Abstract
Although many environmental agents are established male germ cell mutagens, few are known to induce mutations in spermatogonial stem cells. Stem cell mutations are of great concern because they result in a permanent increase in the number of mutations carried in sperm. We investigated mutation induction during mouse spermatogenesis following exposure to benzo(a)pyrene (BaP). MutaMouse males were given 0, 12.5, 25, 50, or 100 mg/kg bw/day BaP for 28 days by oral gavage. Germ cells were collected from the cauda epididymis and seminiferous tubules 3 days after exposure and from cauda epididymis 42 and 70 days after exposure. This design enabled targeted investigation of effects on post-spermatogonia, dividing spermatogonia, and spermatogonial stem cells, respectively. BaP increased lacZ mutant frequency (MF) in cauda sperm after exposure of dividing spermatogonia (4.2-fold at highest dose, P < .01) and spermatogonial stem cells (2.1-fold at highest dose, P < .01). No significant increases in MF were detected in cauda sperm or seminiferous tubule cells collected 3 days post-exposure. Dose-response modelling suggested that the mutational response in male germ cells to BaP is sub-linear at low doses. Our results demonstrate that oral exposure to BaP causes spermatogonial stem cell mutations, that different phases of spermatogenesis exhibit varying sensitivities to BaP, with dividing spermatogonia representing a window of peak sensitivity, and that sampling spermatogenic cells from the seminiferous tubules at earlier time-points may underestimate germ cell mutagenicity. This information is critical to optimize the use of the international test guideline for transgenic rodent mutation assays for detecting germ cell mutagens.
Keywords: polycyclic aromatic hydrocarbon, transgenic rodent mutation assay, germ cell mutation, spermatogonia, de novo mutation
Exposure to environmental agents can cause de novo mutations in the germline, leading to concerns over the genetic health of future generations (recently reviewed in DeMarini, 2012). Extensive evidence suggests that the majority of de novo mutations arise in the male germline (Conrad et al., 2011; Crow, 2000; Kong et al., 2012; Rahbari et al., 2015). Thus, there is a need to characterize the mutagenicity of environmental agents in male germ cells. The Organization for the Economic Co-operation and Development (OECD) Test Guideline for transgenic rodent (TGR) mutation assay (TG 488) is an internationally endorsed method (OECD, 2013) that can be used to quantify induced mutations in somatic tissues and male germ cells (Lambert et al., 2005; O’Brien et al., 2014; Yauk et al., 2015a). Of critical importance is the ability to detect mutations in spermatogonial stem cells because these cells can permanently and continuously produce sperm that carry mutations.
In the mouse, mutations fixed in spermatogonial stem cells can be detected in sperm collected from the cauda epididymis at least 49 days after exposure to a mutagen (O’Brien et al., 2014). This provides sufficient time for exposed stem cells to replicate and daughter cells to progress through spermatogenesis before they are collected as mature sperm. In practice, longer sampling times (ie, 70 days) are preferable to account for pharmacokinetic distribution of the mutagen after an exposure, and to allow stem cells to go through several rounds of replication while being exposed. Although stem cells are critical for detecting permanent effects, differentiated spermatogonia may be more sensitive to mutagens, as they divide more rapidly than stem cells (De Rooij, 2001). Mutations fixed in dividing spermatogonia can be detected in cauda sperm approximately 35–42 days after they are induced. Thus, adjusting the sampling time between mutagen exposure and sperm collection allows the detection of induced mutations in specific germ cell populations.
The sampling times required to detect spermatogonial stem cell mutations in sperm are longer than what is generally recommended for measuring somatic mutation using the TGR assay. TG488 recommends a 28-day exposure period followed by a 3-day sampling time (28 + 3) for measuring mutations in somatic tissues (OECD, 2013). Cauda sperm collected using this sampling time are derived from cells that were in the later phases of spermatogenesis during the exposure period. As germ cells progress through spermatogenesis they progressively lose their DNA repair capacity, and no longer replicate DNA (Baarends et al., 2001; Gunes et al., 2015; Marchetti and Wyrobek, 2008; Olsen et al., 2005; Xu et al., 2005). Because errors in these processes are the primary mechanism of mutation fixation, post-meiotic germ cells are not sensitive to the induction of mutations that can be detected with the TGR assay, although they represent a sensitive window for the induction of lesions that may result in adverse effects after fertilization (Marchetti and Wyrobek, 2005). As a possible compromise, TG488 recommends collecting germ cells at the 3-day sampling time from the seminiferous tubules. However, cells from the seminiferous tubules represent a mixed population not adequately exposed as stem cells and are enriched for post-meiotic germ cells. Therefore, it is presently unknown whether the analysis of cells from seminiferous tubules provides the same sensitivity to detect germ cell mutagens as the analysis of sperm derived from exposed stem cells or dividing spermatogonia.
The mutagenic activity of benzo(a)pyrene (BaP), a ubiquitous environmental pollutant and Group 1 human carcinogen, has been extensively characterized for somatic tissues (IARC Working Group, 2010; Moffat et al., 2015). BaP mutagenesis is less well-characterized in germ cells. BaP exposure via intraperitoneal (i.p.) injection induced dominant lethal mutations in meiotic and post-meiotic spermatogenic cells, but not heritable translocations (Generoso et al., 1982). Results for the specific locus test were negative or inconclusive for pre- and post-spermatogonial germ cells after i.p. injection (Russell et al., 1981). Recent studies using TGR models have reported increases in the mutant frequencies (MF) of lacZ (Verhofstad et al., 2011) and cII reporter genes (Olsen et al., 2010) in mouse sperm collected 42 and 119 days after oral exposure to BaP, respectively. The latter increase was statistically significant compared with concurrent solvent-treated controls, but not significantly distinguishable from the MF in naïve animals. Thus, although BaP does induce mutations in male germ cells, the susceptibility of adult spermatogonial stem cells to BaP-induced mutation remains unclear.
