Abstract
Background and Purpose
The sole FDA approved treatment for stroke is tPA, but its brief therapeutic window and complications of treatment constrain its use. One limitation may be its potential to exacerbate impairment of cerebral autoregulation after stroke. Vasodilation is maintained by elevations in cAMP. However, cAMP levels fall after stroke due to over-activation of NMDA receptors (NMDA-Rs) by toxic levels of glutamate, an effect that is exacerbated by tPA. Binding of wild type (wt-) tPA to the low-density lipoprotein-related receptor (LRP) mediates dilation. We propose that binding of wt-tPA to NMDA-Rs reduces cAMP and impairs vasodilation. We hypothesize that tPA-A296-299, a variant that is fibrinolytic but cannot bind to NMDA-Rs, preferentially binds to LRP and increases cAMP and p38, limiting autoregulation impairment after stroke.
Methods
Stroke was induced by photothrombosis in pigs equipped with a closed cranial window, CBF determined by microspheres, and CSF cAMP and p38 determined by ELISA.
Results
Stroke decreased CBF. CBF was reduced further during hypotension, indicating impairment of autoregulation. Autoregulation was further impaired by wt-tPA, which was prevented by MK801 and tPA-A296-299. Protection by tPA-A296-299 was blocked by anti-LRP Ab, the LRP antagonist RAP, and the p38 inhibitor SB 203580, but not by control IgG. Stroke reduced CSF cAMP, which was reduced further by wt-tPA, but augmented by tPA-A296-299. CSF p38 was unchanged by wt-tPA, increased by tPA-A296-299, and decreased by anti-LRP Ab and RAP.
Conclusions
tPA-A296-299 prevents impairment of cerebral autoregulation after stroke through a LRP-dependent increase in cAMP and p38.
Keywords: cerebral autoregulation, stroke, plasminogen activators, signal transduction
Introduction
The thrombolytic agent tissue-type plasminogen activator (tPA) remains the only approved treatment for ischemic stroke. However, its brief therapeutic window and high incidence of post-treatment complications, including intracranial hemorrhage (ICH), have constrained clinical use of tPA to approximately 5–8% of patients eligible for such therapy.1 Methods to improve the efficacy and safety of tPA remain an unmet need. We propose that one of the deleterious effects of tPA involves exacerbating an uncoupling of cerebral blood flow (CBF) from neuronal metabolism by aggravating pre-existing over-activity of N-methyl-D-aspartate receptors (NMDA-Rs) that occurs post stroke. Glutamatergic hyperactivity has been reported in animal models of stroke even in absence of exogenous tPA2 for which NMDA-R antagonists are protective.3 In healthy brain, wild type (wt) tPA is critical for the full expression and linkage of NMDA-R activation to NO synthesis and functional hyperemia, thereby coupling local metabolism to CBF. In CNS pathology, binding of glutamate to NMDA-Rs may also foster excess excitoxicity.4,5 Although any reduction in CBF might contribute to neurologic damage, little attention has been given to the role NMDA-Rs may play in this process. wt-tPA, given in a clinically relevant timeframe after stroke in the pig, disrupts CBF autoregulation by impairing vasodilation.6,7 In pigs with traumatic brain injury (TBI), we have shown that tPA impairs autoregulation by activating NMDA-Rs,8 but the contribution of NMDA-R activation by tPA to neurotoxicity after ischemic stroke, identification of the pathogenic intracellular signaling pathway and means to limit this damage are unknown. Although the NMDA-R antagonist MK 801 protects against cerebral dysregulation after TBI,9 its toxicity limits usage in humans indicating the need for novel approaches.
wt-tPA may damage brain tissue through breakdown of the blood brain barrier, and ICH, in addition to over-activation of NMDA-Rs.1,2 In this study we focused on the contribution of NMDA-Rs to the toxicity of tPA administered after stroke and asked whether this can be mitigated without loss of fibrinolytic activity. We took advantage of our finding that deletion of the “docking site” in tPA prevents its binding and activation of NMDA-Rs.10,11 Based on this, we constructed a variant, tPA-K296, A/H297, A/R298, A/R299, A (tPA-A296-299),10,11 that prevents the enzyme from binding to NMDA-Rs but fully maintains its fibrinolytic activity. We hypothesize that tPA-A296-299, a variant that is fibrinolytic but cannot bind to NMDA-Rs, preferentially binds to LRP, which increases cAMP and p38, limiting impairment of autoregulation after stroke.
Activation of NMDA-Rs has both vital and neurotoxic effects, but the mechanism(s) involved in this transition are less well understood.12–14 Elevation of cAMP and activation of PKA by glucagon prevents upregulation of endogenous tPA, diminishes NMDA-R excitotoxicity and protects cerebral autoregulation and responsiveness to vasodilator stimuli in the setting of TBI in the pig15, providing support for the hypothesis that interventions that limit tPA-NMDA-R activation and restore cAMP might improve outcome after thrombotic stroke. Phosphorylation (activation) of the cAMP response element (CRE)-binding (CREB) motif can occur by activation of mitogen activated protein kinase (MAPK).16,17 MAPK is a family of at least three kinases, ERK, JNK, and p38; the p38 isoform appears protective in the setting of stroke.7
tPA can bind to either the lipoprotein-related receptor (LRP), which mediates vasodilation, or NMDA-Rs. We propose that binding of wt-tPA binds to NMDA-Rs reduces cAMP and impairs ability of cerebral vessels to vasodilate whereas tPA-A296-299, a variant that is fibrinolytic but cannot bind to NMDA-Rs, preferentially binds to LRP, which increases cAMP and p38 and limits impairment of autoregulation after stroke.
