Abstract
Acute pancreatitis (AP) is considered as major problem around the world and the incidence of AP is increasing. Carvacrol (CAR), a monoterpenic phenol, has good antioxidant activity. This in vivo study was designed to evaluate whether CAR provide protection against AP that developed by pancreas injury. The rats were randomised into groups to receive (I) no therapy; (II) 50 µg/kg cerulein at 1 h intervals by four intraperitonally (i.p.) injections; (III) 50, 100 and 200 mg/kg CAR by one i.p. injection; and (IV) cerulein plus CAR after 2 h of cerulein administration. 12 h later, serum samples were obtained to assess pancreatic function, the lipase and amylase values. The oxidative stress markers were evaluated by changes in the amount of lipid peroxides measured as malondialdehyde (MDA) and changes in main tissue antioxidant enzyme levels including SOD, CAT and GSH-PX. Histopathological examination was performed using scoring systems. Additionally, oxidative DNA damage was determined by measuring the increases of 8-hydroxy-deoxyguanosine (8-OH-dG) formations. We found that the increasing doses of CAR decreased AP-induced MDA and 8-OH-dG levels. Moreover, the pancreas antioxidant enzyme activities were higher than that of the rats in the AP group when compared to the AP plus CAR group. In the treatment groups, the lipase and amylase were reduced. Besides, histopathological findings in the pancreatic tissue were alleviated (p < 0.05). We suggest that CAR could be a safe and potent new drug candidate for treating AP through its antioxidative mechanism of action for the treatment of a wide range of disorders related to pancreas.
Keywords: Experimental acute pancreatitis, Pancreas, Carvacrol, Antioxidant response, Oxidative DNA damage, Histopathology
Introduction
AP is a necroinflammatory pancreatic disease with significant morbidity and mortality due to lack of specific therapy (Petrov et al. 2010). New findings of AP-associated risk have been reported in the peripancreatic tissues and even remote organs (Yang et al. 2014). The development of AP is a multistep process, the exact mechanism of which remains controversial. Recent research efforts demonstrate a major role of oxidative stress risk in the development, and progression of most pancreatic diseases, including recurrent acute and chronic pancreatitis (Robles et al. 2013). Drugs have been shown to increase the risk of developing AP (Stobaugh and Deepak 2014; Giorda et al. 2014; Oliveira et al. 2014). And the supplementation of certain medicinal herbal extracts with drug may reduce the stress caused by drugs (Lata et al. 2014). This new knowledge promises to improve disease management and prevention through alternative treatment. Rationally, it is time to look for alternative truly effective interventions for patients with this dreadful disease.
The exploratory investigations of new natural or synthetic antioxidants becomes a very popular in the world since the last 20 years (Geyikoglu and Turkez 2008); in addition, many efforts have been performed to explore novel antioxidant-natured compounds (Cingolani et al. 2000; Cacciatore et al. 2003, 2005; Rispoli et al. 2004; Turkez and Sisman 2007; Heuking et al. 2009; Turkez and Dirican 2012). At this point, especially, in recent decades plant essential oils (EOs) and their components have attracted increased interest and consequently have been extensively investigated mainly in in vitro and in vivo systems (Turkez et al. 2012a). Monoterpenes are obtained from essential oils and many of them have shown antioxidant activity. Carvacrol (CAR) used as the most active constituent of thyme EOs is a predominant monoterpenoic phenol and exhibits a potent anti-fungal, anti-viral, anti-tumor, anti-diabetic and anti-inflammatory effect (Bayramoglu et al. 2014). It is also recognized as a safe food additive and used as flavoring agent in packed foods, sweets, beverages, and chewing gum (Abdollahi et al. 2012; Gilling et al. 2014). Moreover, CAR shows a high antioxidative effectiveness against DNA lesions (Horvathova et al. 2014) and provides protection against lipid peroxidation (Quiroga et al. 2015). But its antioxidant effect on AP-caused tissue dysfunctions is not yet documented. Oxidative stress induced by reactive oxygen species (ROS) is thought to be critically involved in pancreas dysfunction (Wang and Roper 2014). Paradoxically, according to some papers, well-known antioxidants, including vitamins C and E, may promote or even induce peroxidation (Pinchuk et al. 2012). Hence our observation must be interpreted carefully. Thus, the present study was aimed to elucidate the effectiveness of CAR therapy on pancreas injuries in an AP model, using biochemical and histopathological methods. Also, we analysed 8-hydroxy-deoxyguanosine (8-OH-dG), an important marker of oxidative DNA damage.
Materials and methods
Animals
Seventy adult male Spraque-Dawley rats (weighing 200–250 g) obtained from the Medical Experimental Application and Research Center, Atatürk University (Erzurum, Turkey) were used. Animals were housed inside polycarbonate cages in an air-conditioned room (22 ± 2 °C) with 12 h light–dark cycle. Standard rat feed and water were provided ad libitum. The rats were allowed to acclimatize to the laboratory environment for 7 days before the start of the experiment. All procedures were performed in conformity with the Institutional Ethical Committee for Animal Care and Use at the Atatürk University (protocol number: B.30.2.ATA.0.23.85-11) and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Experimental protocols
Animals were randomly divided into eight groups (n = 7, each): (I) vehicle-treated group (control); (II) AP group; (III, IV and V) CAR-treated groups (50, 100 and 200 mg/kg); (VI, VII and VIII) CAR-treated AP groups. AP was induced by cerulein (Sigma-Aldrich, GmbH, Stenheim, Germany) administered i.p. four times with 1 h intervals at a dose of 50 µg/kg b.w. AP was assessed after last injection of cerulein by measurement of serum amylase and lipase levels. Animals without induction of AP (control) were treated i.p. with saline at the same time when animals were treated with cerulein.
To evaluate the effects of CAR, animals were treated with carvacrol in 10 ml of saline (Peptide International Inc, Louisville, KY, USA). The CAR groups received one i.p. injection of 50, 100 and 200 mg/kg b.w. Therapeutic treatments were administered after 2 h of cerulein injection. The rats were anesthetized with isoflurane after 12 h taking CAR and euthanized by exsanguination with blood retained for serum harvest.
Biochemical analyses
Amylase and lipase measurement for pancreatic function assessment
Serum amylase and lipase levels were determined spectrophotometrically using an automated analyzer (Olympus AU 600, Diamond Diagnostic, Holliston, USA). All chemicals were obtained from Sigma (St. Louis, MO, USA).
Determination of lipid peroxidation
Lipid peroxidation was determined by quantifying malondialdehyde (MDA) concentrations, which was spectrophotometrically measured by the absorbance of a red-colored product with thiobarbituric acid (Ohkawa et al. 1979).
