Abstract
Transplantation is often the only choice many patients have when suffering from end stage organ failure. Although the quality of life improves after transplantation, challenges such as organ shortages, necessary immunosuppression with associated complications and chronic graft rejection limits its wide clinical application. Nanotechnology has emerged in the past two decades as a field with the potential to satisfy clinical needs in the area of targeted and sustained drug delivery, non-invasive imaging, and tissue engineering. In this paper, we provide an overview of popular nanotechnologies and a summary of the current and potential uses of nanotechnology in cell and organ transplantation.
Grand challenges in transplantation
Over the past two decades, through improved surgical procedures and the use of powerful immunosuppressive drugs, cell and organ (i.e., kidney, heart, liver, pancreas) transplantations have become the standard of care for millions of patients with end stage organ failure [1–4]. Unfortunately, organ shortages, graft failure, and life-long administration of immunosuppressants continue to pose as critical obstacles limiting successful transplantation. In the case of kidney transplants, there were only about 17,000 kidneys available while approximately 99,000 patients were on the waiting list in 2014, in the U.S. alone [5]. In addition, an estimated 20% of the patients on the transplant list are those needing a replacement organ due to chronic rejection, even when undergoing broad immunosuppression [1, 2]. While immunosuppressant therapy has proven paramount to transplantation success, strenuous requirements or life-long systemic use, often lead to poor patient compliance causing eventual morbidity and mortality [6, 7].
In an attempt to overcome these existing barriers, promising alternatives are in development to improve transplant techniques. Nanotechnology has contributed immensely to the world of tissue engineering and has demonstrated encouraging results in drug delivery that would benefit the world of transplant therapy [8, 9]. By improving established manufacturing techniques and chemical modifications, many tunable nanotechnologies have been successfully applied in two areas of medicine: i) the localized, sustained, and controlled delivery of drugs and bioactive factors; ii) the imaging of clinically relevant biomarkers and functional parameters for diagnosis and treatment. In this review, we will provide a brief summary of the current achievements of nanotechnology in the field of drug delivery and will discuss some of the recent applications of this technology in organ transplantation (Table 1).
Table 1.
Application of Nanotechnology in Transplantation
| Applications in Transplantation | Platforms | Description |
|---|---|---|
| Delivery of Immunosuppressants and other Drugs |
Nanoparticles | Nanoparticles allow for a targeted, sustained and more controlled drug delivery dosage, reducing the side effects of indiscriminate prolonged used. |
| Liposomes & Peptide Amphiphiles |
The use of lipid-based delivery platforms and Peptide Amphiphiles help in the delivery of water-insoluble therapeutics, increasing drug efficacy. |
|
| Donor Specific Tolerance & Rejection |
Nanochannel Membranes |
Nanochannel membranes offer a constant, sustained release and can be tuned in channel size (2 – 200nm) and density to achieve a clinically relevant, constant delivery of drugs. It has shown constant in vivo delivery for periods ranging from 1 to 6 months. |
| Nanobodies | Nanobodies (therapeutic fragments of antibodies) present advantages in size, stability, and low immunogenic potential and can be used to stimulate inhibitory pathways and shut off immune cells to prevent allograft rejection. |
|
| Biocapsules & Nanoglands |
The use of biocapsules and Nanogland platforms, allows the exchange of nutrients and metabolites while inhibiting the permeation of antibodies and the infiltration of immune cells. They are designed to maintain cell proximity while ensuring sufficient separation to simulate the in vivo environment. |
|
| Imaging, Diagnostics and other uses |
Nanoparticles (e.g., gold, iron oxide, quantum dots) |
Often used to deliver contrast agents to assist in delineating anatomy and physiology for medical imaging, the use of nanoparticles in diagnostic imaging has exhibited a six-fold contrast enhancement compared to the use of free contrast agents. |
Significance and overview of nanotechnology
Nanotechnology has been defined as the science of developing and studying materials and devices that function within the nanometer scale [10]. As such, materials must be synthesized from pre-existing nanoscale building blocks exhibiting unique chemical and physical characteristics proper of the nanoscale. More recently, nanomedicine has emerged as a field which utilizes concepts from nanotechnology and medicine to prevent, diagnose, and treat diseases. As a result, a variety of nanoparticles and nanodevices have been created using a variety of materials including iron, carbon, gold, silica, and silicon [11]. Nanoparticles have been designed to serve multiple functions: drug delivery [12], receptor mediated targeting [13], environmentally-triggered release [14], thermal ablation [15], molecular imaging [16], and magnetism [17]. On the other hand, nano-fluidic systems and nano-membranes have been developed for the selective filtration of fluids [18], diagnoses [19], and sustained delivery of drugs [20].
One of the primary goals of nanomedicine, especially in the case of nanoparticles, is to increase the accumulation of a therapeutic or imaging agent at a target site, while minimizing toxicity to healthy tissue. In the context of cancer treatment, investigations into nanoparticles found significant therapeutic benefits through the utilization of the enhanced permeability and retention effect (i.e. accumulation of nanoparticles ranged 10-100 nm in tumor tissue) [21]. For example, liposomes, one of the simplest forms of nanoparticles approved by the Food and Drug Administration, loaded with doxorubicin, a chemotherapeutic used to treat various cancers including leukemia, were shown to significantly increase accumulation at tumor sites compared to free drug [22]. In their most basic form, liposomes are biocompatible spherical vesicles, with one or more lipid bilayer membranes, used to encapsulate a variety of hydrophobic and hydrophilic drugs [23]. In an effort to further increase their therapeutic potential, conjugations with polyethylene glycol, ligands, antibodies, and proteins have been explored and demonstrated promising results [24]. Other lipid-based nanoparticles, such as micelles (lipid molecules spherically arranged in aqueous solutions), have also been explored for their potential use as drug carriers. Similar to liposomes, micelles have been exploited for their relative ease of production and ability to encapsulate poorly water-soluble drugs [25]. Regardless of the type of lipid-based nanoparticle, the ability to encapsulate biological agents (i.e., siRNA, enzymes) has garnered great interest.
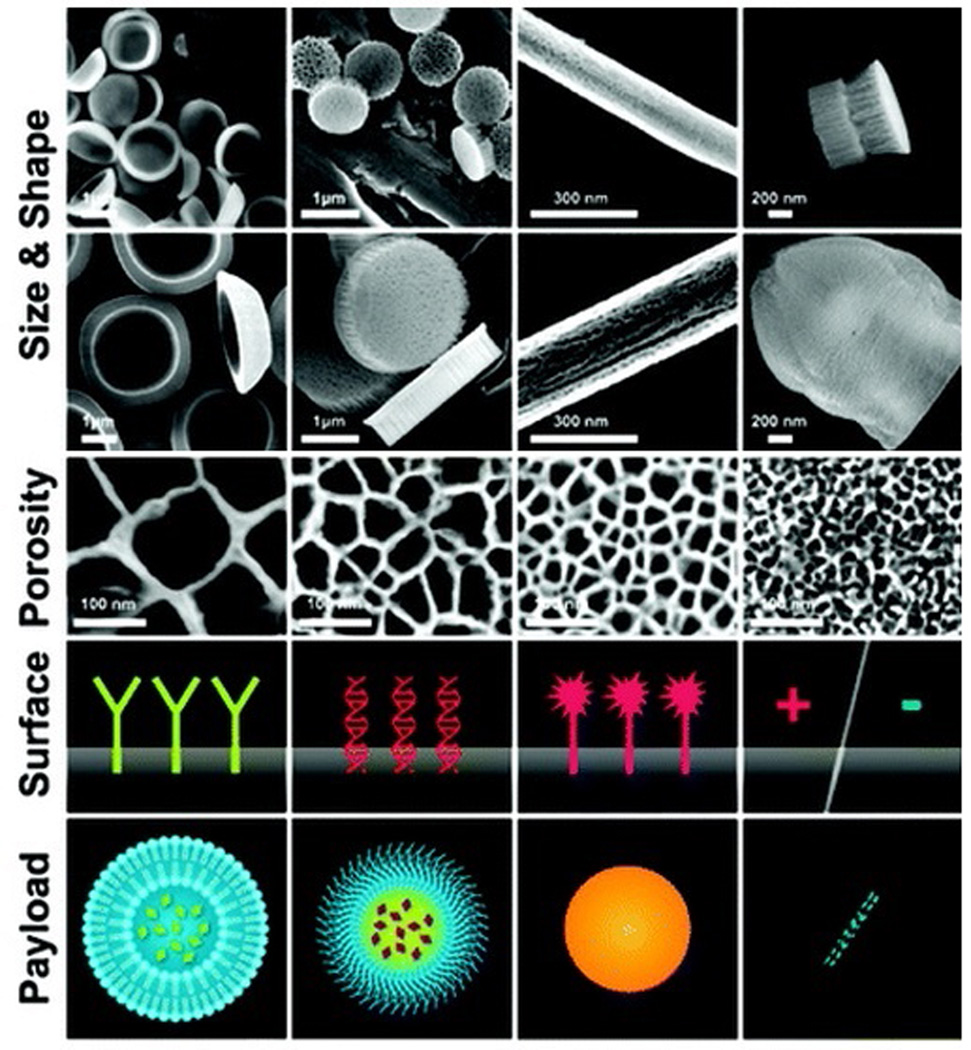
Other groups have explored porous materials, to encapsulate and deliver nanoparticles, which provide space to attach additional targeting moieties, enabling greater tissue penetration [26]. For example, porous silicon has been widely investigated for its biodegradability and biocompatibility [27–29]. Features such as high surface area and tunable shape and size have led to porous silicon being used for a variety of biomedical applications (e.g. tissue engineering [30], biosensors [31], optics [32]). Recently, multistage nanovectors (i.e. disk-shaped porous silicon [33–35]) were developed to strategically overcome the body’s biological barriers through unique size and shape tailoring (Figure 1). Researchers demonstrated that the degradation rates increase significantly as pore size increases [36]. Furthermore, modification of the pore size resulted in prolonged release of a fluorescent payload, and increased loading concentration as pore size increased. Additionally, Decuzzi and coworkers showed that particle geometry and size play a critical role in the biodistribution of particles in different organs after systemic injection. When mice were injected with plateloid-shaped multistage nanovectors, smaller particles (600×200 nm) accumulated at a higher rate in the liver and spleen compared to larger particles (1000×400 nm), while the reverse was observed in the tumor tissue [37]. Others loaded doxorubicin into polymeric micelles, then into multistage nanovectors, and showed that the toxicity to normal cells was significantly reduced while toxicity to breast cancer cells increased in vivo [12]. Furthermore, by conjugating a vascular endothelium growth factor receptor-2 antibody onto multistage nanovectors, particles displayed significant adhesion to inflamed vasculature compared to unconjugated particles [38]. Further functionalization of these nanovectors with cellular membrane proteins isolated from leukocytes [39, 40] gave particles the ability to avoid opsonization and macrophage uptake while increasing particle circulation and accumulation in a melanoma tumor mouse model, with no significant immunological impact [41].
