Significance
Rheumatoid arthritis (RA) is a chronic, prevalent, and disabling autoimmune disease that occurs when inflammation damages joints. Recent advances in neuroscience and immunology have mapped neural circuits that regulate the onset and resolution of inflammation. In one circuit, termed “the inflammatory reflex,” action potentials transmitted in the vagus nerve inhibit the production of tumor necrosis factor (TNF), an inflammatory molecule that is a major therapeutic target in RA. Although studied in animal models of arthritis and other inflammatory diseases, whether electrical stimulation of the vagus nerve can inhibit TNF production in humans has remained unknown. The positive mechanistic results reported here extend the preclinical data to the clinic and reveal that vagus nerve stimulation inhibits TNF and attenuates disease severity in RA patients.
Keywords: vagus nerve, rheumatoid arthritis, inflammatory reflex, tumor necrosis factor, cytokines
Abstract
Rheumatoid arthritis (RA) is a heterogeneous, prevalent, chronic autoimmune disease characterized by painful swollen joints and significant disabilities. Symptomatic relief can be achieved in up to 50% of patients using biological agents that inhibit tumor necrosis factor (TNF) or other mechanisms of action, but there are no universally effective therapies. Recent advances in basic and preclinical science reveal that reflex neural circuits inhibit the production of cytokines and inflammation in animal models. One well-characterized cytokine-inhibiting mechanism, termed the “inflammatory reflex,” is dependent upon vagus nerve signals that inhibit cytokine production and attenuate experimental arthritis severity in mice and rats. It previously was unknown whether directly stimulating the inflammatory reflex in humans inhibits TNF production. Here we show that an implantable vagus nerve-stimulating device in epilepsy patients inhibits peripheral blood production of TNF, IL-1β, and IL-6. Vagus nerve stimulation (up to four times daily) in RA patients significantly inhibited TNF production for up to 84 d. Moreover, RA disease severity, as measured by standardized clinical composite scores, improved significantly. Together, these results establish that vagus nerve stimulation targeting the inflammatory reflex modulates TNF production and reduces inflammation in humans. These findings suggest that it is possible to use mechanism-based neuromodulating devices in the experimental therapy of RA and possibly other autoimmune and autoinflammatory diseases.
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovial inflammation in the musculoskeletal joints resulting in cartilage degradation and bone destruction with consequent disability (1). The prevalence exceeds 1.3 million adult cases in the United States, with attributable medical costs estimated between $19–39 billion (2, 3). Standard therapies include glucocorticoids, methotrexate, monoclonal antibodies, and other pharmacological agents targeting inflammatory mechanisms (4). Despite these treatment options, many RA patients fail to respond, instead persisting with poor health, shortened life span, and significant impairments in quality of life affecting work, leisure, and social functions (5, 6). Thus, there remains a significant need for alternative therapeutic approaches.
Recent advances at the intersection of immunology and neuroscience reveal reflex neural circuit mechanisms regulating innate and adaptive immunity (7, 8). One well-characterized reflex circuit, termed the “inflammatory reflex,” is defined by signals that travel in the vagus nerve to inhibit monocyte and macrophage production of tumor necrosis factor (TNF) and other cytokines (7). Electrical stimulation of the vagus nerve in animals (e.g., mouse, rat, and dog) stimulates choline acetyltransferase-positive T cells to secrete acetylcholine in spleen and other tissues (9). Acetylcholine is the cognate ligand for α-7 nicotinic acetylcholine receptors (α7nAChR) expressed on cytokine-producing monocytes, macrophages, and stromal cells (7, 10, 11). Ligand binding inhibits the nuclear translocation of NF-κB and inhibits inflammasome activation in macrophages activated by exposure to lipopolysaccharide (LPS), other Toll-like receptor (TLR) ligands, and other proinflammatory stimulating factors (12, 13).
Inflammatory reflex signaling, which is enhanced by electrically stimulating the vagus nerve, significantly reduces cytokine production and attenuates disease severity in experimental models of endotoxemia, sepsis, colitis, and other preclinical animal models of inflammatory syndromes (7, 8, 14–16). In experimental collagen-induced arthritis, vagotomy or selective disruption of α7nAChR worsened disease severity, and administration of nicotine or other selective α7nAChR agonists, ameliorated disease severity (17, 18). Vagus nerve stimulation delivered once daily for 60 s with an implanted device attenuated joint swelling, inhibited cytokine production, and conferred significant protection against synovitis and periarticular bone erosions (19, 20). Accordingly, we reasoned that it might be possible to modulate cytokine levels and inflammation using an active implantable medical device in humans (20).
Vagus nerve-stimulating devices have been used for decades in patients with refractory epilepsy and have been used more recently in patients with depression. These devices have been implanted in more than 100,000 patients, are relatively well tolerated, and have not been associated with immunosuppression or long-term complications (21, 22). We implanted a cohort of epilepsy patients with a vagus nerve-stimulating device and observed that transient delivery of electrical current during general anesthesia significantly inhibited TNF production in peripheral blood monocytes. A subsequent study of 17 RA patients in an 84-d open-label trial also revealed significantly decreased TNF production and significantly improved clinical signs and symptoms of disease.
Results
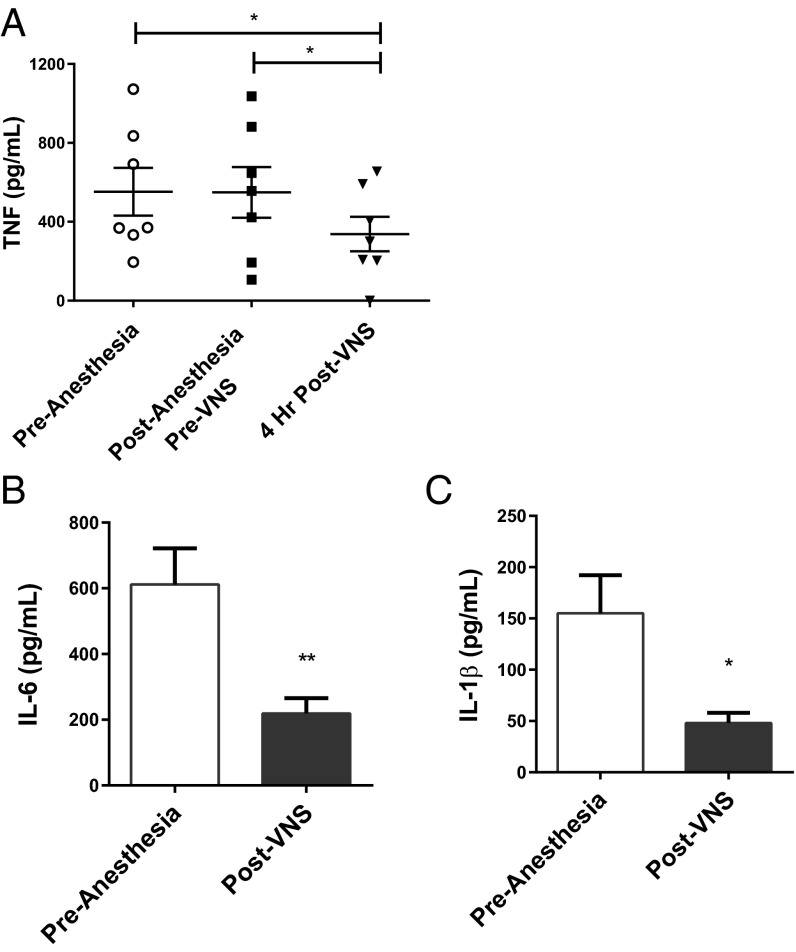
To determine whether vagus nerve stimulation inhibits TNF production in humans, we studied seven epilepsy patients [five male, two female; mean age 35 y (range 25–43 y)] who were implanted with a vagus nerve-stimulating device using a coiled cuff electrode (Cyberonics) on the left cervical vagus nerve. These patients had no history of inflammatory or autoimmune disorders. Peripheral blood was collected before, during, and after vagus nerve stimulator implantation surgery. Endotoxin was added to the whole blood to stimulate the production of TNF by monocytes for 4 h (13, 23). The application of current-controlled electrical pulses (single 30-s stimulation at 1.0-mA output current, 20-Hz pulse frequency, 500-μs pulse duration) significantly inhibited whole-blood TNF production compared with baseline levels before electrical stimulation (Fig. 1A). The inhibition of TNF release following vagus nerve stimulation during general anesthesia cannot be attributed to a placebo effect, because the subjects were unconscious and were not aware of the nerve stimulation. Whole-blood production of interleukin (IL)-6 and IL-1β was also inhibited significantly by vagus nerve stimulation (Fig. 1 B and C). To our knowledge, this is the first report that the delivery of electric current applied directly on the cervical vagus nerve to stimulate the inflammatory reflex inhibits the endotoxin-induced release of TNF, IL-1β, and IL-6 in humans.
