Abstract
Psychoeducation improves adherence and motivates patients to accept a maintenance therapy as recommended by the guidelines. This would mean a daily consumption of at least 300 chlorpromazine (CPZ) units in the long run and should lead to an increase of the antipsychotic dosage in comparison to patients with treatment as usual (TAU). This raises 2 important questions: whether more side effects are provoked and do the patients have a corresponding benefit with a better outcome. A total of 41 patients with a diagnosis of schizophrenic or schizoaffective disorder were randomized at study entry, either to bifocal psychoeducation (21), or to standard treatment (20). They were compared concerning compliance, type of medication, dosage (CPZ equivalents), motor side effects and number of days in hospital. The average daily antipsychotic medication 2 and 7 years after index discharge was 365 and 354 CPZ-units respectively in the intervention group (IG), but 247 and 279, respectively in the control group (CG). The extent of motor side effects was slightly smaller in the IG, but they showed a small and statistically not significant increase in the rate of tardive dyskinesia (TD) after 7 years. At the 7-year follow-up the patients in the IG had spent 74.7 days in hospital compared to 243.4 days for the patients in the CG (P < .05). The course of illness was significantly better in the IG without increasing motor side-effects. Therefore, psychoeducation should be integrated more systematically into the routine treatment. These data are part of a previous study, published 2007, with a sample size of 48 patients. Seven patients—3 of the IG and 4 of the CG—could not be included, because they were not able to complete the very complex “Computer-based kinematic analysis of motor performance.” In this article all conclusions are referred to the new sample size, therefore some results are slightly different in comparison to the previous data.
Key words: schizophrenia, psychoeducation, rehospitalization, side effects, EPMS, tardive dyskinesia
Introduction
A maintenance therapy with antipsychotics during a period of at least 5 years is recommended for schizophrenic patients with repetitive episodes in numerous guidelines.1–4 Relapses can occur in spite of antipsychotic treatment during the outpatient phase due to noncompliance of patients,5–9 as well as to psychiatrists’ administration of an insufficient dosage.10–12
Therefore, the patients of the intervention group (IG) were motivated during their index stay by a psychoeducational approach to accept a maintenance therapy of at least 300 chlorpromazine (CPZ) units daily, as recommended in the guidelines. In reference to the non-inferior hypothesis it should be proven that patients who follow this advice will not have more side effects than patients in the control group (CG) with a probably lower dosage.
One of the most important goals of this study was to train the patients of the IG very successfully to make the best choice concerning medication with a minimal rate of side-effects during maintenance therapy. This can only be realized in close contact with the patients.13–16 Even under treatment with atypicals, drop outs or change of medication will occur among 75% of the patients during a period of 18 months.17 Beyond this aspect, improvement of compliance means being aware of the higher risk of side-effects, especially tardive dyskinesia (TD)18–23; patients have to be very intensively trained in recognizing their side effects in time and adapting treatment in close cooperation with their psychiatrists.24–30
Two questions were interesting in this context: Firstly, can psychoeducation (PE) improve compliance and be helpful in motivating patients to take a sufficient dosage in the long run? And, on the other hand, will PE support the patients, in an appropriate manner, to detect side effects early enough to prevent harm by adapting the medication in time? To answer these questions, the data among the intervention and the CG from the Munich PIP-study (Psychosis Information Project) were reanalyzed and compared at index discharge, as well as 2 and 7 years later: Compliance and days in hospital; type of medication and dosage; extra pyramidal motor symptoms (EPMS) and TD. In order to have the best objective findings concerning EPMS, the additional computer-based kinematic analysis of motor performance was one of the topics of the 7-year follow-up. This method was not available either at baseline nor at 2-years follow-up, therefore data exist only at the endpoint after 7 years.
Munich PIP-Study
Design
Between 1990 and 1994 the randomized multi-centre PIP Study was organized at 3 psychiatric hospitals in Munich (LMU: Ludwig-Maximilians-Universität; BKH Haar: Community Hospital of Munich; TUM: Technical University of Munich). All patients with a schizophrenic or schizoaffective psychosis (DSM III-R: 295.10-94; 297.10/ International Classification of Diseases (ICD)-10: F 20, F22, F25) were screened at admission. Additional inclusion criteria: indication of antipsychotic relapse prevention for a period of at least 12 months; age between 18 and 65 years; patients’ acceptance of an outpatient treatment in the study centre; patients’ agreement to involve a key relative or a friend.
Exclusion Criteria.