Figure 1.
Schematic of synthesis and functionalization of particles. Size, shape and porosity: Mesoporous silicon nanoparticles with various aspect ratios and various pore sizes (e.g. Discoid nanoparticle, semi-spheres, nanorods). Surface modifications of particles: Positive/negative surface charges, peptides, antibodies. Payload nanoparticles: named second-stage carriers (SSNs) are nanoparticles within the approximate size range of 5-100 nm in diameter (e.g. liposomes, micelles, inorganic/metallic nanoparticles, and carbon structures).
As the gap between the availability of and the demand for organs used in transplantation increases, alternative methods need to be explored. Advances in nanomaterial synthesis and modification have played a significant role in tissue engineering and have led to promising results in regenerative medicine, leading to possible avenues for improvements in current transplant therapy [42]. In the following section, we discuss nanotechnology’s current role in the treatment of organ transplantation through drug delivery and imaging techniques [10].
Nanotechnology as a tool in transplant therapy
1. Localized, sustained, and controlled delivery of drugs and bioactive agents
A number of (nanotechnology based) drug delivery strategies are currently being investigated to circumvent the limitations of conventional approaches and to increase the potential of a drug. Targeted and controlled drug delivery carriers play fundamental roles in the individualization of drug-dependent therapies. While targeted delivery relates to the transportation of drugs to a desired location, controlled delivery relates to the release of the drug at a designated time, in an adequate concentration. Drug targeting and controlled administration are widely investigated, employing the novel tools offered by nanotechnology, resulting in a series of implantable and injectable nano-delivery systems [9, 43]. Substantial resources focus on the development of nanotechnologies to capitalize on their potential benefits in personalized treatments for a large number of clinical applications, including transplantation [44]. Recent studies showed that nanotechnology-based devices could deliver drugs within a specific therapeutic range while avoiding overdose and side effects typically associated with conventional treatments [45]. As a result, the adoption of nano-sized drug delivery technologies would improve the efficacy of treatments, reduce the necessary drug dosage, and minimize toxicity. Additionally, the employment of such devices would prevent issues related to patient compliance and significantly improve their quality of life [46]. The nano-channel drug delivery system is an example of an implantable device featuring precision-fabricated nano-channel membranes that achieve constant release over extended timeframes by simply tuning the channel size (2–200 nm) and density [45, 47–49].
In order to maximize the therapeutic indexes and minimize the side effects of therapeutic agents, a constant concentration of drug within the therapeutic range must be delivered to the plasma. This can be done by employing implantable drug delivery devices able to sustain the constant release of drug over long periods of time (i.e. weeks to years). The adoption of implantable delivery strategies allows for the controlled release of therapeutics in a systemic or localized fashion, dramatically reducing required dosages and associated toxicity (Figure 2) [50, 51].
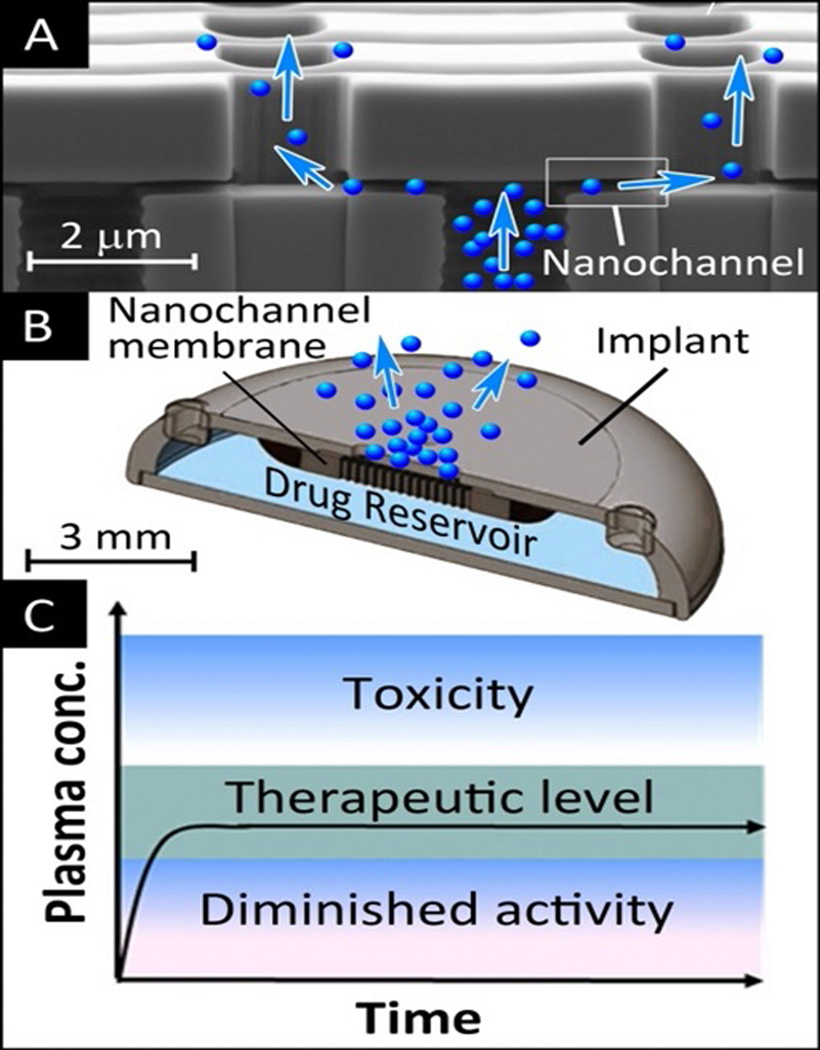
Figure 2.
Scanning electron microscopy image of the cross section of a silicon – silicon nitride nanochannel membrane designed for constant and sustained drug release (A); 3D rendering of the structure of a drug delivery implant incorporating a nanochannel membrane (B); zero-order sustained release can achieve and maintain plasma level of drugs within the therapeutic window for the duration of treatment (C). This has potential for improved efficacy and reduction of adverse side effects of treatment as compared to the conventional bolus administration of therapeutics.