Antioxidant enzymes
The activities of antioxidant enzymes were assayed in pancreas tissue of each group. For this purpose, the tissues were removed, cleaned, and washed in ice-cold normal saline for biochemical studies. All samples were stored at −70 °C until assayed. The 10 % homogenates of tissues were prepared in the phosphate buffer (0.1 M, pH 7.4) containing 1 mmol ethylenediaminetetra acetic acid (EDTA), 0.25 mM sucrose, 10 mM potassium chloride (KCl), and 1 mM phenylmethyl sulfonyl fluoride (PMSF).
The superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich (1972). In this test, the degree of inhibition of pyrogallol auto-oxidation by pancreas homogenate supernatant was measured. The change in absorbance was read at 470 nm against blank every 3 min on a spectrophotometer and the enzyme activity was expressed as 50 % inhibition of adrenaline auto oxidation/min.
The catalase (CAT) activity was measured as follows (Beers and Sizer 1952). For CAT activity, dichromatic acetic acid is reduced to chromic acetate when heated in the presence of H2O2, with the formation of perchloric acid as an unstable intermediate. In the test, the green color development was read at 590 nm against blank in a spectrophotometer. The activity of CAT was expressed as μmol of H2O2 consumed/mg protein/min.
The glutathione peroxidase (GSH-PX) activity was determined essentially as described by Rotruck et al. (1973). The rate of glutathione oxidation by H2O2, as catalyzed by the GSH-PX present in the supernatant is determined and the color developed was read against a reagent blank at 412 nm in a spectrophotometer. In the test, the enzyme activity was expressed as μmol of glutathione oxidized/mg protein/min.
Histopathological examination
The pancreas tissues of rats were fixed in buffered 10 % formalin solution for 24 h and embedded in a paraffin wax. Tissues were then sectioned at 5 μm, stained with hematoxylin eosin (H & E), Masson trichrome and Amyloid methods. A semiquantitative evaluation of pancreas tissue was accomplished by scoring the degree of severity according to the formerly published criteria (Akyazi et al. 2013). For each pancreas section, whole slide was examined for acinar damage, fibrosis vacuolisation, infiltration, edema and congestion were observed under bright field using an Olympus BX60 microscope. In addition, high-resolution pictures (200×) of samples were taken under the same microscope. The pancreas damage was scored with maximum score of 18. The maximum score for the other pathological findings was 3.
Determination of 8-OHdG level
8-hydroxy-2′-deoxyguanosine assay kits were purchased from Cayman Chemical Company (Ann Arbor, MI, USA) for determining 8-OH-dG levels in the pancreas samples. Since it is a competitive assay that can be used for the quantification of 8-OH-dG in homogenates and recognizes both free 8-OH-dG and DNA-incorporated 8-OH-dG, many researches have been performed to use this protocol. This assay depends on the competition between 8-OH-dG and 8-OH-dG-acetylcholinesterase (AChE) conjugate (8-OH-dGTracer) for a limited amount of 8-OH-dG monoclonal antibody. All procedures were carried out in accordance with the provider manual.
Statistical analysis
For statistical analysis, we used SPSS for Windows 18.0 (SPSS Inc., Chicago, IL, USA). The experimental data were analysed using oneway analysis of variance (ANOVA) followed by Tukey post hoc test for multiple comparisons. Results are presented as mean ± standard deviation (SD) and values p < 0.05 were regarded as statistically significant.
Results
Tables 1 and 2 show the effects of CAR on biochemical parameters in all experimental groups. The serum amylase levels in cerulein-induced AP were increased from an average 486 U/L to about 2346 U/L as compared with those of control rats. Similarly, lipase level was increased from 23 to 124 U/L. Following intraperitoneal injection of CAR alone, the amylase and lipase levels were not changed. Moreover, in animals with AP, the increasing dosages of CAR showed positive effects on above parameters and the values were significantly decreased with respect to high dose of CAR (p < 0.05).
Table 1.
Effect of CAR treatment on serum amylase and lipase levels in cerulein-induced AP
| Groups | Amylase (U/L) mean ± SD | Lipase (U/L) mean ± SD |
|---|---|---|
| Control | 486.85 ± 19.98a | 23.52 ± 1.20a,b |
| AP | 2346.56 ± 443.51d | 124.33 ± 9.38d |
| CAR 50 mg/kg | 503.64 ± 26.01a | 24.01 ± 1.67a,b |
| CAR 100 mg/kg | 472.29 ± 35.25a | 22.67 ± 2.03a |
| CAR 200 mg/kg | 478.38 ± 22.85a | 23.89 ± 0.96a,b |
| AP + CAR 50 mg/kg | 1719.51 ± 367.98c | 108.27 ± 5.82c,d |
| AP + CAR 100 mg/kg | 1344.43 ± 291.25b,c | 94.40 ± 11.40c |
| AP + CAR 200 mg/kg | 547.68 ± 41.69a,b | 39.14 ± 3.47b |
Data are presented as mean ± SD (n = 7). The means in the same column marked by different letters are significantly different (p < 0.05) from each other
AP acute pancreatitis, CAR carvacrol
Table 2.
Effects of CAR treatments on pancreas SOD, CAT, GSH-PX activities and MDA levels in cerulein-induced AP
| Groups | SOD (U/mg-protein) mean ± SD | CAT (U/mg-protein) mean ± SD | GSH-PX (U/mg-protein) mean ± SD | MDA (nmol/mg-protein) mean ± SD |
|---|---|---|---|---|
| Control | 4.13 ± 0.18a | 11.10 ± 0.53b,c | 0.63 ± 0.05b,c | 1.80 ± 0.21a |
| AP | 3.11 ± 0.23c | 2.85 ± 0.14e | 0.11 ± 0.07f | 2.99 ± 0.34c |
| CAR 50 mg/kg | 4.15 ± 0.32a | 11.17 ± 0.49a,b | 0.67 ± 0.08a,b | 1.75 ± 0.49a |
| CAR 100 mg/kg | 4.25 ± 0.37a | 11.31 ± 0.33a,b | 0.69 ± 0.11a,b | 1.83 ± 0.27a |
| CAR 200 mg/kg | 4.30 ± 0.46a | 12.20 ± 0.42a | 0.89 ± 0.14a | 1.81 ± 0.19a |
| AP + CAR 50 mg/kg | 3.18 ± 0.15c | 3.24 ± 0.20d,e | 0.20 ± 0.13e | 2.89 ± 0.38c |
| AP + CAR 100 mg/kg | 3.23 ± 0.42b,c | 4.11 ± 0.18d | 0.32 ± 0.05d | 2.79 ± 0.33b,c |
| AP + CAR 200 mg/kg | 4.10 ± 0.24a,b | 10.10 ± 0.43c | 0.58 ± 0.14c | 1.90 ± 0.40a,b |
Data are presented as mean ± SD (n = 7). The means in the same column marked by different letters are significantly different (p < 0.05) from each other. For abbreviations see legend in Table 1
As presented in Table 2, the SOD, CAT, and GSH-PX activities markedly decreased in pancreas of AP rats while MDA increased (p < 0.05) compared to those found in the controls. CAR groups alone showed an increased level of antioxidant enzymes at both dosages (50 and 100 mg/kg) but the best result was observed at a dose of 200 mg/kg of CAR. In the AP + CAR group at high dose, the antioxidant enzymes revealed statistically significant (p < 0.05) increases and oxidative stress returned to the control levels.