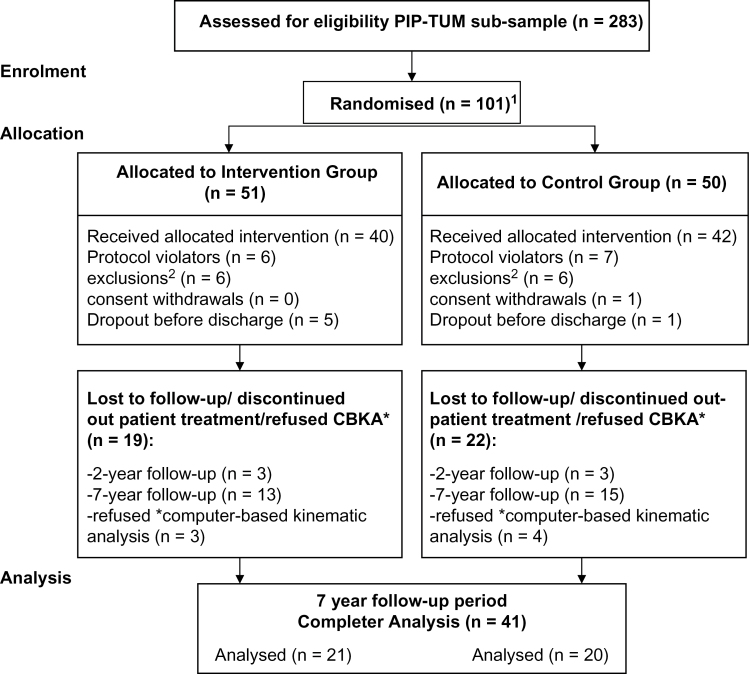
A distance between home and hospital of more than 150 kilometers; less than 30 minutes contact per week with the key relative; drug addiction during the past 6 months prior to admission; pregnancy; IQ < 80; insufficient knowledge of the German language; no remission of the psychotic symptoms during the last 2 years despite a sufficient therapy (figure 1).
Fig. 1.
Consort flow diagram of the progression through the phases of the study (n = 41; Psychosis Information Project- Technical University of Munich [PIP-TUM] sub-sample). 1These 101 patients constitute a sub-sample of the PIP-Study with 236 patients (Pitschel-Walz et al29). 2Change of diagnosis; no indication for antipsychotic relapse prevention; no remission during inpatient stay; distance from patient’s home to hospital more than 150 km.
Details of the screening and randomization process, sample size calculation, statistical analysis and the 2- and 7-year follow-up outcome are published elsewhere.29,31 This article focuses exclusively on the sub-sample of the TUM (n = 101) because only here has the 7-year follow-up investigation been performed.
Subjects
At the study centre of the TUM, 283 patients were screened; 101 could be included into the randomization procedure. Nineteen patients had to be excluded before index-discharge for formal reasons or due to discontinuation of the index-intervention, 34 patients dropped out during the 7-year follow-up period, another 7 patients had to be excluded on account of their refusal to take part in the extended neuropsychological motor test-battery. Therefore, 41 of the original 82 patients (50%) were available for data analyses. These patients showed no significant differences in their sociodemographic and clinical characteristics at the point of study entry (table 1).
Table 1.
Sociodemographic and Clinical Characteristics of Subjects at 7-Year Follow-up (n = 41)
| Intervention Group (n = 21) | Control Group (n = 20) | Test | P (2-tailed) | ||
|---|---|---|---|---|---|
| Age (y) | Mean | 38.1 | 40.7 | t = −0.95 | .35 |
| SD | 7.9 | 9.4 | |||
| Sex | Female % | 52.4 | 65 | χ2 = 0.67 | .53a |
| Education | Low % | 33.3 | 10.0 | χ2 = 3.35 | .19 |
| Medium % | 33.3 | 40.0 | |||
| High % | 33.3 | 50.0 | |||
| Diagnosis (ICD-10) | 20.0 | 71.4 | 75.0 | χ2 = 4.12 | .39 |
| 20.1 | 4.8 | 0.0 | |||
| 20.2 | 4.8 | 0.0 | |||
| 25.1 | 0.0 | 10.0 | |||
| 25.2 | 19.0 | 15.0 | |||
| Duration of illness (y) | Mean | 13.0 | 13.3 | t = −0.22 | .83 |
| SD | 5.2 | 5.0 | |||
| Total number of hospitalizations | Mean | 5.8 | 6.1 | t = −0.28 | .78 |
| SD | 3.3 | 3.3 |
Note: ICD, International Classification of Diseases.
aFisher’s Exact Test.
Index Stay: PE for the IG, Treatment as Usual for the CG
Psychoeducation began as quickly as possible after randomized allocation. There were 4 weekly sessions of 60 minutes each; afterwards, 4 additional monthly sessions were held. Relatives were also invited to 8 weekly sessions, each lasting 90 minutes. The groups were headed by therapists who had not been involved in the routine treatment. In both settings the same psychoeducational modules were presented. Apart from improvement of coping by discussing similar experiences, considerable attention was paid to the interactive evaluation of illness-relevant information. The take-home message of the psychoeducational program was: schizophrenic psychoses are provoked by biological factors in combination with psychosocial stress; therefore they have to be treated with medication and psychotherapeutic interventions. Patients’ empowerment can only be developed successfully on the basis of a sufficient medication and long-term psychosocial treatment elements. Above all, the patients were trained to report their side effects to their therapists immediately and to look together with them for the most suitable medication.31–33 In addition, patients and relatives received an information booklet to sustain the learning process initiated by psychoeducational groups.34 With the exception of the 8 bifocal psychoeducational sessions, the intervention and CGs received the same psychiatric treatment as usual (TAU) during the inpatient period.
Outpatient Treatment: TAU for IG and CG
In the study centre of the TUM it was possible to provide the current outpatient treatment (TAU) for a period of 4 years. After this period, the patients in both groups had to be referred to the general psychiatrists’ practices outside of the hospital for organizational reasons. A thorough assessment of psychopathology, social functioning, medication, compliance and insight was after 2 and 7 years. There are no systematically data concerning the situation after 7 years.