Here, we characterize the dose response in germ cells of male transgenic mice (MutaMouse) exposed subchronically to BaP across various phases of spermatogenesis. We also compared the response in germ cells collected from the seminiferous tubules using a 28 + 3 experimental design with the response in cauda sperm collected at later sampling times to scrutinize the sensitivity of this design for detecting germ cell mutagenicity. Our results clarify the phase-specific sensitivity of male germ cells to the mutagenicity of BaP after subchronic oral exposure and provide important information on the ability to detect germ cell mutation at different sampling times.
MATERIALS AND METHODS
Exposures and tissue collection
All protocols involving animal use were approved by the Health Canada Ottawa Animal Care Committee. Experiments were designed based on the OECD guideline TG488 (OECD, 2013) and conducted as described previously (O’Brien et al., 2014). We used MutaMouse transgenic mice, which are derived from a DBA2 and BALB/c cross (originally obtained from Covance Laboratories, Ltd., and maintained at in-house colony of for >25 years), and harbor ∼29 tandem copies of a recombinant λgt10 phage vector on each copy of chromosome 3 (Shwed et al., 2010). The recombinant phage vector contains a mutation-reporting Escherichia coli lacZ gene. Male MutaMouse mice (8–10 weeks old, n = 5–6 per treated group, 8–12 for controls) were orally exposed to an olive oil vehicle control or 12.5, 25, 50, or 100 mg/kg bw BaP (Sigma-Aldrich, Oakville, ON, Canada) dissolved in olive oil for 28 consecutive days via gavage (volume = 5 µl/g bw). Mice were euthanized by cervical dislocation under isofluorane anesthesia 3, 42, or 70 days after the end of the exposure to assess effects on post-spermatogonia, dividing spermatogonia, and spermatogonial stem cells, respectively. Control groups comprised about 10 animals to provide a robust spontaneous mutant frequency especially for the seminiferous tubules, which had not been previously examined in our lab.
Cauda epididymides were collected for all 3 time points, flash frozen in liquid nitrogen, and stored at −80 °C. Sperm were later collected from the cauda as described previously (O’Brien et al., 2014). Briefly, cauda were defrosted, minced, and suspended in cold phosphate buffered saline. The suspension was filtered through a size 80 mesh filter (#S3770, Sigma-Aldrich). Non-germ cells in the filtrate were lysed with sodium dodecyl sulfate and the unlysed sperm were collected by centrifugation. During method development, analysis by microscopy did not reveal any contaminating somatic cells following this lysis step (Yauk et al., 2002). Seminiferous tubule cells were extracted from freshly excised testes only for the 3-day post-exposure time-point as described previously (O’Brien et al., 2014). Briefly, the epithelial capsule was removed from the testes to release the seminiferous tubules. Germ cells were extracted from the seminiferous tubules by squeezing the tubules in phosphate buffered saline using a tissue roller. Tubules were removed from the suspension by gravity sedimentation, and the remaining cells in suspension were collected by centrifugation, flash frozen in liquid nitrogen, and stored at −80 °C. Table 1 shows the type of germ cells targeted and the phases of spermatogenesis the target germ cells passed through during exposure for each time-point.
TABLE 1.
Cell types and phases of spermatogenesis that are targeted by various experimental designs.
| Sampling time (days) | Tissue | Phase at beginning of exposure | Phases passed during exposure | Phases passed after exposure | Collected cell types |
|---|---|---|---|---|---|
| 3 | Cauda | Spermatocyte | Spermatocyte | Sperm | Sperm |
| Spermatid | |||||
| Sperm | |||||
| Tubules | Stem cell | Stem cell | Stem cell | + Spermatogonia | |
| Spermatogonia | Spermatogonia | Spermatogonia | ++ Spermatocyte | ||
| Spermatocyte | Spermatocyte | Spermatocyte | +++ Spermatid | ||
| Spermatid | Spermatid | +++ Sperm | |||
| 42 | Cauda | Stem cell | Stem cell | Spermatogonia | Sperm |
| Spermatogonia | Spermatocyte | ||||
| Spermatid | |||||
| Sperm | |||||
| 70 | Cauda | Stem cell | Stem cell | Stem cell | Sperm |
| Spermatogonia | |||||
| Spermatocyte | |||||
| Spermatid | |||||
| Sperm |
+ indicates the relative proportion of cell types when a mixture is collected.
LacZ mutation assay
Genomic DNA was isolated from cauda sperm and seminiferous tubule cells as described previously (O’Brien et al., 2014). Germ cells were digested with proteinase K and β-mercaptoethanol (cauda sperm only). Genomic DNA was extracted using phenol/chloroform. The extracted DNA was concentrated by ethanol precipitation and stored at 4 °C in Tris-EDTA buffer, pH 8, then quantified using a NanoDrop spectrophotometer (Thermo Scientific).
The lacZ MF was determined in genomic DNA isolated from germ cells as previously described (O’Brien et al., 2014). Briefly, λgt10 phage vectors were recovered from approximately 1 to 4 µg of DNA using Transpack Packaging Extract kits (Agilent Technologies, Mississauga, ON, Canada) according to the manufacturer’s instructions. The lacZ−/galE− E. coli were infected with the recovered phage and grown on agar containing 0.3% phenyl-β-d-galactopyranoside (P-Gal) to detect mutants, or on agar without P-Gal to determine the total number of plaque-forming units (pfu). A minimum of 125 000 total pfu were scored for each animal (average = 390 000 pfu). When necessary, plaque counts from multiple assays per animal, including some instances of reactions with zero mutant counts, were combined to achieve this minimal total pfu only if replicates did not fail a binomial likelihood ratio test (P < .05).
