioavailability of the intravenous dose is considered as complete, or 100%, because the intestine is by-passed with intravenous administration. When tissues are in equilibrium with plasma, the calculations are valid because the administered dose is not suddenly taken up by tissues. Conversely, if subjects were not in equilibrium for any dose, then absorption and bioavailability calculations could be unreliable. For example, if a subject at equilibrium for a 30 mg/day dose was then given a much larger dose instead, say 200 mg, tissues would suddenly be exposed to higher vitamin C concentrations, plasma concentrations could change unpredictably, and bioavailability calculations therefore become unreliable or of questionable value. Current data on absolute bioavailability pertain to doses of 15 to 1250 mg when the subject was at steady state for each specific dose (Graumlich et al., 1997).
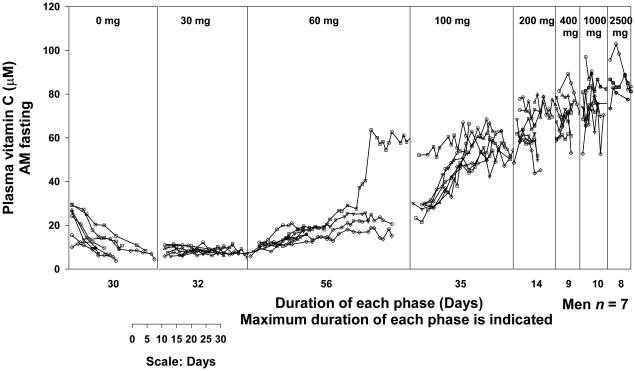
Figure 4. Vitamin C dose concentration relationship.
Vitamin C concentrations in plasma and circulating cells were studied in young healthy men (Levine et al., 1996b) and women (Levine et al., 2001b) each of whom were given six to seven different doses of the vitamin in two depletion-repletion studies. Seven healthy men (4A) and fifteen healthy women (4B), all nonsmokers, age 19-27 years were studied as inpatients. To decrease hospitalization time, outpatient subjects prior to admission were instructed to consume a diet containing < 60 mg of vitamin C. When inpatients, throughout hospitalization they consumed a defined diet containing less than 5 mg of vitamin C daily (King et al., 1997). Deficiencies of other nutrients were prevented by supplementation. When plasma vitamin C concentrations achieved nadir of <10 µM, vitamin C in solution was administered at 15 mg orally in the fasted state twice daily (30 mg total per day) until steady state for the dose was achieved. Vitamin C dose was increased to 30 mg twice daily (60 mg total per day) until steady state was achieved for this dose. In this way subjects received the following doses in mg/day: 30, 60, 100, 200, 400, 1000, and 2500. Vitamin C was measured by HPLC with coulometric electrochemical detection. Doses are indicated at the top of the figure. Each symbol represents a different subject. There is a one-day gap between all doses for bioavailability sampling. Each vertical line represents the start of a new dose. Some subjects reached steady state concentrations earlier than others; the duration shown is longest time taken by a subject to reach steady state. Two subjects were studied at one time and the graphs show data collated at the end of all studies. Modified and reproduced with permission from Biofactors (Levine et al., 2001a) and The Proceedings of the National Academy of Sciences (Levine et al., 2001b).
While useful for understanding absorption, the steady- state condition rarely pertains to real life. When a patient takes oral vitamin C at 30 mg or 1000 mg, current data cannot accurately predict bioavailability for the dose unless that patient consumes the same dose chronically and is at steady- state for that dose (assuming no other vitamin C intake in food). Bioavailability is very high (meaning close to 100%) when small doses of (for example 30 mg/day) are given to subjects with low plasma vitamin C concentrations. In the carefully controlled depletion-repletion studies, pure vitamin C was given as a solution by mouth in the fasting state. In real life, vitamin C in food may not be fully bioavailable because of physical sequestration of the vitamin within foods, or because vitamin C is degraded or its absorption inhibited by other food components. It is also possible that some substances in food can enhance vitamin C absorption. Therefore, published bioavailability data has to be interpreted cautiously when applied to real life clinical practice. A summary of vitamin dose concentration relationships, bioavailability, amount absorbed and the amount excreted in the urine is shown in figure 5.
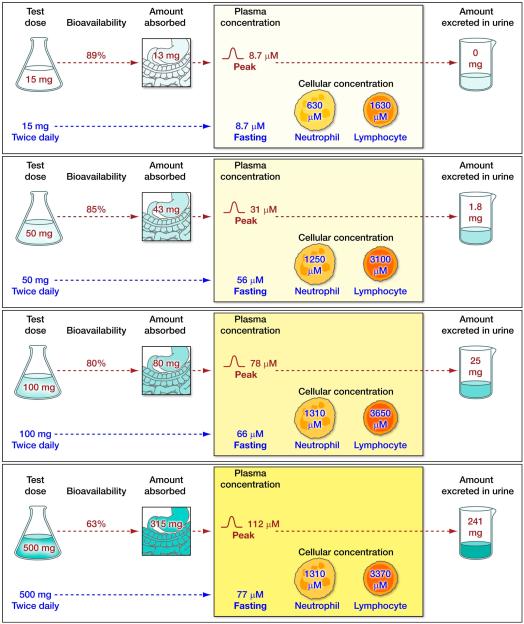
Figure 5. Ascorbate flux in humans.
The relationship between the daily intake of known doses of vitamin C and its absorption, distribution and excretion are shown for four different daily intakes of vitamin C. Data were obtained from studies in seven healthy young men (Levine et al., 1996b) detailed in Fig 4. Ascorbate flux is shown in four panels. The upper part of each panel shows the relationship between an oral dose of vitamin C and: bioavailability; amount absorbed by the gastrointestinal tract; peak plasma concentrations reached; and the amount of the vitamin excreted in urine. Bioavailability studies were performed when patients were at steady-state for that dose (with some modifications, see below). Following each bioavailability study, subjects were given twice daily doses of the vitamin until they reached steady state for that dose. The lower part of each panel shows the steady-state condition for a specific dose. The panels for 50 and 100 mg doses can be taken as examples. When subjects were at steady-state for vitamin C intake of 50 mg twice daily (100mg total daily dose), fasting plasma vitamin C concentrations were 56µM, and neutrophil and lymphocyte vitamin C concentrations were 1250 µM and 3100 µM respectively. At this stage, bioavailability was studied in part by using a test dose of 100mg by mouth (labelled “test dose”). Urine excretion data (25 mg) are shown for this oral dose. Not shown are data for the same dose administered intravenously, to permit bioavailability to be calculated. Bioavailability for the 100 mg test dose was 80%: 80 mg of vitamin C was absorbed, and the resultant peak plasma vitamin C concentration attained was 78 µM. 25 mg of vitamin C was excreted in the urine in the following 24 hours. The upper part of each of the four panels shows bioavailability doses of 15, 50, 100 or 500 mg. The lower part of the four panels show steady state for vitamin C intake of 30 mg/day, 100 mg/day (close to the recommended dietary allowance), 200 mg/day (the approximate amount provided by five servings of fruits and vegetables per day), or 1000mg/day (a dose that is used as a dietary supplement). Data shown are mean values for all patients studied. Amount absorbed was calculated from bioavailability data (Graumlich et al., 1997, Levine et al., 1996b). Separate color schemes, for which the intensity of color is related to the amount or concentration of vitamin C, are used for test doses; amounts absorbed and excreted in the urine; and for fasting steady state intracellular vitamin C concentrations.
Bioavailability studies were performed as follows: bioavailability for 15 mg dose at the end of depletion phase, for 50 mg when the subjects were at steady state for 60 mg, for 100 mg when the subjects were at steady state for 100 mg, and for 500 mg when the subjects were at steady state for 400 mg. Note that the end of the depletion phase was not a true steady-state. Mean fasting plasma vitamin C concentration at the end of depletion was 7.62 +/− 1.64 µM. Fasting steady state plasma vitamin C concentrations at the time of oral bioavailability tests were: for 50 mg dose, 24.8 µM +/− 14.1; for 100 mg dose, 56 µM +/− 4.5; and for 500 mg dose, 70 µM +/− 6.9. Each subject received a total of seven different doses of vitamin C but only data from four bioavailability studies and for four steady state conditions are shown. Details of methods used were previously published (King et al., 1997, Graumlich et al., 1997, Levine et al., 1996b, Levine et al., 2001b).
Vitamin C in disease states
In addition to its deficiency state scurvy, plasma and tissue vitamin C may be altered in many disease states. Vitamin C is the most potent concentration-dependent water soluble antioxidant in the body (Frei et al., 1989) and the principal antioxidant that quenches aqueous peroxyl radicals and lipid per oxidation products in plasma ex vivo (Frei et al., 1990). If in the process ascorbate is irreversibly oxidized, vitamin C will be consumed and dietary requirements for the vitamin increase. In vitro, ascorbate is preferentially oxidized in plasma before other antioxidants (uric acid, tocopherols, and bilirubin). Although antioxidant effects of vitamin C and other antioxidants have been shown in vitro, significance of the findings is unclear unless such effects can be shown in vivo. It has not been possible to definitively attribute pathological processes to antioxidant deficiency, nor to lack of specific antioxidants.
Many common clinical conditions are thought to result in pro-oxidant states that contribute to pathology, including that attributable to cigarette smoking and diabetes. Smokers (Schectman et al., 1991, Lykkesfeldt et al., 2000, Smith & Hodges, 1987, Kallner et al., 1981, Schectman et al., 1989) and diabetic subjects (Dorchy, 1999, Seghieri et al., 1994, Yue et al., 1990, Som et al., 1981, Stankova et al., 1984, Will et al., 1999, Sinclair et al., 1994, Sargeant et al., 2000) (Chen et al 2006; Tu et al 2015) (Kaviarasan et al., 2005) have lower plasma vitamin C concentrations than controls. These findings may be due to low dietary intake and/or increased metabolism of vitamin C. In vitro and in vivo, hyperglycemia reduces red cell vitamin C concentrations (Tu et al., 2015). In vivo, it is unclear whether reductions in plasma vitamin C concentrations are due to direct glucose toxicity or indirectly from other metabolic consequences of diabetes. Vitamin C concentrations may also be low in acute illnesses, including myocardial infarction (Hume et al., 1972, Riemersma et al., 2000), acute pancreatitis (Bonham et al., 1999, Scott et al., 1993), sepsis (Fowler et al., 2014) and in patients with critical illness (Berger & Oudemans-van Straaten, 2015). It is possible that low plasma and tissue vitamin C concentrations contribute to the pathology or is a consequence of the disease process. On the other hand low concentrations may be merely associated with a disease condition but not causal. In these disease conditions, circulating oxidants may be present in the plasma. While such oxidants could have no effect on vitamin C in vivo, they still could oxidize vitamin C in blood once it has been withdrawn from the patient and is awaiting analysis. Other substances that interfere with vitamin C assays may also be present, and it is necessary to take the possibility of measurement artifacts into consideration (Padayatty & Levine, 2000). Disease states are associated with sub-optimal nutrition, and low vitamin C concentrations may simply reflect poor intake (Dallongeville et al., 1998). The role of vitamin C as an antioxidant has been reviewed in detail elsewhere (Padayatty et al., 2003). Because vitamin C is excreted by the kidneys, it can accumulate in renal failure, and also is lost in dialysis fluid during hemodialysis (Sullivan & Eisenstein, 1970, Balcke et al., 1984, Handelman, 2011, Clase et al., 2013). Vitamin C replacement in patients with renal failure has to take both these factors into consideration to avoid both vitamin C deficiency and vitamin C toxicity (Pru et al., 1985, Balcke et al., 1984, Ono, 1986).
Vitamin C in relation to oral health
Vitamin C measurements have been performed in different oral tissues, but clinical interpretation is often problematic. The earliest studies of vitamin C using guinea pig as a model of a scurvy-prone animal showed that teeth take up substantial amounts of administered vitamin C (Burns et al., 1951). C13 labeled vitamin C is accumulated by the parotid and submandibular glands in the ascorbate replete (Hornig et al., 1974) and ascorbate depleted guinea pig (Hornig et al., 1972) (Hornig et al., 1972). Some uptake was noted in the periodontal tissue and pulp of teeth (Hornig et al., 1972) (Hornig et al., 1974). Secretory granules in the acinar cells of rat parotid glands contain millimolar concentrations of vitamin C and it is cosecreted with amylase (Vonzastrow et al., 1984). In hypophysectomized rats compared to normal rats, vitamin C accumulation is reduced in many tissues, including salivary glands (Horning et al., 1972) by as yet unknown mechanisms. Vitamin C is secreted by the salivary glands and is found in low concentrations in saliva. Studies of salivary vitamin C concentrations in humans have shown widely varying results, from undetectable (Feller et al., 1975) to very low concentrations or near or higher than plasma concentrations (Anonymous, 1986, Diab-Ladki et al., 2003, Schock et al., 2004, Liskmann et al., 2007, Rai et al., 2011, Rai et al., 2007, Buduneli et al., 2006, Gumus et al., 2009, Feller et al., 1975, Makila & Kirveskari, 1969, Moore et al., 1994, Bates et al., 1972, Vaananen et al., 1994, Leggott et al., 1986b, Leggott et al., 1986a, Makila, 1968, de Sousa et al., 2015, Saral et al., 2005). On balance, it seems likely that salivary vitamin C concentrations are lower than those of plasma. Salivary vitamin C concentrations increase within hours on consumption of vitamin C (Makila & Kirveskari, 1969). Vitamin C concentrations do not vary between submandibular, lingual and parotid salivary glands (Makila & Kirveskari, 1969), but decrease with increased salivary flow in submandibular but not in parotid glands (Makila & Kirveskari, 1969). In a later study, plasma ascorbic acid concentrations as expected were synchronous with vitamin C depletion and repletion, but salivary ascorbic acid concentrations were unchanged, perhaps because they were at the lower limit of detection of the assay (Leggott et al., 1986b). These widely discordant reports of vitamin C concentrations in saliva may be attributable to assays that were affected by interfering substances (Feller et al., 1975), or because of differences in sample collection and processing. Standardized methodology, meticulous sample processing and modern methods of analysis that are specific and precise may resolve this issue. The function of vitamin C in saliva remains unknown.
In scurvy, oral signs and symptoms are prominent, and include bleeding and spongy gingivae with eventual loosening and loss of teeth. The underlying pathology in scurvy may be an impaired ability to replace collagen or dental tissues lost due to turnover or bacterial action. In the guinea pig, which has continuously growing teeth, teeth growth stops when vitamin C is withdrawn (Dalldorf & Zall, 1930). In the US studies on experimental scurvy in men, signs of scurvy began to appear about 40 days after vitamin C withdrawal (Hodges et al., 1969, Hodges et al., 1971, Hood et al., 1970). Oral lesions consisted of gingival swelling, gingival hemorrhage, and sublingual petechial hemorrhages. The lesions began along the margins of the gingivae and extended to interdental papillae, but periodontal membrane was intact on X-ray. The lesions were more marked in those with gingivitis. There were only minor gingival lesions in a subject with healthy gingivae and none in an edentulous subject. Some subjects also had Sjogren’s syndrome, including xerostomia and enlargement of the parotid and submandibular salivary glands (Hodges et al., 1969, Hodges et al., 1971, Hood et al., 1970). In these studies, signs and symptoms of scurvy occurred later and were less severe than that reported in historical records, probably because scurvy in sailors or other populations occurred under much more adverse conditions. Those affected by either land or sea scurvy most likely suffered from multiple nutritional deficiencies and infections, and were usually involved in strenuous physical work. In self-induced experimental scurvy in a physician investigator, signs of scurvy appeared only after 161 days of consuming a self-reported vitamin C free diet (Crandon et al., 1940). The subject had good oral hygiene and had no nutritional deficiency other than that of vitamin C. No gross changes were seen in the teeth or gingivae except for interruption of lamina seen on x-ray, which may be a reliable sign of scurvy. Gingival biopsy was normal. From these studies, it is clear that the gingival lesions of scurvy only occur in those with teeth, and with preexisting gingivitis and/or dental caries. In a vitamin C depletion-repletion (and a second depletion) study of healthy men, low plasma vitamin C was not associated with periodontal disease, but was associated with gingival inflammation and bleeding (Leggott et al., 1986a).
