Abstract
Expression of α4βδ GABAA receptors (GABARs) increases at the onset of puberty on dendritic spines of CA1 hippocampal pyramidal cells. These receptors reduce activation of NMDA receptors (NMDARs), impair induction of long-term potentiation (LTP) and reduce hippocampal-dependent spatial learning. These effects are not seen in the δ−/− mouse, implicating α4βδ GABARs. Here we show that knock-out of α4 also restores synaptic plasticity and spatial learning in female mice at the onset of puberty (verified by vaginal opening). To this end, field excitatory post-synaptic potentials (fEPSPs) were recorded from the stratum radiatum of CA1 hippocampus in the slice from +/+ and α4−/− pubertal mice (PND 36–44). Induction of LTP, in response to stimulation of the Schaffer collaterals with theta burst stimulation (TBS), was unsuccessful in the +/+ hippocampus, but reinstated by α4 knock-out (~65% potentiation) but not by blockade of α5-GABARs with L-655,708 (50 nM). In order to compare spatial learning in the two groups of mice, animals were trained in an active place avoidance task where the latency to first enter a shock zone is a measure of learning. α4−/− mice had significantly longer latencies by the third learning trial, suggesting better spatial learning, compared to +/+ animals, who did not reach the criterion for learning (120 s latency). These findings suggest that knockout of the GABAR α4 subunit restores synaptic plasticity and spatial learning at puberty and is consistent with the concept that the dendritic α4βδ GABARs which emerge at puberty selectively impair CNS plasticity.
Keywords: GABA-A receptor, alpha-4, alpha-5, delta, hippocampus, puberty, spatial learning, long-term potentiation
1. Introduction
Both human and animal studies show that there is a critical period for optimal learning of numerous cognitive processes, including spatial memory, that decline at the onset of puberty (Johnson and Newport, 1989; Kanit et al., 2000; McGivern et al., 2002; Newman et al., 2001; Subrahmanyam and Greenfield, 1994; Wright and Zecker, 2004). For review, see (Smith, 2013). Impairment of spatial memory at the onset of puberty in female mice appears to be due, at least in part, to the increased expression of δ-containing GABAA receptors (GABARs) in the hippocampus (Shen et al., 2010) because it is not observed in the δ −/− mouse.
1.1. Learning deficits in adolescence
Certain types of learning are facilitated before puberty in humans, an outcome more pronounced in individuals with learning disabilities (Wright and Zecker, 2004). For language acquisition, the onset of puberty may represent the end of a critical period for optimal acquisition, as both adolescents and adults are less likely to learn a second language as quickly or accurately as younger children (Johnson and Newport, 1989; Newman et al., 2001). Structural and functional changes are also apparent in musicians who begin training before puberty compared to later learners and non-musicians (Bailey and Penhune, 2012; Imfeld et al., 2009; Schlaug et al., 1995), suggesting that the pubertal period may have important implications for brain plasticity.
In other cases, impairments in learning are reported to be transient events, limited to the pubertal period. Mismatch detection is selectively impaired at puberty, with greater and longer impairment in girls than in boys (McGivern et al., 2002), but gradual improvement in late adolescence. Both mismatch detection and some types of semantic processing are mediated by the hippocampus (Kumaran and Maguire, 2006).
The hippocampus is best known for its role in spatial memory (Bannerman et al., 2004), and there is evidence that impairment of visuo-spatial learning on a computer game task is seen in early adolescence compared to young adult and pre-pubertal children (Pepin and Dorval, 1986; Shavalier, 2004; Subrahmanyam and Greenfield, 1994). A more recent study suggests that early adolescence is associated with a slowing of the rapid upward trajectory of learning seen in younger children (Gur et al., 2012), which is especially apparent for spatial learning in females, but which then improves later in adolescence. Several studies have suggested that sex differences in spatial memory appear at or after the onset of puberty (Ardila et al., 2011; Gur et al., 2012; Kanit et al., 2000), although there are conflicting reports (Newhouse et al., 2007).
1.2. Hippocampus
The hippocampus is widely known to be the site for encoding and storage of spatial memory in both rodents and humans (Burgess et al., 2002; Maguire et al., 2006; Pastalkova et al., 2006; Tsien et al., 1996). This CNS area is theorized to encode memory through the activity-dependent increase of synaptic transmission between neurons, or long-term potentiation (LTP) (Bliss and Collingridge, 1993; Herron et al., 1986; Larson and Lynch, 1986) that is dependent upon activation of NMDA receptors (Herron et al., 1986), which are localized to dendritic spines (He et al., 1998). It is well established that the ability to induce LTP in vitro serves as a cellular model of learning (Bliss and Collingridge, 1993; Malenka and Nicoll, 1999). We have shown that induction of NMDAR-dependent LTP in CA1 hippocampus through theta burst stimulation (TBS) of the Schaffer collaterals is impaired at pubertal onset in +/+ female mice, as is spatial learning. Both parameters are restored to pre-pubertal levels by knock-out of the δ GABAR subunit (Shen et al., 2010).
