Abstract
Osteoporosis has been reported as a hidden death factor in aged people. So far, prevention and treatment therapies for osteoporosis only slow down the progress but do not treat the disease. Fucoidan has been recognized its roles in anti-tumor, anti-inflammatory, anti-coagulant and antiviral activities. To date, low molecular weight (LMW) fucoidan role in bone loss disease has been not determined yet. Therefore, this study aims to figure out potential effects of LMW fucoidan in osteoporosis in vitro and in vivo. LMW fucoidan was extracted from fresh Sargassum hemiphyllum showing a significant increase in 7F2 cell viability to 150.33 ± 6.50 % relative to normal fucoidan (130.12 ± 5.74 %). The expression of level BMP-2, ALP, osteocalcin significantly increased with 2.28 ± 0.06, 2.18 ± 0.12 and 2.06 ± 0.07 fold, respectively. The RT-PCR assay showed that LMW fucoidan increased mRNA expression of BMP-2, ALP, osteocalcin, COL I, BSP and osteonectin. Furthermore, the bone density and bone ash weight were considerably boosted by the oral administration of 280 mg/kg LMW fucoidan and 100 mg/kg calcium carbonate in C57BL/6J female aged mice. The present finding indicated that LMW fucoidan triggered osteogenic differentiation in vitro, and had an anabolic effect on bone mineralization in vivo. Dietary intake of LMW fucoidan from S. hemiphyllum suggested playing a role in the enhancement of bone loss with increasing age.
Keywords: Low molecular weight fucoidan, Osteoblast, Aged mice, Bone, Mineralization
Introduction
The interactions between osteoblasts and osteoclasts, ensure a proper balance between bone formation and resorption. With the age increasing, a slight unbalance between bone formation and resorption may cause bone loss, and the excessive bone loss may lead to mechanical failure, which can result in osteoporosis (Frost 1992). Osteoporosis is a silent but serious problem in old people, most osteoporotic fractures are associated with higher health care costs, physical disability, impaired quality of life and increased mortality, and there are many synthetic drugs that can prevent and cure osteoporosis. These drugs are all bone resorption inhibitors, which only can retard bone loss passively, but cannot induce new bone formation. Moreover, parts of these drugs bring side effects as abdominal pain, fear (Segal et al. 2003) and increasing risk of breast cancer (Genant et al. 1989). New bone formation is the main function of osteoblasts. Agents that regulate this function are important in reversing osteoporosis. Previous studies showed that sulfated polysaccharides, including fucoidan, heparin and chitosan–chondroitin sulphate, are macromolecules associated with osteogenesis (Park et al. 2011; Santo et al. 2012; Takada et al. 2003). These polysaccharides are associated with osteoblastic cell surface and extracellular matrix, and show beneficial effects on cell differentiation and bone regeneration.
Fucoidan is a class of sulfated, fucosylated polysaccharides found in brown seaweed, it was identified by Kylin (1913). The intensity of biological activities of fucoidan varies with species, molecular weight, composition, structure and the route of extraction (Fitton 2011), and its non-animal origin brings particular pharmacological activities (Senni et al. 2006). Fucoidan has been well studied for its antitumor (Hsu et al. 2013), antiviral (Tengdelius et al. 2014), anti-inflammatory (Hwang et al. 2011), and anticoagulant (Irhimeh et al. 2009) activities. These biological activities are closely related to fucoidan’s molecular weight (Yang et al. 2008) and sulfate content (Soeda et al. 1992). Furthermore, low molecular weight (LMW) fucoidan shows more potent bioactivities compared with that of high molecular weight (HMW) fucoidan (Park et al. 2010). In a recent study, it was reported that LMW fucoidan, <30 kDa, showed potential effects on bone biomaterial osteoconductive properties (Changotade et al. 2008). However, there are no studies that report on the effect of LMW fucoidan (< 1 kDa) on osteogenic differentiation and bone components. In this study, we extracted the fucoidan from Sargassum hemiphyllum and hydrolyzed it with Glycolytic enzyme, which resulted in LMW fucoidan (<1 kDa). Two experiments have been carried out to clarify the role of LMW fucoidan (<1 kDa) from S. hemiphyllum: 1) the effects on the proliferation and differentiation were investigated using 7F2 osteoblastic cell line in vitro; 2) the effects on bone formation were examined in vivo with age-related mice.
Materials and methods
Preparation of fucoidan and low molecular weight fucoidan (LMW fucoidan)
Fresh S. hemiphyllum was collected from the coast of Penghu county, Taiwan during a period from December, 2012 to January, 2013. 100 g of dried S. hemiphyllum was treated with 5 l of distilled water and boiled at 100 °C for 30 min. The hot water extract was centrifuged and the supernatant was lyophilized under the reduced pressure. One part of the lyophilized hot water extract was added to four parts in volume of 95 % ethanol and then allowed to precipitate overnight at 4 °C (Hwang et al. 2011). The precipitated polysaccharides were collected by centrifugation and lyophilized, resulting in the fucoidan sample. For the hydrolysis of fucoidan, 5 g of fucoidan was suspended in 125 ml of distilled water under controlled condition (55 °C and 700 rpm stirring speed). Glycolytic enzyme was added at a concentration of 1 mg/g fucoidan dry weight to the mixture, and the mixture was heated continuously for 6 hours during hydrolysis. After hydrolysis, the hydrolysate was centrifuged at 10,000×g for 20 min at 4 °C to separate undigested residues and solubilized compounds. After centrifugation, the supernatants were collected and lyophilized, resulting in LMW fucoidan sample.
Chemical analysis
Sulfate content was determined according to the gelatin–barium method (Dodgson and Price 1962) using sodium sulfate (1 mg/ml) as standard and after acid hydrolysis of the polysaccharides (6 N HCl, 100 °C, 6 h). Monosaccharide fractions of the polysaccharide extract hydrolysates were separated by HPAEC (Diones BioLC, Taipei, Taiwan) with an anion-exchange column (Carbopac PA-10, 4.6 × 250 mm). The analysis of monosaccharide was carried out at an isocratic NaOH concentration of 18 mM at ambient temperature (Lee et al. 2002).
