Abstract
The intestinal porcine epithelial cell line IPEC-J2 was used as an in vitro model to assess effects of additives on the adhesion and cell toxic effects of a F4-positive (ETEC) and a F4-negative Escherichia coli (DSM 2840) strain. Bacterial adhesion was examined using flow cytometry in IPEC-J2 cells infected with bacteria stained with 5,6-carboxymethyl fluorescein diacetate succinimidyl ester. Measurement of transepithelial electrical resistance (TEER) was performed to characterize the impact on IPEC-J2 monolayer integrity. The feed additives were prepared as aqueous extract and tested in different dilutions and incubation times. The F4-positive ETEC strain had a high adhesion to IPEC-J2 cells and reduced TEER shortly after the in vitro infection. The nonpathogenic E. coli strain DSM 2840 showed only low adhesion capacity and no TEER impairment. Infection with ETEC with added test extracts showed a reduction of bacterial adhesion to IPEC-J2 cells by an autolyzed yeast product (p < 0.05). Bovine colostrum, an additive containing thyme extract and an organic acid mix did not interfere with the ETEC adherence. The TEER decrease of the IPEC-J2 monolayer after ETEC infection was not affected by the added substances. In conclusion, interference with epithelial adhesion might be a protective mechanism of the tested yeast extract, indicating that the cell culture model might be suitable as screening tool to complement in vivo challenge trials with piglets.
Keywords: Diarrhoea, Feed additives, IPEC-J2 cells, Piglets, Weaning
Introduction
In piglet production, infections with enterotoxigenic Escherichia coli (ETEC) cause significant losses due to increased mortality, impaired performance and higher treatment costs. ETEC adhere to receptors of small intestinal enterocytes on the brush border surface and release toxins inducing cell damage and fluid secretion (Heo et al. 2013). Research for feeding strategies to improve piglet health after weaning has a high priority. However, animal testing is demanding and causes issues on animal welfare. Cell culture is considered as alternative to in vivo experiments. The use of human and mouse cell lines was shown to be inappropriate to investigate ETEC pathogenesis in swine (Mariani et al. 2009). A number of porcine cell lines have been characterized. The IPEC-J2 cell line has emerged as the most relevant porcine cell line for in vitro challenge studies with enteric pathogens. It was derived from small intestinal tissue of a neonatal, unsuckled piglet (Berschneider 1989) and has been characterized extensively (Schierack et al. 2006; Koh et al. 2008; Brosnahan and Brown 2012). The adhesion of E. coli strains to IPEC-J2 cells is strain specific (Schierack et al. 2006) and determined by the presence of flagella and fimbriae as shown for F18ab and F4-positive strains (Duan et al. 2012; Zhou et al. 2013).
K 88 (F4) positive Shiga toxin-producing E. coli strains isolated from pigs had a high adhesion capacity to IPEC-J2 cells, while human isolates did not (Sonntag et al. 2005). The sfa/foc gene, being relevant for encoding a subunit of F1C fimbriae, was correlated with the adhesion of porcine E. coli strains to IPEC-J2 cells (Schierack et al. 2013). The presence of the heat labile enterotoxin was also shown to have a positive impact on the adhesion of E. coli to IPEC-J2 cells (Johnson et al. 2009; Fekete et al. 2013). A F4-positive strain similar to the one used in this experiment had a considerably more severe impact on IPEC-J2 cells than an identical strain not expressing the F4-fimbrium, causing a decrease in transepithelial electrical resistance (TEER) and higher IL8 expression (Geens and Niewold 2010).
Several studies were performed to investigate the interaction of nutritional factors with the E. coli adhesion and cellular integrity using in vitro models. Lactobacillus sobrius, a probiotic strain isolated from the pig intestine, reduced enterotoxigenic E. coli adhesion as well as membrane damage in IPEC-J1 cells (Roselli et al. 2007a). Several feed ingredients and additives, including colostrum, bromelain and yeast extract as well as daidzein and allicin prevented a decrease of TEER in IPEC-J1 cells after infection with an enterotoxigenic E. coli strain (Roselli et al. 2007b). A membrane stabilizing effect of carrageenan, a sulfated polysaccharide mimicking epithelial receptor structures, was demonstrated, when heat-stable toxin b (STb) was used in IPEC-J2 cells (Goncalves et al. 2008). The trace element zinc, often used in practice due to its antidiarrheal effect in the post-weaning period, reduced bacterial adhesion and blocked bacterial invasion of ETEC using Caco-2 enterocytes (Roselli et al. 2003). Interestingly it modulated the inflammatory and metabolic response of IPEC-J2 cells infected with enterotoxigenic E. coli (Sargeant et al. 2010, 2011). Extracts from wheat bran, casein glycomacropeptide, mannan-oligosaccharides, locust bean extract and Aspergillus oryzae fermentation product reduced E. coli attachment to IPEC-J2 cells. Wheat bran specifically reduced the inflammatory response (Hermes et al. 2011).