The MF was calculated by dividing the number of mutant plaques by the total pfu count. The proportion of BaP induced mutants to total pfu was compared with the proportion in the control group by logistic regression using the glm function in R (R Core Team, 2015) with a quasibinomial error distribution to account for overdispersion in the data. The resulting P-values were adjusted for multiple comparisons using a Bonferroni correction. Outlier samples (4 out of 137 samples: the cauda and tubules of one animal from the 28 + 3 25 mg/day had unusually high MF, as did one sample from the 42-day 25 mg/kg/day group and one sample from the 70-day control group) were removed from the analysis if their MF were significantly different from the other samples in the same dose group based on the above logistic regression. The highest dose of BaP that did not induce a statistically significant (P < .05) change in MF over controls was identified as the no observable genotoxic effect level (NOGEL).
Dose-response modeling
Characterization of the dose response was conducted following established approaches (Gollapudi et al., 2013; Johnson et al., 2014; O’Brien et al., 2015). This analysis was only performed for time-points that displayed a significant increasing trend in MF with dose, based on the Cochrane Armitage test for trend. These data sets met the minimum recommended number of dose groups for this type of analysis, which is 5 groups including controls (Johnson et al., 2014). Dose-response modelling was performed using the U.S. Environmental Protection Agency’s (EPA) Benchmark Dose Software (BMDS, v2.6.0.1). The BMDS variance likelihood ratio test (LRT) was used to select between a homogeneous or non-constant variance model. A dose–response model was then selected from the linear, quadratic, exponential, and power models based on the lowest Akaike Information Criterion (AIC). An additional goodness-of-fit LRT was used to assess the model fit. The benchmark response (BMR) was set to 10% of the control group above the estimated response of the model at dose = zero in order to determine the benchmark dose (BMD10) and its lower 90% confidence limit (BMDL10).
The “drsmooth” R package (Johnson et al., 2014) was used to assess additional characteristics of the dose–response relationship. We used the “linearity before cut-off dose” (lbcd) function to test whether the slope of the dose response before the identified NOGEL was significantly greater than zero (P < .05). The “segmented” function was used to identify the dose at which the slope of a fitted bilinear model becomes greater than zero (the breakpoint dose, BPD) and its lower 95% confidence limit (BPDL). The “smooth” function was used to identify the point at which the slope of a fitted smoothing regression spline first became significantly greater than zero (the slope transition dose, STD) and its lower 95% confidence limit (STDL).
RESULTS
BaP Exposures and LacZ Mutant Frequencies in Germ Cells
MutaMouse males were treated by oral gavage with an olive oil vehicle control or with BaP (up to 100 mg/kg bw/day) for 28 consecutive days. Germ cells were collected from the cauda epididymis 3, 42 or 70 days after treatment, or from the seminiferous tubules 3 days after treatment. BaP treatment did not have a significant effect on testicular mass relative to body weight (data not shown). The lacZ MFs in BaP-exposed germ cells collected at various times are summarized in Table 2 and shown graphically in Figure 1. Plaque counts for each individual animal are shown in Supplementary Table 1. The lacZ MF in cauda sperm collected 3 days after exposure was not significantly different from control levels for any dose group. The MFs in germ cells from the seminiferous tubules of testes collected at this time-point reached a maximum average of 6.4 × 10−5 (2.7-fold above concurrent controls) in the 100 mg/kg bw/day dose group. However, this increase in MF was not statistically significant (P = .35) compared with the control group due to the high variation in the treated group. Sperm collected from cauda 42 days post-exposure had a significant dose-dependent increase in MF that reached 13.1 × 10−5 (4.2-fold above controls) at 100 mg/kg bw/day. The other doses did not cause significant increases in MF at this time-point. Sperm collected from the cauda 70 days after exposure showed significant increases in both the 50 and 100 mg/kg bw/day dose groups, achieving a maximum induced MF of 5.3 × 10−5 (2.1-fold above controls) in the high dose group. At 100 mg/kg bw/day, the MF at 42 days was significantly higher (P < .01) than all other time-points, including 70 days. Thus, BaP induced significant increases in MF in sperm exposed as dividing spermatogononia and spermatogonial stem cells, whereas no statistically significant increase was observed in cauda sperm cells or cells collected from seminiferous tubules 3 days post-exposure.
TABLE 2.
LacZ mutant frequency in male germ cells of MutaTMmouse mice exposed to benzo[a]pyrene for 28 days and collected at various sampling times.