Methods
Anesthetic regimen, closed cranial window technique, and cerebral photothrombosis
Pigs (4 weeks old) of either sex were studied. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The anesthetic regimen consisted of: pre-medication with dexmedetomidine (20 μg/kg im), induction with isoflurane (2–3%), isoflurane taper to 0% after start of total intravenous anesthesia (TIVA) with fentanyl (100 μg/kg/hr), midazolam (1mg/kg/hr), dexmedetomidine (2 μg/kg/hr), and propofol (2–10 mg/kg/hr), and maintenance of TIVA for the balance of the surgical and experimental portions of the pig preparation. A catheter was inserted into a femoral artery to monitor blood pressure and femoral veins for drug administration. The trachea was cannulated, the animals ventilated with room air, and temperature maintained in the normothermic range (37° – 39° C), monitored rectally. The closed cranial window technique was used to measure pial artery diameter and collect CSF for ELISA analysis.7,8 Hypotension was induced by the rapid withdrawal of either 5–8 or 10–15 ml blood/Kg to induce moderate or severe hypotension (decreases in mean arterial blood pressure of 25 and 45%, respectively). Such drops in blood pressure were maintained constant for 10 min by titration of additional blood withdrawal or blood reinfusion. CBF was measured in the cerebral cortex using radioactively labeled microspheres.18
Induction of photothrombosis was based on that described for the pig19, but in our studies we used the area of the closed cranial window to expose two to three main and 1–3 smaller arteries supplying the middle cerebral artery territory. Arterial occlusion was achieved by photothrombosis, in which a stable thrombus consisting of aggregating platelets, fibrin and other blood components is formed in response to endothelial peroxidative damage. The photochemical reaction occurs due to interaction of iv photosensitizing dye erythrosine B (20 mg/kg iv) and the focused beam of a solid state laser operated at 532 nm, power of 200 mW, average intensity of 60–75 W/cm2, and durations of up to 3–5 minutes using a Snake Creek minilaser (Hallstead, PA).
Protocol
Pial small arteries (resting diameter, 120–160 μm) were examined and similar sized pial arteries were used in male and female pigs. For sample collection, 300 μl of the total cranial window volume of 500 μl was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
Pigs were randomized to one of the following experimental groups (all n=5): (1) sham control, (2) photothrombosis (PTI), (3) PTI + wt-tPA (1 mg/kg iv), (4) PTI + MK 801 (10−5 M), (5) PTI + tPA-A296-299 (1 mg/kg iv), (6) PTI + tPA-A296-299 + IgG (10μg/ml), (7) PTI + tPA-A296-299 + anti LRP Ab (10 μg/ml), (8) PTI + tPA-A296-299 + RAP (10−7 M), and PTI + tPA-A296-299 + SB 203580 (1 mg/kg iv). The vehicle for all agents was 0.9% saline, except for SB 203580 which used dimethyl sulfoxide (100 μl) diluted with 9.9 ml 0.9% saline. In sham control and photothrombotic animals, responses to hypotension and isoproterenol (10−8, 10−6 M) were obtained initially and then again 5h later in the presence of vehicle. In drug treated photothrombotic animals, drugs were administered 4h after injury, and the insult protocol followed. Pial artery reactivity was determined in small pial arteries close to the area of injury (peri-ischemic area) using the closed cranial window technique.18
ELISA
Commercially available ELISA Kits were used to quantity CSF p38 and cAMP (Assay Designs, EMD; Enzo Life Sciences) concentration.
Infarct volume
Staining with 2,3,5-triphenyltetrazolium chloride (TTC) was used to determine infarct volume.20 The brains of pigs (1–5 days old) were first perfused transcardially with heparinized saline, followed by 2% TTC. Brains were stored in TTC for 20 min in the dark, fixed in 4% paraformaldehyde for 48h, and serial 5 mm sections were prepared. The area of ischemic damage of the hemispheres was measured for each brain slice using Image J software. Complete lack of TTC staining was defined as core and viable tissue stained red. Total infarction volume was taken to be the sum of core and penumbra volumes. Damage volume was calculated as percentage of ipsilateral hemisphere volume (Damage/Total Volume X 100). This was done for 3 hemispheric brain slices per animal and the mean value taken as the numeric for that individual pig. Animal groups (n=4–5) were sham control, PTI, PTI + tPA (1 mg/kg iv), and PTI + tPA-A296-299. The drugs wt-tPA and tPA-A296-299 (1 mg/kg iv) were administered at 4h and TTC perfusion was completed at 5h post stroke.
Statistical analysis
Pial artery diameter, CBF, CSF p38, cAMP, and infarct volume values were analyzed using ANOVA for repeated measures. The order of vascular stimuli application was randomized and no data were excluded from analysis for statistical difference. The Investigator was blinded to treatment. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. An α level of p<0.05 was considered significant in all statistical tests. A sample size calculation determined that with an n of 5, statistical determination could be made with p<0.05 and power of 0.87 for hemodynamic values and 0.84 for ELISA values. Values are represented as mean ± SEM of the absolute value or as percentage changes from control value.