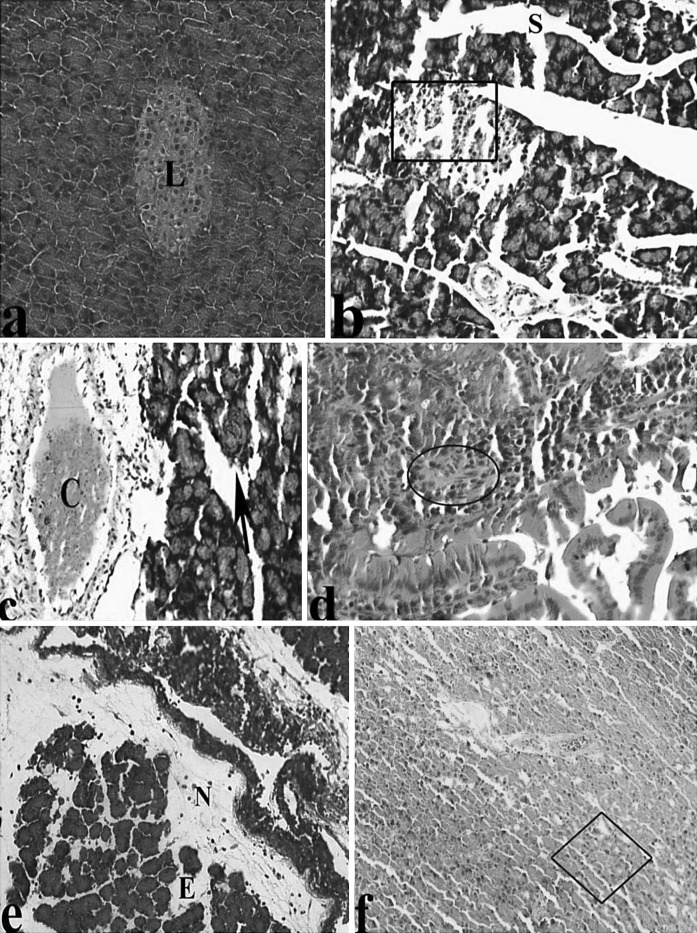
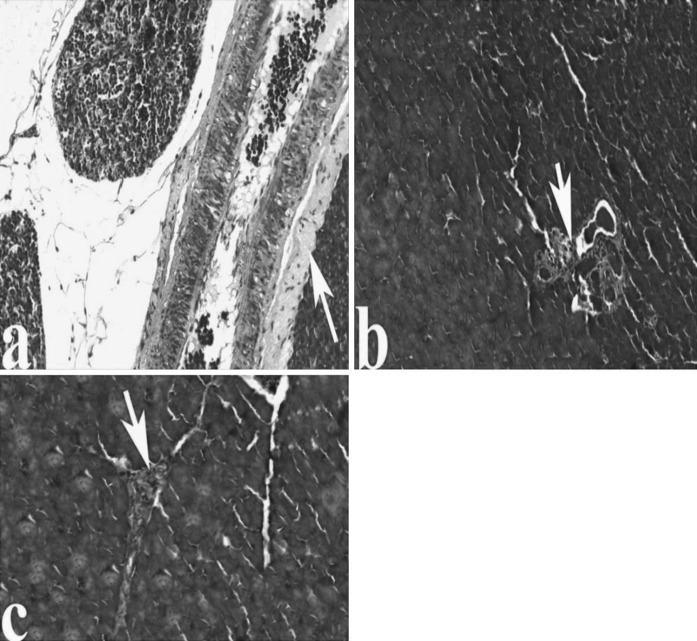
An in-depth characterization of pancreas pathology in AP rats, selected images of different methods-stained AP pancreas are presented in the following Figures. For H&E stains in comparison to the controls, the microscopic observations showed a significant dilatation, Langerhans degeneration, congestion, acinar cell injury, infiltration, fibrosis, fat necrosis, edema and vacuolisation (Fig. 1a–f).
Fig. 1.
Light microscopic appearances of pancreas from control and AP rats. a Pancreas of control group, Langerhans islet (L). Pancreas in AP rats; b major Sinusoidal dilatation (S), degeneration of Langerhans (inside of quadrangle symbol), c congestion (C), asinus necrosis (black arrow), d infiltration (I), fibrosis (inside of circle symbol), portal vein (Pv), e fat necrosis (N), edema (E), f vacuolisation (inside of square symbol), (H&E)
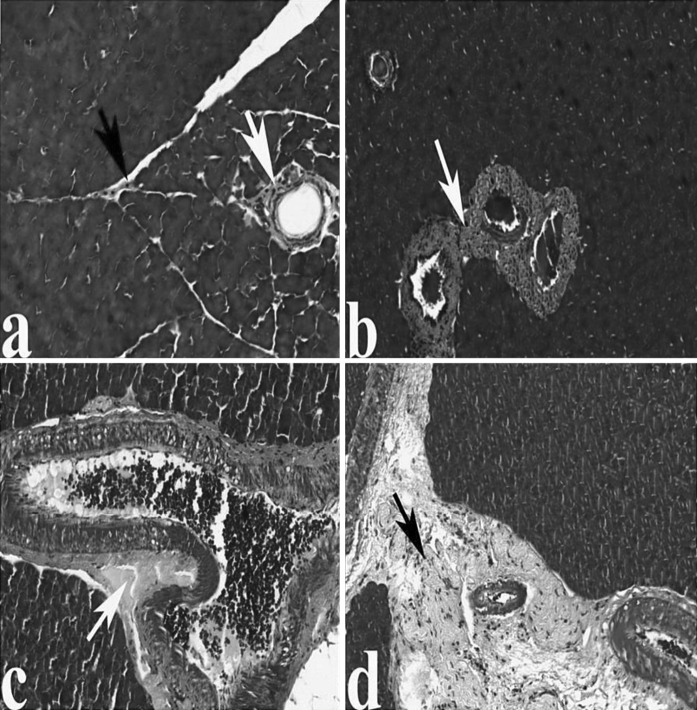
Masson trichrome staining clearly revealed increased intensity of fibrosis in veins and also in intercellular space in AP-induced rats (Fig. 2a–d). Amyloid method showed protein deposits in blood vessels indicating disorders of circulation, respectively (Fig. 3a, b).