Fig. 1.
Inflammatory reflex activation reduces whole-blood LPS-induced TNF production in epilepsy patients. Electrical stimulation of the vagus nerve in humans inhibits whole-blood LPS-induced TNF release. Blood was obtained from epilepsy patients (n = 7) undergoing implantation of a vagus nerve-stimulation device at different time points: before anesthesia induction and before vagus nerve stimulation; after anesthesia induction and before vagus nerve stimulation (pre-VNS); and 4 h after vagus nerve stimulation (post-VNS). Whole blood was incubated with LPS and TNF (A), IL-6 (B), and IL-1β (C) levels in plasma were determined after 4 h in culture. The significance of the differences between mean values at each time point was tested by unpaired ANOVA (*P < 0.05, **P < 0.01). Data are shown as mean ± SEM.
We next studied the effects of vagus nerve stimulation in patients with RA. At enrollment the 18 study patients had active disease, with at least four tender and four swollen joints (of a 28-joint count), despite methotrexate therapy for at least 3 mo on a stable dose. One patient from cohort I, who fulfilled the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification for RA, was later diagnosed with Whipple disease and was excluded from the efficacy analysis. This patient is included in the baseline patient characteristics (Table 1) and adverse-event data (Table S1). The RA patients with active disease were studied in two cohorts. Cohort I (n = 7) included patients with active disease despite therapy with methotrexate. They had never received a biological TNF antagonist or had previously failed treatment with TNF antagonists because of drug toxicity. Cohort II (n = 10) included patients who had failed conventional therapy with methotrexate and also had failed treatment with at least two biological agents differing in mechanisms of action (e.g., anti-TNF, anti–IL-6 receptor, anti-CD20 antibodies, and/or T-cell costimulation inhibitor). There were no deaths, serious adverse events, withdrawals from the study because of adverse events, or infections in either cohort. In agreement with known risks of the procedure, nine patients experienced mild or moderate adverse events associated with implanting the vagus nerve stimulator on the left cervical vagus nerve (Table S1).
Table 1.
RA patient baseline demographics, medication history, and disease severity
| Demographics | Cohort I | Cohort II | Combined |
| Total, n | 8 | 10 | 18 |
| Enrollment by country | |||
| Bosnia | 3 | 0 | 3 |
| Croatia | 2 | 0 | 2 |
| The Netherlands | 3 | 10 | 13 |
| Mean age in years (range) | 55 (36–69) | 48 (36–56) | 51 (36–69) |
| Sex, % female | 50 | 100 | 78 |
| Ethnicity, % Caucasian | 88 | 100 | 94 |
| Mean no. of years since RA diagnosis (SD) | 9.9 (5.7) | 11.8 (6.3) | 11.0 (5.9) |
| No. rheumatoid factor-positive patients (%) | 7 (88) | 5 (50) | 12 (67) |
| No. anti-citrullinated peptide Ab+ patients (%) | 6 (75) | 6 (60) | 12 (67) |
| No. patients receiving prior nonbiologic disease-modifying antirheumatic drugs (%) | |||
| 0 drugs | 1 (13) | 1 (10) | 2 (11) |
| 1 drug | 2 (25) | 3 (30) | 5 (28) |
| 2 drugs | 2 (25) | 2 (20) | 4 (22) |
| 3 or more drugs | 3 (37) | 4 (40) | 7 (39) |
| No. patients receiving prior biologic disease-modifying antirheumatic drugs (%) | |||
| 0 drugs | 3 (38) | 0 | 3 (17) |
| 1 drug | 4 (50) | 0 | 4 (22) |
| 2 drugs | 1 (12) | 0 | 1 (6) |
| 3 drugs | 0 | 3 (30) | 3 (17) |
| 4 drugs | 0 | 4 (40) | 4 (22) |
| 5 drugs | 0 | 2 (20) | 2 (11) |
| 6 drugs | 0 | 1 (10) | 1 (6) |
| DAS28-CRP (SD) | 6.05 (0.87) | 5.94 (0.72) | 5.99 (0.77) |
| High-sensitivity CRP, mg/L (SD) | 17.5 (10.0) | 17.5 (18.5) | 17.5 (14.9) |
Table S1.
Adverse events in RA patients
| Adverse event* | n (%) |
| No. of patients with any adverse event† | 16 (89) |
| Fatigue | 7 (39) |
| Dysphonia | 6 (33) |
| Hypoesthesia | 5 (28) |
| Influenza-like illness | 4 (22) |
| Dizziness | 3 (17) |
| Nasopharyngitis | 3 (17) |
| Nausea | 3 (17) |
| Constipation | 2 (11) |
| Dyspnea | 2 (11) |
| Headache | 2 (11) |
| No. of patients with any implantation procedure-related event‡ | 9 (50) |
| Dysphonia | 5 (28) |
| Hypoesthesia | 4 (22) |
| Dyspnea | 2 (11) |
| Paresthesia | 2 (11) |
| Bradycardia | 1 (6) |
| Constipation | 1 (6) |
| Dry throat | 1 (6) |
| Eructation | 1 (6) |
| Nausea | 1 (6) |
| Oropharyngeal pain | 1 (6) |
| Postprocedural pain | 1 (6) |
An individual patient may have had more than one event.
Events occurring in at least 10% of the combined population.
Events occurring in at least one patient. Implantation procedure-related events are included in the any adverse event totals.
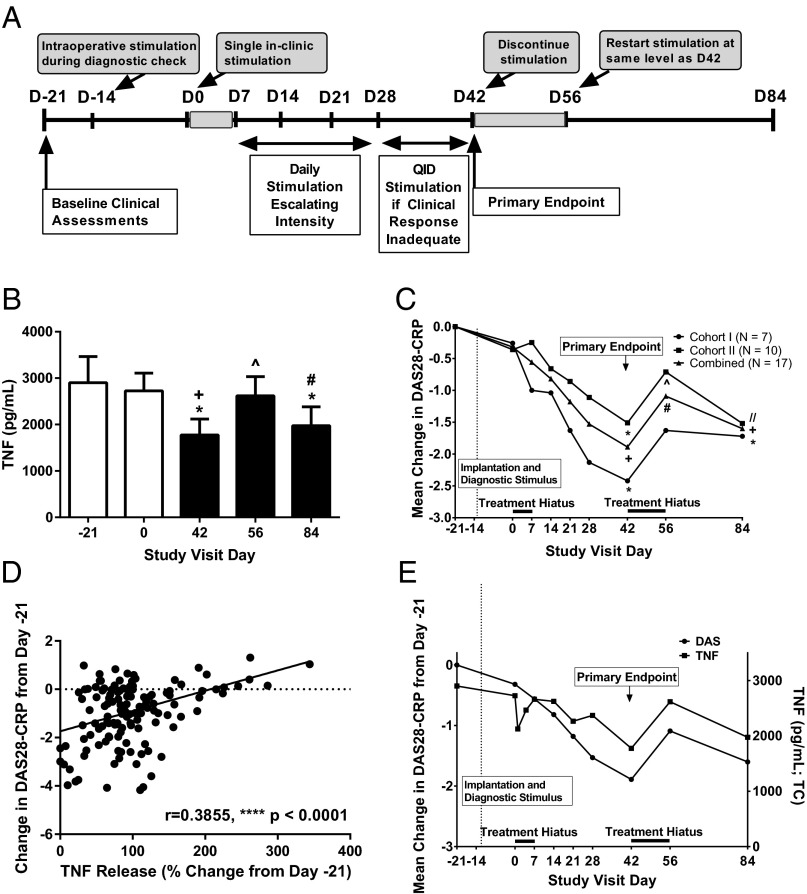
The study design schematic is shown in Fig. 2A. The vagus nerve was stimulated during surgery (day −14) to measure electrode impedance and to verify device function. During the 14-d postoperative recovery period (day −14 to day 0), the device was turned off, and no current was delivered to the vagus nerve. On the first treatment day (study day 0), patients received a single 60-s stimulation with electric current pulses of 250-μs duration at 10 Hz and an output current between 0.25–2.0 mA, as tolerated. No further stimulation was delivered for 7 d. On study day 7, the output current was adjusted to the highest amperage tolerated, up to 2.0 mA; this level of current was subsequently delivered once daily for 60 s in 250-μs pulse widths at 10 Hz. Current escalation up to the highest tolerated amperage (up to 2.0 mA) was repeated weekly until day 28. At that visit the frequency of daily stimulation events was increased to four times daily in patients who had not achieved a moderate or good clinical response according EULAR criteria (24). On day 28, the output current delivered was comparable in both cohorts: cohort I output current was 1.29 ± 0.37 mA (mean ± SD); cohort II output current was 1.60 ± 0.36 mA. In cohort I, two of seven patients received electric current pulses four times daily. In cohort II, 6/10 patients received electric current pulses four times daily.