The role of vitamin C in maintenance of normal dental health, in the absence of scurvy, is unclear. It is possible that adequate tissue, plasma and salivary vitamin C concentrations are essential for the normal health of teeth and gingivae. Low plasma vitamin C is associated with poverty, and it is difficult to attribute poor dental health to any one factor, including plasma vitamin C (Vaananen et al., 1994). It has been hypothesized that low cellular concentration of vitamin C is a cause of dry mouth, (Enwonwu, 1992) or that vitamin C increases production of saliva by increasing prostaglandin production (Horrobin & Campbell, 1980). In an uncontrolled study, treatment of Sjogrens syndrome (not resulting from vitamin C deficiency) with a mixture of substances including vitamin C was reported to improve symptoms (Horrobin et al., 1981) but no improvement was found in another study (McKendry, 1982). An association has recently been found between a rare SNP in SVCT1 and aggressive periodontitis (de Jong et al., 2014). It is too early to draw conclusions from such genetic studies, but it is an area that has promise.
Vitamin C acting locally may have erosive effects on teeth. There are reports of dental erosion from vitamin C consumption (Giunta, 1983, Imfeld, 1996) but these are uncommon considering the wide use of the vitamin. In vitro, prolonged exposure to ascorbic acid causes dental erosions (Giunta, 1983) visible on scanning electron microscopy (Meurman & Murtomaa, 1986). Chewable vitamin tablets (or vitamin C 500mg wafers used experimentally) reduced salivary pH from 7 to about 4.5 in two and a half minutes (Hays et al., 1992). In healthy subjects, no changes in the teeth were seen after one week of vitamin C consumption even in the absence of mechanical cleaning (Meurman & Murtomaa, 1986). However, it is possible that chronic consumption of chewable (Giunta, 1983) or effervescent (Meurman & Murtomaa, 1986) vitamin C may erode dental enamel. It has been suggested that sucrose containing vitamin C syrup may also cause acid damage to teeth (Woods, 1981).
Experimental findings relating to vitamin C
There are numerous experimental phenomena which involve vitamin C in humans or with human cells, but with uncertainty as to how the findings relate to normal physiology, pathophysiology, clinical use, or disease treatment Described below are three such findings and clinically relevant questions for each.
Activated human neutrophils accumulate vitamin C
When exposed to pathogenic bacteria, human neutrophils are activated and produce oxidants that kill the pathogen. These oxidants may cause collateral damage to the neutrophils themselves and to other tissues. When freshly obtained normal human neutrophils are exposed to clinical isolates of E. coli, Enterococcus faecalis, Moraxella catarrhlis, Klebsiella oxytoca, Acinetobacter baumanii or C. albicans, the neutrophils are activated and accumulate vitamin C rapidly from the surrounding culture media until all available external vitamin C is exhausted (Wang et al., 1997) (Washko et al., 1993). Vitamin C concentrations inside the neutrophils increase from starting concentrations of about 1 mM to values as high as 10 to 12 mM (Wang et al., 1997)(Fig. 6). The likely mechanism is via ascorbate recycling (Stankova et al., 1975) (Bigley & Stankova, 1974) (Hemila et al., 1984) (Anderson & Lukey, 1987). As described above, this means that oxidants from neutrophils accelerate oxidation of external ascorbate to dehydroascorbic acid, which is transported on glucose transporters, and immediately reduced internally to ascorbate. Why do vitamin C concentrations increase so much and so rapidly in activated neutrophils? One possibility is that vitamin C is used to protect neutrophils from the oxidants that they make. Conversely, vitamin C could increase oxidant generation, to ensure that pathogens are killed. Answers to these questions may have clinical relevance. Thus, is ascorbate clinically important to enhance neutrophil action in healthy patients? Since the mechanism of increased ascorbate in neutrophils involves glucose transporters, could extra vitamin C be useful for neutrophil action in diabetic subjects? Could extra vitamin C, by its actions in neutrophils, improve outcomes in sepsis? (Fowler et al., 2014) (Voigt et al., 2002) (Fowler et al., 2014, Ferron-Celma et al., 2009) . Would extra ascorbate improve bacterial killing in patients with Chronic Granulomatous Disease? Patients with this disease have recurrent bacterial infections that are uncommon in healthy people, the infections are difficult to treat, and neutrophils from patients with this disease do not increase their ascorbate concentrations in presence of bacteria (Curnutte, 1993, Thrasher et al., 1994, Roos et al., 1996, Holland, 2010, Wang et al., 1997).
Figure 6. Ascorbic acid recycling in Human Neutrophils.
Ascorbic acid (AA) and dehydroascorbic acid (DHA) transport and recycling in human neutrophils in vitro (Stankova et al., 1975) (Bigley & Stankova, 1974) (Hemila et al., 1984) (Anderson & Lukey, 1987, Wang et al., 1997). When normal human neutrophils in vitro were activated by pathogens (E coli, Enterococcus faecalis, Moraxella catarrhlis, Klebsiella oxytoca, Acinetobacter baumanii or C. albicans) (Wang et al., 1997), neutrophils accumulated ascorbic acid. Intracellular vitamin C concentrations increased from ~ 1.3mM to 8mM, and vitamin C in the culture media decreased to undetectable concentrations from 50µM. These findings did not occur when neutrophils were used from patients with Chronic Granulomatous Disease (Wang et al., 1997). Patients with this condition are unable to make superoxide, and have dysfunctional neutrophils that cannot kill certain pathogens. The observed in vitro phenomena can be accounted for by the proposed model described below. Normal human neutrophils maintain internal vitamin C concentrations of about ~ 1.3mM by uptake of AA by sodium-dependent vitamin C transporter 2 (SVCT2). Activated neutrophils secrete reactive oxygen species because membrane associated NADPH oxidase is able to transfer electrons across the cell membrane, using NADPH in the process. H2O2 is formed, which is a precursor for production of reactive oxygen species (ROS and RNOS). These oxidize extracellular AA to DHA. Neutrophil activation with oxidant formation and its consequent results are shown in red. DHA is rapidly transported into the neutrophil by glucose transporters, probably GLUT1 and GLUT3, and immediately reduced to AA by glutaredoxin, producing a 4 to10-fold increase in neutrophil internal AA concentration. When bacteria are engulfed by the neutrophil, a phagolysosome is formed. Myeloperoxidase containing vesicles fuse with the phagolysosome, such that the oxidant generating reactions occur in a sequestered space. Although superoxide cannot cross the plasma membrane, H2O2 can readily diffuse into the cell. Oxidants generated by H2O2 can be reduced by internal AA. Glutathione (GSH), used by glutaredoxin during DHA reduction, is regenerated from glutathione disulfide (GSSG) by glutathione reductase (GRD) and NADPH. NADPH essential for these reactions is derived predominantly from the pentose phosphate pathway. Two molecules of NADPH are produced by the oxidative phase of pentose phosphate pathway (shown in green) when glucose 6 phosphate is converted to ribulose 6 phosphate (shown in green). Ribulose 6 phosphate undergoes further metabolism in the non-oxidative phase of the pentose phosphate pathway. As NADPH is oxidized to NADP, electrons are transferred to GRD so it can reduce GSSG to GSH. Modified and reproduced from (Rumsey & Levine, 1998), with permission of the Journal of Nutritional Biochemistry.
Red blood cells and vitamin C
As discussed above, circulating red blood cells also transport DHA and reduce it intracellularly to AA. There is evidence that plasma ascorbic acid is maintained by ascorbic acid within red blood cells. In clinical samples, vitamin C in plasma is maintained much longer when the plasma is not separated from red blood cells until the time of sample processing (Dhariwal et al., 1991b). Plasma, with red blood cells removed will lose vitamin C fairly rapidly unless special precautions are taken to prevent loss. How red blood cells stabilize plasma vitamin C may be due to transfer of electrons from ascorbic acid within the red cell across the red blood cell membrane by electron transfer proteins (Su et al., 2006) (May et al., 2000) (VanDuijn et al., 2000). These red blood cells proteins are found in species that cannot make vitamin C, such as humans, but are absent from species that synthesize vitamin C such as rodents. It is possible that electron transfer across red blood cells has importance in disease or in conditions where plasma vitamin C is decreased, such as diabetes. Indeed, despite recent intriguing advances discussed above (Tu et al., 2015), the function of vitamin C itself in red blood cells is unknown. If vitamin C has an important function in RBCs, does this have relevance to transfused red cells, either for functional consequences, or as a function of storage of blood for transfusion?
Human Adrenals secrete vitamin C in response to ACTH
Adrenal glands are known to have very high concentrations of vitamin C and Albert Szent-Györgyi first isolated it from ox adrenals. Human adrenal glands in vivo secrete vitamin C in response to ACTH which stimulates adrenal cortex to produce the essential hormone cortisol. Vitamin C secretion is rapid, starting and ending within minutes of ACTH administration in humans (Fig 7), and precedes cortisol secretion (Padayatty et al., 2007). What is the function of vitamin C release from adrenal glands? Vitamin C secretion in response to prostaglandin or hormonal stimulation has also been shown in the testes and ovary in animals (Koba et al., 1971) (Petroff et al., 1998) (Guarnaccia et al., 2000). Does vitamin C release have a similar function in these steroid secreting glands? In humans, vitamin C concentrations in the adrenal vein peak at approximately five times the plasma concentration. Peak plasma vitamin C concentrations approaching values found transiently in the adrenal vein may be attained in the general circulation in those who take very large doses (1- 3 grams/day) of vitamin C supplements (Padayatty et al., 2004). Is mimicking of, or interference with, adrenal function induced by such concentrations?
Figure 7. Vitamin C secretion by human adrenal glands.
Vitamin C concentrations were measured in the adrenal and peripheral veins of 26 patients with primary hyperaldosteronism. Under radiographic guidance, catheters were placed in both adrenal veins, and blood samples were taken after stimulation with adrenocorticotrophic hormone (ACTH). Vitamin C concentrations in each of the adrenal (n = 47) and peripheral (n = 26) veins sampled are shown. In 5 patients, blood samples were obtained from only one adrenal vein because of unusual venous anatomy or difficulties with adrenal vein catheterization. In the adrenal veins, peak vitamin C concentrations (Mean ± SD: 176 ± 71 µmol/L) were reached between 1 and 4 min, and were significantly (P < 0.0001, paired t test) higher than corresponding peripheral plasma vitamin C concentrations (35 ± 15 µmol/L). In patients in whom adrenal vein vitamin C concentration could be measured in only one adrenal gland, that single value was used in the calculation. In patients in whom both adrenals were successfully sampled, the mean of the two adrenal vein vitamin C concentrations was used for calculation, but all values are shown. Modified and reproduced from (Padayatty et al., 2007), with permission from American Journal of Clinical Nutrition.
Recommended Dietary Allowance for vitamin C
The Recommended Dietary Allowance (RDA) for vitamin C in the United States and Canada is 75 mg for women and 90 mg for men (Food and Nutrition Board, 2000b). Many countries have increased the RDA for vitamin C in the past 15 years so that they are now similar to those in the United States and Canada, or even slightly higher.
The early RDA for vitamin C was based on the amount of vitamin C needed to prevent scurvy with an added amount to ensure a margin of safety and to account for individual variability. Human experiments conducted in UK (Peters et al., 1953), Canada (Johnstone et al., 1946) and USA (Hodges et al., 1969, Baker et al., 1969, Baker et al., 1971, Hodges et al., 1971) concluded that 10 mg or less of vitamin C/day was sufficient to prevent scurvy. These studies formed the basis for an RDA for vitamin C for several decades. However these studies had many flaws, including imperfect dietary control, the possibility of multiple nutrient deficiencies, and use of imprecise vitamin C assays. Nevertheless, the effect of the intake of a known amount of vitamin C on observable clinical outcome was shown.
An ideal RDA should be based on an intake that leads to optimum health, and not merely prevent a deficiency state, in this case scurvy. While the goal is laudable, getting there has so far been elusive. This is because we do not quite know how to measure optimum health, for any vitamin. Thus, it is predictable that we do not have data to indicate what intake of- vitamin C is optimal for health.
The Food and Nutrition Board of the Institute of Medicine (of The National Academy of Sciences, USA) revised the RDA for vitamin C in the year 2000 using different guidelines than vitamin C body stores or clinical deficiency criteria used previously. For vitamin C, these guidelines incorporated ability of vitamin C to confer antioxidant protection. In vitro findings from human neutrophils were combined with published clinical data on vitamin C dose concentration relationships in healthy young men to estimate intake values (Food and Nutrition Board, 2000b). Unlike the earlier recommendations that related the amount of vitamin C intake only to a clinical outcome, in vitro data were utilized that described how clinically relevant vitamin C concentrations resulted in inhibition of superoxide production. Unfortunately, we do not know whether the newer criteria apply to health of intact humans or are relevant to in vivo physiology.
How was the current RDA calculated? Neutrophil vitamin C data detailed above consistent with minimal urinary loss was used to calculate the Estimated Average Requirements (EAR) for the vitamin. EAR for vitamin C is that amount of average vitamin C intake per day that is estimated to meet the nutrient requirements of 50% of the healthy population in that age and sex group. EAR was then used to calculate the RDA, taking into consideration the standard deviation of EAR. For vitamin C, a coefficient of variation of 10% is assumed as data on standard deviation of requirements is not available. RDA is the amount of average vitamin C intake per day that would meet the nutritional requirements of 97-98% of the healthy individuals in the particular age and sex group, (Hellwig et al., 2006, Food and Nutrition Board, 2000b). For example, the EAR for vitamin C is 75 mg/day for men aged 19-30 years old, and the RDA for vitamin C is 90 mg/day for this age and sex group (Hellwig et al., 2006, Food and Nutrition Board, 2000b). These criteria are based on intake of the vitamin from foods, and not from supplements.
Basing recommendations in part on in vitro data and extrapolating these data to humans has limitations (Harper, 1975). However, due to absence of key human data, such extrapolations were deemed necessary by the Food and Nutrition Board for vitamin C. A similar rationale was used for vitamin E recommendations (Food and Nutrition Board, 2000a).