To effectively screen candidate substances as inhibitors for ETEC adhesion and their resulting impact on cellular integrity in vitro, the chosen method must be applicable to a high number of substances, i.e. screening of high numbers of possible substances in a short amount of time is desirable. Directly labelling bacteria with fluorescent dyes has been found as an efficient means to rapidly detect a variety of bacterial species (Fuller et al. 2000). This method has also been established for the analysis of the attachment of pathogenic E. coli or viruses to cell cultures (Yan et al. 2014; Brosnahan and Brown 2012). To determine the actual damage of the adhering pathogen to intestinal cells, TEER assays can be used to show if the functional integrity of the cell mono-layer is compromised by adhering pathogens (Geens and Niewold 2011; Schierack et al. 2006).
In this study, aqueous extracts were used in different concentrations to test for protective effects on IPEC-J2 cells against ETEC infection. We hypothesized that bovine colostrum, autolyzed yeast, organic acids, or a phytogenic feed additive containing a thyme extract may limit adhesion of ETEC to IPEC-J2 cells. ETEC were labeled with the fluorescent dye CFDA SE (carboxyfluorescein succinimidyl ester) prior to infection and bacterial adhesion to IPEC-J2 cells was then measured using flow cytometry. In another set of experiments TEER was used to measure the effects of an ETEC challenge on the cellular integrity.
Materials and methods
E. coli strains used in the infection model
The enterotoxigenic E. coli strain Abbotstown, serotype O149:K91:F4ac, was kindly provided by Dr. K. Tedin, (Institute of Microbiology and Epizootics, Freie Universität Berlin). The strain harbors virulence factor genes for F4ac fimbriae, heat stable enterotoxins ST-Ip and ST-II and heat-labile enterotoxin LT-1. Bacteria were obtained from cryo-preservation and grown in Luria–Bertani broth (LB medium, Roth, Karlsruhe, Germany) under constant shaking at 37 °C, starting 2 days prior to infection. Twice a day 5 µl of bacterial solution were transferred to 10 ml of new, sterile LB medium. For the experiments on bacterial adhesion and transepithelial electric resistance (TEER), a non-fimbriated E. coli strain (DSM 2840) was additionally used as negative control (DSMZ, Braunschweig, Germany).
Feed additive extracts
Bovine colostrum (CM) (kindly provided by Veracus GmbH, Bremen, Germany), yeast autolysate (YA) (kindly provided by Biomin Research Center, Tulln, Austria), an organic acid mix (formic acid 99 g, ammonium formate 145 g, acetic acid 141 g per kg; AM), and a phytogenic feed additive containing a thyme extract (thyme essential oil as active ingredient, 35 g per kg; TH) (kindly provided by BIOMIN Research Center, Tulln, Austria) were diluted as 10 %-stock solution in cell culture medium (composition given below) and stirred in a test tube shaker (Multi Reax Shaker, Heidolph Instruments, Schwabach, Germany) for 30 min. After centrifugation at 3900×g for 30 min, the supernatant was filtered through a 0.2 µm filter (VWR, Darmstadt, Germany). This filtrate was used to produce a dilution series with the concentrations 10−2–10−5 (adhesion assay) and 10−2–10−3 (transepithelial resistance) in the final cell culture medium. Higher concentrations induced negative effects on IPEC-J2 cell integrity (data not shown).
Cell culture conditions of IPEC-J2 cells
The IPEC-J2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 (GIBCO™; Invitrogen Corporation, Grand Island, NY, USA), supplemented with 5 % fetal calf serum (FCS, Biochrom AG, Berlin, Germany), 1 % Penicillin–Streptomycin (Biochrom), 1 % insulin–transferrin–selenium solution (ITS, Gibco™), 5 µg/l epidermal growth factor (Biochrom) and 2.5 mmol/L l-glutamine (Biochrom). Cells between passages 30–50 were used for the experiments. They were kept in 175 cm2 Cellstar cell culture flasks (Greiner Bio-One; Frickenhausen, Germany) at 37 °C in a humidified incubator (Salvis Lab, Rotkreuz, Switzerland) in an atmosphere of 5 % CO2 and 95 % air. When reaching about 90 % confluency, cells were used in the experiments. The cell culture was routinely tested free for Mycoplasma spp. contamination.
E. coli adhesion assay using IPEC-J2 cells
IPEC-J2 cells were transferred from cell culture flasks onto 24-well plates (Biochrom, Berlin, Germany) on which experiments were performed. The cell monolayer was washed with phosphate-buffered saline (PBS) and trypsinized with 1 ml trypsin/EDTA (Biochrom); 1 × 105 cells per well were seeded onto 24-well cell culture plates in a volume of 1 ml.