1.3. α4βδ GABARs
α4βδ GABARs occur extrasynaptically (Wei et al., 2003) where they generate a tonic shunting inhibition (Stell and Mody, 2002) in response to ambient levels of GABA (<1 μM) (Wu et al., 2003) due to their high sensitivity to GABA and relative lack of desensitization under steady-state conditions (Brown et al., 2002). They are also capable of high degree of plasticity, especially in response to fluctuations in ovarian hormones (Lovick et al., 2005; Maguire et al., 2005; Maguire et al., 2009; Sabaliauskas et al., 2014; Shen et al., 2005). At puberty, expression of α4 and δ GABAR subunits increases on the dendritic shafts and spines of CA1 hippocampal pyramidal cells (Shen et al., 2007; Shen et al., 2010) from almost undetectable levels before puberty. The presence of these receptors impairs activation of NMDA receptors (NMDARs), likely due to their shunting of current on the spine, because NMDA currents are more robust in the δ−/− hippocampus than in age-matched wildtypes (Shen et al., 2010).
1.4. α4 knock-out and synaptic plasticity
Although pubertal deficits in both LTP induction and spatial learning are not observed in the δ −/− mouse, these parameters have yet to be tested in the α4 −/− mouse. Therefore, the aim of the current study was to determine whether the α4 subunit is obligatory in the diminished spatial learning seen in female mice at the onset of puberty. Previous EM-ICC data showed a concomitant decrease in δ expression on both the spines and dendritic shaft upon knockout of the α4 subunit (Sabaliauskas et al., 2012) with a corresponding decrease in the response of the tonic current to a 100 nM gaboxadol, which is selective for α4βδ GABARs (Brown et al., 2002; Meera et al., 2011), suggesting a decrease in functional α4βδ GABARs. However, δ can still form heteromers with α1 (Glykys et al., 2007) in the α4−/−, suggesting a potential reason to predict that results from α4−/− and δ−/− mice might be different at puberty. Therefore, in the present study we compared recordings from +/+ and α4−/− mice to test whether α4 knock-out reverses the impairment in LTP induction and hippocampal-dependent spatial learning. We hypothesized that α4 −/− mice would not show a reduction in synaptic plasticity at puberty onset, and therefore would not exhibit a pubertal learning deficit.
2. RESULTS
2.1. LTP induction
2.1.1. α4 GABAR knock-out
Because α4 knock-out resulted in robust generation of NMDA-mediated currents at puberty, we predicted that it would also have an impact on synaptic plasticity. To test this hypothesis, we compared the induction of LTP in CA1 hippocampus between pubertal α4 −/− and +/+ mice. It has been established previously that LTP induction is not observed at puberty, an impairment not seen in the δ−/− mouse, where induction of LTP is highly significant (Shen et al., 2010). Therefore, upon knock-out of α4, we also expected to see reinstatement of LTP inducibility because this mouse is also a functional δ knock-out (Sabaliauskas et al., 2012). Indeed, we observed robust LTP induction in hippocampal slices from α4−/− mice, where the average potentiation was ~175%, by 2 h after stimulation of the Schaffer collaterals with theta burst stimulation (TBS, Fig. 1). This is contrast to pubertal +/+ hippocampus, where induction of LTP was not observed.
Figure 1. Pubertal impairments in LTP induction are not observed in α4 −/− hippocampus.
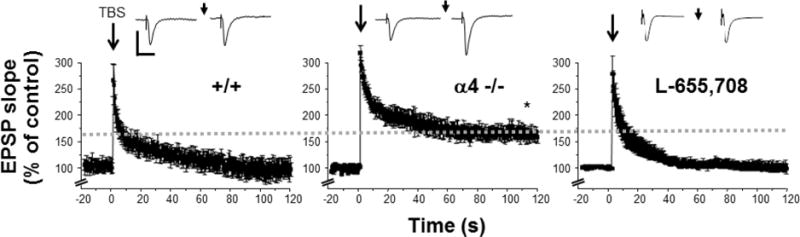
LTP was induced by theta burst stimulation (TBS) of the Schaffer collaterals to the CA1 hippocampus (arrow). LTP was not successfully induced in pubertal +/+ hippocampus (left), but was robustly induced in the α4 −/− at puberty (middle). Bath application of 50 nM L-655,708 to block α5-GABARs (right) did not reinstate LTP induction at puberty. (Dashed line, average potentiation at 2 h post-TBS, α4 −/−) Inset, Representative fEPSPs before and after TBS (arrow). Scale, 0.5 mV, 50 ms; n=10–12 slices/group; *P<0.05 vs. pre-TBS.