The RDA for vitamin C was as low as 10mg/day but has gradually increased over the years so that now it is nearly 100 mg/day. Intake of 10mg/day of vitamin C would result in plasma vitamin C concentrations of about 10 µM while consumption of 100 mg/day of vitamin C will result in plasma vitamin C concentrations of about 50 µM, close to the plasma concentrations above which vitamin C is lost in the urine. Once vitamin C is lost in urine, plasma and tissue levels do not increase much more with oral dosing, except transiently with doses found in supplements. In the NIH depletion repletion studies, fatigue occurred when plasma concentrations were less than about 20 µM (Levine et al., 1996b) (Padayatty & Levine, 2001a). It is likely that some people in the general population will experience fatigue when vitamin C concentrations are between 10 µM and 20 µM, without any other manifestation of vitamin C deficiency. These low concentrations may be avoided by the new higher RDA, which may be an unintended benefit of the higher RDA. Conversely, fatigue is a common symptom in clinical practice, and is rarely due to vitamin C deficiency. The current RDA is higher than that amount for which there is a proven clinical benefit to prevent scurvy, although there may be other benefits that we are currently unable to quantitate clinically.
In summary, how much vitamin C intake is ideal for optimum health is not known. It is likely that requirements for vitamin C will vary (Yew, 1975) with genetically determined variations in vitamin C transport or metabolism, and with disease states. For example, at a time when scurvy was rampant in sailors who were at sea for prolonged periods, not all men developed the disease. All men on board (except the few officers) consumed the same or very similar diets, and while many (as many as half) developed fatal scurvy, others were unaffected or barely affected. Thus there was substantial inter individual variation in susceptibility to scurvy. Since vitamin C is easily acquired from foods, in clinical practice it is perhaps better to consider what foods have general health benefits.
With all of this information, then, how much vitamin C should we consume, and how should we consume it? Consumption of five servings of a variety of fruits and vegetables a day will provide ample amounts of vitamin C (approximately 200 -250 mg of vitamin C). Many observational studies and limited intervention trials have shown the benefits of increased fruit and vegetable intake on reducing cardiovascular risk and all-cause mortality (Wang et al., 2014, Hartley et al., 2013, Woodside et al., 2013) (Hung et al., 2004). A diet rich in fruit and vegetable intake is associated with many factors including high socioeconomic status (in Western countries), education, and a healthy lifestyle. Fruits and vegetables also displace higher calorie foods, provide bulk and fiber, and micronutrients and phytochemicals. Plasma concentrations of many substances, including carotenoids and vitamin C, are elevated in those with higher consumption of a variety of fruits and vegetables. However, association is not causation, and the mechanism of the beneficial effects of fruits and vegetables is not known. In view of available information, the best advice is to eat a wide variety of fruits and vegetables of many colors. Five servings per day will produce near saturation of plasma and tissues by providing 200 to 250 mg/day of vitamin C.
Relevance of experimental studies to ascorbate physiology: remembering Goldilocks
While a large number of studies have shown effects of vitamin C on many physiological parameters or cellular function, the relevance of these findings to clinical physiology and pathophysiology remains unclear. Many such studies used un-physiological doses of vitamin C or attained plasma or tissue concentrations far from the physiological range, either too high or too low. Some of these concerns have been reviewed elsewhere (Levine et al., 2011) and we have limited our discussion to the necessity of careful interpretation of these studies. In essence there are problems that Goldilocks would understand fully, both in experiments in the laboratory and experiments in people. For interpretation to be sound, vitamin C conditions should be “just right”, to reflect physiology. When there is either too little vitamin C, or too much vitamin C, then it is hard to interpret the outcomes.
Interpretation of in vitro studies using vitamin C
Most mammalian cells grow well in cell culture media that contain no ascorbate, and are scorbutic. In contrast, the intact animal cannot survive in the absence of vitamin C, either synthesized by the animal or obtained from food. Cultured cells used in vitro have either adapted to survive and proliferate in the absence of ascorbate, or have subtle abnormalities due to ascorbate deficiency that are not fatal. In either case, these cells may not be representative of normal human physiology in which cells are at all times exposed to ascorbate concentrations in the range of 10 µM to 70 µM (occasionally higher, in those who take vitamin C supplements). Further, vitamin C is the most abundant water soluble antioxidant in circulation. Cell culture studies exploring antioxidant mechanisms that are performed without ascorbate will at the very least be unrepresentative of in vivo physiology. When ascorbate supplementation is undertaken, ideally cells should be exposed to physiological concentrations of ascorbate but not to very high (>100 µM) or very low (<10 µM) vitamin C concentrations that are outside the physiological range. Ascorbate in cell culture conditions is unstable, and a constant concentration of ascorbate can be maintained only by repeated supplementation with appropriate amounts. Unfortunately, there often has been limited appreciation of how to apply ascorbate physiology to cell experiments. As a consequence, physiologic ascorbate concentrations are added to cells for short periods of time, and then effects are determined. Most often this time is insufficient for cells to achieve physiologic intracellular concentrations. Thus, the meaning of findings becomes questionable.
The other side of the spectrum is that for some in vitro experiments, investigators have added very high, or pharmacologic, concentrations of ascorbate, in the mM range. Addition of mM concentrations may result in the generation of ascorbate radical and hydrogen peroxide. These compound, in turn, have many actions on cells that are independent of ascorbate physiology (Levine et al., 2011). Such actions on cells could provide new insights to use of ascorbate in disease treatment, especially cancer treatment. However, it must be remembered that pharmacologic ascorbate actions may not be relevant to ascorbate physiology.
Interpretation of in vivo studies using vitamin C
Similarly, in vivo infusion of mM concentrations of ascorbate have been used to demonstrate many effects including vasodilatation (Levine et al., 1996a, Motoyama et al., 1997, Beckman et al., 2001, Vita et al., 1998, Jackson et al., 1998, Duffy et al., 2001, Kaufmann et al., 2000). The results, even when repeatable, do not represent vitamin C physiology but rather pharmacological use of vitamin C (Padayatty & Levine, 2001b). Such studies are useful to demonstrate specific effects such as anti-oxidant actions or as proof of concept (Kaufmann et al., 2001). Other studies using infused vitamin C do so with the understanding that pharmacologic properties are being studied: for example, in cancer treatment (Levine et al., 2011) (Yun et al., 2015) . While these studies may be very useful for understanding new uses of ascorbic acid as a drug, such studies however are not useful in determining the optimum dietary intake of vitamin C or ascorbate oral supplementation to achieve a desired clinical affect. Therefore, the results of in vitro and in vivo experiments with vitamin C have to be interpreted taking all these of factors into consideration, particularly the dose concentration relationship of vitamin C.
Goldilocks and intervention trials: importance of dose-concentration relationships
Many small and large scale intervention trials have studied the effect of vitamin C supplementation (alone or with other nutrients) on clinical outcome. These have shown either no effect or very small effects. These negative results may indicate that vitamin C had no clinical effect on the condition studied. Alternately, these studies may have had control and treatment groups that were not substantially different with regard to plasma and tissue vitamin C concentrations, (Levine et al 1999a) (Padayatty & Levine, 2009) making it impossible to detect any effect attributable to the vitamin. Such an outcome is likely unless the study is carefully designed, because vitamin C dose concentration relationship is not linear but sigmoidal. In patients who are in a vitamin C depleted state, plasma vitamin C concentrations increases only modestly after small doses of vitamin C (<30mg/day) but much more so after slightly larger doses (>100 mg/day) before reaching a plateau. For example, in men enrolled in the NIH depletion repletion study, fasting steady state plasma vitamin C concentrations were 8.7 µM for a daily vitamin C intake of 30 mg. For a 100 mg/day intake, plasma values increased to 56 µM, and for an intake of 400 mg/day, plasma values increased to 70 µM. Corresponding values for women were: for 30 mg/day-12.6 µM; for 100mg/day-62 µM; and for 400 mg/day-73 µM. Therefore, intakes much higher than the RDA for vitamin C (90 mg/day for men, 75 mg/day for women in the US) resulted in only small increases in plasma vitamin C concentrations (see figures 4A and 4B) (Levine et al., 1996b, Levine et al., 2001b). These findings have several important implications. First, if an intervention study starts with well-nourished control and intervention groups (consuming about 100 mg/day of vitamin C in food), then supplemental vitamin C would only modestly increase plasma and tissue vitamin C concentrations. (Levine et al 1999a) (Padayatty & Levine, 2009). Such minor changes may not have any demonstrable benefit, and predictably so. Second, it is necessary to select populations that have low plasma vitamin C concentrations, so that supplementation (Levine et al 1999a) (Padayatty & Levine, 2009) will produce a clear difference between the control and treatment groups with regard to plasma and tissue vitamin C concentrations (Padayatty & Levine, 2009). Thus the potential for demonstrating benefit (or harm) is greatly increased (Lykkesfeldt & Poulsen, 2010). To ensure this, it is necessary to measure plasma vitamin C concentrations at baseline, and ideally, after supplementation (Padayatty & Levine, 2009) (Padayatty & Levine, 2014). Careful attention to dose concentration relationships is essential to detect any effect from vitamin C supplementation and to ensure that intervention studies have a reasonable chance of detecting benefit or harm (Morris & Tangney, 2011). Finally, these principles apply not just to vitamin C, but to other vitamins. When the goal is to learn whether increase in a vitamin changes outcome, it is essential to select study populations for whom supplementation could change vitamin concentrations more than marginally. These concentrations must be measured both before and after intervention with that vitamin, and in control groups as well.
The importance of the sigmoidal dose concentration response is shown by the following examples. In a study of the effect of antioxidants on eclampsia, 283 pregnant women at increased risk of pre-eclampsia were given either vitamin C 1000 mg/day (with vitamin E) or placebo. The study population had measured plasma vitamin C concentrations of about 30 µM, which increased to ~ 50 µM in the treatment group (Chappell et al., 1999). PAI-1/PAI-2 ratio (PAI-1/2: Plasminogen Activator Inhibitor 1 or 2, a marker of pre-eclampsia) was reduced in the supplemented group, as was pre-eclampsia. To verify the findings, a subsequent study was performed in a larger cohort of 1877 pregnant women at risk of pre-eclampsia and perinatal complications. Vitamin C 1000 mg/day (plus vitamin E) was administered, but plasma vitamin C concentrations were not measured (Rumbold et al., 2006). However, calculated plasma vitamin C concentrations were approximately 70 to 80 µM in both the control and treatment groups, despite supplementation in the treatment group (Padayatty & Levine, 2006). Not surprisingly, the study showed no benefit. In a follow up study of vitamin C 1000 mg/day (plus vitamin E) to prevent hypertension in 10154 pregnant women, plasma vitamin C was not measured and supplementation showed no benefit (Roberts et al., 2010). This outcome was also predictable, because the study population was well nourished at baseline. These, and similar studies cannot reliably distinguish between a true lack of effect of vitamin C and an apparent lack of effect, because the intervention groups were likely already replete in vitamin C and were not substantially different from the control groups with regard to the vitamin, even with supplementation (Rumbold et al., 2006, Padayatty & Levine, 2006, Sesso et al., 2008, Padayatty & Levine, 2009, Fortmann et al., 2013, Padayatty & Levine, 2014, Isanaka et al., 2012, Padayatty & Levine, 2013). In vitamin C supplementation studies, as well as those for other nutrients, consideration of dose-concentration relationships is critical to good study design (Levine et al 1999a) (Padayatty & Levine, 2009) (Morris & Tangney, 2011) (Lykkesfeldt & Poulsen, 2010).
Some stark unknowns
While recent studies have shed new light on many aspects of vitamin C physiology, conundrums remain. A few of these conundrums are listed below.
1) Most animals synthesize vitamin C but some have lost the ability to do so. Those animals that lack vitamin C synthetic ability do not bear any phylogenetic relationship to each other, implying many independent mutations all resulting in the same phenotype. No common environmental influence is apparent. To date, there is no satisfactory evolutionary explanation for the apparent random loss of vitamin C synthetic ability. It remains possible that other animals have not been recognized to have lost the ability to synthesize vitamin C. Identification of all non-synthesizers possibly could enhance recognition of a pattern, but so far none is evident.
2) Vitamin C is concentrated by most tissues in humans and in many mammals. However, only much lower concentrations, sometimes 1-2 orders of magnitude lower, are required for the known enzymatic actions of vitamin C. Why do tissues so avidly acquire vitamin C? A possible explanation is that maintaining concentrations in great excess above required function may be an evolutionary protective or “safety” function, In this case, it would take time for enzymatic consequences, and deficiency, to develop if intake suddenly stopped. Alternatively, intracellular accumulation at much higher concentrations than those needed for enzymatic action may indicate that vitamin C has other, as yet unknown, functions.
3) High concentrations (>5 mM as compared to µM) of vitamin C are produced in extracellular fluid only with intravenous or parenteral administration of ascorbate. Ascorbate at pharmacological concentrations produces hydrogen peroxide (Fig 8). Hydrogen peroxide or oxidant species that result from peroxide damage cancer cells but not normal cells. In blood, these oxidant species are below the threshold of measurement, perhaps due to the large reducing capacity of red blood cells. In nature, pharmacologic concentrations of ascorbate do not occur in the blood or extracellular fluids. But such concentrations occur within cells of many tissues. With administration of ascorbic acid intravenously or parenterally, mM concentrations of ascorbic acid occur in the extracellular fluid as well as within cells. For normal cells, there are redundant intracellular mechanisms to detoxify the harmful oxidants from ascorbate administration as a drug. These mechanisms may also protect cells from oxidants that result from the normal mM intracellular concentrations of ascorbate that are part of physiology. For both examples, which detoxification pathways are the most important ones regarding ascorbate, or is there purposeful redundancy? Which pathways are deficient in the many types of cancer cells that are sensitive to hydrogen peroxide? As ascorbate generates oxidants, are the key damaging pathways initiated within cells or outside them, or both, depending on the cell type? Is intracellular damage initiated at the transcription (DNA) level, translation (RNA) level, or post-translational (protein or organelle or membrane) level? (Parrow et al., 2013).
Figure 8. Ascorbate radical formation in vivo.
Concentrations of ascorbate, ascorbate radical (Asc• −) (radical is denoted by a superscript dot) and H2O2 in blood and extracellular space, and proposed mechanisms that result in measurable Asc• − and H2O2 in extracellular fluid but not in blood. Whether given by oral or parenteral routes, ascorbate rapidly equilibrates between blood and extracellular fluid, possibly through intercellular junctions. When pharmacological doses are administered parenterally, blood and extracellular fluid ascorbate concentrations reached 10 to 20 mM. Concentrations of Asc• − in blood reached 10-30 nM but H2O2 was not detectable in blood. Asc• − and H2O2 reached concentrations of 250-300 nM and 150 µM respectively in extracellular fluid. A proposed scheme that accounts for these observations are as follows: In extracellular fluid, ascorbate loses one electron to form Asc• − (solid lines). The electron reduces a protein-centered metal, for example Fe3+ to Fe2+. Candidate proteins may be either in extracellular fluid, or on cell membranes facing outward. Fe2+ donates an electron to oxygen, forming superoxide (O2 • −) which undergoes dismutation to form H2O2 (Qian & Buettner, 1999). In blood, these reactions are damped or inhibited (dashed lines), or the resultant products diffuse into red blood cells where they are rapidly extinguished/reduced. In blood, the formation of Asc• − may be inhibited by red blood cell membrane bound reducing proteins (May et al., 2001) and/or by large plasma proteins that do not distribute into the extracellular space. H2O2 that is formed in blood will be immediately destroyed by plasma catalase and red blood cell GSH peroxidase, so that no H2O2 is detectable (Chen et al., 2007, Gaetani et al., 1996, Johnson et al., 2005) (Chen et al., 2005). The identities of the metal-centered proteins are not known. H2O2 that diffuses into the blood or tissues from extracellular fluid is inactivated by reducing substances in these compartments. Ascorbate was measured by HPLC, Asc• − by Electron Paramagnetic Resonance (EPR) and H2O2 by fluorescence (Chen et al., 2007). The values shown are approximations from measurements in animals given ascorbate by the intra peritoneal or intravenous routes (Chen et al., 2007)(Chen et al., 2005, Chen et al., 2008). Modified and reproduced from (Chen et al., 2007), with permission from the National Academy of Sciences.