Fig. 2.
The pancreas in control and AP group rats. a The normal architecture of pancreas from control rats, the collagen fibers in veins and intercellular space (arrows). Pancreas in AP group; b, c increased intensity of fibrosis in veins of AP group rat pancreas (arrows), d increased fibrosis in intercellular space (arrow), (Masson trichrome)
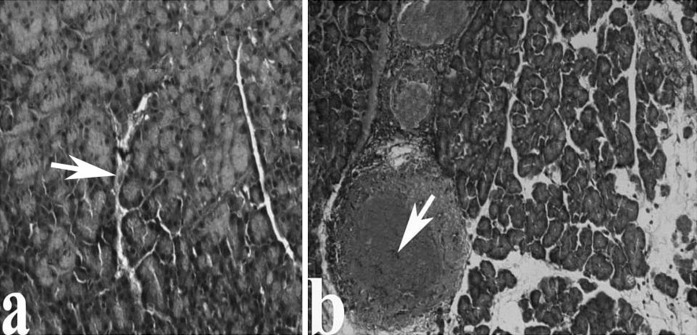
Fig. 3.
The pancreas in control and AP groups, respectively. a Pancreas of control group, The protein (white arrow). b The accumulation of protein in vessels of AP group as compared with the cerulein untreated rats (white arrow), (Amyloid)
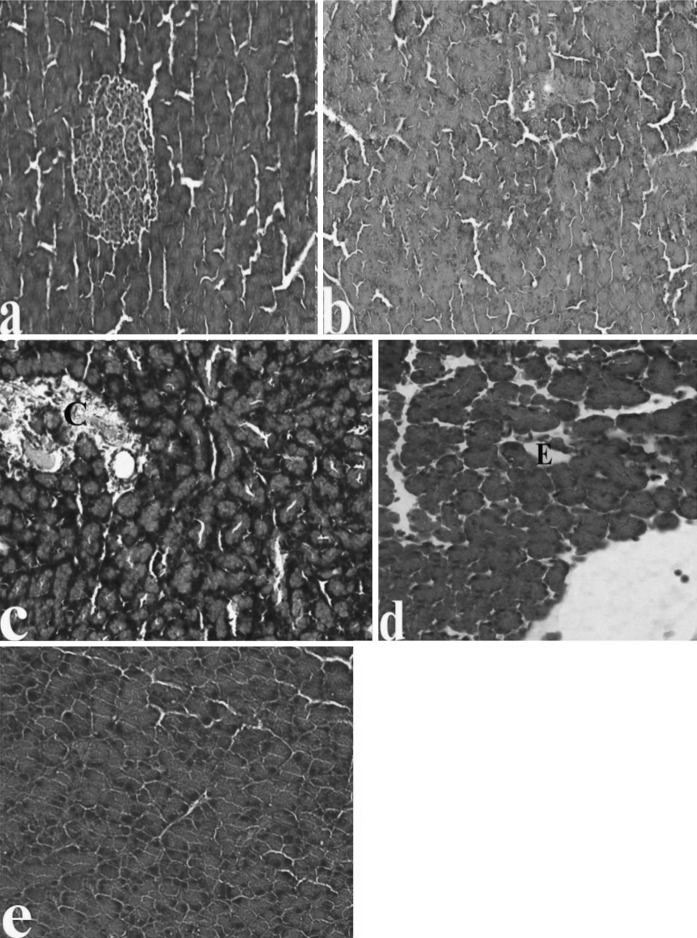
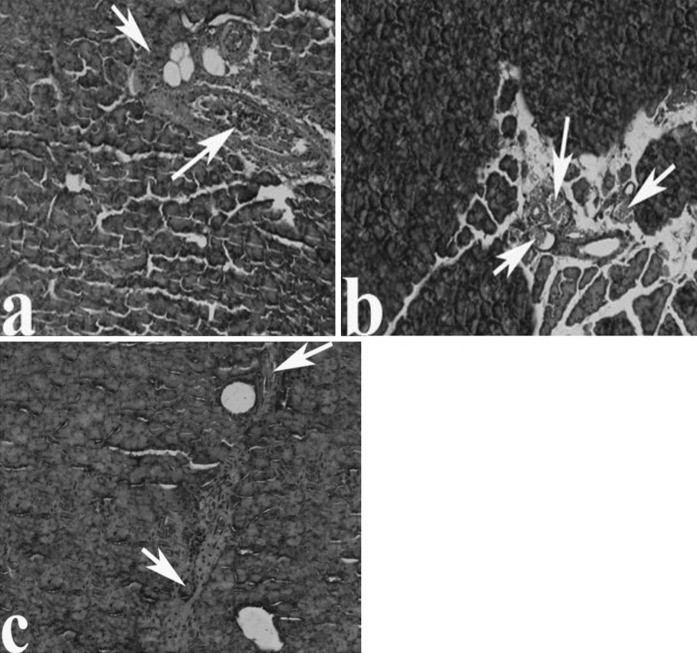
Examination of pancreas sections in CAR groups indicated that the structures of pancreas tissues were similar in the control group and all CAR treated groups. Significant pathological conditions in pancreas tissues were not seen either in the control group or in the 50, 100 and 200 mg/kg CAR-treated groups (data not shown). In the 50 and 100 mg/kg CAR + AP groups, above-mentioned pathological findings were attenuated. There were reduction of sinusoidal dilatations and congestion in a clear dose-dependent manner. Moreover a significant protective effect was observed after treatment with 200 mg/kg CAR (Fig. 4a–e). The increasing CAR doses decreased intensity of fibrosis in the pancreatic tissue of the AP treated animals (Fig. 5a–e). Moreover, abnormal protein accumulations in blood vessels did not occured (Fig. 6a–c). The pancreas tissue showed a normal structure and ordered arrangement and resembled those of control rats. Histopathological scores of the groups are summarized in Table 3. The degree of pathological findings showed a significant difference between groups treated with cerulein and cerulein + CAR (p < 0.05).
Fig. 4.
The pancreas tissue following CAR exposure in AP rats; a Decreased sinusoidal dilatations in the AP + 50 mg/kg CAR group, b–d decreased sinusoidal dilatation, congestion and edema in the AP + 100 mg/kg CAR group as compared with the AP + 50 mg/kg CAR group, respectively. e Normal histological structure of pancreas in the AP + 200 mg/kg CAR group. Abbreviations are as Fig. 1, (H&E)
Fig. 5.
The pancreas tissue following CAR exposure in AP rats; a, b in the 50 and 100 mg/kg CAR groups, decreased collagen fibers in veins, respectively. c The pancreatic structure is similar to controls in the AP + 200 mg/kg CAR group. Fibrosis symbols are as in Fig. 2, (Masson trichrome stain)
Fig. 6.