A constant, single drug concentration in the plasma over an extended length of time is only achievable through zero-order release kinetics [52]. Zero-order release is achieved when the gradient of the drug molecule concentration, throughout a delivery device, stabilizes. Commonly, continuum-based diffusive processes are concentration dependent; the diffusion of molecules out of a delivery device decreases at decreasing concentrations in the reservoir. However, several technologies are now available to control molecule deployment and achieve a concentration-independent release. A zero-order release can be obtained with convective driving mechanisms such as osmotic pressure, mechanical pumping, and through electro-kinetic transport [53]. A constant drug release can also be achieved by tuning the properties of nanofluidic devices. It has been shown that, at the nanoscale, molecular constraints, surface effects, and charge interactions play major roles in molecule transport [54, 55]. Charge exclusion, concentration polarization, and streaming current phenomena have been observed at the nanoscale [56, 57]. Moreover, it was demonstrated that confined fluid at the nanoscale level present anisotropic properties [58]. Nanotechnologies allow for the exquisite control of nanostructure properties and nanoscale effects. This control cannot be obtained at the macroscale, where drug release follows a Fickian exponential profile and is strongly affected by the drug concentration [59]. Consequently, at the nanoscale level, a constant concentration–driven drug release can be achieved, allowing for the enhanced delivery of therapeutics in transplant therapies.
1.1 Liposomes, Nanochannel Membranes and other Nanocarriers
Over the years, immunosuppressive drugs have provided a significant increase in transplant patient survival. However, complications still arise because of the therapies’ potency and pharmacokinetic variability. Therefore, it is critical to modify treatments based on each individual patient to avoid any adverse effects. Unfortunately, poor bioavailability and water solubility also make the administration of immunosuppressants complex. This, coupled with the requirement to combine multiple therapies following organ transplants, has led researchers to devise alternative solutions.
Nanotechnology has provided viable alternatives to combating issues related to increasing drug efficacy and solubility. For example, lipid-based formulations such as emulsions [60], liposomes [23], and polymeric micelles [12, 61] have demonstrated reliable alternatives to transport water-insoluble therapeutics. Following renal transplantation, a patient is typically required to take oral immunosuppressant drugs. However, previous literature reported that a high fat diet can display a pronounced effect on the adsorption of cyclosporine, a common immunosuppressant [62]. This led to the reformulation of cyclosporine into a micro emulsion (i.e. fine dispersion system), improving its pharmacokinetic variability [63]. This formulation was shown to be thermodynamically stable and resulted in a smaller droplet size (i.e. <150 nm). Other immunosuppressive drugs, such as tacrolimus [64] and rapamycin [65], have also demonstrated similar effective results when encapsulated within liposomes. For example, rapamycin demonstrated optimal results when encapsulated within micelles. As demonstrated by Forrest et al., encapsulation within micelles bypasses the need for organic co-solvents or harsh surfactants to solubilize highly concentrated drug solutions [66]. In addition, the micelles were reported to be stable when in contact with serum albumin and exhibited a sustained release over the course of several days.
Although rapamycin has shown to be an effective immunosuppressant, its water insolubility has made it challenging to develop an oral or intravenous formulation. Rapamycin’s solubility in water is 2.6 µg/mL, far below its desired therapeutic concentration of 1 mg/mL [67]. Although some formulations were able to overcome the solubility issue through co-solvent/water mixtures, its poor taste and specific storage conditions made it problematic for patients. The use of nanocrystals as a delivery platform for water-insoluble immunosuppressant drugs, overcame this obstacle by providing improved bioavailability [68]. The nanocrystals provided increased surface area while maintaining increased solubility and decreased thickness of the diffusion boundary layer.
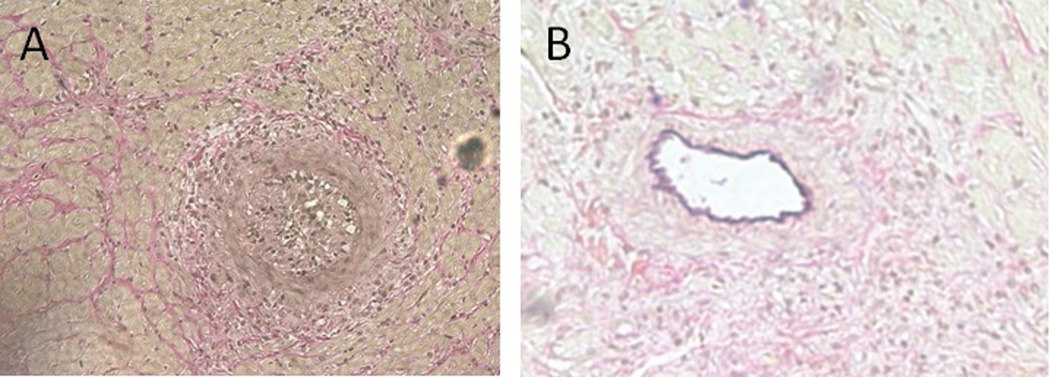
Another area of recent interest is the role of nanoparticles in the disruption of signaling pathways in T cell activation and donor antibody functions. This could demonstrate great potential to treat immunological complications during transplantation [69]. Recent studies have also shown that inflammatory and immune responses are regulated by the small GTPase RhoA pathway via its downstream effector, the Rho-associated protein kinase (ROCK). The inhibition of the RhoA/ROCK pathway should interfere with immune cells and possibly limit or abrogate chronic rejection [70]. Studies in rodent models from various research groups show that chronic rejection of allo-transplants could be ameliorated by the administration of RhoA pathway inhibitors [71–73]. Recent studies showed that the application of nanotechnology in the sustained delivery of a ROCK inhibitor, Y-27632, to the recipients of allografts, in a rat model, resulted in the drastic reduction of collagen deposition, the reduction of tissue fibrosis, and the marked improvement of vascularization in the transplanted heart (Figure 3) [49].
Figure 3.
Sections of transplanted rat hearts VVG stained. Chronically rejecting heart shows fully occluded vessel (A). Recipient treated with RhoA inhibitor delivered from nanochamber shows healthy unoccluded vessels (B).
The central innovation of this sustained delivery technology, is the use of microfabricated nanochannel membranes which, like an hourglass, passively control the release of molecules. Nanochannel membranes bypass the issues of burst and trough release, associated with other delivery technologies and achieve constant drug release by imposing spatial and electrostatic confinement on molecular diffusion. In nanochannels, surface-to-molecule interactions passively control the drug delivery rate, rendering it constant, without the need for complex pumping mechanisms [47, 74]. Nanochannel membranes offer significant advantages as they achieve constant, sustained release and can be easily tuned in channel size (2 – 200nm) and density to achieve a clinically relevant, constant delivery of a broad spectrum of chemotherapeutics [75, 76]. The nanochannel technology has shown constant in vivo delivery of testosterone, leuprolide, interferon, lysozyme, genotropin and octreotide in dog, rat and mouse models for periods ranging from 1 to 6 months [43, 48]. Additionally, this technology demonstrated long-term (more than 6 months), sustained, and constant delivery of therapeutics in an in vitro model (Fig. 2C) [48]. The localized delivery of immunomodulator drugs in the vicinity of transplanted organs or tissues, using a nanochannel drug delivery device, protecting the transplant from immune rejection while eliminating adverse effects associated with systemic immunosuppression, would be the ideal choice in transplant therapy.
Nanocarriers have also proven to be a promising platform to achieve tolerogenic antigen presentation by delivering antigens of interest to specific cell types. Nanocarriers delivering a combination of antigens and immunomodulating agents, such as rapamycin, provide a unique technology platform with the potential to enhance outcomes for the induction of transplant tolerance [77]. Nanobodies, which are therapeutic fragments of antibodies with a single-domain of the antibody variable region, have been developed for cancer therapy with advantages in size, stability, and low immunogenic potential [78, 79]. This formulation can be applied in a similar way to stimulate inhibitory pathways and shut off immune cells to prevent allograft rejection.
1.2 Implantable Devices and Biocapsules
As opposed to constant drug administration, multiple therapies would benefit from the ability to tune drug release according to the circadian cycles. It is well known that the presence of biological rhythms, such as the circadian cycles, affects body metabolism in living organisms over 24 hour cycles and inflammatory markers follow definite circadian cycles. Organs, such as the kidney, liver, and gastrointestinal tract, are very critical to drug metabolism and are highly coupled with circadian rhythms. The pharmacodynamics and efficacy of treatments were demonstrated to relate to the time of administration during the circadian cycle [80]. Therefore, drug delivery strategies should consider the most ideal times for drug administration, in order to reduce toxicity and increase treatment efficacy.