There are many known pathways to detoxify ROS in cells. Ascorbate is sequestered in some secretory vesicles. However, whenever it has been investigated, ascorbate was not found to be in any other intracellular compartment. Such compartmentalization remains possible for some cells types but has not been found.
4) In humans, ascorbate is rapidly released/secreted by the adrenal gland in response to its tropic hormone ACTH. In animals, the testes and ovary also secrete ascorbate. Ascorbate secretion also occurs in the stomach. What functions does ascorbate release serve? What are the mechanisms of ascorbate secretion?
Known, with incomplete evidence
Our understanding of ascorbate physiology has many uncertainties. These extend from its molecular actions to its recommended dietary allowance. Lack of vitamin C inevitably results in scurvy. Many of the signs and symptoms of scurvy match known enzymatic actions of ascorbate. For example, loosening of teeth and tissue breakdown may reflect inability to cross link collagen, leading to structural weakness of connective tissue. Lassitude may reflect impaired nor epinephrine synthesis, defective amidation of peptide hormones or impaired carnitine synthesis (or all three). However, there is no experimental evidence to conclusively link specific enzyme defects to the signs and symptoms of scurvy. Additionally the role of ascorbate in many of these enzyme actions has not been clearly demonstrated in vivo. In vitro, specificity data are incomplete regarding ascorbate as the required electron donor (as opposed to other reducing agents). Nevertheless, scurvy cannot be cured without administering vitamin C, and therefore some biochemical pathways must have a specific requirement for vitamin C.
Vitamin C is by its chemical nature an electron donor, commonly called an antioxidant. However the widely held assumption that vitamin C has an important role as an antioxidant in humans is unproven. To date, supplementation with vitamin C has shown no benefit in disease conditions proposed to be caused by, or resulting in, oxidant stress (Padayatty et al., 2003). Besides, many supplement trials are plagued by the problem of inadequate differences between the treatment and controls groups with regard to vitamin C concentrations, addressed in detail above.
Some common questions about vitamin C
1- Is vitamin C effective in the prevention or treatment of common cold? Cochrane Reviews (Douglas et al., 2000, Hemilä et al., 2007) (Hemila & Chalker, 2013) conducted meta-analyses of published studies in which vitamin C was used for the prophylaxis or the treatment of common cold. 29 placebo (but not necessarily randomized) controlled trials were analyzed, in which oral vitamin C was used at doses of 200 mg or more per day. In the general population, vitamin C supplementation taken prophylactically had no effect on the incidence of common cold, but had a modest effect in reducing the duration (by 8% in adults, 13% in children) and severity of symptoms of cold. In those who underwent severe exercise (five trials), such as in Marathon runners, the risk of cold was halved. Treatment of common cold with vitamin C (seven trials) was ineffective. In summary, vitamin C probably will not prevent colds for most people. In some people, there may be mild or modest effect on duration, especially those who are heavy exercisers. Because of its low cost and safety, and consistent though modest benefit, vitamin C supplementation can be considered on an individual basis in patients with cold who have no contra indications to vitamin C supplementation (Hemila & Chalker, 2013).
2 - Is there evidence for or against the use of gram doses of vitamin C taken as supplements? Vitamin C bioavailability declines rapidly with dose. When 1250 mg of vitamin C is administered orally to healthy young men at steady state for that dose, bioavailability is 46%. Almost all the absorbed dose is excreted in urine within 24 hours (Levine et al., 1996b, Graumlich et al., 1997). No clinical benefits have been shown for consumption of vitamin C above the recommended dietary allowance. Current recommendations are that doses above 2 g/day should be avoided to prevent side effects, particularly bloating and osmotic diarrhea. Whether these doses lead to hyperoxaluria is unclear (Food and Nutrition Board, 2000b, Handelman, 2007, Auer et al., 1998). Supplemental vitamin C can cause hemolysis in those with glucose 6 phosphate dehydrogenase deficiency (Mehta et al., 1990) and in patients with Paroxysmal Nocturnal Hemoglobinuria (Iwamoto et al., 1994b) (Iwamoto et al., 1994a). Vitamin C consumption may also predispose to oxalate accumulation in those with impaired renal function (Canavese et al., 2005). In those without contraindications, supplemental vitamin C appears to be safe (Levine et al., 1999a, Food and Nutrition Board, 2000b). It is possible that vitamin supplements may have local effects such as erosion of teeth (Giunta, 1983). Other side effects may occur rarely. There have been case reports of oesophageal erosions, renal impairment (Nakamoto et al., 1998, Nasr et al., 2006, Rathi et al., 2007, Mashour et al., 2000) and unexpected interactions with other drugs or supplements (Bromley et al., 2005).
3 - What are the therapeutic uses of high dose intravenously administered vitamin C? Intravenously administered vitamin C produces pharmacologic concentrations not possible with oral intake. Vitamin C, given intravenously in doses of up to 100 g have been used for several decades in treating infections, cancer and other conditions (Padayatty et al., 2010). Rigorous trials to test benefit have not yet been undertaken. Intravenous ascorbate is widely used by integrative medicine (complementary and alternate medicine) practitioners. Intravenously administered vitamin C (Padayatty et al., 2004) has a favorable safety profile without any apparent ill effect (Hoffer et al., 2008) Padayatty et al., 2010). At mM plasma concentrations, or pharmacologic concentrations, vitamin C acts as a pro- oxidant. Animal studies show that only these pharmacologic concentrations of ascorbate produce ascorbate radical and hydrogen peroxide in the extracellular fluid (Fig 8) (Chen et al., 2005). Pharmacologic ascorbate concentrations have antitumor activity in vitro and in animal studies (Chen et al., 2008, Chen et al., 2007) (Parrow et al., 2013, Yun et al., 2015). In small phase I clinical studies, intravenously administered ascorbate was safe. Survival was doubled in patients with metastatic pancreatic cancer, but only retrospective controls were used. Phase II and Phase III studies have not yet been conducted to test benefit of intravenously administered vitamin C in patients with cancer. Conducting such trials rigorously, with controls, and considering dose size and frequency are necessary. Such trials will address questions about ascorbate efficacy as an anticancer drug (Parrow et al., 2013).
Acknowledgments
SJP thanks Drs. Michael Espey, Peter Eck and Nermi Parrow for helpful suggestions; Dr. Eck for information on SVCT SNPs deposited in databases; and Ethan Tyler, NIH Medical Arts, for new and modified illustrations.
This work was supported by the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Project no: 1ZIADK053211-08).
List of abbreviations
- AA
Ascorbic Acid
- ACTH
Adrenocorticotrophic Hormone
- CGD
Chronic Granulomatous Disease
- DHA
Dehydroascorbic Acid
- EAR
Estimated Average Requirements
- FIH
Factor Inhibiting HIF
- GLUT
Glucose Transporter
- GRD
Glutathione Reductase
- GSH
Glutathione
- GSSG
Glutathione Disulfide
- HIF
Hypoxia Inducible Factor
- HPLC
High Performance Liquid Chromatography
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- RDA
Recommended Dietary Allowance
- RNOS
Reactive Nitrogen Oxide Species
- ROS
Reactive Oxygen Species
- SNP
Single Nucleotide Polymorphism
- SVCT
Sodium dependent Vitamin C Transporter
Footnotes
Author Contributions SJ Padayatty composed the initial draft of the manuscript and figures; SJ Padayatty and M Levine reviewed and modified the figures and table, extended the text, and edited the final manuscript.
References
- Aditi A, Graham DY. Vitamin C, gastritis, and gastric disease: a historical review and update. Dig Dis Sci. 2012;57:2504–15. doi: 10.1007/s10620-012-2203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus DB, Gambhir SS, Pardridge WM, Spielholz C, Baselga J, Vera JC, Golde DW. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–8. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir Shaghaghi M, Bernstein CN, Serrano Leon A, El-Gabalawy H, Eck P. Polymorphisms in the sodium-dependent ascorbate transporter gene SLC23A1 are associated with susceptibility to Crohn disease. Am J Clin Nutr. 2014;99:378–83. doi: 10.3945/ajcn.113.068015. [DOI] [PubMed] [Google Scholar]
- Anderson R, Lukey PT. A biological role for ascorbate in the selective neutralization of extracellular phagocyte-derived oxidants. Ann N Y Acad Sci. 1987;498:229–47. doi: 10.1111/j.1749-6632.1987.tb23764.x. [DOI] [PubMed] [Google Scholar]
- Anonymous Ascorbic acid intake and salivary ascorbate levels. Nutr Rev. 1986;44:328–30. doi: 10.1111/j.1753-4887.1986.tb07559.x. [DOI] [PubMed] [Google Scholar]
- Auer BL, Auer D, Rodgers AL. Relative hyperoxaluria, crystalluria and haematuria after megadose ingestion of vitamin C. Eur J Clin Invest. 1998;28:695–700. doi: 10.1046/j.1365-2362.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- Baker EM, Hodges RE, Hood J, Sauberlich HE, March SC. Metabolism of ascorbic-1-14C acid in experimental human scurvy. Am J Clin Nutr. 1969;22:549–58. doi: 10.1093/ajcn/22.5.549. [DOI] [PubMed] [Google Scholar]
- Baker EM, Hodges RE, Hood J, Sauberlich HE, March SC, Canham JE. Metabolism of 14C- and 3H-labeled L-ascorbic acid in human scurvy. Am J Clin Nutr. 1971;24:444–54. doi: 10.1093/ajcn/24.4.444. [DOI] [PubMed] [Google Scholar]
- Balcke P, Schmidt P, Zazgornik J, Kopsa H, Haubenstock A. Ascorbic acid aggravates secondary hyperoxalemia in patients on chronic hemodialysis. Ann Intern Med. 1984;101:344–5. doi: 10.7326/0003-4819-101-3-344. [DOI] [PubMed] [Google Scholar]
- Banhegyi B, Loewus FA. Ascorbic acid catabolism: breakdown pathways in animals and plants. In: Asard H, May J, Smirnoff N, editors. Vitamin C: function and biochemistry in animals and plants. BIOS Scientific Publishers; London: 2004. pp. 31–48. [Google Scholar]
- Barkhan P, Howard AN. Distribution of ascorbic acid in normal and leukaemic human blood. Biochem J. 1958;70:163–8. doi: 10.1042/bj0700163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JF, Hughes RE, Hurley RJ. Ascorbic acid status in man: measurements of salivary, plasma and white blood cell concentration. Arch Oral Biol. 1972;17:1017–20. doi: 10.1016/0003-9969(72)90175-6. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103:1618–23. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- Berger MM, Oudemans-van Straaten HM. Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care. 2015;18:193–201. doi: 10.1097/MCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- Berger TM, Rifai N, Avery ME, Frei B. Vitamin C in premature and full-term human neonates. Redox Rep. 1996;2:257–62. doi: 10.1080/13510002.1996.11747058. [DOI] [PubMed] [Google Scholar]
- Bernardino VR, Mendes-Bastos P, Noronha C, Henriques CC. 2011: the scurvy Odyssey. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-02-2012-5819. doi:10.1136/bcr-02-2012-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuttner GR, Schafer FQ. Ascorbate as an antioxidant. In: Asard H, May J, Smirnoff N, editors. Vitamin C: function and biochemistry in animals and plants. BIOS Scientific Publishers; London: 2004. pp. 173–188. [Google Scholar]
- Bigley RH, Stankova L. Uptake and reduction of oxidized and reduced ascorbate by human leukocytes. J Exp Med. 1974;139:1084–92. doi: 10.1084/jem.139.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J. Depletion and repletion kinetics of vitamin C in humans. J Nutr. 1991a;121:170–6. doi: 10.1093/jn/121.2.170. [DOI] [PubMed] [Google Scholar]
- Blanchard J. Effects of gender on vitamin C pharmacokinetics in man. J Am Coll Nutr. 1991b;10:453–9. doi: 10.1080/07315724.1991.10718171. [DOI] [PubMed] [Google Scholar]
- Blanchard J, Conrad KA, Watson RR, Garry PJ, Crawley JD. Comparison of plasma, mononuclear and polymorphonuclear leucocyte vitamin C levels in young and elderly women during depletion and supplementation. Eur J Clin Nutr. 1989;43:97–106. [PubMed] [Google Scholar]
- Blanchard MS, Romero JM, Hoang MP. Case records of the Massachusetts General Hospital. Case 1-2014. A 32-year-old man with loss of vision and a rash. N Engl J Med. 2014;370:159–66. doi: 10.1056/NEJMcpc1214217. [DOI] [PubMed] [Google Scholar]
- Bonham MJ, Abu-Zidan FM, Simovic MO, Sluis KB, Wilkinson A, Winterbourn CC, Windsor JA. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br J Surg. 1999;86:1296–301. doi: 10.1046/j.1365-2168.1999.01182.x. [DOI] [PubMed] [Google Scholar]
- Borsi E, Terragna C, Brioli A, Tacchetti P, Martello M, Cavo M. Therapeutic targeting of hypoxia and hypoxia-inducible factor 1 alpha in multiple myeloma. Transl Res. 2015;165:641–50. doi: 10.1016/j.trsl.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Bromley J, Hughes BGM, Leong DCS, Buckley NA. Life-threatening interaction between complementary medicines: Cyanide toxicity following ingestion of amygdalin and vitamin C. Ann Pharmacother. 2005;39:1566–1569. doi: 10.1345/aph.1E634. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Buduneli N, Kardesler L, Isik H, Willis CS, 3rd, Hawkins SI, Kinane DF, Scott DA. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33:159–64. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- Burns JJ, Burch HB, King CG. The metabolism of 1-C14-L-ascorbic acid in guinea pigs. J Biol Chem. 1951;191:501–14. [PubMed] [Google Scholar]
- Burzle M, Hediger MA. Functional and physiological role of vitamin C transporters. Curr Top Membr. 2012;70:357–75. doi: 10.1016/B978-0-12-394316-3.00011-9. [DOI] [PubMed] [Google Scholar]
- Butler AM, Cushman M. Distribution of Ascorbic Acid in the Blood and Its Nutritional Significance. J Clin Invest. 1940;19:459–67. doi: 10.1172/JCI101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill LE, El-Sohemy A. Vitamin C transporter gene polymorphisms, dietary vitamin C and serum ascorbic acid. J Nutrigenet Nutrigenomics. 2009;2:292–301. doi: 10.1159/000314597. [DOI] [PubMed] [Google Scholar]