The pancreas tissue following CAR exposure in AP rats; a the protein deposits in the 50 mg/kg CAR group, b in the 100 mg/kg CAR group, decreased protein deposits in veins, c the pancreatic structure is similar to controls in the AP + 200 mg/kg CAR group. The symbols are as in Fig. 3, (Amyloid stain)
Table 3.
Histopathological scores of pancreas pathology in cerulein-induced AP
| Groups | Pancreas degeneration (maximum score 18) mean ± SD | Edema (maximum score: 3) mean ± SD | Vacuolisation (maximum score: 3) mean ± SD | Necrosis (maximum score: 3) mean ± SD | Fibrosis (maximum score: 3) mean ± SD | İnfiltration (maximum score: 3) mean ± SD | Congestion (maximum score: 3) mean ± SD |
|---|---|---|---|---|---|---|---|
| Control | 0.25 ± 0.12a | 0.25 ± 0.12a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| AP | 8.36 ± 0.52d | 2.84 ± 0.19c | 1.94 ± 0.11d | 1.27 ± 0.14d | 0.63 ± 0.14c | 0.89 ± 0.17c | 0.68 ± 0.13c |
| CAR 50 mg/kg | 0.23 ± 0.11a | 0.08 ± 0.02a | 0.02 ± 0.02a | 0.03 ± 0.02a | 0.02 ± 0.01a | 0.03 ± 0.02a | 0.04 ± 0.01a |
| CAR 100 mg/kg | 0.16 ± 0.08a | 0.04 ± 0.01a | 0.02 ± 0.01a | 0.03 ± 0.01a | 0.04 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a |
| CAR 200 mg/kg | 0.18 ± 0.06a | 0.08 ± 0.01a | 0.01 ± 0.01a | 0.02 ± 0.01a | 0.01 ± 0.01a | 0.01 ± 0.01a | 0.01 ± 0.01a |
| AP + CAR 50 mg/kg | 6.64 ± 0.42c | 2.50 ± 0.10bc | 1.39 ± 0.12c | 1.05 ± 0.08c | 0.38 ± 0.11b | 0.69 ± 0.10b | 0.39 ± 0.11b |
| AP + CAR 100 mg/kg | 6.01 ± 0.37c | 2.24 ± 0.12b | 1.22 ± 0.13c | 0.92 ± 0.10c | 0.31 ± 0.14b | 0.61 ± 0.13b | 0.33 ± 0.07b |
| AP + CAR 200 mg/kg | 0.74 ± 0.14b | 0.32 ± 0.07a | 0.17 ± 0.02b | 0.13 ± 0.04b | 0.05 ± 0.02a | 0.04 ± 0.02a | 0.03 ± 0.01a |
Data are presented as mean ± SD (n = 7). The means in the same column marked by different letters are significantly different (p < 0.05) from each other. For abbreviations see legend in Table 1
The levels of 8-OHdG, a hallmark of oxidative DNA damage, were measured using an 8-OH-dG detection kit. There were no significant differences between the levels of 8-OHdG in the control and all CAR treated groups (Table 4). On the contrary, the level of 8-OHdG was significantly higher in AP as compared to the control group. However, treatment with CAR decreased the 8-OHdG levels that were increased by cerulein-induced AP in a clear dose dependent manner.
Table 4.
Effect of CAR treatment on pancreas 8-OH-dG levels in cerulein-induced AP
| Groups | 8-OH-dG level (as pg/ml) mean ± SD |
|---|---|
| Control | 1.15 ± 0.14a |
| AP | 3.99 ± 0.55c |
| CAR 50 mg/kg | 0.97 ± 0.12a |
| CAR 100 mg/kg | 1.16 ± 0.19a |
| CAR 200 mg/kg | 1.09 ± 0.15a |
| AP + CAR 50 mg/kg | 2.25 ± 0.22b |
| AP + CAR 100 mg/kg | 1.58 ± 0.18b |
| AP + CAR 200 mg/kg | 1.17 ± 0.23a |
Data are presented as mean ± SD (n = 7). The means in the same column marked by different letters are significantly different (p < 0.05) from each other. For abbreviations see legend in Table 1
Discussion
AP refers to afflicted inflammation of pancreas with unfavorable adverse effects. Unfortunately, there is no therapeutic method for this disease. Oxidative stress has a serious role in the pathogenesis of AP. Thus, decreasing of oxidative stress may prevent induction and progression of AP (Minaiyan et al. 2014). Antioxidatives are widely used and recommended in common clinical praxis (Turkez and Togar 2010; Turkez et al. 2012b, c; Patockova et al. 2014). Our aim was to evaluate the role of CAR in prevention of induced oxidative stress in AP.
Different causes of protective effects of natural compounds have been considered and discussed by various scientists. We reveal that the DNA-protective effect of CAR in pancreas represents induction of antioxidative enzymes (SOD, CAT and GSH-PX) and also modulation of oxidative stress. Targets of accumulating ROS include proteins involve in antioxidant response (Geyikoglu and Turkez 2008; Turkez et al. 2010). Endogenous antioxidant defense systems regulate the levels of ROS to maintain normal physiological homeostasis. Free radical scavengers, CAT and SOD provide significant protection in pancreas (Jaworek et al. 2012). SOD removes superoxide radical by converting it into H2O2 that is rapidly converted to water by CAT. Cell membranes are protected by GSH-PX from ROS-mediated oxidative damage resulting from the formation of H2O2 during normal metabolism in the cells’ mitochondria. In this context, GSH-PX converts H2O2 into water before it can produce damaging ROS. Moreover, GSH-PX reduces lipid hydroperoxides to alcohols. Therefore, any alteration in the activity of these enzymes may result in a number of deleterious effects due to accumulation of superoxide radicals and hydrogen peroxide (Romeu et al. 2002; Turkez and Geyikoglu 2011; Zhou and Yao 2013). In our study, lipid peroxidation was elevated in the pancreas of rat after exposure to cerulein, as evidenced by increased MDA production. However, CAR was capable of preventing lipid peroxidation, and significantly increasing the endogenous antioxidant defense mechanisms of pancreatic tissue of rats. In the respiratory system, CAR treatment decreases MDA and shows a significant protective role (Boskabady and Jalali 2013). Again, CAR exerts greater levels of SOD and GSH-PX in the rat brain than the untreated controls (Youdim and Deans 2000) and it also decreases MDA level in mouse liver (Akkol et al. 2009). Thus, in recent years, considerable research has been undertaken in an effort to establish the biological actions of CAR for its potential use in clinical applications. CAR has been qualified as natural antioxidants due to its ability to reduce free radical formation and scavenge free radicals (Karioti et al. 2006). However, much of its antioxidant properties have been demonstrated in experimental in vitro systems (Aydin et al. 2014). Carvacrol decreases peroxidation of phospholipid liposomes and is found to be a good scavenger of peroxyl radicals in the β-carotene bleaching (BCB) test and in DPPH and TBARS assays (Aeschbach et al. 1994; Kulisic et al. 2004). It has also been reported that isoprenoid such as, CAR, via post-transcriptional actions, suppressed 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase activity in liver, the rate limiting step for the synthesis of MDA (Akkol et al. 2009). In another study, CAR improved H2O2-induced injury on isolated rat pancreas cells (Dagli Gul et al. 2013). According to our in vivo findings, the decrease in the MDA level in the groups of rat treated with increasing CAR doses (especially 200 mg/kg) might be a significant indicator of defense mechanisms in pancreatic tissue. Surely, the high content of CAR could be reckoned as an important property that affects the antioxidant capacity (Saei-dehkordi et al. 2010). Indeed, the hydroxyl group and the presence of a system of delocalized electrons are important for the antioxidant activity of phenolic compounds, such as CAR. Such a particular structure would allow the compound to act as reducing agents, hydrogen or electron donators, and singlet oxygen quenchers (Oke et al. 2009).
