Abstract
Human skin has continuous self-renewal potential throughout adult life and serves as first line of defence. Its cellular components such as human epidermal keratinocytes (HEKs) and dermal mesenchymal stromal cells (DMSCs) are valuable resources for wound healing applications and cell based therapies. Here we show a simple, scalable and cost-effective method for sequential isolation and propagation of HEKs and DMSCs under defined culture conditions. Human skin biopsy samples obtained surgically were cut into fine pieces and cultured employing explant technique. Plated skin samples attached and showed outgrowth of HEKs. Gross microscopic examination displayed polygonal cells with a granular cytoplasm and H&E staining revealed archetypal HEK morphology. RT–PCR and immunocytochemistry authenticated the presence of key HEK markers including trans-membrane protein epithelial cadherin (E-cadherin), keratins and cytokeratin. After collection of HEKs by trypsin–EDTA treatment, mother explants were left intact and cultured further. Interestingly, we observed the appearance of another cell type with fibroblastic or stromal morphology which were able to grow up to 15 passages in vitro. Growth pattern, expression of cytoskeletal protein vimentin, surface proteins such as CD44, CD73, CD90, CD166 and mesodermal differentiation potential into osteocytes, adipocytes and chondrocytes confirmed their bonafide mesenchymal stem cell like status. These findings albeit preliminary may open up significant opportunities for novel applications in wound healing.
Keywords: Human skin, Primary keratinocytes, Mesenchymal stem cells and co-culture
Introduction
The skin is the largest organ of the integumentary system in human composed of multiple layers of tissues such as epidermis and dermis. The principal cell type of the epidermis is the keratinocyte, which makes up the stratified epidermis and stratum corneum (Parenteau et al. 1991). The keratinocytes grow and stratify to form unceasing epithelial layer and serve as mechanical support (Hennings et al. 1983). Keratinocytes have been the focus of extensive research as they can endure many normal processes ex vivo. Keratinocytes expand as they differentiate and terminal differentiation requires calcium for stratification in vitro (Watt et al. 1984).
Methods of in vitro culture and expansion of primary keratinocytes were established in the 1975 by James Rheinwald and Howard Green (Rheinwald and Green 1975). It is now possible to grow keratinocytes on mouse irradiated fibroblast as feeder-layer. Rheinwald and Green protocols are extensively used today with some modifications (Green et al. 1979; Hefton et al. 1983). Concommitant with the widespread efforts to ensure that cells grown for human therapeutic use are free of potential harmful agents like bovine spongio encephalopathy and murine viruses; new defined serum-free and feeder-free techniques have been developed in the last decade (Higham et al. 2003; Sun et al. 2004; Bullock et al. 2006; Coolen et al. 2007; De Corte et al. 2012). In addition, synthetic substrates have been reported to support the growth of keratinocytes in vitro (Mazlyzam et al. 2007).
Fibroblasts are the other major cell type found in skin tissue and exhibit considerable functional diversity. Two distinct lineages of skin connective tissue such as upper and lower dermis are the sources for these stromal cell populations. Recent literature showed the derivation of stromal cells from human dermis exhibits mesenchymal stem cell (MSC) like characteristics (Feisst et al. 2014). Earlier our group showed isolation of foreskin MSCs expressing mesenchymal marker profile and retention of their mesodermal differentiation potential (Mamidi et al. 2011). However, sequential isolation of two different cell types from both dermis and epidermis still remains elusive. In the present study, we report isolation and characterization of human epidermal keratinocytes (HEKs) and dermal mesenchymal stromal cells (DMSCs) in a sequential manner under defined culture conditions. To achieve this goal, we applied the traditional approach of explant culture of skin biopsies but with a novel angle. Our findings may have significant implications in cell therapies for dermatological problems.
Materials and methods
Surgical isolation of skin tissues
This study was conducted in accordance with the protocol approved by Institutional Ethics Committee (IEC) of Manipal Hospital (Bangalore, India). Human skin samples were obtained from unaffected area of vitiligo patients (Table 1) using the curette method by experienced dermatologists. This surgery was performed using a 5 mm curette (Tejco Vision, Mumbai, India) under 2 % lignocaine anaesthesia infiltration and a total area of 2 cm2 was curetted (Fig. 1b–d). Wound site was dressed with antibiotic ointment and vaseline gauze after the surgery. Skin biopsy samples were collected in a 50 ml sterile centrifuge tube (Falcon, BD Biosciences, Bedford, MA, USA) in phosphate buffered saline (PBS) containing 10 % penicillin–streptomycin (Gibco, Grand Island, NY, USA) and the samples were sent to the lab for subsequent processing.