Medication Regimen
All patients were encouraged to accept a maintenance therapy with antipsychotic medication during a period of at least 1 year (first episode patients). In the case of recurrent psychoses, the patients were told to take the medication for a period of 5 years and longer. The kind of the drug and the dosage were adapted individually according to clinical needs; 300 CPZ-units were recommended as the optimal dosage.10,35 Co-medication was not restricted. All changes of medication were documented during the clinical visits.
After 4 years, the patients could no longer be treated in our outpatient department. Their psychiatrists were told to inform external therapeutic colleagues concerning the most significant details. They were instructed, in particular, not to make any distinction between patients of the psychoeducational group and the CG. Both samples should be treated in the same manner.
Clinical Rating Scales
Compliance, type of medication, the average number of consumed CPZ-units and side-effects were the main outcome criteria. These data were assessed 2 and 7 years after index-discharge. Compliance was rated by the treating psychiatrists on a 4-step ordinal scale (1 = very good/ 2 = good/ 3 = moderate/ 4 = bad). Plasma drug level measurements were performed in order to validate the psychiatrists’ compliance ratings; the results of this procedure revealed a very high and statistically significant concordance as published in a former article.29 Blood samples to test the drug levels were taken every 4 to 8 weeks only during the first 2 years and at the end of the 7-year follow-up for reasons of costs. The samples were frozen and the results were evaluated at the end of these 2 years and respectively 7 years later. Doctors’ compliance ratings were therefore not based on the objective values of drug levels. These were later found to have a very high concordance between both methods.29
CPZ-units were calculated as proposed by Jahn and Mussgay.10 The newer atypicals were transformed into CPZ-units following Woods.36
Great attention was paid to the detection of neuroleptic side effects such as extrapyramidal symptoms and TD.22,37,38 During the first 2 years there was a thorough investigation of possible side effects every 4 weeks by means of the scales developed by Simpson and Angus39 and Guy.40
Computer-Based Kinematic Analysis of Motor Performance
To obtain more objective indicators of neurological symptoms such as EPMS, TD, and psychomotor retardation, a series of computer-based kinematic analyses of motor performance was made. These examinations can detect mild psychomotoric disturbances much earlier and more precisely than the clinically based rating scales.
This procedure was one of the topics of the 7-year follow-up, because the patients were motivated by the psychoeducational approach to accept a maintenance therapy of at least 300 CPZ units daily, as recommended in the guidelines. In reference to the non-inferior hypothesis, it should be proven that patients who are following this advice will not have more side effects than patients in the CG with a probably lower dosage. This investigation was only possible at the time of the 7-year follow-up. The experimental setup of these motor tasks and the micro-behavioral kinematic analyses of the stored movement trajectories have been described in full detail elsewhere.41–43 Several motor tasks were given44; 3 of them will be reported here:
(1) Repetitive drawing of superimposed circles with the dominant hand (“as quickly as possible”; 7s) as a measure of hypokinesia and motor automaticity. (2) Finger steadiness during bilateral finger spreading (“please stretch your arms and fingers out and spread your fingers lightly; hold this position as still as possible”; 30s), as well as during bilateral pincer grip (“please stretch your arms out and hold these pens as still as possible”; 30s) as a measure of tremor. (3) Bilateral arm dropping (“let your arms fall against your thighs”; 3 times) as a measure of rigidity.
Task 1 was performed on a digitizer tablet (TDS Numonics ZedPen+), tasks 2 and 3 were performed while the patient was standing in front of or beside an ultrasound measurement system (Zebris CMS 50).
To be brief here, only 2 measures from each task will be presented in the following (this restriction of data was not done to eliminate less favorable findings, other categories showed the same trend): The frequency (Hz) and the intra-individual variability (coefficients of variation) of the peak velocities of the up and down movements of the stylus during repetitive circle drawing (task 1); the successive difference mean squares from the acceleration curves of the index finger tips during finger steadiness and pincer grip, respectively (task 2, mean of both hands); the mean dropping time (ms) and the mean peak velocity during arm dropping (task 3, dominant arm only). While the 2 measures from task 1 reflect speed and intra-individual variability as more general aspects of fine motor control, measures from tasks 2 and 3 reflect decreased motor steadiness (tremor) and increased muscle tone (rigidity) as 2 central aspects of possible neuroleptic motor side effects and motor automaticity.
Data Analysis and Statistical Methods
Fisher’s exact test was employed for all comparisons between the study groups involving dichotomous variables, Chi-square tests according to Pearson were used for group comparisons involving categorical variables with several alternative answers. For continuous variables, equal variance t tests for independent samples were used; in the case of inhomogeneity of variance, t tests with adjusted degrees of freedom (df).
Results
Compliance and Long-term Outcome (Days in Hospital)
At index discharge and 7 years later, both groups showed a “good” or “very good” compliance. After 2 years there was a trend for a better compliance in the IG (P = .09), but this is not a statistically significant result.
The corresponding numbers of days in the hospital up to the point of 7 years showed a significant (P < .03) better outcome in the IG: 74.7 days vs 243.4 days (table 2).
Table 2.
