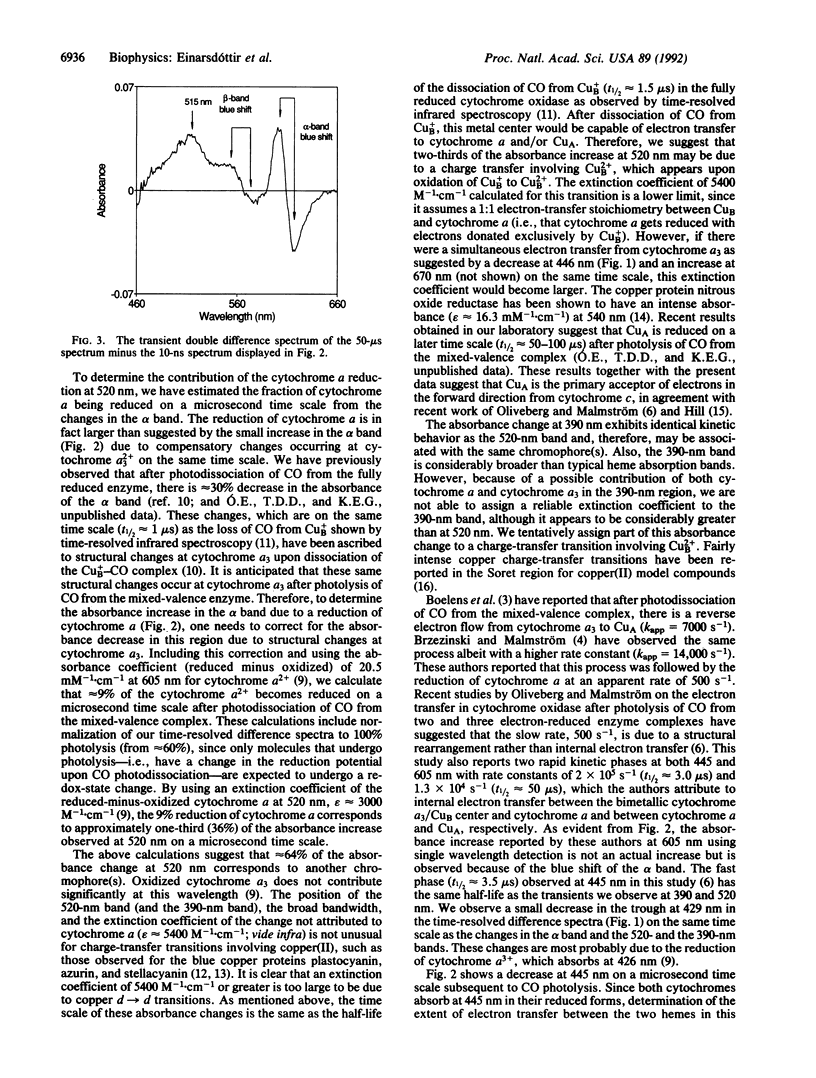
Abstract
Electron transfer following photolysis of CO from mixed-valence (cytochrome a3+ Cu2+A cytochrome a2+3-CO Cu+B) cytochrome oxidase (ferrocytochrome-c; oxygen oxidoreductase, EC 1.9.3.1) was studied on time scales of nanoseconds to milliseconds by multichannel time-resolved optical absorption spectroscopy. In this method, the optical absorption was measured at many wavelengths simultaneously by using an optical spectrometric multichannel analyzer system. The high-quality time-resolved difference spectra showed a large increase on a microsecond time scale in the visible region centered at approximately 520 nm and in the UV region centered at approximately 390 nm. These absorbance changes were not observed after photodissociation of CO from the fully reduced enzyme and therefore are attributed to intramolecular electron transfer. Simultaneously, there was a blue shift and a small increase in the alpha band, which is attributed to the reduction of cytochrome alpha. Approximately one-third of the absorbance change at 520 nm can be attributed to reduction of cytochrome a. The absorbance changes associated with the 520- and the 390-nm bands are on the same time scale (t1/2 approximately 2 microseconds) as the dissociation of CO from Cu+B reported previously by time-resolved infrared spectroscopy. The position and shape of these bands are reasonable for charge-transfer transitions involving copper(II). We suggest that the absorbance increase at 520 nm, which cannot be attributed to a reduction of cytochrome a, may represent a charge transfer involving Cu2+B accompanying the oxidation of Cu+B to Cu2+B. The absorbance increase at 390 nm is also partially attributed to this transition. These results suggest that Cu2+B may be observed spectrophotometrically in the electron-transfer dynamics of cytochrome oxidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amundsen A. R., Whelan J., Bosnich B. Biological analogues. On the nature of the binding sites of copper-containing proteins. J Am Chem Soc. 1977 Sep 28;99(20):6730–6739. doi: 10.1021/ja00462a042. [DOI] [PubMed] [Google Scholar]
- Boelens R., Wever R. Electron-transfer processes in carboxy-cytochrome c oxidase after photodissociation of cytochrome a3 2+ . CO. Biochim Biophys Acta. 1979 Aug 14;547(2):296–310. doi: 10.1016/0005-2728(79)90012-4. [DOI] [PubMed] [Google Scholar]
- Boelens R., Wever R., Van Gelder B. F. Electron transfer after flash photolysis of mixed-valence carboxycytochrome c oxidase. Biochim Biophys Acta. 1982 Nov 15;682(2):264–272. doi: 10.1016/0005-2728(82)90107-4. [DOI] [PubMed] [Google Scholar]