Table 1.
Represents the details of human skin samples used for the study
| S. No. | Patients | Age | Gender | Biopsy site |
|---|---|---|---|---|
| 1 | P1 | 25 years | Male | Leg |
| 2 | P2 | 34 years | Male | Leg |
| 3 | P3 | 38 years | Male | Leg |
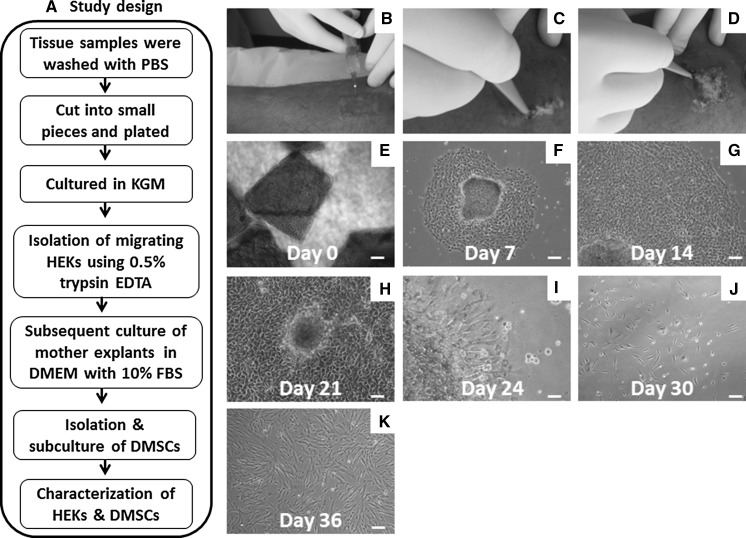
Fig. 1.
Isolation and propagation of HEKs and DMSCs from human skin biopsies: a Flow chart describes study design. b–d Surgical procedures for obtaining skin biopsy samples. Phase contrast photographs showing; e initial plating of minced skin samples with growth medium. f–h Outgrowth of HEKs showing polygonal morphology. i secondary outgrowth of stromal phenotypes from mother explant which were left intact after trypsinization. j–k Scattered growth of stromal cells with spindle morphology. Scale bars 100 μm (e–k). (Color figure online)
Sample processing and organotypic explant cultures
Skin biopsy samples were transported from the surgical suite to the laboratory in a sterile container on ice within 1–2 h after excision. Samples were washed 4–5 times with PBS consisting of 10 % antibiotic and anti-mycotic solution (Gibco). The partial thickness skin samples were cut into small fragments using surgical scissors and the resulting small tissue pieces were explanted on 35 mm tissue culture dishes (Falcon) in keratinocyte growth medium (KGM; Lonza, Mumbai, India). The cultures were maintained at 37 °C and 5 % CO2 incubator (Binder, Bohemia, NY, USA) and observed for appearance of cellular outgrowth. The migrating keratinocytes (sheet-like) were harvested using 0.5 % trypsin EDTA (25 mmol/L) and the mother explants were then allowed to grow in Knockout Dulbecco’s modified Eagles medium (KO-DMEM; Gibco) supplemented with 10 % fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 1 mM l-glutamine (Gibco) and 1 % penicillin and streptomycin (Gibco) and incubated at 37 °C and 5 % CO2 in air (Binder, USA). After 48 h explants were found to give rise to spindle shaped fibroblastic cells. This second cell type was further harvested by trypsin treatment and subcultured in the same medium. Fibroblast like cells preferentially proliferate in DMEM based medium owing to the higher concentration of Ca2+ (1 mM) compared to KGM.
Growth kinetics
Dermal mesenchymal stromal cells were plated at a density of 5000 cells/cm2 in T25 flask (BD Pharmingen, San Jose, CA, USA). Cells were trypsinized, counted, and re-plated once they reached 70–80 % confluency. Culture was terminated when the cell population failed to double after 2 weeks in culture. Population doubling (PD) time was calculated using the formula PD¼tplg2/(lgNH–lgNI). NI: the inoculum cell number; NH is the cell harvest number and t is the time of the culture (in hours).
