Abstract
Purpose
Limited mechanistic understanding of diabetic retinopathy (DR) has hindered therapeutic advances. Berberine, an isoquinolone alkaloid, has shown favorable effects on glucose and lipid metabolism in animal and human studies, but effects on DR are unknown. We previously demonstrated intraretinal extravasation and modification of LDL in human diabetes, and toxicity of modified LDL to human retinal Müller cells. We now explore pathogenic effects of modified LDL on Müller cells, and the efficacy of berberine in mitigating this cytotoxicity.
Methods
Confluent human Müller cells were exposed to in vitro–modified ‘highly oxidized, glycated (HOG-) LDL versus native-LDL (N-LDL; 200 mg protein/L) for 6 or 24 hours, with/without pretreatment with berberine (5 μM, 1 hour) and/or the adenosine monophosphate (AMP)-activated protein kinase (AMPK) inhibitor, Compound C (5 μM, 1 hour). Using techniques including Western blots, reactive oxygen species (ROS) detection assay, and quantitative real-time PCR, the following outcomes were assessed: cell viability (CCK-8 assay), autophagy (LC3, Beclin-1, ATG-5), apoptosis (cleaved caspase 3, cleaved poly-ADP ribose polymerase), oxidative stress (ROS, nuclear factor erythroid 2-related factor 2, glutathione peroxidase 1, NADPH oxidase 4), angiogenesis (VEGF, pigment epithelium-derived factor), inflammation (inducible nitric oxide synthase, intercellular adhesion molecule 1, IL-6, IL-8, TNF-α), and glial cell activation (glial fibrillary acidic protein).
Results
Native-LDL had no effect on cultured human Müller cells, but HOG-LDL exhibited marked toxicity, significantly decreasing viability and inducing autophagy, apoptosis, oxidative stress, expression of angiogenic factors, inflammation, and glial cell activation. Berberine attenuated all the effects of HOG-LDL (all P < 0.05), and its effects were mitigated by AMPK inhibition (P < 0.05).
Conclusions
Berberine inhibits modified LDL-induced Müller cell injury by activating the AMPK pathway, and merits further study as an agent for preventing and/or treating DR.
Keywords: angiogenesis, apoptosis, autophagy, berberine, diabetic retinopathy, GFAP, inflammation, Müller cell, oxidative stress, oxidized LDL
Limited understanding of the pathogenic mechanisms underlying diabetic retinopathy (DR) has hindered development of new therapies. Both vascular and neuronal changes are believed to play critical roles, but subclinical retinal injury is thought to precede clinically detectable vascular injury.1 Müller cells are the major glia of the retina; they form processes around retinal vessels and provide support to retinal neurons, controlling blood flow, modulating neuronal activity and glucose metabolism, and maintaining the blood–retinal barriers (BRBs).2,3
Previously we have demonstrated that in people with diabetes, LDL becomes extravasated through leaking BRBs, and subsequently modified by oxidation and glycation even in the ‘preclinical' stages of DR.4–7 Therefore, we have postulated that in addition to hyperglycemia, extravasation, and modification of plasma lipoproteins, with resultant cytotoxicity, are important drivers of DR.8 We found that among other adverse effects, modified LDL induces death in several types of human retinal cells.5,6,9 Specifically, Müller cell culture studies revealed that modified LDL causes oxidative and endoplasmic reticulum (ER) stresses, and apoptotic death, but the detailed underlying mechanisms of cellular injury are not yet fully understood.9 Agents that could protect Müller cells (and other retinal cells) from the toxic effects of modified lipoproteins may hold therapeutic promise, and also shed light on the mechanisms for DR.
Berberine (benzyltetrahydroxyquinoline; Fig. 1A) is natural compound originally extracted from an herb, Huanglian (Copis chinensis). It is a quaternary ammonium salt from the protoberberine group of isoquinoline alkaloids, and has been used in China for millennia as an antimicrobial agent.10,11 Long-term clinical trials defining the safety of berberine are still not available, but small, short-term studies show no evidence of hepatic or renal toxicity.12,13 Recently, some favorable pharmacological actions of berberine have been demonstrated, including anti-inflammatory, antioxidant, antidiabetic, and cholesterol-lowering effects,14–18 as reviewed by Pang et al.18 Thus, in a study of newly diagnosed type 2 diabetes mellitus, participants were randomly assigned to treatment with berberine or metformin for three months19: berberine exhibited similar glucose-lowering effects as metformin, and had greater beneficial effects on plasma lipid profiles. Similar effects were shown in another study in which berberine significantly lowered fasting blood glucose, hemoglobin A1c, triglyceride, and insulin levels in type 2 diabetic patients.20 These favorable effects have been explored in vitro, and various signalling pathways have been implicated.21–23 Importantly, many, perhaps most, effects of berberine may be mediated through activation of the adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway. Little is known about possible effects of berberine in DR, but some cell culture and animal studies suggest possible benefit.24–28 In the current study, we aimed to test whether berberine can protect cultured human retinal Müller cells against cytotoxicity induced by oxidized-glycated LDL, and to explore further the underlying pathogenic effects of the lipoprotein, and the mechanisms of action of berberine.
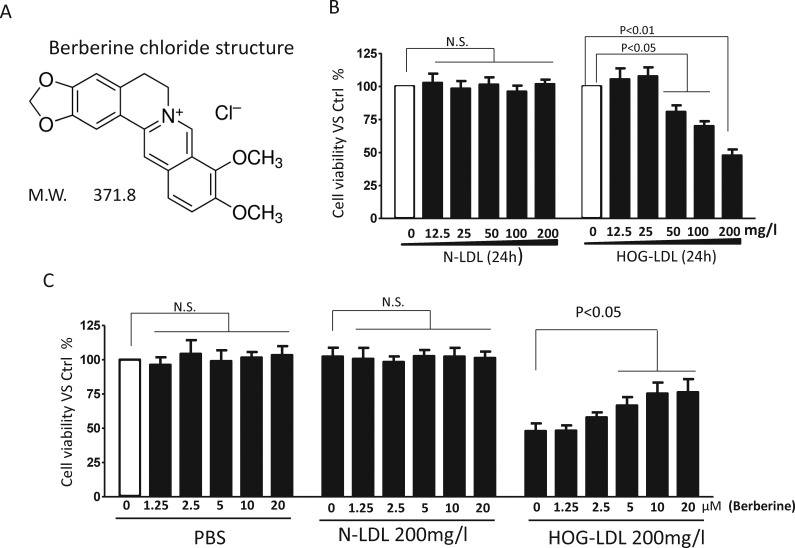
Figure 1.