Molecular weight distribution
Sample was passed through a 30 kDa molecular weight cut-off membrane (ProStream™ PP, TangenX Technology Co., Shrewsbury, MA, USA) and the filtrate was passed through an additional 1 kDa molecular weight cut-off membrane. To determine the molecular weight distribution, sample was injected into a high performance size exclusion chromatography (Waters, Milford, MA, USA) with Ultrahydrogel 120 and Ultrahydrogel 500 column (7.8 × 300 mm, Waters). Elution was carried out at 35 °C with 0.1 M NaNO3 at 0.6 ml/min, and the eluate was monitored using a refractive index detector (Waters). The standard kit contains eight pullulan standards with molecular weights in the range 35–200 kDa.
Cell culture and viability assay
7F2 cell, a murine preosteoblast was obtained from the American Type Culture Collection, Manassas, VA, USA. 7F2 cells were cultured in α-minimum essential medium supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate and 10 % fetal bovine serum (FBS) (Invitrogen Gibco BRL, Rand Island, NY, USA) at 37 °C in an atmosphere of 5 % CO2. The medium was renewed every 2 days.
7F2 cells were seeded into 96-well plates at a density of 1 × 105 cells/well and cultured for 24 h. Then, cells were treated with LWM fucoidan at concentrations of 0.125, 0.25, 0.5, 1, 2 and 4 mg/ml and without LWM fucoidan (Control) under normal cell culture condition for 48 h. The cell viability of cells was determined by MTT colorimetric assay (Vittimberga Jr et al. 1999). Cells were reacted with MTT (1 mg/ml) for 4 h, and absorbance was recorded at 570 nm (Mosmann 1983). The cell viability (%) was determined as (A1/A0) × 100 %, where A0 and A1 were the absorbances in the absence of extracts and presence of extracts, respectively.
Alkaline phosphatase (ALP) activity assay
7F2 cells (1 × 105) were inoculated with medium and treated with 10 μl of different concentrations [0 (control), 0.25, 0.5, 1 and 2 mg/ml] of LMW fucoidan for 48 h. After incubation, the cells were washed three times with phosphate buffered saline (PBS) and homogenized in 25 mM carbonate buffer containing 0.1 % Triton X-100 (pH 10.3). The cellular ALP activity was measured by incubating for 1 h at 37 °C in 250 mM carbonate buffer containing 1.5 mM MgCl2 and 15 mM p-nitrophenyl phosphate. The reaction was stopped by adding 1 M NaOH before measuring absorbance at 405 nm. The ALP activity was determined from the absorbance at 405 nm using a spectrophotometer (Ryu et al. 2009).
Osteocalcin secretion assay
7F2 cells (1 × 105) were inoculated with medium and treated with 10 μl different concentrations (0 (control), 0.25, 0.5, 1 and 2 mg/ml) of LMW fucoidan for 48 h. The cell culture supernatants were collected and measured for osteocalcin by BTI Mouse Osteocalcin (Stoughton, Massachusetts, USA) according to manufacturers’ instruction.
Mineralization assay
7F2 cells (1 × 105) were inoculated with medium and treated with 10 μl different concentrations (0 (control), 0.25, 0.5, 1 and 2 mg/ml) of LMW fucoidan for 7 days. Next, the cells were washed three times with PBS, fixed with cold 70 % ethanol for 1 h and then were stained with 40 mM Alizarin Red S (pH 4.2) for 1 h at room temperature. After removing Alizarin Red S, cells were washed with PBS. The cells were subsequently destained for 15 min with 10 % cetylpyridium chloride in 10 mM of sodium phosphate buffer (pH 7.0). The mineralization was determined by measuring the absorbance at 562 nm (Gregory et al. 2004).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
7F2 cells (1 × 105) were inoculated with medium and treated with 10 μl different concentrations (0 (control), 0.25, 0.5, 1 and 2 mg/ml) of LMW fucoidan for 48 h. Total RNA was isolated by RNAzol B (Amersham Pharmacia Biotech, Uppsala, Sweden), and the concentration of total RNA was detected by spectrophotometer (Hitachi, Tokyo, Japan). The synthesis of cDNA was performed using Improm-II TM Reverse Transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s instructions. PCR was performed on the reverse transcribed cDNA product to determine the expression of bone morphorogenic protein (BMP)-2, BMP-4, collagen type (COL) I, COL II, ALP, bone sialoprotein (BSP), osteocalcin, osteonectin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (as an internal control) using a thermal cycler (biometra, UNO-Thermoblock, Wisbech, UK). Primer sequences used to amplify the desired cDNA were as Table 1. The reactions were carried out in a volume of 25 μl containing 1 unit of Taq DNA polymerase (Dong-Sheng Biotech Co. Yanling Road, Tianhe District, GZ 510507, China), 0.2 mM dNTP, ×10 reaction buffers and 100 pmol of sense and antisense primers. After the initial 1 min of 95 °C denaturation, the amplification sequence protocol of BMP-2 was 1.0 min of 55 °C annealing and 1.0 min of 72 °C extension; that of BMP-4 was 1.0 min of 60 °C annealing and 1.5 min of 72 °C extension; that of COL I was 1.0 min of 56 °C annealing and 1.5 min of 72 °C extension; that of COL II was 1.5 min of 60 °C annealing and 2.5 min of 72 °C extension; that of ALP was 1.0 min of 56 °C annealing and 1.5 min of 72 °C extension; that of BSP and osteocalcin were 1.5 min of 49 °C annealing and 1.0 min of 72 °C extension; that of osteonectin was 1.0 min of 65 °C annealing and 1.5 min of 72 °C extension; and that of GAPDH was 1.0 min of 60 °C annealing and 1.0 min of 72 °C extension, 30 cycles. The sense, antisense PCR primers, and amplification sequence protocol were summarized in Table 1. Above primers were purchased from Mission Biotech Co., Ltd. (Taipei, Taiwan). The PCR products were separated by electrophoresis on 1.2 % agarose gels and visualized by ethidium bromide staining under UV irradiation (Kim et al. 2007). The image of resulting gel was captured and analyzed by ImageMaster VDS and ImageMaster 1D Elite software (Amersham Pharmacia Biotech).