RNA isolation, cDNA synthesis and semi-quantitative PCR
Cell pellets were collected and total RNA was isolated by the Trizol method (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. After estimation of the RNA (Nanodrop), 1 μg of RNA treated with RNase-OUT ribonuclease inhibitor (Invitrogen) was used for cDNA synthesis. Reverse transcription using Superscript reverse transcriptase-II (Invitrogen) and Oligo dT (Invitrogen) to prime the reaction was carried out in 20 μL of reaction mix. PCR cycles consisted of initial denaturation at 95 °C for 5 min followed by 30–35 amplification cycles of denaturation at 94 °C for 45 s, annealing for 45 s, and extension at 72 °C for 45 s and final extension at 72 °C for 10 min. β-actin was used as the housekeeping gene and 100-bp ladder (Invitrogen) was used as molecular weight markers. The primer sequences used in this study are represented in Table 2.
Table 2.
Represents the list of genes primers along with the forward and reverse sequences, Annealing conditions and region of amplification
| Gene symbol | Primer sequences (5′-3′) | Tm | Size |
|---|---|---|---|
| β-actin | 5′-TCACCCACACTGTGCCCATCTACGA-3′ 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ |
58 °C | 294 |
| Keratin-19 | 5′-TGAGGTCATGGCCGAGCAGAAC-3′ 5′-CATGAGCCGCTGGTACTCCTGA-3′ |
58 °C | 331 |
| Keratin-8 | 5′-CAGATCAAGTATGAGGAGCTGCA-3′ 5′-AGCTGGTGCGGCTGAAGGAT-3′ |
58 °C | 329 |
| Keratin-18 | 5′-CCATGCGCCAGTCTGTGGAG-3′ 5′-GTGGTGCTCTCCTCA ATCTGCT-3′ |
58 °C | 322 |
Indirect immunofluorescence
For immunostaining, HEKs and DMSCs were plated in two-well chamber slides (Nunc, Roskilde, Denmark). Briefly, attached cells were washed with PBS and then fixed in 4 % paraformaldehyde at 4 °C for 30 min; permeabilized with 0.2 % Triton X-100 for 10 min at 25 °C and probed with primary antibodies (Table 2) overnight at 4 °C. The cells were then washed and incubated with secondary antibody Alexa Fluor 488 (Life Technologies, Carlsbad, CA, USA) at room temperature for 1.0 h. Finally the cells were counter stained with 4,6-diamidino-2-phenylindole (DAPI, Sigma, St. Louis, MO, USA), mounted and examined under NIKON ECLIPSE 90i microscope (NIKON Corporation, Tokyo, Japan) and fluorescence images were captured using Qcapture Software. Negative controls without primary antibodies were used for each marker in the study.
Flow cytometry analysis
Immunophenotyping of DMSCs was performed using flow cytometry with an objective to identify the presence of MSC-specific cell surface antigens at passage 5 (early) and 15 (late). Conjugated antibodies CD44-PE, CD73-PE, CD90-PE, CD105-PE, CD34-FITC, CD45-FITC, HLA-DR-FITC (BD Pharmingen; Table 3) were analysed with these cells. DMSCs were dissociated with 0.25 % trypsin–EDTA upon reaching 90 % confluency and re-suspended in DPBS (Invitrogen) at a concentration of 1 × 106 cells/mL. Samples were stained with conjugated antibodies for 1 h at 4 °C and washed with FACS buffer for 5 min. Cells were identified by light scatter for 10,000 gated events and analysed using BD Cell Quest Pro software (BD). Appropriate isotype matched controls were used to set the instrument parameters.
Table 3.