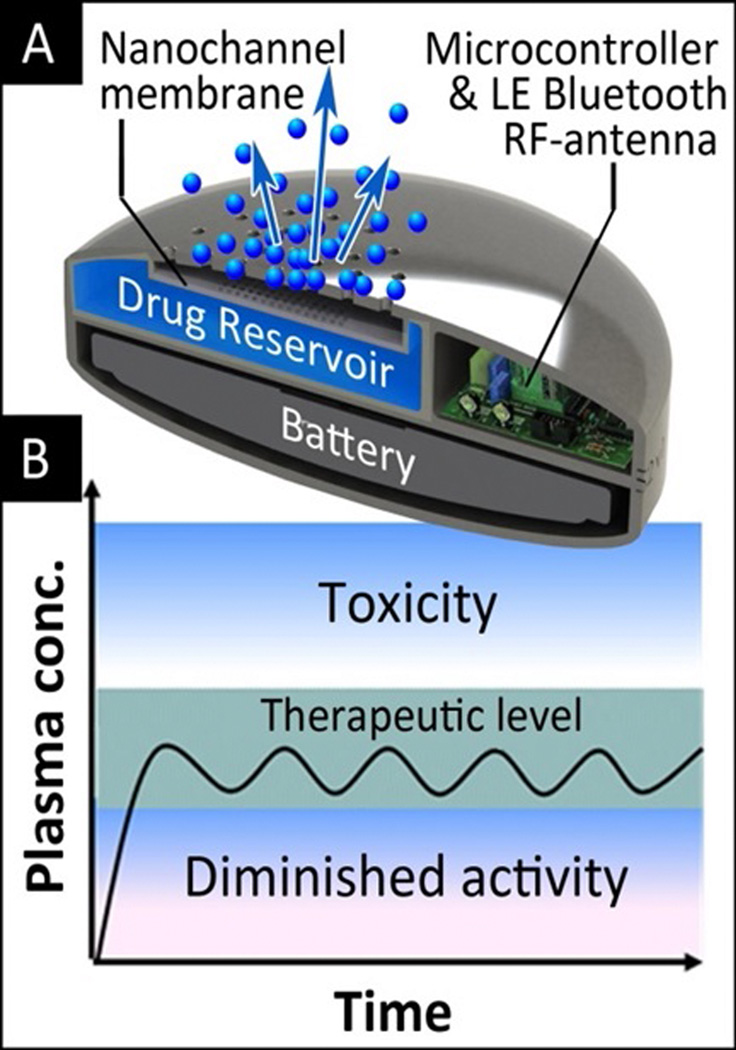
Nanotechnology-based, tunable implant devices have the potential to adjust drug release based on the circadian rhythms of inflammatory markers. The synchronization of drug delivery to bio-cycles using these devices represents an additional step toward individualized medicine. Consequently, some attempts have been made to achieve chrono-therapy with implantable drug delivery systems [57] based on degradable polymers and osmotic devices [81]. Here, researchers present a nanofluidic membrane technology capable of achieving active and tunable control of molecular transport through nanofluidic channels. By applying an electric field between two platinum electrodes positioned on either surface of a 5.7 nm nanochannel membrane, designed for zero-order drug delivery, temporal, reproducible tuning, and interruption of dendritic fullerene 1 (DF-1) transport, was obtained over multi-day release experiments [57]. This ability to actively control and tune delivery of drugs and particles from a subcutaneous implant device has broad applicability to various current and emerging therapeutics and clinical situations including organ and tissue transplantation. The tunable nanochannel drug delivery system (Fig. 4) presents a nanofluidic membrane technology capable of achieving active and tunable control of molecular transport through nanofluidic channels.
Figure 4.
3D rendering of a drug delivery implant for the remotely controlled administration of therapeutics (A). The implant comprises an electrode-coated nanochannel membrane for tuning a low-power applied electric field and tune drug release according to need. Drug administration can be synchronized to the biological clock to maximize the efficacy of treatment (B).
A promising approach for protecting cell transplantation from immune-rejection was proposed back in the 1980’s. Microencapsulated islets implantation was used in vivo as bioartificial endocrine pancreas resulting in the correction of the diabetic state up to three weeks [82]. By enclosing cells within a physical barrier, the biocapsule allows the exchange of nutrients and metabolites while inhibiting the permeation of antibodies and the infiltration of immune cells. This type of technology would enable pancreatic islet cell transplantation, overcoming their immune-rejection without the need for immunosuppressive drugs and, in principle, restore normal glycaemia in diabetic patients, as demonstrated on numerous in vivo studies where experimental animals have recovered for more than 100 days [83–85].
Although progress has been made in the field of cell encapsulation, scientists still seek more favorable synthetic and naturals materials to help overcome previous obstacles such as chemical stability, functional performance, or the production of uniform capsules [86]. The use of photolithographic techniques in the fabrication of micro silicon membranes have helped to overcome some of these challenges by allowing precise control over the pore size and distribution in the range of 20 to 100 nm [87, 88]. In vitro studies showed that rat pancreatic islets cells could maintain their functionality and viability in the three-dimensional encapsulated environment and maintain their glucose-stimulated insulin secretion [89]. Moreover, the silicon-based biocapsule allowed the diffusion of essential nutrients while blocking the permeation of immune molecules. In vivo studies in mice showed biocompatibility of the biocapsule, the viability of the encapsulated cell lines without immunosuppressants, and the secretion of insulin in response to both basal and stimulatory conditions [90].
1.3 Nanoglands and Nanoparticles in Transplant
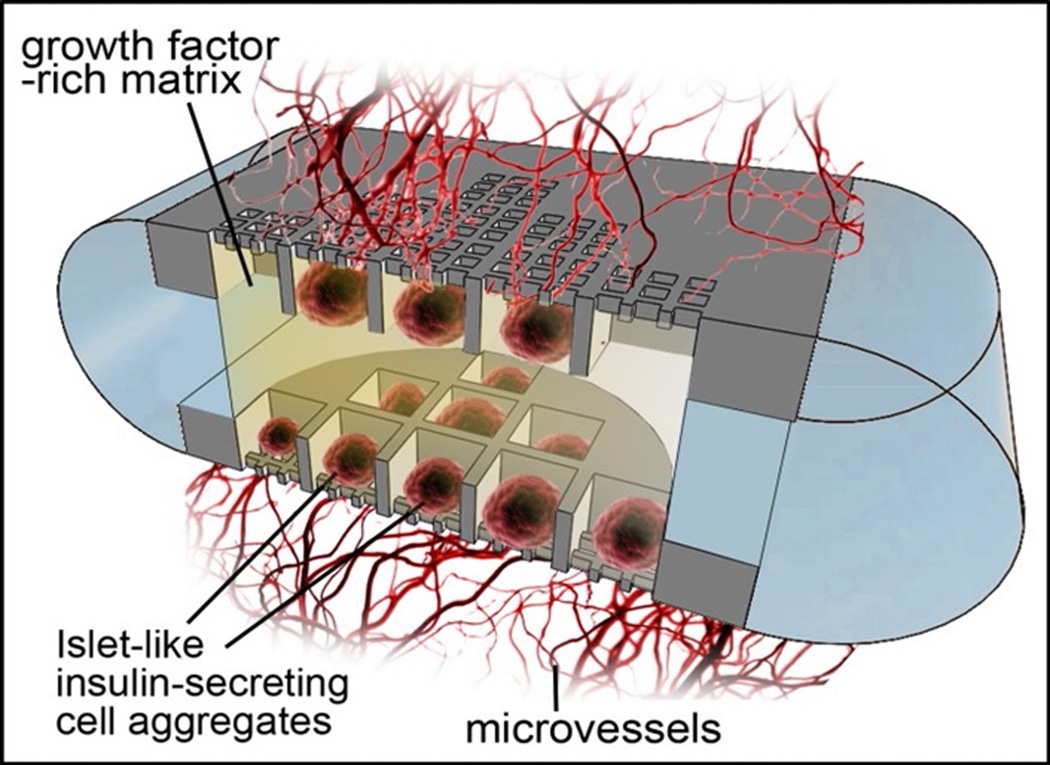
Nanotechnology-based encapsulation systems such as Nanogland (Figure 5) have successfully supported the engraftment of pancreatic islets in animal models [91]. These encapsulation systems protect the transplanted cells from immune attack and provide a physiological environment promoting cell survival and vascularization. The new generation of Nanogland is made with biocompatible, bioinert polymers (PLA/PCL). It is used to house pancreatic islets or islet-like insulin-producing cells in wells. Designed to maintain cell proximity while ensuring sufficient separation to simulate the in vivo environment, the Nanogland, houses cells in a growths factor-rich matrix and presents surface modified microchannels that allow for rapid neovascularization of the graft. This is imperative to assure long-term transplant survival and viability.
Figure 5.
