Abstract
Migraine and other primary headache disorders affect a large population and cause debilitating pain. Establishing animal models that display behavioral correlates of long-lasting and ongoing headache, the most common and disabling symptom of migraine, is vital for the elucidation of disease mechanisms as well as the identification of drug targets. We have developed a mouse model of headache, using dural application of capsaicin along with a mixture of inflammatory mediators (IScap) to simulate the induction of a headache episode. This elicited intermittent head-directed wiping and scratching as well as the phosphorylation of c-Jun N-terminal kinase in trigeminal ganglion neurons. Interestingly, dural application of IScap preferentially induced FOS protein expression in the excitatory but not inhibitory cervical/medullary dorsal horn neurons. The duration of IScap-induced behavior and the number of FOS-positive neurons correlated positively in individual mice; both were reduced to the control level by the pretreatment of anti-migraine drug sumatriptan. Dural application of CGRP(8–37), the calcitonin gene-related peptide (CGRP) receptor antagonist, also effectively blocked IScap-induced behavior, suggesting that the release of endogenous CGRP in dura is necessary for IScap-induced nociception. These data suggest that dural IScap-induced nocifensive behavior in mice may be mechanistically related to the ongoing headache in humans. In addition, dural application of IScap increased resting time in female mice.. Taken together, we present here the first detailed study using dural application of IScap in mice. This headache model can be applied to genetically modified mice to facilitate the research for the mechanisms and therapeutic targets for migraine headache.
1. Introduction
Migraine and other primary headache disorders affect a large proportion of the general population and cause debilitating pain that substantially compromises patients’ quality of life. Despite the high prevalence, the disease mechanisms are still unclear and the treatment options remain limited. The development of animal models that recapitulate the symptoms and pathophysiology of migraine are vital for the elucidation of disease mechanisms as well as the identification of new diagnostic biomarkers and potential drug targets [2, 52]. In rats, chemical stimulation of the dura is a well-established preclinical model of headache. It produces several behavioral correlates of headache and its accompanying symptoms, including a decrease in feeding and exploratory behavior [13, 37, 39], the establishment of facial and hindpaw mechanical allodynia [14, 43, 64], an increase in facial grooming and resting behavior [39] as well as an aversive state of cephalic pain [11]. Administration of nitroglycerin (NTG), a well-established migraine trigger in humans, also evokes cutaneous hyperalgesia in rats [49, 59].
Various genetically modified mouse lines offer great tools to understand migraine pathophysiology. It is thus pertinent to develop preclinical models that provide behavioral correlates of headache and related symptoms in mice, especially the objective and quantifiable measurements of mouse behaviors that may be mechanistically related to the ongoing headache, the major symptom of migraine in humans. Systemic injection of NTG in mice elicits hindpaw allodynia, light aversion and conditioned place aversion [5, 38, 47, 48]. Intracerebroventricular injection of calcitonin gene-related peptide (CGRP) also evokes light-aversion in mice [29, 50]. Repetitive cortical spreading depression induces an increase in mouse grimace scale [30]. Multiple spontaneous behaviors including head-directed grooming, lateralized eye blink and whole-body shuddering are considered suggestive of spontaneous head pain in mice [8].
Building upon these previous works, we mimic the induction of a migraine episode by stimulating the dura with a mixture of capsaicin and inflammatory mediators (IScap) in awake, freely moving mice and observe their home cage behavior. We show here that dural application of IScap elicits robust head-directed nocifensive behavior in mice. Using GAD67-GFP mice to selectively label inhibitory neurons, we find that dural IScap induces FOS protein expression predominantly in excitatory neurons in the cervical/medullary dorsal horn (trigeminocervical complex, TCC). Both IScap-induced behavior and FOS expression are reduced to the control level by the pretreatment of anti-migraine drugs sumatriptan and the CGRP receptor antagonist, suggesting that IScap-induced behavior may be mechanistically related to the ongoing headache during a migraine episode. Activation of the c-Jun N-terminal kinase (JNK) signaling pathway is also necessary for the manifestation of IScap-induced behavior. Interestingly, only female mice exhibit an increase in resting time in response to dural application of IScap. Taken together, we have developed a preclinical mouse model of headache and provided the first detailed characterization of the effects of dural application of IScap in mice. This provides a valuable tool to use mouse as a model system to better understand the mechanisms underlying migraine, especially the ongoing headache, as well as to screen for novel therapeutics.
Portions of this manuscript have been published previously in abstract form [26].
2. Methods
2.1. Mice
All experiments in this study adhered to the guidelines of the Committee for Research and Ethical Issues of IASP [69]. All procedures were approved by the Animal Studies Committee at Washington University in St. Louis, in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 86–23). Mice were housed (2–5 per cage, same sex) in the animal facility of Washington University in St. Louis on a 12-hr light/dark cycle with constant temperature (23–24°C), humidity (45–50%) as well as food and water ad libitum.
Adult Swiss Webster and CD-1 mice (8–12 weeks old, both male and female, Charles River) were used in the experiments. Adult male heterozygous mice expressing the green fluorescent protein (GFP) from one glutamate decarboxylase 67 (GAD67) locus (GAD67-GFP) were maintained on C57BL/6 background and the genotype was determined by PCR of tail DNA as described [58]. The GABAergic inhibitory neurons are selectively labeled by the GFP signal in these mice [58].
2.2. Surgical preparation and behavioral tests
All mice were housed in the animal facility for at least 5–7 days before acclimation. Mice were transported to the testing room and were habituated individually in a clean cage (with bedding, food and water ad libitum) for 3–5 days (> 3 hrs per day) before the surgery and behavioral tests. Mice were gently handled at least 5 times during each habituation period until they show no signs of freezing or rapid escaping when approached by the experimenter.
On the test day, mice (adult male and female Swiss Webster, adult male GAD67-GFP heterozygotes) were acclimated individually in a clean cage (with bedding, food and water ad libitum) for 1 hr. Subsequently, mice were anesthetized with 3–4% isoflurane in oxygen in an induction chamber until loss of the righting reflex and were mounted on a Stoelting stereotaxic apparatus. Anesthesia was maintained by 1.5–2% isoflurane through a nose cone. Body temperature was maintained by placing mice on a 37°C circulating water warming pad. A small amount of ophthalmic ointment was placed in the eyes to prevent the corneas from drying. Lidocaine hydrochloride jelly (2%) was applied on the skin for 5–10 min before a longitudinal skin incision was made to expose the cranium. Topical lidocaine solution (2%) was applied on the skull before the muscle and periosteal sheath were removed in the area overlying the superior sagittal sinus (SSS) between bregma and lambda. A craniectomy (~2.5 mm diameter) was made with a cooled dental drill within this area, leaving the underlying dura exposed but intact. Topical lidocaine solution (2%) was repetitively applied on the skull during the craniectomy to prevent the activation and/or sensitization of the primary afferent neurons. A sterile polypropylene ring (3.5 mm diameter, 3.2 mm length, holds ~30 µl solution) was sealed to the skull surrounding the exposed dura by a mixture of dental cement powder (Stoelting 51459) and superglue adhesive to prevent the spreading of the solution to other peripheral sites. The viscosity of dental cement/superglue mix kept it from spreading to the exposed dura. After waiting 5–10 min for the mix to solidify, we applied 25 µl of solutions (see below) onto the exposed dura. Subsequently, a sterile polypropylene cap was secured over the ring by applying the dental cement/superglue mix on the outer surface of the cap and the ring. The skin incision was closed with 5-0 silk suture.
After recovery from anesthesia, mice were returned to the clean cage and their behaviors were recorded by digital video cameras for 2 hrs before euthanization. A diagram of the recording setup was shown in Fig. S1. The cage was placed in front of a 3-way mirror to ensure that the head-directed behavior be recorded at all body positions. Mice were recorded one at a time in the absence of the experimenter and other mice. Digital video files were quantified off-line by experimenters blinded to the treatments that mice received. The entire 2-hr video was watched and scored. Time spent on forepaw wiping and hindpaw scratching within the mouse V1 dermatome (including the scalp and periorbital area) was quantified as nocifensive behavior.
To estimate the spread of solutions on the dura and the skull, we applied the dye fast green (FG, 1% in 20 µl ACSF with 2% DMSO) to the polypropylene ring and examined the spread of dye 2 hrs later [35]. The average diameter of the FG-stained area on the skull (4.2 ± 0.2 mm) was smaller than the outer diameter of the ring (4.5 mm, Fig. S2), indicating that the spread of solution on the skull was well confined by the polypropylene ring. Conversely, the average diameter of the FG-stained area on the dura was 8.6 ± 0.5 mm (Fig. S2), suggesting that the solution spread to the dura well beyond the exposed area after 2-hr incubation.
In some experiments, mice (adult male Swiss Webster and GAD67-GFP heterozygotes) were perfused for tissue collection at the end of the recording session. To compare the pattern of FOS protein expression between awake and anesthetized mice, we maintained some mice under anesthesia (1.5–2% isoflurane) for 2 hrs after dural application of solutions and skin suture. Mice were then perfused for tissue collection.
To minimize the effects of surgery and anesthesia, we allowed some adult CD-1 mice (both male and female) to recover from surgery for 7 days before testing the behavior. After sealing the sterile polypropylene ring onto the skull to surround the exposed dura, we applied 20 µl of sterile artificial cerebrospinal fluid (ACSF, see below) onto the dura. Subsequently, a sterile polypropylene cap was secured over the ring with bone wax to cover the exposed dura. The skin incision was closed with 5-0 silk suture, leaving the cap exposed. After recovery from anesthesia, mice were housed individually in the animal facility for 7 days and were transported to the testing room for acclimation every day. On post-surgery day 7, we habituated the mouse in a clean cage (with bedding, food and water ad libitum) for 1 hr and recorded their behavior for 2 hrs. The next day, the mouse was briefly anesthetized with isoflurane after 1 hr habituation in a clean cage. The polypropylene cap was removed without disturbing the skin suture and 20 µl of vehicle was applied (see below) onto the exposed dura. Subsequently, a new sterile polypropylene cap was secured over the ring with bone wax to cover the dura. After recovery from anesthesia, the mouse was returned to the clean cage and its behavior was recorded for 2 hrs. Two days later (post-surgery day 10), the procedure was repeated to record mouse behavior after the application of 20 µl of IM (see below) or ACSF onto the dura. In addition to head-directed behavior, we also recorded time spent resting (immobile, with eye closed or open but not freezing) or in these mice. The freezing behavior rarely occurred in all groups of mice.
The facial formalin test was modified as described previously [36]. In brief, adult male GAD67-GFP mice were acclimated individually in a clean cage (with bedding, food and water ad libitum) in the testing room for 3–5 days. The skin around the periorbital region (between the two eyes) was shaved 24 hrs before the test. The dilute formalin solution (1% in 20 µl saline) or saline was injected intradermally in the periorbital region. Mice were immediately returned to the clean cage and were recorded by digital video cameras for 1 hr. Digital video files were quantified off-line. Time spent on forepaw wiping and hindpaw scratching of the injected area and the forehead (within the V1 dermatome) was quantified as nocifensive behavior. Two hrs after formalin injection, mice were perfused for tissue collection.
The mouse cheek models of itch and pain were conducted in adult male Swiss Webster mice as described previously [3, 55]. In brief, mice were acclimated individually in clear Plexiglas containers (11×11×16 cm height) surrounded by 3-way mirrors in the testing room for 3–5 days. The cheeks were shaved 2 days before the experiments. On the test day, mice were habituated in the clean containers for an hour and then received intraperitoneal (i.p.) injections of saline or sumatriptan (10 mg/kg in saline). Fifteen min later, mice were injected with 20 µl solutions intradermally in the cheeks, immediately returned to the containers and were recorded by digital video cameras for 15 min. Digital video files were quantified off-line. To test itch responses, mice received 10 µg serotonin (5-HT, in 20 µl saline) or 20 µl saline injections in the cheeks. Total bouts of ipsilateral hindpaw scratching of the injected area were quantified by a single observer blinded to the experimental groups. To test pain responses, mice received 10 µg capsaicin (in 20 µl saline with 1% DMSO) or 20 µl vehicle (1% DMSO in saline) injections in the cheeks. Total numbers of ipsilateral forepaw wiping of the injected area were quantified by a single observer blinded to the experimental groups.
2.3. Tissue preparation and immunohistochemistry (IHC)
Adult male mice (wild-type Swiss Webster and heterozygous GAD67-GFP) were euthanized with i.p. injection of barbiturate (200 mg/kg) and were transcardiacally perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.2) followed by 4% formaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for fixation. Trigeminal ganglion (TG) as well as the brain and spinal cord tissues were dissected out, post-fixed for 4 hours, and protected overnight in 30% sucrose in 0.1 M PB.