Compliance and Long-term Outcome (Days in Hospital)
| Point of Time | Intervention Group, n | Control Group, n | Test | P (2-tailed) |
|---|---|---|---|---|
| Compliance “good”/ “very good” at Index discharge (20/19) | 90.0% | 94.7% | χ2 = 0.31 | 1.0a |
| Compliance “good”/ “very good” 2 years later (20/19) | 95.0% | 73.7 % | χ2 = 3.40 | .09a |
| Compliance “good”/ “very good” 7 years later (21/20) | 85.7% | 85.0% | χ2 = 0.00 | 1.0a |
| Number of rehospitalizations during follow-up | 1.6 | 3.0 | .06 | |
| Days in hospital 7 years later (mean) (20/19) | 74.7 (SD: 99.5) | 243.4 (SD: 309.0) | t = −2.27 | .03b |
Note: aFisher’s Exact Test.
b t test with adjusted degrees of freedom (df) due to inhomogeneity of variance.
Type of Antipsychotic Medication and Dosage at Index-Discharge, as well as 2 and 7 Years Later
At the point of index-discharge in the early 90s, only 10% of the patients in the IG and 15% of the patients in the CG received an exclusive treatment with atypicals. Two and 7 years later the extent of a treatment with atypicals was 25% respectively 57% in the IG; the patients in the CG remained at a clearly lower level of 25%, respectively 30%.
During the period of 24 months after index-discharge, as well as during the period of 6 months before the 7-year follow-up, the average daily consumption of CPZ-units was higher in the IG, but nevertheless these differences did not reach statistically significance (table 3).
Table 3.
Dosage of Antipsychotic Medication (CPZ—Units)
| Date of Investigation | Intervention Group | Control Group | Test | P (2-tailed) | |
|---|---|---|---|---|---|
| Year 2 (average of the last 24 months) | mean | 365.1 | 247.3 | t = 1.32 | .20 |
| SD | 359.7 | 154.8 | |||
| n | (n = 20) | (n = 19) | |||
| Year 7 (average of the last 6 months) | Mean | 354.2 | 279.2 | t = 0.85 | .40 |
| SD | 313.7 | 244.2 | |||
| n | (n = 21) | (n = 20) |
EPMS and TD at Index-Discharge, as well as 2 and 7 Years Later
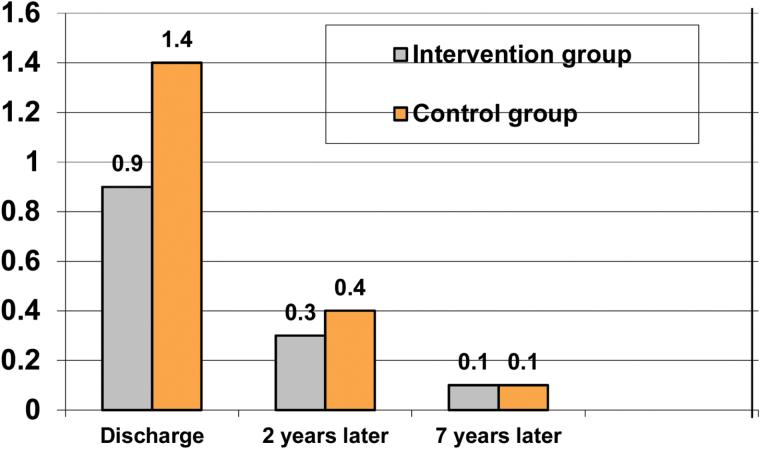
At the time of index-discharge the rigor-score (average of items 1–7 of the EPMS-scale) was 0.9 in the IG and 1.4 in the CG; 2 and 7 years later there was a continuing decrease in both groups, the difference did not reach a significant level (figure 2).
Fig. 2.
Extra pyramidal motor symptoms (EPMS)-rigor (mean of items 1–7).
The amount of TD was measured by item 8 of the Abnormal Involuntary Movement Scale (AIMS)-scale (global severity score). At the time of index-discharge, the percentage of patients without any signs of TD was 71.4% in the intervention and 85% in the CG. Seven years later a decrease to 42.9% in the IG was found, the corresponding value in the CG was 65%. “Severe” hints of TD were absent in both groups 7 years later, but “questionable” or “minimal” signs were more often seen in the IG, but these values did not reach statistical significance (table 4).
Table 4.
Tardive Dyskinesia: AIMS (Item 8: Global Severity Score in %)
| Intervention | IG | CG | IG | CG | IG | CG | IG | CG |
|---|---|---|---|---|---|---|---|---|
| Date of Investigation | No | Questionable (1) or Minimal (2) | Medium (3) | Severe (4) | ||||
| Index discharge (21/20) | 71.4 (n = 21) | 85.0 (n = 20) | 19.0 (n = 21) | 15.0 (n = 20) | 4.8 (n = 21) | 0 (n = 20) | 4.8 (n = 21) | 0 (n = 20) |
| 2 years later (20/19) | 85.0 (n = 20) | 78.9 (n = 19) | 10.0 (n = 20) | 21.3 (n = 19) | 5.0 (n = 20) | 0 (n = 19) | 0 (n = 20) | 0 (n = 19) |
| 7 years later (21/20) | 42.9 (n = 21) | 65.0 (n = 20) | 42.8 (n = 21) | 35.0 (n = 20) | 14.5 (n = 21) | 0 (n = 20) | 0 (n = 21) | 0 (n = 20) |
Note: AIMS, Abnormal Involuntary Movement Scale; IG, intervention group; CG, control group.