Represents the list of antibodies used to characterize the HSKs and DMSCs
| Antigen | Antibody | Dilution used | Brand and cat. no. | Application |
|---|---|---|---|---|
| E-cadherin | E-Cadherin, mouse IgG2a | 1:50 | BD transduction laboratories (610181) | Cultured cells (in situ staining) and flow cytometry |
| Keratin-5 | Anti-cytokeratin 5 antibody (Keratin-5) | 1:50 | Millipore (Temecula, CA, USA) (MAB1620) | Immunohistochemistry, immunofluorescence and flow cytometry |
| Keratin-8 | Anti-keratin epithelial antibody (Keratin-8) | 1:50 | Millipore (MAB1611) | Immunohistochemistry and western blotting |
| Cytokeratin-7 | Cytokeratin-7, mouse IgG1 | 1:100 | BD Pharmingen (550507) | Immunohistochemistry and western blotting |
| Vimentin | Monoclonal mouse immunoglobulin | 1:100 | Millipore (MAB3400) | Cultured cells (in situ staining) and flow cytometry |
| CD-44 | Anti-human CD44 | 1:50 | BD Pharmingen (550989) | Immunophenotyping by flow cytometry |
| CD-73 | Anti-human CD73 | 1:50 | BD Pharmingen (550257) | Immunophenotyping by flow cytometry |
| CD-90 | Anti-human CD90 | 1:50 | BD Pharmingen (555596) | Immunophenotyping by flow cytometry |
| CD-166 | Anti-human CD166 | 1:50 | BD Pharmingen (559263) | Immunophenotyping by flow cytometry |
| HLA-DR | Anti-human HLA-DR | 1:50 | BD Pharmingen (347363) | Immunophenotyping by flow cytometry |
| CD-45 | Anti-human CD45 | 1:50 | BD Pharmingen (555482) | Immunophenotyping by flow cytometry |
| CD-34 | Anti-human CD34 | 1:50 | BD Pharmingen (555821) | Immunophenotyping by flow cytometry |
Assessment of cell viability by 7-amino actinomycin D (7-AAD) staining
In order to evaluate the cell survival, we employed a biologically relevant cytometric method of staining the non-viable cells by dye exclusion using the fluorescent, DNA binding probe 7-AAD. Staining of non-viable (membrane permeable) cells is broadly used most likely due to the simple procedure, and the stained cells are bright red and easy to identify. After harvesting, the cells were stained by ready-to-use 7-AAD staining solution (BD Pharmingen) as per manufacturer’s instructions. The cells were analysed on a flow cytometer (BD).
In vitro differentiation potential
To assess the mesodermal differentiation potential of DMSCs, the cultures were initiated at a density of 3000 cells/cm2 in six welled plates (Nunc) and were allowed to reach confluence. Thereafter cells were induced for differentiation towards osteogenic, chondrogenic and adipogenic potential and specific tissue staining was performed using published protocols (Mamidi et al. 2011). Briefly, cells were cultured up to 3 weeks at 37 °C and 5 % CO2 in air (Binder, USA) with prepared osteo-, chondro- and adipogenic differentiation media. Osteogenic induced cells were stained with Von Kossa to observe calcium deposits. Chondrogenic medium treated cells were evaluated by Alcian blue staining for proteoglycan accumulation. Oil Red O stain was used to identify the lipid droplets formation to confirm adipogenic differentiation.
Statistical analysis
Data are presented as mean ± SEM. Results were analyzed by student’s t test. Differences were considered statistically significant when P < 0.05.
Results
Isolation of keratinocytes from human skin biopsies
We examined whether small fragments of skin tissues upon plating for explant culture can yield keratinocytes or not. Interestingly most of the tissues pieces attached after 48 h and showed the outgrowth of primary keratinocytes (Fig. 1e, f) with epithelial morphology like pavement stones (Fig. 1g). Complete culture was filled with large number of evenly distributed primary keratinocytes by 14–21 days (Fig. 1h).