- Canavese C, Petrarulo M, Massarenti P, Berutti S, Fenoglio R, Pauletto D, Lanfranco G, Bergamo D, Sandri L, Marangella M. Long-term, low-dose, intravenous vitamin C leads to plasma calcium oxalate supersaturation in hemodialysis patients. Am. J. Kidney Dis. 2005;45:540–549. doi: 10.1053/j.ajkd.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Carr AC, Bozonet SM, Pullar JM, Simcock JW, Vissers MC. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am J Clin Nutr. 2013;97:800–7. doi: 10.3945/ajcn.112.053207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrina SB. Impaired hypoxia-inducible factor (HIF) regulation by hyperglycemia. J Mol Med (Berl) 2014;92:1025–34. doi: 10.1007/s00109-014-1166-x. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Bucciarelli L, Rondinelli M, Genovese S. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care. 2013;36:4104–8. doi: 10.2337/dc13-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ, Poston L. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354:810–6. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- Chatterjee IB. Evolution and the biosynthesis of ascorbic acid. Science. 1973;182:1271–2. doi: 10.1126/science.182.4118.1271. [DOI] [PubMed] [Google Scholar]
- Chaudhuri CR, Chatterjee IB. L-ascorbic acid synthesis in birds: phylogenetic trend. Science. 1969;164:435–6. doi: 10.1126/science.164.3878.435. [DOI] [PubMed] [Google Scholar]
- Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon Iii RO, Wang Y, Katz A, Levine M, Quon MJ. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol. 2006;290:H137–45. doi: 10.1152/ajpheart.00768.2005. [DOI] [PubMed] [Google Scholar]
- Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shaded E, Levine M. Pharamacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissuse. Proc Natl Acad Sci U S A. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104:8749–54. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clase CM, Ki V, Holden RM. Water-soluble vitamins in people with low glomerular filtration rate or on dialysis: a review. Semin Dial. 2013;26:546–67. doi: 10.1111/di.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemeston CAB. Vitamin C. CRC Press Inc.; Roca Raton, Florida: 1989. Classical scurvy: a historical review. [Google Scholar]
- Corpe CP, Eck P, Wang J, Al-Hasani H, Levine M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J Biol Chem. 2013;288:9092–101. doi: 10.1074/jbc.M112.436790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, Levine M. 6-Bromo-6-deoxy-L-ascorbic acid: An ascorbate analog specific for Na +-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem. 2005;280:5211–5220. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Y, Nussbaum RL, Espey MG, Levine M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120:1069–83. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandon JH, Lund CC, Dill DB. Experimental human scurvy. N Engl J Med. 1940:353–69. [Google Scholar]
- Cueto GR, Allekotte R, Kravetz FO. Scurvy in capybaras bred in captivity in Argentine. J Wildl Dis. 2000;36:97–101. doi: 10.7589/0090-3558-36.1.97. [DOI] [PubMed] [Google Scholar]
- Curnutte JT. Chronic Granulomatous Disease: The Solving of a Clinical Riddle at the Molecular Level. Clin Immunol Immunopathol. 1993;67:S2–15. doi: 10.1006/clin.1993.1078. [DOI] [PubMed] [Google Scholar]
- Dalldorf G, Zall C. Tooth Growth in Experimental Scurvy. J Exp Med. 1930;52:57–64. doi: 10.1084/jem.52.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallongeville J, Marecaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr. 1998;128:1450–7. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- Dann CE, 3rd, Bruick RK, Deisenhofer J. Structure of factor-inhibiting hypoxia-inducible factor 1: An asparaginyl hydroxylase involved in the hypoxic response pathway. Proc Natl Acad Sci U S A. 2002;99:15351–6. doi: 10.1073/pnas.202614999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruwala R, Song J, Koh WS, Rumsey SC, Levine M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999;460:480–4. doi: 10.1016/s0014-5793(99)01393-9. [DOI] [PubMed] [Google Scholar]
- de Jong TM, Jochens A, Jockel-Schneider Y, Harks I, Dommisch H, Graetz C, Flachsbart F, Staufenbiel I, Eberhard J, Folwaczny M, Noack B, Meyle J, Eickholz P, Gieger C, Grallert H, Lieb W, Franke A, Nebel A, Schreiber S, Doerfer C, Jepsen S, Bruckmann C, van der Velden U, Loos BG, Schaefer AS. SLC23A1 polymorphism rs6596473 in the vitamin C transporter SVCT1 is associated with aggressive periodontitis. J Clin Periodontol. 2014;41:531–40. doi: 10.1111/jcpe.12253. [DOI] [PubMed] [Google Scholar]
- de Sousa MC, Vieira RB, Dos Santos DS, Carvalho CA, Camargo SE, Mancini MN, de Oliveira LD. Antioxidants and biomarkers of oxidative damage in the saliva of patients with Down's syndrome. Arch Oral Biol. 2015;60:600–5. doi: 10.1016/j.archoralbio.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Dengler VL, Galbraith MD, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014;49:1–15. doi: 10.3109/10409238.2013.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhariwal KR, Black CD, Levine M. Semidehydroascorbic acid as an intermediate in norepinephrine biosynthesis in chromaffin granules. J Biol Chem. 1991a;266:12908–14. [PubMed] [Google Scholar]
- Dhariwal KR, Hartzell WO, Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr. 1991b;54:712–6. doi: 10.1093/ajcn/54.4.712. [DOI] [PubMed] [Google Scholar]
- Dhariwal KR, Washko P, Hartzell WO, Levine M. Ascorbic acid within chromaffin granules. In situ kinetics of norepinephrine biosynthesis. J Biol Chem. 1989;264:15404–9. [PubMed] [Google Scholar]
- Diab-Ladki R, Pellat B, Chahine R. Decrease in the total antioxidant activity of saliva in patients with periodontal diseases. Clin Oral Investig. 2003;7:103–7. doi: 10.1007/s00784-003-0208-5. [DOI] [PubMed] [Google Scholar]
- Dorchy H. Lower plasma vitamin C levels in young type I diabetic patients with microalbuminuria. J Diabetes Complications. 1999;13:119. doi: 10.1016/s1056-8727(99)00035-5. [DOI] [PubMed] [Google Scholar]
- Dostalova L. Correlation of the vitamin status between mother and newborn during delivery. Dev Pharmacol Ther. 1982;4:45. doi: 10.1159/000457358. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold (Cochrane Review) Cochrane Databse. 2000:CD000980. doi: 10.1002/14651858.CD000980. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, Gokce N, Holbrook M, Hunter LM, Biegelsen ES, Huang A, Keaney JF, Vita JA. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am J Physiol Heart Circ Physiol. 2001;280:H528–34. doi: 10.1152/ajpheart.2001.280.2.H528. [DOI] [PubMed] [Google Scholar]
- Dunn WA, Rettura G, Seifter E, Englard S. Carnitine biosynthesis from gamma-butyrobetaine and from exogenous protein-bound 6-N-trimethyl-L-lysine by the perfused guinea pig liver. Effect of ascorbate deficiency on the in situ activity of gamma-butyrobetaine hydroxylase. J Biol Chem. 1984;259:10764–70. [PubMed] [Google Scholar]
- Eck P, Erichsen HC, Taylor JG, Yeager M, Hughes AL, Levine M, Chanock S. Comparison of the genomic structure and variation in the two human sodium-dependent vitamin C transporters, SLC23A1 and SLC23A2. Hum Genet. 2004;115:285–94. doi: 10.1007/s00439-004-1167-x. [DOI] [PubMed] [Google Scholar]
- Eck P, Kwon O, Chen S, Mian O, Levine M. The human sodium-dependent ascorbic acid transporters SLC23A1 and SLC23A2 do not mediate ascorbic acid release in the proximal renal epithelial cell. Physiol Rep. 2013;1:e00136. doi: 10.1002/phy2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper BA, Mains RE. The role of ascorbate in the biosynthesis of neuroendocrine peptides. Am J Clin Nutr. 1991;54:1153S–1156S. doi: 10.1093/ajcn/54.6.1153s. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu Rev Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986;6:365–406. doi: 10.1146/annurev.nu.06.070186.002053. [DOI] [PubMed] [Google Scholar]
- ENSEMBL 2015a http://uswest.ensembl.org/Homo_sapiens/Gene/Variation_Gene/Table?db=core;g=ENSG00000170482;r=5:139367196-139384553 (accessed on 22 Dec 2015)
- ENSEMBL 2015b http://uswest.ensembl.org/Homo_sapiens/Gene/Variation_Gene/Table?db=core;g=ENSG00000089057;r=20:4852356-5010293 (accessed on 23 Dec 2015)
- Enwonwu CO. Ascorbate status and xerostomia. Med Hypotheses. 1992;39:53–7. doi: 10.1016/0306-9877(92)90140-8. [DOI] [PubMed] [Google Scholar]
- Erichsen HC, Engel SA, Eck PK, Welch R, Yeager M, Levine M, Siega-Riz AM, Olshan AF, Chanock SJ. Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. Am J Epidemiol. 2006;163:245–54. doi: 10.1093/aje/kwj035. Epub 2005 Dec 15. [DOI] [PubMed] [Google Scholar]
- Erichsen HC, Peters U, Eck P, Welch R, Schoen RE, Yeager M, Levine M, Hayes RB, Chanock S. Genetic variation in sodium-dependent vitamin C transporters SLC23A1 and SLC23A2 and risk of advanced colorectal adenoma. Nutr Cancer. 2008;60:652–9. doi: 10.1080/01635580802033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr. 1982;47:473–482. doi: 10.1079/bjn19820059. [DOI] [PubMed] [Google Scholar]
- Feller RP, Black HS, Shannon IL. Evidence for absence of ascorbic acid in human saliva. Arch Oral Biol. 1975;20:563–6. doi: 10.1016/0003-9969(75)90075-8. [DOI] [PubMed] [Google Scholar]
- Ferron-Celma I, Mansilla A, Hassan L, Garcia-Navarro A, Comino AM, Bueno P, Ferron JA. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J Surg Res. 2009;153:224–30. doi: 10.1016/j.jss.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Flashman E, Davies SL, Yeoh KK, Schofield CJ. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem J. 2010;427:135–42. doi: 10.1042/BJ20091609. [DOI] [PubMed] [Google Scholar]
- Fleming PJ, Kent UM. Cytochrome b561, ascorbic acid, and transmembrane electron transfer. Am J Clin Nutr. 1991;54:1173S–1178S. doi: 10.1093/ajcn/54.6.1173s. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board Panel on Dietary Antioxidants and Related Compounds . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Beta Carotene, and other Carotenoids. National Academy Press; Washington DC: 2000a. pp. 186–283. [Google Scholar]
- Food and Nutrition Board Panel on Dietary Antioxidants and Related Compounds . Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. National Academy Press; Washington, D.C.: 2000b. pp. 95–185. [PubMed] [Google Scholar]
- Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:824–34. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- Fowler AA, 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S, Fisher BJ, Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12 doi: 10.1186/1479-5876-12-32. doi10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Gassmann M, Wielockx B. Erythrocytosis: the HIF pathway in control. Blood. 2013;122:1122–8. doi: 10.1182/blood-2013-01-478065. [DOI] [PubMed] [Google Scholar]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–81. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B, Stocker R, England L, Ames BN. Ascorbate: the most effective antioxidant in human blood plasma. Adv Exp Med Biol. 1990;264:155–63. doi: 10.1007/978-1-4684-5730-8_24. [DOI] [PubMed] [Google Scholar]
- Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87:1595–9. [PubMed] [Google Scholar]
- Geesin JC, Darr D, Kaufman R, Murad S, Pinnell SR. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J Invest Dermatol. 1988;90:420–4. doi: 10.1111/1523-1747.ep12460849. [DOI] [PubMed] [Google Scholar]
- Gentili A, Caretti F, D'Ascenzo G, Marchese S, Perret D, Di Corcia D, Rocca LM. Simultaneous determination of water-soluble vitamins in selected food1 matrices by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:2029–43. doi: 10.1002/rcm.3583. [DOI] [PubMed] [Google Scholar]
- Giunta JL. Dental erosion resulting from chewable vitamin C tablets. J Am Dent Assoc. 1983;107:253–6. doi: 10.14219/jada.archive.1983.0239. [DOI] [PubMed] [Google Scholar]
- Glembotski CC. The characterization of the ascorbic acid-mediated alpha-amidation of alpha-melanotropin in cultured intermediate pituitary lobe cells. Endocrinology. 1986;118:1461–8. doi: 10.1210/endo-118-4-1461. [DOI] [PubMed] [Google Scholar]
- Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR, Jr., Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997;14:1133–9. doi: 10.1023/a:1012186203165. [DOI] [PubMed] [Google Scholar]
- Gregory JF. Ascorbic acid bioavailability in foods and supplements. Nutr Rev. 1993;51:301–3. doi: 10.1111/j.1753-4887.1994.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Guarnaccia MM, Takami M, Jones EE, Preston SL, Behrman HR. Luteinizing hormone depletes ascorbic acid in preovulatory follicles. Fertil Steril. 2000;74:959–63. doi: 10.1016/s0015-0282(00)01550-8. [DOI] [PubMed] [Google Scholar]
- Gumus P, Buduneli N, Cetinkalp S, Hawkins SI, Renaud D, Kinane DF, Scott DA. Salivary antioxidants in patients with type 1 or 2 diabetes and inflammatory periodontal disease: a case-control study. J Periodontol. 2009;80:1440–6. doi: 10.1902/jop.2009.090159. [DOI] [PubMed] [Google Scholar]
- Hallberg L, Brune M, Rossander-Hulthen L. Is there a physiological role of vitamin C in iron absorption? Ann N Y Acad Sci. 1987;498:324–32. doi: 10.1111/j.1749-6632.1987.tb23771.x. [DOI] [PubMed] [Google Scholar]
- Handelman GJ. Vitamin C neglect in hemodialysis: sailing between Scylla and Charybdis. Blood Purif. 2007;25:58–61. doi: 10.1159/000096399. [DOI] [PubMed] [Google Scholar]
- Handelman GJ. New insight on vitamin C in patients with chronic kidney disease. J Ren Nutr. 2011;21:110–2. doi: 10.1053/j.jrn.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Harper AE. The recommended dietary allowances for ascorbic acid. Ann N Y Acad Sci. 1975;258:491–7. doi: 10.1111/j.1749-6632.1975.tb29307.x. [DOI] [PubMed] [Google Scholar]
- Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, Stranges S, Hooper L, Rees K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;6:CD009874. doi: 10.1002/14651858.CD009874.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays GL, Bullock Q, Lazzari EP, Puente ES. Salivary pH while dissolving vitamin C-containing tablets. Am J Dent. 1992;5:269–71. [PubMed] [Google Scholar]
- Hellwig JP, Otten JJ, Meyers LD. Dietary Reference Intakes:: The Essential Guide to Nutrient Requirements. National Academies Press; Washington DC: 2006. pp. 19–33. 216-224. [Google Scholar]