Structure of berberine, and dose response on HOG-LDL–induced Müller cell death. (A) Berberine structure. (B) Müller cells were treated with N- or HOG-LDL for 24 hours at various concentrations as indicated, or (C) pretreated with berberine (1.25–20 μM) for 1 hour followed by N- or HOG-LDL (200 mg/L) exposure for 24 hours. Untreated cells served as control in this and the following figures (unless specifically indicated). Cell viability was assessed by CCK-8 assay. Data are expressed as means ± SD, n = 3. Statistical significance was determined by Student's t-test, or 1-way ANOVA, and P < 0.05 indicated significance in this and the following Figures. NS, not significant.
Materials and Methods
LDL Preparation, Modification, and Characterization
Preparation of native-LDL (N-LDL) and ‘highly oxidized, glycated' LDL (HOG-LDL) was as described previously.5,29 Briefly, N-LDL (density = 1.019–1.063) was isolated by sequential ultracentrifugation of pooled plasma from 4 to 6 healthy fasted volunteers. Glycated LDL was prepared by incubating LDL with freshly-prepared 50 mM glucose (72 hours, 37°C) under antioxidant conditions (1 mM diethylenetriaminepentaacetic acid [DTPA] with 270 μM EDTA, under nitrogen). HOG-LDL was prepared by oxidizing the glycated LDL in the presence of 10 μM copper chloride (24 hours, 37°C), followed by repeated dialysis (4°C, 24 hours). Protein concentration of the LDL preparations was determined by the Pierce BCA assay (Thermo Scientific, Rockford, IL, USA). Low-density lipoprotein preparations were further characterized by fluorescence (excitation: 360 nm; emission: 430 nm), agarose gel electrophoresis (Paragon Lipo Gel; Beckman, Fullerton, CA, USA) and absorbance at 234 nm. LDL preparations were stored in the dark under nitrogen at 4°C, in the presence of 270 μM EDTA, and used within 6 weeks. Experiments were repeated using different LDL preparations.
Human Retinal Müller Cell Culture
Moorfields/Institute of Ophthalmology-Müller 1 (MIO-M1) cells, from a spontaneously immortalized human Müller cell line, were provided by G. Astrid Limb (Institute of Ophthalmology, University College London, London, UK). Passages 22 to 25 were used for the current study. The cells were maintained in Dulbecco's modified Eagle's medium/F12 (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) at 37°C under 5% CO2 and 95% air. Experiments were conducted on cells that had reached 80% to 85% confluence. Berberine chloride, 3-methyladenine (3-MA), Z-VAD-fmk, and N-acetylcysteine (NAC) were obtained from Sigma-Aldrich, and 4-hydroxynonenal (4-HNE) was from Cayman Chemical (Ann Arbor, MI, USA).
Cell Viability Assay
Human MIO-M1 Müller cells were seeded in 96-well plates (10,000 cells/well) and cultured to 80% to 85% confluence, then treated as described below. Cell viability was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Rockville, MD, USA). In this indirect method, the water-soluble tetrazolium salt, WST-8, is added to culture medium, where as a result of intracellular dehydrogenase activity, it is reduced to a highly colored, nontoxic formazan dye. Absorbance at 450 nm was used to determine cell viability per manufacturer's instructions. All experiments were performed in triplicate.
Western Blotting
Cells were homogenized with complete lysis buffer (Roche, Indianapolis, IN, USA). Protein concentrations were measured using Pierce BCA Assay Kit (Thermo Scientific). Thirty micrograms of protein were loaded onto each lane of a gel. Proteins were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed with specific primary antibodies at dilutions of 1:1000 unless indicated otherwise. Antibodies against β-actin (1:3000), autophagy-related homologue 5 (ATG-5), Beclin-1, LC3, cleaved poly-ADP ribose polymerase (PARP), cleaved caspase-3, glial fibrillary acidic protein (GFAP), phospho-AMPK (p-AMPK) and total AMPK (t-AMPK) were purchased from Cell Signalling Technology (Danvers, MA, USA); antibodies against NADPH oxidase 4 (Nox4), nuclear factor erythroid 2-related factor 2 (Nrf2), glutathione peroxidase 1 (Gpx-1), VEGF, pigment epithelium-derived factor (PEDF), inducible nitric oxide synthase (iNOS), and intercellular adhesion molecule 1 (ICAM-1) were purchased from Abcam (Cambridge, MA, USA). β-actin was used as a loading control. All experiments were repeated three times independently and quantified by densitometry.
Measurement of Intracellular Reactive Oxygen Species (ROS)
Intracellular ROS were measured by the DCFDA Cellular ROS detection Assay Kit (Abcam), according to the manufacturer's instructions. Briefly, Müller cells were seeded in 96-well plates at 1× 104 cells per well. At 70% to 80% confluence, cells were washed, then incubated with 20 μM DCFDA in phenol red-free medium (37°C, 45 minutes). After washing twice more, cells were exposed to experimental conditions as described below. Fluorescence signals were read at excitation 485 nm/emission 535 nm (VICTOR3 microplate reader; PerkinElmer, Waltham, MA, USA). Data were presented as fold change versus control after background subtraction.
Quantitative Real-Time PCR
RNA was extracted from Human MIO-M1 Müller cells (RNeasy Mini Kit; Qiagen, Valencia, CA, USA). Complementary (c)DNA was synthesized using Superscript III (Invitrogen Ltd., Paisley, UK). Semiquantitative real-time PCR was performed using gene specific Real Time ready Custom Single assays for IL-6, IL-8, TNF-α, and β-actin (Roche). Relative quantitative values were obtained using the ΔΔCt method.
Statistical Analysis
Data are expressed as means ± SD. Statistical significance was determined by Student's t-test, or 1-way ANOVA followed by post hoc Bonferroni test as appropriate (Prism 4; Graphpad, La Jolla, CA, USA). P values less than 0.05 were considered significant.
Results
Berberine Attenuated HOG-LDL–Induced Müller Cell Death
As expected, over 24 hours, HOG-LDL, but not N-LDL, decreased Müller cell viability at concentrations greater than or equal to 50 mg protein/L in a dose-dependent manner (Fig. 1B). To evaluate the effect of berberine on LDL-cell interactions, we employed N-LDL and HOG-LDL at 200 mg/L, a concentration we consider to be pathophysiologically relevant in DR.8 Native-LDL did not affect cell viability, while HOG-LDL reduced it by approximately 50% (Fig. 1B). Berberine (0–20 μM) either alone, or added as a pretreatment before exposure to N-LDL, had no effect on Müller cell viability (Fig. 1C). However, pretreatment with berberine for 1 hour significantly improved viability following 24-hour exposure to HOG-LDL in a dose-dependent manner (Fig. 1C). The lowest effective concentration, 5 μM, was used in all the experiments described below.
