Abstract
Background: Insomnia is associated with several adverse health outcomes. Small clinical studies have suggested that an inferior nutrition status is a potential explanation, but to our knowledge, this possibility has not been examined in a large-scale, population-based cohort study.
Objective: We examined whether individuals with probable insomnia and individual insomnia symptoms had greater energy intake and a lower diet quality as assessed with the use of the Alternate Healthy Eating Index (AHEI) 2 y later.
Design: A cohort study of 15,273 US men aged 58–93 y who were free of cancer, cardiovascular diseases, and diabetes and were participating in the Health Professionals Follow-Up Study reported information on insomnia symptoms in 2004. Dietary intake was assessed with the use of a food-frequency questionnaire in 2002 and 2006. We calculated the adjusted mean differences of total energy intake in 2006 and the AHEI-component scores and their 95% CIs between subjects with and without probable insomnia in 2004 and also across categories for each insomnia symptom while adjusting for related covariates.
Results: After dietary intake in 2002, major chronic conditions, and other potential confounders were controlled for, men with probable insomnia had a mean higher consumption of 35.8 kcal/d (95% CI: 17.4, 54.1 kcal/d) and had lower scores in 3 individual AHEI components (trans fat, vegetables, and sodium), which denoted higher consumption of trans fat and sodium and lower intake of vegetables (P ≤ 0.01 for all). For individual insomnia symptoms, nonrestorative sleep and a difficulty maintaining sleep were associated with higher energy intake (P-trend ≤ 0.007 for both). A similar trend was observed in men who had difficulty initiating sleep (P-trend = 0.07). We also observed a significant association between the difficulty of initiating sleep and a lower AHEI score 2 y later (P-trend = 0.004).
Conclusion: Probable insomnia is associated with higher intakes of total energy, trans fat, and sodium and lower intake of vegetables.
Keywords: cohort, diet quality, energy intake, insomnia, sleep disorders
INTRODUCTION
Insomnia affects ∼10–30% of Americans and is characterized by a difficulty initiating sleep, difficulty maintaining sleep, early-morning awakenings, and nonrestorative sleep (1, 2). There is a growing body of literature that suggests that individuals with insomnia may have increased risk of adverse health outcomes, including metabolic syndrome (3), hypertension (4), myocardial infarction (5), and diabetes (6). In a large-scale prospective study, we previously reported that insomnia and certain insomnia symptoms, especially a difficulty initiating sleep and nonrestorative sleep, were associated with higher risk of mortality in the HPFS (Health Professionals Follow-Up Study)8 (7). However, the reasons for these associations remain unclear. One potential hypothesis is that individuals with such symptoms may have greater energy intake and lower dietary quality, which are known factors for inferior health outcomes (8, 9). Although this hypothesis has been supported by some small clinical studies (10–12), to our knowledge it has yet to be investigated in a larger-scale, population-based cohort study. Of current sleep-quality studies, most focused on the individual macronutrient or micronutrient as the outcome (13–16). Because most individuals consume a combination of nutrients, it is important to consider the overall dietary quality [e.g., the Alternate Healthy Eating Index (AHEI)]. A better understanding of this relation could help to explain the observed association between insomnia and other diseases and provide support for implementing an early intervention as a potential strategy to address this issue. In addition, the 2015 Dietary Guidelines Advisory Committee determined that “sleep patterns was an emerging area with an insufficient body of evidence” (17). Thus, we conducted an analysis to examine whether individuals with probable insomnia had greater energy intake and lower diet quality 2 y later as assessed with the AHEI-2010 in the HPFS cohort while accounting for the effects of a variety of lifestyle factors and medical comorbidities that are known to affect nutrition behavior and outcomes.