Results
tPA-A296-299 prevents, whereas wt-tPA aggravates, impairment of cerebral autoregulation when administered 4h after stroke
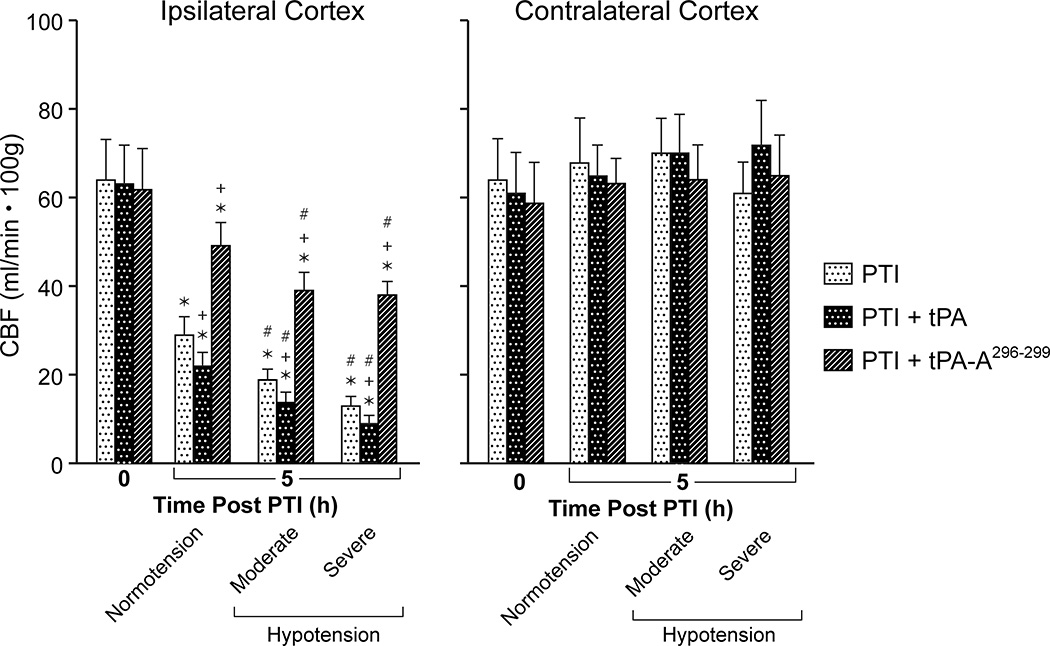
CBF was unchanged during hypotension (moderate 25%, severe 45% decrease in mean arterial blood pressure, MAP), in sham control animals, indicating cerebral autoregulation was intact (data not shown). Values for MAP during normotension, moderate and severe hypotension were 75 ± 9, 56 ± 6, and 42 ± 5 mm Hg, respectively, n =5. CBF was reduced in brain tissue surrounding the infarct at 5h post thrombotic stroke and reduced further during hypotension, indicating impairment of autoregulation (Fig 1). Administration of wt-tPA (at 4h post stroke) elicited a short lived normalization of CBF7, but blood flow returned to a level below that observed in untreated pigs by 5h post stroke (Fig 1). In fact, administration of tPA at 4h further aggravated reductions in CBF during normotension and hypotension compared to vehicle at 5h post stroke (Fig 1). In contrast, administration of tPA-A296-299 prevented impairment of autoregulation when administered at 4h post stroke (Fig 1). CBF was unchanged by stroke or drug treatment in the contralateral cortex, demonstrating the focal effects of stroke induction and limiting the possibility that drug-induced effects were an epiphenomenon (Fig 1). There was no significant effect of either wt-tPA or tPA-A296-299 on MAP (78 ± 10 vs 76 ± 9 mm Hg and 74 ± 9 vs 78 ± 10 mm Hg, respectively, n=5).
Figure 1.
Effect of photothrombosis (PTI) on CBF (ml/min .100g) during normotension and hypotension (moderate, severe) at 5h post insult in the ipsilateral and contralateral cortex in the absence and presence of wt-tPA and tPA-A296-299 (1 mg/kg iv), administered at 4h post insult, n=5. *p<0.05 versus corresponding 0 time value +p<0.05 versus corresponding PTI alone value #p<0.05 versus corresponding normotension value.
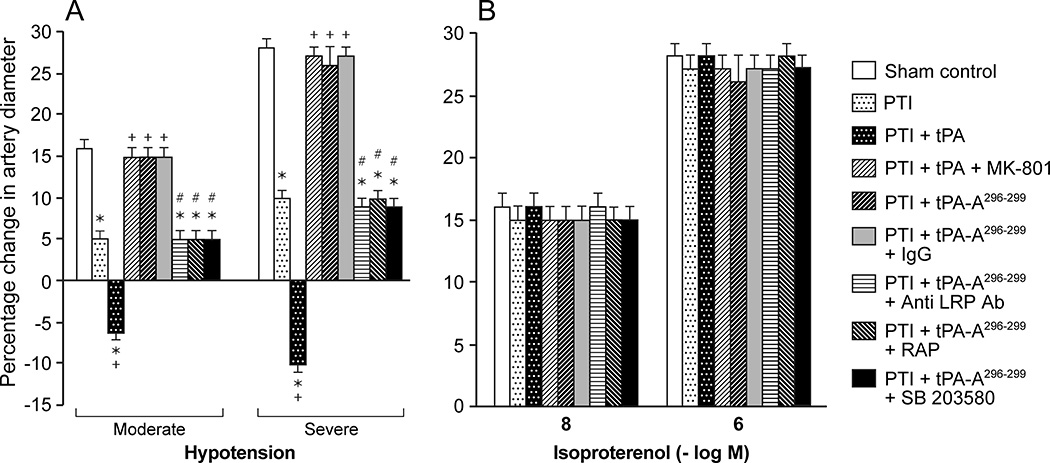
Pial artery dilation in response to hypotension is reproducible over time in sham control animals (data not shown), indicating intact autoregulation. However, dilation of pial arteries during hypotension was blunted at 5h after stroke (Fig 2), indicating disturbed autoregulation. Administration of wt-tPA 4h post insult reversed pial artery dilation to vasoconstriction, whereas tPA-A296-299 protected autoregulatory vasodilation during hypotension (Fig 2). Since isoproterenol-induced dilation was unchanged by thrombotic stroke and by wt-tPA or by tPA-A,296-299 these data show that impairment of autoregulation after stroke is not an epiphenomenon. Binding of tPA to NMDA-R vs LRP alters outcome of impairment of cerebral autoregulation after stroke.