2.1.2. α5 GABAR blockade
The most abundant extrasynaptic GABAR in CA1 hippocampus is α5β3γ2 (Caraiscos et al., 2004). Thus, we blocked its effect with 50 nM L-655,708 to test whether the tonic inhibition generated by this receptor could also reinstate LTP induction. In fact, LTP induction was not significantly changed after bath administration of L-655,708 (Fig. 1), suggesting that the impairment of synaptic plasticity at puberty is exclusively due to α4βδ GABARs.
2.2. α4 KO eliminates the spatial learning deficit seen at puberty in wildtypes
As hippocampal LTP is an accepted model for in vivo learning, we would expect α4−/− mice to show better performance on a hippocampal-dependent spatial learning task as compared to +/+ mice at the onset of puberty. Our new data confirm the previous findings (Shen et al., 2010) that +/+ pubertal mice are severely compromised in their ability to perform a spatial learning task. In contrast, α4 −/− animals do not exhibit a learning deficit at puberty, relative to +/+ pubertal mice. For the active avoidance task that we used, the latency to first enter the shock zone on a trial-by-trial basis is a measure of spatial learning, where longer latencies indicate better learning. Best latencies of pubertal α4−/− mice were more than 4-fold longer than those of +/+ mice at puberty (p<0.01, Fig. 2): The average best latency for α4 −/− mice was 235 seconds, compared to 53 seconds for +/+ mice. Only ~7% of the +/+ pubertal mice were able to achieve the 120-second learning criterion, whereas ~70% of α4 −/− animals achieved this criterion. When broken down by trial, α4−/− mice begin outperforming their +/+ counterparts by the second training trial, showing a 127% longer latency to first entry (109 seconds versus 43 seconds; p<0.05). By the third trial, α4−/− mice show a 343% increase in latency compared to WT mice (p<0.01).
Figure 2. Pubertal impairments in spatial learning are not observed in α4 −/− mice.
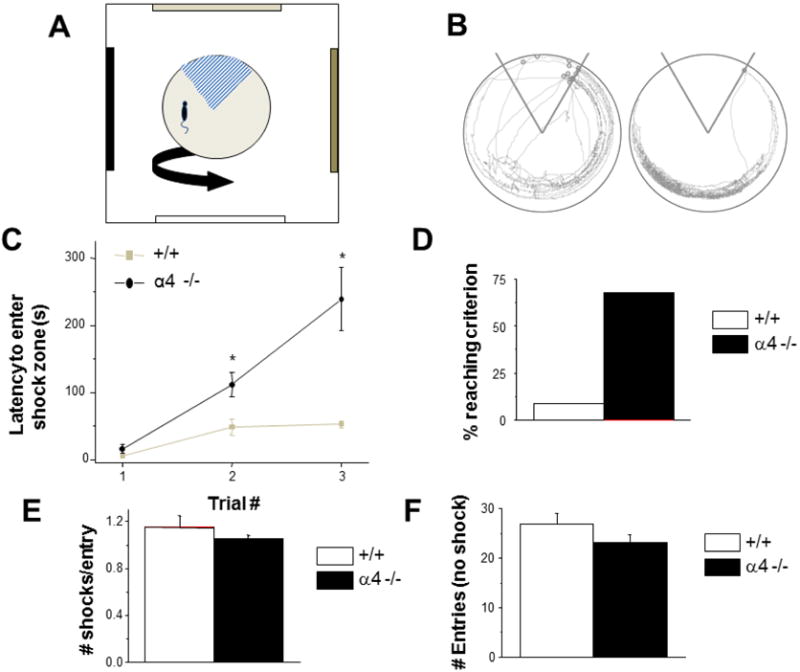
A, Spatial learning was assessed by the latency to enter the shock zone (hatched sector) on a rotating arena in an active avoidance task (longer latency=improved learning) across 3 10-min learning trials. B, Representative traces of mouse trajectory during the final training trial for +/+ (left) and α4 −/− (right) pubertal mice, reflecting a greater number of shocks (open circles, 13) for the +/+ compared to the α4 −/− (1). C, Latency to first entry for training trials #1–3. Latencies were significantly longer in pubertal α4 −/− mice compared to wild-type (WT), signifying improved learning by learning trial #2. *P<0.05 vs. +/+. D, A greater percentage of α4 −/− mice reached learning criterion (120 s latency) compared to +/+. E, #shocks/entry was unaltered across groups suggesting that the shock was equally aversive for all WT and α4 −/− mice. F, #entries for the acclimation trial (no shock), a measure of locomotor activity. n=14 mice/group.