Treatments were (1) non-infected cells cultivated in plain cell culture medium, (2) non-infected cells cultivated under influence of the additive in various dilutions, (3) infected cells cultivated in plain cell medium, (4) infected cells cultivated under influence of the additive at various dilutions. Results from treatments 1 and 2 were used to exclude a potential impact of the test substances on the non-infected cells (data not reported). Cells were incubated without or with the test substances from day 2 for the remaining experimental period. Experiments were repeated in triplicate, one experimental run was 3 days. On day one, cells were transferred from the 175 cm2 cell culture flasks onto the 24-well plates. On day 2 the cell medium was removed and cell monolayers were washed with PBS to remove traces of antibiotics. New antibiotic free cell medium containing additives or plain cell medium were added to treatment and control wells, respectively. On day 3 the cells were infected with the ETEC strain using a multiplicity of infection (MOI) of 100 as described previously (Sargeant et al. 2011), equivalent to 1 × 107 bacteria per well.
Staining procedure and flow cytometry to determine E. coli adhesion
Staining and detection of E. coli strains were done based on the methods optimized by Fuller et al. (2000). Fluorescent stain used was 5,6-carboxymethyl fluorescein diacetate succinimidyl ester (CFDA-SE) (Sigma Aldrich, Steinheim, Germany). CFDA-SE was dissolved at 20 µl (100 mM) in 380 µl of dimethyl sulfoxide (DMSO, Sigma Aldrich, Steinheim, Germany) and 100 µl of this staining solution were added to 10 ml of bacterial suspension and incubated at 37 °C for 120 min. Bacteria were then centrifuged (13,000g, 4 min, 4 °C), re-suspended in PBS, counted and used for infection. After an exposure time of 90 min, the cell monolayers were washed with PBS three times to remove non-adherent bacteria. Cell monolayers were trypsinized with 20 µl trypsin/EDTA (Biochrom AG, Berlin, Germany), incubated at 37 °C for 5 min until cell monolayers detached from surface. Cells were suspended in 1 ml of cell medium and transferred to BD Falcon Round-Bottom Polystyrene Tubes (Becton–Dickinson, Heidelberg, Germany) and centrifuged at 390×g for 5 min at 4 °C. While the supernatant was discarded, the pellet was suspended in 300 µl of antibiotic free cell medium.
Flow cytometric measurement was carried out using a FACSCalibur™ instrument (Becton–Dickinson, San José, CA, USA) equipped with a 15 mW argon ion laser emitting light at a fixed wavelength of 488 nm. Data were analyzed using the CellQuest™ software (Becton–Dickinson. The forward scattered (FSC) and side scattered (SSC—90° side angle) light was displayed according to size and granularity using scatter plots. A gate region was drawn to specify characteristics of desired cells. Cells were analyzed at a rate of 500–750 events per second until 10,000 events in the region of interest were collected. Noise, cell debris and non-adherent bacteria outside of the gate were discriminated.
Gated particles were then displayed in dot plots and histograms. Histograms provided information about the mean relative fluorescence intensity of measured cells, fluorescence of the negative control cells was used as threshold. Similarly, dot plots were used to determine percentage of infected cells. Fluorescence of negative control cells was used as a threshold and fluorescence of infected cells exceeding this autofluorescence was attributed to bacterial adhesion.
Effects on transepithelial resistance
Cell culture on permeable filters
Basic cell culturing was performed as described above. After 3–5 days the cell monolayer was washed with PBS and treated with trypsin–EDTA. The detached cells were pelleted at 200×g for 5 min and re-suspended in 10 ml of cell culture medium with 1 % Penicillin–Streptomycin (Biochrom). Cells (105 in 300 µl cell medium) were seeded onto Transwell® permeable support filters (polystyrene inserts, 12 mm diameter, 0.4 µm pore size, Corning, Corning, NY, USA) coated with rat tail collagen (BD Biosciences, Bedford, USA) and applied to 6 well plates (Corning™ Transwell®). After sowing, 4 ml of cell medium with antibiotics was added to each well. The medium was changed every second day.
Measurement of transepithelial electrical resistance
Measurement of TEER was carried out according to methodes published by Geens and Niewold (2010). Measurement was performed using an epithelial Voltmeter adapted to a sterile Endohm chamber (World Precision Instruments, Sarasota, FL, USA). The TEER-value reflects the confluence and integrity of the cell monolayer. Three different experiments were performed using the test substances in combination without or with ETEC infection. Within two experiments performed in triplicate (1) the influence of the test substances, and (2) the influence of test substances added over 24 h to the IPEC-J2 cells and ETEC infection were tested for a total of 3 days.
Cells were cultured with cell culture medium with antibiotics for 3–5 days. The increase in TEER was used as indicator for confluency (Schierack et al. 2006). When TEER of the cell monolayer reached values over 1 kΩ × cm2, the medium in the inner (apical) compartment of the devices were exchanged and the different substance dilutions were applied. The medium contained the substance extracts as described above at final dilutions of 10−2–10−5 or was unsupplemented. The medium outside the chamber was exchanged every second day. The TEER was measured every second day.
For the infection experiments with ETEC, the cells were pre-cultured in antibiotic free media. TEER measurements were performed immediately after feed additive addition, and after 6 and 24 h. The F4-fimbriated enterotoxigenic E. coli strain (Abbotstown) strain and the non-fimbriated E.coli strain (DSM 2840) were added to the snapwells at a calculated MOI of three bacteria per one epithelial cell. After infection, TEER was measured after 24, 48, and 72 h.