Table 1.
Oligonucleotide sequences of osteoblastic differentiation makers
| Gene name | Oligonucleotide sequences | Product size (bp) |
|---|---|---|
| BMP-2 | Fa: GACGGACTGCGGTCTCCTAAAG | 472 |
| Rb: TCTGCAGATGTGAGAAACTCGTCA | ||
| BMP-4 | F: CGCCGTCATTCCGGATTACAT | 574 |
| R: GGCCCAATCTCCACTCCCTT | ||
| COL I | F: GAGGCATAAAGGGTCATCGTGG | 716 |
| R: CATTAGGCGCAGGAAGGTCAGC | ||
| COL II | F: ACACTGGTAAGTGGGGCAAGACC | 172 |
| R: GGATTGTGTTGTTTCAGGGTTCGGG | ||
| ALP | F: ACTCAGGGCAATGAGGTCAC | 506 |
| R: GAGAGCGAAGGGTCAGTCAG | ||
| BSP | F: AAGTAAAAGTTGGCCTTAGCGACC | 516 |
| R: GCTGAAACCCGTTCAGA | ||
| Osteocalcin | F: GGGGAAGGG ACA ACACATGA | 412 |
| R: TCCTGGACATGGGGATTGA | ||
| Osteonectin | F: GAATTTGAGGACGGTGCA | 419 |
| R: TTCTGCTTCTCAGTGAGGA | ||
| GAPDH | F: TGGTGTCTTCACCACCA | 515 |
| R: TGGTGTCTTCACCACCA |
aForward primer for sequence
bReverse primer for sequence
Animal and LWM fucoidan treatment
The influence of LMW fucoidan on bone formation of aged mice was investigated using 36 weeks old C57BL/6J female mice. These mice were purchased from the National Taiwan University, College of Medicine, Laboratory Animal Center (Taipei, Taiwan).
All animals aged 40 weeks at the start of the experiment and were housed in a normal, environmentally controlled animal room with free access to pathogen-free feed and water ad libitum. Mice were divided into five groups with 5 mice of each. Mice in different groups were treated with saline solution (control), 100 mg/kg calcium carbonate body weight (BW) (Positive control), 70 mg/kg BW LMW fucoidan and 100 mg/kg BW calcium carbonate (low dose + Ca), 140 mg/kg BW LMW fucoidan and 100 mg/kg BW calcium carbonate (middle dose + Ca) or 280 mg/kg BW LMW fucoidan and 100 mg/kg BW calcium carbonate (high dose + Ca). Calcium carbonate or LMW fucoidan was dissolved into redistilled water to its desired concentration, then mixed and given by gavage to mice every other day for four consecutive weeks. 24 h after the last supplementation, mice were anaesthetized and rapidly sacrificed. After sacrifice, femurs were dissected free and were kept in −20 °C freezer until analysis. All procedures were approved by Institutional Animal Care and Use National Taiwan University College of Medicine.
Bone density measurement
Bone densities of femurs were measured as described by Martin and Gutman (1978). Briefly, femurs were placed in an 85 °C water bath for 1 week, in order to remove excess flesh, dehydrated and defatted in graded alcohols, 50 % alcohol/50 % ether, 100 % ether, 50 % ether/50 % acetone and 100 % acetone, and finally dried in a vacuum oven. Using a Roller-Smith torsion balance, bones were weighted in air (μ), weighted submerged in water (μs), and the bone density = μ × pH2O/μ − μs, and the pH2O is the density of water.
Bone ash weight measurement
Femora were ashed in a Thermolyne Furnace at 800 °C for 24 h and weighted while hot.
Statistical analysis
All data obtained from the experiments were analyzed to determine the significance of different treatment by one-way ANOVA. Significant differences were reported at p value <0.05.
Results and discussion
Molecular weight distribution of LMW fucoidan from S. hemiphyllum
After hydrolysis of fucoidan from S. hemiphyllum using glycolytic enzyme, the hydrolyte was separated by 30 and 1 kDa molecular weight cut-off membrane. Two fractions were obtained after filtration: 1–30 and <1 kDa, and the yields of two fractions were 8.7 and 91.3 % (dry weight). Two major peaks demonstrated molecular weights of 12 and 760 Da by high performance size exclusion chromatography. It was shown that LMW fucoidan primarily consisted of 760 Da oligosaccharide and fucoidan primarily consisted of 270 kDa polysaccharide. The sulfate and fucose content of LMW fucoidan and fucoidan were similar, which demonstrated that the hydrolysis process was mild and did not damage the major components in fucoidan (Table 2).
Table 2.