| Sampling time | Tissue | Dose (mg/kg/day) | n | # mutants | # pfu | Averagea MF × 10−5 | SDa | P-value |
|---|---|---|---|---|---|---|---|---|
| 3 | Cauda | 0 | 12 | 138 | 4 446 661 | 3.1 | 1.7 | – |
| 12.5 | 5 | 14 | 1 066 397 | 1.4 | 0.9 | .0987 | ||
| 25 | 4 | 24 | 913 653 | 2.6 | 0.9 | 1.0000 | ||
| 50 | 5 | 33 | 1 101 683 | 3.1 | 1.3 | 1.0000 | ||
| 100 | 5 | 87 | 3 669 186 | 2.5 | 0.7 | .5539 | ||
| 3 | Tubules | 0 | 8 | 51 | 2 194 254 | 2.4 | 1.1 | – |
| 12.5 | 5 | 52 | 1 452 400 | 3.4 | 1.7 | 1.0000 | ||
| 25 | 5 | 38 | 1 449 087 | 2.6 | 1.2 | 1.0000 | ||
| 50 | 6 | 85 | 2 173 052 | 3.9 | 1.5 | .6645 | ||
| 100 | 6 | 102 | 2 341 201 | 6.4 | 4.0 | .3501 | ||
| 42 | Cauda | 0 | 11 | 102 | 3 716 402 | 3.1 | 2.5 | – |
| 12.5 | 6 | 67 | 2 007 879 | 3.3 | 0.8 | 1.0000 | ||
| 25 | 5 | 30 | 1 239 188 | 2.4 | 0.6 | 1.0000 | ||
| 50 | 6 | 104 | 1 937 802 | 5.4 | 2.5 | .0738 | ||
| 100 | 5 | 447 | 3 523 564 | 13.1 | 3.4 | <.0001 | ||
| 70 | Cauda | 0 | 9 | 130 | 5 118 604 | 2.5 | 0.7 | – |
| 12.5 | 6 | 75 | 3 095 980 | 2.7 | 1.1 | 1.0000 | ||
| 25 | 5 | 65 | 2 261 681 | 2.3 | 1.2 | 1.0000 | ||
| 50 | 5 | 86 | 1 842 379 | 4.7 | 1.2 | .0045 | ||
| 100 | 5 | 186 | 3 598 943 | 5.3 | 1.2 | .0001 |
aAverage mutant frequency (MF) and standard deviation (SD) based on the arithmetic mean of individual animals.
pfu: plaque forming units.
Bold P-values indicate statistical significance (P < .05).
FIG. 1.
LacZ mutant frequency (MF) dose response in spermatogenic cells exposed to benzo[a]pyrene: A, sperm collected from the cauda epididymis 3 days after a 28 day exposure; B, germ cells collected from the seminiferous tubules 3 days after exposure; C, sperm collected from the cauda epididymis 42 days after exposure; and D, sperm collected from the cauda epididymis 70 days after exposure.
Dose–Response Modeling
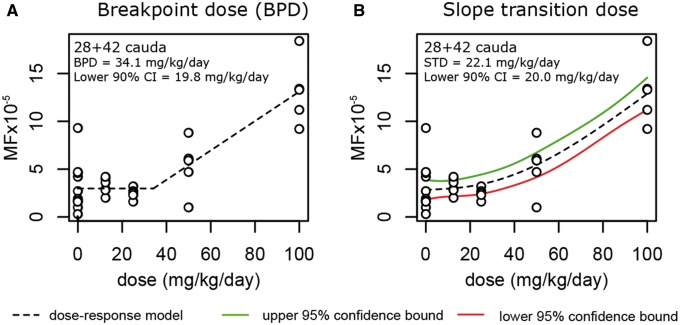
Highly significant trends for increasing MF with dose were observed for all time-points (Cochrane Armitage test for trend, P < .001), except for the 28 + 3 cauda sperm (P = .922). As such, the 28 + 3 cauda sperm data were omitted from further dose-response analysis. Dose–response data for all other data sets were modeled using the U.S. EPA BMDS software. Data were fit to 4 different dose–response models (linear, quadratic, exponential, or power models) each with 2 different variance models (constant or non-constant variance) for a total of 8 possible models. The best fitting model was selected for each time-point based on the lowest AIC score. The models selected for each time-point are shown graphically in Figure 1. Goodness-of-fit LRT P-values are shown in Supplementary Table 2. Only the data from the 42 day time-point had poor fit (P < .05) for either variance model. This was not improved after applying a log or square-root transformation, which are often recommended (Gollapudi et al., 2013) (data not shown). Because none of the variance models in BMDS had ideal fits (P > .1) for this data set, we used the constant variance model because it produced the lowest AIC value and best model fit P-value. The selected dose–response models showed acceptable fit (P > .1) for all data sets. BMDs based on a 10% response were derived using the best fit models and are shown graphically in Figure 1, and with their 90% lower confidence limits in Supplementary Table 2. We also tested whether the slope of the dose response was significantly different from zero up to and including the NOGEL. Although no single dose group has a response that was significantly greater than controls for the tubules at 28 + 3, a statistically significant trend was observed, that was best described by the exponential model (and therefore not linear up to the NOGEL of 100 mg/kg bw/day), with a BMD10 of 10.4 mg/kg bw/day. Sperm from the 42-day time-point were best described by a polynomial dose response, with a BMD10 of 16.7 mg/kg bw/day. The slope of this dose response was not significantly different than zero up to the NOGEL of 50 mg/kg bw/day, although only marginally so (P = .053). The lacZ MF in the 70-day group was best described by an exponential dose response. The highest BMD10 (43.0 mg/kg bw/day) was found for this post-exposure time point. The slope of this dose response was also not significantly different than zero up to the NOGEL of 25 mg/kg bw/day (P = .752). A significant BPD and STD was only detected for the day 42 sperm data (shown graphically in Figure 2, and with their 95% lower confidence limits in Supplementary Table 2). These values were 34.1 mg/kg bw/day and 22.1 mg/kg bw/day, respectively. The slopes of lacZ MF response in all 3 time points at which dose-dependent increases were observed (28 + 3 tubules, 42-day, and 70 day sperm) were not significantly different than zero up to and including 25 mg/kg bw/day (P > .05). In summary, sperm derived from exposed dividing spermatogonia (42 days post-exposure) were more sensitive to BaP-induced mutation following subchronic oral exposure compared with sperm from exposed spermatogonial stem cells (based on BMD analysis). Despite having no single dose with a significantly elevated MF (based on logistic regression), a significant trend was observed (based on the Cochrane Armitage test) in germ cells from the 28 + 3 seminiferous tubules that was comparable to the trends observed in cauda sperm. Collectively, the dose-response analyses from all 3 of these data sets suggest that the mutational response in male germ cells to BaP is sub-linear in the low dose range.