The whole experiment was run in triplicate at different days. One well per plate was used as non infected control. Another 6 well plate was used to test the different feed additives in combination with the ETEC strain as positive infection control. The non fimbriated E. coli strain DSM 2840 was tested like the positive infection control on separate 6 well plates.
Statistical analysis
Statistical analysis was conducted using the statistical program PASW 21.0 (IBM, Somers, NY, USA). Distribution of data was tested using the Kolmogorov–Smirnov test. Normally distributed data were subjected to one-way analysis of variance (ANOVA) with treatments as fixed factors, followed by post hoc Tukey test. A p value <0.05 was considered significant.
Results and discussion
E. coli adhesion assays to IPEC-J2 cells and subsequent infection as measured by TEER
The porcine IPEC-J2 cell line has been extensively characterized and has been shown to retain its epithelial nature (Mariani et al. 2009), expressing a variety of histological and physiological characteristics of the original tissue (Geens and Niewold 2011). As a prerequisite for this study, IPEC-J2 cells have also been shown to express the F4-receptor (Rasschaert et al. 2007). Expression of F4-fimbria among pathogenic E. coli strains was found to be of paramount importance for successful adherence and subsequent infection of IPEC-J2 cells (Hermes et al. 2011). Generally, the adhesion of pathogenic E. coli to small intestinal cells via fimbriae is a prerequisite for infection. We therefore hypothesized that the addition of feed additives to the porcine small intestinal cell culture IPEC-J2 influences the adhesion potential of pathogenic E. coli strains and may thus be useful in the prevention of diarrhea in piglets.
First, we compared the adhesion capacity of a pathogenic E. coli that carries a F4 fimbrium (strain E. coli Abbotstown) with a non-pathogenic E. coli strain DSM 2840. The results show that the percentage of fluorescent IPEC-J2 cells (i.e. cells with adhering E. coli cells) after infection was 54 % for the pathogenic strain versus 3.7 % for the non-pathogenic strain. This indicates that the F4-fimbrium of the pathogenic E. coli is required for efficient adhesion to IPEC-J2 cells. However, bacterial adhesion is a complex process and unspecific binding has to be considered. For instance, both flagella and F4-fimbriae were required for efficient F4ac+ ETEC adhesion in vitro after infection with an enterotoxigenic E. coli strain (Zhou et al. 2013). Non-fimbrial binding was not specifically investigated in this study. However, yeast cell walls and their fractions are long known as specific binding agent for F4 fimbriae (Shoaf-Sweeney and Hutkins 2002; Zopf and Roth 1996). Furthermore, the difference between the two test strains underlines that the major factor for the binding process is most probably a F4-fimbrium-mediated adhesion.
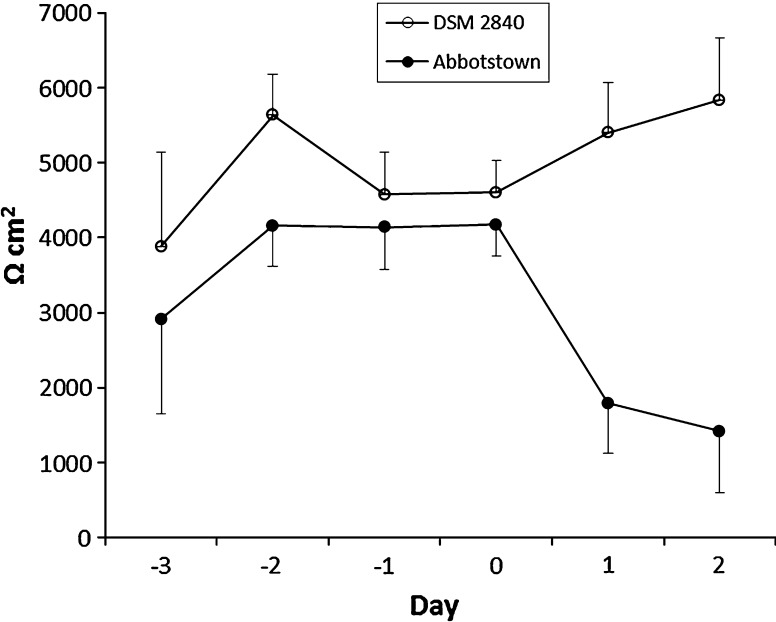
TEER was used as a marker for E. coli infection, i.e. the second step after successful adhesion to epithelial cells. After adhesion, pathogenic E. coli have to initiate the secretion of toxins to finally generate diarrhea. Interestingly, expression of heat-labile enterotoxins, which are also present in the pathogenic strain used here, was also found to promote adherence of pathogenic E. coli to IPEC-J2 cells (Johnson et al. 2009). TEER represents changes in transcellular- and paracellular resistance, which can be considered as parallel resistors. Thus, TEER decreases indicate disruption of cell integrity or impaired tight junction function of cell monolayers (von Bonsdorff et al. 1985, Wilson et al. 1990, Winter et al. 1990, Fromm et al. 2009). Our results showed that reduced TEER values were only achieved by the pathogenic E. coli strain (Fig. 1).