3D rendering of a nanochannel encapsulation of insulin secreting cells. The encapsulation creates a protective environment to improve graft survival and to promote rapid vascularization post transplantation. The encapsulation may supply the graft with oxygen, nutrients, growth factor and immunosuppressive agents in situ, to promote long term viability and abrogate rejection.
Additionally, transplanted cells are protected from immune attack by local, constant, and sustained delivery of immunomodulator agents (e.g., CTLA4Ig). CTLA4Ig, delivered into the cell reservoir, slowly diffuses outside of the implant, generating a local concentration gradient, thereby protecting the transplanted tissue from immune attack. Constant and sustained CTLA4Ig delivery is achieved from an internal reservoir by means of a biocompatible, bioinert, and microfabricated silicon nanochannel membrane. The cell and drug reservoirs are separately fabricated by 3D-printing and assembled by polymeric welding [91, 92].
Nanotechnology has also been considered as a tool to address the poor viability and engraftment following pancreatic islet transplantation [93]. Specifically, these limitations led researchers to investigate peptide amphiphiles (PA) as a potential solution. PA, are peptide molecules that incorporate a hydrophobic domain on one end and a hydrophilic oligosequence on the other end. This promotes self-assembly into nanofibers, exposing the bioactive region on the outer surface to interact with the cell or protein of interest [94].
With this technology, Stendahl et al. explored the use of heparin-binding PA scaffolds for the delivery of angiogenic growth factors (i.e. vascular endothelial growth factor and fibroblast growth factor-2) to mitigate the adverse effects typically encountered with islet transplantation [95]. Remarkably, heparin-binding PA, combined with the angiogenic growth factors, displayed significantly superior vascularization in the omentum (interperitoneal fat mass). In addition, this led to higher cure percentages of diabetic mice and significantly decreased time to achieve normoglycemia.
As is the case for organ rejection, corneal rejection is also subject to a strict regimen of immunosuppressants typically administered systemically or through eye drops [96]. Currently, the two-year survival rate for those receiving an uncomplicated transplant is 90%, but this number can reach as low as 50% for those with neovascularization in the cornea or who have previously experienced graft failure [97, 98]. Although corticosteroids are typically administered to minimize graft rejection, administration can often be required as often as 1 h immediately following transplantation [99]. This strenuous requirement leads to unsatisfactory patient compliance and, eventually, increased rejection rates [100]. Efforts have been made to address this concern including the administration of corticosteroids via a subconjunctival injection immediately following surgery. Unfortunately, rapid clearance of small molecules (i.e. drugs) from the ocular tissue significantly impacts the extent of their therapeutic effects.
Nano- and microparticles have presented a viable strategy to overcome the rapid clearance of small molecules from the occular tissue and improve therapeutic drug levels. Specifically, polymer particles are being employed for the delivery of therapeutic agents to the eye by harnessing various routes of administration, such as intravenous, subconjunctival, and topical administration [101]. For example, Pan, et al., demonstrated that dexamethasone sodium phosphate-loaded nanoparticles provided sustained release in vitro and resulted in effective prevention of corneal graft rejection when injected subconjunctivally in a rat animal model [102]. Conversely, when injected with free drug, rejection occurred as soon as three weeks following transplantation, with all mice experiencing rejection at four weeks.
2. Imaging and functional parameters for diagnosis
Nanotechnology has made substantial progress in the world of medical imaging. Similar to their ability to deliver therapeutics, nanoparticles can be used to deliver contrast agents to assist in delineating anatomy and physiology for medical imaging. Examples include the use of iodine-encapsulated liposomes for x-ray computed tomography [103], gadolinium within mesoporous silica nanoparticles for magnetic resonance imaging (MRI) [104], and perfluorocarbons within polymer nanocapsules for ultrasound [105]. Nanoparticles have transformed the way we use complex contrast agents. Here we expand on one example, gadolinium, and its contrast enhancement for MRI. Magnevist is a clinically available, and widely used, agent for MRI comprised of gadolinium chelated with an aminopolycarboxylic acid-based agent. This formulation suffers from rapid clearance from the blood and limits their use for MR-based angiography [106]. A possible solution was to encapsulate gadolinium into PEGylated liposomes, which produced significant contrast enhancement of tissue vasculature enabling high spatial resolution [106]. Furthermore, excessive chelation of gadolinium and other contrast agents can substantially reduce their contrast enhancement. In this case, investigators used carbon nanostructures to enclose gadolinium ions within fullerene cages [107] or gadolinium ion clusters within nanotubes [108, 109] and achieved 10 and 40 times greater contrast enhancement, respectively. The loading of these agents within nanoporous silicon particles yielded a 6-fold contrast enhancement of the embedded payloads (Magnevist, gadofullerenes, gadonanotubes) attributed to their nanoscale confinement [110, 111].
In addition to the delivery of contrast agents, some nanoparticles can serve as imaging agents due to their unique nano-scaled features. For example, gold nanoparticles can serve as contrast agents for computed tomography [112], iron oxide nanoparticles for spin-spin relaxation (T2-weighed imaging, MRI) [113–115], quantum dots for near infrared (NIR) fluorescence-based imaging [116, 117], and carbon nanotubes for NIR and ultrasound imaging [118, 119]. In general, these imaging properties are size-dependent, so MRI contrast enhancement increases with increasing diameters of iron oxide nanoparticles. However, large nanoparticles tend to aggregate and are more readily recognized by the immune system, therefore, a certain balance must be reached depending on their intended application [120]. Quantum dots (2–15 nm) also show a size-dependent correlation; their fluorescence emission can be tuned from blue to red by increasing their size, representing a powerful alternative to traditional dyes, permitting broad excitation spectra, high quantum yield, and remarkable resistance to photobleaching [121]. Due to the high surface area of nanoparticles, they can also be decorated with various recognition moieties (e.g. antibodies, aptamers, peptides, etc.) to target and enhance the imaging of cancer [122], apoptosis [123], hypoxia [124], angiogenesis [125], atherosclerosis [126], and inflammation [127]. In addition, therapeutics and other diagnostics can be added to their surfaces to create particles able to provide both therapy and imaging (i.e. theranostics), including radioactive probes for positron emission tomography imaging [128, 129]. However, when using metallic and semiconductor nanoparticles, one needs to be cautious of possible adverse immunological and toxicity effects.
Clinical approval of iron oxide nanoparticles to diagnose lymph node metastases and liver lesions with MRI was obtained in 1996 for Feridex (iron oxide nanoparticles decorated with dextran) [130]. Following this, other agents (e.g. Resovist, Combidex, Clariscan, and Gastromark) received approval or were in development for clinical use [131]. However, the production of these agents was discontinued due to safety concerns. High false-positive rates, and minimal market representation (penetration?), and thus, have been phased out of use [131, 132]. Several promising nanoparticle-based imaging applications are currently in clinical trials, or expected to be in the near future, including nanoparticles for MRI contrast that target an integrin commonly found on the surface of newly developed vessels, and applying carbon nanotubes as possible x-ray sources for a new type of computed tomography scanner [133]. Nanoparticles with metallic components can be used as biosensors, for imaging capability with CT (such as super-paramagnetic iron oxide) in the attempt to better visualize cancer masses [134].
With respect to the transplant field, nanoparticle approaches for imaging have predominately been used to monitor transplanted grafts [135, 136], track distribution (dispersion) of administered stem cells [137–139], gauge viability of implanted cells within scaffolds [140, 141] or within tissues [142, 143], and to evaluate drug release from scaffolds [144]. In summary, nanotechnology has the potential to provide powerful solutions to permit noninvasive imaging of organs and tissues before and after transplantation and as means to visualize the vasculature and enhance the resolution for superior medical imaging.
Summary and perspectives
Nanotechnology presents novel ways to approach the different barriers that organ and cell transplantations present today. The implementation of nanotechnology has demonstrated various successes including the recent use of nanocomposite polymer as scaffolding for the synthesis of a successfully implanted artificial trachea [145]. In addition, nanotechnology has been shown to play a significant role in ensuring successful transplants for patients with high risk of chronic rejection by providing targeted and controlled delivery of immunosuppressive drugs. The use of these platforms has also provided viable alternatives to combating issues related to drug solubility and increasing drug efficacies. Nanoformulated emulsions [60], liposomes [23], and polymeric micelles [12, 61] have been shown as reliable alternatives to transport water-insoluble therapeutics. In tissue engineering, nanomedicine has been employed to regenerate healthy tissue using a variety of composites, nanodelivery systems, implantable nanochannels, and nanoencapsulation platforms. New developments in nanomaterials such as the inclusion of bioactive properties, able to enhance cell growth and function, offer a promising future for today’s transplant therapies and could improve the prognosis of transplant patients.