METHODS
Participants
The HPFS was established in 1986 when 51,529 male US health professionals aged 40–75 y completed a mailed questionnaire about their medical histories and lifestyles. Follow-up questionnaires were mailed to participants every 2 y to update information on potential risk factors and to ascertain newly diagnosed diseases. Food consumption was collected every 4 y via a validated semiquantitative food-frequency questionnaire (FFQ) (18, 19). Follow-up rates have averaged 94% for each 2- or 4-y cycle.
In 2004, 34,884 men responded to the 2004 questionnaire, which included questions about insomnia. We excluded men with a physician diagnosis of cancer [other than nonmelanoma skin cancer; n = 7592)], cardiovascular disease (n = 3311), or diabetes (n = 2142) because individuals with these conditions generally change their diets and sleep habits. We also excluded men (n = 3847) with implausible FFQ data (<800 or >4200 kcal/d or >70 food items with missing data) and missing values for insomnia questions (n = 1483) and the AHEI in 2002 (n = 1236), which left 15,273 men for this analysis (Supplemental Figure 1). Individuals who were excluded, compared with included subjects, tended to be older (mean: 71.7 compared with 67.5 y; P < 0.001) and more likely to have probable insomnia (29% compared with 25%; P < 0.001), but baseline and follow-up AHEI scores (52.2 compared with 52.0, and 55.8 compared with 56.1, respectively) and baseline BMI (in kg/m2; 25.2 compared with 25.6) were similar (P > 0.05). This study was approved by the institutional review board at Brigham and Women’s Hospital and Harvard School of Public Health.
Assessment of insomnia symptoms
In 2004, participants in the HPFS were asked how often (rarely or never; sometimes; or most of the time) they 1)“have difficulty falling asleep” (referred to as difficulty initiating sleep in the current article), 2)“have trouble with waking up during the night” (referred to as difficulty maintaining sleep), 3)“are troubled by waking up too early and not being able to fall asleep again” (referred to as early-morning awakenings), and 4)“feel really rested when waking up in the morning” [we coded nonrestorative sleep frequency as the opposite of feeling rested when waking up in the morning (referred to nonrestorative sleep)]. Excessive daytime sleepiness was also assessed in 2004 with a question of “get so sleepy during the day or the evening that have to take a nap.” Note that these questions did not refer to a specific time period (e.g., previous month or 3 mo).
We defined probable insomnia as having nonrestorative sleep plus one or more of the 3 nocturnal insomnia symptoms (difficulty initiating sleep, difficulty maintaining sleep, and early-morning awakenings) as was done previously (2, 7).
Ascertainment of energy intake and diet-quality scores
Dietary intake was assessed in 2002 and 2006 with the use of a validated semiquantitative FFQ (18, 19). In brief, participants were asked to indicate their average frequency of consumption with 9 possible categories ranging from never or <1 time/mo” to ≥6 times/d over the past year in regards to a specific amount for each food item. We calculated total energy intake from the FFQ with the use of nutrient information obtained from the Harvard University Food Composition Database which is derived from the USDA and other sources.
We used AHEI-2010 criteria to assess dietary intake quality in 2002 and 2006. The AHEI-2010 was developed on the basis of “foods and nutrients predictive of chronic disease risk” (9), and studies have confirmed its association with chronic diseases (9) and mortality (20, 21). The AHEI-2010 is comprised the following 11 components: nuts and legumes, red and processed meats, trans fat, long-chain (n–3) fats (EPA and DHA), PUFAs, vegetables, fruit, whole grains, sugar-sweetened beverages and fruit juice, sodium, and alcohol. Each of the 11 components had a score that ranged from 0 to 10, which totaled up to 110 as the maximum overall diet-quality score. For 6 of the components (nuts and legumes; n–3 fats; PUFAs; vegetables; fruit; and whole grains), higher score represented higher consumption. For alcohol, a higher score was given to moderate intake. As for the other 4 components (red and processed meats, trans fat, sugar-sweetened beverages and fruit juice, and sodium), higher scores represented lower consumption. In sum, a higher total AHEI score indicated better overall dietary quality. We adjusted for the AHEI-2010 and total energy intake in 2002 because dietary intake was not assessed in 2004.