Fig. 2.
The effects of inflammatory reflex activation on whole-blood LPS-induced TNF production and disease activity in RA patients. (A) Schematic of the RA study design. D −21 to D84 indicate study visit days. The stimulation schedule and timing of assessments are shown. (B) Mean LPS-induced TNF production in the combined RA cohort (n = 17) at study days −21, 0, 42, 56, and 84; visit means are designated by bars, and error bars indicate SEM. Differences in means were tested for significance by paired t test: *P < 0.05 vs. d −21; +P < 0.01 vs. d 0; ^P < 0.01 vs. d 42; #P < 0.01 vs. d 56. (C) The mean change in DAS2 8-CRP from baseline by study visit day for cohort I (patients failing methotrexate treatment), cohort II (patients failing treatment by multiple biologic agents), and combined cohorts. The significance of the mean change by paired t test between visits is shown: *P < 0.05 vs. d −21; //P < 0.01 vs. d −21; +P < 0.001 vs. d −21; #P < 0.001 vs. d 42; ^P < 0.05 vs. d 42). (D) Linear regression analysis comparing the changes in the DAS28-CRP and the percent change in TNF release from study day −21 measured at each individual visit for each patient in the combined cohort. Changes in the DAS28-CRP and TNF release are significantly correlated by Pearson’s test (r = 0.384, P < 0.0001). (E) Mean change in the DAS28-CRP and mean LPS-induced TNF release over time by study visit day. Changes in the DAS28-CRP and TNF release follow a similar temporal pattern in response to initial simulation, stimulation withdrawal, and stimulation reinitiation.
We observed that TNF production in cultured peripheral blood obtained from the combined RA study cohort on day 42 was significantly reduced from baseline day −21 (TNF = 2,900 ± 566 pg/mL on day −21 vs. 1,776 ± 342 pg/mL on day 42, P < 0.05) (Fig. 2B). On day 42 the vagus nerve stimulator was turned off. After a 14-d hiatus, it was restarted on day 56, and patients were followed through day 84. After the vagus nerve stimulator was turned off, TNF production increased significantly by day 56; when the stimulator was turned on again, TNF production again decreased significantly by day 84 (1,776 ± 342 pg/mL on day 42 vs. 2,617 ± 342 pg/mL on day 56 and 1,975 ± 407 pg/mL on day 84, P < 0.01 for both). This finding indicates that active electrical stimulation of the vagus nerve inhibits TNF production in patients with RA.
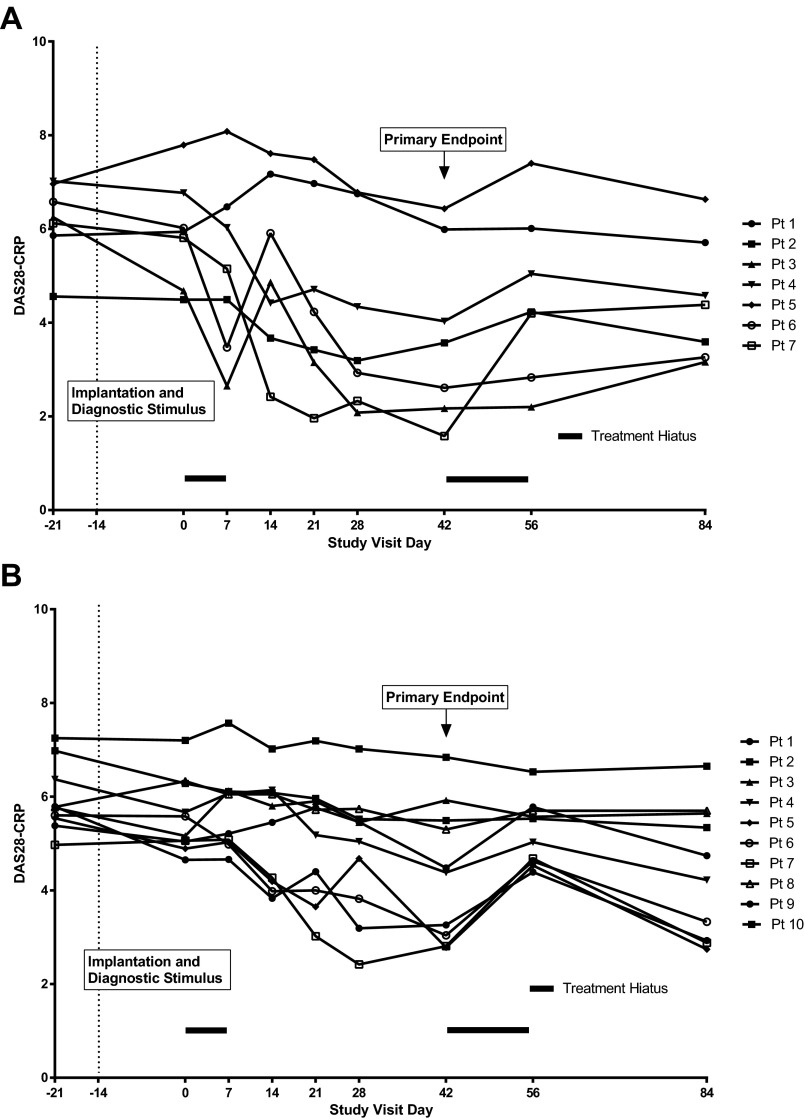
RA signs and symptoms are measured using a standard disease activity composite score [the 28-joint C-reactive protein (CRP)-based disease activity score, DAS28-CRP] derived from counting swollen joints and tender joints, a patient-defined visual analog score of disease activity, and serum CRP levels (25). We observed that DAS28-CRP values at day 42 were significantly improved (i.e., lower) from baseline day −21 in the combined cohorts (DAS28-CRP = 6.05 ± 0.18 on day −21 vs. 4.16 ± 0.39 on day 42, P < 0.001), when the device was delivering current (Fig. 2C). Within days after receiving electrical stimulation of the vagus nerve, the DAS28-CRP improved significantly in some patients (Fig. S1). When the device was turned off (at day 42), the DAS28-CRP increased significantly within 14 d (4.16 ± 0.39 on day 42 vs. 4.96 ± 0.31 on day 56, P = 0.001). Restarting the device (day 56) significantly reduced the DAS28-CRP (Fig. 2C). Linear regression analysis comparing the mean change in the DAS28-CRP and the percentage change in TNF release from baseline day −21 to day 42 revealed a highly significant correlation (r = 0.384, P < 0.0001) (Fig. 2D). The temporal pattern of TNF production in the combined cohort correlated with the DAS28-CRP (Fig. 2E).
Fig. S1.
Individual RA patient DAS28-CRP. Individual patient DAS28-CRP over time are shown for cohorts I (7 patients) (A) and II (10 patients) (B).