Berberine Attenuated HOG-LDL–Induced Autophagic Cell Death
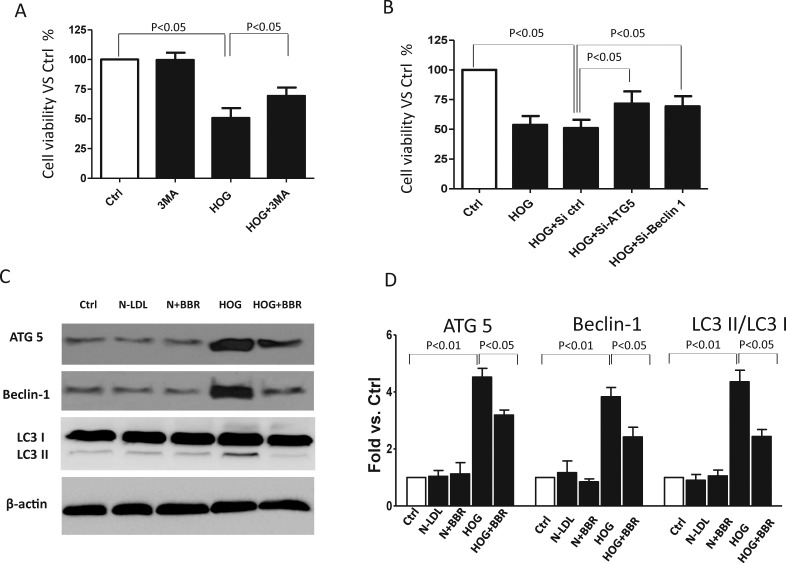
We previously showed that both autophagic cell death and apoptosis contribute to HOG-LDL–induced retinal pericyte loss,5 and that HOG-LDL can cause apoptosis in Müller cells.8 To determine if autophagy is also implicated in Müller cell responses to HOG-LDL, we pretreated cells for 1 hour with 3 MA, a specific inhibitor of phosphoinositide 3-kinase, before exposure to lipoproteins (200 mg/L in this and following experiments) for 24 hours. 3 MA inhibits the initial phase of the autophagic process, and it reduced HOG-LDL–induced autophagy (Supplementary Fig. S1). As shown in Figure 2A, loss of Müller cell viability was partially prevented by 3 MA pretreatment, implicating autophagy in HOG-LDL–induced cell death. We also genetically inhibited autophagy by knocking down ATG-5 or Beclin-1, two key autophagy-related genes (Supplementary Figs. S2A, S2B). Again, HOG-LDL–induced cell death was attenuated (Fig. 2B). HOG-LDL–induced autophagy was confirmed by Western blot, which showed significantly increased protein levels of ATG-5, Beclin-1, and the ratio of LC3II/LC3I. HOG-LDL–induced autophagy was attenuated by berberine (Figs. 2C, 2D).
Figure 2.
Berberine attenuated HOG-LDL induced autophagic death in Müller cell. (A) Müller cells were pretreated with 3 MA (5 mM) for 1 hour, or (B) transfected with Si-ATG-5 or Si-Beclin-1 for 36 hour then treated (in this and following experiments) with N- or HOG-LDL (200 mg/L) for 24 hour. Cell viability was expressed as percentage versus control (mean ± SD, n = 3). (C, D) Müller cells were pretreated with berberine (5 μM) for 1 hour then exposed to N- or HOG-LDL for 24 hours. ATG-5, Beclin-1, LC3I, and LC3II were detected by Western blot and quantified by densitometry (mean ± SD; n = 3).
Berberine Attenuated HOG-LDL–Induced Apoptosis
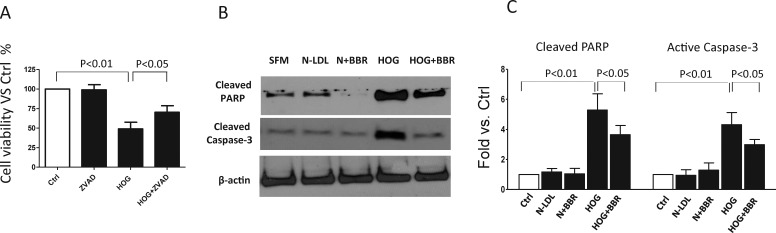
As expected, we found, as before,8 that apoptosis was implicated in HOG-LDL–induced Müller cell death over 24 hours, evidenced by the protective effect of ZAD-fmk, an inhibitor of caspase-dependent apoptosis (Fig. 3A), and by increased cleavage of PARP (cleaved PARP, molecular weight: 89 kD, Fig. 3B) and activation of caspase 3 (cleaved caspase, molecular weight: 18 kD, Fig. 3C). Again, a protective effect of berberine was observed (Figs. 3B, 3C).
Figure 3.
Berberine attenuated HOG-LDL–induced Müller cell apoptosis. (A) Müller cells were exposed to HOG-LDL for 24 hours with/without 1 hour pretreatment with Z-VAD-fmk (100 μM). Cell viability was expressed as percentage versus control (mean ± SD, n = 3). (B, C) Müller cells were exposed to N- or HOG-LDL for 24 hours with/without berberine (5 μM) pretreatment for 1 hours. Cleaved PARP and cleaved caspase-3 were detected by Western blot and quantified by densitometry (mean ± SD; n = 3).
Berberine Attenuated HOG-LDL–Induced Oxidative Stress, and Oxidative Stress may be Implicated in Both Autophagy and Apoptosis
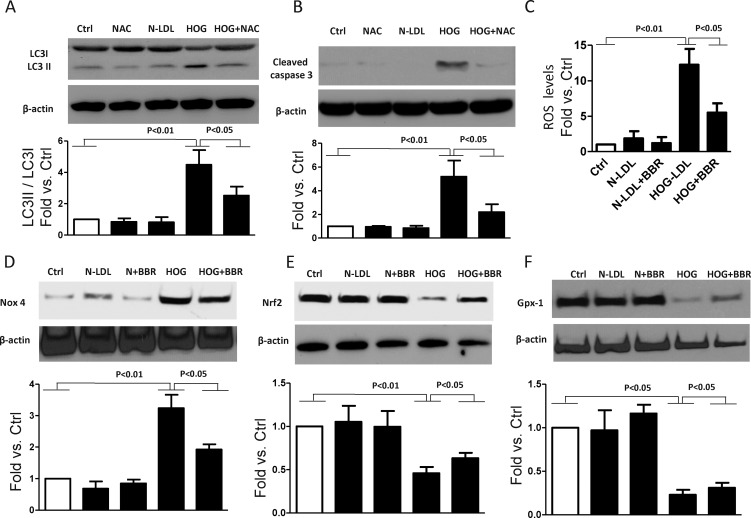
Previously, we have shown that oxidative stress is implicated in HOG-LDL–induced apoptosis in retinal cells, including Müller cells.5,6,9 We hypothesize that the protective role of berberine against cell death, whether autophagic or apoptotic, might operate through inhibition of oxidative stress. To test this, cells were pretreated with NAC (100 μM, 1 hour) before exposure to HOG-LDL for 24 hours, then markers for autophagy and apoptosis were measured. NAC, a scavenger of ROS, significantly mitigated HOG-LDL–induced LC3 conversion and caspase activation, supporting a role for oxidative stress in HOG-LDL–induced autophagy and apoptosis (Figs. 4A, 4B). We also measured intracellular levels of ROS and proteins related to oxidative stress. We found that berberine ameliorated excess ROS production by Müller cells (Fig. 4C). Moreover, oxidative stress–induced expression of Nox4 and decreased expression of Nrf2 and Gpx-1 were also mitigated by berberine (Figs. 4D, 4F). All these data suggest that the protective mechanism of berberine involves mitigation of oxidative stress.