Sulfate, fucose and molecular weight distribution of fucoidan and LMW fucoidan
| Sulfate % (w/w) | Fucose (μmol/g) | Fraction (kDa) | % (w/w, dry weight) | Molecular weight (Da) | |
|---|---|---|---|---|---|
| Fucoidan | 39.4 ± 2.1 | 211.23 ± 1.57 | >30 | 100 | 270 k |
| LMW fucoidan | 40.8 ± 0.4 | 207.91 ± 0.72 | 1–30 | 8.7 | 12 k |
| <1 | 91.3 | 760 |
Effect of LMW fucoidan and fucoidan on cell viability
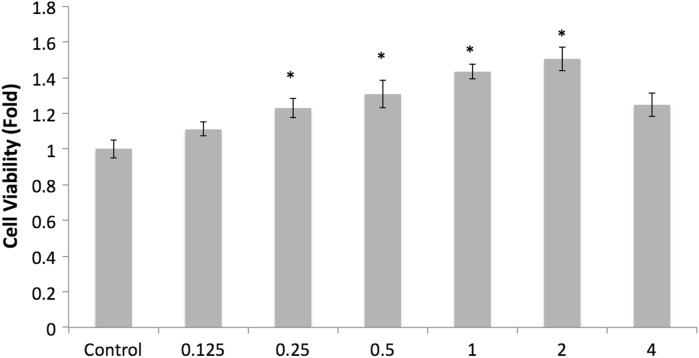
Fucoidan’s polyanionic structure is expected to allow it to bind to various proteins, thus exerting its biological activities. However, it is different to use fucoidan because of its high molecular weight causing the bioavailability to be lower (Shimizu et al. 2005). Therefore, reducing the molecular weight of fucoidan has been a topic of research (Colliec et al. 1991). In order to determine whether the difference in the molecular weight of fucoidan affects the cell viability in osteoblast cell, 7F2 cells treated with LMW fucoidan and fucoidan. Both LMW fucoidan and fucoidan could increase cell viability to as high as 150.33 ± 6.50 % and 130.12 ± 5.74 %, respectively, when cells were treated with 2 mg/ml. Moreover, the cell viability was significantly greater in the LMW fucoidan group than in the fucoidan group at 0.25–2 mg/ml concentration (Fig. 1). This results agrees with that obtained for LMW fucoidan extracted from pheophicae cell wall increasing cell proliferation of osteoblast cells and improving bioavailability (Changotade et al. 2008). Zhang et al. (2011) also demonstrated that enzymatically hydrolyzed 500 Da fucoidan is expected to facilitate rapid absorption into blood vessels (Zhang et al. 2011). So, it was suggested that LMW fucoidan may have contributed to the change in chemical composition and the structure of fucoidan, which may lead to the different activities of fucoidan according to their molecular weight.
Fig. 1.
Effects of LMW fucoidan on the viability of 7F2 cells for 48 h (n = 3). Cells were treated with different concentrations (0.125, 0.25, 0.5, 1, 2 and 4 mg/ml) and cell viabilities were detected by MTT assay. *p < 0.05 when compared with the control group
Effect of LMW fucoidan on ALP activity, osteocalcin secretion and mineralization
Osteoblast differentiation can be characterized in three stages, first stage: cell proliferation; second stage: matrix maturation; third stage: matrix mineralization. During the first stage, several extracellular matrix proteins, such as: procollagen I, transforming growth factor beta (TGF)-β, and fibronectin can be detected. The second stage is characterized by maximal expression of ALP. And during the third stage, genes of proteins such as osteocalcin, bone sialoprotein, and osteopontin are expressed and once mineralization is completed (Kasperk et al. 1995). Previous data reported that LMW fucoidan increased the cell proliferation of 7F2 cells, in order to assess whether LMW fucoidan is able to enhance osteoblast differentiation, we measured ALP and osteocalcin as the phenotype marker of the second and third stage, respectively.
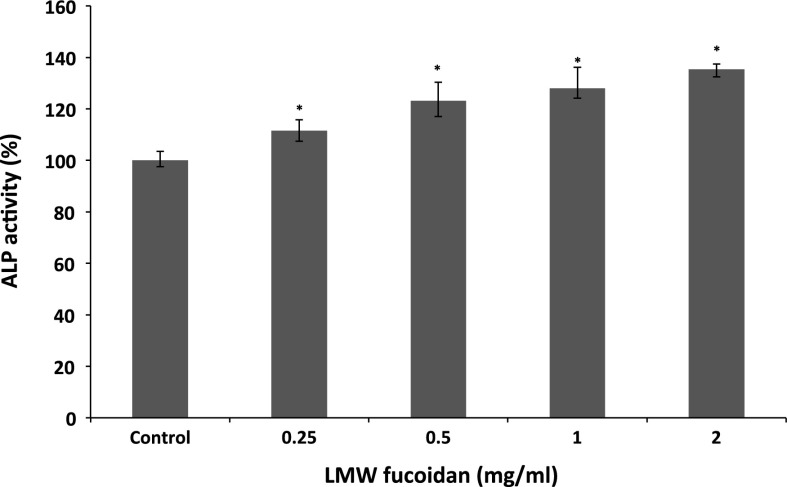
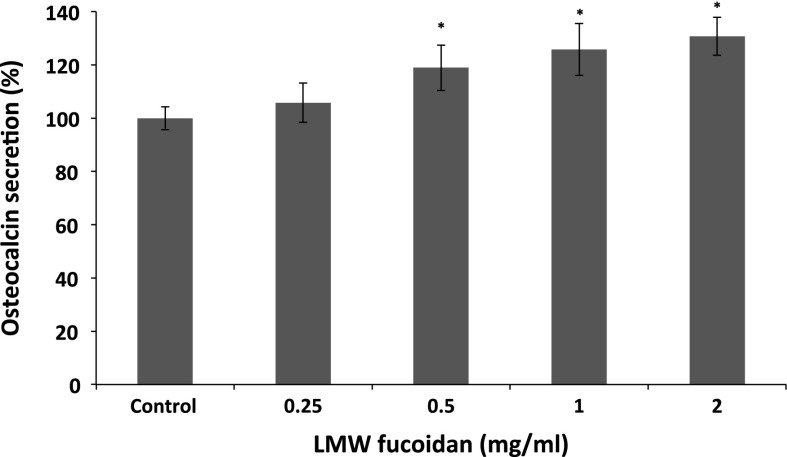
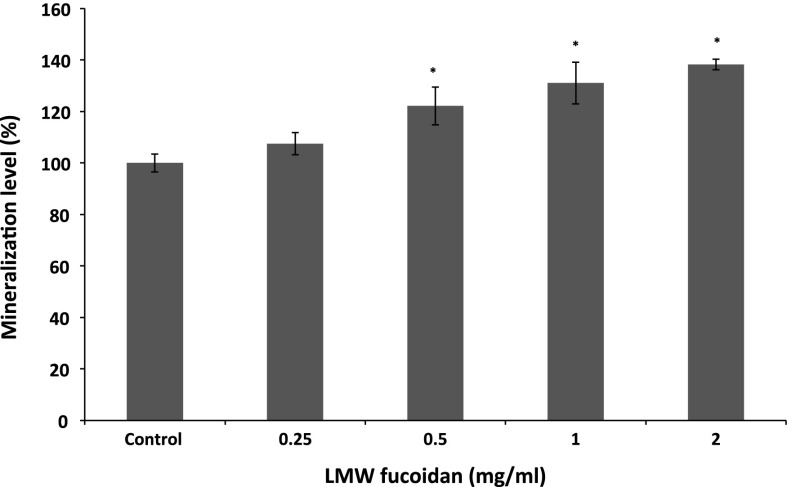