2.2.1. Non-specific behavior
To verify that both groups of animals were equally able to perform the task, we measured the ratio of the number of shocks to the number of entrances. This value is a measure of escape behavior, but also shows if the shock is equally aversive to each group. We found no significant difference between the shock-to-entrance ratio for α4 −/− and +/+ pubertal mice (Fig. 2). Locomotor activity, assessed by the number of entries in the first acclimation trial (no shock), was also similar between groups (Fig. 2). Thus, these data suggest that the results reflect differences in spatial learning, rather than differences in pain threshold or sensorimotor performance.
3. Discussion
3.1. Overview
Previous data have shown that knock-out of the α4 GABAR subunit is a functional knock-out of δ subunit expression (Sabaliauskas et al., 2012), where expression was barely detectable on both the dendritic shafts and spines of CA1 hippocampal pyramidal cells at puberty. In this study, our findings are consistent with a lack of α4βδ GABAR expression on dendritic spines because we observed robust induction of LTP and rapid learning of a hippocampal-dependent spatial learning task as previously observed for the δ−/− mouse. These findings suggest that expression of hippocampal α4βδ GABARs at puberty are necessary and sufficient for the decline in plasticity and learning observed at puberty in female mice.
3.2. GABA inhibition effects on synaptic plasticity and learning
It is well known that reducing GABA inhibition facilitates synaptic plasticity (Paulsen and Moser, 1998; Wigstrom and Gustafsson, 1983), while GABA agonists impair LTP (Whissell et al., 2013): Positive GABA modulators which enhance GABA inhibition, including benzodiazepines and anesthetics, are known to be amnestic in humans (Veselis et al., 2009). These drugs, and others such as alcohol, impair hippocampal synaptic plasticity and spatial learning in rodents (del Cerro et al., 1992; Izumi et al., 2005; Matthews et al., 2002; Nagashima et al., 2005; Saab et al., 2010).
3.3. Effect of α5-GABARs on LTP
The predominant extrasynaptic GABAR in pre-pubertal and adult hippocampus is α5β3γ2, which is a target for the amnestic effects of anesthetics (Cheng et al., 2006). In the present study, in contrast to the full recovery of LTP induction in response to TBS after α4 knock-out, blockade of α5-GABARs did not improve the impairment in LTP induction at puberty. Previous studies have shown that α5 knock-out does not alter TBS-induced LTP in the adult (Collinson et al., 2002), although it lowers the threshold for LTP induction to 10 Hz (Martin et al., 2010), suggesting that α5-GABARs may have a more subtle effect on synaptic plasticity than α4βδ GABARs. This may be due to the fact that α4βδ are exclusively localized to the spine (Shen et al., 2010), while α5-GABARs are localized to the dendritic shaft (Brunig et al., 2002). Thus, α4βδ GABARs would play a greater role in shunting excitatory current to impair NMDA receptor activation, necessary for LTP induction (Herron et al., 1986).
3.4. Effect of α5-GABARs on learning
However, α5-GABARs play a specific role in memory and learning; an α5 inverse agonist improves encoding and recall but not consolidation in the hippocampal-dependent Morris Water Maze task (Chambers et al., 2003; Collinson et al., 2006). Knock-out of α5 also improves weak hippocampus-dependent associative fear memory tasks. Trace fear conditioning, but not delay conditioning or contextual conditioning, is facilitated in α5 −/− mice (Crestani et al., 2002). For delay conditioning, the tone co-terminates with the foot shock. Trace fear conditioning differs from delay conditioning in that the tone and footshock are separated by a time interval. The facilitation of trace fear conditioning by α5 knock-out suggests that α5-containing GABARs serves as control elements of the temporal association of threat cues (Crestani et al., 2002). This suggests a significant but pattern-specific role for α5β3γ2 in contrast to the more global inhibitory effect of α4βδ GABARs on synaptic plasticity and learning at puberty.