Computer-Based Kinematic Analysis of Motor Performance at 7-Year Follow-up
As can be seen from table 5, none of the experimental motor tasks showed significant differences between the 2 patient groups with the exception of the coefficient of variation of the peak velocities during repetitive circle drawing (CV of Vmax in %), which exhibited a tendency to be higher in the CG, indicating increased intra-individual motor variability in the less compliant and with a trend of lower medication and, in the meantime, longer hospitalized patient group (table 5).
Table 5.
Computer-Based Kinematic Analysis of Motor Performance at 7-Year Follow-up
| Intervention Group | Control Group | Test | P (2-tailed) | ||
|---|---|---|---|---|---|
| Circle drawing (21/20) | |||||
| Frequency (Hz) | Mean (SD) | 4.24 (0.59) | 4.19 (0.89) | t = 0.22 | .83 |
| CV of Vmax (%) | Mean (SD) | 10.62 (1.97) | 12.82 (4.52) | t = −2.04 | .05 |
| Finger steadiness (20/20) | |||||
| Finger spreading (sdms) | Mean (SD) | 64.35 (23.03) | 65.58 (21.92) | t = −0.17 | .86 |
| Pincer grip (sdms) | Mean (SD) | 53.57 (19.42) | 49.14 (10.83) | t = 0.89 | .38 |
| Arm dropping (17/20) | |||||
| Dropping time (ms) | Mean (SD) | 670.49 (87.29) | 685.63 (116.32) | t = −0.44 | .66 |
| Vmax (º/ ms) | Mean (SD) | 259.12 (50.59) | 244.60 (51.00) | t = 0.87 | .39 |
Note: CV, coefficient of variation; Vmax, maximum velocity; HZ, Hertz; sdms, successive differences mean square.
Discussion
The main focus of the Munich PIP-Study was to motivate the patients to accept an adequate antipsychotic maintenance therapy in line with the recommendations of Brügge.3 The positive consequences of this motivating program were still found to be working in the IG 2 years later.29
In the 7-year follow-up published in 2007 already,31 including the whole sample size of 48 patients—24 in the IG and 24 in the CG—a significant lower rehospitalization rate—54% vs 88%—and a significant lower amount of hospital days in the meantime—75 vs 225—was found. The non-inferior hypothesis, that patients of the IG would not suffer from a higher rate of motor side effects, despite a higher dosage of antipsychotic medication, was to be tested. Therefore at the 7-year investigation a computer-based kinematic analysis of motor performance was used to find even minimal signs of motor side effects, which are usually not detected by exclusively clinical based examinations. As it was a very complex and long enduring examination, unfortunately 7 patients—3 of the IG and 4 of the CG—did not agree with this procedure in the motor lab. Therefore, the actual article focuses only on the 41 completers of the motor lab and the outcome data are adapted to this new sample size with the consequence of slightly deviating results in comparison with the previous article of 2007.31
The rehospitalization rate per patient during the 7-year follow-up of this slightly smaller sample of 41 patients was 1.6 in the intervention and 3.0 in the CG (P = .06). The number of days in hospital in the interim totaled 243 days in the CG as compared with only 74 days in the IG (P < .03). In comparison with the CG, the patients in the IG had a higher, but not significant, medication dosage after 2 and 7 years.
If this positive outcome was completely caused by psychoeducational long-term effects cannot be proven, because the ongoing treatment and assessment of both groups in the outpatient department of the study center had to be stopped after 4 years owing to a lack of therapeutic capacity. Therefore exact data concerning the therapeutic behavior during the years 5 to 7 of both patient groups are lacking. But these findings would be in line with the long-term outcomes of the psychoeducational studies of Hornung et al45 and Tarrier et al.46
The quota of patients who were treated exclusively with atypicals 7 years later was 57% in the IG; in comparison with the CG this amount had nearly doubled. The external therapists who took over had not been instructed to treat the intervention patients preferentially with atypicals, neither during the 4 years of the treatment in the index facility, nor during the outpatient phase in the following 3 years. In the early and mid 90s, only a minority of schizophrenic patients were treated with atypicals in Germany. Meanwhile, the majority of schizophrenic patients gets atypicals.21,47 It is to be assumed that patients with psychoeducation were more successful in receiving the very best medication strategy. These findings are in agreement with results of the adherence literature.25 According to the shared decision-making concepts,26,30 patients were presumably more actively involved in the selection of their medication. Yet the resulting improvement of compliance 2 years after index-discharge was not significant, probably due to the small sample size of the 2 and 7 follow-up group; patients from the total sample of the PIP-Study did have significant differences.29 Seven years later, there was an equalization of the compliance-rates. It is assumed that the recurrent rehospitalizations of the control patients with repeated psychiatric and psychotherapeutic treatment gradually led to more insight and a better compliance in the long run.
The rate of EPMS was nearly the same in both groups at all investigation points.