FIG. 2.
LacZ mutant frequency (MF) dose-response models used to determine: A, the breakpoint dose; and B, the slope transition dose for sperm collected 42 days after exposure to benzo[a]pyrene.
DISCUSSION
We used the lacZ mutation assay to characterize the dose response of various phases of spermatogenesis in transgenic mice exposed subchronically to BaP. Our results demonstrate that BaP induces mutations in spermatogonial stem cells of adult mice, but that the strongest mutational effect is observed in dividing spermatogonia. Comparison of the response in germ cells collected from the seminiferous tubules 3 days after a 28 day exposure (MF ranging from 2.4 to 6.4 × 10−5) with that in cauda sperm collected 70 days after exposure (ranging from 2.5 to 5.3 × 10−5) shows that, although comparable MFs were obtained in the 2 systems, statistically significant effects were observed only in cauda sperm.
Our results strengthen the existing body of evidence that BaP is a spermatogonial stem cell mutagen in mice. We observed a dose-dependent increase in lacZ MF that was statistically significant at the 2 top dose groups (50 mg/kg bw/day and 100 mg/kg bw/day) in sperm collected 70 days after the end of the 28-day exposure. One study previously reported an increased MF in sperm of mice collected 119 days after 3 daily intraperitoneal (i.p.) exposures of 50 mg/kg bw BaP (150 mg/kg bw total) (Olsen et al., 2010). The stem cell effect was statistically significant when compared with concurrent solvent-treated controls, but not when compared with naïve animals. More definitive stem cell effects were observed in pre-pubescent mice exposed to BaP (Xu et al., 2014); increased lacI MFs were observed in isolated pachytene spermatocytes, round spermatids, and spermatozoa collected 35 days after juvenile mice (7 days old) were exposed to a single i.p. injection of up to 300 mg/kg/bw BaP. This effect was less apparent in older mice (25 and 60 days old) using a similar experimental design, where significant effects were only detected in pachytene spermatocytes at the highest dose. These findings and the results from the present study collectively demonstrate that BaP is a spermatogonial stem cell mutagen in mice and that exposure to this chemical can permanently increase the production of mutated sperm throughout the reproductive life of the animal.
Dividing spermatogonia represent the most sensitive spermatogenic population to BaP-induced mutations following oral exposure. In fact, the MF for the highest BaP dose was significantly higher than that observed after stem cell exposure (P < .01). An elevated MF was also detected at 50 mg/kg bw/day, but this was only marginally significant (P = .074), likely due to the unusually high variability in the control group at this time-point (Table 2). The strong MF response observed at 42 days post-exposure correlates with the higher rate of division in dividing spermatogonia relative to spermatogonial stem cells (De Rooij, 2001). In addition, it has been shown that there is approximately a one week delay in the formation of BaP-induced DNA adducts in sperm after an oral exposure (Verhofstad et al., 2010). Therefore, BaP may continue to be delivered to the testes several days after the final oral dose and additional mutations may have been produced during the last week of active spermatogonial divisions before the cells commit to meiosis. Dividing spermatogonia represent a sensitive window for induction for other types of genomic alterations such as tandem repeat mutations (Beal et al., 2015b; Somers, 2006). Therefore, dividing spermatogonia may be the most useful spermatogenic population to determine whether a chemical is a germ cell mutagen.
Important data gaps exist on whether the analysis of cells from seminiferous tubules at the time-point commonly used for detecting mutations in somatic tissues (ie, 28 + 3) provides the same sensitivity to detect germ cell mutagens as the analysis of sperm derived from exposed spermatogonia (Yauk et al., 2015a). As expected, we observed no change in the lacZ MF in cauda sperm collected at this time-point (Figure 1), which represent germ cells that were spermatocytes or spermatids during the exposure period. These cell types do not synthesize DNA and lose their DNA repair capacity, processes that are required to fix mutations (Marchetti and Wyrobek, 2008). They are also on the lumen (closed) side of the blood-testes barrier, which can differentially affect the distribution of toxicants to the various germ cell phases (Yauk et al., 2015a). Conversely, the analysis of germ cells from seminiferous tubules at the 28 + 3 time point targets a mixture of cells with varying capacity for DNA synthesis and repair during exposure (Marchetti and Wyrobek, 2008; Olsen et al., 2005; Xu et al., 2005). The MF obtained from seminiferous tubule cells were comparable with those observed in sperm collected 70 days after the end of exposure (Table 2) and showed an apparent dose response (Figure 1). However, no statistically significant increase above controls was found. Similarly, Verhofstad et al. (2011) detected a significant increase in plasmid-born lacZ MF in sperm collected 42 days after BaP exposure, but not in the testes at this same time point. The apparent signal suppression in the testes is likely due to the mixed nature of the exposed and sampled cell population, which contains germ cells from various phases of spermatogenesis that are enriched for post-meiotic germ cells, and a small amount of somatic (Leydig and Sertoli) cells. These non-target cells may dilute the mutational effect, thereby decreasing assay sensitivity. Therefore, simultaneously measuring induced mutations in somatic tissues and germ cells at 28 + 3 may not represent an optimal approach for detecting germ cell mutagens, especially weak mutagens. A time-point that would allow more cells exposed as dividing spermatogonia to be collected (eg, 28 + 28) may provide a stronger signal in the tubules.