Ascertainment of other covariates
Age, ethnicity, smoking status, weight, height, physical activity, marriage status, living status, medication use (e.g., of antidepressants, benzodiazepine, and melatonin), depression symptoms (being “sad, blue, or depressed” ≥2 wk in the past 2 y), elevated laboratory values (e.g., presence of elevated total cholesterol), and high blood pressure were collected in 2004 via biennial self-reported questionnaires. Caffeine intake was assessed through the FFQ. Information on sleep duration (i.e., “Please indicate total hours of actual sleep in a typical 24-hour period: 5 hours or less, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, or 11+ hours”) and snoring frequency (i.e., “Do you snore? Every night, most nights, a few nights a week, occasionally, or almost never”) was collected in the 2000 self-reported questionnaire. BMI was calculated as weight divided by the square of height).
Statistical analysis
We used regression and general linear models to calculate the adjusted mean differences of total energy intake and the AHEI-2010 score in 2006 (also for the individual AHEI item for probable insomnia) and their 95% CIs between subjects with and without probable insomnia and also across categories for each insomnia symptom as assessed in 2004 after adjustment for age, ethnicity (Caucasian; yes or no), smoking status, alcohol drinking, BMI, physical activity, marriage status, living status (alone or not), AHEI-2010 (2002), total energy intake (2002), caffeine (quintiles), regular use of aspirin, depression symptoms, use of antidepressant drugs, the presence of elevated total cholesterol, high blood pressure, elevated triglycerides, sleep duration, and snoring frequency. Participants without probable insomnia were used as the reference group. As for individual insomnia symptom, we assigned a numeric value to insomnia-symptom categories [0 = rarely or never have an insomnia complaint (reference); 1 = sometimes; and 2 = most of the time] and treated it as a continuous variable for trend testing.
We investigated the joint effect of probable insomnia and depression on total energy intake and diet quality because insomnia could result in depression symptoms (22), and having depression symptoms is related to an unhealthy diet (23). Because the American Academy of Sleep Medicine included daytime impairment as a characteristic of insomnia (24), we also examined the joint effect of probable insomnia and daytime sleepiness to parse out their effects on the aforementioned outcomes. To minimize the possibility that the potential insomnia-diet relation could have been due to the use of common hypnotics, we conducted a sensitivity analysis by excluding participants who reported the use of benzodiazepine and melatonin. All analyses were performed with the SAS statistical package (version 9; SAS Institute).
RESULTS
Basic characteristics
Of 15,273 men, 3816 individuals (25%) met the criteria of having probable insomnia at baseline (Table 1). In general, probable insomnia was associated with lower physical activity, higher BMI, and greater prevalences of frequent snoring, depression symptoms, elevated cholesterol and triglycerides concentrations, and high blood pressure (Table 1).
TABLE 1.
Baseline characteristics of participants according to probable insomnia status in 2004 in the HPFS1
| Insomnia |
|||
| No | Yes | P | |
| Participants, n | 11,457 | 3816 | — |
| Age, y | 67.6 ± 7.82 | 67.0 ± 8.1 | <0.001 |
| BMI, kg/m2 | 25.5 ± 4.3 | 25.8 ± 4.4 | <0.001 |
| Caucasian, % | 97.0 | 96.2 | 0.02 |
| Marriage status (married), % | 89.4 | 87.6 | 0.001 |
| Living alone, % | 7.7 | 9.4 | <0.001 |
| Past smoker, % | 49.7 | 51.3 | 0.13 |
| Current smoker, % | 3.3 | 3.1 | 0.40 |
| Alcohol, g/d | 14.1 ± 16.8 | 12.7 ± 15.7 | <0.001 |
| Physical activity, MET-h/wk | 50.8 ± 48.7 | 42.1 ± 42.1 | <0.001 |
| Alternate Healthy Eating Index | 52.2 ± 11.4 | 51.4 ± 11.4 | <0.001 |
| Total calories, kcal/d | 2049.0 ± 617.3 | 2049.0 ± 631.4 | 0.98 |
| Use of aspirin, % | 58.3 | 58.6 | 0.96 |
| Sleep duration, h/d | 7.1 ± 1.0 | 6.9 ± 1.0 | <0.001 |
| Frequent snoring, % | 33.2 | 37.9 | <0.001 |
| Depression symptom,3 % | 6.3 | 17.5 | <0.001 |
| Elevated cholesterol, % | 55.4 | 60.2 | <0.001 |
| Elevated triglycerides, % | 36.9 | 42.2 | <0.001 |
| High blood pressure,4 % | 47.2 | 53.6 | <0.001 |
| Caffeine, mg/d | 153.4 ± 152.5 | 155.3 ± 151.0 | 0.57 |
Values were standardized to the age distribution of the study population except for age. HPFS, Health Professionals Follow-Up Study; MET-h, metabolic equivalent task hours from recreational and leisure-time activities.