- Helser MA, Hotchkiss JH, Roe DA. Influence of fruit and vegetable juices on the endogenous formation of N-nitrosoproline and N-nitrosothiazolidine-4-carboxylic acid in humans on controlled diets. Carcinogenesis. 1992;13:2277–80. doi: 10.1093/carcin/13.12.2277. [DOI] [PubMed] [Google Scholar]
- Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H, Chalker E, Treacy B, Douglas B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2007;3:CD000980. doi: 10.1002/14651858.CD000980.pub3. [DOI] [PubMed] [Google Scholar]
- Hemila H, Roberts P, Wikstrom M. Activated polymorphonuclear leucocytes consume vitamin C. FEBS Lett. 1984;178:25–30. doi: 10.1016/0014-5793(84)81232-6. [DOI] [PubMed] [Google Scholar]
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895–906. doi: 10.1016/s0190-9622(99)70244-6. [DOI] [PubMed] [Google Scholar]
- Hitomi K, Tsukagoshi N. Role of ascorbic acid in modulation of gene expression. Subcell Biochem. 1996;25:41–56. doi: 10.1007/978-1-4613-0325-1_3. [DOI] [PubMed] [Google Scholar]
- Hodges RE, Baker EM, Hood J, Sauberlich HE, March SC. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535–48. doi: 10.1093/ajcn/22.5.535. [DOI] [PubMed] [Google Scholar]
- Hodges RE, Hood J, Canham JE, Sauberlich HE, Baker EM. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432–43. doi: 10.1093/ajcn/24.4.432. [DOI] [PubMed] [Google Scholar]
- Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH., Jr Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19:1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- Hood J, Burns CA, Hodges RE. Sjogren's syndrome in scurvy. N Engl J Med. 1970;282:1120–4. doi: 10.1056/NEJM197005142822003. [DOI] [PubMed] [Google Scholar]
- Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci. 1975;258:103–18. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- Hornig D, Weber F, Wiss O. Tissue distribution of labelled material in vitamin C-deficient guinea pigs after intravenous injection of 1- 14 C) ascorbic acid or (1- 14 C) dehydroascorbic acid. Int J Vitam Nutr Res. 1972;42:511–23. [PubMed] [Google Scholar]
- Hornig D, Weber F, Wiss O. Studies on the distribution of (1-14C) ascorbic acid and (1-14C) dehydroascorbic acid in guinea pigs after oral application. Int J Vitam Nutr Res. 1974;44:217–29. [PubMed] [Google Scholar]
- Horning D, Gallo-Torres HE, Weiser H. Tissue distribution of labelled ascorbic acid in normal and hypophysectomized rats. Int J Vitam Nutr Res. 1972;42:487–96. [PubMed] [Google Scholar]
- Horrobin DF, Campbell A. Sjogren's syndrome and the sicca syndrome: the role of prostaglandin E1 deficiency. Treatment with essential fatty acids and vitamin C. Med Hypotheses. 1980;6:225–32. doi: 10.1016/0306-9877(80)90120-6. [DOI] [PubMed] [Google Scholar]
- Horrobin DF, Campbell A, McEwen CG. Treatment of the Sicca syndrome and Sjogren's syndrome with E.F.A., pyridoxine and vitamin C. Prog Lipid Res. 1981;20:253–4. doi: 10.1016/0163-7827(81)90048-5. [DOI] [PubMed] [Google Scholar]
- Hume R, Weyers E, Rowan T, Reid DS, Hillis WS. Leucocyte ascorbic acid levels after acute myocardial infarction. Br Heart J. 1972;34:238–43. doi: 10.1136/hrt.34.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–84. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- Ichiki T, Sunagawa K. Novel roles of hypoxia response system in glucose metabolism and obesity. Trends Cardiovasc Med. 2014;24:197–201. doi: 10.1016/j.tcm.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Iggo B, Owen JA, Stewart CP. The determination of vitamin C in normal human plasma and erythrocytes. Clin Chim Acta. 1956;1:167–77. doi: 10.1016/0009-8981(56)90032-8. [DOI] [PubMed] [Google Scholar]
- Iheanacho EN, Hunt NH, Stocker R. Vitamin C redox reactions in blood of normal and malaria-infected mice studied with isoascorbate as a nonisotopic marker. Free Radic Biol Med. 1995;18:543–52. doi: 10.1016/0891-5849(94)00182-j. [DOI] [PubMed] [Google Scholar]
- Imfeld T. Dental erosion. Definition, classification and links. Eur J Oral Sci. 1996;104:151–5. doi: 10.1111/j.1600-0722.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- Isanaka S, Mugusi F, Hawkins C, Spiegelman D, Okuma J, Aboud S, Guerino C, Fawzi WW. Effect of high-dose vs standard-dose multivitamin supplementation at the initiation of HAART on HIV disease progression and mortality in Tanzania: a randomized controlled trial. JAMA. 2012;308:1535–44. doi: 10.1001/jama.2012.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto N, Kawaguchi T, Horikawa K, Nagakura S, Hidaka M, Kagimoto T, Takatsuki K, Nakakuma H. Haemolysis induced by ascorbic acid in paroxysmal nocturnal haemoglobinuria. Lancet. 1994a;343:357. doi: 10.1016/s0140-6736(94)91195-9. [DOI] [PubMed] [Google Scholar]
- Iwamoto N, Nakakuma H, Ota N, Shimokado H, Takatsuki K. Ascorbic acid-induced hemolysis of paroxysmal nocturnal hemoglobinuria erythrocytes. Am J Hematol. 1994b;47:337–8. doi: 10.1002/ajh.2830470426. [DOI] [PubMed] [Google Scholar]
- Jackson TS, Xu A, Vita JA, Keaney JF., Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–22. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- Jacob RA, Skala JH, Omaye ST. Biochemical indices of human vitamin C status. Am J Clin Nutr. 1987;46:818–26. doi: 10.1093/ajcn/46.5.818. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Johnson RM, Goyette G, Jr., Ravindranath Y, Ho YS. Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic Biol Med. 2005;39:1407–17. doi: 10.1016/j.freeradbiomed.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Johnstone WM, Drake TGH, Tisdall FF, Harvie FH. Ascorbic Acid Metabolism of Healthy Young Canadians. CMAJ. 1946;55:581–5. [PubMed] [Google Scholar]
- Jorde LB, Watkins WS, Bamshad MJ, Dixon ME, Ricker CE, Seielstad MT, Batzer MA. The distribution of human genetic diversity: a comparison of mitochondrial, autosomal, and Y-chromosome data. Am J Hum Genet. 2000;66:979–88. doi: 10.1086/302825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek M, Timofeeva OA, Karaczyn A, Malyguine A, Kasprzak KS, Salnikow K. The role of ascorbate in the modulation of HIF-1alpha protein and HIF-dependent transcription by chromium(VI) and nickel(II) Free Radic Biol Med. 2007;42:1246–57. doi: 10.1016/j.freeradbiomed.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallner A, Hartmann D, Hornig D. Steady-state turnover and body pool of ascorbic acid in man. Am J Clin Nutr. 1979;32:530–9. doi: 10.1093/ajcn/32.3.530. [DOI] [PubMed] [Google Scholar]
- Kallner AB, Hartmann D, Hornig DH. On the requirements of ascorbic acid in man: steady-state turnover and body pool in smokers. Am J Clin Nutr. 1981;34:1347–55. doi: 10.1093/ajcn/34.7.1347. [DOI] [PubMed] [Google Scholar]
- Kassan RJ, Roe JH. The preservation of ascorbic acid in drawn samples of blood. J Biol Chem. 1940;133:579–584. [Google Scholar]
- Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schafers KP, Luscher TF, Camici PG. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–8. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schafers KP, Luscher TF, Camici PG. Vitamin C and Coronary Microcirculation. Circulation. 2001;103:E117. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- Kaviarasan K, Arjunan MM, Pugalendi KV. Lipid profile, oxidant-antioxidant status and glycoprotein components in hyperlipidemic patients with/without diabetes. Clin Chim Acta. 2005;362:49–56. doi: 10.1016/j.cccn.2005.05.010. [DOI] [PubMed] [Google Scholar]
- King CG, Waugh WA. The chemical nature of vitamin C. Science. 1932;75:357. doi: 10.1126/science.75.1944.357-a. [DOI] [PubMed] [Google Scholar]
- King J, Wang Y, Welch RW, Dhariwal KR, Conry-Cantilena C, Levine M. Use of a new vitamin C-deficient diet in a depletion-repletion clinical trial. Am J Clin Nutr. 1997;65:1434–1440. doi: 10.1093/ajcn/65.5.1434. [DOI] [PubMed] [Google Scholar]
- Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18:281–8. doi: 10.1016/8756-3282(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Kivirikko KI, Myllyla R. Post-translational processing of procollagens. Ann N Y Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–8. [PubMed] [Google Scholar]
- Koba H, Kawao K, Yamashita K. Effect of human chorionic gonadotrophin on the discharge of ascorbic acid from the canine testis. Tohoku J Exp Med. 1971;104:65–71. doi: 10.1620/tjem.104.65. [DOI] [PubMed] [Google Scholar]
- Koliakos GG, Konstas AGP, Schlötzer-Schrehardt U, Bufidis T, Georgiadis N, Ringvold A. Ascorbic acid concentration is reduced in the aqueous humor of patients with exfoliation syndrome. Am J Ophthalmol. 2002;134:879–883. doi: 10.1016/s0002-9394(02)01797-x. [DOI] [PubMed] [Google Scholar]
- Kuiper C, Dachs GU, Currie MJ, Vissers MC. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic Biol Med. 2014;69:308–17. doi: 10.1016/j.freeradbiomed.2014.01.033. [DOI] [PubMed] [Google Scholar]
- Kumar D, Mains RE, Eipper E. 60 YEARS OF POMC: From POMC and alphaMSH to PAM, molecular oxygen, copper and vitamin C. J Mol Endocrinol. 2015 doi: 10.1530/JME-15-0266. doi:10.1530/jme-15-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari M, Rapola J. Reversible pulmonary hypertension associated with vitamin C deficiency. Chest. 2012;142:225–7. doi: 10.1378/chest.11-1857. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–6. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- Leggott PJ, Robertson PB, Rothman DL, Murray PA, Jacob RA. The effect of controlled ascorbic acid depletion and supplementation on periodontal health. J Periodontol. 1986a;57:480–5. doi: 10.1902/jop.1986.57.8.480. [DOI] [PubMed] [Google Scholar]
- Leggott PJ, Robertson PB, Rothman DL, Murray PA, Jacob RA. Response of lingual ascorbic acid test and salivary ascorbate levels to changes in ascorbic acid intake. J Dent Res. 1986b;65:131–4. doi: 10.1177/00220345860650020801. [DOI] [PubMed] [Google Scholar]
- Leveque N, Mary S, Humbert P, Makki S, Muret P, Kantelip J-P. Ascorbic acid assessment in human dermis by a microdialysis technique associated with gas chromatography-mass spectrometry. Eur J Mass Spectrom. 2000;6:397–404. [Google Scholar]
- Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr., Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996a;93:1107–13. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996b;93:3704–9. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Dhariwal KR, Washko PW, Butler JD, Welch RW, Wang YH, Bergsten P. Ascorbic acid and in situ kinetics: a new approach to vitamin requirements. Am J Clin Nutr. 1991;54:1157S–1162S. doi: 10.1093/ajcn/54.6.1157s. [DOI] [PubMed] [Google Scholar]
- Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2:78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Padayatty SJ, Wang Y, Corpe C, Lee L, Wang J, Chen Q, Zhang L. Vitamin C. In: Stipanuk M, Caudill M, editors. Biochemical; Physiological; and Molecular Aspects of Human Nutrition. W B Saunders; St. Louis, MO, USA: 2006. pp. 760–796. [Google Scholar]
- Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999a;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- Levine M, Wang Y, Katz A, Eck P, Kwon O, Chen S, Lee JH, Padayatty SJ. Ideal vitamin C intake. Biofactors. 2001a;15:71–4. doi: 10.1002/biof.5520150203. [DOI] [PubMed] [Google Scholar]
- Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001b;98:9842–6. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Wang Y, Rumsey SC. Analysis of ascorbic acid and dehydroascorbic acid in biological samples. Methods Enzymol. 1999b;299:65–76. doi: 10.1016/s0076-6879(99)99009-2. [DOI] [PubMed] [Google Scholar]
- Levine SZ, Gordon HH, Marples E. A Defect in the Metabolism of Tyrosine and Phenylalanine in Premature Infants. Ii. Spontaneous Occurrence and Eradication by Vitamin C. J Clin Invest. 1941a;20:209–19. doi: 10.1172/JCI101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SZ, Marples E, Gordon HH. A Defect in the Metabolism of Tyrosine and Phenylalanine in Premature Infants. I. Identification and Assay of Intermediary Products. J Clin Invest. 1941b;20:199–207. doi: 10.1172/JCI101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin S. Vitamin C: Its Molecular Biology and Medical Potential. Academic Press; London: 1976. [Google Scholar]
- Li H, Tu H, Wang Y, Levine M. Vitamin C in mouse and human red blood cells: an HPLC assay. Anal Biochem. 2012;426:109–17. doi: 10.1016/j.ab.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J. The diagnostics, or signs. In: Stewart CP, Guthrie D, editors. Lind's Treatise on Scurvy. Edinburgh University Press; Edinburgh: 1953a. pp. 113–132. [Google Scholar]
- Lind J. In: Lind's Treatise on Scurvy. Stewart CP, Guthrie D, editors. Edinburgh University Press; Edinburgh: 1953b. [Google Scholar]
- Lindblad B, Lindstedt G, Lindstedt S. The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J Am Chem Soc. 1970;92:7446–9. doi: 10.1021/ja00728a032. [DOI] [PubMed] [Google Scholar]
- Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- Liskmann S, Vihalemm T, Salum O, Zilmer K, Fischer K, Zilmer M. Characterization of the antioxidant profile of human saliva in peri-implant health and disease. Clin Oral Implants Res. 2007;18:27–33. doi: 10.1111/j.1600-0501.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- Lubschez R. Studies in Ascorbic Acid with Especial Reference to the White Layer. I. Description of Method and Comparison of Ascorbic Acid Levels in Whole Blood, Plasma, Red Cells, and White Layer. J Clin Invest. 1945;24:573–8. doi: 10.1172/JCI101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000;71:530–6. doi: 10.1093/ajcn/71.2.530. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr. 2010;103:1251–9. doi: 10.1017/S0007114509993229. [DOI] [PubMed] [Google Scholar]
- Makila E. Salivary vitamins. Int Z Vitaminforsch. 1968;38:260–9. [PubMed] [Google Scholar]
- Makila E, Kirveskari P. A study of ascorbic acid in human saliva. Arch Oral Biol. 1969;14:1285–92. doi: 10.1016/0003-9969(69)90201-5. [DOI] [PubMed] [Google Scholar]
- Mangels AR, Block G, Frey CM, Patterson BH, Taylor PR, Norkus EP, Levander OA. The bioavailability to humans of ascorbic acid from oranges, orange juice and cooked broccoli is similar to that of synthetic ascorbic acid. J Nutr. 1993;123:1054–61. doi: 10.1093/jn/123.6.1054. [DOI] [PubMed] [Google Scholar]
- Mann GV, Newton P. The membrane transport of ascorbic acid. Ann N Y Acad Sci. 1975;258:243–52. doi: 10.1111/j.1749-6632.1975.tb29285.x. [DOI] [PubMed] [Google Scholar]