Fig. 1.
TEER of IPEC-J2 cell culture infected on day 0 with the F4-positive E. coli strain (ETEC, Abbotstown) and the F4-negative E. coli strain DSM 2480
Therefore we can conclude that the assay established in this study is able to monitor E. coli adhesion and infection as it would occur under in vivo conditions. Consequently, the combination of adhesion and TEER measurement allowed us to test the E. coli potential for infection under conditions similar to those found in the living animal.
The impact of feed additives on adhesion and infection of IPEC-J2 cells by the pathogenic E. coli strain
It is known in animal nutrition, that feed additives can have a positive impact on the prevention of diarrhea in piglets. Besides direct antimicrobial effects on pathogens the prevention of pathogen interaction with intestinal epithelia also plays a role. When considering the results of the different test substances in the present study, it has to be taken into account, that the materials needed to be extracted and sterilized before use in the experiment. Thermal sterilization would have been an option, but this was not used due to the risk for destroying the intrinsic structure of the extracts. The aqueous extracts were therefore sterile-filtered. Components of complex substrates are differently soluble in aqueous media, and thus bioactive compounds could either be not dissolved in the aqueous medium due to the lipophilic character. Consequently, the results for the tested extracts relate to the filtered water-soluble fraction only and might therefore deviate from the additives effect when included in a diet as entirety.
The impact of the different feed additives on the adhesion of E. coli cells was evaluated as the percentage of cells with increased fluorescence (Table 1) and the arbitrary fluorescence intensity (Table 2). Both indicate the highest reduction of fluorescence by the YA. Dose–response relationships were not always clear for the different feed additives. Cells grown with supplementation of bovine colostrum (CM) even showed increased fluorescence, indicating higher bacterial adhesion. Furthermore, AM containing a blend of organic acids could not be tested at higher concentrations as cell integrity was disrupted due to a strong pH reduction.
Table 1.
Percentage of infected IPEC-J2 cells after treatment with different extracts and infection with the enterotoxigenic E. coli straina
| Extract dilution | ||||
|---|---|---|---|---|
| 10−2 | 10−3 | 10−4 | 10−5 | |
| Control | 49.8 | |||
| AM | n.a.a | 65.0b | 49.7 | 47.7 |
| CM | 56.5b | 66.1b | 54.3 | 37.4 |
| TH | 49.7b | 39.7bc | 31.8 | 30.6 |
| YA | 2.2c# | 8.1c# | 28.3 | 28.5 |
| SEM | 9.6 | 8.3 | 6.8 | 6.9 |
| p value | 0.010 | 0.010 | 0.500 | 0.816 |
AM acid mix, CM colostrum, TH thyme, YA yeast autolysate
#Indicates difference to the control samples at p < 0.05
aNot analyzed, concentration of the extracts impaired cell integrity
bcMeans within the same column not sharing the same superscripts differ at p < 0.05
Table 2.
Fluorescence intensity of infected IPEC-J2 cells after treatment with different extracts and infection with the enterotoxigenic E. coli strain (arbitrary units)
| Extract dilution | ||||
|---|---|---|---|---|
| 10−2 | 10−3 | 10−4 | 10−5 | |
| Control | 13.6 | |||
| AM | n.a.a | 19.3b | 14.6 | 14.6 |
| CM | 15.9b | 16.7bc | 13.2 | 16.3 |
| TH | 15.3b | 11.4bc | 9.8 | 9.5 |
| YA | 6.0c# | 6.4c# | 9.0 | 9.1 |
| SEM | 1.6 | 1.8 | 1.2 | 1.7 |
| p value | 0.011 | 0.025 | 0.303 | 0.336 |
AM acid mix, CM colostrum, TH thyme, YA autolyzed yeast
#Indicates difference to the control samples at p < 0.05
aNot analyzed, concentrations of the extract impaired cell integrity
bcMeans within the same column not sharing the same superscripts differ at p < 0.05
Regarding the effect of the YA, preparations from Saccharomyces cerevisiae have been described to adhere to IPEC-J2 cells and to have protective effects after exposure to a Shiga-like toxin 2e producing E. coli strain (van der Aa Kühle et al. 2005). The protective effect seems to be related to the blocking of F4-receptors, thus making the epithelium less available for ETEC adhesion. Generally, yeast extracts or their cell wall components are considered as alternatives to antibiotics with respect to the promotion of health and performance in livestock (Ganner and Schatzmayr 2012).