The number of patients who had no signs of TD at the 7-year follow-up diminished by about 20 % in the CG and 28.5% in the IG. “Questionable” or “minimal” signs were more often seen in the IG, but you should take into consideration that at index-discharge more patients in the IG had TD in general and severe TD in particular. The higher level of medication in the IG did not result in a statistically significant increase of TD. If this was an effect of the higher rate of atypicals cannot be answered at the moment. These findings agree with data in the literature.22,23,48,49
The results from the clinical rating scales are confirmed by the results of the experimental motor tasks. The kinematic indices derived from those motor tasks have been demonstrated to be very sensitive to even the subtlest dysfunctions in motor control that may easily be overlooked by clinical observation alone.41 The only really significant difference was the higher intra-individual motor variability during repetitive circle drawing in the CG. Increased motor variability in repetitive (diadochokinetic) movements may be interpreted as a neurological soft sign intrinsic to the neurobiological basis of schizophrenia itself rather than an adverse medication side effect.50,51 On the other hand, the higher rate of relapses and the more intensive oscillations of the course of the illness with a more frequent stop-and-go of medication may also be responsible for this significantly worse result in the CG. These findings must be replicated by further studies.
As in the case of other authors,45,46 the 7-year follow-up outcome was significantly better in the IG.31,52 The advantage concerning rehospitalizations due to good compliance did not provoke a higher rate of discrete motor disturbances. Whether the higher rate of atypicals has induced this positive outcome cannot be answered by the present data, but we can assume that the higher rate of atypicals is responsible for the lower level of side-effects in spite of a higher amount of CPZ-units. Other findings in the literature,13,52–55 confirm that patients with psychoeducation usually exert more influence on the kind of treatment they receive, and this could be the reason for more atypicals, as found in this study. The higher rate of atypicals could be seen as the only reason for the better outcome of the IG in general, but atypicals alone cannot explain the enormous difference of almost 168 days in hospital during the 7 years (74.7 vs 243.4); this reduction of hospital days can be seen as a consequence of improvement of relapse prevention by the bifocal psychoeducational intervention.
These findings are encouraging concerning the improvement of adherence by psychoeducation. Psychoeducation can lead to a significant improvement of long-term outcome and save treatment costs, above all, by the reduced number of hospital days.56 Being better informed and thus better empowered, patients can positively influence their medical treatment and reduce side effects in the long run. In particular we have to realize, that improvement of compliance means an increase of CPZ units and dosage. Patients who trust us and follow our advice can avoid the risk irresponsible harm in comparison to patients with non-adherence. We can never promise a total guarantee that the prescribed medication will do no harm, but we have to guarantee that our patients are well informed concerning their individual risk and that we provide them with the necessary information to make an informed consent concerning their way of treatment. This must sometimes include the possibility to ignore our recommendations. Psychoeducation should make sure by providing a convincing empowerment that this will be the smaller part of patients. Psychoeducation can be recommended as an important part of the routine treatment of patients with schizophrenia.
Limitations
The sample size 7 years after discharge was rather small in both groups and a selection bias of higher motivated and more healthy patients cannot be excluded. The type of medication used was not documented over a period of 6.5 years. It was only during the previous 6 months prior to follow-up that the medication was exactly explored. The higher rate of atypicals in the IG may have influenced the better outcome in this patient group. Severe TD at discharge was only observed in the IG, therefore presumably at the 7-year follow-up, the patients of the IG continued to show a higher incidence of TD. The study patients could only be treated in the index facility for a period of 4 years, the rest of the 3 follow-up years was more or less uncontrolled. It cannot be excluded that unknown confounders have differently influenced the outcome in both groups.
Funding
The first 2 years of the PIP-study were supported by a grant from the Bundesministerium für Forschung und Technologie (BMFT), Registration Number: 325-4007-07018548; the long-term follow-up of the sub-sample of the TUM was supported by a grant from the DORIST-Fond, in Kreuzlingen, Switzerland.
Acknowledgments