- Mashour S, Turner JF, Jr., Merrell R. Acute renal failure, oxalosis, and vitamin C supplementation: a case report and review of the literature. Chest. 2000;118:561–3. doi: 10.1378/chest.118.2.561. [DOI] [PubMed] [Google Scholar]
- May JM. Famine From Feast: Low Red Cell Vitamin C Levels in Diabetes. EBioMedicine. 2015 doi: 10.1016/j.ebiom.2015.10.023. doi:10.1016/j.ebiom.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JM, Qu Z, Cobb CE. Extracellular reduction of the ascorbate free radical by human erythrocytes. Biochem Biophys Res Commun. 2000;267:118–23. doi: 10.1006/bbrc.1999.1906. [DOI] [PubMed] [Google Scholar]
- May JM, Qu Z, Cobb CE. Recycling of the ascorbate free radical by human erythrocyte membranes. Free Radic Biol Med. 2001;31:117–24. doi: 10.1016/s0891-5849(01)00566-4. [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC, Cobb CE. Human erythrocyte recycling of ascorbic acid: relative contributions from the ascorbate free radical and dehydroascorbic acid. J Biol Chem. 2004;279:14975–82. doi: 10.1074/jbc.M312548200. [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC, Whitesell RR, Cobb CE. Ascorbate recycling in human erythrocytes: role of GSH in reducing dehydroascorbate. Free Radic Biol Med. 1996;20:543–51. doi: 10.1016/0891-5849(95)02130-2. [DOI] [PubMed] [Google Scholar]
- McKendry RJ. Treatment of Sjogren's syndrome with essential fatty acids, pyridoxine and vitamin C. Prostaglandins Leukot Med. 1982;8:403–8. doi: 10.1016/0262-1746(82)90064-6. [DOI] [PubMed] [Google Scholar]
- Mehta JB, Singhal SB, Mehta BC. Ascorbic-acid-induced haemolysis in G-6-PD deficiency. Lancet. 1990;336:944. doi: 10.1016/0140-6736(90)92317-b. [DOI] [PubMed] [Google Scholar]
- Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med. 1998;24:789–97. doi: 10.1016/s0891-5849(97)00351-1. [DOI] [PubMed] [Google Scholar]
- Meurman JH, Murtomaa H. Effect of effervescent vitamin C preparations on bovine teeth and on some clinical and salivary parameters in man. Eur J Oral Sci. 1986;94:491–499. doi: 10.1111/j.1600-0722.1986.tb01791.x. [DOI] [PubMed] [Google Scholar]
- Michels AJ, Hagen TM, Frei B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu Rev Nutr. 2013;33:45–70. doi: 10.1146/annurev-nutr-071812-161246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–48. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- Montero D, Tachibana C, Rahr Winther J, Appenzeller-Herzog C. Intracellular glutathione pools are heterogeneously concentrated. Redox Biol. 2013;1:508–13. doi: 10.1016/j.redox.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radic Res. 1994;21:417–25. doi: 10.3109/10715769409056594. [DOI] [PubMed] [Google Scholar]
- Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305:1348–9. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Yasue H. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: effect of vitamin C. Am J Physiol. 1997;273:H1644–50. doi: 10.1152/ajpheart.1997.273.4.H1644. [DOI] [PubMed] [Google Scholar]
- Musicki B, Kodaman PH, Aten RF, Behrman HR. Endocrine regulation of ascorbic acid transport and secretion in luteal cells. Biol Reprod. 1996;54:399–406. doi: 10.1095/biolreprod54.2.399. [DOI] [PubMed] [Google Scholar]
- Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003;22:15–24. doi: 10.1016/s0945-053x(03)00006-4. [DOI] [PubMed] [Google Scholar]
- Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med. 2008;40:402–417. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]
- Nakamoto Y, Motohashi S, Kasahara H, Numazawa K. Case report. Irreversible tubulointerstitial nephropathy associated with prolonged, massive intake of vitamin C. Nephrol Dial Transplant. 1998;13:754–756. doi: 10.1093/ndt/13.3.754. [DOI] [PubMed] [Google Scholar]
- Nasr SH, Kashtanova Y, Levchuk V, Markowitz GS. Secondary oxalosis due to excess vitamin C intake. Kidney Int. 2006;70:1672–1672. doi: 10.1038/sj.ki.5001724. [DOI] [PubMed] [Google Scholar]
- NCBI d, NCBI, SLC23A1 2015a http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=9963 (accessed on 22 Dec 2015)
- NCBI d, NCBI, SLC23A2 2015b http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=9962 (accessed on 23 Dec 2015)
- Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, Vera JC. Recycling of vitamin C by a bystander effect. J Biol Chem. 2003;278:10128–33. doi: 10.1074/jbc.M210686200. [DOI] [PubMed] [Google Scholar]
- Okamura M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin Chim Acta. 1980;103:259–68. doi: 10.1016/0009-8981(80)90144-8. [DOI] [PubMed] [Google Scholar]
- Omaye ST, Schaus EE, Kutnink MA, Hawkes WC. Measurement of vitamin C in blood components by high-performance liquid chromatography. Implication in assessing vitamin C status. Ann N Y Acad Sci. 1987;498:389–401. doi: 10.1111/j.1749-6632.1987.tb23776.x. [DOI] [PubMed] [Google Scholar]
- Ono K. Secondary hyperoxalemia caused by vitamin C supplementation in regular hemodialysis patients. Clin Nephrol. 1986;26:239–43. [PubMed] [Google Scholar]
- Padayatty SJ, Doppman JL, Chang R, Wang Y, Gill J, Papanicolaou DA, Levine M. Human adrenal glands secrete vitamin C in response to adrenocorticotrophic hormone. Am J Clin Nutr. 2007;86:145–9. doi: 10.1093/ajcn/86.1.145. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Vitamin C and myocardial infarction: the heart of the matter. Am J Clin Nutr. 2000;71:1027–8. doi: 10.1093/ajcn/71.5.1027. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. CMAJ. 2001a;164:353–5. [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Vitamin C and coronary microcirculation. Circulation. 2001b;103:E117. doi: 10.1161/01.cir.103.23.e117. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Vitamins C and E and the prevention of preeclampsia. N Engl J Med. 2006;355:1065. doi: 10.1056/NEJMc061414. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Antioxidant supplements and cardiovascular disease in men. JAMA. 2009;301:1336. doi: 10.1001/jama.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Standard-dose vs high-dose multivitamin supplements for HIV. JAMA. 2013;309:545–6. doi: 10.1001/jama.2012.216991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer. Ann Intern Med. 2014;160:654. doi: 10.7326/L14-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5:e11414. doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533–7. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- Parrow NL, Leshin JA, Levine M. Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Antioxid Redox Signal. 2013;19:2141–56. doi: 10.1089/ars.2013.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons KK, Maeda N, Yamauchi M, Banes AJ, Koller BH. Ascorbic acid-independent synthesis of collagen in mice. Am J Physiol Endocrinol Metab. 2006;290:E1131–9. doi: 10.1152/ajpendo.00339.2005. [DOI] [PubMed] [Google Scholar]
- Pekkala M, Hieta R, Kursula P, Kivirikko KI, Wierenga RK, Myllyharju J. Crystallization of the proline-rich-peptide binding domain of human type I collagen prolyl 4-hydroxylase. Acta Crystallogr D Biol Crystallogr. 2003;59:940–2. doi: 10.1107/s0907444903005420. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S–1140S. doi: 10.1093/ajcn/54.6.1135s. [DOI] [PubMed] [Google Scholar]
- Peters R, Barnes AE, Bartley W, Frankau IM, Higgins GA, Pemberton J, Roberts GL, Vickers HR, Krebs HA. Vitamin C Requirement of Human Adults. A Report by the Vitamin C Sub-committee of the Accessory Food Factors Committee. HM Stationary Office; London: 1953. [Google Scholar]
- Petroff BK, Ciereszko RE, Dabrowski K, Ottobre AC, Pope WF, Ottobre JS. Depletion of vitamin C from pig corpora lutea by prostaglandin F2 alpha-induced secretion of the vitamin. J Reprod Fertil. 1998;112:243–7. doi: 10.1530/jrf.0.1120243. [DOI] [PubMed] [Google Scholar]
- Piotrovskij VK, Kallay Z, Gajdos M, Gerykova M, Trnovec T. The use of a nonlinear absorption model in the study of ascorbic acid bioavailability in man. Biopharm Drug Dispos. 1993;14:429–42. doi: 10.1002/bdd.2510140509. [DOI] [PubMed] [Google Scholar]
- Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Mecocci P, Levine M, Frei B. Short-term and long-term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation. Arch Biochem Biophys. 2004;423:109–15. doi: 10.1016/j.abb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Kolhekar AS, Eipper BA, Mains RE, Amzel LM. Substrate-mediated electron transfer in peptidylglycine alpha-hydroxylating monooxygenase. Nat Struct Biol. 1999;6:976–83. doi: 10.1038/13351. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol Life Sci. 2000;57:1236–59. doi: 10.1007/PL00000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Pru C, Eaton J, Kjellstrand C. Vitamin C intoxication and hyperoxalemia in chronic hemodialysis patients. Nephron. 1985;39:112–6. doi: 10.1159/000183353. [DOI] [PubMed] [Google Scholar]
- Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: an electron paramagnetic resonance spin trapping study. Free Radic Biol Med. 1999;26:1447–1456. doi: 10.1016/s0891-5849(99)00002-7. [DOI] [PubMed] [Google Scholar]
- Qiao H, May JM. Regulation of the human ascorbate transporter SVCT2 exon 1b gene by zinc-finger transcription factors. Free Radic Biol Med. 2011;50:1196–209. doi: 10.1016/j.freeradbiomed.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai B, Kaur J, Catalina M, Anand SC, Jacobs R, Teughels W. Effect of simulated microgravity on salivary and serum oxidants, antioxidants, and periodontal status. J Periodontol. 2011;82:1478–82. doi: 10.1902/jop.2011.100711. [DOI] [PubMed] [Google Scholar]
- Rai B, Kharb S, Jain R, Anand SC. Salivary vitamins E and C in oral cancer. Redox Rep. 2007;12:163–4. doi: 10.1179/135100007X200245. [DOI] [PubMed] [Google Scholar]
- Rathbone BJ, Johnson AW, Wyatt JI, Kelleher J, Heatley RV, Losowsky MS. Ascorbic acid: a factor concentrated in human gastric juice. Clin Sci. 1989;76:237–41. doi: 10.1042/cs0760237. [DOI] [PubMed] [Google Scholar]
- Rathi S, Kern W, Lau K. Vitamin C-induced hyperoxaluria causing reversible tubulointerstitial nephritis and chronic renal failure: a case report. J Med Case Rep. 2007;1:155. doi: 10.1186/1752-1947-1-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebouche CJ. Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr. 1991a;54:1147S–1152S. doi: 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ. Quantitative estimation of absorption and degradation of a carnitine supplement by human adults. Metabolism. 1991b;40:1305–10. doi: 10.1016/0026-0495(91)90033-s. [DOI] [PubMed] [Google Scholar]
- Reed FA, Tishkoff SA. African human diversity, origins and migrations. Curr Opin Genet Dev. 2006;16:597–605. doi: 10.1016/j.gde.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Richards JC, Crecelius AR, Larson DG, Dinenno FA. Acute ascorbic acid ingestion increases skeletal muscle blood flow and oxygen consumption via local vasodilation during graded handgrip exercise in older adults. Am J Physiol Heart Circ Physiol. 2015;309:H360–8. doi: 10.1152/ajpheart.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma RA, Carruthers KF, Elton RA, Fox KA. Vitamin C and the risk of acute myocardial infarction. Am J Clin Nutr. 2000;71:1181–6. doi: 10.1093/ajcn/71.5.1181. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr., Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–91. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D, de Boer M, Kuribayashi F, Meischl C, Weening RS, Segal AW, Ahlin A, Nemet K, Hossle JP, Bernatowska-Matuszkiewicz E, Middleton-Price H. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–81. [PubMed] [Google Scholar]
- Rossi AC, Mullin PM. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;158:9–16. doi: 10.1016/j.ejogrb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2015;9:CD004072. doi: 10.1002/14651858.CD004072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- Rumsey SC, Daruwala R, Al-Hasani H, Zarnowski MJ, Simpson IA, Levine M. Dehydroascorbic acid transport by GLUT4 xenopus oocytes and isolated rat adipocytes. J Biol Chem. 2000a;275:28246–28253. doi: 10.1074/jbc.M000988200. [DOI] [PubMed] [Google Scholar]
- Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997;272:18982–9. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- Rumsey SC, Levine M. Absorption,transport, and disposition of ascorbic acid in humans. J Nutr Biochem. 1998;9:116–130. [Google Scholar]
- Rumsey SC, Wang Y, M. L. Ascorbic acid and dehydroascorbic acid analyses in biological samples. In: Song WO, Beecher GR, Eitenmiller RR, editors. Modern Analytical Methodologies on Fat and Water Soluble Vitamins. John Wiley and Sons; New York: 2000b. pp. 411–455. [Google Scholar]
- Rumsey SC, Zarnowski M, Simpson IA, Levine M. DHA transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. FASEB Journal. 1998;12:2667. doi: 10.1074/jbc.M000988200. [DOI] [PubMed] [Google Scholar]
- Saral Y, Coskun BK, Ozturk P, Karatas F, Ayar A. Assessment of salivary and serum antioxidant vitamins and lipid peroxidation in patients with recurrent aphthous ulceration. Tohoku J Exp Med. 2005;206:305–312. doi: 10.1620/tjem.206.305. [DOI] [PubMed] [Google Scholar]
- Sargeant LA, Wareham NJ, Bingham S, Day NE, Luben RN, Oakes S, Welch A, Khaw KT. Vitamin C and hyperglycemia in the European Prospective Investigation into Cancer--Norfolk (EPIC-Norfolk) study: a population-based study. Diabetes Care. 2000;23:726–32. doi: 10.2337/diacare.23.6.726. [DOI] [PubMed] [Google Scholar]
- Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347–55. doi: 10.1007/s00726-007-0555-7. [DOI] [PubMed] [Google Scholar]
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158–62. doi: 10.2105/ajph.79.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schectman G, Byrd JC, Hoffmann R. Ascorbic acid requirements for smokers: analysis of a population survey. Am J Clin Nutr. 1991;53:1466–70. doi: 10.1093/ajcn/53.6.1466. [DOI] [PubMed] [Google Scholar]
- Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES) Am J Clin Nutr. 2009;90:1252–63. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- Schock BC, Koostra J, Kwack S, Hackman RM, Van Der Vliet A, Cross CE. Ascorbic acid in nasal and tracheobronchial airway lining fluids. Free Radic Biol Med. 2004;37:1393–401. doi: 10.1016/j.freeradbiomed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Scott P, Bruce C, Schofield D, Shiel N, Braganza JM, McCloy RF. Vitamin C status in patients with acute pancreatitis. Br J Surg. 1993;80:750–4. doi: 10.1002/bjs.1800800632. [DOI] [PubMed] [Google Scholar]