Mean ± SD (all such values).
Defined as the use of antidepressant medications or an affirmative answer to the question “feel blue, sad or depressed?”
Considered as either a physician diagnosis of hypertension or use of an antihypertensive medication.
Probable insomnia, energy intake, and diet quality
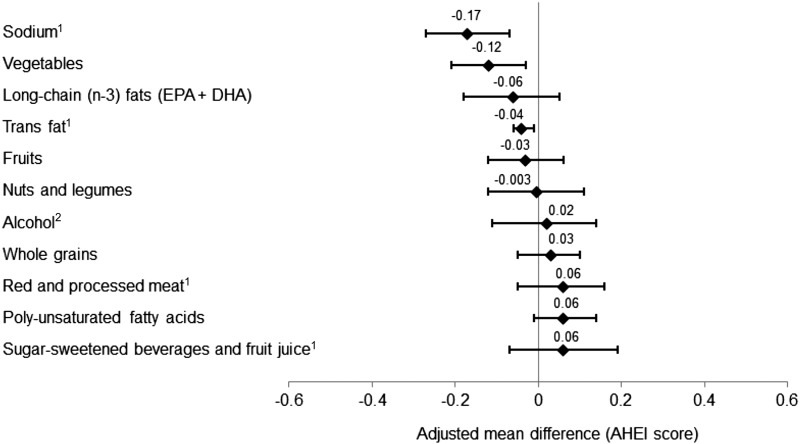
Men with probable insomnia had significantly higher energy intake than did men with no probable insomnia, which was independent of other covariates (P ≤ 0.01) (Table 2). As for diet quality, we did not observe a trend for a lower AHEI score in men with probable insomnia. When we examined the associations between probable insomnia and individual AHEI items, participants with probable insomnia had lower scores in trans fat, vegetables, and sodium (P ≤ 0.01 for all) (Figure 1).
TABLE 2.