Characterization of keratinocytes
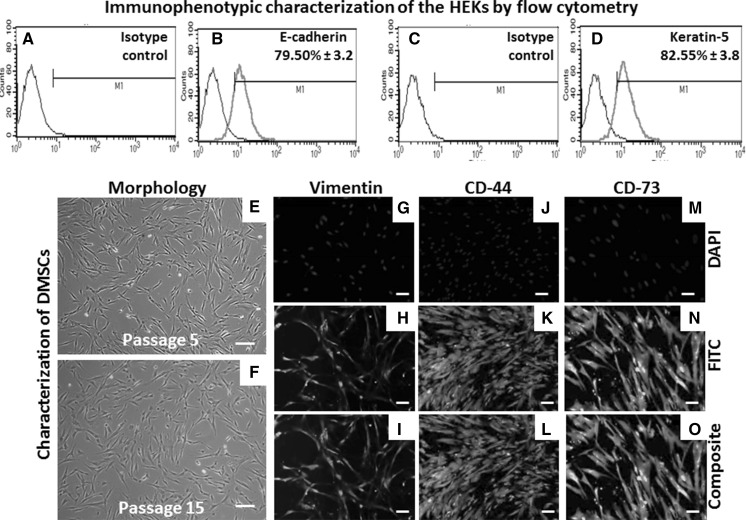
Morphologically HEKs displayed honeycomb pattern of cohesive polygonal cells filled with granular cytoplasm (Fig. 2a). Cytological studies by H&E staining revealed keratinocyte architecture (Fig. 2b). Gene expression analysis by RT–PCR showed expression of key keratinocyte markers including keratin-19, 8 and 18 (Fig. 2c). These findings were further supported by immunocytochemistry results demonstrating expression of essential keratinocyte markers such as transmembrane protein E-cadherin (Fig. 2d–f), keratin-5 (Fig. 2g–i), keratin-8 (Fig. 2j–l), cytokeratin-7 (Fig. 3m–o). Next, the quantitative expression of the ketatinocyte markers including E-cadherin (79.50 % ± 3.2; Fig. 3a, b) and keratin-5 (82.55 % ± 4.3; Fig. 3c, d) was shown by flow cytometry. Taken together, these data sets prove that the authenticity of initial population of these epithelial cells as keratinocytes.
Fig. 2.
Molecular and cellular characterization of HEKs: a morphology of HEKs shows cohesive polygonal cells filled with granular cytoplasm. b H&E staining showing polygonal architecture of primary keratinocytes. c RT–PCR studies revealed the expression key HEK markers. Immunocytochemistry of HEKs demonstrated uniform fluorescence when immunostained against d–f E-cadherin, g–i Keratn-5, j–l Keratin-8, and m–o cytokeratin-7 using specific antibodies. 4,6-diamino-2-phenylindole (DAPI) was used for each sample as a nuclear stain. Green colour represents FITC conjugate. Scale bars 100 μm (a, b, j–o), 200 μm (g–i), 400 μm (d–f). (Color figure online)
Fig. 3.
Immunophenotyping of the HEKs: flow cytometry revealed the positive expression of these ketatinocyte markers such as (a, b) E-cadherin along with its specific isotype control and (c, d) keratin-5 with isotype control. Expansion and characterization of dermal-derived mesenchymal-like stromal cells (DMSCs): e Phase contrast photographs of DMSCs at early passage (P5); f late passages (P15), respectively. Immunofluorescence analysis of DMSCs using: g–i anti-vimentin; j–l anti-CD44; m–o anti-CD73 antibodies. Vimentin being a cytoskeletal protein shows cytoskeletal localization while CD44 and CD73 being cell surface antigens demonstrate surface localization. DAPI was used as a nuclear stain. Scale bars 100 μm (e–o). (Color figure online)
Stromal cells derived next from explant cultures reveal distinct growth kinetics and other features easily distinguishable from keratinocytes
After harvesting the primary keratinocytes by trypsinization, mother explants when continued in culture further showed the outgrowth of stromal cells (Fig. 1i) and reached confluence within 2 weeks (Fig. 1j–k). These cells demonstrated small spindle-shaped cell morphology (Fig. 3e) and were maintained in the culture for up to 15 passages (Fig. 3f). Further these cells stained positive for cytoskeletal proteins vimentin (Fig. 3g–i), the cell surface antigen CD44 (Fig. 3j–l) and CD73 (Fig. 3m–o).