Patients and their relatives from the following institutes were involved in the PIP-Study: BKH Haar, Universitätsnervenklinik Nußbaumstraße (LMU), Klinikum rechts der Isar der TU München (TUM). We wish to thank them all for their willingness to take part in this study. We are pleased to be able to extend our very special thanks to the following colleagues for their excellent collaboration: Lempa G, Peuker-Schulz I, Welschehold M, Bender W, Walter A, Perro I, Börner R, Mayer C, Messer T, Wagner M, Naber D, Engel R, Hippius H, Möller H-J, Schlag K, Buttner P, Lauter H. To support the implementation of psychoeducation in routine treatment, the society Deutsche Gesellschaft für Psychoedukation” (DGPE) was founded in Germany in October 2005. Contact mail: J.Baeuml@lrz.tum.de. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. APA. Guidelines for Treatment of Schizophrenia. Washington, DC: American Psychiatric Association; 2004. [Google Scholar]
- 2. DGPPN. S-3-Behandlungsleitlinie Schizophrenie. Darmstadt, Germany: Steinkopff Verlag; 2006. [Google Scholar]
- 3. Kissling W. Guidelines for Neuroleptic Relapse Prevention in Schizophrenia. Berlin, Germany: Springer Verlag; 1991. [Google Scholar]
- 4. Harrow M, Jobe TH. Does long-term treatment of schizophrenia with antipsychotic medications facilitate recovery? Schizophr Bull. 2013;39:962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henriksen MD, Parnas J. Self-disorders and schizophrenia: a phenomenological reappraisal of poor insight and noncompliance. Schizophr Bull. 2015;40:542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fenton W, Bleyler C, Heinssen R. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23:637–651. [DOI] [PubMed] [Google Scholar]
- 7. Knapp M, King D, Pugner K, et al. Non-adherence to antipsychotic medication regimes: associations with resource use and costs. Brit J Psychiatry. 2004;184:509–516. [DOI] [PubMed] [Google Scholar]
- 8. Lacro J, Dunn L, Dolder C, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892–909. [DOI] [PubMed] [Google Scholar]
- 9. McEvoy J. The relationship between insight into psychosis and compliance with medications. In: Amador X, David A, eds. Insight and Psychosis. Oxford, UK: Oxford University Press; 2004:311–333. [Google Scholar]
- 10. Jahn T, Mussgay L. Die statistische Kontrolle möglicher Medikamenteneinflüsse in experimentalpsychologischen Schizophrenie-Studien: ein Vorschlag zur Berechnung von Chlorpromazin-Äquivalenten. Zeitschrift für Klinische Psychologie. 1989;18:257–267. [Google Scholar]
- 11. Leucht S, Pitschel-Walz G, Abraham D, Kissling W. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. [DOI] [PubMed] [Google Scholar]
- 12. Schooler N, Keith S, Severe J, et al. Relapse and rehospitalisation during maintenance treatment of schizophrenia. Arch Gen Psychiatry. 1997;54:453–463. [DOI] [PubMed] [Google Scholar]
- 13. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of one-year studies. Am J Psychiatry. 2004;161:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346:16–22. [DOI] [PubMed] [Google Scholar]
- 15. Leucht S, Barnes TRE, Kissling W, et al. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry. 2003;160:1209–1222. [DOI] [PubMed] [Google Scholar]
- 16. Möller HJ. Course and long-term treatment of schizophrenic psychoses. Pharmacopsychiatry. 2004;37:126–135. [DOI] [PubMed] [Google Scholar]
- 17. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. [DOI] [PubMed] [Google Scholar]
- 18. Young AS, Niv N, Cohen AN, Kessler C, McNagny K. The appropriateness of routine medication treatment for schizophrenia. Schizophr Bull. 2010;36:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeuchi H, Suzuki T, Remington G, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophr Bull. 2013;39:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stübner S, Rustenbeck E, Grohmann R, et al. Severe and uncommon involuntary movement disorders due to psychotropic drugs. Pharmacopsychiatry. 2004;37:54–64. [DOI] [PubMed] [Google Scholar]
- 21. Woods SW, Morgenstern H, Saska JR, et al. Incidence of tardive dyskinesia with atypical and conventional antipsychotic medications: prospective cohort study. J Clin Psychiatry. 2010;71:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willems AE, Tenback DE, Ingenhoven TJ, van Harten PN. Acute movement disorders associated with the use of second-generation antipsychotics in borderline personality disorder: a meta-analysis. J Clin Psychopharmacol. 2014;34:520–522. [DOI] [PubMed] [Google Scholar]
- 23. Gopal S, Xu H, Bossie C, et al. Incidence of tardive dyskinesia: a comparison of long-acting injectable and oral paliperidone clinical trial databases. Int J Clin Pract. 2014;68:1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bäuml J, Froböse T, Kraemer S, Rentrop M, Pitschel-Walz G. Psychoeducation: a basic psychotherapeutic intervention of patients with schizophrenia and their families. Schizo Bull. 2006;32:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamann J, Pfeiffer H, Leucht S, Kissling W. Are patients with schizophrenia under-treated with second-generation antipsychotics? A pilot study of the prescription practices of German psychiatrists. Pharmacopsychiatry. 2003;36:309–312. [DOI] [PubMed] [Google Scholar]
- 26. Jahn T, Pitschel-Walz G, Gsottschneider A, Froböse T, Kraemer S, Bäuml J. Neurocognitive prediction of illness knowledge after psychoeducation in schizophrenia: results from the Munich COGPIP study. Psychol Med. 2011;41:533–544. [DOI] [PubMed] [Google Scholar]
- 27. Lincoln TM, Wilhelm K, Nestoriuc Y, Lincoln TM, Wilhelm K, Nestoriuc Y. Effectiveness of psychoeducation for relapse, symptoms, knowledge, adherence and functioning in psychotic disorders: a meta-analysis. Schizophr Res. 2007;96:232–245. [DOI] [PubMed] [Google Scholar]