Adjusted mean differences of energy intake and the AHEI score according to insomnia symptoms and probable insomnia status (n = 15,273)1
| Energy intake, kcal |
AHEI score |
|||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Probable insomnia | ||||||
| No (n = 11,457) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| Yes (n = 3816) | 33.2 (15.3, 51.1)** | 31.7 (13.5, 49.9)** | 35.8 (17.4, 54.1)** | −0.18 (−0.50, 0.13) | −0.13 (−0.46, 0.19) | −0.20 (−0.52, 0.13) |
| P-trend | 0.0003 | 0.0006 | 0.0001 | 0.26 | 0.42 | 0.23 |
| Difficulty initiating sleep | ||||||
| Rarely or never (n = 11,217) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| Sometimes (n = 3534) | 8.07 (−10.4, 26.5) | 6.98 (−11.6, 25.6) | 9.32 (−9.33, 28.0) | −0.32 (−0.65, 0.01) | −0.30 (−0.63, 0.03) | −0.35 (−0.68, −0.02)* |
| Most of the time (n = 522) | 35.4 (−7.30, 78.2) | 33.1 (−10.0, 76.2) | 42.2 (−1.31, 86.7) | −0.89 (−1.64, −0.13)* | −0.80 (−1.57, −0.04)* | −0.91 (−1.69, −0.14)* |
| P-trend | 0.11 | 0.15 | 0.07 | 0.005 | 0.01 | 0.004 |
| Difficulty maintaining sleep | ||||||
| Rarely or never (n = 4531) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| Sometimes (n = 6983) | 4.91 (−13.3, 23.1) | 4.55 (−13.7, 22.8) | 4.50 (−13.7, 22.7) | −0.20 (−0.52, 0.13) | −0.18 (−0.51, 0.14) | −0.19 (−0.52, 0.13) |
| Most of the time (n = 3759) | 29.2 (7.96, 50.4)** | 28.1 (6.76, 49.4)** | 30.5 (9.08, 51.9)** | 0.08 (−0.30, 0.46) | 0.11 (−0.27, 0.49) | 0.07 (−0.31, 0.45) |
| P-trend | 0.009 | 0.01 | 0.007 | 0.76 | 0.65 | 0.82 |
| Early-morning awakenings | ||||||
| Rarely or never (n = 7557) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| Sometimes (n = 6628) | 18.0 (1.95, 34.0)* | 17.4 (1.38, 33.5)* | 19.2 (3.01, 35.3)* | 0.06 (−0.22, 0.34) | 0.07 (−0.21, 0.36) | 0.02 (−0.26, 0.31) |
| Most of the time (n = 1088) | −10.2 (−41.1, 20.6) | −12.8 (−43.9, 18.2) | −5.65 (−37.1, 25.8) | 0.02 (−0.53, 0.57) | 0.06 (−0.49, 0.61) | −0.06 (−0.61, 0.50) |
| P-trend | 0.36 | 0.44 | 0.23 | 0.77 | 0.65 | 0.97 |
| Nonrestorative sleep | ||||||
| Rarely or never (n = 11,007) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| Sometimes (n = 3421) | 26.4 (7.60, 45.1)** | 25.4 (6.52, 44.3)** | 29.3 (10.3, 48.4)** | −0.23 (−0.57, 0.10) | −0.19 (−0.53, 0.14) | −0.26 (−0.59, 0.08) |
| Most of the time (n = 845) | 25.2 (−8.91, 59.3) | 20.9 (−13.7, 55.4) | 25.3 (−9.36, 60.0) | −0.19 (−0.80, 0.41) | −0.11 (−0.72, 0.51) | −0.18 (−0.79, 0.44) |
| P-trend | 0.006 | 0.01 | 0.005 | 0.20 | 0.35 | 0.19 |
All values are adjusted mean differences (95% CIs). Model 1 was adjusted for age, ethnicity (Caucasian; yes or no), smoking status (never smoker, former smoker, or current smoker), alcohol drinking (0, 0.1–9.9, 10.0–19.9, 20.0–29.9, or ≥30 g/d), BMI (in kg/m2; <23, 23–24.9, 25–26.9, 27–29.9, or ≥30), physical activity (quintiles), marriage status (married; divorced, separated, or single; or widowed), living status (alone or not), AHEI (2002), total energy intake (2002), and caffeine (quintiles). Model 2 was adjusted as for model 1 plus for regular use of aspirin (yes or no); feeling sad, blue or depressed for ≥2 wk (yes or no); use of antidepressant drugs (yes or no); and presence of elevated total cholesterol, high blood pressure, and elevated triglyceride (yes or no). Model 3 was adjusted as for model 2 plus for sleep duration (≤5, 6, 7, 8, or ≥9 h) and snoring frequency (every night, most nights, a few nights per week, occasionally, ≤1 time/wk, or missing). *,**Relative to the reference group: *P < 0.05, **P < 0.01. AHEI, Alternate Healthy Eating Index.