Flow cytometric analysis and in vitro differentiation capacity of DMSCs
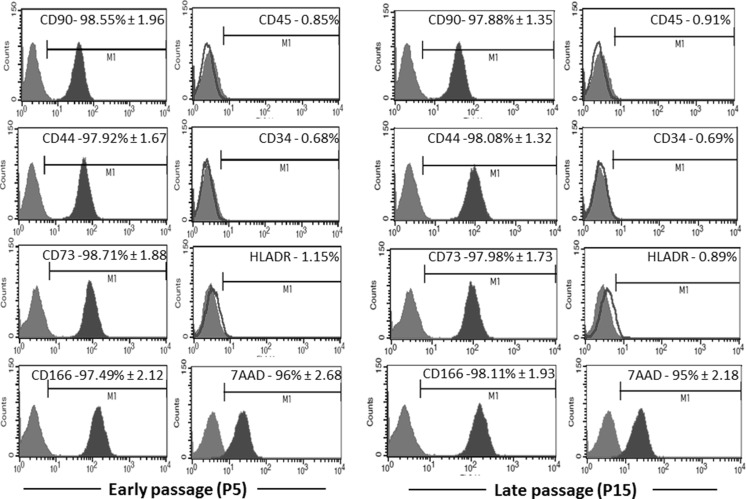
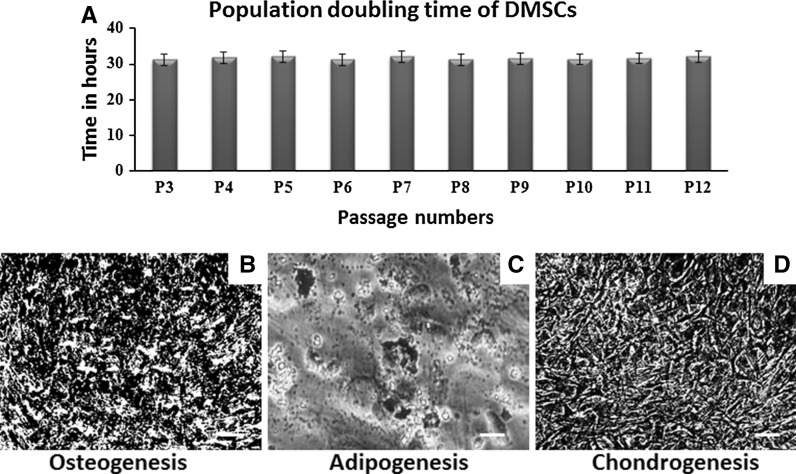
Flow cytometric analysis carried out using stromal cells at passage 5 and 15. These cells showed more than 95 % positive expression against MSC-associated surface markers including CD90, CD44, CD73, and CD166 (Fig. 4). As expected, the expression of CD34, CD45, and HLA-DR in these stromal cells were found to be negative (Fig. 4). Cell viability of DMSCs at passage 5 and 15 was found to be 96 % and 95 %, respectively (Fig. 4). Population doubling time was found to be 31 h over the period of 12 passages (Fig. 5a). As to the multilineage differentiation potential, our results demonstrate that these stromal cells cultured in proper media were able to initiate differentiation into osteoblasts (Fig. 5b), adipocytes (Fig. 5c) and chondrocytes (Fig. 5d). Altogether, these results confirm the minimum criteria for qualifying these stromal cells are MSCs as per International Society for Cellular Therapy (ISCT) recommendations (Dominici et al. 2006).
Fig. 4.
Stromal marker expression and viability of DMSCs: flow cytometric analysis of cell surface markers and cell viability in DMSCs at early (P5) and late passages (P15). Experiments were carried out in triplicates and data are expressed as mean of standard deviation (SD). Red lines indicate isotype control. (Color figure online)
Fig. 5.
Population doubling time and mesodermal differentiation potential of DMSCs: a population doubling time of DMSCs as determined over a period of 12 passages. b Von Kossa stained the calcium deposits resulted due to osteogenic differentiation. c Lipid droplets formed as a result of adipogenic differentiation was confirmed with Oil Red O stain. d Alcian blue staining was applied to detect chondrogenic differentiation. Scale bars 100 μm (b–d). (Color figure online)
Discussion
The explant culture is an established age old method in tissue culture as pioneered by classical experiments of Harrison in 1907 (Harrison et al. 1907). We exploited this traditional technique to reveal the hidden face of explant cultures in derivation of HEK and DMSC together. Our analysis of HEK progeny displayed the hallmarks of epithelial cell type and exhibited important keratinocyte markers both at gene and protein level similar to other studies (Hartmann-Fritsch et al. 2013). However, there are limitations in the expansion of these HEKs. These challenges can be addressed either via cellular reprogramming by overexpression of self-renewal gene like telomerase or employment of DMSCs as feeder layer for expansion of HEKs. Further, it has been shown that the keratinocytes without paracrine or mechanical support from MSCs or in the absence of fibroblasts formed only an irregular epidermal layer emphasizing the importance of a cross-talk between mesenchyme and epithelium for epidermal stratification (Hartmann-Fritsch et al. 2013; Markowicz et al. 2005). Our results demonstrating successive growth and proliferation of keratinocytes and stromal cells reinforce the co-existence of these two cell types in the intact skin explant in a stratified manner thereby supporting each other via paracrine signalling, precisely the phenomenon which we are capitalized on.