ALP is an important component in bone formation, it acts both to increase the local concentration of inorganic phosphate, a mineralization promoter, and to decrease the concentration of extracellular pyrophosphate, an inhibitor of mineral formation (Golub and Boesze-Battaglia 2007). The increase in the activity was significant at a LMW fucoidan concentration of 0.25–2 mg/ml, and the activity increased to 135.35 ± 2.91 % at 2 mg/ml when compared with control group (Fig. 2). In addition, we examined whether LMW fucoidan has a positive effect on stimulating osteocalcin secretion. Osteocalcin has been known to be a key mineral component of bone and regulated bone crystal growth, which binds with high affinity to hydroxyapatite crystals (Boskey et al. 1998). As shown in Fig. 3, 7F2 cells cultured with LMW fucoidan show enhanced osteocalcin secretion at concentration from 0.5 to 2 mg/ml. LMW fucoidan biological activities are concentration-dependent. Next, to confirm the beneficial effect of LMW fucoidan, we used Alizarine Red-S staining assay in 7F2 cells. LMW fucoidan (0.25–2 mg/ml) significantly increased the amounts of hydroxyapatite in the cells. The amount of the mineralization level of extracellular matrix was higher in a concentration-dependent manner (Fig. 4). Fucoidan extracted from Undaria pinnatifida also can increase the ALP activity, osteocalcin secretion and mineralization level (Cho et al. 2009). Furthermore, >30 kDa fucoidan was reported to stimulate osteogenic differentiation of human adipose-derived stem cell (Park et al. 2011), and <30 kDa fucoidan promoted human osteoblast proliferation, ALP activity, osteocalcin secretion and mineral deposition (Changotade et al. 2008).
Fig. 2.
Effects of LMW fucoidan on ALP activity of 7F2 cells for 48 h (n = 3). Cells were treated with different concentrations (0.25, 0.5, 1 and 2 mg/ml) and ALP activities were detected by generated p-nitrophenol. *p < 0.05 when compared with control group
Fig. 3.
Effects of LMW fucoidan on osteocalcin secretion by 7F2 cells for 48 h (n = 3). Cells were treated with different concentrations (0.25, 0.5, 1 and 2 mg/ml) and osteocalcin secretions were detected by EIA kit. *p < 0.05 when compared with control group
Fig. 4.
Effects of LMW fucoidan on mineralization of 7F2 cells for 7 days (n = 3). Cells were treated with different concentrations (0.25, 0.5, 1 and 2 mg/ml) and mineralizations were detected by Alizarin Red staining. *p < 0.05 when compared with control group
The smaller molecular weight fucoidan, 4.5 kDa, also can enhance the osteogenic capacity of human adipose-derived stromal cells (Pereira et al. 2013). Our data demonstrated that LMW fucoidan from S. hemiphyllum increased ALP activity, osteocalcin secretion, cell proliferation, and matrix mineralization in the process of osteogenic differentiation.
Effect of LMW fucoidan on various gene expression levels of osteoblastic differentiation markers
Osteogenesis is a complex process that involves the differentiation of osteoblast and leads to synthesis and deposition of bone matrix protein (Lian et al. 2004). To determine the mechanism underlying the promotion of mineralization by LMW fucoidan, the gene expression of markers of osteoblastic differentiation was studied using cultured 7F2 cells in the absence or presence of LMW fucoidan for 48 h. BMPs are a group of growth factors also known as cytokines and as metabologens, which stimulate osteoblast cell differentiation mainly by increased expression of ALP and osteocalcin (Xiao et al. 2004). Our data showed that the expression of BMP-2 significantly increased 2.28 ± 0.06 fold upon treatment with LMW fucoidan at 2 mg/ml (Fig. 5a), but did not increase BMP-4 mRNA level (data not shown). Furthermore, LMW fucoidan significantly increased the mRNA level of ALP and osteocalcin for 2.18 ± 0.12 and 2.06 ± 0.07 fold upon treatment with LMW fucoidan at 2 mg/ml (Fig. 5b, c). BMP-2 and BMP-4 are highly homologous, they interact with BMP receptors with comparable affinities (Sebald et al. 2004), and loss of both BMP-2 and BMP-4 results in a severe impairment of osteogenesis (Bandyopadhyay et al. 2006). Thus, it was demonstrated that LMW fucoidan played an enhancing role specifically on BMP-2, and then BMP-2 increased of ALP and osteocalcin gene expression.
Fig. 5.