3.5. α4βδ GABAR localization
The α4βδ GABAR plays a greater role in regulating synaptic plasticity at puberty than at other developmental stages due to the unique localization of α4βδ on dendritic spines at puberty (Shen et al., 2010) where it can reduce the depolarizing current necessary for NMDAR activation. In the adult and pre-pubertal rodent, most GABARs in CA1 hippocampus localize to the soma (80%) or dendritic shaft (20%) (Megias et al., 2001), unlike layer 1 of the neocortex and stratum lacunosum-moleculare of the hippocampus, where GABAergic innervation directly targets the dendritic spine (Kubota et al., 2007). In fact, earlier studies reported that GABAergic inhibition limited LTP induction around the time of puberty (Paulsen and Moser, 1998), although α4βδ GABARs were not yet identified as the mediating factor. For this reason, many LTP studies have been carried out using pre-pubertal animals. Surpisingly, blockade of the synaptic GABARs does not restore induction of LTP at puberty (Shen et al., 2010), suggesting that α4βδ GABARs selectively impair LTP induction at puberty.
3.6. α4βδ GABAR impairment of NMDAR activation
Activation of NMDARs on the spine is the trigger for synaptic plasticity, which requires sufficient depolarization to unblock the receptor from Mg2+ (Nowak et al., 1984), which normally prevents receptor activation (Herron et al., 1986). The development of a shunting inhibition on the spines would be expected to selectively impair activation of NMDARs relative to activation of the AMPARs, which do not require local depolarization to trigger receptor activation. Thus, expression of α4βδ GABARs on dendritic spines would be expected to decrease the NMDA/AMPA ratio which is observed in the pubertal +/+ mouse (Shen et al., 2010). Recent reports have suggested that local changes in depolarization have a bigger impact on synaptic plasticity than events mediated via the dendritic shaft such as back-propagating action potentials, although both would contribute to the general excitability level of the neuron (Hardie and Spruston, 2009). In fact, estrous cycle-correlated changes in α4βδ GABAR expression are localized to the dendritic shaft, rather than to the spine (Sabaliauskas et al., 2014). The impact of increased α4βδ expression on proestrus is to reduce the degree of LTP, but not abolish it, as seen in pubertal hippocampus, suggesting that inhibition of the dendritic shaft has less of an impact on synaptic plasticity.
3.7. The dentate gyrus and learning
In contrast to the CA1 hippocampus, the dentate gyrus normally exhibits high expression levels of extrasynaptic α4βδ GABARs (Peng et al., 2014; Wei et al., 2003). This CNS region plays a pivotal role in certain types of learning, such as context-dependent fear conditioning (Saxe et al., 2006). Knock-out of either α4 or δ subunits has also been shown to improve this type of learning (Moore et al., 2010; Wiltgen et al., 2005) although sex differences were observed in which trace conditioning or delay conditioning were selectively affected, in female versus male mice, respectively (Moore et al., 2010). Other studies have shown that acute administration of gaboxadol, to activate δ-containing GABARs, impairs both LTP and spatial learning in the hippocampus (Whissell et al., 2013).
3.8. Knock-out of α4 versus δ GABAR subunits
Previous immunohistochemical findings demonstrate that knockout of the δ subunit leads to a concomitant decrease in α4 subunit immunoreactivity in adult males in areas of the brain which normally express high levels of this receptor, such as dentate gyrus and thalamic relay nuclei (Peng et al., 2002). However, α4 readily co-expresses with γ2, which increases its expression in the δ−/−, consistent with a re-partnering of α4 with γ2, which is likely synaptic because the tonic current is reduced (Mihalek et al., 1999). More recent studies investigating the α4−/− suggest that δ expression is concomitantly reduced in thalamic relay nuclei, and the tonic current is also reduced, suggesting that re-partnering of δ does not occur (Peng et al., 2014).
3.9. Plasticity of α4βδ GABAR expression
Unlike the thalamus and dentate gyrus, the CA1 hippocampal pyramidal cell does not normally express a high level of α4βδ GABARs (Benke et al., 1997; Wisden et al., 1992). However, these receptors exhibit a high degree of plasticity. Their expression is influenced by ovarian and stress steroids (Kuver et al., 2012; Sabaliauskas et al., 2014; Shen et al., 2005), and are increased by up to 8-fold at the onset of puberty in the female mouse (Shen et al., 2010). At puberty, α4βδ GABARs express on the dendritic shaft and spines, for a period of about 10 days before declining to low levels in adulthood (Aoki et al., 2012; Shen et al., 2010). Recent studies from our labs demonstrate that the α4−/− is a functional δ knock-out at puberty (Sabaliauskas et al., 2012). However, intracellular δ expression was not reduced, suggesting that co-expression of α4 and δ subunits is necessary for surface expression of δ. Knock-out of α4 reduced the tonic current at puberty and greatly diminished the response to 100 nM gaboxadol, which is selective for α4βδ GABARs at this concentration. These are outcomes similar to those observed in the pubertal δ−/− hippocampus (Shen et al., 2010), suggesting that knock-out of either subunit has a similar impact to decrease inhibition of CA1 hippocampal pyramidal cells.