Figure 4.
Berberine decreased HOG-LDL–induced oxidative stress. Müller cells were pretreated with/without NAC (100 μM) or berberine (5 μM) for 1 hour, then exposed to N- or HOG-LDL for 6, 12, or 24 hours: (A) autophagy marker, LC3, 24 hours; (B) apoptosis marker cleaved caspase-3, 24 hours; (C) cellular ROS levels, 6 hours, and (D–F) oxidative stress-related proteins, Nox4, Nrf2, and Gpx-1, 12 hours, were measured by DCFDA-cellular ROS detection assay or by Western blot and quantified by densitometry (mean ± SD; n = 3).
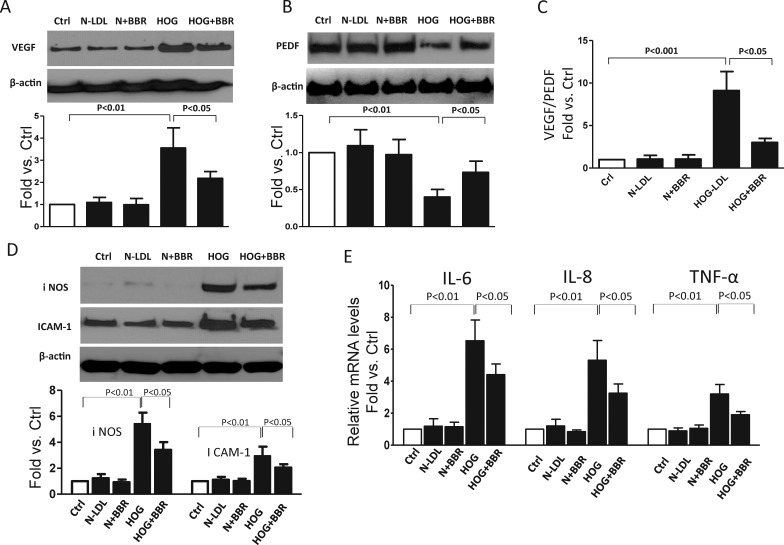
Berberine Attenuated HOG-LDL–Induced Angiogenesis and Inflammation
Increased angiogenesis and inflammation are known features of DR. Proangiogenic VEGF and antiangiogenic PEDF are two major proteins balancing angiogenesis in the retina, and both are perturbed in DR.30,31 Using Western blots, we confirmed that over 24 hours, HOG-LDL increased VEGF and decreased PEDF expression (Figs. 5A, 5B). This HOG-LDL–induced shift in angiogenic balance (increased VEGF/PEDF ratio) was mitigated by berberine (Fig. 5C). Berberine also reduced expression of inflammatory cytokines: by Western blot, it attenuated the HOG-LDL–induced increases in protein levels of iNOS and ICAM-1 (Fig. 5D), and by quantitative (q)PCR, it mitigated HOG-LDL–induced expression for IL-6, IL-8, and TNF-α (Fig. 5E).
Figure 5.
Berberine attenuated HOG-LDL–induced Müller cell angiogenesis and inflammation. Müller cells were exposed to HOG-LDL for 24 hours with/without 1 hour pretreatment with berberine (5 μM). (A–C) Angiogenesis related proteins VEGF and PEDF and VEGF:PEDF ratio; (D) proinflammation cytokines iNOS and ICAM-1, detected by Western blot and quantified by densitometry (mean ± SD; n = 3). (E) mRNA levels for IL-6, IL-8, and TNF-α, measured by real-time PCR and expressed as fold change versus control (mean ± SD; n = 3).
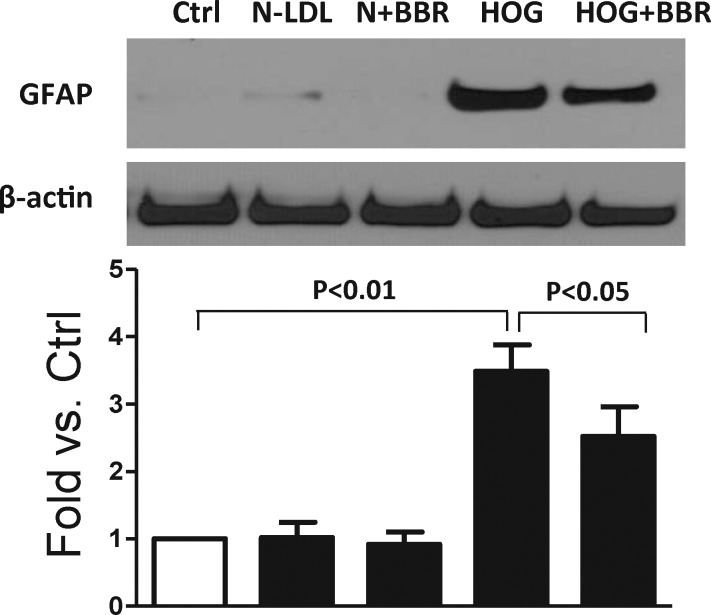
Berberine Blunted HOG-LDL–Induced GFAP Upregulation
Glial fibrillary acidic protein is a structural protein which is predominantly expressed in Müller cells, and is upregulated in response to retinal injury or stress. Over 24 hours, HOG-LDL, but not N-LDL, significantly increased GFAP expression, and berberine inhibited the increase (Fig. 6).
Figure 6.
Berberine blunted HOG-LDL–induced Müller cell activation. Müller cells were exposed to HOG-LDL for 24 hours with/without berberine (5 μM) pretreatment for 1 hour. Müller cell activation marker, GFAP, was detected by Western blot and quantified by densitometry (mean ± SD; n = 3).