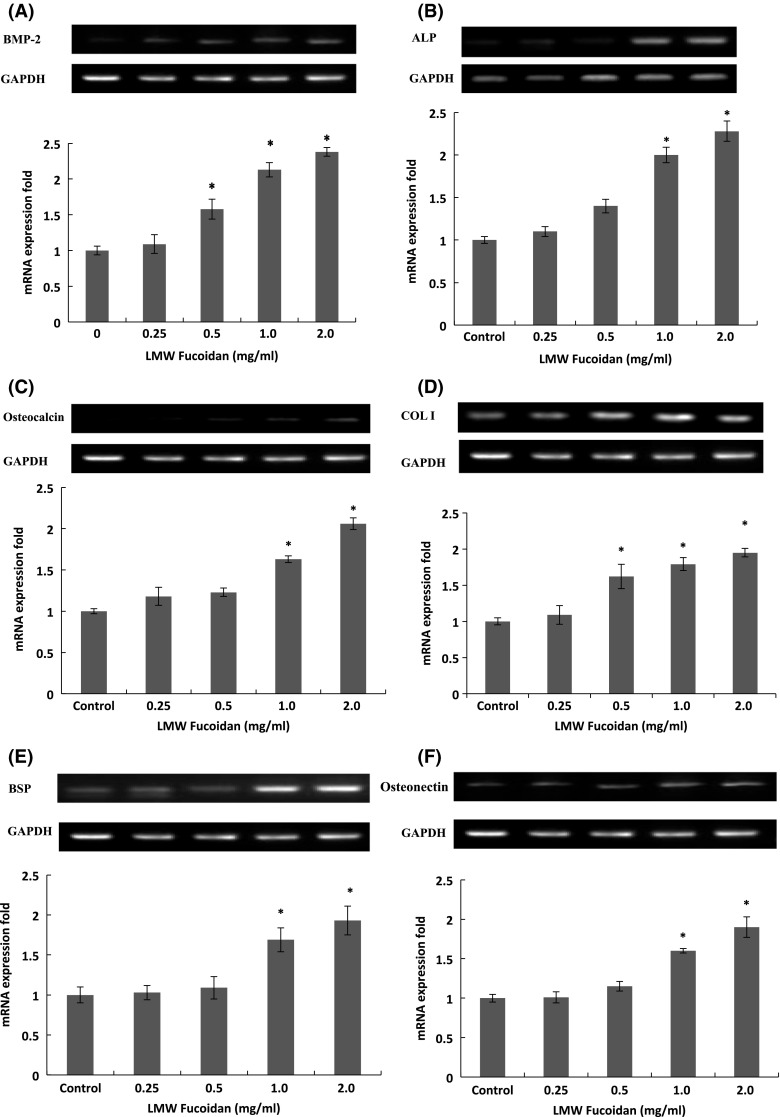
Effects of LMW fucoidan at different concentrations [0 (control), 0.25, 0.5, 1 and 2 mg/ml] on gene expression of markers of osteoblastic differentiation as BSP-2 (a), ALP (b), Osteocalcin (c), COL I (d), BSP (e) and steonectin (f) in 7F2 cells for 48 h (n = 3). mRNA expression fold = mRNA expression of the given gene/mRNA expression of GAPDH expression
The bone extracellular matrix is composed of collagens, mostly COL I, which constitutes 90 % of the total organic extracellular matrix in mature bone (Boskey et al. 1999) and noncollagenous proteins, such as BSP (Bianco et al. 1991). The addition of LMW fucoidan at 0.25–2 mg/ml resulted in a significant increase in COL I mRNA level (Fig. 5d), but not in COL II (data not shown), and also stimulated BSP mRNA expression in the 7F2 cells at 1–2 mg/ml (Fig. 5e). Similar effects were also observed by Cho et al. (2009), Changotade et al. (2008) and Park et al. (2011) that fucoidan can increase COL I levels in osteoblast. Osteonectin is a glycoprotein in the bone that binds calcium, hydroxyapatite and COL I. It is secreted by osteoblasts during bone formation, initiating mineralization and promoting mineral crystal formation (Bellahcene and Castronovo 1995). The expression of the mRNA level of osteonectin significantly increased 1.74 ± 0.23 fold upon treatment with LMW fucoidan at 2 mg/ml (Fig. 5f).
The RT-PCR assay showed that LMW fucoidan increased mRNA expression of BMP-2, ALP, osteocalcin, COL I, BSP and osteonectin, but did not increase BMP-4 and COL II mRNA levels (Data not shown). It was suggested that BMP-2 was originally identified as a molecule that promotes the differentiation of osteoblasts followed by induction of the osteoblastic makers, such as ALP (Lo et al. 2010). In addition, an increased ALP level by the addition of fucoidan in osteoblasts is an important process for bone tissue formation in vivo (Cho et al. 2009).
Effect of administration of LMW fucoidan on bone component in aged female rats
We showed that LMW fucoidan promoted the in vitro osteogenic differentiation of 7F2 cell, inducing an increase in cell proliferation, ALP activity, mineralization of the extracellular matrix, and expression of osteoblastic lineage-specific genes. To assess the effect of LMW fucoidan in vivo, LMW fucoidan and/or calcium carbonate were orally administered to aged female mice for 4 weeks. The body weight of aged mice was not significantly altered by the oral administration of LMW fucoidan and/or calcium carbonate. Bone density and bone ash weight were also not significantly altered by the oral administration of 100 mg/kg BW calcium carbonate alone (Table 3). Absorption of calcium fell with age after about 60 years and people over 80 years have significant malabsorption (Bullamore et al. 1970) that may be caused by the decline in the active calcium transport (Armbrecht et al. 1979). Thus, daily supplement with calcium may not be able to improve bone formation in the elderly. However, bone density and bone ash weight were significantly increased by oral administration of 280 mg/kg BW LMW fucoidan and 100 mg/kg BW calcium carbonate (Table 3). Sargassum horneri extract has been shown to stimulate bone formation in vitro (Uchiyama and Yamaguchi 2002b) and in vivo (Uchiyama and Yamaguchi 2003), and it exhibits an anabolic effect on bone components of young and aged rats (Uchiyama and Yamaguchi 2002a). Furthermore, seaweed given alone with Ca can increase intestinal calcium absorption and bone mineral density in rats (Fujita et al. 2000). This observation suggested that LMW fucoidan stimulated osteoblastic bone formation in bone density and bone ash weight of aged mice.
Table 3.