Mutant frequency (MF) data from the TGR mutation assay often suffer from overdispersion (ie, greater variability than predicted by the theoretically binomial error distribution), which can negatively impact assay sensitivity. One of the primary causes of this increased variability is that the clonal expansion of mutations in some samples is greater than in others. This is of particular concern for control samples, where recovered mutations may have occurred before the experimental time-frame. Overdispersion can be accounted for, to a limited degree, during statistical analysis (for example, by assuming a quasi-binomial error distribution). Alternatively, assay sensitivity can be greatly improved by correcting the MF for clonality, which can only be done by sequencing the recovered mutants to quantify the degree of clonal expansion in each sample. Previously, such an endeavor would be rather costly and time-consuming, especially for the large lacZ gene. However, technological advances have made sequencing much more amenable to regulatory testing, and may soon make routine clonal correction of TGR MF data more practical (Beal et al., 2015a; Besaratinia et al., 2012).
Dose–response modeling showed that the BMD10 was lower in dividing spermatogonia than spermatogonial stem cells. Our result supports the previous observation that actively dividing germ cells are more susceptible to BaP-induced mutations than spermatogonial stem cells. These BMDs are higher (10.4–43.0 mg/kg bw/day in germ cells, present study) than what has been reported for mutation in somatic tissues (0.5–7.2 mg/kg bw/day, Moffat et al., 2015). The BMD10 of germ cells collected from the tubules was very similar to the BMD10 of dividing spermatogonia. However, caution should be used when interpreting the BMD10 derived from tubule cells, considering the mixed nature of the target cell population and the fact that significant effects were not detected at any dose tested. Differences in the pharmacokinetic distribution of BaP and its metabolites in the testes and somatic tissues may contribute to the variation in response. BPDE-DNA adducts are detected in somatic tissues and testes within days of exposure (Lemieux et al., 2011; Olsen et al., 2010; Verhofstad et al., 2010). However, no studies have compared adduct levels between these tissues after the typical OECD-recommended 28-day exposure regimen. Such information would help clarify how much of the BaP metabolites reach the testes relative to other tissues, enabling comparison of mutagenic efficiency in these cell types. It is thought that germ cells in general may have superior resistance to spontaneous and environmentally induced mutation (Murphey et al., 2012), which may also have contributed to the reduced levels of BaP-induced mutation in sperm.
The findings from dose-response modelling suggest a sub-linear dose response for the induction of mutations by BaP after spermatogonial stem cell exposure. In fact, slope analysis in the low dose region of the curves indicated that the mutagenic effect of BaP did not have a slope significantly greater than zero at doses below 25 mg/kg bw/day for all cell types targeted. A significant BPD and STD were also identified at the 42-day time-point, further supporting a sub-linear response. There is increasing evidence for sub-linear dose responses for DNA reactive mutagens, such as ethyl methanesulfonate, and N-ethyl-N-nitrosourea in both somatic tissues and germ cells (Bryce et al., 2010; Doak et al., 2007; Gocke and Muller, 2009; Lynch et al., 2011; O’Brien et al., 2015; Pottenger et al., 2009). This is indicative of a saturable repair response, where at lower doses the DNA damage is repaired, but repair processes are overwhelmed as doses increase (Julien et al., 2009; Yauk et al., 2015b). Because BaP induces mutations primarily through DNA adduct-forming metabolites, it is possible that the sub-linear dose response is attributable to a saturable repair mechanism for BaP adducts. Data with knockout mice suggest that nucleotide excision repair (NER) is essential to BaP-induced mutation (Verhofstad et al., 2011) and that NER activity varies among spermatogenic cell types (Jansen et al., 2001; Xu et al., 2005).
In conclusion, we demonstrated that oral exposure to BaP causes spermatogonial stem cell mutations in mice and that dividing spermatogonia represent a window of peak sensitivity for the induction of mutations. Evaluation of MFs in cells from the cauda and seminiferous tubules at the time-point recommended in the OECD test guideline for measuring mutations in somatic tissues (28 + 3) may not be sufficiently sensitive to detect germ cell mutagens, suggesting that data derived from this design be interpreted with caution. We advise that a later time point after the exposure (eg, 28 days for tubules, 42 days for cauda sperm) provides a more sensitive measure of germ cell mutagenic response for chemical assessment.
SUPPLEMENTARY DATA
Supplementary data are available online athttp://toxsci.oxfordjournals.org/.
ACKNOWLEDGEMENTS
The authors would like to thank John Gingerich and Lynda Soper for their work on the animal exposures, tissue collections and lacZ assay portions of this project. We would also like to thank Andrew Williams for his guidance on statistical analysis and Alexandra Long and Christine Lemieux for helpful comments.
FUNDING
This work was supported by Health Canada intramural funds and the Chemicals Management Plan initiative. Stipend support for JMO’B and MAB was provided by the Canadian Institute of Health Research Training Program in Reproduction, Early Development, and the Impact on Health. Stipend support for MAB was also provided by the Natural Sciences and Engineering Research Council.