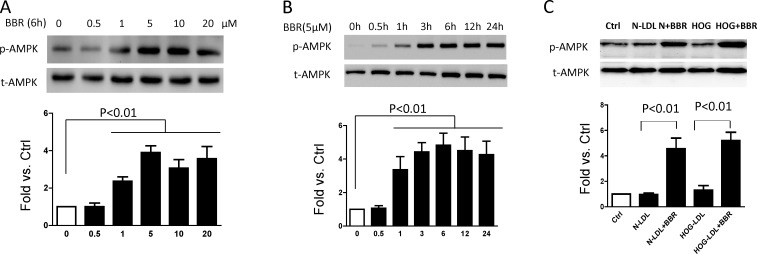
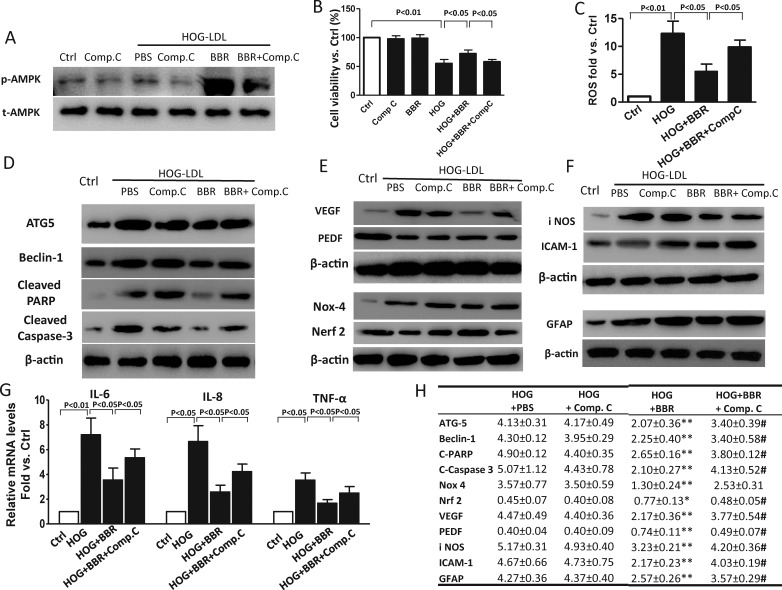
AMPK Activation is Implicated in the Protective Role of Berberine on Müller Cells
Adenosine monophosphate-activated protein kinase (AMPK) is a sensor of cellular energy status. Previously, it has been implicated in the favorable metabolic effects of berberine in diabetes and cardiovascular disease.32,33 We found that in Müller cells, berberine activates the AMPK pathway in a dose-dependent (0–20 μM) and time-dependent (0–24 hours) manner (Figs. 7A, 7B): its effect was maximal at 5 μM and was observed within 1 hour. Similar activation by berberine was observed in the presence of N- or HOG-LDL (cells treated with berberine for 1 hour, then lipoproteins added for 6 hours), although neither lipoprotein alone activated AMPK (Fig. 7C). Compound C, a pharmacological inhibitor of AMPK, was used to pretreat Müller cells, with or without berberine, before exposure to LDL. The efficacy of Compound C as an inhibitor of AMPK in Müller cells was confirmed by Western blot (Fig. 8A). Compound C reduced the protective effects of berberine on cell viability, autophagy, apoptosis, oxidative stress, angiogenesis, inflammation, and GFAP activation, implicating AMPK activation in the mechanism of action of berberine (at time points as defined in Figs. 8B–G). Quantitative analysis of the effects of berberine and berberine combined with Compound C on HOG-LDL–induced effects are shown in tabular form (Fig. 8H). The data shown are fold-changes in expression of the various parameters compared with HOG-LDL alone: in every case, a potentially beneficial effect of berberine is inhibited by Compound C.
Figure 7.
Berberine activated the AMPK pathway in a time- and dose-dependent manner. (A) Müller cells were treated with berberine at various concentrations for 6 hours. (B) Cells were treated with 5 μM berberine for times indicated. (C) Cells were pretreated with berberine (5 μM, 1 hour) before being exposed to N- or HOG-LDL (200 mg/L) for 6 hours: untreated cells served as controls, and p-AMPK and t-AMPK were detected by Western blot. p-AMPK was quantified by densitometry. For all experiments, data shown as mean ± SD; n = 3.
Figure 8.
Adenosine monophosphate–activated protein kinase activation was implicated in the protective role of berberine on Müller cells. Müller cells were treated with HOG-LDL for 6, 12, or 24 hours with/without berberine (5 μM) or berberine + Compound C (5 μM) pretreatment for 1 hour. Untreated cells served as control, and DMSO as ‘vehicle control'. (A) Western blots for p-AMPK and t-AMPK, 6 hours ± pretreatment with Compound C, 1 hour. (B) Viability expressed as percentage versus control, 24 hours (mean ± SD; n = 3). (C) Cellular ROS levels, 6 hours (mean ± SD; n = 3). (D) Western blots for ATG-5, Beclin-1, cleaved PARP, and cleaved caspase-3, 24 hours. (E) Western blot for VEGF and PEDF (24 hours); Nox4 and Nrf2, 12 hours. (F) Western blot for iNOS and ICAM-1, 24 hours; GFAP, 24 hours. (G) mRNA levels for IL-6, IL-8, and TNF-α, 24 hours (mean ± SD; n = 3). (H) Tabular presentation to show effects (Western blots) of berberine pretreatment (5 μM, 1 hour) or 'berberine plus Compound C' pretreatment (each 5 μM, 1 hour) prior to exposure to HOG-LDL (200 mg/L, 12 or 24 hours). In each case, data show fold changes normalized to the response to “no treatment” control. *P < 0.05, **P < 0.01 versus HOG+PBS, #P < 0.05 versus HOG+BBR treatment (mean ± SD; n = 3).
4-HNE Replicates Most Effects of HOG-LDL on Müller Cells
4-HNE is a major fatty acid oxidation product, and has previously been shown to replicate some effects of HOG-LDL on Müller cells.9 In this study, to explore further the use of 4-HNE as a surrogate for HOG-LDL, we repeated the experiments described above using 4-HNE in place of HOG-LDL. Using conditions defined previously,9 Müller cells were treated with 4-HNE at 40 μM for 6 hours. As shown in Supplementary Figure S3A, 4-HNE decreased cell viability by 50% versus no treatment control. Berberine attenuated cell death dose-dependently from 0 to 5 μM, but had no additional effect up to 20 μM. As it did with HOG-LDL, berberine ameliorated 4-HNE–induced autophagy, apoptosis, oxidative stress, angiogenesis, GFAP activation, and inflammatory responses (Supplemental Figs. 3B–G). Taken together, these data support the use of 4-HNE as a surrogate for HOG-LDL in studies of Müller cell function.
Discussion
Previously, we demonstrated toxicity of HOG-LDL toward Müller cells, and showed that it causes oxidative and ER stresses, leading to apoptosis.9 We now confirm and extend these findings, showing that HOG-LDL also activates GFAP, has proangiogenic and proinflammatory effects in Müller cells, and autophagic as well as apoptotic cell death is implicated. Further, we demonstrate that the harmful effects of HOG-LDL on Müller cells can be mitigated by berberine, through a mechanism partially dependent on AMPK activation (Fig. 9). We conclude that berberine merits further study as a potential agent to prevent or treat DR.