- 28. Xia J, Merinder LB, Belgamwar MR. Psychoeducation for schizophrenia. Cochrane Database Syst Rev. 2011;6:CD002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pitschel-Walz G, Bäuml J, Engel R, Voss A, Kissling W. Psychoeducation and Compliance in the treatment of schizophrenia: results of the Munich Psychosis Information Project-Study. J Clin Psychiatry. 2006;67:443–452. [DOI] [PubMed] [Google Scholar]
- 30. Huhn M, Tardy M, Spineli LM, et al. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry. 2014;71:706–715. [DOI] [PubMed] [Google Scholar]
- 31. Bäuml J, Pitschel-Walz G, Volz A, Engel R, Kissling W. Psychoeducation in schizophrenia: rehospitalisation and hospital days – 7 year follow-up of the Munich Psychosis Information Project - Study. J Clin Psychiatry. 2007;68:854–861. [PubMed] [Google Scholar]
- 32. Bäuml J, Pitschel-Walz G, Berger H, Gunia H, Juckel G, Heinz A. Arbeitsbuch PsychoEdukation bei Schizophrenien. Stuttgart, Germany: Schattauer Verlag; 2005. [Google Scholar]
- 33. Fiszdon JM, Kurtz MM, Choi J, Bell MD, Martino S. Motivational interviewing to increase cognitive rehabilitation adherence in schizophrenia. Schizophr Bull. 2015. doi:10.1093/schbul/sbv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bäuml J. Psychosen aus dem schizophrenen Formenkreis. Ein Ratgeber für Patienten und Angehörige. Heidelberg, Germany: Springer Verlag; 1994. [Google Scholar]
- 35. Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 37. Kane J. Pharmacologic treatment of schizophrenia. Biol Psychiatry. 1999;46:1396–1408. [DOI] [PubMed] [Google Scholar]
- 38. Ryu S, Yoo JH, Kim JH, et al. Tardive dyskinesia and tardive dystonia with second-generation antipsychotics in non-elderly schizophrenic patients unexposed to first-generation antipsychotics: a cross-sectional and retrospective study. J Clin Psychopharmacol. 2015;35:13–21. [DOI] [PubMed] [Google Scholar]
- 39. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 40. Guy W, ed. ECDEU Assessment Manual. Rockville, MD: US Department of Health, Education and Welfare; 1976:534–537. [Google Scholar]
- 41. Jahn T. Diskrete motorische Störungen bei Schizophrenie. Weinheim, Germany: Beltz/ Psychologie Verlags Union; 1999. [Google Scholar]
- 42. Jahn T. ed. Bewegungsstörungen bei psychischen Erkrankungen. Berlin, Germany: Springer; 2004. [Google Scholar]
- 43. Jahn T, Cohen R. Kinematische Analysen motorischer Störungen in der Psychiatrie: Einige Prinzipien und Befunde. In Bräunig P, ed. Motorische Störungen bei Schizophrenen Psychosen. Stuttgart, Germany: Schattauer; 1999:17–40. [Google Scholar]
- 44. Wiesholler C. Kinematische Analyse fein- und grobmotorischer Koordinationsleistungen bei schizophrenen Patienten sieben Jahre nach einer pharmakologisch-psychoedukativen Kombinationsbehandlung. Ludwig-Maximilians-Universität München; 2000. [Google Scholar]
- 45. Hornung P, Feldmann R, Klingberg S, et al. Long-term-effects of a psychoeducational psychotherapeutic intervention for schizophrenic outpatients and their key-persons – results of a five-year follow-up. Eur Arch Psychiatry Clin Neurosci. 1999;249:162–167. [DOI] [PubMed] [Google Scholar]
- 46. Tarrier N, Barrowclough C, Porceddu K, Fitzpatrick E. The Salford Family Intervention Project: relapse rates of schizophrenia at five and eight years. Brit J Psychiatry. 1994;165:829–832. [DOI] [PubMed] [Google Scholar]
- 47. Percudani M, Barbui C, Fortino I, Petrovich L. Epidemiology of first- and second-generation antipsychotic agents in Lombardy, Italy. Pharmacopsychiatry. 2005;38:128–131. [DOI] [PubMed] [Google Scholar]
- 48. Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-Generation Antipsychotics and Extrapyramidal Adverse Effects. Bio Med research International 2014. Hindawi Publishing Corporation; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Falkai P, Schmitt A. [Surveillance in the group of schizophrenia: key to understanding the etiology]. Nervenarzt. 2013;84:1091–1092. [DOI] [PubMed] [Google Scholar]
- 50. Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145:11–18. [DOI] [PubMed] [Google Scholar]
- 51. Jahn T, Cohen R, Hubmann W, et al. The Brief Motor Scale (BMS) for the assessment of motor soft signs in schizophrenic psychoses and other psychiatric disorders. Psychiatry Res. 2006;142:177–189. [DOI] [PubMed] [Google Scholar]
- 52. Pitschel-Walz G, Leucht S, Bäuml J, Kissling W, Engel RR. The effect of family interventions on relapse and rehospitalisation in schizophrenia – a meta-analysis. Schizophr Bull. 2001;27:73–92. [DOI] [PubMed] [Google Scholar]
- 53. Guo JJ, Wu J, Kelton CM, et al. Exposure to potentially dangerous drug-drug interactions involving antipsychotics. Psychiatr Serv. 2012. doi:10.1176/appi.ps.201100443. [DOI] [PubMed] [Google Scholar]
- 54. Schaub A, Neubauer N, Mueser KT, Engel R, Möller HJ. Neuropsychological functioning in inpatients with major depression or schizophrenia. BMC Psychiatry. 2013;13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao S, Sampson S, Xia J, Jayaram MB. Psychoeducation (brief) for people with serious mental illness. Cochrane Database Syst Rev. 2015;4:CD010823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pitschel-Walz G, Gsottschneider A, Froböse T, Kraemer S, Bäuml J, Jahn T. Neuropsychologie der Psychoedukation bei Schizophrenie. Ergebnisse der Münchner COGPIPStudie. Nervenarzt. 2013;84:79–90. [DOI] [PubMed] [Google Scholar]