Figure 2.
Influence of hypotension (moderate, severe) and isoproterenol (10−8,10−6 M) on pial artery diameter in pigs before (sham control), and after photothrombotic injury (PTI), or treated at 4h post injury with wt-tPA (1 mg/kg iv), tPA + MK801 (10−5 M), tPA-A296-299 (1 mg/kg iv), tPA-A296-299 + IgG (10 μg/ml), tPA-A296-299+ anti-LRP Ab (10 μg/ml), tPA-A296-299 + RAP (10−7 M), or tPA-A296-299 + SB203580 (1mg/kg iv), n=5. *p<0.05 versus corresponding sham control value +p<0.05 versus corresponding untreated PTI value #p<0.05 versus tPA-A296-299 alone.
MK 801 (10−5 M) blocked tPA-mediated impairment of pial artery dilation during hypotension to the same extent as tPA-A296-299 (Fig 2). On the other hand, co-administration of anti-LRP antibody (10 μg/ml) or the LRP antagonist RAP21,22 (10−7M), but not control IgG (10 μg/ml), along with tPA-A296-299 blocked its ability to protect pial artery dilation during hypotension after stroke (Fig 2).
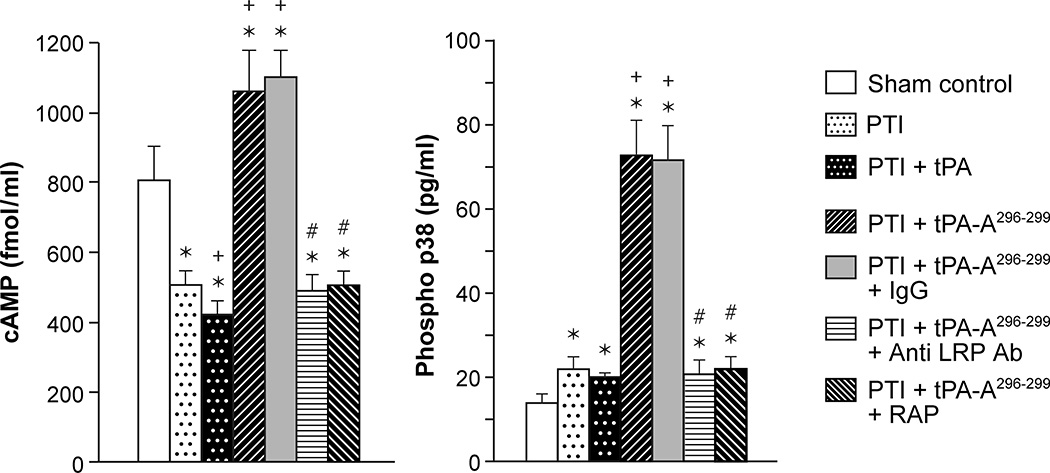
Thrombotic stroke in the pig reduces CSF cAMP, reduced further by wt-PA, but prevented by tPA-A296-299. cAMP and p38 MAPK are augmented by tPA-A296-299 in an LRP dependent manner
We next explored the intracellular signaling pathways activated by wt-tPA via LRP. Drugs were administered 4h post stroke as above and the concentration of cAMP and p38 MAPK in CSF was measured 1h later. The concentrations of substances in CSF reflects those in brain parenchyma.6,7 Thrombotic stroke reduced the concentration of cAMP, which was further reduced by administration of wt-tPA, but cAMP was augmented above levels in sham (control) animals after treatment with tPA-A296-299 (Fig 3). In contrast, p38 MAPK was robustly augmented in animals given tPA-A296-299 (Fig 3). wt-tPA administered after stroke modestly, though not significantly, decreased cAMP compared to post stroke pigs not receiving tPA. Co-administration of anti-LRP Ab or RAP but not control IgG with tPA-A296-299 blocked upregulation of cAMP and p38, indicating that the tPA variant modulates cAMP and p38 concentration in an LRP-dependent manner (Fig 3).
Figure 3.
CSF cAMP and phosphorylated p38 MAPK prior to thrombotic stroke (sham control), after stroke (PTI), or treated at 4h post injury with wt-tPA (1 mg/kg iv), tPA-A296-299 (1 mg/kg iv), tPA-A296-299 + IgG (10 μg/ml), tPA-A296-299+ anti LRP Ab (10 μg/ml), or tPA-A296-299 + RAP (10−7 M), n=5. *p<0.05 versus corresponding sham control value +p<0.05 versus corresponding untreated PTI value #p<0.05 versus tPA-A296-299 alone.