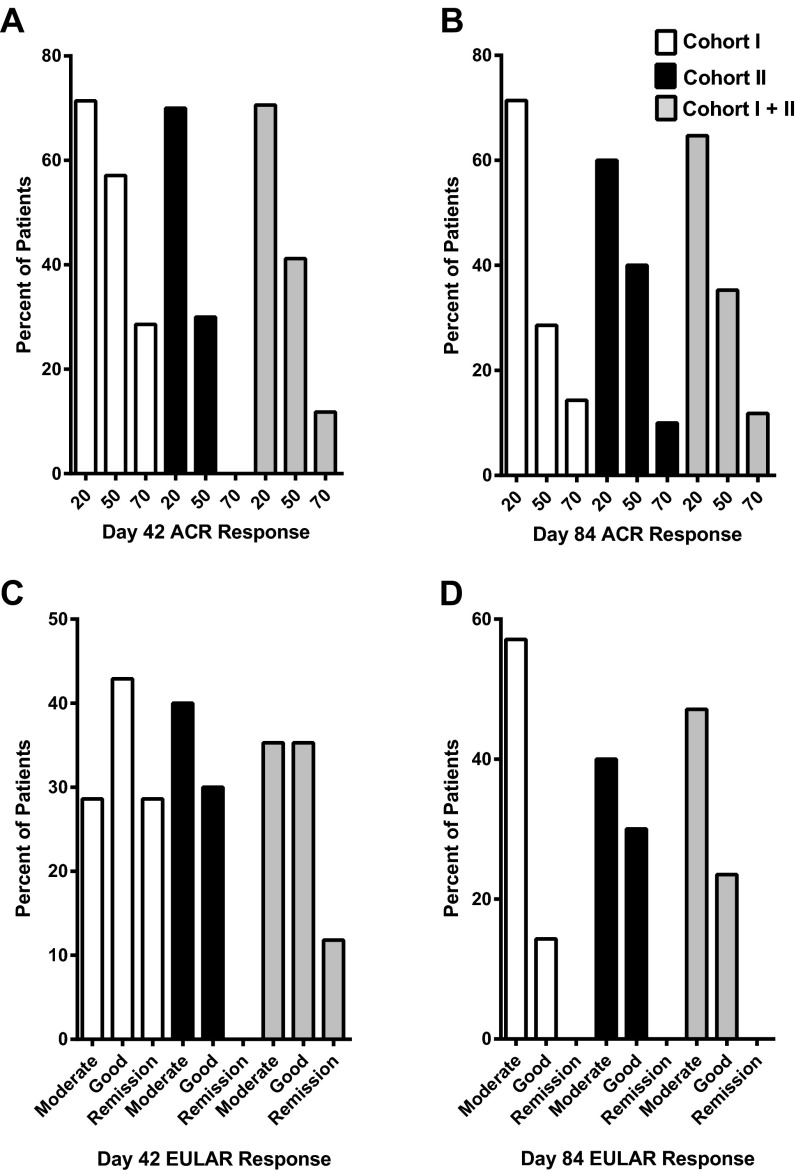
We assessed the fraction of patients who improved from baseline to achieve ACR 20%, 50%, and 70% clinical responses and also the number of patients who improved from baseline sufficiently to meet the definition for EULAR response and remission. The ACR response is defined as the percentage improvement in disease activity between two time points (ACR20 is ≥20%, ACR 50 is ≥50%, and ACR70 is ≥70% improvement). The EULAR response depends on the change in the DAS28-CRP and the absolute level achieved after treatment (24). As shown in Fig. S2, the ACR and EULAR response criteria were fulfilled in a large subset of patients in both cohorts. At the primary endpoint (day 42) the percentages of patients fulfilling the ACR response criteria for 20%, 50%, and 70% improvement were 71.4%, 57.1%, and 28.6%, respectively, for cohort I and were 70.0%, 30.0%, and 0.0%, respectively, for cohort II. The percentages of patients achieving DAS28 remission (DAS28-CRP <2.6) on day 42 in cohorts I and II were 28.6% and 0.0%, respectively. Improvement was observed in all constituent components of the composite end points (tender joint count, swollen joint count, patient’s assessment of pain, patient’s global assessment, physician’s global assessment, and CRP) (Table S2). Together, these data indicate that vagus nerve stimulation inhibits TNF and significantly attenuates RA disease severity.
Fig. S2.
Clinical response and remission rates of RA patients with respect to screening day −21. (A and B) The ACR20, ACR50, and ACR70 response rates in cohort I, cohort II, and the combined cohorts are shown at study day 42 (A), and study day 84 (B). (C and D) The rates of EULAR moderate and good response and remission in cohorts I and II and the combined cohorts are shown at study day 42 (C) and study day 84 (D). The patient with Whipple disease excluded from efficacy analysis had an ACR20 response and a good EULAR response on day 42 and no ACR response and a moderate EULAR response on day 84.
Table S2.
Individual disease component scores and DAS28-CRP in RA patients
| Cohort | Day −21 | Day 0 | Day 42 | Day 56 | Day 84 |
| Cohort I, n = 7 | |||||
| Tender joint count, range 0–28 | 20.0 (8.4) | 16.7 (8.2) | 6.0 (7.6) | 8.4 (9.1) | 7.0 (6.8) |
| Swollen joint count, range 0–28 | 14.0 (6.6) | 14.7 (7.0) | 5.4 (5.9) | 8.7 (7.0) | 6.1 (5.2) |
| Patient’s assessment of pain, VAS 100 mm | 69.1 (17.2) | 55.1 (29.2) | 27.1 (22.9) | 48.7 (26.7) | 36.3 (17.8) |
| Patient’s global assessment of disease activity, VAS 100 mm | 58.1 (17.5) | 50.6 (25.8) | 29.7 (22.0) | 44.3 (25.9) | 35.3 (18.6) |
| Physician’s global assessment of disease activity, VAS 100 mm | 70.7 (13.4) | 58.1 (19.0) | 29.1 (18.0) | 46.9 (27.9) | 36.1 (21.7) |
| CRP, mg/L | 15.7 (9.3) | 21.5 (16.2) | 13.7 (15.6) | 12.3 (8.3) | 12.2 (14.2) |
| HAQ-DI, range 0–3 | 1.857 (0.891) | 1.750 (0.800) | 1.196 (0.935) | 1.321 (0.889) | 1.321 (0.946) |
| DAS28-CRP | 6.19 (0.84) | 5.39 (1.14) | 3.77 (1.86) | 4.56 (1.79) | 4.47 (1.30) |
| Cohort II, n = 10 | |||||
| Tender joint count, range 0–28 | 15.9 (4.7) | 15 (4.6) | 9.3 (6.7) | 12.8 (5.9) | 9.4 (6.5) |
| Swollen joint count, range 0–28 | 8.9 (3.8) | 8.0 (4.0) | 4.4 (4.6) | 5.7 (3.5) | 4.4 (4.0) |
| Patient’s assessment of pain, VAS 100 mm | 72 (12.4) | 62.8 (14.6) | 38.2 (26.6) | 59.3 (19.1) | 36.8 (24.4) |
| Patient’s global assessment of disease activity, VAS 100 mm | 75.4 (11.2) | 64.2 (13.8) | 39.4 (26.2) | 62.2 (19.1) | 40.5 (24.3) |
| Physician’s global assessment of disease activity, VAS 100 mm | 66.8 (7.6) | 65.9 (13.0) | 34.2 (18.1) | 53.6 (13.7) | 37.7 (19.1) |
| CRP, mg/L | 17.5 (18.5) | 15.2 (20.5) | 12.6 (13.2) | 13.3 (13.1) | 12.4 (14.9) |
| HAQ-DI, range 0–3 | 1.888 (0.406) | 1.988 (0.498) | 1.613 (0.532) | 1.763 (0.484) | 1.588 (0.500) |
| DAS28-CRP | 5.94 (0.72) | 5.59 (0.80) | 4.43 (1.44) | 5.24 (0.70) | 4.42 (1.40) |
| Combined, n = 17 | |||||
| Tender joint count, range (0–28 | 17.6 (6.6) | 15.7 (6.2) | 7.9 (7.1) | 11.0 (7.5) | 8.4 (6.5) |
| Swollen joint count, range 0–28 | 11.0 (5.6) | 10.8 (6.3) | 4.8 (5.0) | 6.9 (5.2) | 5.1 (4.4) |
| Patient’s assessment of pain, VAS 100 mm | 70.8 (14.2) | 59.6 (21.3) | 33.6 (25.0) | 54.9 (22.4) | 36.6 (21.3) |
| Patient’s global assessment of disease activity, VAS 100 mm | 68.3 (16.2) | 58.6 (20.1) | 35.4 (24.3) | 54.8 (23.2) | 38.4 (21.7) |
| Physician’s global assessment of disease activity, VAS, 100 mm | 68.4 (10.2) | 62.7 (15.7) | 32.1 (17.7) | 50.8 (202) | 37.1 (19.5) |
| CRP, mg/L | 16.8 (15.0) | 17.8 (18.6) | 13.1 (13.7) | 12.9 (11.1) | 16.1 (14.8) |
| HAQ-DI, range 0–3 | 1.875 (0.625) | 1.890 (0.628) | 1.441 (0.729) | 1.581 (0.692) | 1.478 (0.703) |
| DAS28-CRP | 6.05 (0.75) | 5.73 (0.94) | 4.16 (1.60) | 4.96 (1.26) | 4.44 (1.32) |
All values are mean (SD). HAQ-DI, Health Assessment Questionnaire Disability Index; VAS 100 mm, Visual Analog Scale 100 mm in length.