Figure 9.
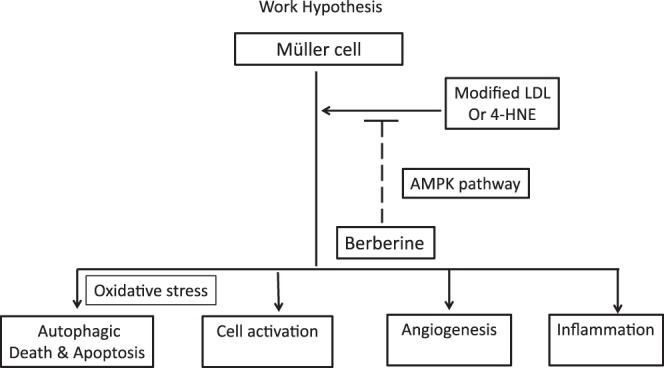
Hypothesis to explain beneficial effects of berberine on HOG-LDL–induced Müller cell dysfunction.
Currently, therapies for DR (e.g., laser treatment, intraocular angiogenic inhibitors) are only applicable to advanced disease; specific early treatments are lacking. Emerging evidence from cell culture, animal studies, and clinical trials suggests beneficial effects of berberine on type 2 diabetes and its complications.18 In this study, we hypothesized that berberine protects Müller cells against modified LDL-induced cytotoxicity. Our findings support the hypothesis but raise new questions that are not yet answered: does the protection apply to other retinal cells species exposed to modified LDL; is this a general effect on cell-modified lipoprotein interaction with possible benefits beyond DR; and will the in vitro findings translate to clinical benefit? These questions await answers.
Favorable effects of berberine on various retinal cells have been demonstrated previously, in vitro, in vivo, and ex vivo. In human retinal pigment epithelial cells, berberine inhibited IL-1β–induced BRB breakdown, improving transepithelial electric resistance and reducing permeability,24 and reduced TNF-α–induced proinflammatory cytokine production by downregulating the ERK pathway.25 In STZ- and high fat diet-induced diabetic rats, berberine improved retinal thickness.26 Another recent in vivo study showed that in mice, by reducing oxidative stress, berberine can protect against light-induced photoreceptor degeneration.27 In an ex vivo study, it inhibited toxicity of leukocytes derived from diabetic patients toward retinal endothelial cells.28 However, our study is the first to address the effect of berberine on toxicity of modified LDL toward retinal cells. We have accumulated much evidence that modified LDL is implicated in the progression of DR.4–9 We now provide in vitro evidence that berberine can protect Müller cells against modified LDL-induced cell death and dysfunction, and we report new detail of its action, and of the pathophysiologic effects of modified LDL. We show that berberine acts in part through AMPK pathway activation, mitigating oxidative stress, autophagic and apoptotic cell death, Müller cell activation, angiogenesis, and inflammation.
Interest in berberine has increased dramatically in the past decade.18 It has been shown to decrease blood glucose, enhance insulin sensitivity and improve cholesterol levels in type 2 diabetic rodent models.14,32 It has also shown protective effects against diabetic complications, including nephropathy, neuropathy, and cardiomyopathy.23,34–37 In diabetic nephropathy, berberine treatment was shown to improve kidney function in mice via AMPK activation.35 In a study of diabetic hypercholesterolemic rats, positive effects of berberine on cardiac dysfunction were shown, and activation of the peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ pathways were implicated.36
In the present study, we showed that berberine protected Müller cells against HOG-LDL–induced death. We employed berberine concentrations ranging from 1.25 to 20 μM, with most experiments being conducted at 5 μM. We saw no evidence of any toxic effect of the agent. However, in studies by others using higher concentrations, toxic effects in cell culture have been observed38,39: berberine showed toxicity toward HepG2 cells at 100 μM and toward MHCC97-L cells at 250 μM.38 In another study with primary pancreatic islet cells from Institute for Cancer Research (ICR; Philadelphia, PA) mice, berberine protected cells against STZ-induced death at 10 μM, but at greater than 30 μM, it induced cell death39; similar findings were with human RPE-19 cells, where berberine became toxic at 50 μM.25 Although berberine has been used medicinally for three millennia, rigorous studies are needed to define dosage and safety before any possible long-term use.
As in previous studies, our data suggest multiple actions of berberine. Previously, we have demonstrated that HOG-LDL induced apoptosis through oxidative stress in retinal pericytes, RPE, and Müller cells.5,6,9 The present study confirms the finding in Müller cells, and we now show that berberine can inhibit ROS production. Others have shown that berberine can significantly reduce cellular ROS and thereby protect cells against oxidative stress,40,41 so we measured expression of relevant proteins. Nox4 is a key enzyme for ROS generation; Nrf2 is a major component of the ROS signalling pathway regulating expression of antioxidant proteins; and Gpx-1 plays an important protective role via ROS detoxification. HOG-LDL increased Nox4 and reduced Nrf2 and Gpx-1. These changes were blunted by berberine, which therefore may act both by reducing ROS generation and increasing ROS removal.
Reactive gliosis is a general response to inflammation and neuron injury, and in Müller cells is reflected by increased GFAP expression. We have demonstrated for the first time that this activation was inhibited by berberine in Müller cells exposed to HOG-LDL. Neuroprotective effects of berberine have been shown in other studies.42,43 In gerbils, berberine ameliorated ischemia–induced activation of microglia in the hippocampus, evidenced by decreased GFAP expression,42 and in STZ-induced diabetic rats, berberine attenuated upregulation of cerebral GFAP.43 Interestingly, the latter study showed that berberine also reduced oxidative stress, consistent with our findings. The positive effects of berberine on GFAP regulation may be mediated by its antioxidant effects, but the mechanism(s) still needs further elucidation.