MSCs isolated from various pre- and post-natal tissues have evolved as promising drug for various cell therapies due to their excellent safety records, relative resilience in ex vivo expansion, differentiation ability, multiple donor tissue types and immunomodulatory effects during transplants (Uccelli et al. 2011; Trounson 2009; Mamidi et al. 2012; Badylak and Nerem 2010; Nuschke 2014). Nonetheless recently skin has emerged as an attractive source for MSCs as it can be obtained abundantly. Here, the naive DMSCs could be easily propagated up to 15 passages. In addition, they retained their multipotent status over a long period of time. Interestingly our data are supported by the recent findings by Vaculik et al. (2012) showing multilineage differentiation potential of stromal cells derived from human skin. The authentic MSC nature of these cells was confirmed by morphology, growth kinetics, stromal markers expression and mesodermal differentiation potential. We envisage that this alternative source of MSCs can be applied for biological dressings to promote wound healing.
Skin explant culture is known to give rise to either epithelial cells or fibroblast like outgrowth. In the present study too we obtained two types of cells in the explant culture of human skin viz. HEKs and DMSCs. However, contamination of dermal fibroblasts in our culture cannot be ruled out since the characteristics of MSCs and fibroblast are largely similar. The growth of fibroblasts in our culture might have been inhibited due to high glucose concentration of KO-DMEM which selectively permitted the growth of DMSCs. Fibroblasts are highly susceptible to high glucose concentration, and thus delay wound healing in diabetic models (Loots et al. 1999). On the contrary MSCs have been shown to be resistant to high glucose concentrations (Weil et al. 2009).
The use of skin explant culture is limited due to its short term survival in vitro. However, based on our present data we hypothesize several advantages with this current protocol such as: (a) explant cultures are cost effective, easy to handle and less time consuming; (b) offers sequential isolation of two important cell types HEKs and DMSCs which holds both clinical as well as commercial potential: (c) cellular outgrowth can be obtained from the mother explants at regular intervals by repeated enzymatic digestions to enhance the cell yield; (d) explant culture has been considered as a method of choice to get cellular outgrowth from mother explant whenever the quantity of the tissue sample is very limited. The essence of our present study is to obtain sequential expansion of two important cellular components of human skin viz. HEKs and DMSCs from a small biopsy tissue.
Conclusion
This culture system to a reasonable extent mimics in vivo scenario and generates two most valuable cell types HEKs and DMSCs by a simple methodology just by taking advantage of their coexistence in situ. These two cell types either together or separately hold promise in basic aspects of dermatological research as well as regenerative medicine.
Acknowledgments
The authors gratefully acknowledge Manipal University for the continuous support and also acknowledge the paramedical staff of Dermatology department, Manipal Hospital, Bangalore for providing the skin samples.
Footnotes
Shyam Mahabal and Vijay Bhaskar Reddy Konala have contributed equally to this work.
References
- Badylak SF, Nerem RM. Progress in tissue engineering and regenerative medicine. Proc Natl Acad Sci USA. 2010;107:3285–3286. doi: 10.1073/pnas.1000256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock AJ, Higham MC, MacNeil S. Use of human fibroblasts in the development of a xenobiotic-free culture and delivery system for human keratinocytes. Tissue Eng. 2006;12:245–255. doi: 10.1089/ten.2006.12.245. [DOI] [PubMed] [Google Scholar]
- Coolen NA, Verkerk M, Reijnen L, Vlig M, van den Bogaerdt AJ, Breetveld M, Gibbs S, Middelkoop E, Ulrich MM. Culture of keratinocytes for transplantation without the need of feeder layer cells. Cell Transpl. 2007;16:649–661. doi: 10.3727/000000007783465046. [DOI] [PubMed] [Google Scholar]