tPA-A296-299 reduces infarct volume after thrombotic stroke
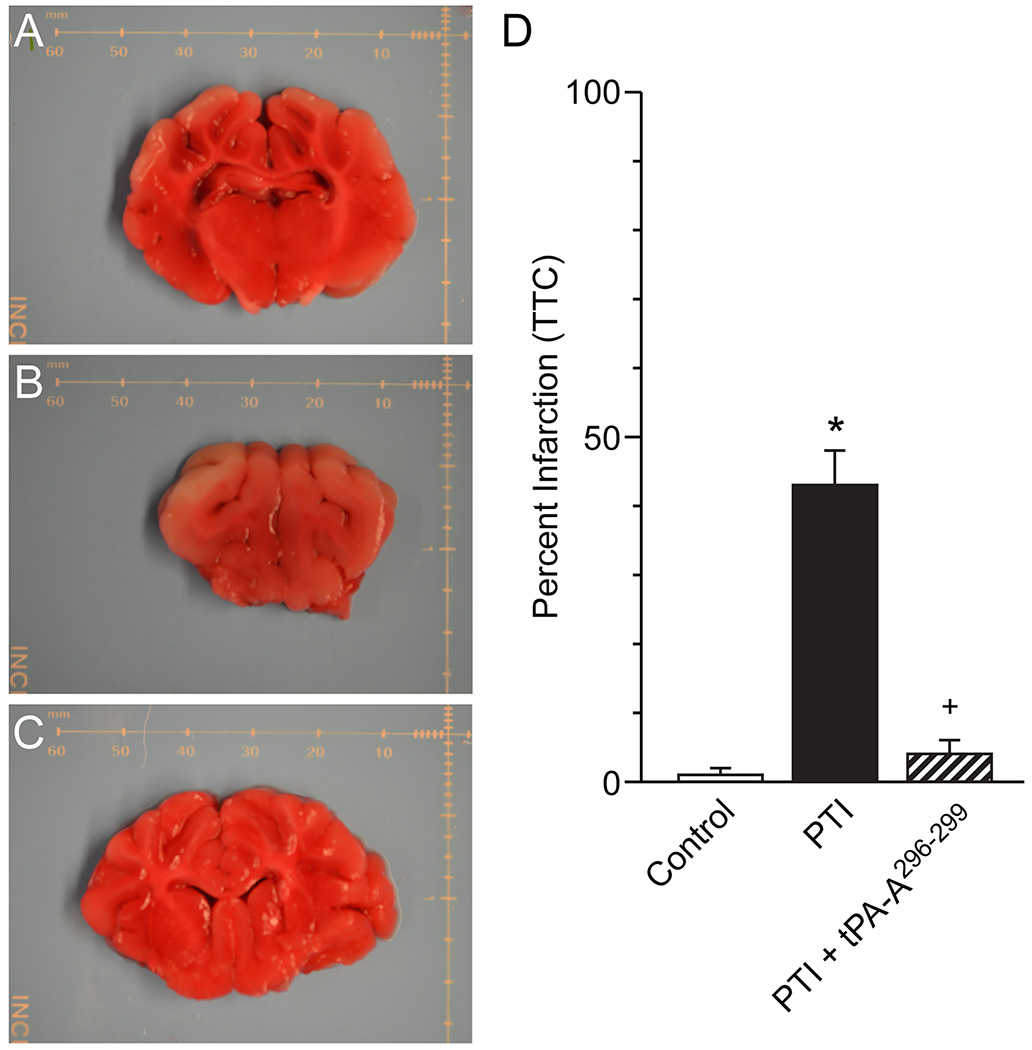
The amount of brain tissue stained with TTC was reduced after stroke ipsilateral, but not contralateral, to the insult consistent with the ability of this stain to differentiate healthy from damaged brain tissue (Fig 4). On a percentage basis, the volume of damaged tissue increased after stroke to the same extent in vehicle and wt-tPA (1 mg/kg iv) treated animals but was reduced to a value statistically no different than control value in animals treated with tPA-A296-299 (Fig 4).
Figure 4.
(A) Representative sham control brain slice stained with TTC, (B) corresponding typical brain slice from a photothrombotic stroke (PTI) pig (left hemisphere) treated with vehicle and (C) typical brain slice from a pig post stroke (left hemisphere) treated with tPA-A296-299, distance marker is in mm. (D) Summary data showing on a percentage basis the volume of damaged tissue volume in sham control, PTI stroke and animals post stroke treated with tPA-A296-299 (1 mg/kg iv), n=4–5. *p<0.05 versus corresponding sham control value +p<0.05 versus corresponding untreated PTI value.
Blood chemistry
Blood chemistry values were collected before and after all experiments. There were no statistically significant differences between sham control, tPA, and tPA-A296-299-treated animals. Specifically, the values were 7.44 ± 0.05, 38 ± 4, and 94 ± 11 and 7.43 ± 0.06, 37 ± 5, and 95 ± 11 mm Hg for pH, pCO2, and pO2 at the beginning and end of the experiments in sham controls, while pH, pCO2, and pO2 values were 7.45 ± 0.04, 37 ± 5, and 91 ± 11 and 7.43 ± 0.03, 39 ± 5, and 93 ± 11 mm Hg for pH, pCO2, and p O2 at the beginning and end of the experiments in tPA-A296-299 -treated animals, respectively.
Discussion
An important new finding of translational relevance in this study is that the tPA variant tPA-A296-299 prevents reductions in CBF and impairment of cerebral autoregulation when administered in a clinically relevant timeframe (4h) after thrombotic stroke. In contrast, standard of care administration of wt-tPA in the same timeframe only transiently restores CBF6,7 and blood flow values are in the ischemic range when determined at 1h post drug administration. Further, wt-tPA did not prevent impairment of cerebral autoregulation after stroke. The functional significance of these observations is that cognitive outcome (GCS) and risk of death have been shown to correlate with the severity of cerebral autoregulatory impairment in other CNS ischemic conditions, such as human traumatic brain injury.23 Since isoproterenol-induced dilation was unchanged by thrombotic stroke and by tPA or tPA-A,296-299 these data show that impairment of autoregulation after stroke is not an epiphenomenon. Additionally, the finding that infarct volume was reduced by tPA-A296-299 to a value statistically no different than sham control, indicates that this tPA variant both protects cerebral hemodynamics and prevents histopathology associated with the development of stroke.