Also, bovine colostrum preparations were discerned as successful candidates in a study with IPEC-J2 cells (Roselli et al. 2007a). However, the reported findings could not be reproduced in the IPEC-J2 infection used in this study. Supposedly, bovine colostrum possesses protective factors such as immunoglobulins or antimicrobial compounds like lactoferrin, lysozyme and lactoperoxidase, that have been shown to inhibit the growth of several pathogenic bacteria (Solomons 2002) or contain oligosaccharides and glycoproteins that may interfere with bacterial adhesion to epithelial cells (Gopal and Gills 2000). However, heat or other processing treatments impair the bioactivity of bovine colostrum (Gosch et al. 2013). The colostrum used in this study was defatted and pasteurized and may therefore have lost its original potential to inhibit E. coli adhesion. On the contrary, as colostrum offers a rich and diversified substrate spectrum for bacteria, the observed increased adhesion may have been due to the additional growth factors for the E. coli strain.
Organic acids are often used in practice as feed additives to improve health and performance of pigs especially in the weaning period (Partanen and Mroz 1999). However, the acid effect was apparently not a deciding factor to inhibit E. coli adhesion in this study. As higher concentrations of AM actually destroyed cell integrity, lower concentrations may have negatively impacted cell cultures without visible loss of cell integrity.
Finally, thyme is used as a phytogenic feed additive on the basis of the antibacterial activity of its essential oils (mainly thymol). However, it is also considered to stimulate physiological processes in the pig (Colombo et al. 2014). As the presumed bioactive ingredient in thyme is an essential oil, watery extracts may contain only very low amounts and therefore low bioactivity was expected.
In conclusion, of the four feed additives tested, only the YA was able to reduce the adhesion of the pathogenic E. coli strain. However, as adhesion is just the first step of the infection process, it is of high importance to also validate the second step, i.e. the ability of feed additives to reduce or even eliminate infection altogether.
None of the feed additives were able to reduce the impairment of IPEC-J2 cell integrity after E. coli challenge. TEER measurements over a period of 15 days revealed no significant impact of the various extracts and concentration ranges upon incubation of the cells (Table 3). Even the YA that was able to reduce adherence of the pathogenic E. coli cells could not suppress the destruction of the cell monolayer as shown by the TEER decrease. Thus, a reduction of E. coli adhesion is not a decisive factor to estimate the potential of a feed additive to prevent infection.
Table 3.
Transepithelial resistance (Ωcm2) of non-infected IPEC-J2 cells after treatment with different extracts over 15 days at dilutions 10−2 and 10−3
| Day | |||||
|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | |
| Control | 209 | 1554 | 3930 | 5708 | 8057 |
| Extract dilution 10−2 | |||||
| AM | 282 | 2702 | 4094 | 4820 | 6541 |
| CM | 243 | 3285 | 5943 | 7043 | 9320 |
| TH | 260 | 3198 | 5917 | 8638 | 10,260 |
| YA | 277 | 3018 | 4620 | 7590 | 8219 |
| SEM | 32.9 | 432 | 681 | 891 | 758 |
| p valuea | 0.926 | 0.957 | 0.878 | 0.483 | 0.434 |
| Extract dilution 10−3 | |||||
| AM | 162 | 2470 | 4095 | 5771 | 7353 |
| CM | 173 | 1934 | 4611 | 7775 | 8666 |
| TH | 192 | 1704 | 2545 | 5529 | 7292 |
| YA | 352 | 2519 | 3485 | 5337 | 6901 |
| SEM | 28.6 | 482 | 628 | 868 | 809 |
| p valuea | 0.049 | 0.807 | 0.694 | 0.962 | 0.922 |
AM acid mix, CM colostrum, TH thyme, YA yeast autolysate
aComparison of means within the same column
A final experiment was conducted to examine the hypothesis that cell cultures may need a preincubation for the feed additives to act. In vivo, feed additives could be present before pathogenic E. coli reach the intestine and thus, the physiology of small intestinal epithelial cells may have been modified as shown by Colombo et al. (2014) for thymol. However, a 24 h preincubation with the tested feed additives did not change the outcome. TEER was comparatively uniform in a range of 3000–3400 Ωcm2 prior to the implementation of the infection with the pathogenic E. coli strain. Shortly after infection, there was a significant reduction of resistance, in the case of the infected control wells to 55 % of the initial value. No test extract showed a significant effect and resistance values were reduced to 56–83 % compared to the non infected control values (Table 4).
Table 4.
Transepithelial resistance (Ωcm2) of IPEC-J2 cells before infection with an enterotoxigenic E. coli strain cells after 24 h treatment with different extracts (dilution 10−2) and decrease of TEER after infection
| Treatment | TEER before infection (Ωcm2) | TEER decrease after infection (%a) |
|---|---|---|
| Control, non infected | 3.360 | – |
| Control, infected | 3.043 | 73 |
| AM | 3.120 | 72 |
| CM | 3.121 | 83 |
| TH | 3.559 | 56 |
| YA | 3.151 | 57 |
| SEM | 308 | 7.03 |
| p valueb | 0.579 | 0.068 |
AM acid mix, CM colostrum, TH thyme, YA yeast autolysate
aCompared to the non-infected control
bComparison of means within the same column
In conclusion, the ability of the F4-positive pathogenic E. coli strain to adhere to IPEC-J2 cells and its destructive effect on cell integrity could be demonstrated by using flow cytometry and TEER measurements. For a YA feed additive, the potential to interfere with the adhesion process could be demonstrated. This study has also shown that this cell culture model is suitable as an in vitro screening tool that can complement the much more expensive feeding trials with piglets.