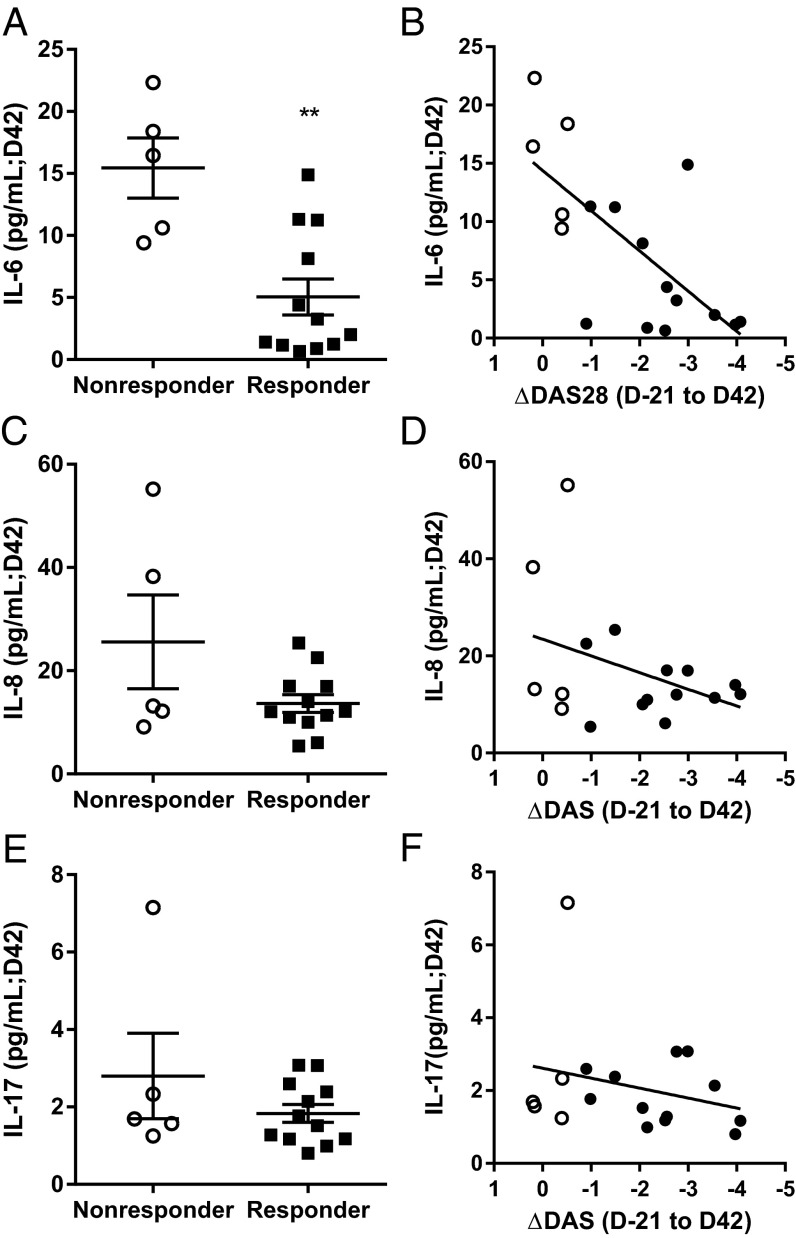
We measured a panel of serum cytokines to assess further the mechanisms of this experimental therapeutic intervention. Most, including serum TNF, IL-10, IL-12p70, IL-13, IL-1α, IL-1β, IL-2, IL-4, IL-5, and TNF-β, were below 1 pg/mL (unreliable limits of detection). Serum IL-6 levels in subjects who improved by EULAR criteria were significantly decreased compared with subjects who failed to improve: IL-6 levels were 15.4 ± 2.4 pg/mL in nonresponders (n = 5) vs. 5.0 ± 1.4 pg/mL in responders (n = 12) (P = 0.001) (Fig. 3A). Decreased IL-6 levels in the patients who responded to therapy correlated with improvement in disease severity between day −21 and day 42 (r = 0.707, P = 0.002) (Fig. 3B). The IL-6 responses are specific, because IL-8 and IL-17 levels did not change significantly [IL-8: 25.6 ± 9.1 pg/mL in nonresponders (n = 5) vs. 13.7 ± 1.7 pg/mL in responders (n = 12), P = 0.29 (Fig. 3C); IL-17: 2.8 ± 1.1 pg/mL in nonresponders (n = 5) vs. 1.8 ± 0.2 pg/mL in responders (n = 12), P = 0.18 (Fig. 3E)] and did not correlate to clinical response (Fig. 3 D and F).
Fig. 3.
Modulation of serum cytokines. Serum from each patient in the combined cohort was analyzed for multiple analytes at day 42. (A, C, and E) Individual patient values for EULAR nonresponders and responders are shown for IL-6 (A), IL-8 (C), and IL-17 (E) levels. The significance of differences between mean values at each time point was tested by unpaired t test (**P < 0.01). Horizontal bars indicate mean ± SEM. (B, D, and F) Linear regression analysis comparing analyte level at day 42 to the change in the DAS28-CRP from study day −21 to day 42. The change in the DAS28-CRP is significantly correlated to IL-6 release (r = 0.707, P = 0.002) (B) but not to IL-8 release (r = 0.261, P = 0.31) (D) or IL-17 release (r = 0.384, P = 0.07) (F).
Discussion
To our knowledge, this study is the first to assess whether stimulating the inflammatory reflex by directly implanting an electronic device modulates TNF and other cytokines in humans. Historically the development of electrically active implantable medical devices has been primarily empiric, based upon observing effects of devices that deliver electrical current to depolarize neuronal or cardiac tissue. Absent appropriate biomarkers or mechanistic understanding, it has been difficult or impossible to develop or optimize the device parameters for current delivery, physiological effect in the targeted organ system, and clinical efficacy. Direct and accessible surrogate molecular markers of disease mechanism targeted by active implantable medical devices are uncommon. The discovery of the inflammatory reflex affords a unique opportunity for developing a neuromodulating device to regulate immune cell function by targeting a neural pathway that regulates cytokine production, a surrogate marker of molecular mechanism (26).
RA patients in cohort I are in early stages of disease not responding to therapy with methotrexate. These patients are frequently candidates for subsequent therapy with a biological agent that inhibits TNF. Cohort II patients are in later stages of disease, having failed multiple biological disease-modifying antirheumatic drugs. After electrical stimulation of the vagus nerve the DAS28-CRP improved significantly in both cohorts, and withdrawal of treatment significantly worsened the severity of disease. Reactivating the device on day 56 restored significant clinical improvement. The clinical responses were accompanied by significant reductions in TNF release during periods of disease remission and significant increases in TNF release during disease exacerbation. A large body of preclinical evidence has delineated the molecular and physiological mechanisms of the inflammatory reflex modulating TNF, IL-6, HMGB1, and other cytokines (7–9, 11–20). The molecular mechanisms of cytokine inhibition implicate acetylcholine derived from TChAt cells, the subset of choline acetyltransferase-positive T cells that we identified in the inflammatory reflex (9). In future clinical trials it should be interesting to study whether TChAt cells participate in mediating anti-inflammatory reflex mechanisms.