- De Corte P, Verween G, Verbeken G, Rose T, Jennes S, De Coninck A, Roseeuw D, Vanderkelen A, Kets E, Haddow D, Pirnay JP. Feeder layer- and animal product-free culture of neonatal foreskin keratinocytes: improved performance, usability, quality and safety. Cell Tissue Bank. 2012;13:175–189. doi: 10.1007/s10561-011-9247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Feisst V, Brooks AE, Chen CJ, Dunbar PR. Characterization of mesenchymal progenitor cell populations directly derived from human dermis. Stem Cells Dev. 2014;23:631–642. doi: 10.1089/scd.2013.0207. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci USA. 1979;76:5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG, Greenman MJ, Mall FP, Jackson CM. Observations of the living developing nerve fiber. Anat Rec. 1907;1:116–128. doi: 10.1002/ar.1090010503. [DOI] [Google Scholar]
- Hartmann-Fritsch F, Hosper N, Luginbühl J, Biedermann T, Reichmann E, Meuli M. Human amniotic fluid derived cells can competently substitute dermal fibroblasts in a tissue-engineered dermo-epidermal skin analog. Pediatr Surg Int. 2013;29:61–69. doi: 10.1007/s00383-012-3207-2. [DOI] [PubMed] [Google Scholar]
- Hefton JM, Madden MR, Finkelstein JL, Shires GT. Grafting of burn patients with allografts of cultured epidermal cells. Lancet. 1983;2:428–430. doi: 10.1016/S0140-6736(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Hennings H, Holbrook KA, Yuspa SH. Potassium mediation of calcium-induced terminal differentiation of epidermal cells in culture. J Investig Dermatol. 1983;81:50–55s. doi: 10.1111/1523-1747.ep12540491. [DOI] [PubMed] [Google Scholar]
- Higham MC, Dawson R, Szabo M, Short R, Haddow DB, MacNeil S. Development of a stable chemically defined surface for the culture of human keratinocytes under serum-free conditions for clinical use. Tissue Eng. 2003;9:919–930. doi: 10.1089/107632703322495565. [DOI] [PubMed] [Google Scholar]
- Loots MA, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res. 1999;291:93–99. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- Mamidi MK, Pal R, Mori NA, Arumugam G, Thrichelvam ST, Noor PJ, Abdullah HM, Gupta PK, Das AK, Zakaria Z, Bhonde R. Co-culture of mesenchymal-like stromal cells derived from human foreskin permits long term propagation and differentiation of human embryonic stem cells. J Cell Biochem. 2011;112:1353–1363. doi: 10.1002/jcb.23052. [DOI] [PubMed] [Google Scholar]
- Mamidi MK, Nathan KG, Singh G, Thrichelvam ST, Mohd Yusof NA, Fakharuzi NA, Zakaria Z, Bhonde R, Das AK, Majumdar AS. Comparative cellular and molecular analyses of pooled bone marrow multipotent mesenchymal stromal cells during continuous passaging and after successive cryopreservation. J Cell Biochem. 2012;113:3153–3164. doi: 10.1002/jcb.24193. [DOI] [PubMed] [Google Scholar]
- Markowicz M, Koellensperger E, Neuss S, Pallua N (2005) Adult bone marrow mesenchymal stem cells as feeder cells for human keratinocytes: new approaches in bilayered skin replacements. Top Tissue Eng 4(2):1–12
- Mazlyzam AL, Aminuddin BS, Fuzina NH, Norhayati MM, Fauziah O, Isa MR, Saim L, Ruszymah BH. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns. 2007;33:355–363. doi: 10.1016/j.burns.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10:29–37. doi: 10.4161/org.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenteau NL, Nolte CM, Bilbo P, Rosenberg M, Wilkins LM, Johnson EW, Watson S, Mason VS, Bell E. Epidermis generated in vitro: practical considerations and applications. J Cell Biochem. 1991;45:245–251. doi: 10.1002/jcb.240450304. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/S0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Sun T, Higham M, Layton C, Haycock J, Short R, MacNeil S. Developments in xenobiotic-free culture of human keratinocytes for clinical use. Wound Repair Regen. 2004;12:626–634. doi: 10.1111/j.1067-1927.2004.12609.x. [DOI] [PubMed] [Google Scholar]
- Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009;7:29. doi: 10.1186/1741-7015-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- Vaculik C, Schuster C, Bauer W, Iram N, Pfisterer K, Kramer G, Reinisch A, Strunk D, Elbe-Bürger A. Human dermis harbors distinct mesenchymal stromal cell subsets. J Investig Dermatol. 2012;132:563–574. doi: 10.1038/jid.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Mattey DL, Garrod DR. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J Cell Biol. 1984;99:2211–2215. doi: 10.1083/jcb.99.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil BR, Abarbanell AM, Herrmann JL, Wang Y, Meldrum DR. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1735–R1743. doi: 10.1152/ajpregu.90876.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]