The caudal medulla (~1.5 mm rostral of obex) and upper cervical spinal cord (to C3) was removed as a single block, frozen in OCT media and cut transversely into 30 µm free-floating sections. One in every 3 sections was processed for each IHC experiment. Sections were washed 3 times in 0.01 M PBS and incubated in blocking buffer consisting of 0.01 M PBS, 10% normal goat serum (NGS) and 0.3% triton X-100 at room temperature (RT) for 1 hr. Sections were then incubated with an antibody for FOS protein (EMD, 1:5000 dilution in blocking buffer) for 48 hrs at 4°C with gentle agitation. Following 3 washes by 0.01 M PBS with 1% NGS and 0.3% triton for 10 min and blocking for 1hr, sections were incubated with the AlexaFluor 568- or 488-conjugated goat anti-rabbit secondary antibody (Invitrogen,1:1000 dilution in blocking buffer) at RT for 2 hrs and were washed 3 times in 0.01 M PBS. The sections were mounted on gelatin-coated slides and cover-slipped using Fluoromount-G mounting medium (Electron Microscopy), sealed with nail polish and stored at 4°C.
The number of neurons containing FOS-immunoreactivity (FOS-ir) in TCC was quantified under a 20x objective on a Nikon TE2000S epifluorescence microscope by a single observer blinded to the experimental groups. We counted sections (10–12 per mouse) from the caudal portion of the trigeminal nucleus caudalis (Vc) to C1 cervical spinal cord. We defined the rostral border of the caudal Vc as ~270 µm caudal of the obex (the appearance of the central canal) in the coronal sections of adult mouse brain. The caudal border of C1 was defined according to a previous study [41]. To avoid counting FOS-ir in neurons of the trigeminal nucleus interplaris (Vi), we did not analyze FOS-ir in the rostral portion of Vc (90 – 270 µm caudal of obex in adult mouse) [56]. In some experiments, the dorsal horn was divided into laminae I-V [56] and the number of neurons containing FOS-ir in laminae I-II, III-IV and V was quantified separately.
TG tissues were frozen in OCT media and sectioned at 20 µm using a cryostat, mounted on Superfrost Plus glass slides and stored at −20°C. One in every 3 TG sections was processed for each IHC experiment. The sections were dried at RT, washed three times in 0.01 M PBS and incubated in blocking buffer for 1 hr. Sections were then incubated with primary antibodies (Cell Signaling Technologies) against the phosphorylated forms of extracellular signal-regulated kinase (pERK, 1:1000), p38 (p-p38, 1:200) and JNK (pJNK, 1:100) for 24 hrs at 4°C, respectively. The AlexaFluor 568-conjugated goat anti-rabbit secondary antibody (Invitrogen) was used at 1:1000 dilution. The number of TG neurons containing pERK-, p-p38- and pJNK-immunoreactivities (pERK-ir, p-p38-ir, and pJNK-ir) was quantified under a 20x objective on a Nikon TE2000S epifluorescence microscope by a single observer blinded to the experimental groups.
The low-power images were captured by the Olympus NanoZoomer Whole-Slide Imaging System in the Alafi neuroimaging core facility of Washington University Medical School. The high-power images were captured through a 20x objective on a Nikon TE2000S inverted epifluorescence microscope equipped with a CoolSnapHQ2 camera (Photometrics). Representative images were contrast and brightness adjusted using the same parameter within individual experiments. No other manipulations were made to the images.
2.4. Drug application
The ACSF consists of (in mM) 125 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 1 MgCl2, 1 CaCl2, and 25 glucose. The composition of IScapwas modified from previous studies [40, 43, 57]. The IScap solution contained 0.5 mM capsaicin and a mixture of proinflammatory reagents including 1 mM bradykinin, 1 mM histamine, 1 mM 5-HT and 0.1 mM prostaglandin E2 (PGE2) in 0.01 M PBS (pH 7.2) with 2% DMSO. The acidic (pH 5.0) IM solution contained an additional 10 mM Hepes [14]. The vehicle control consists of ACSF (pH 7.2) with 2% DMSO. All chemicals were purchased from Sigma, dissolved in H2O (bradykinin, histamine and 5-HT) or DMSO (PGE2 and capsaicin) at 100x concentrations and stored at −80°C in aliquots. IScap was freshly prepared from the stock solution on each day of surgery and behavioral test.
S-Nitroso-N-acetyl-penicillamine (SNAP, Sigma) was dissolved in ACSF to generate 50 mM stock and was diluted in ACSF to 500 µM prior to dural application. This concentration of SNAP induced significant cerebral vasodilation in a previous report [20]. Another vasodilator, rat α-CGRP (Bachem), was dissolved in ACSF and was applied to the dura at 1 µM or 100 µM in 25 µl ACSF. The CGRP receptor antagonist CGRP(8–37) (Bachem) was dissolved in ACSF as 100 µM stock solution and was diluted in ACSF on the test day. We applied 25 µl CGRP(8–37) (1 µM) to the dura for 15 min, removed the solution, and then applied IM or vehicle in the presence of 1 µM CGRP(8–37) to the dura. The control groups received 15 min ACSF pretreatment.
Sumatriptan succinate (Sigma) was dissolved in saline as 10 mg/ml stock solution and was injected (i.p. 3–10 mg/kg in saline, [5, 40]) 15 min prior to the dural application of IScap or vehicle. Naproxen (Sigma) was injected (i.p. 100 mg/kg in saline) 15 min prior to the dural application of IScap or vehicle [10].
2.5. Statistical analysis
All data are reported as mean ± standard error of the mean. Shapiro-Wilk test was used to check data normality. Statistical significance between experimental groups was assessed by the two-tailed t-test or analysis of variance (ANOVA, one-way or two-way, with or without repeated measures [RM]) with post hoc Bonferroni test where appropriate, using Origin and Statistica (from OriginLab and StatSoft, respectively). Differences with p < 0.05 were considered to be statistically significant.
3. Results
3.1. Dural application of IScap elicits trigeminal V1-directed nocifensive behavior in mice
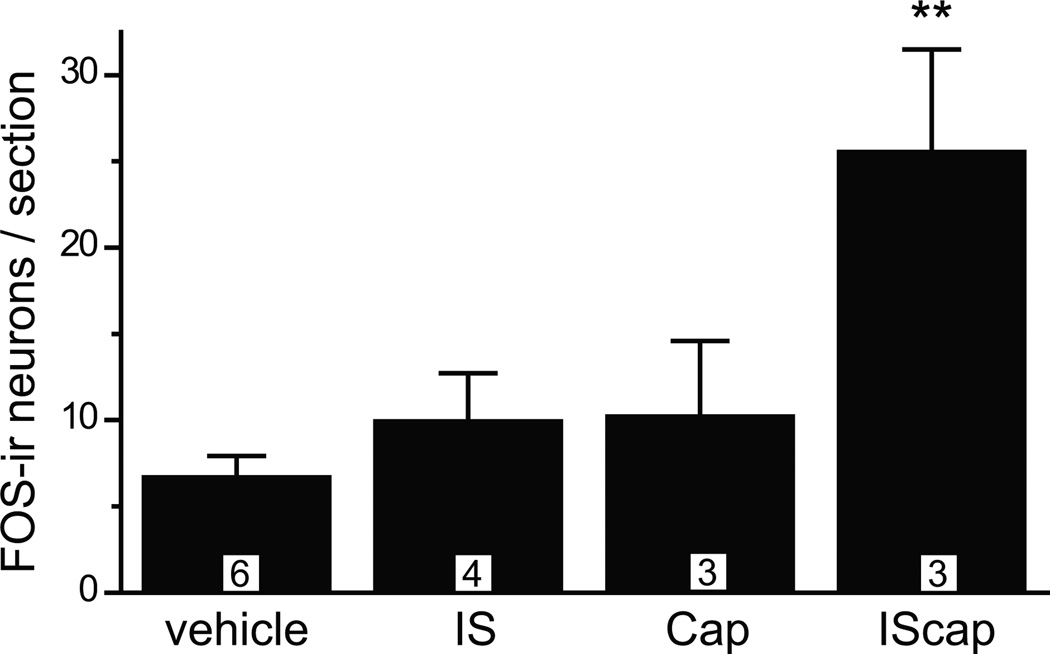
First, we screened for the chemical stimulus that robustly activates the trigeminovascular pathway in mice, using the expression of the immediate-early gene product FOS protein as a marker for neuronal activation. Previous studies show that FOS expression in TCC neurons can be induced by intracisternal administration of capsaicin in mice and dural application of a mixture of proinflammatory reagents (IS) in rats, respectively [14, 40]. We have shown by retrograde labeling that around 25% of dural afferent neurons in mice express the capsaicin-sensitive channel TRPV1 [25]. We exposed the dura above a section of SSS in anesthetized adult male Swiss Webster mice and applied various chemical stimuli 10 min after craniectomy. Dural application of capsaicin or IS for 2 hours did not significantly increase the number of TCC neurons containing FOS-ir in anesthetized mice (Fig. 1). On the contrary, dural application of both IS and capsaicin (IScap) resulted in a more than 3-fold increase in the number of FOS-positive neurons in mouse TCC (Fig. 1, 7 ± 1 and 26 ± 6 FOS-ir neurons/section in vehicle and IScap groups, respectively, p < 0.01, one-way ANOVA with post hoc Bonferroni test).
Fig. 1. Dural application of IScap induces FOS protein expression in TCC neurons in anesthetized mice.
Average number of TCC neurons exhibiting FOS-ir in adult male Swiss Webster mice that received 25 µl dural application of vehicle (2% DMSO in ACSF, pH 7.2), IS (1 mM bradykinin, 1 mM histamine, 1 mM 5-HT and 0.1 mM PGE2), Cap (0.5 mM capsaicin) and IScap (IS plus capsaicin) 10 min after the craniectomy. Mice were kept under anesthesia for 2 hours. Tissue sections were incubated with FOS antibody (1:5000) at 4°C overnight (**p < 0.01 compared with the vehicle group; one-way ANOVA with post hoc Bonferroni test). The number of mice in each group is indicated in the figure.
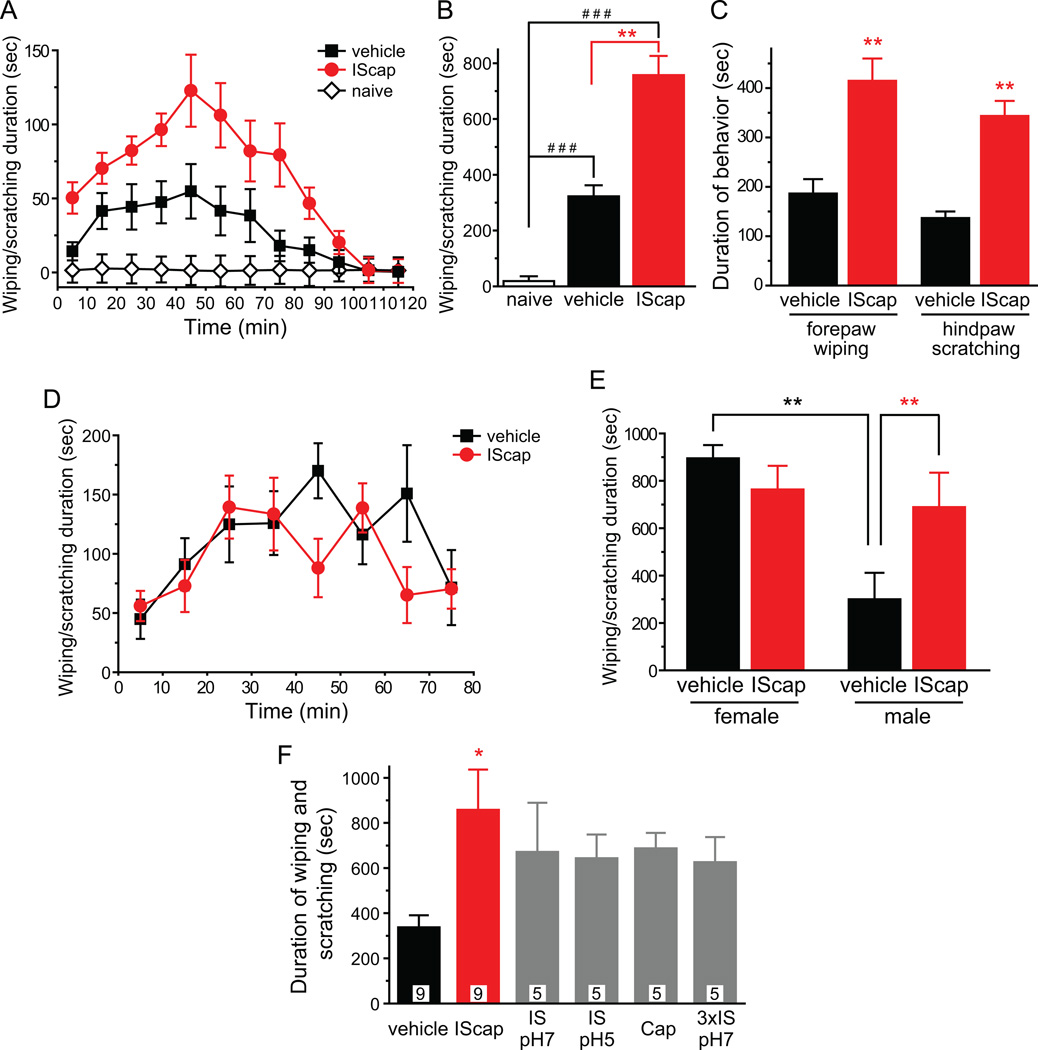
Next, we tested whether dural application of IScap causes behavioral changes in awake mice. Naïve, well habituated mice spent little time (20 ± 3 sec) wiping and scratching the skin around the scalp and periorbital area during the 2 hour observation period (Fig. 2A). We then applied IScap or vehicle onto the dura above a section of SSS 10 min after the craniectomy, let mice recover from anesthesia and recorded their behavior for 2 hours. In IScap -treated group, we observed bouts of forepaw wiping and hindpaw scratching around the scalp and periorbital area within the trigeminal V1 dermatome. The duration of wiping and scratching reached peak 40–50 min after mice recovered from anesthesia and gradually subsided to baseline level within 2 hours (Fig. 2A). Mice underwent surgery and dural vehicle-treatment spent significantly less time on wiping and scratching compared with IScap -treated mice (Fig. 2A, B, 322 ± 39 sec and 758 ± 69 sec, respectively; p < 0.01, one-way ANOVA with post hoc Bonferroni test). We also quantified wiping and scratching duration separately in dural IScap - and vehicle-treated mice, as a previous study indicates that they measure pain and itch respectively in the mouse cheek injection model [55]. Both forepaw wiping and hindpaw scratching around the scalp and periorbital area were significantly more robust in mice receiving dural application of IScap than in vehicle-treated mice (Fig. 2C, p < 0.01, two-tailed t-test). In later experiments, we investigated whether dural IScap-induced scratching likely reflects pain or itch in this model (Fig. 6F, G).