Body weights, bone densities and bone ash weight of C57BL/6J mice following 4 weeks of LMW fucoidan treatment
| Group | Dosage | Body weight (g) | Bone density (g/cc) | Bone ash weight (mg) |
|---|---|---|---|---|
| Control | 18.2 ± 0.3 | 0.92 ± 0.01 | 50.01 ± 3.13 | |
| Positive control | 100 mg/kg BW calcium carbonate | 17.9 ± 0.2 | 0.91 ± 0.08 | 51.61 ± 2.58 |
| Low dose | 70 mg/kg BW LMW fucoidan + 100 mg/kg BW calcium carbonate | 18.0 ± 0.2 | 0.95 ± 0.14 | 51.39 ± 0.75 |
| Middle dose | 140 mg/kg BW LMW fucoidan + 100 mg/kg BW calcium carbonate | 18.1 ± 0.3 | 1.12 ± 0.23 | 54.44 ± 0.67* |
| High dose | 280 mg/kg BW LMW fucoidan + 100 mg/kg BW calcium carbonate | 17.9 ± 0.3 | 1.25 ± 0.17* | 54.06 ± 1.60* |
Values are expressed as mean ± SD. Means with asterisk were significantly different from control (p < 0.05)
Conclusions
In conclusion, it has been demonstrated that LMW fucoidan stimulated osteogenic differentiation in vitro, and had an anabolic effect on bone mineralization in vivo. It was suggested that dietary intake of LMW fucoidan from S. hemiphyllum may have a role in the improvement of bone loss with increasing age.
Conflict of interest
Authors declare no conflict of interest in this manuscript.
Footnotes
Pai-An Hwang, Yu-Lan Hung and Nam Nhut Phan have contributed equally to this work.
References
- Armbrecht HJ, Zenser TV, Bruns ME, Davis BB. Effect of age on intestinal calcium absorption and adaptation to dietary calcium. Am J Physiol Endocrinol Metab. 1979;236:E769. doi: 10.1152/ajpendo.1979.236.6.E769. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellahcene A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol. 1995;146:95. [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int. 1991;49:421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–196. doi: 10.1016/S8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Wright TM, Blank RD. Collagen and bone strength. J Bone Miner Res. 1999;14:330–335. doi: 10.1359/jbmr.1999.14.3.330. [DOI] [PubMed] [Google Scholar]
- Bullamore JR, Wilkinson R, Gallagher JC, Nordin BEC, Marshall DH. Effect of age on calcium absorption. Lancet. 1970;296:535–537. doi: 10.1016/S0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- Changotade S, Korb G, Bassil J, Barroukh B, Willig C, Colliec-Jouault S, Durand P, Godeau G, Senni K. Potential effects of a low-molecular-weight fucoidan extracted from brown algae on bone biomaterial osteoconductive properties. J Biomed Mater Res, Part A. 2008;87:666–675. doi: 10.1002/jbm.a.31819. [DOI] [PubMed] [Google Scholar]
- Cho Y-S, Jung W-K, Kim J, Choi IWL, Kim S-K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009;116:990–994. doi: 10.1016/j.foodchem.2009.03.051. [DOI] [Google Scholar]
- Colliec S, Fischer AM, Tapon-Bretaudiere J, Boisson C, Durand P, Jozefonvicz J. Anticoagulant properties of a fucoidan fraction. Thromb Res. 1991;64:143–154. doi: 10.1016/0049-3848(91)90114-C. [DOI] [PubMed] [Google Scholar]
- Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962;84:106. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton JH. Therapies from fucoidan; multifunctional marine polymers. Marine drugs. 2011;9:1731–1760. doi: 10.3390/md9101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost HM. Perspectives: the role of changes in mechanical usage set points in the pathogenesis of osteoporosis. J Bone Miner Res. 1992;7:253–261. doi: 10.1002/jbmr.5650070303. [DOI] [PubMed] [Google Scholar]
- Fujita T, Fujii Y, Goto B, Miyauchi A, Takagi Y, Kobayashi S, Kamoshita K, Mikuni N, Kurihara Y, Shikauchi I. Increase of intestinal calcium absorption and bone mineral density by heated algal ingredient (HAI) in rats. J Bone Miner Metab. 2000;18:165–169. doi: 10.1007/s007740050108. [DOI] [PubMed] [Google Scholar]
- Genant HK, Baylink DJ, Gallagher JC. Estrogens in the prevention of osteoporosis in postmenopausal women. Am J Obstet Gynecol. 1989;161:1842–1846. doi: 10.1016/S0002-9378(89)80004-3. [DOI] [PubMed] [Google Scholar]
- Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18:444–448. doi: 10.1097/BCO.0b013e3282630851. [DOI] [Google Scholar]
- Gregory CA, Grady Gunn W, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Lin TY, Hwang PA, Tseng LM, Chen RH, Tsao SM, Hsu J (2013) Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGFβ receptor degradation in breast cancer. Carcinogenesis 34:874–884 [DOI] [PubMed]
- Hwang P-A, Chien S-Y, Chan Y-L, Lu M-K, Wu C-H, Kong Z-L, Wu C-J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J Agric Food Chem. 2011;59:2062–2068. doi: 10.1021/jf1043647. [DOI] [PubMed] [Google Scholar]
- Irhimeh MR, Fitton JH, Lowenthal RM. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul Fibrinolysis. 2009;20:607–610. doi: 10.1097/MBC.0b013e32833135fe. [DOI] [PubMed] [Google Scholar]
- Kasperk C, Wergedal J, Strong D, Farley J, Wangerin K, Gropp H, Ziegler R, Baylink DJ. Human bone cell phenotypes differ depending on their skeletal site of origin. J Clin Endocrinol Metab. 1995;80:2511–2517. doi: 10.1210/jcem.80.8.7629252. [DOI] [PubMed] [Google Scholar]
- Kim J-B, Han A-R, Park E-Y, Kim J-Y, Cho W, Lee J, Lee K-T. Inhibition of LPS-Induced iNOS, COX-2 and cytokines expression by poncirin through the NF-. KAPPA. B inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull. 2007;30:2345–2351. doi: 10.1248/bpb.30.2345. [DOI] [PubMed] [Google Scholar]