REFERENCES
- Baarends W. M., van der Laan R., Grootegoed J. A. (2001). DNA repair mechanisms and gametogenesis. Reproduction 121, 31–39. [DOI] [PubMed] [Google Scholar]
- Beal M. A., Gagné R., Williams A., Marchetti F., Yauk C. L. (2015a). Characterizing Benzo[a]pyrene-induced lacZ mutation spectrum in transgenic mice using next-generation sequencing. BMC Genomics 16, 812.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. A., Rowan-Carroll A., Campbell C., Williams A., Somers C. M., Marchetti F., Yauk C. L. (2015b). Single-molecule PCR analysis of an unstable microsatellite for detecting mutations in sperm of mice exposed to chemical mutagens. Mutat. Res. 775, 26–32. [DOI] [PubMed] [Google Scholar]
- Besaratinia A., Li H., Yoon J. I., Zheng A., Gao H., Tommasi S. (2012). A high-throughput next-generation sequencing-based method for detecting the mutational fingerprint of carcinogens. Nucleic Acids Res. 40, e116.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce S. M., Avlasevich S. L., Bemis J. C., Phonethepswath S., Dertinger S. D. (2010). Miniaturized flow cytometric in vitro micronucleus assay represents an efficient tool for comprehensively characterizing genotoxicity dose-response relationships. Mutat. Res. 703, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad D. F., Keebler J. E., DePristo M. A., Lindsay S. J., Zhang Y., Casals F., Idaghdour Y., Hartl C. L., Torroja C., Garimella K. V., et al. (2011). Variation in genome-wide mutation rates within and between human families. Nat. Genet. 43, 712–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow J. F. (2000). The origins, patterns and implications of human spontaneous mutation. Nat. Rev. 1, 40–47. [DOI] [PubMed] [Google Scholar]
- De Rooij D. G. (2001). Proliferation and differentiation of spermatogonial stem cells. Reproduction 121, 347–354. [DOI] [PubMed] [Google Scholar]
- DeMarini D. M. (2012). Declaring the existence of human germ-cell mutagens. Environ. Mol. Mutagen. 53, 166–172. [DOI] [PubMed] [Google Scholar]
- Doak S. H., Jenkins G. J., Johnson G. E., Quick E., Parry E. M., Parry J. M. (2007). Mechanistic influences for mutation induction curves after exposure to DNA-reactive carcinogens. Cancer Res. 67, 3904–3911. [DOI] [PubMed] [Google Scholar]
- Generoso W. M., Cain K. T., Hellwig C. S., Cacheiro N. L. (1982). Lack of association between induction of dominant-lethal mutations and induction of heritable translocations with benzo[a]pyrene in postmeiotic germ cells of male mice. Mutat. Res. 94, 155–163. [DOI] [PubMed] [Google Scholar]
- Gocke E., Muller L. (2009). In vivo studies in the mouse to define a threshold for the genotoxicity of EMS and ENU. Mutat. Res. 678, 101–107. [DOI] [PubMed] [Google Scholar]
- Gollapudi B. B., Johnson G. E., Hernandez L. G., Pottenger L. H., Dearfield K. L., Jeffrey A. M., Julien E., Kim J. H., Lovell D. P., Macgregor J. T., et al. (2013). Quantitative approaches for assessing dose-response relationships in genetic toxicology studies. Environ. Mol. Mutagen. 54, 8–18. [DOI] [PubMed] [Google Scholar]
- Gunes S., Al-Sadaan M., Agarwal A. (2015). Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod. Biomed. Online 31, 309–319. [DOI] [PubMed] [Google Scholar]
- IARC Working Group (2010) Benzo[a]pyrene. IARC Monogr. 100F, 2005, 111–144.
- Jansen J., Olsen A. K., Wiger R., Naegeli H., de Boer P., van Der Hoeven F., Holme J. A., Brunborg G., Mullenders L. (2001). Nucleotide excision repair in rat male germ cells: Low level of repair in intact cells contrasts with high dual incision activity in vitro. Nucleic Acids Res. 29, 1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. E., Soeteman-Hernandez L. G., Gollapudi B. B., Bodger O. G., Dearfield K. L., Heflich R. H., Hixon J. G., Lovell D. P., Macgregor J. T., Pottenger L. H., et al. (2014). Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment. Environ. Mol. Mutagen. 55, 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien E., Boobis A. R., Olin S. S. and ILSI Research Foundation Threshold Working Group. (2009). The Key Events Dose-Response Framework: A cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Crit. Rev. Food Sci. Nutr. 49, 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A., Frigge M. L., Masson G., Besenbacher S., Sulem P., Magnusson G., Gudjonsson S. A., Sigurdsson A., Jonasdottir A., Jonasdottir A., et al. (2012). Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert I. B., Singer T. M., Boucher S. E., Douglas G. R. (2005). Detailed review of transgenic rodent mutation assays. Mutat. Res. 590, 1–280. [DOI] [PubMed] [Google Scholar]
- Lemieux C. L., Douglas G. R., Gingerich J., Phonethepswath S., Torous D. K., Dertinger S. D., Phillips D. H., Arlt V. M., White P. A. (2011). Simultaneous measurement of benzo[a]pyrene-induced Pig-a and lacZ mutations, micronuclei and DNA adducts in Muta Mouse. Environ. Mol. Mutagen. 52, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., Giddings A., Custer L., Gleason C., Henwood A., Aylott M., Kenny J. (2011). International Pig-a gene mutation assay trial (stage III): Results with N-methyl-N-nitrosourea. Environ. Mol. Mutagen. 52, 699–710. [DOI] [PubMed] [Google Scholar]
- Marchetti F., Wyrobek A. J. (2005). Mechanisms and consequences of paternally-transmitted chromosomal abnormalities. Birth Defects Res. C, Embryo Today Rev. 75, 112–129. [DOI] [PubMed] [Google Scholar]