FIGURE 1.
Adjusted mean (95% CI) score difference for the AHEI item by probable insomnia status (n = 15,273). Analyses were adjusted for age; ethnicity (Caucasian; yes or no); smoking status (never smoker, former smoker, or current smoker); alcohol drinking (0, 0.1–9.9, 10.0–19.9, 20.0–29.9, or ≥30 g/d); BMI (in kg/m2: <23, 23–24.9, 25–26.9, 27–29.9, or ≥30); physical activity (quintiles); marriage status (married; divorced, separate, or single; or widowed); living status (alone or not alone); AHEI (2002); total energy intake (2002); caffeine intake (quintiles); regular use of aspirin (yes or no); feeling sad, blue, or depressed ≥2 wk (yes or no); use of antidepressant drugs (yes or no); presence of elevated total cholesterol, high blood pressure, or elevated triglyceride (yes or no); sleep duration (≤5, 6, 7, 8, or ≥9 h), and snoring frequency (every night, most nights, a few nights per week, occasionally, ≤1 time/wk, or missing). 1A higher score represents lower consumption. 2A higher score represents lower-to-moderate consumption. AHEI, Alternate Healthy Eating Index.
We observed significant relations between the frequency of specific insomnia symptoms (difficulty initiating sleep and nonrestorative sleep) and energy intake (P-trend ≤ 0.007 for both) (Table 2). A similar pattern was observed in men with difficulty initiating sleep (P-trend = 0.07) but not for early-morning awakenings. We noted a significant trend in a decreasing AHEI score as the frequency of difficulty initiating sleep increased but not for other insomnia symptoms (P-trend = 0.004) (Table 2).
When we restricted analyses to those who did not use benzodiazepine and melatonin, we obtained similar significant results between probable insomnia and higher consumption of energy, trans fat, vegetables, and sodium (data not shown). Also, because sleep duration and insomnia symptoms are closely related, we conducted a sensitivity analysis without adjustment for sleep duration, but the results remained consistent (data not shown).
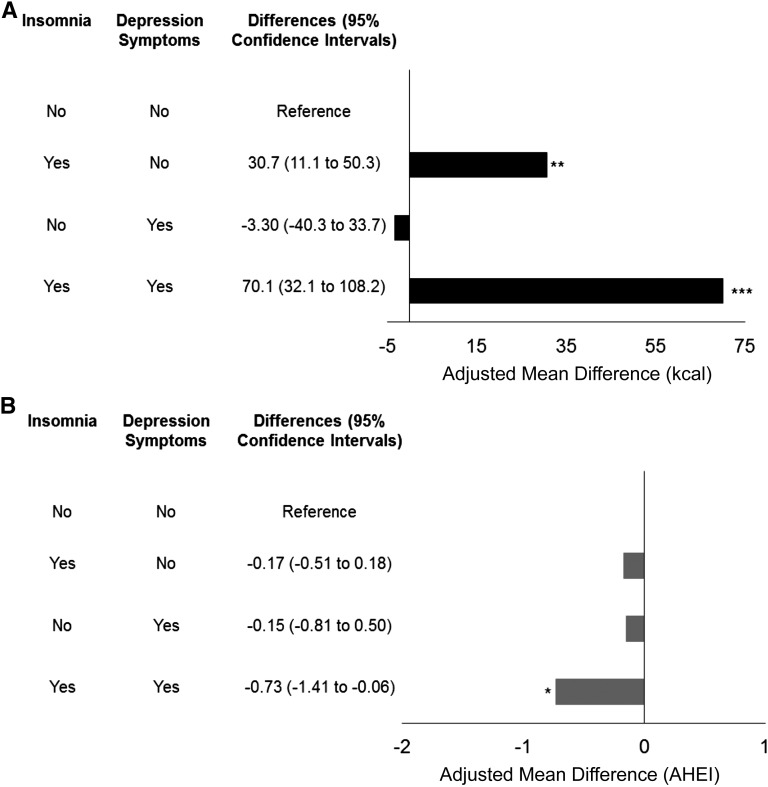
Compared with men with no probable insomnia and no depression symptoms, those with 1) only probable insomnia and 2) both probable insomnia and depression symptoms had significantly higher energy intake (Figure 2A). After adjustment for other covariates, participants with both probable insomnia and depression symptoms had highest energy intake followed by individuals with only probable insomnia (P < 0.01 for both). In terms of diet quality, men with both probable insomnia and depression symptoms had a significantly lower AHEI score than did those who were free of those symptoms (Figure 2B).