- Seghieri G, Martinoli L, Miceli M, Ciuti M, D'Alessandri G, Gironi A, Palmieri L, Anichini R, Bartolomei G, Franconi F. Renal excretion of ascorbic acid in insulin dependent diabetes mellitus. Int J Vitam Nutr Res. 1994;64:119–24. [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–47. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilkumari S, Talwar B, Dharmalingam K, Ravindran RD, Jayanthi R, Sundaresan P, Saravanan C, Young IS, Dangour AD, Fletcher AE. Polymorphisms in sodium-dependent vitamin C transporter genes and plasma, aqueous humor and lens nucleus ascorbate concentrations in an ascorbate depleted setting. Exp Eye Res. 2014;124:24–30. doi: 10.1016/j.exer.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JAE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaghaghi MA, Kloss O, Eck P. Genetic Variation in Human Vitamin C Transporter Genes in Common Complex Diseases. Adv Nutr. 2016;7:1–12. doi: 10.3945/an.115.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry BS. SNPs: impact on gene function and phenotype. Methods Mol Biol. 2009;578:3–22. doi: 10.1007/978-1-60327-411-1_1. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Laurie SS. HIF and pulmonary vascular responses to hypoxia. J Appl Physiol (1985) 2014;116:867–74. doi: 10.1152/japplphysiol.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–6. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AJ, Taylor PB, Lunec J, Girling AJ, Barnett AH. Low plasma ascorbate levels in patients with type 2 diabetes mellitus consuming adequate dietary vitamin C. Diabet Med. 1994;11:893–8. doi: 10.1111/j.1464-5491.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA. Biosynthesis of Ascorbic Acid in Plants: A Renaissance. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:437–467. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- Smith JL, Hodges RE. Serum levels of vitamin C in relation to dietary and supplemental intake of vitamin C in smokers and nonsmokers. Ann N Y Acad Sci. 1987;498:144–52. doi: 10.1111/j.1749-6632.1987.tb23758.x. [DOI] [PubMed] [Google Scholar]
- Sobala GM, Schorah CJ, Sanderson M, Dixon MF, Tompkins DS, Godwin P, Axon AT. Ascorbic acid in the human stomach. Gastroenterology. 1989;97:357–63. doi: 10.1016/0016-5085(89)90071-1. [DOI] [PubMed] [Google Scholar]
- Som S, Basu S, Mukherjee D, Deb S, Choudhury PR, Mukherjee S, Chatterjee SN, Chatterjee IB. Ascorbic acid metabolism in diabetes mellitus. Metabolism. 1981;30:572–7. doi: 10.1016/0026-0495(81)90133-5. [DOI] [PubMed] [Google Scholar]
- Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, Nussbaum RL. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–7. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- Sram RJ, Binkova B, Rossner P., Jr. Vitamin C for DNA damage prevention. Mutat Res. 2012;733:39–49. doi: 10.1016/j.mrfmmm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Stankova L, Riddle M, Larned J, Burry K, Menashe D, Hart J, Bigley R. Plasma ascorbate concentrations and blood cell dehydroascorbate transport in patients with diabetes mellitus. Metabolism. 1984;33:347–53. doi: 10.1016/0026-0495(84)90197-5. [DOI] [PubMed] [Google Scholar]
- Stankova L, Rigas DA, Bigley RH. Dehydroascorbate uptake and reduction by human blood neutrophils, erythrocytes, and lymphocytes. Ann N Y Acad Sci. 1975;258:238–42. doi: 10.1111/j.1749-6632.1975.tb29284.x. [DOI] [PubMed] [Google Scholar]
- Stewart LC, Klinman JP. Dopamine beta-hydroxylase of adrenal chromaffin granules: structure and function. Annu Rev Biochem. 1988;57:551–92. doi: 10.1146/annurev.bi.57.070188.003003. [DOI] [PubMed] [Google Scholar]
- Stubbe J. Identification of two alpha-ketoglutarate-dependent dioxygenases in extracts of Rhodotorula glutinis catalyzing deoxyuridine hydroxylation. J Biol Chem. 1985;260:9972–5. [PubMed] [Google Scholar]
- Su D, May JM, Koury MJ, Asard H. Human erythrocyte membranes contain a cytochrome b561 that may be involved in extracellular ascorbate recycling. J Biol Chem. 2006;281:39852–9. doi: 10.1074/jbc.M606543200. [DOI] [PubMed] [Google Scholar]
- Sullivan JF, Eisenstein AB. Ascorbic acid depletion in patients undergoing chronic hemodialysis. Am J Clin Nutr. 1970;23:1339–46. doi: 10.1093/ajcn/23.10.1339. [DOI] [PubMed] [Google Scholar]
- Sullivan TA, Uschmann B, Hough R, Leboy PS. Ascorbate modulation of chondrocyte gene expression is independent of its role in collagen secretion. J Biol Chem. 1994;269:22500–6. [PubMed] [Google Scholar]
- Svirbely J, Szent-Gyorgyi A. The chemical nature of vitamin C. Biochem J. 1932;26:865–870. doi: 10.1042/bj0260865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szultka M, Buszewska-Forajta M, Kaliszan R, Buszewski B. Determination of ascorbic acid and its degradation products by high-performance liquid chromatography-triple quadrupole mass spectrometry. Electrophoresis. 2014;35:585–92. doi: 10.1002/elps.201300439. [DOI] [PubMed] [Google Scholar]
- Taabazuing CY, Hangasky JA, Knapp MJ. Oxygen sensing strategies in mammals and bacteria. J Inorg Biochem. 2014;133:63–72. doi: 10.1016/j.jinorgbio.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot NP, Croft QP, Curtis MK, Turner BE, Dorrington KL, Robbins PA, Smith TG. Contrasting effects of ascorbate and iron on the pulmonary vascular response to hypoxia in humans. Physiol Rep. 2014;2:e12220. doi: 10.14814/phy2.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Jacques PF, Nowell T, Perrone G, Blumberg J, Handelman G, Jozwiak B, Nadler D. Vitamin C in human and guinea pig aqueous, lens and plasma in relation to intake. Curr Eye Res. 1997;16:857–64. doi: 10.1076/ceyr.16.9.857.5039. [DOI] [PubMed] [Google Scholar]
- Thoma WJ, Henderson LM. Effect of vitamin C deficiency on hydroxylation of trimethylaminobutyrate to carnitine in the guinea pig. Biochim Biophys Acta. 1984;797:136–9. doi: 10.1016/0304-4165(84)90392-1. [DOI] [PubMed] [Google Scholar]
- Thrasher AJ, Keep NH, Wientjes F, Segal AW. Chronic granulomatous disease. Biochim Biophys Acta. 1994;1227:1–24. doi: 10.1016/0925-4439(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Timpson NJ, Forouhi NG, Brion MJ, Harbord RM, Cook DG, Johnson P, McConnachie A, Morris RW, Rodriguez S, Luan J, Ebrahim S, Padmanabhan S, Watt G, Bruckdorfer KR, Wareham NJ, Whincup PH, Chanock S, Sattar N, Lawlor DA, Davey Smith G. Genetic variation at the SLC23A1 locus is associated with circulating concentrations of L-ascorbic acid (vitamin C): evidence from 5 independent studies with >15,000 participants. Am J Clin Nutr. 2010;92:375–82. doi: 10.3945/ajcn.2010.29438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff SA, Verrelli BC. Patterns of human genetic diversity: implications for human evolutionary history and disease. Annu Rev Genomics Hum Genet. 2003;4:293–340. doi: 10.1146/annurev.genom.4.070802.110226. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Williams SM. Genetic analysis of African populations: human evolution and complex disease. Nat Rev Genet. 2002;3:611–21. doi: 10.1038/nrg865. [DOI] [PubMed] [Google Scholar]
- Toth I, Rogers JT, McPhee JA, Elliott SM, Abramson SL, Bridges KR. Ascorbic acid enhances iron-induced ferritin translation in human leukemia and hepatoma cells. J Biol Chem. 1995;270:2846–52. doi: 10.1074/jbc.270.6.2846. [DOI] [PubMed] [Google Scholar]
- Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H, Bates CJ. Comparison of vitamin C deficiency with food restriction on collagen cross-link ratios in bone, urine and skin of weanling guinea-pigs. Br J Nutr. 2003;89:303–10. doi: 10.1079/BJN2002775. [DOI] [PubMed] [Google Scholar]
- Tsukada Y, Zhang Y. Purification of histone demethylases from HeLa cells. Methods. 2006;40:318–26. doi: 10.1016/j.ymeth.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–5. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- Tu H, Li H, Wang Y, Niyyati M, Wang Y, Leshin J, Levine M. Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid. EBioMedicine. 2015 doi: 10.1016/j.ebiom.2015.09.049. doi: 10.1016/j.ebiom.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaananen MK, Markkanen HA, Tuovinen VJ, Kullaa AM, Karinpaa AM, Luoma H, Kumpusalo EA. Dental caries and mutans streptococci in relation to plasma ascorbic acid. Scand J Dent Res. 1994;102:103–8. doi: 10.1111/j.1600-0722.1994.tb01163.x. [DOI] [PubMed] [Google Scholar]
- VanDuijn MM, Tijssen K, VanSteveninck J, Van Den Broek PJ, Van Der Zee J. Erythrocytes reduce extracellular ascorbate free radicals using intracellular ascorbate as an electron donor. J Biol Chem. 2000;275:27720–5. doi: 10.1074/jbc.M910281199. [DOI] [PubMed] [Google Scholar]
- Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- Vinson JA, Bose P. Comparative bioavailability to humans of ascorbic acid alone or in a citrus extract. Am J Clin Nutr. 1988;48:601–4. doi: 10.1093/ajcn/48.3.601. [DOI] [PubMed] [Google Scholar]
- Vissers MC, Gunningham SP, Morrison MJ, Dachs GU, Currie MJ. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic Biol Med. 2007;42:765–72. doi: 10.1016/j.freeradbiomed.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Vita JA, Frei B, Holbrook M, Gokce N, Leaf C, Keaney JF., Jr. L-2-Oxothiazolidine-4-carboxylic acid reverses endothelial dysfunction in patients with coronary artery disease. J Clin Invest. 1998;101:1408–14. doi: 10.1172/JCI1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vobecky JS, Vobecky J, Shapcott D, Demers PP, Cloutier D, Blanchard R, Fisch C. Biochemical indices of nutritional status in maternal, cord, and early neonatal blood. Am J Clin Nutr. 1982;36:630–42. doi: 10.1093/ajcn/36.4.630. [DOI] [PubMed] [Google Scholar]
- Voigt K, Kontush A, Stuerenburg HJ, Muench-Harrach D, Hansen HC, Kunze K. Decreased plasma and cerebrospinal fluid ascorbate levels in patients with septic encephalopathy. Free Radic Res. 2002;36:735–9. doi: 10.1080/10715760290032557. [DOI] [PubMed] [Google Scholar]
- Vonzastrow M, Tritton TR, Castle JD. Identification of L-ascorbic-acid in Secretion Granules of the Rat Parotid-gland. J Biol Chem. 1984;259:1746–1750. [PubMed] [Google Scholar]
- Wade KH, Forouhi NG, Cook DG, Johnson P, McConnachie A, Morris RW, Rodriguez S, Ye Z, Ebrahim S, Padmanabhan S, Watt G, Bruckdorfer KR, Wareham NJ, Whincup PH, Chanock S, Sattar N, Lawlor DA, Davey Smith G, Timpson NJ. Variation in the SLC23A1 gene does not influence cardiometabolic outcomes to the extent expected given its association with L-ascorbic acid. Am J Clin Nutr. 2015;101:202–9. doi: 10.3945/ajcn.114.092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dutta B, Huang W, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human Na(+)-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta. 1999;1461:1–9. doi: 10.1016/s0005-2736(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun. 2000;267:488–94. doi: 10.1006/bbrc.1999.1929. [DOI] [PubMed] [Google Scholar]
- Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, Levine M. Ascorbate recycling in human neutrophils: induction by bacteria. Proc Natl Acad Sci U S A. 1997;94:13816–9. doi: 10.1073/pnas.94.25.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washko PW, Wang Y, Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem. 1993;268:15531–5. [PubMed] [Google Scholar]
- Washko PW, Welch RW, Dhariwal KR, Wang Y, Levine M. Ascorbic acid and dehydroascorbic acid analyses in biological samples. Anal Biochem. 1992;204:1–14. doi: 10.1016/0003-2697(92)90131-p. [DOI] [PubMed] [Google Scholar]
- Westerman MP, Zhang Y, McConnell JP, Chezick PA, Neelam R, Freels S, Feldman LS, Allen S, Baridi R, Feldman LE, Fung LW. Ascorbate levels in red blood cells and urine in patients with sickle cell anemia. Am J Hematol. 2000;65:174–5. doi: 10.1002/1096-8652(200010)65:2<174::aid-ajh15>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Will JC, Byers T. Does diabetes mellitus increase the requirement for vitamin C? Nutr Rev. 1996;54:193–202. doi: 10.1111/j.1753-4887.1996.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Will JC, Ford ES, Bowman BA. Serum vitamin C concentrations and diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 1999;70:49–52. doi: 10.1093/ajcn/70.1.49. [DOI] [PubMed] [Google Scholar]
- Witherspoon DJ, Wooding S, Rogers AR, Marchani EE, Watkins WS, Batzer MA, Jorde LB. Genetic similarities within and between human populations. Genetics. 2007;176:351–9. doi: 10.1534/genetics.106.067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondrack LM, Hsu CA, Abbott MT. Thymine 7-hydroxylase and pyrimidine deoxyribonucleoside 2' -hydroxylase activities in Rhodotorula glutinis. J Biol Chem. 1978;253:6511–5. [PubMed] [Google Scholar]
- Woods RG. Vitamin C syrup. Aust Dent J. 1981;26:336. [PubMed] [Google Scholar]
- Woodside JV, Young IS, McKinley MC. Fruit and vegetable intake and risk of cardiovascular disease. Proc Nutr Soc. 2013;72:399–406. doi: 10.1017/S0029665113003029. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Yang M, Chowdhury R, Ge W, Hamed RB, McDonough MA, Claridge TD, Kessler BM, Cockman ME, Ratcliffe PJ, Schofield CJ. Factor-inhibiting hypoxia-inducible factor (FIH) catalyses the post-translational hydroxylation of histidinyl residues within ankyrin repeat domains. FEBS J. 2011a;278:1086–97. doi: 10.1111/j.1742-4658.2011.08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ge W, Chowdhury R, Claridge TD, Kramer HB, Schmierer B, McDonough MA, Gong L, Kessler BM, Ratcliffe PJ, Coleman ML, Schofield CJ. Asparagine and aspartate hydroxylation of the cytoskeletal ankyrin family is catalyzed by factor-inhibiting hypoxia-inducible factor. J Biol Chem. 2011b;286:7648–60. doi: 10.1074/jbc.M110.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew ML. Biological variation in ascorbic acid needs. Ann N Y Acad Sci. 1975;258:451–7. doi: 10.1111/j.1749-6632.1975.tb29303.x. [DOI] [PubMed] [Google Scholar]
- Young VR. Evidence for a recommended dietary allowance for vitamin C from pharmacokinetics: a comment and analysis. Proc Natl Acad Sci U S A. 1996;93:14344–8. doi: 10.1073/pnas.93.25.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;275:L818–26. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- Yue DK, McLennan S, McGill M, Fisher E, Heffernan S, Capogreco C, Turtle JR. Abnormalities of ascorbic acid metabolism and diabetic control: differences between diabetic patients and diabetic rats. Diabetes Res Clin Pract. 1990;9:239–44. doi: 10.1016/0168-8227(90)90051-t. [DOI] [PubMed] [Google Scholar]
- Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391–6. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]