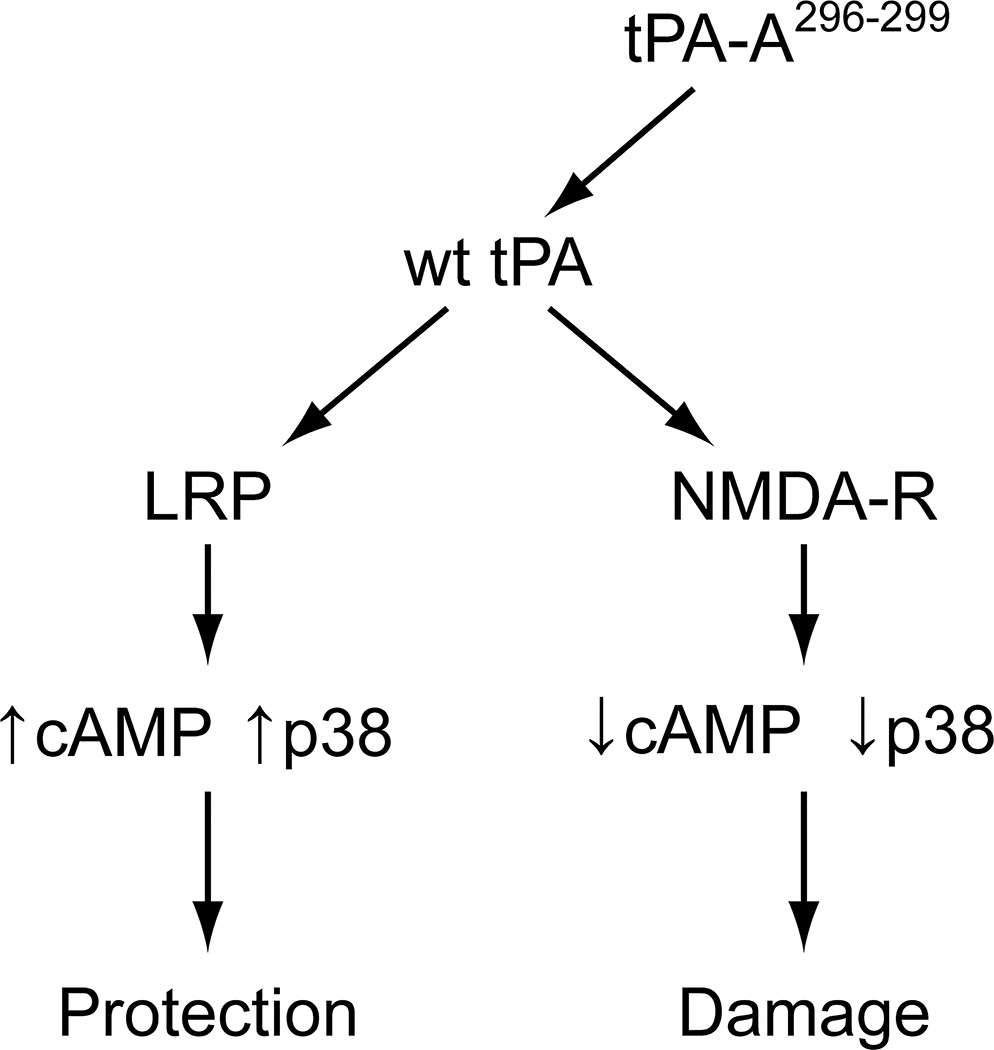
wt-tPA can bind to either LRP, important in mediating vasodilation to vasoactive stimuli, or to NMDA-Rs (Fig 5). We propose that after stroke, the pathway shown on the right side predominates based on release of endogenous tPA and is markedly accelerated by therapeutic administration of wt-tPA. This directly impacts the mechanisms responsible for compensatory vasodilation. For example, elevation of cAMP and the cAMP response element (CRE)-binding (CREB) motif is an important mechanism contributing to vasodilation. Phosphorylation (activation) of CREB can occur by activation of MAPK.16,17 However, toxic levels of glutamate and over-activation of NMDA-Rs in the setting of stroke activate phosphodiesterases that decrease cAMP (Fig 5). MAPK is a family of at least 3 isoforms: ERK, p38, and JNK. The JNK isoform is upregulated robustly and contributes to tPA-mediated impairment of autoregulation after thrombotic stroke in the pig.6,7 Indeed, results of the present study show that co-administration of the NMDA-R antagonist with wt-tPA prevented impairment of cerebral autoregulation after stroke, supporting the predominance of the pathway shown on the right side in Figure 5, wherein wt-tPA acts primarily on NMDA-Rs to contribute to damage after stroke, presumably, in part, via upregulation of JNK MAPK.6,7
Figure 5.
Proposed signaling pathways and outcomes after stroke and administration of wt tPA or tPA-A.296-299 wt-tPA drives pathway toward the right leading to damage whereas tPA-A296-299 drives pathway towards the left and providing protection.