Oxidative damage to DNA is quantitated in studied tissues of experimental animals by measuring the increases of 8-O-HdG formations (Neophytou et al. 2014). 8-OHdG is also used for risk assessment of cancers and degenerative diseases (Valavanidis et al. 2009; Turkez et al. 2015). Our study found that the increasing doses of CAR in rat pancreas decreased pancreatitis-induced 8-OHdG levels. The results obtained in the present study showed a statistically significant DNA-protective effect of the antioxidant CAR. Moreover, no detrimental effect was observed for CAR alone at any concentration of exposure according to the histopathological scores. In vitro results suggest that CAR tested could prevent ROS from reaching biomolecules such as lipoproteins, polyunsaturated fatty acids, DNA, amino acids, proteins, and sugars. CAR prevents 8-OH-dG formations in lung cancer cell lines and protects the cells against oxidative stress (Ozkan and Erdogan 2012). Moreover, the resistance against hydrogen peroxide-induced DNA damage in hepatic and testicular tissues is higher in rats given CAR (Slamenova et al. 2008). Besides, the potent free radical scavenger activity of CAR is demonstrated in hepatocellular carcinogenesis (Jayakumar et al. 2011).
According to histopathological findings of this study the induction of AP resulted in a significant increase in pancreatic necrosis. In a significant number of pathological conditions, including disease states as AP, ROS production prevails over the antioxidant defense mechanisms leading to chronic oxidative stress thus causing injury to cellular components. Thus, ROS-associated lipid peroxidation causes pancreatic damage during AP (Müller et al. 2014). Consistent with the enhanced necrosis response in the AP rat compared to their control counterparts, we found a striking increase in both interstitial and perivascular fibrosis in the AP pancreas. Pancreatic necrosis has been defined as loss of acinar cells followed by the development of fibrosis (Hyun and Lee 2014). This is most likely the primary cause of the significant deterioration of pancreas function and microcirculation disorders (Banks et al. 1996; Alhan et al. 2001; Sugimoto et al. 2004). In our study, the efficacy of CAR treatment in reducing fibrosis and improving regeneration was firstly determined in AP rat pancreas. Our results also indicated that CAR treatment resulted in (1) significant attenuation of pancreas dysfunction (decreased amylase and lipase levels); and (2) significant attenuation of pancreas congestion and inflammation. Previous studies support our findings. CAR has long been used medicinally, proving to be beneficial in treatment of circulatory problems and improved the pancreatic blood flow during AP (Karkabounas et al. 2006; Esmaeili 2013). In diabetic animals, the lesions of the pancreas and liver are decreased after CAR exposure (Akkol et al. 2009; Ezhumalai et al. 2014). And CAR is shown to be the effective antioxidant in protecting the d-galactosamine-induced lipid peroxidation of the erythrocytes, liver and kidney membranes (Aristatile et al. 2009). CAR also depicts a preventive effect on leukocyte migration (in vivo and in vitro) and shows anti-inflammatory effects in respirator system. In addition, CAR is able to control receptors that play an important role in inflammation (Hotta et al. 2010). For this reason, antioxidants as CAR are potentially useful in positively influencing the systemic redox balance and in consequence tissue inflammation and MDA (Fabian et al. 2013).
From the results, it is evident that CAR is capable of modulating the levels of MDA and significantly increases the endogenous antioxidant defense mechanisms in AP-induced pancreatic damage. Our results also show that the significant increase in the levels of serum markers is prevented by CAR treatment. Moreover, CAR does not present negative effects in the rat pancreas. Thus, we suggest that CAR might be developed as an effective therapeutic agent for preservation of pancreas in AP.
References
- Abdollahi A, Hassani A, Ghosta Y, Bernousi I, Meshkatalsadat MH, Shabani R, Ziaee SM. Evaluation of essential oils for maintaining postharvest quality of Thompson seedless table grape. Nat Prod Res. 2012;26:77–83. doi: 10.1080/14786419.2010.541887. [DOI] [PubMed] [Google Scholar]
- Aeschbach R, Loliger J, Scott BC, Murcia A, Butler J, Halliwell B, Aruoma OI. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol. 1994;32:31–36. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]
- Akkol EK, Avci G, Küçükkurt I, Keleş H, Tamer U, Ince S, Yesilada E. Cholesterol-reducer, antioxidant and liver protective effects of Thymbra spicata L. var. spicata. J Ethnopharmacol. 2009;126:314–319. doi: 10.1016/j.jep.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Akyazi I, Eraslan E, Gülçubuk A, Ekiz EE, Cırakli ZL, Haktanir D, Bala DA, Ozkurt M, Matur E, Ozcan M. Long-term aspirin pretreatment in the prevention of cerulein-induced acute pancreatitis in rats. World J Gastroenterol. 2013;19:2894–2903. doi: 10.3748/wjg.v19.i19.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhan E, Kücüktülü U, Ercin C, Deger O, Cicek R. The effects of dopexamine on acute necrotizing pancreatitis in rats. Eur J Surg. 2001;167:761–767. doi: 10.1080/11024150152707743. [DOI] [PubMed] [Google Scholar]
- Aristatile B, Al-Numair KS, Veeramani C, Pugalendi KV. Effect of carvacrol on hepatic marker enzymes and antioxidant status in d-galactosamine-induced hepatotoxicity in rats. Fundam Clin Pharmacol. 2009;23:757–765. doi: 10.1111/j.1472-8206.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- Aydin E, Turkez H, Keleş MS. The effect of carvacrol on healthy neurons and N2a cancer cells: some biochemical, anticancerogenicity and genotoxicity studies. Cytotechnology. 2014;66:149–157. doi: 10.1007/s10616-013-9547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks PA, Tenner S, Noordhoek EC, Sica G, Feng S, Zinner M. Does pancreatic necrosis predict severity in patients with acute necrotizing pancreatitis? Digestion. 1996;5:218–229. [Google Scholar]