Fig. 2. Dural application of IScap elicits trigeminal V1-directed nocifensive behavior in adult male mice.
(A) Time spent on forepaw wiping and hindpaw scratching around the scalp and periorbital area within trigeminal V1 dermatome in 10 min bins in response to dural application of vehicle or IScap 10 min after the craniectomy in adult male mice (n = 8 in each group). Naïve mice (n = 4) were habituated to the test room and recording cage as mice in other groups but were not subjected to anesthesia exposure, surgery or drug application.
(B) Total duration of nocifensive behavior in naïve mice and in mice receiving dural application of vehicle or IScap during the 120 min recording period (same mice as in A, one-way ANOVA with post hoc Bonferroni test, ###p < 0.001, compared with the naïve group, **p < 0.01 between the vehicle and IScap groups).
(C) Total duration of forepaw wiping and hindpaw scratching in response to dural application of vehicle or IScap during the 120 min recording period, respectively (same mice as in A, **p < 0.01, two-tailed t-test between the corresponding vehicle and IScap groups).
(D) Time spent on forepaw wiping and hindpaw scratching around the scalp and periorbital area in 10 min bins in response to dural application of vehicle or IScap in adult female mice (n = 9 in each group).
(E) Comparison of the total duration of nocifensive behavior in female and male mice after dural application of vehicle and IScap during the first 80 min of recording period (same mice as in A and D, **p < 0.01, two-way ANOVA with post hoc Bonferroni test).
(F) Total duration of nocifensive behavior in response to dural application of vehicle and various chemical stimuli during the 120 min recording period. The number of mice in each group is indicated in the figure (*p < 0.05 compared with the vehicle group, one-way ANOVA and post hoc t-test with Bonferroni correction).
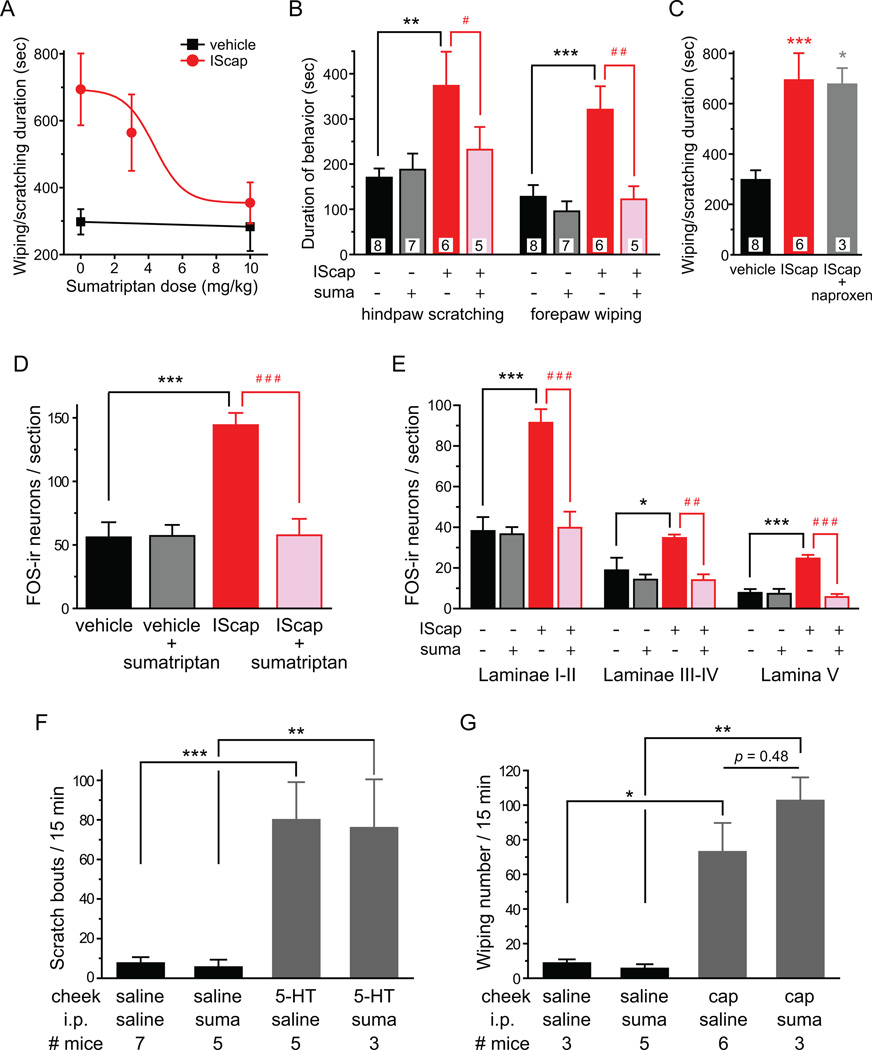
Fig. 6. Sumatriptan pretreatment blocks the effects of dural application of IScap.
(A) Sumatriptan dose-dependently inhibits nocifensive behavior elicited by dural application of IScap 10 min after the craniectomy. Adult male Swiss Webster mice were i.p. injected with sumatriptan or 100 µl saline (n = 4 mice in the 3 mg/kg sumatriptan group, mice in other groups are the same as in B). The red line represents the fitted dose-response curve (IC50 = 4.34 mg/kg).
(B) Sumatriptan (suma, 10 mg/kg i.p.) reduces both IScap-induced forepaw wiping and hindpaw scratching to the control level. The number of mice in each group is indicated in the figure (**p < 0.01, ***p < 0.001, between dural vehicle- and IScap-treated groups receiving i.p. saline; ##p < 0.01, between dural IScap-treated groups receiving i.p. saline and sumatriptan; two-way ANOVA with post hoc Bonferroni test).
(C) Naproxen (100 mg/kg i.p.) does not reduce the duration of dural IScap-induced behavior. The number of mice in each group is indicated in the figure (*p < 0.05, ***p < 0.001, compared with the vehicle group; one-way ANOVA with post hoc Bonferroni test).
(D) Sumatriptan (10 mg/kg i.p.) reduces the number of FOS-positive TCC neurons in mice that received dural application of IScap (n = 4 mice in each group; ***p < 0.001, between dural vehicle- and IScap-treated groups receiving i.p. saline; ###p < 0.001, between dural IScap-treated groups receiving i.p. saline and sumatriptan; two-way ANOVA with post hoc Bonferroni test).
(E) Average number of FOS-positive neurons per TCC section in mice received dural application of vehicle and IScap, respectively. Separate counts of FOS-positive neurons are shown for dorsal horn laminae I-II, III-IV and V. Sumatriptan (suma, 10 mg/kg i.p.) reduces IScap-induced FOS-ir in all dorsal horn laminae to the control level (same mice as in D; *p < 0.05, ***p < 0.001, between the corresponding dural vehicle- and IScap-treated groups receiving i.p. saline; ##p < 0.01, ###p < 0.001, between the corresponding dural IScap-treated groups receiving i.p. saline and sumatriptan; two-way ANOVA with post hoc Bonferroni test).
(F) Mean total number of bouts of hindpaw scratching after the injection of saline or 10 µg 5-HT into the mouse cheek (**p < 0.01, ***p < 0.001, between the corresponding saline and 5-HT groups, two-way ANOVA with post hoc Bonferroni test). Sumatriptan pretreatment (10 mg/kg i.p., 15 min before cheek injection) does not affect vehicle- or 5-HT-induced scratching (p = 0.79, two-way ANOVA for i.p. drug effects).
(G) Mean total number of forepaw wiping after the injection of saline or 10 µg capsaicin (cap) into the mouse cheek (*p < 0.05, **p < 0.01, between the corresponding saline and capsaicin groups, two-way ANOVA with post hoc Bonferroni test). Sumatriptan pretreatment (10 mg/kg i.p., 15 min before cheek injection) does not affect vehicle- or capsaicin-induced wiping (p = 0.29, two-way ANOVA for i.p. drug effects).
We went on to test the effects of IScap in adult female Swiss Webster mice, as more women suffer from migraine than men. Interestingly, dural application of vehicle 10 min after the craniectomy evoked much longer duration of wiping and scratching within the trigeminal V1 dermatome in female mice than in vehicle-treated male mice (Fig. 2D, E, female versus male vehicle groups, p < 0.01, two-way ANOVA with post hoc Bonferroni test). Dural application of IScap in female mice 10 min after the craniectomy did not further increase the duration of V1-directed behavior (Fig. 2D, E, female vehicle versus IScap groups), suggesting that the effect of IScap may be masked by surgery-induced behavior. In subsequent experiments, we used adult male mice to further characterize IScap -induced behavior and the activation of TG and TCC neurons (Fig. 2–8). In the last set of experiments, we let male and female mice recover from surgery for 7 days before testing the effects of dural application of vehicle and IScap on their home-cage behavior (Fig. 9, 10). In addition, we also tested the effects of dural IScap on other mouse strains to see if this headache model can be applied to mice of various genetic backgrounds (Fig. 4, 9, 10).