Acknowledgments
The authors would like to thank Matthew Landry for excellent graphical support, Sebastian Powell and Megan Livingston for helpful discussions. This work was supported financially by the following sources: the Department of Defense (W81XWH-09-1-0212 and W81XWH-12-1-0414), the National Institute of Health (U54CA143837 and U54CA151668), the Cancer Prevention and Research Institute of Texas (RP121071), the Brown Foundation, the Cullen Trust for Health Care Foundation (Project ID: 18130014), The Hearst Foundation and the Ernest Cockrell Jr. Distinguished Endowed Chair.
Footnotes
Ennio Tasciotti: Participated in the writing, critical discussion and editing of the paper.
Fernando J. Cabrera: Participated in the writing, critical discussion and editing of the paper.
Michael Evangelopoulos: Participated in the writing and editing of the paper
Jonathan O. Martinez: Participated in the writing and editing of the paper
Malgorzata Kloc: Participated in the writing of the paper.
Usha Thekkedath: Participated in the writing, critical discussion and editing of the paper.
Rafik M. Ghobrial: Participated in the mentorship, critical discussion of the paper.
Xian C. Li: Participated in the mentorship, critical discussion of the paper.
Alessandro Grattoni: Participated in the mentorship, critical discussion of the paper.
Mauro Ferrari: Corresponding Author. Participated in the mentorship, critical discussion and editing of the paper.
REFERENCES
- 1.Transplant, N.B.a. Survival Rates Following Transplantation. 2014 Available from: http://www.organdonation.nhs.uk/statistics/.
- 2.Recipients, S.R.o.T. 2012 Annual Data Report. 2014 Available from: http://www.srtr.org/.
- 3.Gondos A, et al. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation. 2013;95(2):267–274. doi: 10.1097/TP.0b013e3182708ea8. [DOI] [PubMed] [Google Scholar]
- 4.Dawwas MF, et al. Survival after liver transplantation in the United Kingdom and Ireland compared with the United States. Gut. 2007;56(11):1606–1613. doi: 10.1136/gut.2006.111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins AJ, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63(1 Suppl):A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. American journal of transplantation. 2006;6(2):262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 7.Kasiske BL, et al. Diabetes mellitus after kidney transplantation in the United States. American journal of transplantation. 2003;3(2):178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 8.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharmaceutical research. 2006;23(7):1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, et al. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano letters. 2010;10(9):3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theis T, et al. nan'o.tech.nol'o.gy n. Nature nanotechnology. 2006;1(1):8–10. doi: 10.1038/nnano.2006.77. [DOI] [PubMed] [Google Scholar]
- 11.Sanvicens N, Marco MP. Multifunctional nanoparticles--properties and prospects for their use in human medicine. Trends in biotechnology. 2008;26(8):425–433. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Martinez JO, et al. Multistage Nanovectors Enhance the Delivery of Free and Encapsulated Drugs. Curr Drug Targets. 2014 doi: 10.2174/1389450115666141015113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thierry B, et al. Immunotargeting of Functional Nanoparticles for MRI detection of Apoptotic Tumor Cells. Advanced materials. 2009;21(5):541–545. doi: 10.1002/adma.200800998. [DOI] [PubMed] [Google Scholar]
- 14.Muhammad F, et al. pH-Triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. Journal of the American Chemical Society. 2011;133(23):8778–8781. doi: 10.1021/ja200328s. [DOI] [PubMed] [Google Scholar]
- 15.Letfullin RR, Iversen CB, George TF. Modeling nanophotothermal therapy: kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticles. Nanomedicine : nanotechnology, biology, and medicine. 2011;7(2):137–145. doi: 10.1016/j.nano.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Hedlund A, et al. Gd(2)O(3) nanoparticles in hematopoietic cells for MRI contrast enhancement. International journal of nanomedicine. 2011;6:3233–3240. doi: 10.2147/IJN.S23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yigit MV, Moore A, Medarova Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharmaceutical research. 2012;29(5):1180–1188. doi: 10.1007/s11095-012-0679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Fu J, Schoch RB. Molecular sieving using nanofilters: past, present and future. Lab on a Chip. 2008;8(1):23–33. doi: 10.1039//b714128a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, et al. Nanodevices in diagnostics. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2011;3(1):11–32. doi: 10.1002/wnan.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine D, et al. A robust nanofluidic membrane with tunable zero-order release for implantable dose specific drug delivery. Lab on a Chip. 2010;10(22):3074–3083. doi: 10.1039/c0lc00013b. [DOI] [PubMed] [Google Scholar]
- 21.Maeda H, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 22.Perez AT, et al. Pegylated liposomal doxorubicin (Doxil) for metastatic breast cancer: the Cancer Research Network, Inc., experience. Cancer Invest. 2002;20(Suppl 2):22–29. doi: 10.1081/cnv-120014883. [DOI] [PubMed] [Google Scholar]
- 23.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 24.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Drugs. 1997;54(4):15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Advanced drug delivery reviews. 2001;47(1):113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 26.Yazdi IK, et al. Physicochemical properties affect the synthesis, controlled delivery, degradation and pharmacokinetics of inorganic nanoporous materials. Nanomedicine (Lond) 2015 doi: 10.2217/nnm.15.133. [DOI] [PubMed] [Google Scholar]
- 27.Martinez JO, et al. Degradation and biocompatibility of multistage nanovectors in physiological systems. J Biomed Mater Res A. 2014;102(10):3540–3549. doi: 10.1002/jbm.a.35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahbazi MA, et al. The mechanisms of surface chemistry effects of mesoporous silicon nanoparticles on immunotoxicity and biocompatibility. Biomaterials. 2013;34(31):7776–7789. doi: 10.1016/j.biomaterials.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 29.Martinez JO, et al. Short and long term, in vitro and in vivo correlations of cellular and tissue responses to mesoporous silicon nanovectors. Small. 2013;9(9–10):1722–1733. doi: 10.1002/smll.201201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan D, et al. The role of nanostructured mesoporous silicon in discriminating in vitro calcification for electrospun composite tissue engineering scaffolds. Nanoscale. 2011;3(2):354–361. doi: 10.1039/c0nr00550a. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez GA, Hu S, Weiss SM. Porous silicon ring resonator for compact, high sensitivity biosensing applications. Opt Express. 2015;23(6):7111–7119. doi: 10.1364/OE.23.007111. [DOI] [PubMed] [Google Scholar]
- 32.Riley M, et al. Nanostructured porous silicon films for terahertz optics. Nanotechnology. 2012;23(32):325301. doi: 10.1088/0957-4484/23/32/325301. [DOI] [PubMed] [Google Scholar]
- 33.Martinez JO, et al. Multifunctional to multistage delivery systems: The evolution of nanoparticles for biomedical applications. Chin Sci Bull. 2012;57(31):3961–3971. doi: 10.1007/s11434-012-5387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tasciotti E, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature nanotechnology. 2008;3(3):151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 35.Wolfram J, Shen H, Ferrari M. Multistage vector (MSV) therapeutics. Journal of Controlled Release. 2015;219:406–415. doi: 10.1016/j.jconrel.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez JO, et al. Engineering multi-stage nanovectors for controlled degradation and tunable release kinetics. Biomaterials. 2013;34(33):8469–8477. doi: 10.1016/j.biomaterials.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Ven AL, et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J Control Release. 2012;158(1):148–155. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez JO, et al. The effect of multistage nanovector targeting of VEGFR2 positive tumor endothelia on cell adhesion and local payload accumulation. Biomaterials. 2014;35(37):9824–9832. doi: 10.1016/j.biomaterials.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbo C, et al. Proteomic profiling of a biomimetic drug delivery platform. Curr Drug Targets. 2014 doi: 10.2174/1389450115666141109211413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evangelopoulos M, et al. Cell source determines the immunological impact of biomimetic nanoparticles. Biomaterials. doi: 10.1016/j.biomaterials.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parodi A, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nature nanotechnology. 2013;8(1):61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4(1):66–80. [Google Scholar]
- 43.Grattoni A, et al. Nanochannel systems for personalized therapy and laboratory diagnostics. Current pharmaceutical biotechnology. 2010;11(4):343–365. doi: 10.2174/138920110791233280. [DOI] [PubMed] [Google Scholar]