A precise balance between pro- and anti-angiogenic proteins, represented by VEGF and PEDF respectively, is essential for retinal function. This balance is disrupted in diabetes, and we have hypothesized that exposure of retinal tissues to modified LDL may be implicated. We now demonstrate for the first time that in Müller cells, HOG-LDL triggers a proangiogenic response, and that this is inhibited by berberine. The antiangiogenic activity of berberine has previously been demonstrated in melanoma and lung cancer cells, where it dramatically decreased VEGF protein and mRNA expression.44,45
Vascular endothelial growth factor may also promote inflammation, and increased expression of proinflammatory cytokines (e.g., IL-6, IL-8, and TNF-α) is characteristic of DR.46,47 Inhibition of Müller cell–derived VEGF significantly decreased retinal expression of TNF-α, ICAM-1, and nuclear factor-kappa B (NF-κB) in diabetic mice.48 We now show that HOG-LDL increased iNOS and ICAM-1, and transcriptionally increased the proinflammatory factors IL-6, IL-8, and TNF-α in Müller cells, and that all of these responses were inhibited by berberine. This is consistent with anti-inflammatory effects seen in other studies of macrophages and cancer cells.49–51
Adenosine monophosphate-activated protein kinase is a key signalling system that controls cellular energy balance and metabolism. Its activation is an important defensive response to stress and inflammation.52 Berberine is known to activate AMPK; this effect has been implicated in its beneficial effects in diabetes53–55 and may lie upstream of many of the effects described above. In a study of type 2 diabetic mice, berberine downregulated proinflammatory gene expression; inhibition of AMPK abolished the effect.49 In the retina, a role for AMPK was suggested in a study using AICAR, a specific AMPK activator, which significantly reduced retinal leukocyte adhesion and VEGF expression.56 Consistent with this, AMPK pathway was activated by berberine in Müller cells in a time- and dose-dependent manner, and pharmacologic inhibition of AMPK with Compound C partially but significantly attenuated the protective effects of berberine. Thus Compound C weakened protective effects of berberine against cell death (apoptosis and autophagy), angiogenesis, and inflammation, suggesting a role for AMPK as an upstream regulator of HOG-LDL–induced Müller cell dysfunction. A similar role for AMPK was shown in a study of metformin, another AMPK activator that is widely used oral antihyperglycemic agent. Metformin significantly mitigated albuminuria in diabetic mice.55 Thus, AMPK activation may have therapeutic benefits in the treatment and prevention of diabetic complications, including DR, but with the clear and important caveat that animal and cell culture work may not translate to human disease. It must also be noted that in our study, inhibition AMPK did not completely block the protective effect of berberine, suggesting that other pathways (e.g., PPARα, MAPK) may also be implicated.16,17,26,36,57 Indeed, it is likely that the harmful intraretinal effects of extravasated, modified lipoproteins involve several, or many, pathways. Further work is needed to define their relative importance in different settings, and agents with more than one effect, or combinations of agents, may be needed to block them.
In conclusion, we demonstrate in vitro beneficial effects of berberine on HOG-LDL–induced Müller cell death (by inhibition of autophagic or apoptotic pathways) and dysfunction (by inhibition of cytotoxicity, oxidative stress, angiogenesis, inflammation, and GFAP activation). The detailed mechanisms require further exploration, as do the effects of berberine in other retinal cell species, but AMPK activation appears to contribute. The safety of berberine for long-term human use in either oral or topical preparations needs to be fully established, and human clinical studies are needed to define any real benefit for patients. If berberine is capable of mitigating pathologic effects of modified lipoproteins on other cell types, it could have benefits that extend beyond diabetic retinopathy. The present findings support the case for further studies: there is an urgent need for agents that can block the development of diabetic complications, and for agents that can block or mitigate the toxic effects of extravasated, modified lipoproteins on vascular and neuronal tissues.
Supplementary Material
Acknowledgments
Supported by the Oklahoma Center for the Advancement of Science and Technology (HR08-067; Oklahoma City, OK, USA), by the Centers of Biomedical Research Excellence Program of the National Center for Research Resources (P20 RR 024215; Bethesda, MD, USA), and by the LINJO fund (Oklahoma City, OK, USA).
Disclosure: D. Fu, None; J.Y. Yu, None; A.R. Connell, None; S. Yang, None; M.B. Hookham, None; R. McLeese, None; T.J. Lyons, None
References
- 1. Engerman RL,, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987; 36: 808–812. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad I,, Del Debbio CB,, Das AV,, Parameswaran S. Müller glia: a promising target for therapeutic regeneration. Invest Ophthalmol Vis Sci. 2011; 52: 5758–5764. [DOI] [PubMed] [Google Scholar]
- 3. Canning P,, Glenn JV,, Hsu DK,, Liu FT,, Gardiner TA,, Stitt AW. Inhibition of advanced glycation and absence of galectin-3 prevent blood-retinal barrier dysfunction during short-term diabetes. Exp Diabetes Res. 2007; 2007: 51837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu M,, Chen Y,, Wilson K,, et al. Intraretinal leakage and oxidation of LDL in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008; 49: 2679–2685. [DOI] [PubMed] [Google Scholar]
- 5. Fu D,, Wu M,, Zhang J,, et al. Mechanisms of modified LDL-induced pericyte loss and retinal injury in diabetic retinopathy. Diabetologia. 2012; 55: 3128–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du M,, Wu M,, Fu D,, et al. Effects of modified LDL and HDL on retinal pigment epithelial cells: a role in diabetic retinopathy? Diabetologia. 2013; 56: 2318–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu D,, Yu JY,, Wu M,, et al. Immune complex formation in human diabetic retina enhances toxicity of oxidized LDL towards retinal capillary pericytes. J Lipid Res. 2014; 55: 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu JY,, Lyons TJ. Modified lipoproteins in diabetic retinopathy: a local action in the retina. J Clin Exp Ophthalmol. 2013; 4: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu M,, Yang S,, Elliott MH,, et al. Oxidative and endoplasmic reticulum stresses mediate apoptosis induced by modified LDL in human retinal Müller cells. Invest Ophthalmol Vis Sci. 2012; 53: 4595–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho E. Berberini hydrochloride. Pharmacopoeia of the People's Republic of China. Beijing, China; 1990: 437–439. [Google Scholar]
- 11. Luo LJ. Experience of berberine in the treatment of diarrhea. Chin J Med. 1955; 41: 452–455. [Google Scholar]
- 12. Lan J,, Zhao Y,, Dong F,, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015; 161: 69–81. [DOI] [PubMed] [Google Scholar]
- 13. Yin J,, Xing H,, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008; 57: 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kong W,, Wei J,, Abidi P,, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004; 10: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 15. Bhutada P,, Mundhada Y,, Bansod K,, et al. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011; 220: 30–41. [DOI] [PubMed] [Google Scholar]