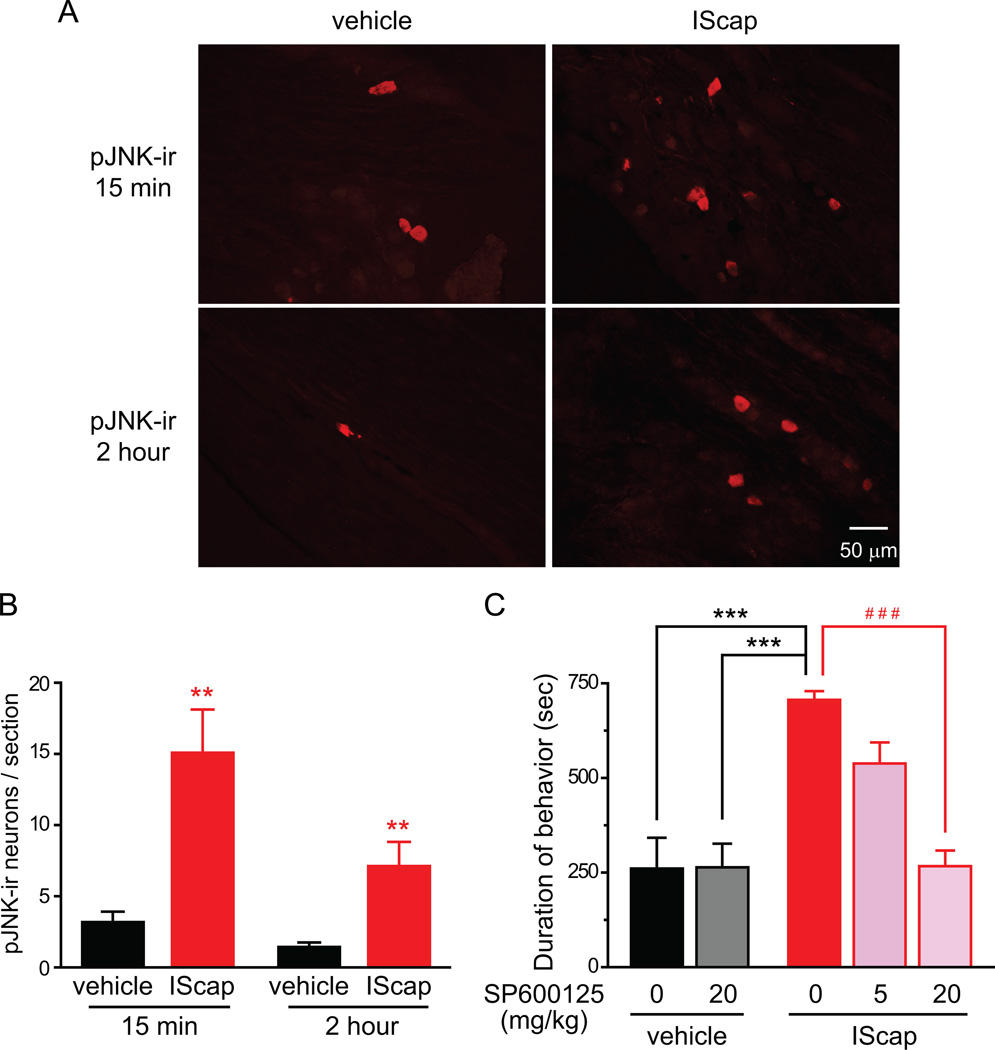
Fig. 8. JNK inhibitor blocks dural IScap-induced behavior.
(A) Representative images of pJNK-ir in TG neurons from adult male Swiss Webster mice that received vehicle and IScap treatment on the dura (10 min after the craniectomy) for 15 min and 2 hr, respectively.
(B) Average number of pJNK-positive neurons per TG section at 15 min and 2 hr after dural application of vehicle and IScap, respectively (n = 4 mice in each group; **p < 0.01 compared with the corresponding vehicle group; two-way ANOVA with post hoc Bonferroni test).
(C) JNK inhibitor SP600125 dose-dependently reduces dural IScap-induced nocifensive behavior. Mice were i.p. injected with SP600125 or 100 µl saline 15 min before dural application of vehicle or IScap (IC50 = 7.88 mg/kg; n = 4–5 mice in each group; ***p < 0.001, between the dural IScap-treated group receiving i.p. saline and the dural vehicle-treated groups; ###p < 0.001, between dural IScap-treated groups receiving i.p. saline and 20 mg/kg SP600125; two-way ANOVA with post hoc Bonferroni test).
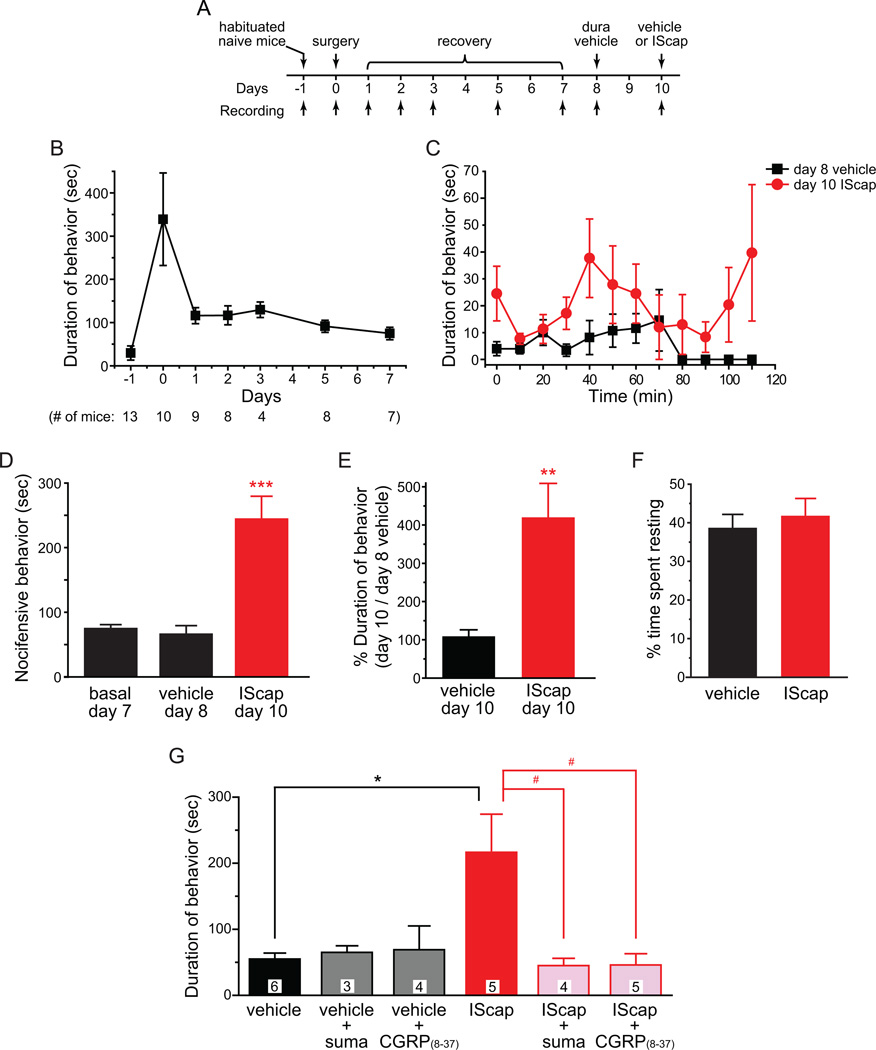
Fig. 9. Improvement of the model by minimizing the effects of surgery and anesthesia on adult male CD-1 mice.
(A) Timeline of experiments
(B) Duration of nocifensive behavior within the 120 min recording period (numbers in the parenthesis indicate the number of mice observed on each day). Naïve, habituated adult male CD-1 mice were recorded one day prior to the surgery. On the surgery day (0), mice received dural application of 20 µl ACSF and were recorded after recovery from anesthesia as in previous experiments. In subsequently days, mice were recorded at around the same time of the day.
(C) Time spent on forepaw wiping and hindpaw scratching within trigeminal V1 dermatome in 10 min bins in response to dural application of vehicle or IScap in adult male mice (n = 6 mice in each group).
(D) Total duration of nocifensive behavior during the 120 min recording period. Each mouse was recorded on day 7, 8 and 10 post-surgery for basal activity, response to dural vehicle application and response to dural IScap application, respectively (same mice as in C***p < 0.001 , compared with the basal and vehicle groups; one-way RM ANOVA with post hoc Bonferroni test).
(E) The duration of V1-directed behavior on day 10 in response to dural application of vehicle (n = 5 mice) or IScap (same mice as in C) versus the duration of behavior on day 8 (dural vehicle treatment) in individual mice (**p < 0.01, two-tailed t-test).
(F) The percentage of time spent resting during the 120 min recording period was comparable between the vehicle and IScap groups (same mice as in C).
(G) Dural IScap-induced nocifensive behavior is effectively blocked by sumatriptan and CGRP(8–37) (*p < 0.05, between dural vehicle- and IScap-treated groups; #p < 0.05, between dural IScap-treated groups with and without sumatriptan or CGRP(8–37) treatment; two-way ANOVA with post hoc Bonferroni test). Mice were i.p. injected with sumatriptan (suma, 10 mg/kg) 15 min before the dural application of vehicle or IScap. CGRP(8–37) (1 µM) was applied onto the dura for 15 min and was then replaced by 1 µM CGRP(8–37) along with vehicle or IScap on the dura. The duration of nocifensive behavior in the dural vehicle group is not affected by sumatriptan or CGRP(8–37). The number of mice in each group is indicated on the figure.
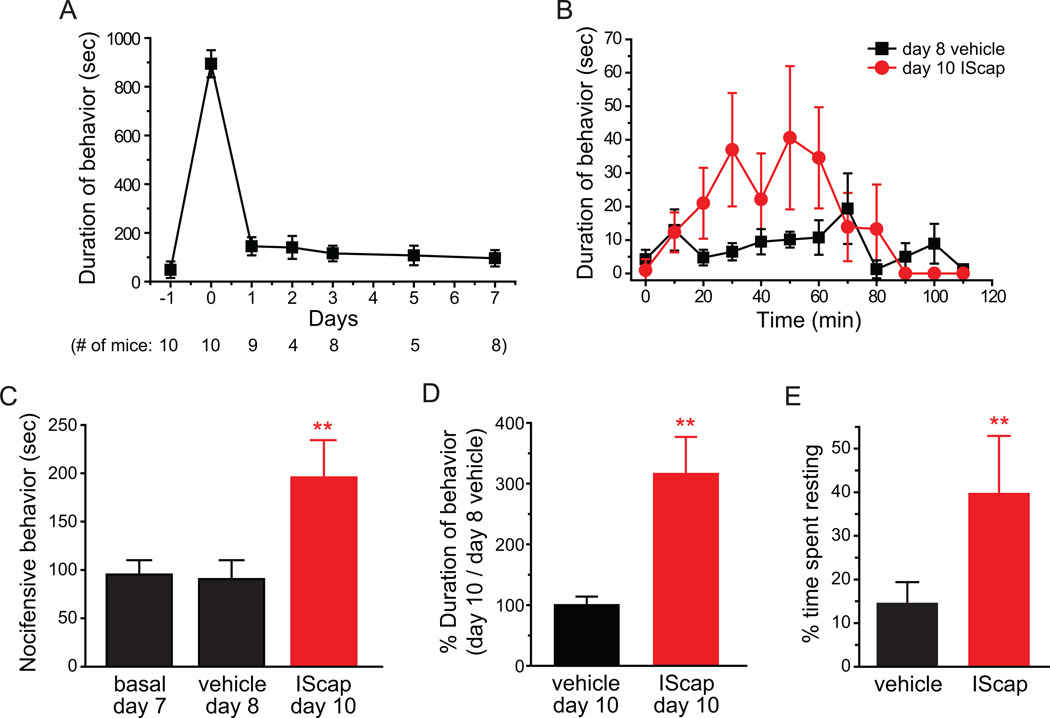
Fig. 10. Dural vehicle- or IScap-induced behavior in adult female CD-1 mice in the improved model.
(A) Duration of nocifensive behavior within the 120 min recording period (numbers in the parenthesis indicate the number of mice observed on each day). Naïve, habituated adult female CD-1 mice were recorded one day prior to the surgery. On the surgery day (0), mice received dural application of 20 µl ACSF and were recorded after recovery from anesthesia as in previous experiments. In subsequently days, mice were recorded at around the same time of the day.
(B) Time spent on forepaw wiping and hindpaw scratching within trigeminal V1 dermatome in 10 min bins in response to dural application of vehicle or IScap in adult female mice (n = 7 mice in each group).
(C) Total duration of nocifensive behavior during the 120 min recording period. Each mouse was recorded on day 7, 8 and 10 post-surgery for basal activity, response to dural vehicle application and response to dural IScap application, respectively (same mice as in B, ***p < 0.01 , compared with the basal and vehicle groups; one-way RM ANOVA with post hoc Bonferroni test).
(D) The duration of V1-directed behavior on day 10 in response to dural application of vehicle (n = 5 mice) or IScap (same mice as in B) versus the duration of behavior on day 8 (dural vehicle treatment) in individual female mice (**p < 0.01, two-tailed t-test).
(E) The percentage of resting time in adult female mice receiving dural application of vehicle and IScap, respectively (**p < 0.01, two-tailed t-test).