- 44.Desai TA, et al. Nanopore technology for biomedical applications. Biomedical microdevices. 1999;2(1):11–40. [Google Scholar]
- 45.Grattoni A, et al. Nanotechnologies and regenerative medical approaches for space and terrestrial medicine. Aviation, space, and environmental medicine. 2012;83(11):1025–1036. doi: 10.3357/asem.3307.2012. [DOI] [PubMed] [Google Scholar]
- 46.Nicolov E, et al. MP43-20 NANOTECHNOLOGY-BASED IMPLANT FOR LONG TERM TESTOSTERONE REPLACEMENT. The Journal of Urology. 2014;191(4):e485–e486. [Google Scholar]
- 47.Celia C, et al. Sustained Zero-Order Release of Intact Ultra-Stable Drug-Loaded Liposomes from an Implantable Nanochannel Delivery System. Advanced healthcare materials. 2014;3(2):230–238. doi: 10.1002/adhm.201300188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrati S, Nicolov E, Zabre E, Geninatti T, Shirkey BA, Hudson L, Hosali S, Crawley M, Khera M, Palapattu G, Grattoni A. The Nanochannel Delivery System for Constant Testosterone Replacement Therapy. Journal of Sexual Medicine. 2015 doi: 10.1111/jsm.12897. [DOI] [PubMed] [Google Scholar]
- 49.Ferrati S, et al. Leveraging nanochannels for universal, zero-order drug delivery in vivo. Journal of Controlled Release. 2013;172(3):1011–1019. doi: 10.1016/j.jconrel.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 50.Serda RE, et al. Multi-stage delivery nano-particle systems for therapeutic applications. Biochimica et Biophysica Acta (BBA)-General Subjects. 2011;1810(3):317–329. doi: 10.1016/j.bbagen.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tasciotti E, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature nanotechnology. 2008;3(3):151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 52.Ziemys A, et al. Hierarchical modeling of diffusive transport through nanochannels by coupling molecular dynamics with finite element method. Journal of Computational Physics. 2011;230(14):5722–5731. [Google Scholar]
- 53.Fine D, et al. A low-voltage electrokinetic nanochannel drug delivery system. Lab on a Chip. 2011;11(15):2526–2534. doi: 10.1039/c1lc00001b. [DOI] [PubMed] [Google Scholar]
- 54.Pimpinelli A, Ferrari M, Grattoni A. Scaling and crossovers in molecular transport in nano-fluidic systems. Applied Physics Letters. 2013;103(11):113104. [Google Scholar]
- 55.Grattoni A, et al. Gated and near-surface diffusion of charged fullerenes in nanochannels. ACS nano. 2011;5(12):9382–9391. doi: 10.1021/nn2037863. [DOI] [PubMed] [Google Scholar]
- 56.Kim SJ, Li LD, Han J. Amplified electrokinetic response by concentration polarization near nanofluidic channel. Langmuir. 2009;25(13):7759–7765. doi: 10.1021/la900332v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruno G, et al. Leveraging electrokinetics for the active control of dendritic fullerene-1 release across a nanochannel membrane. Nanoscale. 2015 doi: 10.1039/c4nr06209d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziemys A, et al. Confinement effects on monosaccharide transport in nanochannels. The Journal of Physical Chemistry B. 2010;114(34):11117–11126. doi: 10.1021/jp103519d. [DOI] [PubMed] [Google Scholar]
- 59.Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert opinion on drug delivery. 2010;7(4):429–444. doi: 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hippalgaonkar K, Majumdar S, Kansara V. Injectable lipid emulsions-advancements, opportunities and challenges. AAPS PharmSciTech. 2010;11(4):1526–1540. doi: 10.1208/s12249-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12(36):4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 62.Mueller EA, Kovarik JM, Kutz K. Minor influence of a fat-rich meal on the pharmacokinetics of a new oral formulation of cyclosporine. Transplant Proc. 1994;26(5):2957–2958. [PubMed] [Google Scholar]
- 63.Ritschel WA. Microemulsion technology in the reformulation of cyclosporine: the reason behind the pharmacokinetic properties of Neoral. Clin Transplant. 1996;10(4):364–373. [PubMed] [Google Scholar]
- 64.McAlister VC, Keshavamurthy M, Lee TD. Oral delivery of liposomal tacrolimus: increased efficacy and reduced toxicity. Transplant Proc. 1999;31(1–2):1110. doi: 10.1016/s0041-1345(98)01923-x. [DOI] [PubMed] [Google Scholar]
- 65.Alemdar AY, et al. Liposomal formulations of tacrolimus and rapamycin increase graft survival and fiber outgrowth of dopaminergic grafts. Cell Transplant. 2004;13(3):263–271. doi: 10.3727/000000004783983936. [DOI] [PubMed] [Google Scholar]
- 66.Forrest ML, et al. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J Control Release. 2006;110(2):370–377. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Simamora P, Alvarez JM, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001;213(1–2):25–29. doi: 10.1016/s0378-5173(00)00617-7. [DOI] [PubMed] [Google Scholar]
- 68.Shen LJ, Wu FL. Nanomedicines in renal transplant rejection--focus on sirolimus. International journal of nanomedicine. 2007;2(1):25–32. doi: 10.2147/nano.2007.2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hom C, et al. Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small. 2010;6(11):1185–1190. doi: 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olson MF. Applications for ROCK kinase inhibition. Current opinion in cell biology. 2008;20(2):242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kloc M GR. Chronic allograft rejection: a significant hurdle to transplant success. Burns and Trauma. 2014;2:3–10. doi: 10.4103/2321-3868.121646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skelton TS, et al. Downregulation of RhoA and changes in T cell cytoskeleton correlate with the abrogation of allograft rejection. Transplant immunology. 2010;23(4):185–193. doi: 10.1016/j.trim.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang LKM, Tejpal N, You J, Cordero-Reyes AM, Youker KA, Ghobrial RM. ROCK1 inhibitor abrogates chronic rejection in rat cardiac model system. Open J Organ Transp Surg. 2012;2:46–51. [Google Scholar]
- 74.Sakamoto JH, et al. Enabling individualized therapy through nanotechnology. Pharmacological Research. 2010;62(2):57–89. doi: 10.1016/j.phrs.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grattoni A, et al. Nanochannel technology for constant delivery of chemotherapeutics: beyond metronomic administration. Pharmaceutical research. 2011;28(2):292–300. doi: 10.1007/s11095-010-0195-6. [DOI] [PubMed] [Google Scholar]
- 76.Zanni MT, Wright JC, Fulmer EC. Nonlinear spectroscopic methods for identifying and characterizing molecular interactions. Google Patents; 2010. [Google Scholar]
- 77.Hlavaty KA. Biomaterial Approaches for Immunomodulation to Enhance Transplantation Tolerance. NORTHWESTERN UNIVERSITY; 2015. [Google Scholar]
- 78.Muyldermans S. Nanobodies: natural single-domain antibodies. Annual review of biochemistry. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 79.Kijanka M, et al. Nanobody-based cancer therapy of solid tumors. Nanomedicine : nanotechnology, biology, and medicine. 2015;10(1):161–174. doi: 10.2217/nnm.14.178. [DOI] [PubMed] [Google Scholar]
- 80.Smolensky MH, Peppas NA. Chronobiology, drug delivery, and chronotherapeutics. Advanced drug delivery reviews. 2007;59(9):828–851. doi: 10.1016/j.addr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Bisht R. Chronomodulated drug delivery system: A comprehensive review on the recent advances in a new sub-discipline of’chronopharmaceutics’. Asian journal of pharmaceutics. 2011;5(1):1. [Google Scholar]
- 82.Lim F, Sun A. Microencapsulated islets as bioartificial endocrine pancreas. Science (New York, NY) 1980;210(4472):908. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 83.de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends in molecular medicine. 2002;8(8):363–366. doi: 10.1016/s1471-4914(02)02381-x. [DOI] [PubMed] [Google Scholar]
- 84.Kobayashi T, et al. Indefinite islet protection from autoimmune destruction in nonobese diabetic mice by agarose microencapsulation without immunosuppression1. Transplantation. 2003;75(5):619–625. doi: 10.1097/01.TP.0000053749.36365.7E. [DOI] [PubMed] [Google Scholar]
- 85.Korbutt G, et al. Improved survival of microencapsulated islets during in vitro culture and enhanced metabolic function following transplantation. Diabetologia. 2004;47(10):1810–1818. doi: 10.1007/s00125-004-1531-3. [DOI] [PubMed] [Google Scholar]
- 86.Orive G, et al. Cell encapsulation: promise and progress. Nat Med. 2003;9(1):104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 87.Ferrari M, et al. Microfabricated silicon biocapsule for immunoisolation of pancreatic islets. Proceedings of the fourth international conference on Advanced manufacturing systems and technology; Udine, Italy: Springer-Verlag New York, Inc.; 1996. pp. 559–567. [Google Scholar]
- 88.Chu W, et al. Silicon-micromachined direct-pore filters for ultrafiltration. 1997 [Google Scholar]
- 89.Desai T, et al. Investigating Islet Immunoisolation Parameters Using Microfabricated Membranes. Materials Research Society; 1998. [Google Scholar]
- 90.Desai TA, et al. Microfabricated immunoisolating biocapsules. Biotechnology and Bioengineering. 1998;57(1):118–120. doi: 10.1002/(sici)1097-0290(19980105)57:1<118::aid-bit14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 91.Sabek OM, et al. Characterization of a nanogland for the autotransplantation of human pancreatic islets. Lab on a Chip. 2013;13(18):3675–3688. doi: 10.1039/c3lc50601k. [DOI] [PubMed] [Google Scholar]