Vagus nerve stimulation has been used to treat medically refractory epilepsy in more than 100,000 patients, and it is generally well tolerated (21, 22). The adverse events reported here were mild to moderate in severity and were comparable in type and frequency to those seen in prior studies of vagus nerve stimulation therapy in epilepsy patients. These adverse events included transient hoarseness, postoperative hoarseness from neuropraxis, and transient intraoperative bradycardia during surgery. None of the patients developed infection. Larger clinical trials can be designed to determine the risk/benefit ratio for implantable electronic devices compared with the toxicity and side effects of pharmacological and targeted therapies for RA.
The electrical stimulation parameters used in this study were previously established to stimulate the inflammatory reflex in preclinical studies and differ significantly from the stimulation protocols used in epilepsy (19, 27). Here, electrical current (up to 2.0 mA) was delivered to the cervical vagus nerve for 60 s one to four times daily; the maximum time of electrical current flow for any patient in this study was 4 min daily. This stimulation protocol differs significantly from the protocols for treating epilepsy, in which current (up to 2.25 mA) is delivered at 60-s intervals, followed by an OFF interval of 5–180 min, repeated continuously. Thus, epilepsy patients may receive electrical current delivery for up to 240 min daily. Preclinical studies have established that stimulation of the inflammatory reflex for as little as 60 s confers significant inhibition of cytokine production for up to 24 h. The present study was not designed or powered to evaluate the relationship between specific electrical current dose–response and clinical outcomes or the longer-term durability of therapeutic benefit, and the effects of under- or overstimulation of the inflammatory reflex are also an important area for future study.
The primary objective of this study was to determine whether activating the inflammatory reflex with an implanted electronic device inhibits cytokine production in humans. It is reasonable to consider whether placebo mechanisms contribute to these findings, because some patients are aware when the device is delivering current. There are several arguments against a placebo effect explaining the observed inhibition of TNF and IL-6 and the significant clinical improvements. First, we observed that intraoperative vagus nerve stimulation significantly inhibited TNF release in epilepsy patients who were unconscious during the implantation. These patients could not be aware of the stimulation, indicating that the suppression of cytokine release immediately following vagus nerve stimulation cannot be attributed to a placebo effect. Second, we also observed that the suppression of TNF release during vagus nerve stimulation in RA patients occurred only when the device was functioning. It has been established previously that biomarkers are not modifiable by placebo effects in RA studies of this duration (28, 29). Third, we observed reduced TNF and IL-6 production and positive clinical responses in the subset of therapy-resistant patients who had failed both methotrexate therapy and treatment with multiple biologic agents with differing mechanisms of action. It has been established in prior studies that placebo response rates in drug-resistant cohorts are extremely low (ACR20 responses 5–11%). The findings here of significantly higher ACR20 responses (between 70% and 71.4%) argue strongly against a placebo effect being the mechanism. Fourth, a recent study reported clinical improvement using vagus nerve-stimulation therapy to treat another disease mediated by TNF, Crohn’s disease (30). Although the investigators in that study did not measure the activity of the inflammatory reflex or cytokine production, they did examine endoscopic biopsies and observed that vagus nerve stimulation significantly inhibited inflammation in the colonic tissues, an objective histological tissue response that cannot be attributed to placebo effects. Finally, our recent prospective observational studies indicate that impaired constitutive vagus nerve activity precedes the development of clinically manifest RA (31). Therefore, when considered together with extensive preclinical data that identify molecular and neurophysiological mechanisms, the inhibition of TNF during electrical stimulation and the significant clinical responses shown give evidence that the clinical mechanism is mediated by the inflammatory reflex.
This first-in-class study supports a conceptual framework for further studies of electronic medical devices in diseases currently treated with drugs, an approach termed “bioelectronic medicine” (32). Larger clinical trials in RA can be designed and powered to assess clinical efficacy, but our findings encourage pursuing this strategy in RA and in other cytokine-mediated autoimmune and auto-inflammatory disorders.
Materials and Methods
Study of Vagus Nerve Stimulation in Epilepsy Patients.
The study of vagus nerve stimulation in epilepsy patients was performed at the Hofstra Northwell School of Medicine and was approved by the Clinical Research Center (CRC) and the Institutional Review Board. All patients provided informed consent before participation. The study population consisted of seven epilepsy patients being implanted with a Cyberonics Vagus Nerve Stimulation System (Cyberonics) according to the manufacturer’s instructions as part of their standard care for the treatment of refractory epilepsy (Fig. S3). During the intraoperative diagnostic procedure, the pulse generator produces a 30-s stimulation at a 1.0-mA output current with pulse frequency of 20 Hz and pulse width of 500 μs. Blood samples were taken before anesthesia induction, after anesthesia induction but before intraoperative vagus nerve stimulation, and 4 h after intraoperative vagus nerve stimulation.
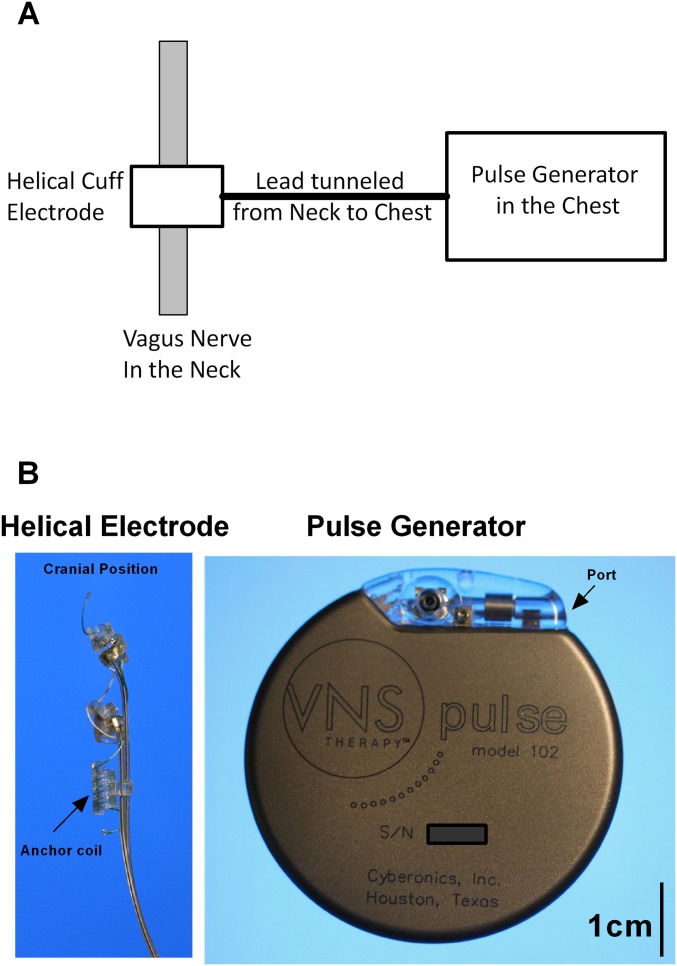
Fig. S3.
The Cyberonics vagus nerve-stimulation system. The implanted system has two components: a bipolar lead containing two helical coil electrodes and a helical anchor tether that were wrapped around the cervical vagus nerve, and the pulse generator that is placed within a subcutaneous pocket in the chest wall. (A) Schematic diagram of the system. The lead was tunneled subcutaneously from the neck to the pulse generator and inserted into the lead attachment port on the generator. (B) Images of the helical electrode and pulse generator.
The LPS-induced cytokine release assay was performed as previously described (33). Cytokine levels were analyzed using the MSD multiplex cytokine assay (Meso Scale Discovery) per the manufacturer’s instructions. TNF, IL-6, and IL-1β release across time points was analyzed using the Prism analytical software package (GraphPad).