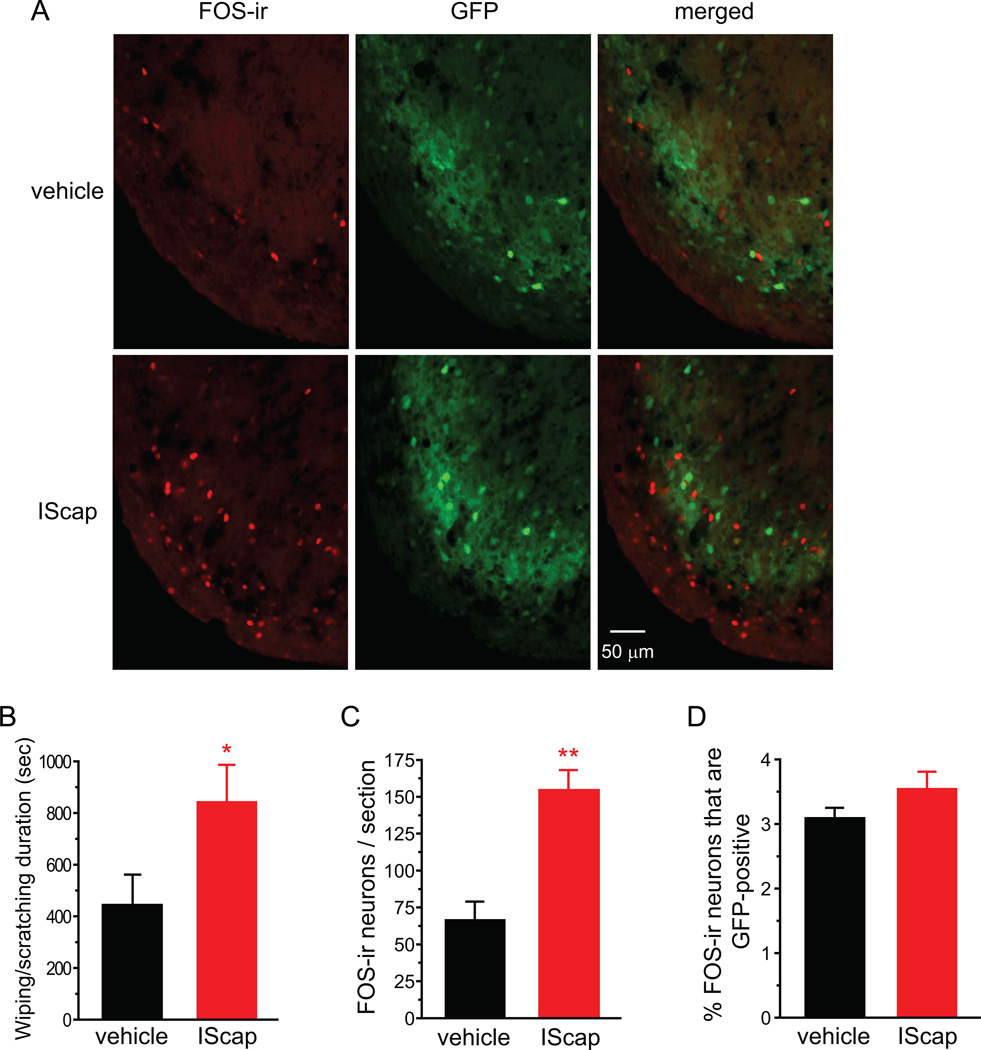
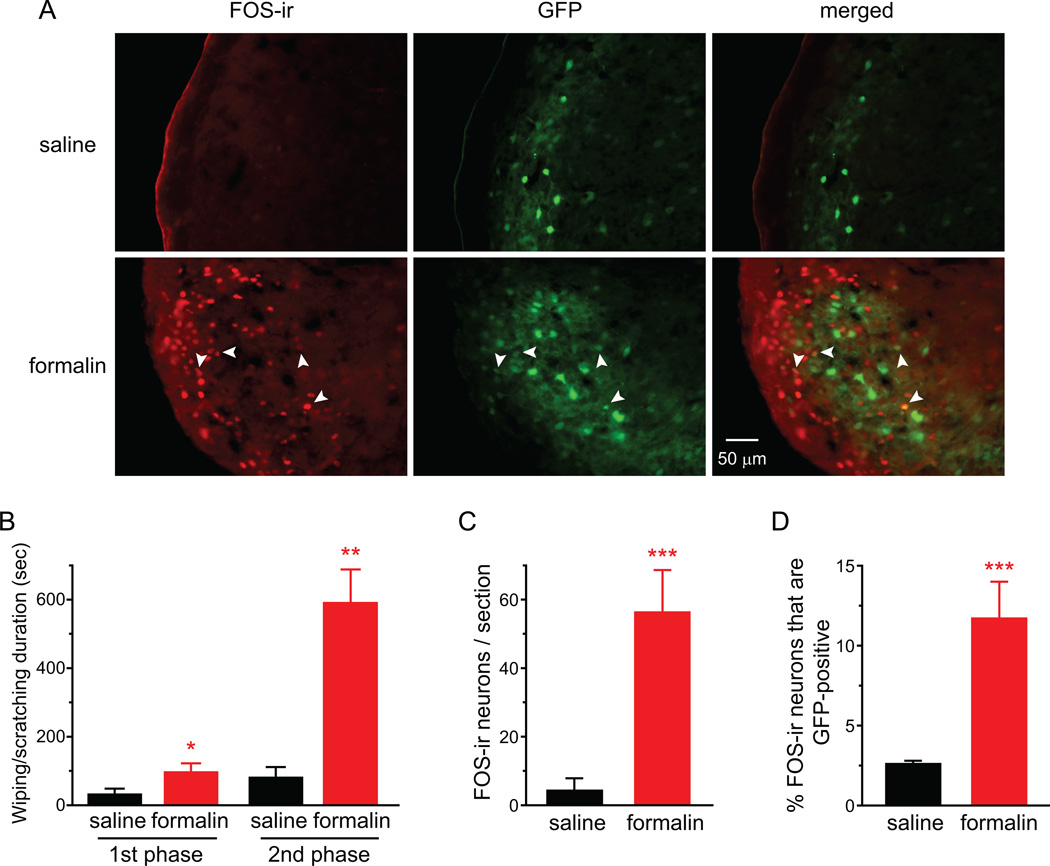
Fig. 4. Dural application of IScap preferentially induces FOS protein expression in the excitatory neurons in TCC.
(A) Representative images of FOS-positive cells and GFP-positive cells in the ventrolateral part of the caudal Vc in heterozygous GAD67-GFP mice that received vehicle and IScap treatment on the dura 10 min after the craniectomy, respectively. There is little overlap between FOS-ir and GFP signals.
(B) Total duration of the nocifensive behavior in response to dural application of vehicle or IScap during the 120 min recording period in adult male heterozygous GAD67-GFP mice on C57BL/6 background (n = 6 and 5 mice, respectively; *p < 0.05, two-tailed t-test).
(C) Average number of FOS-positive TCC neurons in GAD67-GFP mice that received dural application of vehicle and IScap, respectively (same mice as in B; **p < 0.01, two-tailed t-test).
(D) The percentage of FOS-positive neurons that are GFP-positive (same mice as in B).
In rats, cutaneous allodynia can be induced by dural application of IS, capsaicin or acidic solution alone [14, 43, 63–65]. We varied the components of dural stimuli and measured the duration of V1-directed wiping and scratching in adult male mice. All stimuli induced longer duration of nocifensive behavior than the vehicle group when applied to dura 10 min after the craniectomy. However, only the IScap -treated group reached statistical significance compared with the vehicle-treated group (Fig. 2F, p < 0.05, one-way ANOVA and post hoc t-test with Bonferroni correction). Thus, we used IScap to stimulate the dura in all subsequent experiments, as it consistently elicits robust V1-directed nocifensive behavior as well as significantly increases the number of FOS-positive neurons in TCC.
3.2. Dural application of IScap preferentially induces FOS protein expression in the excitatory neurons in TCC
Does the expression of FOS protein in TCC correlate with the duration of V1-directed behavior in individual mice? To address this question, we recorded dural vehicle- or IScap-induced behavior for 2 hours and then quantified FOS-ir in TCC neurons in the same mice. Similar to what we have observed in anesthetized mice (Fig. 1), dural application of IScap 10 min after the craniectomy induced significantly higher number of FOS-ir neurons than the vehicle in awake mice (Fig. 3A, B). The FOS-ir was most densely distributed in laminae I-II of the ventrolateral Vc that receives inputs from the V1 ophthalmic and some of the maxillary branches of the trigeminal nerve (Fig. 3A). FOS-positive cells were present at much lower density in the dorsomedial part of Vc that receives inputs from the mandibular and maxillary branches of the trigeminal nerve.
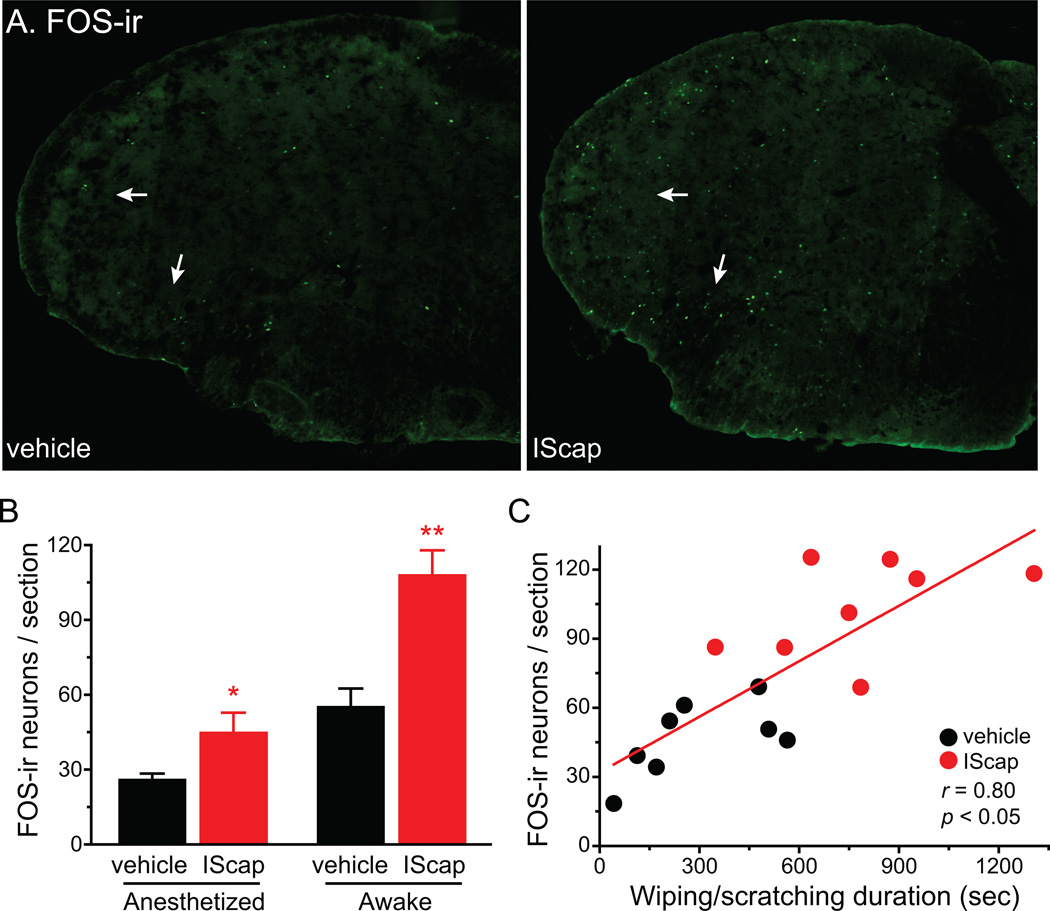
Fig. 3. The number of FOS-positive neurons in TCC correlates with the duration of V1-directed nocifensive behavior.
(A) Representative images of FOS-ir in the caudal Vc in mice that received vehicle and IScap treatment on the dura 10 min after the craniectomy, respectively. Adult male Swiss Webster mice were recorded for spontaneous behavior for 2 hours before tissue collection. Arrows indicate FOS-ir in the ventrolateral medullary dorsal horn.
(B) Average number of FOS-positive neurons per TCC section in mice received dural application of vehicle or IScap. Left columns (anesthetized): mice were maintained under anesthesia for 2 hours before tissue collection (n = 4 in each group). Right columns (awake): mice were recovered from anesthesia and recorded for spontaneous behavior for 2 hour before tissue collection (n = 8 in each group). Tissue sections were incubated with the FOS antibody (1:5000) at 4°C for 48 hours for this and all subsequent experiments (*p < 0.05, **p < 0.01 compared with the corresponding vehicle group; two-way ANOVA with post hoc Bonferroni test).
(C) Scatterplot of the number of FOS-positive TCC neurons versus the duration of wiping and scratching behavior in individual mice that received dural application of vehicle or IScap. The regression line represents the positive correlation between the two parameters (correlation coefficient r = 0.80, p < 0.05, same mice as in B awake groups).