- 16. Chen FL,, Yang ZH,, Liu Y,, et al. Berberine inhibits the expression of TNFalpha, MCP-1, and IL-6 in AcLDL-stimulated macrophages through PPARgamma pathway. Endocrine. 2008; 33: 331–337. [DOI] [PubMed] [Google Scholar]
- 17. Gao N,, Zhao TY,, Li XJ. The protective effect of berberine on beta-cell lipoapoptosis. J Endocrinol Invest. 2011; 34: 124–130. [DOI] [PubMed] [Google Scholar]
- 18. Pang B,, Zhao LH,, Zhou Q,, et al. Application of berberine on treating type 2 diabetes mellitus. Internat J Endocrinol. 2015; 2015: 905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y,, Li X,, Zou D,, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008; 93: 2559–2565. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H,, Wei J,, Xue R,, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010; 59: 285–292. [DOI] [PubMed] [Google Scholar]
- 21. Liu W,, Liu P,, Tao S,, et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch Biochem Biophys. 2008; 475: 128–134. [DOI] [PubMed] [Google Scholar]
- 22. Wang LH,, Yu CH,, Fu Y,, Li Q,, Sun YQ. Berberine elicits anti-arrhythmic effects via IK1/Kir2.1 in the rat type 2 diabetic myocardial infarction model. Phytother Res. 2011; 25: 33–37. [DOI] [PubMed] [Google Scholar]
- 23. Sun SF,, Zhao TT,, Zhang HJ,, et al. Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2015; 42: 662–670. [DOI] [PubMed] [Google Scholar]
- 24. Cui HS,, Hayasaka S,, Zhang XY,, Hayasaka Y,, Chi ZL,, Zheng LS. Effect of berberine on barrier function in a human retinal pigment epithelial cell line. Jpn J Ophthalmol. 2007; 51: 64–67. [DOI] [PubMed] [Google Scholar]
- 25. Wang Q,, Qi J,, Hu R,, Chen Y,, Kijlstra A,, Yang P. Effect of berberine on proinflammatory cytokine production by ARPE-19 cells following stimulation with tumor necrosis factor-alpha. Invest Ophthalmol Vis Sci. 2012; 53: 2395–2402. [DOI] [PubMed] [Google Scholar]
- 26. Zhou JY,, Zhou SW. Effect of berberine on PPARalpha/delta/gamma expression in type 2 diabetic rat retinae. Yao Xue Xue Bao. 2007; 42: 1243–1249. [PubMed] [Google Scholar]
- 27. Song D,, Song J,, Wang C,, Li Y,, Dunaief JL. Berberine protects against light-induced photoreceptor degeneration in the mouse retina. Exp Eye Res. 2015; 145: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tian P,, Ge H,, Liu H,, et al. Leukocytes from diabetic patients kill retinal endothelial cells: effects of berberine. Mol Vis. 2013; 19: 2092–2105. [PMC free article] [PubMed] [Google Scholar]
- 29. Jenkins AJ,, Velarde V,, Klein RL,, et al. Native and modified LDL activate extracellular signal-regulated kinases in mesangial cells. Diabetes. 2000; 49: 2160–2169. [DOI] [PubMed] [Google Scholar]
- 30. Ogata N,, Nishikawa M,, Nishimura T,, Mitsuma Y,, Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002; 134: 348–353. [DOI] [PubMed] [Google Scholar]
- 31. Duh EJ,, Yang HS,, Haller JA,, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol. 2004; 137: 668–674. [DOI] [PubMed] [Google Scholar]
- 32. Lee YS,, Kim WS,, Kim KH,, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006; 55: 2256–2264. [DOI] [PubMed] [Google Scholar]
- 33. Wang Q,, Liang B,, Shirwany NA,, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS One. 2011; 6: e17234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang LQ,, Ni WJ,, Cai M,, Ding HH,, Liu S,, Zhang ST. The renoprotective effects of berberine and its potential impact on the expression of beta-arrestins and ICAM-1/VCAM-1 in streptozocin induced-diabetic nephropathy rats [ published online ahead of print November 30, 2015]. J. Diabetes. doi: http://dx.doi.org/10.1111/1753-0407.12349. [DOI] [PubMed]
- 35. Zhao L,, Sun LN,, Nie HB,, Wang XL,, Guan GJ. Berberine improves kidney function in diabetic mice via AMPK activation. PLoS One. 2014; 9: e113398. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Dong SF,, Hong Y,, Liu M,, et al. Berberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic rats. Eur J Pharmacol. 2011; 660: 368–374. [DOI] [PubMed] [Google Scholar]
- 37. Hsu YY,, Tseng YT,, Lo YC. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol App Pharmacol. 2013; 272: 787–796. [DOI] [PubMed] [Google Scholar]
- 38. Wang N,, Feng Y,, Zhu M,, et al. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem. 2010; 111: 1426–1436. [DOI] [PubMed] [Google Scholar]
- 39. Chueh WH,, Lin JY. Berberine an isoquinoline alkaloid, inhibits streptozotocin-induced apoptosis in mouse pancreatic islets through down-regulating Bax/Bcl-2 gene expression ratio. Food Chem. 2012; 132: 252–260. [DOI] [PubMed] [Google Scholar]
- 40. Cheng F,, Wang Y,, Li J,, et al. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int J Cardiol. 2013; 167: 936–942. [DOI] [PubMed] [Google Scholar]
- 41. Sarna LK,, Wu N,, Hwang SY,, Siow YL, O K. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can J Physiol Pharmacol. 2010; 88: 369–378. [DOI] [PubMed] [Google Scholar]
- 42. Kim M,, Shin MS,, Lee JM,, et al. Inhibitory effects of isoquinoline alkaloid berberine on ischemia-induced apoptosis via activation of phosphoinositide 3-kinase/protein kinase B signaling pathway. Internat Neurology J. 2014; 18: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moghaddam HK,, Baluchnejadmojarad T,, Roghani M,, et al. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol Neurobiol. 2014; 49: 820–826. [DOI] [PubMed] [Google Scholar]
- 44. Hamsa TP,, Kuttan G. Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem Toxicol. 2012; 35: 57–70. [DOI] [PubMed] [Google Scholar]
- 45. Fu L,, Chen W,, Guo W,, et al. Berberine targets AP-2/hTERT, NF-kappaB/COX-2, HIF-1alpha/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One. 2013; 8: e69240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang J,, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011; 30: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zong H,, Ward M,, Madden A,, et al. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia. 2010; 53: 2656–2666. [DOI] [PubMed] [Google Scholar]
- 48. Wang J,, Xu X,, Elliott MH,, Zhu M,, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010; 59: 2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeong HW,, Hsu KC,, Lee JW,, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009; 296: E955–E964. [DOI] [PubMed] [Google Scholar]
- 50. Hu JP,, Nishishita K,, Sakai E,, et al. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-kappaB and Akt pathways. Eur J Pharmacol. 2008; 580: 70–79. [DOI] [PubMed] [Google Scholar]
- 51. Kuo CL,, Chi CW,, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004; 203: 127–137. [DOI] [PubMed] [Google Scholar]
- 52. O'Neill LA,, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013; 493: 346–355. [DOI] [PubMed] [Google Scholar]
- 53. Li Z,, Geng YN,, Jiang JD,, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Altern Med. 2014; 2014: 289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coughlan KA,, Valentine RJ,, Ruderman NB,, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014; 7: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dugan LL,, You YH,, Ali SS,, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013; 123: 4888–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kubota S,, Ozawa Y,, Kurihara T,, et al. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest Ophthalmol Vis Sci. 2011; 52: 9142–9148. [DOI] [PubMed] [Google Scholar]
- 57. Cui G,, Qin X,, Zhang Y,, Gong Z,, Ge B,, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009; 284: 28420–28429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.