Study of Vagus Nerve Stimulation in RA Patients.
The study of vagus nerve stimulation in RA patients was performed at one center in The Netherlands (the Academic Medical Center of the University of Amsterdam), at two centers in Bosnia and Herzegovina (the University Clinical Hospital in Mostar and Sarajevo University Clinical Center in Sarajevo), and at in one center in Croatia (Clinical Hospital Center Sestre Milosrdnice, Zagreb) and was approved by the respective national and institutional Ethics Committees. All patients provided informed consent before participation. The investigational study device was a Cyberonics Vagus Nerve Stimulation System, implanted as described above. The systems were treated as investigational study devices because of their off-label use in patients with RA. The study recruited two separate patient cohorts. Cohort I consisted of RA patients who had failed to respond to methotrexate and who were either TNF-antagonist naive or had previously failed treatment with a TNF antagonist because of safety reasons rather than lack of efficacy. Cohort II included patients who had not responded adequately to at least two biologic agents with at least two different mechanisms of action. Major inclusion and exclusion criteria are given in SI Materials and Methods. The use of prednisone at a stable daily dose of less than 10 mg and other nonbiological disease-modifying antirheumatic drugs at stable doses was allowed.
The design schematic of this single-arm study is shown in Fig. 2A. At the conclusion of the study, patients were offered the options of having the device surgically removed or left in place and inactivated or continuing treatment in a long-term extension study. All recruited subjects opted to continue in the extension study, which will be reported separately.
The primary study end point was mean change in the DAS28-CRP between visits on baseline day −21 and day 42 (25). Mean changes in the DAS28-CRP between day −21 and day 42 or day 84, and between day 42 and day 56 also were assessed for significance at P < 0.05 using a Student’s paired t test in the SAS 9.2 statistical analysis package (SAS). Because this was an exploratory study, no formal statistical power calculations were performed, and no adjustments for multiple comparisons were made. Adverse events were collected from the day of implantation through the day 84 visit, coded using the Medical Dictionary for Regulatory Activities (MedDRA), and presented by MedDRA term as subject incidence rates.
Whole-Blood Cytokine Release Assay in the RA Study.
The TruCulture system (Myriad RBM), an assay system suitable for use at clinical sites and scalable to larger studies, was used. Venous blood was drawn into tubes containing endotoxin at 100 ng/mL and was incubated at 37 °C for 24 h. Supernatant TNF was measured by ELISA (R&D Systems). Comparisons of changes in TNF release between baseline and subsequent visits [with three statistical outlier exclusions; robust regression and outlier removal (ROUT) method with the maximum false discovery rate at 1%] by paired t test and linear regression analysis of relationships between changes in the DAS28-CRP and TNF release were performed using the Prism analytical software package.
Serum Cytokines in the RA Study.
Serum cytokine levels from day 42 were analyzed using the MSD multiplex cytokine assay as above. Analysis of IL-6, IL-8, and IL-17 release on day 42 and linear regression analysis of relationships (Pearson’s test) between the change in the DAS28-CRP and serum cytokine release at day 42 were performed using the Prism analytical software package.
SI Materials and Methods
Study of Vagus Nerve Stimulation in Epilepsy Patients.
The study of vagus nerve stimulation in epilepsy patients was performed at the Hofstra Northwell Medical Center and was approved by the CRC and the Institutional Review Board. All patients provided informed consent before participation. The study population consisted of six epilepsy patients being implanted with a standard, commercially available Cyberonics Vagus Nerve Stimulation System (Cyberonics) as part of their standard care for the treatment of refractory epilepsy. The implantation procedure was performed as recommended in the manufacturer’s instructions for use, with the patient under general endotracheal anesthesia. A 2- to 3-cm horizontal left paramedian neck incision was used to expose the carotid sheath. The left vagus nerve was isolated within the sheath between the carotid artery and internal jugular vein. A short segment of perineurium was dissected, and a standard 2-mm Cyberonics vagus nerve stimulation lead with three helical coiled cuffs was placed around the vagus nerve. The lead then was tunneled subcutaneously from the neck and connected to a pulse generator placed in a subcutaneous pocket created on the chest wall, after which the recommended standard diagnostic testing procedure was performed to ensure proper system function and lead integrity and impedance. During the diagnostic procedure, the pulse generator automatically produces a 30-s stimulation at a 1.0-mA output current with pulse frequency of 20 Hz and pulse width of 500 μs. Following this diagnostic stimulation, the surgical incisions were closed, and the patient was allowed to recover. Blood samples were taken before anesthesia induction, after anesthesia induction but before intraoperative vagus nerve stimulation, and at 4 h after intraoperative vagus nerve stimulation.
The LPS-induced cytokine release assay was performed as previously described (33). Briefly, LPS was added to heparinized whole blood at a final concentration of 1 ng/mL; samples were incubated for 4 h at 37 °C, and plasma was collected by centrifugation and stored frozen at −20 °C until analysis. Cytokine levels were analyzed using the MSD multiplex cytokine assay (MSD Sector Imager 2400; Meso Scale Discovery) per the manufacturer’s instructions. Analysis of TNF, IL-6, and IL-1β release across time points was performed using the Prism analytical software package.
Study of Vagus Nerve Stimulation in RA Patients.
The study of vagus nerve stimulation in RA patients was performed at two centers in Bosnia and Herzegovina, one center in Croatia, and one center in The Netherlands as detailed in Materials and Methods in the main text. At each center the study was approved by the responsible Ethics Committee and had regulatory approval by the national Competent Authority. All patients provided informed consent before participation. The investigational study device was a standard, commercially purchased Cyberonics vagus nerve stimulation system, including a model 102 pulse generator and a model 302 lead (Cyberonics). The systems were treated as investigational study devices because of their off-label use in patients with RA. The vagus nerve-stimulation systems were implanted as described above.
The study recruited two separate patient cohorts. Cohort I consisted of patients who had not responded to methotrexate and who were either TNF antagonist naive or had previously failed treatment with a TNF antagonist because of safety reasons rather than lack of efficacy. Cohort II included patients who had not responded adequately to at least two biologic agents with at least two different mechanisms of action. Apart from the differing requirements for prior RA drug treatments, inclusion and exclusion criteria were the same for both cohorts, and both cohorts underwent identical study procedures and assessments. Other major inclusion criteria were age of 18–75 y, a diagnosis of adult-onset RA as defined by the 2010 ACR/EULAR criteria (34), a functional status of I, II, or III according to the ACR 1991 revised criteria (35), and active disease as defined by at least four tender and four swollen joints (in a 28-joint count) despite at least 3 mo of treatment with a stable dose of methotrexate (which could not be changed during the course of the study). Major exclusion criteria were related mainly to restrictions pertaining to vagus nerve stimulation and included prior vagotomy, vasovagal syncope history, pharyngeal dysfunction, preexisting vocal cord damage or dysfunction, uncontrolled asthma or obstructive lung disease, peptic ulcer disease, significant cardiac rhythm disturbances, sleep apnea, or the use of other electrically active medical devices. The use of prednisone at a stable daily dose of less than 10 mg and of other nonbiological disease-modifying antirheumatic drugs at stable doses was allowed.
The design schematic of this single-arm study is shown in Fig. 2A. Patients had screening assessments and baseline clinical and biomarker assessments at the visit on day −21 and were implanted with the vagus nerve stimulator under general endotracheal anesthesia at the visit on day −14. During the implantation procedure, before wound closure, the patients received a single stimulus as part of the standard intraoperative diagnostic testing to check system function and lead integrity and impedance, as described above. The device then was inactivated, and the patient was allowed to recover from surgery for at least 14 d. On the day 0 visit, patients had postoperative clinical assessments and were given a single 60-s active stimulation at a pulse frequency of 10 Hz, a 250-μs pulse duration, and an output current as maximally tolerated between a minimum of 0.25 mA and a maximum of 2.0 mA. The patients received no stimulation between the visits on days 0 and 7. On the days 7, 14, and 21 visits patients had clinical and biomarker assessments, and the stimulation output current to be delivered by the device was incremented as tolerated at each visit to a maximum of 2.0 mA. During each of these visits and on the intervening days patients received daily stimulations for 60 s at their tolerated output current, with a frequency of 10 Hz, and pulse width of 250 μs, using magnet actuation with the device set to deliver stimulation in magnet mode only. At the day 28 visit, if the patient had not achieved a moderate or good clinical response according to EULAR classification criteria (24), the stimulation frequency was increased from once daily to four times daily with other stimulation parameters remaining the same. The primary end point of the study was the day 42 visit, on which patients had clinical and biomarker assessments. On day 42 all subjects had their device inactivated and entered a 14-d treatment-withdrawal period. On the day 56 visit, stimulation was reinitiated at the same level and on the same schedule as the patient was receiving at the day 42 visit, and this stimulation was continued through the final study visit at day 84. At the conclusion of the study, patients were offered the options of having the device surgically removed, having the device left in place and inactivated, or continuing treatment in a long-term extension study. All recruited subjects opted to continue in the extension, which will be reported separately.