Prolonged exposure to anesthetics significantly reduced the number of FOS-positive TCC neurons in both vehicle- and IScap-treated mice (Fig. 3B, p < 0.001 for the anesthesia effect, two-way ANOVA). Nevertheless, the fold increase in FOS-positive TCC neurons of IScap- versus vehicle-treated mice was comparable between anesthetized (1.7 ± 0.3 fold) and awake groups (2.0 ± 0.2 fold, p = 0.30, two-tailed t-test). For mice in the awake groups, we observed a positive correlation between the number of FOS-positive neurons per TCC section and the total duration of wiping and scratching (correlation coefficient r = 0.80, p < 0.05, Fig. 3C), suggesting that V1-directed nocifensive behavior reflects the activation of the trigeminal nociceptive pathway.
We went on to investigate whether dural application of IScap induces FOS protein expression in both excitatory and inhibitory neurons in TCC, using heterozygous GAD67-GFP knock-in mice [58]. The GAD67-GFP mice have been used for the identification of GABAergic neurons in the central nervous system (CNS) including spinal dorsal horn [17, 42]. In addition, since nearly all glycinergic dorsal horn neurons in laminae I-III also contain GABA, the GFP signal is a reliable marker for the identification of inhibitory neurons in this region [46]. Heterozygous GAD67-GFP mice on C57BL/6 background have slightly lower GABA content in the brain (84% of the wild-type mice) and exhibit grossly normal behavior [58]. Adult male heterozygous GAD67-GFP mice that received dural application of IScap 10 min after the craniectomy exhibited more robust forepaw wiping and hindpaw scratching than vehicle-treated mice (Fig. 4B, p < 0.05, two-tailed t-test). The number of FOS-positive TCC neurons in IScap-treated GAD67-GFP heterozygotes was also significantly higher than that in vehicle-treated mice (Fig. 4A, C, p < 0.01, two-tailed t-test). Thus, dural IScap induced robust head-directed nocifensive behavior and TCC FOS expression in mice on C57BL6 as well as Swiss Webster backgrounds. Interestingly, only a very small fraction of FOS-positive neurons were GFP-positive (3.1 ± 0.2% and 3.5 ± 0.3% in vehicle- and IScap-treated groups, respectively; Fig. 4D), indicating that dural application of IM preferentially activates excitatory neurons in TCC.
An alternative explanation is that dural IScap activates inhibitory TCC neurons but does not induce FOS expression. To test this possibility, we examined cutaneous formalin-induced FOS-ir in TCC neurons in heterozygous GAD67-GFP mice. In rats that receive hindpaw injection of capsaicin or formalin, more than 20% of the FOS-positive neurons in the dorsal horn of the lumbar spinal cord are GABAergic [23, 70, 71]. We injected heterozygous GAD67-GFP mice with saline or 1% dilute formalin into the skin of the periorbital region and recorded behavior for 1 hour. Consistent with previous reports [36, 55, 61], formalin induced robust biphasic responses, mostly forepaw wiping directed to the periorbital area, in GAD67-GFP mice (Fig. 5B). The 1st phase started immediately after injection and subsided in 5–10 min; and the 2nd phase lasted between 10–60 min after the injection. We quantified FOS-positive neurons in TCC 2 hrs after formalin injection. In saline-injected mice, only 2.6 ± 0.2% of FOS-positive neurons in TCC contained GFP signal (Fig. 5A, D, saline groups). Sections from formalin-injected mice exhibited many FOS-positive neurons in the cervical/medullary dorsal horn (Fig. 5A, C, p < 0.001, two-tailed t-test). Importantly, a substantial fraction of FOS-positive neurons were GFP-positive (11.7 ± 2.3%; Fig. 5D, p < 0.001), indicating that noxious chemical stimuli can induce FOS protein expression in inhibitory TCC neurons. Taken together, our results suggest that chemical stimulation of the dura preferentially activates the excitatory neurons in TCC, as manifested by the selective increase in FOS-ir in these neurons.
Fig. 5. Facial formalin injection induces FOS expression in inhibitory neurons in TCC.
(A) Representative images of FOS-positive cells and GFP-positive cells in caudal Vc of heterozygous GAD67-GFP mice that received saline or 1% formalin injection into the periorbital skin. Arrows indicate FOS-ir in GFP-positive cells.
(B) Total duration of nocifensive behavior in response to intradermal injection of 20 µl saline or 1% dilute formalin at the periorbital region of heterozygous GAD67-GFP mice. The 1st and 2nd phases include behavior 0–10 min and 10–60 min after injection, respectively (n = 3 mice in each group; *p < 0.05, **p < 0.01, two-tailed t-test, compared with the corresponding saline group).
(C) Average number of FOS-positive TCC neurons in heterozygous GAD67-GFP mice that received periorbital injection of saline and formalin, respectively (same mice as in B; ***p < 0.001, two-tailed t-test).
(D) The percentage of FOS-positive neurons that are GFP-positive (same mice as in B; ***p < 0.001, two-tailed t-test).
3.3. Dural IScap-induced nocifensive behavior and TCC FOS expression are blocked by anti-migraine drugs
Is dural IScap-induced forepaw wiping and hindpaw scratching in mice a potential behavioral correlate of the ongoing headache in human? To address this question, we tested whether IScap-induced nocifensive behavior can be blocked by drugs that ameliorate migraine in humans. First, we pretreated mice with sumatriptan or saline before applying vehicle or IScap on the dura 10 min after the craniectomy. Systemic injection of sumatriptan (10 mg/kg, i.p.) did not alter the responses in dural vehicle-treated mice (Fig. 6A, B). Conversely, sumatriptan dose-dependently blocked the nocifensive behavior elicited by dural application of IScap, with a half maximal inhibitory concentration (IC50) of 4.34 mg/kg (Fig. 6A). Both IScap-induced forepaw wiping and hindpaw scratching were reduced to the level comparable to that in vehicle-treated mice (Fig. 6B). On the other hand, pretreatment of naproxen (100 mg/kg i.p., [10, 14]), a nonsteroidal anti-inflammatory drug (NSAID), did not reduce the duration of nocifensive behavior elicited by dural application of IScap (Fig. 6C). Higher dose of naproxen (300 mg/kg i.p.) was not tested as it caused sedation in naïve mice in the pilot study (data not shown).
Next, we tested the effect of sumatriptan on IScap-induced FOS expression in TCC neurons. Dural application of IScap induced FOS protein expression at all TCC laminae. Compared with the vehicle-treated group, the number of FOS-positive neurons in IScap-treated mice increased 2.4 and 3.2 fold in laminae I-II and V that receive nociceptive inputs, respectively (Fig. 6E, p < 0.001, two-way ANOVA with post hoc Bonferroni test). The increase in FOS-positive neurons was less prominent (1.8-fold) in laminae III-IV that primarily process non-nociceptive information (Fig. 6E, p < 0.05). Systemic injection of sumatriptan (10 mg/kg, i.p.) completely blocked IScap-induced FOS expression in all TCC laminae (Fig. 6D, E). Thus, both dura IScap-induced nocifensive behavior and FOS expression in TCC neurons can be prevented by the anti-migraine drug sumatriptan.
In mouse cheek models of itch and pain, intradermal injection of pruritogens and algogens predominantly evokes hindpaw scratching and forepaw wiping of the cheek, respectively [3, 55]. We therefore consider the alternative explanation that dural IScap-induced scratching may be a measure of itch and sumatriptan may have previously unidentified anti-itch function. We tested whether sumatriptan blocks pruritogen-induced scratching in the mouse cheek model of itch. Intradermal injection of pruritogen 5-HT in mouse cheek elicited robust site-directed hindpaw scratching (Fig. 6F, p < 0.001, two-way ANOVA with post hoc Bonferroni test). However, sumatriptan pretreatment has no effect on 5-HT-induced cheek scratching (Fig. 6F, two-way ANOVA p = 0.79 for i.p. drug effects), indicating that sumatriptan is has no anti-itch function.
In humans, sumatriptan is mainly used to treat migraine and cluster headache, not cutaneous pain [27]. Similarly, in the mouse cheek model of pain, sumatriptan pretreatment did not attenuate forepaw wiping of the cheek in response to intradermal injection of algogen capsaicin in mouse cheek (Fig. 6G, two-way ANOVA p = 0.29 for i.p. drug effects). Taken together, our data suggest that sumatriptan preferentially inhibits pain originated from stimulation of deep tissue as opposed to cutaneous pain and itch. Furthermore, these experiments excluded the possibility that sumatriptan’s effect on IScap-induced behavior is not due to impairment of locomotor function.
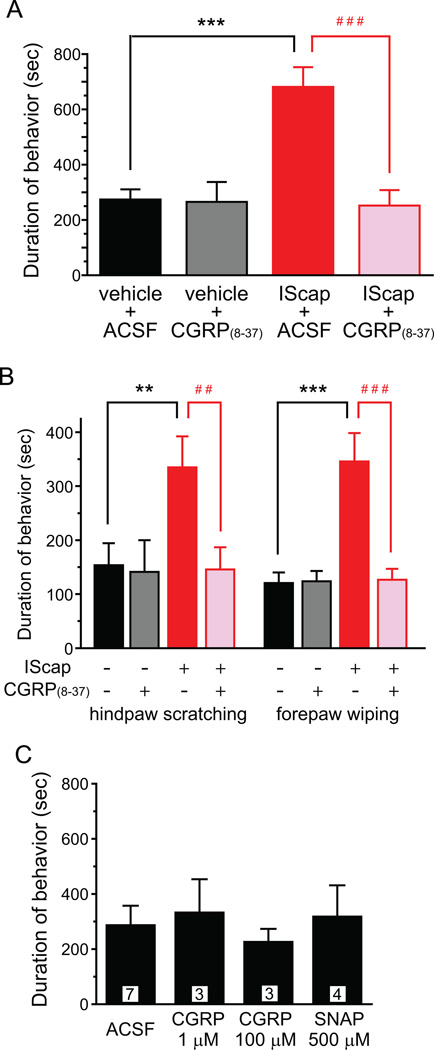
The contribution of neuropeptide CGRP to migraine pathogenesis is well established [22, 53, 62]. The level of CGRP in serum and saliva is elevated during a migraine episode [22], likely resulting from the perivascular release of CGRP from the peripheral terminals of TG neurons. CGRP receptor antagonists are effective for migraine headache in clinical trials [15]. In rats, stimulation of the dural afferent terminals releases CGRP, which plays a role in the sensitization of the trigeminovascular system [22, 53, 62]. First, we tested whether IScap-induced CGRP released to the dura is necessary for the manifestation of the nocifensive behavior. We pretreated the dura with CGRP receptor antagonist CGRP(8–37) (1 µM, [16]) for 15 min. We then co-applied 1 µM CGRP(8–37) along with the vehicle or IScap onto the dura and recorded the duration of nocifensive behavior. Both IScap-induced hindpaw scratching and forepaw wiping was significantly reduced to the control level by the dural application of CGRP(8–37) (Fig. 7A, B, p < 0 .01 and p < 0.001, two-way ANOVA with post hoc Bonferroni test). CGRP(8–37) did not alter the behavioral responses in dural vehicle-treated mice (Fig. 7A, B).
Fig. 7. Dural application of CGRP(8–37) inhibits IScap-induced behavior.
(A) Dural application of CGRP(8–37) reduces IScap-induced nocifensive behavior to the control level in adult male Swiss Webster mice. The dura was pretreated with CGRP(8–37) (1 µM in 25 µl ACSF, applied 10 min after the craniectomy) for 15 min and then was treated with IScap or vehicle along with 1 µM CGRP(8–37) for 2 hrs. The control groups received 15 min ACSF pretreatment. (n = 5–6 mice in each group; ***p < 0.001, between dural vehicle- and IScap-treated groups; ###p < 0.001, between dural IScap-treated groups with and without CGRP(8–37) treatment; two-way ANOVA with post hoc Bonferroni test).
(B) Dural application of CGRP(8–37) reduces both IScap-induced forepaw wiping and hindpaw scratching to the control level (same mice as in A; **p < 0.01, ***p < 0.001, between dural vehicle- and IScap-treated groups; ##p < 0.01, ###p < 0.001, between dural IScap-treated groups with and without CGRP(8–37) treatment; two-way ANOVA with post hoc Bonferroni test).
(C) Dural application of vasodilators CGRP and SNAP did not induce head-directed nocifensive behavior. The number of mice in each group is indicated in the figure.