Acknowledgments
The project was supported by the FFG Austria, project Preventive Veterinary Medicine and Biomin Holding GmbH (Herzogenburg, Austria) and Veracus (Bremen, Germany).
References
- Berschneider M. Development of a normal cultured small intestinal epithelial cell line which transport Na and Cl. Gastroenterology. 1989;96:A41. [Google Scholar]
- Brosnahan AJ, Brown DR. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol. 2012;156:229–237. doi: 10.1016/j.vetmic.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Priori D, Gandolfi G, Boatto G, Nieddu M, Bosi P, Trevisi P. Effect of free thymol on differential gene expression in gastric mucosa of the young pig. Animal. 2014;8:786–791. doi: 10.1017/S1751731114000172. [DOI] [PubMed] [Google Scholar]
- Duan QD, Zhou MX, Zhu XF, Bao WB, Wu SL, Ruan XS, Zhang WP, Yang A, Zhu J, Zhu GQ. The flagella of F18ab Escherichia coli is a virulence factor that contributes to infection in a IPEC-J2 cell model in vitro. Vet Microbiol. 2012;160:132–140. doi: 10.1016/j.vetmic.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Fekete PZ, Mateo KS, Zhang WP, Moxley RA, Kaushik RS, Francis DH. Both enzymatic and non-enzymatic properties of heat-labile enterotoxin are responsible for LT-enhanced adherence of enterotoxigenic Escherichia coli to porcine IPEC-J2 cells. Vet Microbiol. 2013;164:330–335. doi: 10.1016/j.vetmic.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Fromm M, Krug SM, Zeissig S, Richter JF, Rosenthal R, Schulzke JD, Gunzel D. High-resolution analysis of barrier function. Ann NY Acad Sci. 2009;1165:74–81. doi: 10.1111/j.1749-6632.2009.04047.x. [DOI] [PubMed] [Google Scholar]
- Fuller ME, Streger SH, Rothmel RK, Mailloux BJ, Hall JA, Onstott TC, Fredrickson JK, Balkwill DL, DeFlaun MF. Development of a vital fluorescent staining method for monitoring bacterial transport in subsurface environments. Appl Environ Microbiol. 2000;66:4486–4496. doi: 10.1128/AEM.66.10.4486-4496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganner A, Schatzmayr G. Capability of yeast derivatives to adhere enteropathogenic bacteria and to modulate cells of the innate immune system. Appl Microbiol Biotechnol. 2012;95:289–297. doi: 10.1007/s00253-012-4140-y. [DOI] [PubMed] [Google Scholar]
- Geens MM, Niewold TA (2010) Preliminary characterization of the transcriptional response of the porcine intestinal cell line IPEC-J2 to enterotoxigenic Escherichia coli, Escherichia coli, and E. coli lipopolysaccharide. Comp Funct Genomics. http://www.hindawi.com/journals/ijg/2010/469583/cta/ [DOI] [PMC free article] [PubMed]
- Geens MM, Niewold TA. Optimizing culture conditions of a porcine epithelial cell line IPEC-J2 through a histological and physiological characterization. Cytotechnology. 2011;63:415–423. doi: 10.1007/s10616-011-9362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves C, Berthiaume F, Mourez M, Dubreuil JD. Escherichia coli STb toxin binding to sulfatide and its inhibition by carragenan. FEMS Microbiol Lett. 2008;281:30–35. doi: 10.1111/j.1574-6968.2008.01075.x. [DOI] [PubMed] [Google Scholar]
- Gopal PK, Gill HS. Oligosaccharides and glycoconjugates in bovine milk and colostrum. Br J Nutr. 2000;84:S69–S74. doi: 10.1017/S0007114500002270. [DOI] [PubMed] [Google Scholar]
- Gosch T, Apprich S, Kneifel W, Novalin S. Improved isolation of bioactive components of bovine colostrum using cross-flow microfiltration. Int J Dairy Technlo. 2013;66:175–181. doi: 10.1111/1471-0307.12027. [DOI] [Google Scholar]
- Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Hermes RG, Manzanilla EG, Martín-Orúe SM, Pérez JF, Klasing KC. Influence of dietary ingredients on in vitro inflammatory response of intestinal porcine epithelial cells challenged by an enterotoxigenic Escherichia coli (K88) Comp Immunol Microbiol Infect Dis. 2011;34:479–488. doi: 10.1016/j.cimid.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Kaushik RS, Francis DH, Fleckenstein JM, Hardwidge PR. Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells. J Bacteriol. 2009;191:178–186. doi: 10.1128/JB.00822-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SY, George S, Brozel V, Moxley R, Francis D, Kaushik RS. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet Microbiol. 2008;130:191–197. doi: 10.1016/j.vetmic.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Mariani V, Palermo S, Fiorentini S, Lanubile A, Giuffra E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet Immunol Immunopathol. 2009;131:278–284. doi: 10.1016/j.vetimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Partanen KH, Mroz Z. Organic acids for performance enhancement in pig diets. Nutr Res Rev. 1999;12:117–145. doi: 10.1079/095442299108728884. [DOI] [PubMed] [Google Scholar]