3.10. Comparison with the δ−/− mouse
The effects of α4 knock-out to restore synaptic plasticity and spatial learning which are impaired at puberty are virtually identical to those observed in the δ−/− mouse (Shen et al., 2010). This suggests that expression of both the α4 and δ subunits play an equally important role in producing extrasynaptic receptors which impair NMDAR activation and reduce synaptic plasticity. Thus, knock-out strains of both of these receptor subunits are important genetic tools for use in elucidating the mechanism of learning.
3.11. Summary
Collectively, these data confirm the role of the α4 subunit in mediating altered NMDAR activation at puberty, and how this translates into synaptic plasticity and learning. The GABAR has been implicated in defining critical periods for other systems (Aoki and Erisir, 2012), such as in the auditory cortex (Sarro et al., 2008) and visual cortex (Fagiolini and Hensch, 2000), the latter of which also involves tonic inhibition (Iwai et al., 2003). This inhibitory system may provide the limit for changes in plasticity of CNS areas when appropriate for developmental time-points.
4. Experimental Procedures
4.1. Experimental subjects
α4 +/− mice (C57BL6, supplied by G. Homanics, Univ. Pittsburgh) were bred to yield first generation α4 +/+ and −/− mice, as described previously (Chandra et al., 2006). Tails were genotyped to verify the genetic identity of the offspring. Because +/+ and C57BL6 mice (Jackson Labs, Bar Harbor, Maine) did not exhibit different characteristics, these groups were pooled. Pubertal (~35 to 43 days old) female mice were housed on a 12-hour reverse light-dark cycle, with lights off at 11:30 AM and had free access to food and water. All mice were tested in the early AM before the onset of the dark cycle. Vaginal opening was used to determine the onset of puberty (Shen et al., 2007). All procedures involving live animals were in accordance with NIH guidelines and the Institutional Animal Care and Use Committees of SUNY Downstate Medical Center, University of Pittsburgh, and New York University Washington Square Campus.
4.2. Hippocampal slice electrophysiology
4.2.1. Slice preparation
Mice were rapidly decapitated; the brains were removed and cooled using an ice cold solution of artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 124, KCl 3, CaCl2 2, KH2PO4 1.25, MgSO4 2, NaHCO3 26, and glucose 10, saturated with 95% O2, 5% CO2 and buffered to a pH of 7.4. Following sectioning at 400 μm, slices were incubated for one hour in oxygenated aCSF.
4.2.2. LTP studies
Field EPSPs (fEPSPs) were recorded extracellularly from the stratum radiatum of CA1 hippocampus using an aCSF-filled glass micropipet (1–5 mΩ) in response to stimulation of the Schaffer collateral-commissural pathway using a pair of insulated tungsten bipolar electrodes as we have described (Shen et al., 2010). The intensity of the stimulation was adjusted to produce 50% of the maximal response. LTP was induced using theta burst stimulation (TBS 8–10 trains of 4 pulses at 100 Hz, delivered at 200 ms intervals, repeated 3 times at 30 s intervals). EPSP responses were recorded at 30 s intervals with an Axoprobe-1A amplifier (Axon Instruments) and pClamp 10 (Axon Instruments) for 20 min before and 120 min after TBS (producing 1–4 mV EPSPs). In some cases, the α5 selective inverse agonist (Caraiscos et al., 2004), L-655,708 (50 nM) was bath applied to test the effect of α5 blockade on LTP induction.
4.3. Spatial learning task
4.3.1. Spatial learning apparatus
Mice were placed on a 48-cm diameter metal disk with a 40-cm high transparent wall and an electrifiable grid floor (Bio-Signal Group, DE). The apparatus was located in a rectangular room with many visible landmarks. Animal position and movement were tracked by PC-based software that analyzes images from an overhead camera acquired at 60 Hz. This spatial learning task has been successfully used as a measure of hippocampal synaptic plasticity in a number of studies (Cimadevilla et al., 2001; Sabaliauskas et al., 2014; Shen et al., 2010).