Next, we tested whether the increase in dural CGRP is sufficient to elicit nocifensive behavior in mice. Application of 1 µM or 100 µM CGRP onto the mouse dura 10 min after the craniectomy did not increase the duration of the nocifensive behavior (Fig. 7C), consistent with previous reports that CGRP at this dose range induces considerable vasodilation and histamine release in the dura but does not excite or sensitize meningeal nociceptors [16, 34, 54]. Likewise, dural application of the nitric oxide donor SNAP (500 µM) 10 min after the craniectomy did not increase the duration of nocifensive behavior beyond the control level, despite the fact that SNAP has been shown to elicit vasodilation, CGRP release as well as a delayed mechanical sensitization of meningeal nociceptors [7, 20, 67]. Taken together, we conclude that the increase in dural CGRP is necessary but not sufficient for the manifestation of IScap-induced nocifensive behavior in our model.
3.4. Activation of JNK signaling pathway contributes to dural IScap-induced nocifensive behavior
The activation of mitogen-activated protein (MAP) kinase pathways in primary afferent neurons has been implicated in nociceptive sensitization under various persistent pain conditions, including migraine headache [12, 18, 28, 33, 66, 68]. First, we quantified the number of TG neurons expressing phosphorylated MAP kinase proteins ERK, p38 and JNK in control and dural IScap-treated mice, respectively. We found no significant increase in the number of TG neurons containing pERK-ir or p-p38-ir between 15 min and 24 hrs after dural application of IScap 10 min after the craniectomy (data not shown). Conversely, there was a nearly 5-fold increase in the number of TG neurons containing pJNK-ir relative to the control group 15 min after dural IScap treatment (Fig. 8A, B). The effect of IScap on pJNK increase persisted for at least 2 hrs (Fig. 8B, p < 0.01 compared with the corresponding vehicle groups; two-way ANOVA with post hoc Bonferroni test).
Next, we tested the effect of JNK inhibitor SP600125 on IScap-induced behavior. Systemic injection of SP600125 (20 mg/kg, i.p.) did not alter the responses in dural vehicle-treated mice (Fig. 8C). On the contrary, pretreatment with SP600125 dose-dependently blocked dural IScap-induced nocifensive behavior, with an IC50 of 7.88 mg/kg (Fig. 8C), suggesting that the activation of JNK signaling pathway is necessary for the manifestation of dural IScap-evoked nocifensive behavior.
3.5. Improvement of the model by minimizing the effects of surgery and anesthesia
Here, we let adult male CD-1 mice recover from the surgery and anesthesia for 7 days before testing the effects of dural IScap application (Fig. 9A). The duration of head-directed nocifensive behavior was already substantially reduced 1 day post-surgery, from 337 ± 107 sec to 116 ± 18 sec. By day 7 post-surgery, the time spent on spontaneous wiping and scratching within the trigeminal V1 dermatome was further decreased to 75 ± 6 sec (Fig. 9B). Application of vehicle onto the dura on day 8 did not alter the duration of V1-directed behavior (66 ± 12 sec, Fig. 9C, D). Conversely, dural application of IScap on day 10 to the same mice significantly increased the duration of V1-directed nocifensive behavior to 244 ± 35 sec (Fig. 9C, D; p < 0.001, one-way RM ANOVA with post hoc Bonferroni test). On average, the duration of dural IScap-induced behavior on day 10 was 418 ± 91% of the duration of vehicle-induced behavior on day 8 in individual mice (Fig. 9E). To test the possibility that the increase in behavior on day 10 may be due to repeated dural application of solution per se, we applied vehicle onto the dura on both day 8 and 10 in individual mice. In this case, the duration of behavior on day 10 was 107 ± 9% of that on day 8 (Fig. 9E, p < 0.001, two-tailed t-test), confirming that dural application of IScap elicits robust nocifensive behavior towards the trigeminal V1 dermatome in adult male mice relative to the vehicle treatment.
In a rat model of migraine, dural application of IScap during the awake period of the circadian cycle causes a decrease in physical activity and a concomitant increase in resting behavior in addition to facial grooming [39]. On the contrary, dural IScap treatment in adult male mice during the sleep period did not alter the duration of resting behavior, which accounted for 40% of the observation time in both vehicle and IM groups (Fig. 9F).
We went on to test whether IScap-induced behavior can be blocked by anti-migraine drugs in the improved model. We let adult male CD-1 mice recover from surgery for 7 days and then pretreated mice with sumatriptan (10 mg/kg, i.p.) or CGRP(8–37) (1 µM on the dura) for 15 min. We applied vehicle or IScap to the dura of sumatriptan pretreated mice and co-applied 1 µM CGRP(8–37) along with vehicle or IScap to the dura of CGRP(8–37)-pretreated mice. Both sumatriptan and CGRP(8–37) effectively reduced IScap-induced behavior to the level comparable to that in vehicle-treated mice (Fig. 9G, p < 0 .05, two-way ANOVA with post hoc Bonferroni test). On the other hand, sumatriptan or CGRP(8–37) pretreatment did not alter the behavioral responses in dural vehicle-treated mice (Fig. 9G).
In adult female mice, surgery per se induced robust nocifensive behavior that masked the effects of IScap (Fig. 2D, E). We found that the duration of nocifensive behavior in females was also substantially reduced during post-surgery recovery period. By day 7 post-surgery, female CD-1 mice on average spent 95 ± 12 sec spontaneously wiping and scratching within the trigeminal V1 dermatome during the 2 hour observation period (Fig. 10A), a number comparable to that of male mice after recovery (Fig. 9B). We went on to test the effects of dural application of vehicle and IScap in individual female mice on post-surgery day 8 and 10, respectively. Application of vehicle onto the dura did not significantly alter the duration of V1-directed behavior (91 ± 19 sec, Fig. 10B, C). Conversely, dural application of IScap to the same mice evoked 196 ± 39 sec of V1-directed nocifensive behavior (Fig. 10B, C; p < 0.01, one-way RM ANOVA with post hoc Bonferroni test). The duration of IScap-induced behavior on day 10 was 316 ± 61% of the duration of vehicle-induced behavior on day 8 in individual female mice (Fig. 10D). When female mice received dural vehicle application on both day 8 and 10, the duration of behavior on day 10 was 100 ± 14% of that on day 8 (Fig. 10D, p < 0.01, two-tailed t-test). We conclude that, after minimizing the effects of surgery and anesthesia, dural application of IScap elicits robust nocifensive behavior towards the trigeminal V1 dermatome in adult female mice relative to the vehicle treatment.
In addition to V1-directed wiping and scratching, we also observed that dural application of IScap significantly increased the resting time in female mice, from 14 ±5% of the total observation time in the vehicle group to 40 ± 13% of the time (Fig. 10E, p < 0.01, two-tailed t-test), an almost 3-fold increase. Taken together, the improved model allows us to quantify meningeal inflammation-induced behavioral changes in both male and female mice.
4. Discussion
Long-lasting, ongoing headache is the most common and disabling symptom of migraine and other primary headache disorders. The onset of headache likely results from the activation and sensitization of primary afferent neurons innervating the dura and cerebral blood vessels [19, 45, 57]. Establishing behavioral endpoints for ongoing head pain in animal models is important for understanding the basic mechanisms of headache and the identification of new therapeutic targets. Here, we report the first detailed characterization of the effects of dural application of inflammatory mediators in mice. Stimulation of mouse dura with IScap elicits intermittent wiping and scratching within the trigeminal V1 dermatome. Pretreatment of anti-migraine drugs sumatriptan and CGRP receptor antagonist effectively blocks IScap-induced behavior, suggesting that it may be mechanistically related to the ongoing headache during a migraine episode in humans. The effective dose of sumatriptan is higher than that required to inhibit facial allodynia in rat models of migraine [14, 44]. It is possible that larger dose of systemic sumatriptan is needed to block pain-related behavior in mice [5]. The lack of effect of naproxen may result from the presence of exogenous PGE2 in IScap. The effect of other NSAIDs also needs to be tested in this model in the future. Importantly, dural application of IScap induces head-directed behavior in mice on C57BL6 (the background of GAD67-GFP mice), CD-1 and Swiss Webster backgrounds, suggesting that this mouse model of headache can be applied to other inbred and outbred strains of mice. Since we did not replicate all experiments in individual strains, further optimization may be needed to adapt this model to mice on other genetic backgrounds.
In the mouse cheek assay, hindpaw scratching is regarded as a measure of itch [55], raising the concern that dural IScap-evoked scratching may indicate itch, not pain. Several lines of evidence argue against this possibility. First, in humans, itch is readily evoked from skin, but is never felt in deep tissues [60]. Secondly, sumatriptan effectively blocks dural IScap-induced scratching but show no effect in the mouse cheek model of itch. Likewise, dural application of IS in male Wistar rats elicits a brief hindpaw facial grooming that is inhibited by zolmitriptan [39]. Finally, injection of inflammatory reagents into the mouse masseter muscle produces both forepaw rubbing and hindpaw scratching of the face that can be blocked by opioids [51]. It is likely that both forepaw wiping and hindpaw scratching are associated with deep-tissue orofacial pain in rodents; whereas they represent pain and itch in response to cutaneous stimuli, respectively.
In the majority of our experiments, mouse behavior was measured immediately after the mice recovered from surgery and anesthesia, both of which may attenuate the effectiveness of dural chemical stimulation. This may explain why IS elicits behavior and TCC FOS expression in rats several days after surgery [13, 14, 37, 39, 43, 64], whereas IScap is required to achieve a similar phenotype in mice when applied shortly after craniectomy. Nonetheless, both sumatriptan and CGRP(8–37) effectively reduce IScap-induced behavior and FOS expression to the control level, but exhibit no effects on the vehicle groups. This suggests that the surgery-induced behavior is by and large of somatic origin, and the difference between vehicle and IScap groups likely results from IScap-induced activation/sensitization of dural afferent neurons. After minimizing the effects of surgery and anesthesia, dural application of IScap not only elicits robust head-directed nocifensive behavior in both male and female mice, but also significantly increases the duration of resting behavior in female mice. In rats, the suppression of feeding, locomotor and/or exploratory behavior in response to chemical stimulation of the dura is recognized as behavioral correlates of ongoing pain [13, 37, 39]. Similarly, both IScap-induced nocifensive behavior and the increase in resting time can be used as endpoints for assessing ongoing pain in our model. In male rats, dural application of IScap during the awake period of the circadian cycle causes a decrease in physical activity and a concomitant increase in resting behavior [39]. Difference in species, IScap components as well as the circadian cycle during which the behavior is observed may account for the discrepancy between our data and the previous study.
The immediate-early gene product FOS protein is a well-established marker for neuronal activation in TCC [14, 40, 56]. Using heterozygous GAD67-GFP mice, we show here that dural application of IScap induces little FOS expression in GFP-positive GABAergic TCC neurons. Thus, chemical stimulation of the dura preferentially activates the excitatory neurons in TCC, in contrast to chemical stimulation of other facial or somatic areas which activates both excitatory and inhibitory dorsal horn neurons (Fig.5D, [23, 70, 71]). This unexpected finding suggests that the organization of the headache circuits at the level of TCC may be different from those encoding cutaneous orofacial pain and/or somatic pain. More work is needed to confirm and expand this observation as well as to investigate the underlying mechanisms and functional implications. Of note, the duration of IScap-induced nocifensive behavior positively correlates with the number of FOS-positive TCC neurons in individual mice. Pretreatment with sumatriptan blocks both IScap-induced behavior and TCC FOS expression. These data further validate that the magnitude of IM-induced nocifensive behavior reflects the activation status of the neuronal circuits encoding headache. In addition to systemic sumatriptan, dural application of CGRP(8–37) also effectively blocks IScap-induced nocifensive behavior, suggesting that targeting peripheral CGRP receptors is sufficient to prevent the activation of the headache circuits in response to chemical stimulation of the dura. This is consistent with the human positron emission tomography study indicating that doses of the CGRP receptor antagonist telcagepant that are maximally efficacious in aborting migraine attacks only result in low receptor occupancy in the brain [24]. It is possible that chemical stimulation of the dura may cause opening of the blood brain barrier [6], allowing some CGRP(8–37) to enter CNS. However, the amount of CGRP(8–37) in CNS may be functionally negligible in our study, given that it is topically applied to the dura. Conversely, dural application of CGRP at doses that induce considerable vasodilation and histamine release [16, 54] does not elicit nocifensive behavior in mice, consistent with previous reports that CGRP does not excite or sensitize meningeal nociceptors in rodents and intravenous infusion of CGRP rarely precipitates migraine attack in normal human volunteers [21, 32, 34]. That dural application of SNAP does not evoke V1-directed behavior in mice is also consistent with the human studies indicating that migraine headache may not be caused by meningeal vasodilation [1, 4, 9]. Collectively, our results suggest that CGRP release in dura is necessary but may not be sufficient for the manifestation of IScap-induced nocifensive behavior in normal mice. In addition to CGRP, IScap may result in the release of other neurotransmitters/ neuropeptides from dural afferent terminals. Other cells in the meninges may also be activated by IScap and contribute to the manifestation of nocifensive behavior. On the other hand, intravenous infusion of CGRP in migraineurs causes delayed headache and migraine several hours after the administration [21, 32]. It will be interesting to investigate whether CGRP exhibits a delayed effect in mice with previous experience of ‘migraine’-like episodes (e.g. repetitive dural application of IScap or systemic NTG treatment [39, 43, 47]) in future experiments. In addition to primary afferent activation/sensitization, further work is needed to assess the contribution of the descending pain-facilitating pathway to IScap-induced nocifensive behavior in mice, given its established role in the rat headache model [14].