- 92.Grattoni A, Parazynski S, Hussain F. Building Nanoglands. Mechanical engineering. 2011;133(2):22–26. [Google Scholar]
- 93.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350(7):694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 94.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 95.Stendahl JC, et al. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86(3):478–481. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. Int Ophthalmol. 2008;28(3):223–232. doi: 10.1007/s10792-007-9100-7. [DOI] [PubMed] [Google Scholar]
- 97.Al-Swailem SA. Graft failure: II. Ocular surface complications. Int Ophthalmol. 2008;28(3):175–189. doi: 10.1007/s10792-007-9127-9. [DOI] [PubMed] [Google Scholar]
- 98.Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28(3):209–222. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 99.Design and methods of The Collaborative Corneal Transplantation Studies. The Collaborative Corneal Transplantation Studies Research Group. Cornea. 1993;12(2):93–103. doi: 10.1097/00003226-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 100.Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17(2):163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 101.Wadhwa S, et al. Nanocarriers in ocular drug delivery: an update review. Curr Pharm Des. 2009;15(23):2724–2750. doi: 10.2174/138161209788923886. [DOI] [PubMed] [Google Scholar]
- 102.Pan Q, et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015;201:32–40. doi: 10.1016/j.jconrel.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kao CY, et al. Long-residence-time nano-scale liposomal iohexol for X-ray-based blood pool imaging. Acad Radiol. 2003;10(5):475–483. doi: 10.1016/s1076-6332(03)80055-7. [DOI] [PubMed] [Google Scholar]
- 104.Li C, et al. Multifunctional upconversion mesoporous silica nanostructures for dual modal imaging and in vivo drug delivery. Small. 2013;9(24):4150–4159. doi: 10.1002/smll.201301093. [DOI] [PubMed] [Google Scholar]
- 105.Yang P, et al. Stimuli-responsive biodegradable poly(methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials. 2014;35(6):2079–2088. doi: 10.1016/j.biomaterials.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 106.Ayyagari AL, et al. Long-circulating liposomal contrast agents for magnetic resonance imaging. Magn Reson Med. 2006;55(5):1023–1029. doi: 10.1002/mrm.20846. [DOI] [PubMed] [Google Scholar]
- 107.Toth E, et al. Water-soluble gadofullerenes: toward high-relaxivity, pH-responsive MRI contrast agents. Journal of the American Chemical Society. 2005;127(2):799–805. doi: 10.1021/ja044688h. [DOI] [PubMed] [Google Scholar]
- 108.Sitharaman B, et al. Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem Commun (Camb) 2005;(31):3915–3917. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
- 109.Hartman KB, et al. Gadonanotubes as ultrasensitive pH-smart probes for magnetic resonance imaging. Nano Lett. 2008;8(2):415–419. doi: 10.1021/nl0720408. [DOI] [PubMed] [Google Scholar]
- 110.Ananta JS, et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nature nanotechnology. 2010;5(11):815–821. doi: 10.1038/nnano.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sethi R, et al. Enhanced MRI relaxivity of Gd(3+) -based contrast agents geometrically confined within porous nanoconstructs. Contrast Media Mol Imaging. 2012;7(6):501–508. doi: 10.1002/cmmi.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Popovtzer R, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8(12):4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee H, et al. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. Journal of the American Chemical Society. 2006;128(22):7383–7389. doi: 10.1021/ja061529k. [DOI] [PubMed] [Google Scholar]
- 114.Gizzatov A, et al. Hierarchically-Structured Magnetic Nanoconstructs with Enhanced Relaxivity and Cooperative Tumor Accumulation. Adv Funct Mater. 2014;24(29):4584–4594. doi: 10.1002/adfm.201400653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Serda RE, et al. Cellular association and assembly of a multistage delivery system. Small. 2010;6(12):1329–1340. doi: 10.1002/smll.201000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281(5385):2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 117.Kim S, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22(1):93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Welsher K, et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature nanotechnology. 2009;4(11):773–780. doi: 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delogu LG, et al. Functionalized multiwalled carbon nanotubes as ultrasound contrast agents. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(41):16612–166127. doi: 10.1073/pnas.1208312109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu F, et al. The magnetophoretic mobility and superparamagnetism of core-shell iron oxide nanoparticles with dual targeting and imaging functionality. Biomaterials. 2010;31(22):5842–58428. doi: 10.1016/j.biomaterials.2010.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Medintz IL, et al. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4(6):435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 122.Toy R, et al. Targeted nanotechnology for cancer imaging. Adv Drug Deliv Rev. 2014;76:79–97. doi: 10.1016/j.addr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Quinti L, Weissleder R, Tung CH. A fluorescent nanosensor for apoptotic cells. Nano Lett. 2006;6(3):488–490. doi: 10.1021/nl0524694. [DOI] [PubMed] [Google Scholar]
- 124.Liu J, et al. Ultrasensitive nanosensors based on upconversion nanoparticles for selective hypoxia imaging in vivo upon near-infrared excitation. Journal of the American Chemical Society. 2014;136(27):9701–9709. doi: 10.1021/ja5042989. [DOI] [PubMed] [Google Scholar]
- 125.Winter PM, et al. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res. 2003;63(18):5838–5843. [PubMed] [Google Scholar]
- 126.Marrache S, Dhar S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(23):9445–9450. doi: 10.1073/pnas.1301929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farr TD, et al. Imaging early endothelial inflammation following stroke by core shell silica superparamagnetic glyconanoparticles that target selectin. Nano Lett. 2014;14(4):2130–2134. doi: 10.1021/nl500388h. [DOI] [PubMed] [Google Scholar]
- 128.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine (Lond) 2008;3(2):137–140. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 129.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62(11):1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imaging. 2010;9(6):291–310. [PMC free article] [PubMed] [Google Scholar]
- 131.Wang YX. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg. 2011;1(1):35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014;13(2):125–138. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 133.Cancer, N.A.f.N.i. [cited 2015 October 2nd];Nanotechnology in Clinical Trials. Available from: http://nano.cancer.gov/learn/now/clinical-trials.asp.
- 134.Abakumov MA, et al. VEGF-targeted magnetic nanoparticles for MRI visualization of brain tumor. Nanomedicine: nanotechnology, biology, and medicine. 2015 doi: 10.1016/j.nano.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 135.Evgenov NV, et al. In vivo imaging of islet transplantation. Nat Med. 2006;12(1):144–148. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 136.Toso C, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8(3):701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 137.Meng Y, et al. MRI of auto-transplantation of bone marrow-derived stem-progenitor cells for potential repair of injured arteries. PLoS One. 2012;7(2):e31137. doi: 10.1371/journal.pone.0031137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jendelova P, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76(2):232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 139.Bhirde A, et al. Nanoparticles for cell labeling. Nanoscale. 2011;3(1):142–153. doi: 10.1039/c0nr00493f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ko IK, et al. In vivo MR imaging of tissue-engineered human mesenchymal stem cells transplanted to mouse: a preliminary study. Ann Biomed Eng. 2007;35(1):101–108. doi: 10.1007/s10439-006-9204-7. [DOI] [PubMed] [Google Scholar]
- 141.Terrovitis JV, et al. Magnetic resonance imaging of ferumoxide-labeled mesenchymal stem cells seeded on collagen scaffolds-relevance to tissue engineering. Tissue Eng. 2006;12(10):2765–2775. doi: 10.1089/ten.2006.12.2765. [DOI] [PubMed] [Google Scholar]
- 142.Cai J, et al. In vivo MR imaging of magnetically labeled mesenchymal stem cells transplanted into rat liver through hepatic arterial injection. Contrast Media Mol Imaging. 2008;3(2):61–66. doi: 10.1002/cmmi.231. [DOI] [PubMed] [Google Scholar]
- 143.Rosen AB, et al. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25(8):2128–2138. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- 144.Choi J, et al. Multimodal imaging of sustained drug release from 3-D poly(propylene fumarate) (PPF) scaffolds. J Control Release. 2011;156(2):239–245. doi: 10.1016/j.jconrel.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jungebluth P, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. The Lancet. 2011;378(9808):1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]