The primary study end point was mean change in the DAS28-CRP between the visits on baseline day −21 and day 42 (25). Secondary clinical end points included ACR 20%, 50%, and 70% response rate, EULAR moderate or good response rate, EULAR remission rate at day 42, change in DAS28-CRP during the treatment-withdrawal period between the visits on day 42 and day 56; and DAS28, ACR response rate, and EULAR response rate at the day 84 visit compared with baseline (24). The EULAR response depends on the change in the DAS28-CRP and on the absolute level achieved after treatment: A change of ≤0.6 is classified as no response, a change >0.6 and ≤1.2 is a moderate response when the DAS28-CRP after treatment is ≤5.1; and a change of >1.2 is a good response when the DAS28-CRP after treatment is ≤3.2 but is a moderate response when the DAS28-CRP after treatment is >3.2 (24).
Other secondary end points included serum cytokines and whole-blood LPS-induced TNF release. The mean changes in DAS28-CRP between day −21 and day 42 or day 84 and between day 42 and day 56 were assessed for significance at a P value of <0.05 using a paired Student’s t test in the SAS 9.2 statistical analysis package (SAS). Because this was an exploratory study, no formal statistical power calculations were performed, and no adjustments for multiple comparisons were made. Adverse events were collected from the day of implantation through the day 84 visit, coded using the MedDRA, and are presented by MedDRA term as subject incidence rates.
Whole-Blood Cytokine Release Assay in the RA Study.
Because the standard whole-blood LPS-induced cytokine release assay described above involves techniques and equipment not readily available at most clinical trial centers, we sought to develop an assay system suitable for use at clinical sites and scalable to larger studies. The TruCulture system (Myriad RBM) is a self-contained collection tube that fits in a small tabletop heating block and connects to standard phlebotomy equipment. The tube contains culture medium and a plunger for separation of cells without centrifugation. Venous blood was drawn into tubes containing LPS at 100 ng/mL and incubated at 37 °C for 24 h, and supernatants were separated, frozen, and shipped to a central laboratory (SetPoint Medical) where supernatant TNF was measured by ELISA (R&D Systems). Comparisons of changes in TNF release between baseline and subsequent visits (with three statistical outlier exclusions; ROUT, the maximum false discovery rate at 1%) by paired t test, and linear regression analysis of relationships between changes in DAS28-CRP and TNF release were performed using the Prism analytical software package.
Study of Serum Cytokines in the RA Study.
Serum cytokine levels from day 42 were analyzed using the MSD multiplex cytokine assay (MSD Sector Imager 2400; Meso Scale Discovery) per the manufacturer’s instructions. Analyses of IL-6, IL-8, and IL-17 release on day 42 and linear regression analysis of relationships (Pearson’s test) between the change in DAS28-CRP and serum cytokine release at day 42 were performed using the Prism analytical software package.
Acknowledgments
We thank the patients for their participation, Dr. Martin Lesser for his biostatistical input, and April Caravaca and Anna Drake for technical assistance.
Footnotes
Conflict of interest statement: M.F., Y.A.L., and R.Z. are employees of and equity holders in SetPoint Medical Corporation. K.J.T. is an equity holder in and has received consulting fees from SetPoint Medical Corporation. S.M., S.G., S.S., P.R.S., and P.P.T. received research grants from SetPoint Medical Corporation to support the clinical study reported here. P.P.T. has received consulting fees from SetPoint Medical Corporation and is currently an employee of GlaxoSmithKline, which holds an equity interest in SetPoint Medical Corporation.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605635113/-/DCSupplemental.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. May 3, 2016 doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum H, et al. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90. doi: 10.1185/03007990903422307. [DOI] [PubMed] [Google Scholar]
- 3.Helmick CG, et al. National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 4.Tak PP, Kalden JR. Advances in rheumatology: New targeted therapeutics. Arthritis Res Ther. 2011;13(Suppl 1):S1–S5. doi: 10.1186/1478-6354-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 6.Chorus AM, Miedema HS, Boonen A, Van Der Linden S. Quality of life and work in patients with rheumatoid arthritis and ankylosing spondylitis of working age. Ann Rheum Dis. 2003;62(12):1178–1184. doi: 10.1136/ard.2002.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum. 2008;58(11):3439–3449. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Maanen MA, et al. The alpha7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: A possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 2009;60(5):1272–1281. doi: 10.1002/art.24470. [DOI] [PubMed] [Google Scholar]
- 12.Lu B, et al. α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol Med. 2014;20:350–358. doi: 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas-Ballina M, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15(7-8):195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 15.Huston JM, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203(7):1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meregnani J, et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160(1-2):82–89. doi: 10.1016/j.autneu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 17.van Maanen MA, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60(1):114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 18.van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: Aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis. 2010;69(9):1717–1723. doi: 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 19.Levine YA, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One. 2014;9(8):e104530. doi: 10.1371/journal.pone.0104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Maanen MA, Vervoordeldonk MJ, Tak PP. The cholinergic anti-inflammatory pathway: Towards innovative treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(4):229–232. doi: 10.1038/nrrheum.2009.31. [DOI] [PubMed] [Google Scholar]
- 21.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27(2):130–138. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol. 2001;18(5):415–418. doi: 10.1097/00004691-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Huston JM, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35(12):2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 24.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol. 2005;23(5) Suppl 39:S93–S99. [PubMed] [Google Scholar]
- 25.Fransen J, Stucki G, van Riel PLCM. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI) Arthritis Care Res. 2003;49(S5):S214–S224. [Google Scholar]
- 26.Levine YA, Koopman F, Faltys M, Zitnik R, Tak P-P. Using traditional preclinical models to guide development of an untraditional inflammation therapy: neurostimulation of the cholinergic anti-inflammatory pathway in rheumatoid arthritis and inflammatory bowel disease. Bioelectronic Medicine. 2014;1:34–43. [Google Scholar]
- 27.Olofsson PS, Levine YA, et al. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectronic Medicine. 2015;2:37–42. [Google Scholar]
- 28.Choi IY, Gerlag DM, Holzinger D, Roth J, Tak PP. From synovial tissue to peripheral blood: Myeloid related protein 8/14 is a sensitive biomarker for effective treatment in early drug development in patients with rheumatoid arthritis. PLoS One. 2014;9(8):e106253. doi: 10.1371/journal.pone.0106253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerlag DM, et al. Effects of oral prednisolone on biomarkers in synovial tissue and clinical improvement in rheumatoid arthritis. Arthritis Rheum. 2004;50(12):3783–3791. doi: 10.1002/art.20664. [DOI] [PubMed] [Google Scholar]
- 30.Bonaz B, et al. Chronic vagus nerve stimulation in Crohn’s disease: A 6-month follow-up pilot study. Neurogastroenterol Motil. 2016;28(6):948–953. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 31.Koopman FA, et al. Autonomic dysfunction precedes development of rheumatoid arthritis: A prospective cohort study. EBioMedicine. 2016;6:231–237. doi: 10.1016/j.ebiom.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tracey KJ. Shock medicine. Sci Am. 2015;312(3):28–35. [Google Scholar]
- 33.Bruchfeld A, et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med. 2010;268(1):94–101. doi: 10.1111/j.1365-2796.2010.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aletaha D, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 35.Hochberg MC, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35(5):498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]