- Bayramoglu G, Senturk H, Bayramoglu A, Uyanoglu M, Colak S, Ozmen A, Kolankaya D. Carvacrol partially reverses symptoms of diabetes in STZ-induced diabetic rats. Cytotechnology. 2014;66:251–257. doi: 10.1007/s10616-013-9563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Boskabady MH, Jalali S. Effect of carvacrol on tracheal responsiveness, inflammatory mediators, total and differential WBC count in blood of sensitized guinea pigs. Exp Biol Med. 2013;238:200–208. doi: 10.1177/1535370212474604. [DOI] [PubMed] [Google Scholar]
- Cacciatore I, Caccuri AM, Di Stefano A, Luisi G, Nalli M, Pinnen F, Ricci G, Sozio P. Synthesis and activity of novel glutathione analogues containing an urethane back-bone linkage. Farmaco. 2003;58:787–793. doi: 10.1016/S0014-827X(03)00135-6. [DOI] [PubMed] [Google Scholar]
- Cacciatore I, Caccuri AM, Cocco A, De Maria F, Di Stefano A, Luisi G, Pinnen F, Ricci G, Sozio P, Turella P. Potent isozyme-selective inhibition of human glutathione S-transferase A1-1 by a novel glutathione S-conjugate. Amino Acids. 2005;29:255–261. doi: 10.1007/s00726-005-0232-7. [DOI] [PubMed] [Google Scholar]
- Cingolani GM, Di Stefano A, Mosciatti B, Napolitani F, Gior-gioni G, Ricciutelli M, Claudi F. Synthesis of L-(+)-3-(3-hydroxy-4-pivaloyloxybenzyl)-2,5-diketomorpholine as potential prodrug of L-dopa. Bioorg Med Chem Lett. 2000;10:1385–1388. doi: 10.1016/S0960-894X(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Dagli Gul AS, Fadillioglu E, Karabulut I, Yesilyurt A, Delibasi T. The effects of oral carvacrol treatment against H2O2-induced injury on isolated pancreas islet cells of rats. Islets. 2013;5:149–155. doi: 10.4161/isl.25519. [DOI] [PubMed] [Google Scholar]
- Esmaeili A. Biological activities and chemical composition of the stems and roots of Helichrysum oligocephalum DC grown in Iran. Pak J Pharm Sci. 2013;26:599–604. [PubMed] [Google Scholar]
- Ezhumalai M, Radhiga T, Pugalendi KV. Antihyperglycemic effect of carvacrol in combination with rosiglitazone in high-fat diet-induced type 2 diabetic C57BL/6J mice. Mol Cell Biochem. 2014;385:23–31. doi: 10.1007/s11010-013-1810-8. [DOI] [PubMed] [Google Scholar]
- Fabian E, Pölöskey P, Kósa L, Elmadfa I, Rethy LA. Nutritional supplements and plasma antioxidants in childhood asthma. Central Eur J Med. 2013;125:309–315. doi: 10.1007/s00508-013-0359-6. [DOI] [PubMed] [Google Scholar]
- Geyikoglu F, Turkez H. Boron compounds reduce vanadium tetraoxide genotoxicity in human lymphocytes. Environ Toxicol Pharmacol. 2008;26:342–347. doi: 10.1016/j.etap.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Gilling DH, Kitajima M, Torrey JR, Bright KR. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J Appl Microbiol. 2014;116:1149–1163. doi: 10.1111/jam.12453. [DOI] [PubMed] [Google Scholar]
- Giorda CB, Nada E, Tartaglino B, Marafetti L, Gnavi R. A systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs. Diabetes Obes Metab. 2014;16:1041–1047. doi: 10.1111/dom.12297. [DOI] [PubMed] [Google Scholar]
- Heuking S, Iannitelli A, Di Stefano A, Borchard G. Toll-like receptor-2 agonist functionalized biopolymer for mucosal vaccination. Int J Pharm. 2009;381:97–105. doi: 10.1016/j.ijpharm.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Horvathova E, Navarova J, Galova E, Sevcovicova A, Chodakova L, Snahnicanova Z, Melusova M, Kozics K, Slamenova D. Assessment of antioxidative, chelating, and dna-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact rosmarinus officinalis oil. J Agric Food Chem. 2014;62:6632–6639. doi: 10.1021/jf501006y. [DOI] [PubMed] [Google Scholar]
- Hotta M, Nakata R, Katsukawa M, Hori K, Takahashi S, Inoue H. Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J Lipid Res. 2010;51:132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun JJ, Lee HS. Experimental models of pancreatitis. Clin Endosc. 2014;47:212–216. doi: 10.5946/ce.2014.47.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworek J, Szklarczyk J, Jaworek AK, Nawrot-Porąbka K, Leja-Szpak A, Bonior J, Kot M. Protective effect of melatonin on acute pancreatitis. Int J Inflam. 2012;2012:173675. doi: 10.1155/2012/173675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar S, Madankumar A, Asokkumar S, Raghunandhakumar S, Gokula Dhas K, Kamaraj S, Josephine Divya MG, Devaki T. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2011;360:51–60. doi: 10.1007/s11010-011-1043-7. [DOI] [PubMed] [Google Scholar]
- Karioti A, Vrahimi-Hadjilouca T, Droushiotis D, Rancic A, Hadjipavlou-Litina D, Skaltsa H. Analysis of the essential oil of Origanum dubium growing wild in Cyprus. Investigation of its antioxidant capacity and antimicrobial activity. Planta Med. 2006;72:1330–1334. doi: 10.1055/s-2006-947255. [DOI] [PubMed] [Google Scholar]
- Karkabounas S, Kostoula OK, Daskalou T, Veltsistas P, Karamouzis M, Zelovitis I, Metsios A, Lekkas P, Evangelou AM, Kotsis N, Skoufos I. Anticarcinogenic and antiplatelet effects of carvacrol. Exp Oncol. 2006;28:121–125. [PubMed] [Google Scholar]
- Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- Lata S, Singh S, NathTiwari K, Upadhyay R. Evaluation of the antioxidant and hepatoprotective effect of phyllanthus fraternus against a chemotherapeutic drug cyclophosphamide. Appl Biochem Biotechnol. 2014;173:2163–2173. doi: 10.1007/s12010-014-1018-8. [DOI] [PubMed] [Google Scholar]
- Minaiyan M, Zolfaghari B, Taheri D, Gomarian M. Preventive effect of three pomegranate (Punica granatum L.) seeds fractions on cerulein-induced acute pancreatitis in mice. Int J Prev Med. 2014;5:394–404. [PMC free article] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the auto oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Müller S, Kaiser H, Krüger B, Fitzner B, Lange F, Bock CN, Nizze H, Ibrahim SM, Fuellen G, Wolkenhauer O, Jaster R. Age-dependent effects of UCP2 deficiency on experimental acute pancreatitis in mice. PLoS One. 2014;9:e94494. doi: 10.1371/journal.pone.0094494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou AM, Hart JE, Chang Y, Zhang JJ, Smith TJ, Garshick E, Laden F. Short-term traffic related exposures and biomarkers of nitro-PAH exposure and oxidative DNA damage. Toxics. 2014;2:377–390. doi: 10.3390/toxics2030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Oke F, Aslim B, Ozturk S, Altundag S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009;112:874–879. doi: 10.1016/j.foodchem.2008.06.061. [DOI] [Google Scholar]