Additional mechanistic studies were designed to address our hypothesis that the administration of tPA-A296-299 will drive the pathway depicted in Figure 5 to the left which would protect autoregulation. We decided to investigate the p38 isoform of MAPK because prior studies showed that interventions that selectively upregulate this isoform protect autoregulation in the setting of thrombotic stroke in the pig.7 Indeed, we observed that protection by tPA-A296-299 was blocked by anti-LRP Ab, the LRP antagonist RAP,21,22 the p38 inhibitor SB 203580, but not by control IgG, supportive of activity mediated primarily through LRP rather than NMDA-Rs. Additionally, we observed that stroke decreased the concentration of cAMP in the CSF, which was reduced further by administration of wt-tPA, but the levels were increased robustly by tPA-A.296-299 These data support the hypothesis that activation of NMDA-Rs by tPA leads to decreased cAMP, but driving the pathway to the left by administration of tPA-A296-299 results in elevation of CSF cAMP concentration (Fig 5). In other studies, we investigated effects of pharmacologic intervention on cAMP and the p38 MAPK isoform. In particular, we noted that tPA-A296-299 increased phosphorylated (activated) p38 but wt-tPA did not. Elevations of cAMP and p38 by tPA-A296-299 were blocked by anti-LRP antibody and RAP, but not by control IgG. Taken together with the vascular response data, these observations indicate that tPA-A296-299 preserves cerebral autoregulation after stroke via LRP-mediated upregulation of cAMP and p38, whereas wt-tPA impairs vascular responsiveness by opposing these two signaling pathways through activating NMDA-Rs (Fig 5). We measured p38 in the CSF because in prior studies6–8,15,18 we observed that its concentration parallels change in magnitude and direction in brain parenchyma in response to vascular stimuli.
Historically, the neurotoxic effects of wt-tPA in the brain have received considerable attention. tPA activity increases rapidly in ischemic tissue after middle cerebral artery occlusion (MCAO)24,25 and mice genetically deficient in tPA (tPA−/−) have reduced infarct volumes after MCAO.24,26 More recently, studies have focused on mechanisms that link the neurotoxic effects of wt-tPA to NMDA-R signaling. For example, tPA may enhance NMDA-R mediated calcium influx and activate the ERK isoform of MAPK to cause neurotoxicity.27–32 However, additional studies are needed to establish the exact role of wt-tPA neurotoxicity in the clinical setting.
Indeed, wt-tPA may also provide neuroprotection in some settings.33 Enhancement of NMDA-R signaling by tPA may depend on the type of neuron or the absence of astrocytes.30–32 It is possible that activation of NMDA-R subunits within the synapse may support neuronal survival whereas high or sustained levels of activation of extrasynaptic NMDA-Rs may promote cell death.34,35 This, in turn, may depend on the expression level or distribution of NR2A-D subunits that must interact with NR1 for NMDA-Rs to be functional. The level and subcellular localization of wt-tPA may also help to determine whether it promotes cell death or survival via a plasminogen-independent proteolytic cleavage of an NR subunit28 or non-proteolytic signaling through LRP via activation of mTOR, accumulation of HIF-1a, recruitment of glucose GLUT3 to the plasma membrane, and GLUT3-mediated uptake of glucose by neurons in the ischemic brain.28,30
Finally, there are a few experimental caveats that should be discussed regarding our data in the present study. Additional work is needed to identify the anatomical location of tPA and NMDA-R and their interaction observed in the present study. Others have observed the presence of tPA in cerebrovascular endothelial cells and oligodendrocytes, but not in pericytes, microglial cells, or astrocytes.36 NMDA-Rs have been identified demonstrated on microglia in murine and human CNS.37 We have evidence that the NR1 subunit of the NMDA-R is expressed in brain vascular smooth muscle cells (unpublished observations), but more rigorous testing is needed. Additionally, the mechanism linking binding to LRP to elevation of CSF cAMP and p38 concentration will need to be investigated. We speculate that p38 may increase cAMP16,17 which may, in turn, cause further phosphorylation of p38 in a feed forward cycle. The opposite sequence (p38 causing decrease in cAMP) may occur via activation of NMDA-Rs by wt-tPA. It is known that the JNK isoform of MAPK is upregulated after stroke and further upregulated by wt-tPA in the setting of stroke, contributing to impairment of cerebral autoregulation.7 However, mechanism(s) that mediate interactions between p38 and JNK and alter their profile in the setting of stroke to foster protection or damage will also require additional study. Further, there may be additional downstream mechanism(s) that link p38 to protection and JNK to damage after stroke (and their manipulation by wt-tPA and tPA-A296-299) that have not been identified. Future experiments will be conducted to address these and other unresolved relevant issues.
Conclusions
The results of the present study show that tPA-A296-299 blocks the increase in TTC staining associated with infarct volume generation and prevents impairment of cerebral autoregulation after stroke through an LRP-dependent increase in cAMP and p38.
Acknowledgments
We thank Hugh Hekierski for expert analysis of infarct volume with use of TTC staining.
Sources of Funding
We thank the University of Pennsylvania Institute for Translational Medicine and Therapeutics and NIH UL1RR024134 (WMA), P01 HL40387, R01 HL116916, and R21 NS080014 (DBC) for its generous funding of this research.
Footnotes
Conflicts of Interest/Disclosure
Nothing to declare.