- Marchetti F., Wyrobek A. J. (2008). DNA repair decline during mouse spermiogenesis results in the accumulation of heritable DNA damage. DNA Repair (Amst). 7, 572–581. [DOI] [PubMed] [Google Scholar]
- Moffat I., Chepelev N. L., Labib S., Bourdon-lacombe J., Kuo B., Buick J. K., Lemieux F., Williams A., Halappanavar S., Malik A. I., et al. (2015). Comparison of toxicogenomics and traditional approaches to inform mode of action and points of departure in human health risk assessment of benzo[a]pyrene in drinking water. Crit. Rev. Toxicol. 8444, 1–43., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey P., McLean D. J., McMahan C. A., Walter C. A., McCarrey J. R. (2012). Enhanced Genetic Integrity in Mouse Germ Cells. Biol. Reprod. 88, 6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. M., Beal M. A., Gingerich J. D., Soper L., Douglas G. R., Yauk C. L., Marchetti F. (2014). Transgenic rodent assay for quantifying male germ cell mutant frequency—Material list. J. Vis. Exp. 3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. M., Walker M., Sivathayalan A., Douglas G. R., Yauk C. L., Marchetti F. (2015). Sublinear response in lacZ mutant frequency of Muta TM Mouse spermatogonial stem cells after low dose subchronic exposure to N-ethyl-N-nitrosourea. Environ. Mol. Mutagen. 56, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2013). Test No. 488: Transgenic rodent somatic and germ cell gene mutation assays In OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris. [Google Scholar]
- Olsen A. K., Andreassen Å., Singh R., Wiger R., Duale N., Farmer P. B., Brunborg G. (2010). Environmental exposure of the mouse germ line: DNA adducts in spermatozoa and formation of De Novo mutations during spermatogenesis. PLoS One 5, e11349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A. K., Lindeman B., Wiger R., Duale N., Brunborg G. (2005). How do male germ cells handle DNA damage? Toxicol. Appl. Pharmacol. 207, 521–531. [DOI] [PubMed] [Google Scholar]
- Pottenger L. H., Schisler M. R., Zhang F., Bartels M. J., Fontaine D. D., McFadden L. G., Bhaskar Gollapudi B. (2009). Dose-response and operational thresholds/NOAELs for in vitro mutagenic effects from DNA-reactive mutagens, MMS and MNU. Mutat. Res. 678, 138–147. [DOI] [PubMed] [Google Scholar]
- R Core Team (2015) R: A language and environment for statistical computing R Foundation for Statistical Computung, Vienna, Austria.
- Rahbari R., Wuster A., Lindsay S. J., Hardwick R. J., Alexandrov L. B., Al Turki S., Dominiczak A., Morris A., Porteous D., Smith B., et al. (2015). Timing, rates and spectra of human germline mutation. Nat. Genet. 48, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B., Selby P. B., von Halle E., Sheridan W., Valcovic L. (1981). The mouse specific-locus test with agents other than radiations: Interpretation of data and recommendations for future work. Mutat. Res. 86, 329–354. [DOI] [PubMed] [Google Scholar]
- Shwed P. S., Crosthwait J., Douglas G. R., Seligy V. L. (2010). Characterisation of MutaMouse lambdagt10-lacZ transgene: Evidence for in vivo rearrangements. Mutagenesis 25, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers C. M. (2006). Expanded simple tandem repeat (ESTR) mutation induction in the male germline: Lessons learned from lab mice. Mutat. Res. 598, 35–49. [DOI] [PubMed] [Google Scholar]
- Verhofstad N., van Oostrom C. T., van Benthem J., van Schooten F. J., van Steeg H., Godschalk R. W. (2010). DNA adduct kinetics in reproductive tissues of DNA repair proficient and deficient male mice after oral exposure to benzo(a)pyrene. Environ. Mol. Mutagen. 51, 123–129. [DOI] [PubMed] [Google Scholar]
- Verhofstad N., van Oostrom C. T. M., Zwart E., Maas L. M., van Benthem J., van Schooten F. J., van Steeg H., Godschalk R. W. L. (2011). Evaluation of benzo(a)pyrene-induced gene mutations in male germ cells. Toxicol. Sci. 119, 218–223. [DOI] [PubMed] [Google Scholar]
- Xu G., McMahan C. A., Walter C. A. (2014). Early-life exposure to benzo[a]pyrene increases mutant frequency in spermatogenic cells in adulthood. PLoS One 9, e87437.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Spivak G., Mitchell D. L., Mori T., McCarrey J. R., McMahan C. A., Walter R. B., Hanawalt P. C., Walter C. A. (2005). Nucleotide excision repair activity varies among murine spermatogenic cell types. Biol. Reprod. 73, 123–130. [DOI] [PubMed] [Google Scholar]
- Yauk C. L., Aardema M. J., Benthem J., van Bishop J. B., Dearfield K. L., DeMarini D. M., Dubrova Y. E., Honma M., Lupski J. R., et al. (2015a). Approaches for identifying germ cell mutagens: Report of the 2013 IWGT workshop on germ cell assays. Mutat. Res. Toxicol. Environ. Mutagen. 783, 36–54. [DOI] [PubMed] [Google Scholar]
- Yauk C. L., Dubrova Y. E., Grant G. R., Jeffreys A. J. (2002). A novel single molecule analysis of spontaneous and radiation-induced mutation at a mouse tandem repeat locus. Mutat. Res. 500, 147–156. [DOI] [PubMed] [Google Scholar]
- Yauk C. L., Lambert I. B., Meek M. E. B., Douglas G. R., Marchetti F. (2015b). Development of the adverse outcome pathway ‘alkylation of DNA in male premeiotic germ cells leading to heritable mutations’ using the OECD’s users' handbook supplement. Environ. Mol. Mutagen. 56, 724–750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.