- Brzezinski P., Malmström B. G. The mechanism of electron gating in proton pumping cytochrome c oxidase: the effect of pH and temperature on internal electron transfer. Biochim Biophys Acta. 1987 Oct 29;894(1):29–38. doi: 10.1016/0005-2728(87)90209-x. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Clore G. M., Andréasson L. E., Karlsson B., Aasa R., Malmström B. G. Characterization of the low-temperature intermediates of the reaction of fully reduced soluble cytochrome oxidase with oxygen by electron-paramagnetic-resonance and optical spectroscopy. Biochem J. 1980 Jan 1;185(1):139–154. doi: 10.1042/bj1850139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle C. L., Zumft W. G., Kroneck P. M., Körner H., Jakob W. Nitrous oxide reductase from denitrifying Pseudomonas perfectomarina. Purification and properties of a novel multicopper enzyme. Eur J Biochem. 1985 Dec 16;153(3):459–467. doi: 10.1111/j.1432-1033.1985.tb09324.x. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. Reactions of cytochrome oxidase with oxygen and carbon monoxide. Biochem J. 1963 Mar;86:541–554. doi: 10.1042/bj0860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C., Greenwood C., Nicholls P. Intermediate steps in the reaction of cytochrome oxidase with molecular oxygen. Biochim Biophys Acta. 1986;853(2):91–113. doi: 10.1016/0304-4173(86)90006-6. [DOI] [PubMed] [Google Scholar]
- Hill B. C. The reaction of the electrostatic cytochrome c-cytochrome oxidase complex with oxygen. J Biol Chem. 1991 Feb 5;266(4):2219–2226. [PubMed] [Google Scholar]
- Morgan J. E., Li P. M., Jang D. J., el-Sayed M. A., Chan S. I. Electron transfer between cytochrome a and copper A in cytochrome c oxidase: a perturbed equilibrium study. Biochemistry. 1989 Aug 22;28(17):6975–6983. doi: 10.1021/bi00443a030. [DOI] [PubMed] [Google Scholar]
- Oliveberg M., Malmström B. G. Internal electron transfer in cytochrome c oxidase: evidence for a rapid equilibrium between cytochrome a and the bimetallic site. Biochemistry. 1991 Jul 23;30(29):7053–7057. doi: 10.1021/bi00243a003. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- Woodruff W. H., Einarsdóttir O., Dyer R. B., Bagley K. A., Palmer G., Atherton S. J., Goldbeck R. A., Dawes T. D., Kliger D. S. Nature and functional implications of the cytochrome a3 transients after photodissociation of CO-cytochrome oxidase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2588–2592. doi: 10.1073/pnas.88.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