References
- 1.Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:1–6. doi: 10.1007/s11910-002-0051-0. [DOI] [PubMed] [Google Scholar]
- 2.Lipton P. Ischemic cell death in brain neurons. Physiolog Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 3.Armstead WM. NOC/oFQ and NMDA contribute to piglet hypoxic ischemic hypotensive cerebrovasodilation impairment. Ped Res. 2002;51:586–591. doi: 10.1203/00006450-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 5.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Armstead WM, Riley J, Kiessling JW, Cines DB, Higazi AAR. Novel plasminogen activator inhibitor-1 derived peptide protects against impairment of cerebrovasodilation after photothrombosis through inhibition of JNK MAPK. Am J Physiol. 2010;299:R480–R485. doi: 10.1152/ajpregu.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstead WM, Ganguly K, Riley J, Kiessling JW, Cines DB, Higazi AAR, et al. RBC-coupled tPA prevents impairment of cerebral vasodilatory responses through inhibition of JNK and potentiation of p38 MAPK after cerebral photothrombosis. Ped Crit Care Med. 2011;12:369–375. doi: 10.1097/PCC.0b013e3181fe40a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstead WM, Kiessling JW, Riley J, Cines DB, Higazi AAR. tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNK MAPK. Neurological Research. 2011;33:726–733. doi: 10.1179/016164110X12807570509853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstead WM. Age dependent NMDA contribution to impaired hypotensive cerebral hemodynamics following brain injury. Develop Brain Res. 2002;139:19–28. doi: 10.1016/s0165-3806(02)00511-4. [DOI] [PubMed] [Google Scholar]
- 10.Nassar T, Yarovoi S, Abu Fanne R, Akkawi S, Jammal M, Allen T, et al. Regulation of airway contractility by plasminogen activators through NMDA receptor-1. Am J Respir Cell Molec Biol. 2010;43:703–711. doi: 10.1165/rcmb.2009-0257OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassar T, Bdeir K, Yarovoi S, Fanne RA, Murciano, Idell S, et al. tPA regulates pulmonary vascular activity through NMDA receptors. Am J Physiol. 2011;301:L307–L314. doi: 10.1152/ajplung.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collingridge G, Lester R. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989;41:143–210. [PubMed] [Google Scholar]
- 13.Nakanishi S, Masu M. Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol. 1994;23:319–348. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- 14.Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 15.Armstead WM, Kiessling JW, Cines DB, Higazi AAR. Glucagon protects against impaired NMDA-mediated cerebrovasodilation and cerebral autoregulation during hypotension after brain injury by activating cAMP protein kinase A and inhibiting upregulation of tPA. J Neurotrauma. 2011;28:451–457. doi: 10.1089/neu.2010.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaywitz A, Greenberg M. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 17.Ginty D, Kornhauser J, Thompson M, Bading H, Mayo KE, Takahashi JS, et al. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 18.Armstead WM, Riley J, Kiessling JW, Cines DB, Higazi AAR. Novel plasminogen activator inhibitor-1 derived peptide protects against impairment of cerebrovasodilation after photothrombosis through inhibition of JNK MAPK. Am J Physiol. 2010;299:R480–R485. doi: 10.1152/ajpregu.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuluz JW, Prado R, He D, Zhao W, Dietrich WD, Watson B. New pediatric model of ischemic stroke in infant piglets by photothrombosis. Stroke. 2007;38:1932–1937. doi: 10.1161/STROKEAHA.106.475244. [DOI] [PubMed] [Google Scholar]
- 20.Kramer M, Dang J, Baertling F, Denecke B, Clarner T, Kirsch C, et al. TTC staining of damaged brain areas after MCA occlusion in the rat does not constrict quantitative gene and protein analyses. J of Neuroscience Methods. 2010;187:84–89. doi: 10.1016/j.jneumeth.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Bu G, Williams S, Strickland DR, Schwartz Al. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassar T, Haj-Yehia A, Akkawi S, Kuo A, Bdeir K, Mazar A, et al. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem. 2002;277:40499–40504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- 23.Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology. 2008;108:588–595. doi: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- 24.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild type and tPA-deficient mice. Nat Med. 1998;4:2–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 25.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Colleen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 27.Parcq J, Bertrand T, Montagne A, Baron AF, Macrez R, Billard JM, et al. Unveiling an exceptional zymogen: the single chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity. Cell Death and Differentiation. 2012;19:1983–1991. doi: 10.1038/cdd.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F, Echeverry R, Wu J, An J, Haile WB, Cooper DS, et al. Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ErK1/2 CREB-ATF3 signaling pathway. Mol Cell Neurosci. 2013;52:9–19. doi: 10.1016/j.mcn.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu F, Wu J, Nicholson AD, Echeverry R, Haile WB, Catano M, et al. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J Neurosci. 2012;32:9848–9858. doi: 10.1523/JNEUROSCI.1241-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertrand T, Lesept F, Chevilley A, Lenoir S, Aimable M, Briens A, et al. Conformations of tissue plasminogen activator (tPA) orchestrate neuronal survival by a cross talk between EGFR and NMDAR. Cell Death and Disease. 2015;6:e1924. doi: 10.1038/cddis.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rijken DC, Colleen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981;256:7035–7041. [PubMed] [Google Scholar]
- 32.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 33.Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the central nervous system. Frontiers in Cellular Neuroscience. 2015;9:304. doi: 10.3389/fncel.2015.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Wong T, Aarts M, Rooyakkers A, Liu L, Lai TW, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signaling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louessard M, Lacroix A, Martineau M, Mondielli G, Montagne A, Lesept F, et al. Tissue plasminogen activator expression is restricted to subsets of excitatory pyramidal glutamatergic neurons. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9432-7. [DOI] [PubMed] [Google Scholar]
- 37.Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, et al. Activation of microglial N-methyl-D-Aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–549. doi: 10.1002/ana.23626. [DOI] [PubMed] [Google Scholar]