Activation of all three MAP kinases has been implicated in migraine pathogenesis. Both ERK and p38 activation contribute to the sensitization of meningeal afferent neurons in response to chemical stimuli [34, 66–68]. In TG neurons, MAP kinases stimulate the CGRP enhancer region and increase CGRP expression [12]. CGRP, in turn, induces cytokine expression in TG organ culture through the activation of JNK pathway [31]. In our model, dural application of IScap in mice preferentially increases the phosphorylation of JNK, but not ERK and p38, in TG neurons. The increase in pJNK-positive neurons correlates with the onset of IScap-induced behavior and persists after it subsides to the basal level. Moreover, activation of the JNK signaling pathway is necessary for the expression of IScap-induced behavior in mice, as suggested by the inhibitory effect of systemically administered JNK inhibitor SP600125. Future experiments are needed to test whether inhibition of the JNK signaling pathway in TG neurons is sufficient to prevent the manifestation of behavioral responses to chemical stimulation of dura.
In summary, we have developed and characterized a mouse model of headache that exhibits ongoing head-directed nocifensive behavior in response to chemical stimulation of dura. Dural application of IScap results in concomitant increase in pJNK-positive primary afferent neurons in TG and FOS-positive excitatory neurons in TCC. IScap-induced behavior can be effectively blocked by the pretreatment of anti-migraine drugs sumatriptan and the CGRP receptor antagonist, suggesting that it may be mechanistically related to the ongoing headache in humans. The establishment of this model allows us to take full advantage of mouse as a model system to better understand the mechanisms underlying migraine, especially the contributions of primary afferent inputs to ongoing headache, as well as to screen for novel therapeutic targets.
Supplementary Material
Acknowledgments
The authors thank Ms Tripti Soni and Iqra Khan for the technical help. This study is supported by the NIH-NINDS grants (NS074210 and NS083698 to YQC).
Footnotes
Conflict of interest statement
The authors report no conflict of interest.
References
- 1.Ahn AH. On the temporal relationship between throbbing migraine pain and arterial pulse. Headache. 2010;50(9):1507–1510. doi: 10.1111/j.1526-4610.2010.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerman S, Holland PR, Hoffmann J. Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia. 2013;33(8):577–592. doi: 10.1177/0333102412472071. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and micro-opoid modulation in mice. Acta dermato-venereologica. 2010;90(6):575–581. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- 4.Amin FM, Asghar MS, Hougaard A, Hansen AE, Larsen VA, de Koning PJ, Larsson HB, Olesen J, Ashina M. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. The Lancet Neurology. 2013;12(5):454–461. doi: 10.1016/S1474-4422(13)70067-X. [DOI] [PubMed] [Google Scholar]
- 5.Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptacek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2009;30(2):170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beggs S, Liu XJ, Kwan C, Salter MW. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Molecular pain. 2010;6:74. doi: 10.1186/1744-8069-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. The European journal of neuroscience. 2006;23(8):2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanda ML, Tuttle AH, Baran I, Atlin C, Guindi D, Hathaway G, Israelian N, Levenstadt J, Low D, Macrae L, O'Shea L, Silver A, Zendegui E, Mariette Lenselink A, Spijker S, Ferrari MD, van den Maagdenberg AM, Mogil JS. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154(8):1254–1262. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Charles A. Migraine is not primarily a vascular disorder. Cephalalgia. 2012;32(5):431–432. doi: 10.1177/0333102412441717. [DOI] [PubMed] [Google Scholar]
- 10.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153(4):876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Annals of neurology. 2013 doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durham PL, Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci. 2003;23(3):807–815. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153(9):1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Annals of neurology. 2009;65(2):184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edvinsson L. The Journey to Establish CGRP as a Migraine Target: A Retrospective View. Headache. 2015;55(9):1249–1255. doi: 10.1111/head.12656. [DOI] [PubMed] [Google Scholar]
- 16.Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8–37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. British journal of pharmacology. 2007;150(5):633–640. doi: 10.1038/sj.bjp.0707134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukushima T, Ohtsubo T, Tsuda M, Yanagawa Y, Hori Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. Journal of neurophysiology. 2009;102(3):1459–1471. doi: 10.1152/jn.91160.2008. [DOI] [PubMed] [Google Scholar]
- 18.Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neuroscience letters. 2008;437(3):180–183. doi: 10.1016/j.neulet.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161(2):327–341. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, Cirrito JR, Milner E, Holtzman DM, Zipfel GJ. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J Neurosci. 2008;28(50):13542–13550. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 22.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nature reviews. 2010;6(10):573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 23.Hossaini M, Duraku LS, Sarac C, Jongen JL, Holstege JC. Differential distribution of activated spinal neurons containing glycine and/or GABA and expressing c-fos in acute and chronic pain models. Pain. 2010;151(2):356–365. doi: 10.1016/j.pain.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Hostetler ED, Joshi AD, Sanabria-Bohorquez S, Fan H, Zeng Z, Purcell M, Gantert L, Riffel K, Williams M, O'Malley S, Miller P, Selnick HG, Gallicchio SN, Bell IM, Salvatore CA, Kane SA, Li CC, Hargreaves RJ, de Groot T, Bormans G, Van Hecken A, Derdelinckx I, de Hoon J, Reynders T, Declercq R, De Lepeleire I, Kennedy WP, Blanchard R, Marcantonio EE, Sur C, Cook JJ, Van Laere K, Evelhoch JL. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. The Journal of pharmacology and experimental therapeutics. 2013;347(2):478–486. doi: 10.1124/jpet.113.206458. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Li S, Dhaka A, Story GM, Cao YQ. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Molecular pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang DY, Liu P, Yanagawa Y, Cao YQ. Activation of trigeminal nociceptive pathway by dural application of inflammatory mediators in mice. Society for Neuroscience Meeting Abstract. 2011 [Google Scholar]
- 27.Humphrey PP. The discovery and development of the triptans, a major therapeutic breakthrough. Headache. 2008;48(5):685–687. doi: 10.1111/j.1526-4610.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 28.Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain research reviews. 2009;60(1):135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci. 2012;32(44):15439–15449. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 31.Kristiansen KA, Edvinsson L. Neurogenic inflammation: a study of rat trigeminal ganglion. J Headache Pain. 2010;11(6):485–495. doi: 10.1007/s10194-010-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(1–2):166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Annals of neurology. 2005;58(5):698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- 35.Liao YJ, Safa P, Chen YR, Sobel RA, Boyden ES, Tsien RW. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2705–2710. doi: 10.1073/pnas.0710771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luccarini P, Childeric A, Gaydier AM, Voisin D, Dallel R. The orofacial formalin test in the mouse: a behavioral model for studying physiology and modulation of trigeminal nociception. J Pain. 2006;7(12):908–914. doi: 10.1016/j.jpain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(17):9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiology of disease. 2012;45(1):633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia. 2013 doi: 10.1177/0333102413486320. [DOI] [PubMed] [Google Scholar]
- 40.Mitsikostas DD, Sanchez del Rio M, Waeber C. 5-Hydroxytryptamine(1B/1D) and 5-hydroxytryptamine1F receptors inhibit capsaicin-induced c-fos immunoreactivity within mouse trigeminal nucleus caudalis. Cephalalgia. 2002;22(5):384–394. doi: 10.1046/j.1468-2982.2002.00382.x. [DOI] [PubMed] [Google Scholar]
- 41.Molander C, Xu Q, Rivero-Melian C, Grant G. Cytoarchitectonic organization of the spinal cord in the rat: II. The cervical and upper thoracic cord. The Journal of comparative neurology. 1989;289(3):375–385. doi: 10.1002/cne.902890303. [DOI] [PubMed] [Google Scholar]
- 42.Nowak A, Mathieson HR, Chapman RJ, Janzso G, Yanagawa Y, Obata K, Szabo G, King AE. Kv3.1b and Kv3.3 channel subunit expression in murine spinal dorsal horn GABAergic interneurones. Journal of chemical neuroanatomy. 2011;42(1):30–38. doi: 10.1016/j.jchemneu.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47(7):1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oshinsky ML, Sanghvi MM, Maxwell CR, Gonzalez D, Spangenberg RJ, Cooper M, Silberstein SD. Spontaneous trigeminal allodynia in rats: a model of primary headache. Headache. 2012;52(9):1336–1349. doi: 10.1111/j.1526-4610.2012.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annual review of physiology. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 46.Polgar E, Durrieux C, Hughes DI, Todd AJ. A quantitative study of inhibitory interneurons in laminae I-III of the mouse spinal dorsal horn. PloS one. 2013;8(10):e78309. doi: 10.1371/journal.pone.0078309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155(2):269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pradhan AA, Smith ML, Zyuzin J, Charles A. delta-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. British journal of pharmacology. 2014;171(9):2375–2384. doi: 10.1111/bph.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramachandran R, Bhatt DK, Ploug KB, Olesen J, Jansen-Olesen I, Hay-Schmidt A, Gupta S. A naturalistic glyceryl trinitrate infusion migraine model in the rat. Cephalalgia. 2012;32(1):73–84. doi: 10.1177/0333102411430855. [DOI] [PubMed] [Google Scholar]
- 50.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29(27):8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero-Reyes M, Akerman S, Nguyen E, Vijjeswarapu A, Hom B, Dong HW, Charles AC. Spontaneous behavioral responses in the orofacial region: a model of trigeminal pain in mouse. Headache. 2013;53(1):137–151. doi: 10.1111/j.1526-4610.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romero-Reyes M, Ye Y. Pearls and pitfalls in experimental in vivo models of headache: Conscious behavioral research. Cephalalgia. 2013;33(8):566–576. doi: 10.1177/0333102412472557. [DOI] [PubMed] [Google Scholar]
- 53.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annual review of pharmacology and toxicology. 2015;55:533–552. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwenger N, Dux M, de Col R, Carr R, Messlinger K. Interaction of calcitonin gene-related peptide, nitric oxide and histamine release in neurogenic blood flow and afferent activation in the rat cranial dura mater. Cephalalgia. 2007;27(6):481–491. doi: 10.1111/j.1468-2982.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 55.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139(3):681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14(6):3725–3735. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384(6609):560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 58.Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. The Journal of comparative neurology. 2003;467(1):60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 59.Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G. Nitroglycerin induces hyperalgesia in rats--a time-course study. European journal of pharmacology. 2003;464(2–3):159–162. doi: 10.1016/s0014-2999(03)01421-3. [DOI] [PubMed] [Google Scholar]
- 60.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM : monthly journal of the Association of Physicians. 2003;96(1):7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 61.Vos BP, Hans G, Adriaensen H. Behavioral assessment of facial pain in rats: face grooming patterns after painful and non-painful sensory disturbances in the territory of the rat's infraorbital nerve. Pain. 1998;76(1–2):173–178. doi: 10.1016/s0304-3959(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 62.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64(10 Suppl 2):S9–S15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 63.Wei X, Edelmayer RM, Yan J, Dussor G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia. 2011;31(16):1595–1600. doi: 10.1177/0333102411427600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wieseler J, Ellis A, Sprunger D, Brown K, McFadden A, Mahoney J, Rezvani N, Maier SF, Watkins LR. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. Journal of neuroscience methods. 2010;185(2):236–245. doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152(1):106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Molecular pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Kainz V, Zhao J, Strassman AM, Levy D. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Annals of neurology. 2013;73(6):741–750. doi: 10.1002/ana.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011;152(1):140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 70.Zou X, Lin Q, Willis WD. NMDA or non-NMDA receptor antagonists attenuate increased Fos expression in spinal dorsal horn GABAergic neurons after intradermal injection of capsaicin in rats. Neuroscience. 2001;106(1):171–182. doi: 10.1016/s0306-4522(01)00175-0. [DOI] [PubMed] [Google Scholar]
- 71.Zou X, Lin Q, Willis WD. The effects of sympathectomy on capsaicin-evoked fos expression of spinal dorsal horn GABAergic neurons. Brain research. 2002;958(2):322–329. doi: 10.1016/s0006-8993(02)03621-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.