- Rasschaert K, Verdonck F, Goddeeris BM, Duchateau L, Cox E. Screening of pigs resistant to F4-enterotoxigenic Escherichia coli (ETEC) infection. Vet Microbiol. 2007;123:249–253. doi: 10.1016/j.vetmic.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J Nutr. 2003;133:4077–4082. doi: 10.1093/jn/133.12.4077. [DOI] [PubMed] [Google Scholar]
- Roselli M, Britti MW, Le Huerou-Luron I, Marfaing H, Zhu WY, Mengheri E. Effect of different plant extracts and natural substances (PENS) against membrane damage induced by enterotoxigenic Escherichia coli K88 in pig intestinal cells. Toxicol In Vitro. 2007;21:224–229. doi: 10.1016/j.tiv.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Roselli M, Finamore A, Britti MS, Konstantinov SR, Smidt H, de Vos WM, Mengheri E. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J Nutr. 2007;137:2709–2716. doi: 10.1093/jn/137.12.2709. [DOI] [PubMed] [Google Scholar]
- Sargeant HR, Shaw MA, AbuOun M, Collins JW, Woodward MJ, La Ragione MR, Miller HM. The metabolic impact of zinc oxide on porcine intestinal cells and enterotoxigenic Escherichia coli K88. Livest Sci. 2010;133:45–48. doi: 10.1016/j.livsci.2010.06.021. [DOI] [Google Scholar]
- Sargeant HR, Miller HM, Shaw MA. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol Immunol. 2011;48:2113–2121. doi: 10.1016/j.molimm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Schierack P, Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, Lodemann U, Jores J, Tachu B, Kleta S, Blikslager A, Tedin K, Wieler LH. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- Schierack P, Rodiger S, Kuhl C, Hiemann R, Roggenbuck D, Li G, Weinreich J, Berger E, Nolan LK, Nicholson B, Romer A, Frommel U, Wieler LH, Schroder C. Porcine E. coli: virulence-associated genes, resistance genes and adhesion and probiotic activity tested by a new screening method. PLoS One. 2013;8:e59242. doi: 10.1371/journal.pone.0059242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaf-Sweeney KD, Hutkins RW. Adherence, anti-adherence, and oligosaccharides preventing pathogens from sticking to the host. Adv Food Nutr Res. 2002;55:101–161. doi: 10.1016/S1043-4526(08)00402-6. [DOI] [PubMed] [Google Scholar]
- Solomons NW. Modulation of the immune system and the response against pathogens with bovine colostrum concentrates. Eur J Clin Nutr. 2002;56:S24–S28. doi: 10.1038/sj.ejcn.1601480. [DOI] [PubMed] [Google Scholar]
- Sonntag AK, Bielaszewska M, Mellmann A, Dierksen N, Schierack P, Wieler LH, Schmidt MA, Karch H. Shiga toxin 2e-producing Escherichia coli isolates from humans and pigs differ in their virulence profiles and interactions with intestinal epithelial cells. Appl Env Microbiol. 2005;71:8855–8863. doi: 10.1128/AEM.71.12.8855-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Aa Kühle A, Skovgaard K, Jespersen L. In vitro screening of probiotic properties of Saccharomyces cerevisiae var. boulardii and food-borne Saccharomyces cerevisiae strains. Int J Food Microbiol. 2005;101:29–39. doi: 10.1016/j.ijfoodmicro.2004.10.039. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff CH, Fuller SD, Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985;4:2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, Winter M, Shasby DM. Oxidants, ATP depletion, and endothelial permeability to macromolecules. Blood. 1990;76:2578–2582. [PubMed] [Google Scholar]
- Winter M, Wilson JS, Bedell K, Shasby DM. The conductance of cultured epithelial cell monolayers: oxidants, adenosine triphosphate, and phorbol dibutyrate. Am J Resp Cell Mol Biol. 1990;2:355–363. doi: 10.1165/ajrcmb/2.4.355. [DOI] [PubMed] [Google Scholar]
- Yan M, Zhu L, Yang Q. Infection of porcine circovirus 2 (PCV2) in intestinal porcine epithelial cell line (IPEC-J2) and interaction between PCV2 and IPEC-J2 microfilaments. Virol J. 2014;11:193. doi: 10.1186/s12985-014-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Duan Q, Zhu X, Guo Z, Li Y, Hardwidge PR, Zhu G. Both flagella and F4-fimbriae from F4ac+ enterotoxigenic Escherichia coli contribute to attachment to IPEC-J2 cells in vitro. Vet Res. 2013;44:30. doi: 10.1186/1297-9716-44-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf D, Roth S. Oligosaccharide anti-infective agents. Lancet. 1996;347:1017–1021. doi: 10.1016/S0140-6736(96)90150-6. [DOI] [PubMed] [Google Scholar]