- Kylin H. Zur Biochemie der Meeresalgen. Hoppe-Seyler’s Zeitschrift für physiologische Chemie. 1913;83:171–197. doi: 10.1515/bchm2.1913.83.3.171. [DOI] [Google Scholar]
- Lee IHRL, Huang R-L, Chen C-T, Chen H-C, Hsu W-C, Lu M-K. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. FEMS Microbiol Lett. 2002;209:63–67. doi: 10.1111/j.1574-6968.2002.tb11110.x. [DOI] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1. doi: 10.1615/CritRevEukaryotGeneExpr.v14.i12.10. [DOI] [PubMed] [Google Scholar]
- Lo Y-C, Chang Y-H, Wei B-L, Huang Y-L, Chiou W-F. Betulinic acid stimulates the differentiation and mineralization of osteoblastic MC3T3-E1 cells: involvement of BMP/Runx2 and β-catenin signals. J Agric Food Chem. 2010;58:6643–6649. doi: 10.1021/jf904158k. [DOI] [PubMed] [Google Scholar]
- Martin RB, Gutman W. The effect of electric fields on osteoporosis of disuse. Calcif Tissue Res. 1978;25:23–27. doi: 10.1007/BF02010747. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Park S-B, Chun K-R, Kim J-K, Suk K, Jung Y-M, Lee W-H. The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother Res. 2010;24:1384–1391. doi: 10.1002/ptr.3140. [DOI] [PubMed] [Google Scholar]
- Park S-j, Lee KW, Lim D-S, Lee S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cells Dev. 2011;21:2204–2211. doi: 10.1089/scd.2011.0521. [DOI] [PubMed] [Google Scholar]
- Pereira J, Portron S, Dizier B, Vinatier C, Masson M, Sourice S, Galy-Fauroux I, Corre P, Weiss P, Fischer AM, Guicheux J. The in vitro and in vivo effects of a low-molecular-weight fucoidan on the osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A. 2013;20:275–284. doi: 10.1089/ten.tea.2013.0028. [DOI] [PubMed] [Google Scholar]
- Ryu B, Li Y, Qian Z-J, Kim M-M, Kim S-K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: implication for chronic articular disease. Chem Biol Interact. 2009;179:192–201. doi: 10.1016/j.cbi.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Santo VE, Gomes ME, Mano JF, Reis RL. Chitosan–chondroitin sulphate nanoparticles for controlled delivery of platelet lysates in bone regenerative medicine. J Tissue Eng Regen Med. 2012;6:s47–s59. doi: 10.1002/term.1519. [DOI] [PubMed] [Google Scholar]
- Sebald W, Nickel J, Zhang J-L, Mueller TD. Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol Chem. 2004;385:697–710. doi: 10.1515/BC.2004.086. [DOI] [PubMed] [Google Scholar]
- Segal E, Tamir A, Ish-Shalom S. Compliance of osteoporotic patients with different treatment regimens. IMAJ-RAMAT GAN. 2003;5:859–862. [PubMed] [Google Scholar]
- Senni K, Gueniche F, Foucault-Bertaud A, Igondjo-Tchen S, Fioretti F, Colliec-Jouault S, Durand P, Guezennec J, Godeau G, Letourneur D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch Biochem Biophys. 2006;445:56–64. doi: 10.1016/j.abb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Wada-Funada U, Mano H, Matahira Y, Kawaguchi M, Wada M. Proportion of murine cytotoxic T cells is increased by high molecular-weight fucoidan extracted from Okinawa mozuku (Cladosiphon okamuranus) J Health Sci. 2005;51:394–397. doi: 10.1248/jhs.51.394. [DOI] [Google Scholar]
- Soeda S, Sakaguchi S, Shimeno H, Nagamatsu A. Fibrinolytic and anticoagulant activities of highly sulfated fucoidan. Biochem Pharmacol. 1992;43:1853–1858. doi: 10.1016/0006-2952(92)90721-T. [DOI] [PubMed] [Google Scholar]
- Takada T, Katagiri T, Ifuku M, Morimura N, Kobayashi M, Hasegawa K, Ogamo A, Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003;278:43229–43235. doi: 10.1074/jbc.M300937200. [DOI] [PubMed] [Google Scholar]
- Tengdelius M, Lee CJ, Grenegård M, Griffith M, Påhlsson P, Konradsson P (2014) Synthesis and biological evaluation of fucoidan-mimetic glycopolymers through cyanoxyl-mediated free-radical polymerization. Biomacromolecules 15:2359–2368 [DOI] [PubMed]
- Uchiyama S, Yamaguchi M. Anabolic effect of marine alga sargassum horneri extract on bone components in the femoral-diaphyseal and-metaphyseal tissues of young and aged rats in vivo. J Health Sci. 2002;48:325–330. doi: 10.1248/jhs.48.325. [DOI] [Google Scholar]
- Uchiyama S, Yamaguchi M. Stimulatory effect of sargassum horneri extract on bone formation in rat femoral-diaphyseal and-metaphyseal tissues in vitro. J Health Sci. 2002;48:148–153. doi: 10.1248/jhs.48.148. [DOI] [Google Scholar]
- Uchiyama S, Yamaguchi M. Preventive effect of marine alga sargassum horneri extract on bone loss in streptozotocin-diabetic rats in vivo. J Health Sci. 2003;49:149–155. doi: 10.1248/jhs.49.149. [DOI] [Google Scholar]
- Vittimberga FJ, Jr, Frank J, McDade TP, Perugini RA, Callery MP. Sodium salicylate inhibits macrophage TNF-α production and alters MAPK activation. J Surg Res. 1999;84:143–149. doi: 10.1006/jsre.1999.5630. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Haase H, Young WG, Bartold M. Development and transplantation of a mineralized matrix formed by osteoblasts in vitro for bone regeneration. Cell Transplant. 2004;13:15–25. doi: 10.3727/000000004772664851. [DOI] [PubMed] [Google Scholar]
- Yang C, Chung D, Shin I-S, Lee H, Kim J, Lee Y, You S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int J Biol Macromol. 2008;43:433–437. doi: 10.1016/j.ijbiomac.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Teruya K, Eto H, Shirahata S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE. 2011;6:e27441. doi: 10.1371/journal.pone.0027441. [DOI] [PMC free article] [PubMed] [Google Scholar]