- Oliveira NM, Ferreira FA, Yonamine RY, Chehter EZ. Antiretroviral drugs and acute pancreatitis in HIV/AIDS patients: is there any association? A literature review. Einstein (Sao Paulo) 2014;12:112–119. doi: 10.1590/S1679-45082014RW2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan A, Erdogan A. A comparative study of the antioxidant/prooxidant effects of carvacrol and thymol at various concentrations on membrane and DNA of parental and drug resistant H1299 cells. Nat Prod Commun. 2012;7:1557–1560. [PubMed] [Google Scholar]
- Patockova J, Sliva J, Marhol P, Crkovska J, Stipek S. The influence of vitamin-rich diet on the extent of lipoperoxidation in brain of mice during an acute post-insulin hypoglycaemia. Eur J Pharmacol. 2014;740:641–644. doi: 10.1016/j.ejphar.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterol. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Pinchuk I, Shoval H, Dotan Y, Lichtenberg D. Evaluation of antioxidants: scope, limitations and relevance of assays. Chem Phys Lipids. 2012;165:638–647. doi: 10.1016/j.chemphyslip.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Quiroga PR, Asensio CM, Nepote V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J Sci Food Agric. 2015;95:471–479. doi: 10.1002/jsfa.6744. [DOI] [PubMed] [Google Scholar]
- Rispoli V, Rotiroti D, Carelli V, Liberatore F, Scipione L, Marra R, Giorgioni G, Di Stefano A. Choline pivaloyl esters improve in rats cognitive and memory performances impaired by scopolamine treatment or lesions of the nucleus basalis of Meynert. Neurosci Lett. 2004;356:199–202. doi: 10.1016/j.neulet.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Robles L, Vaziri ND, Ichii H. Role of oxidative stress in the pathogenesis of pancreatitis: effect of antioxidant therapy. Pancreat Disord Ther. 2013;3:112. doi: 10.4172/2165-7092.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeu M, Mulero M, Giralt M, Folch J, Nogués MR, Torres A, Fortuño A, Sureda FX, Cabré M, Paternáin JL, Mallol J. Parameters related to oxygen free radicals in erythrocytes, plasma and epidermis of the hairless rat. Life Sci. 2002;71:1739–1749. doi: 10.1016/S0024-3205(02)01946-X. [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48:1562–1567. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Slamenova D, Horvathova E, Marsalkova L, Wsolova L. Carvacrol given to rats in drinking water reduces the level of DNA lesions induced in freshly isolated hepatocytes and testicular cells by H2O2. Neoplasma. 2008;55:394–399. [PubMed] [Google Scholar]
- Stobaugh DJ, Deepak P. Effect of tumor necrosis factor-α inhibitors on drug-induced pancreatitis in inflammatory bowel disease. Ann Pharmacother. 2014;48:1282–1289. doi: 10.1177/1060028014540869. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Takada T, Yasuda H. A new experimental pancreatitis by incomplete closed duodenal loop: the influence of pancreatic microcirculation on the development and progression of induced severe pancreatitis in rats. Pancreas. 2004;28:e112–e119. doi: 10.1097/00006676-200405000-00023. [DOI] [PubMed] [Google Scholar]
- Turkez H, Dirican E. A modulator against mercury chloride-induced genotoxic damage: dermatocarpon intestiniforme (L.) Toxicol Ind Health. 2012;28:58–63. doi: 10.1177/0748233711404036. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. The efficacy of bismuth subnitrate against genotoxicity and oxidative stress induced by aluminum sulphate. Toxicol Ind Health. 2011;27:133–142. doi: 10.1177/0748233710381894. [DOI] [PubMed] [Google Scholar]
- Turkez H, Sisman T. Anti-genotoxic effect of hydrated sodium calcium aluminosilicate on genotoxicity to human lymphocytes induced by aflatoxin B1. Toxicol Ind Health. 2007;23:83–89. doi: 10.1177/0748233707076738. [DOI] [PubMed] [Google Scholar]
- Turkez H, Togar B. The genotoxic and oxidative damage potential of olanzapine in vitro. Toxicol Ind Health. 2010;26:583–588. doi: 10.1177/0748233710373090. [DOI] [PubMed] [Google Scholar]
- Turkez H, Tatar A, Hacimuftuoglu A, Ozdemir E. Boric acid as a protector against paclitaxel genotoxicity. Acta Biochim Pol. 2010;57:95–97. [PubMed] [Google Scholar]
- Turkez H, Aydin E, Aslan A. Xanthoria elegans (Link) (lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology. 2012;64:679–686. doi: 10.1007/s10616-012-9447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Mokhtar YI, Togar B. Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology. 2012;64:15–25. doi: 10.1007/s10616-011-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Yousef MI. Ameliorative effect of docosahexaenoic acid on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced histological changes, oxidative stress, and DNA damage in rat liver. Toxicol Ind Health. 2012;28:687–696. doi: 10.1177/0748233711420475. [DOI] [PubMed] [Google Scholar]
- Turkez H, Togar B, Di Stefano A, Taspinar N, Sozio P. Protective effects of cyclosativene on H2O2-induced injury in cultured rat primary cerebral cortex cells. Cytotechnology. 2015;67:299–309. doi: 10.1007/s10616-013-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2-deoxyguanosine (8-OH-dG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- Wang X, Roper MG. Measurement of DCF fluorescence as a measure of reactive oxygen species in murine islets of Langerhans. Anal Methods. 2014;6:3019–3024. doi: 10.1039/C4AY00288A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CJ, Chen J, Phillips AR, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Dig Liver Dis. 2014;46:446–451. doi: 10.1016/j.dld.2014.01.158. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Deans SG. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br J Nutr. 2000;83:87–93. [PubMed] [Google Scholar]
- Zhou X, Yao Y. Unexpected nephrotoxicity in male ablactated rats induced by Cordyceps militaris: the involvement of oxidative changes. Evid Based Complement Alternat Med. 2013;2013:786528. doi: 10.1155/2013/786528. [DOI] [PMC free article] [PubMed] [Google Scholar]