4.3.2. Spatial learning training
Mice were habituated to the rotating arena (1 rotation per minute). Mice were trained for four 10-minute trials on an hourly basis, to avoid a foot shock (0.2 mA, 500 ms) administered upon entrance in a 60° sector of the rotating disk. The position of the electrified sector remained stationary with respect to the room frame of reference, but rotated relative to the platform, thus requiring the animal to perform active avoidance behavior. If the animal failed to exit the 60° sector, additional shocks were administered every 1.5 seconds until the mouse left the shock zone. The time to first entry into the 60° sector was recorded from each trial as a measure of spatial memory acquisition. The criterion for learning was set at a minimum latency of 120 s (Shen et al., 2010). This task is hippocampus-dependent, as performance has been shown to worsen upon inactivation of the hippocampus (Cimadevilla et al., 2001). It has also recently been shown to correlate with measures of LTP, assessed in vivo, together with task performance (Pastalkova et al., 2006).
This shock intensity used is subthreshold for release of the stress hormone corticosterone (Harrison et al., 2009), suggesting that this paradigm is not highly stressful. As a measure for pain sensitivity, the number of shocks per entry into the shock zone was recorded. If this value is equivalent between experimental groups, it indicates that the shock is equally aversive for all animals, and that they are equally capable of escape behavior. Similar shock-to-entrance ratios are indicative of equivalent pain sensitivity and sensorimotor function between groups.
Highlights.
Pubertal impairment of synaptic plasticity is restored by α4 knock-out
Pubertal impairment of synaptic plasticity is not restored by α5 blockade
Pubertal impairment of spatial learning is restored by α4 knock-out
Pubertal α4βδ GABARs impair CNS plasticity and learning
Acknowledgments
The authors are grateful to A. Fenton for use of the APA device, G. Homanics for supplying the α4+/− mice and to C. Ferguson for genotyping the animals. The authors also thank N. Zeak and J. Molla for helpful technical assistance. This work was supported by NIH grants DA09618, AA12968 and MH100561 to SSS and The Klarman Foundation Grant Program in Eating Disorders Research, R21MH091445-01, R01NS066019-01A1, R01NS047557-07A1, NEI Core grant EY13079, and R21 MH105846 to CA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki C, Erisir A. Activity-dependent synaptic plasticity in in the developing cortex. In: Virginia JEJ, Picke M, Segal Menahem Jr, editors. Structure and Function of the Synapse. Vol. 3. Neuroscience.com; 2012. Managing Editor. http://www.neuroscience.com. [Google Scholar]
- Aoki C, et al. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse. 2012;66:391–407. doi: 10.1002/syn.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A, et al. Gender differences in cognitive development. Dev Psychol. 2011;47:984–990. doi: 10.1037/a0023819. [DOI] [PubMed] [Google Scholar]
- Bailey J, Penhune VB. A sensitive period for musical training: contributions of age of onset and cognitive abilities. Ann NY Acad Sci. 2012;1252:163–170. doi: 10.1111/j.1749-6632.2011.06434.x. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, et al. Regional dissociations within the hippocampus–memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Benke D, Michel C, Mohler H. GABAA receptors containing the a4-subunit: prevalence, distribution, pharmacology, and subunit architecture in situ. J Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brown N, et al. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, et al. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MS, et al. Identification of a novel, selective GABA(A) alpha-5 receptor inverse agonist which enhances cognition. J Med Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Chandra D, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15230–5. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng VY, et al. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, et al. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociaiton of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci. 2001;98:3532–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABA-A receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, et al. An inverse agonist selective for alpha5 subunit containing GABA-A receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharm. 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Crestani F, et al. Trace fear conditioning involves hippocampal alpha-5 GABA(A) receptors. Proc Natl Acad Sci. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro S, Jung M, Lynch G. Benzodiazepines block long-term potentiation in slices of hippocampal and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Glykys J, et al. A new naturally occurring GABA-A receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gur RC, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J, Spruston N. Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons. J Neurosci. 2009;29:3233–3241. doi: 10.1523/JNEUROSCI.6000-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Janssen WG, Morrison JH. Synaptic coexistence of AMPA and NMDA receptors in the rat hippocampus: a postembedding immunogold study. J Neurosci Res. 1998;54:444–9. doi: 10.1002/(SICI)1097-4547(19981115)54:4<444::AID-JNR2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Herron CE, et al. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Imfeld A, et al. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage. 2009;46:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Iwai Y, et al. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, et al. Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience. 2005;136:509–517. doi: 10.1016/j.neuroscience.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: The influence of maturational state on the acquisition of English as a second language. Cognitive Psychology. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Kanit L, et al. Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res Bull. 2000;52:243–248. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, et al. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J Neurosci. 2007;27:1139–1150. doi: 10.1523/JNEUROSCI.3846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. An unexpected sequence of events: Mismatch detection in the human hippocampus. PLOS Biology. 2006;4:2372–2382. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuver A, Shen H, Smith SS. Regulation of the surface expression of alpha4beta2delta GABA(A) receptors by high efficacy states. Brain Res. 2012;1463:1–20. doi: 10.1016/j.brainres.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Lovick TA, et al. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129:2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- Maguire JL, et al. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire JL, et al. Excitability changes related to GABA-A receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Martin LJ, et al. Alpha5 GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci. 2010;30:5269–5282. doi: 10.1523/JNEUROSCI.4209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, et al. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alc Clin Exp Res. 2002;26:1747–1751. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- McGivern RF, et al. Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain Cogn. 2002;50:73–89. doi: 10.1016/s0278-2626(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Meera P, M W, Otis T. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. J Neurophysiology. 2011;106:2057–2011. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megias M, et al. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neurosci. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, et al. Attenuated sensitivity to neuroactive steroids in g-aminobutyrate type A receptor delta subunit knockout mice. PNAS. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MD, et al. Trace and contextual fear conditioning is enhanced in mice lacking the alpha4 subunit of the GABA(A) receptor. Neurobiol Learn Mem. 2010;93:383–387. doi: 10.1016/j.nlm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, CF Z, Izumi Y. Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology. 2005;103:318–326. doi: 10.1097/00000542-200508000-00015. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Newman AJ, et al. A critical period for right hemispheric recruitment in American Sign Language processing. Nature Neuroscience. 2001;5:76–80. doi: 10.1038/nn775. [DOI] [PubMed] [Google Scholar]
- Nowak L, et al. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–5. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- Peng Z, et al. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Peng Z, et al. Altered localization of the delta subunit of the GABAA receptor in the thalamus of alpha4 subunit knockout mice. Neurochem Res. 2014;39:1104–17. doi: 10.1007/s11064-013-1202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin M, Dorval M. Effect of playing a video game on adults’ and adolescents’ spatial visualization. Educational Resources Information Center. 1986;ED273266:2–18. [Google Scholar]
- Saab BJ, et al. Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type A receptor inverse agonist L-655,708. Anesthesiology. 2010;113:1061–1071. doi: 10.1097/ALN.0b013e3181f56228. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, et al. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaliauskas N, et al. Neurosteroid effects at alpha4betadelta GABA receptors alter spatial learning and synaptic plasticity in CA1 hippocampus across the estrous cycle of the mouse. Brain Res. 2014 doi: 10.1016/j.brainres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, et al. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb Cortex. 2008;18:2855–67. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, et al. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Shavalier M. The effects of CAD-like software on the spatial ability of middle school students. J Educat Comput Res. 2004;31:37–49. [Google Scholar]
- Shen H, et al. Short-term steroid treatment increases delta GABA-A receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, et al. Reversal of neurosteroid effects at alpha4-beta2-delta GABA-A receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, et al. A critical role for alpha4-beta-delta GABA-A receptors in shaping learning deficits at puberty in mice. Science (Supp Online Material) 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS. The influence of stress at puberty on mood and learning: role of the alpha4betadelta GABAA receptor. Neuroscience. 2013;249:192–213. doi: 10.1016/j.neuroscience.2012.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam K, Greenfield P. Effect of video game practice on spatial skills in girls and boys. J Applied Devel Psychol. 1994;15:15–32. [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Veselis RA, et al. Propofol and midazolam inhibit conscious memory processes very soon after encoding: an event-related potential study of familiarity and recollection in volunteers. Anesthesiology. 2009;110:295–312. doi: 10.1097/ALN.0b013e3181942ef0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, et al. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, et al. Acutely increasing δ-GABA-A receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus. Frontiers in Neural Circuits. 2013 doi: 10.3389/fncir.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, et al. Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABAA receptor. Learn Mem. 2005;12:327–333. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, et al. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Zecker SG. Learning problems, delayed development, and puberty. Proc Natl Acad Sci. 2004;101:9942–9946. doi: 10.1073/pnas.0401825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson G. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89:2021–2034. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]