FIGURE 2.
Joint effect of probable insomnia and depression symptoms on energy intake (kcal) (A) and diet quality (AHEI) (B). *P < 0.05, **P < 0.01, ***P < 0.001. AHEI, Alternate Healthy Eating Index.
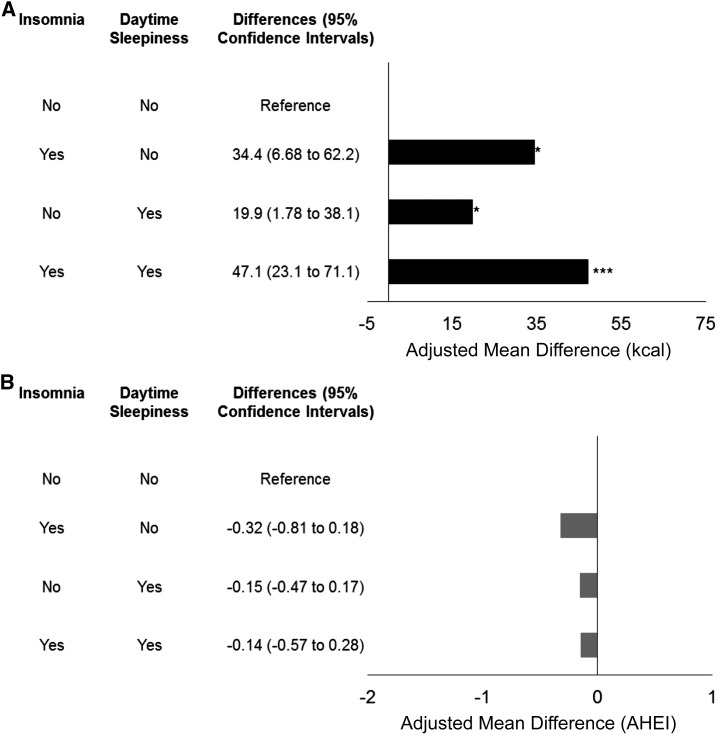
We also investigated the joint effect between probable insomnia and daytime sleepiness and showed that men with 1) only probable insomnia, 2) only daytime sleepiness, or 3) both probable insomnia and daytime sleepiness all had significantly greater energy intakes relative to those of participants without those conditions (Figure 3A). Men with both probable insomnia and daytime sleepiness had the highest energy intake followed by men with only probable insomnia and only daytime sleepiness (P < 0.05 for all). In contrast, we did not observe significant associations between the joint effects of probable insomnia and daytime sleepiness and the AHEI score (Figure 3B).
FIGURE 3.
Joint effect of probable insomnia and daytime sleepiness on energy intake (kcal) (A) and diet quality (AHEI) (B). *P < 0.05, ***P < 0.001. AHEI, Alternate Healthy Eating Index.
DISCUSSION
In this large cohort, we observed that men with probable insomnia had higher energy intake and lower scores for 3 individual AHEI items (trans fat, vegetables, and sodium). In addition, difficulty maintaining sleep, nonrestorative sleep, and difficulty initiating sleep were associated with greater energy intake, whereas difficulty initiating sleep was related to a lower AHEI score. These associations were independent of a variety of lifestyle factors and medical comorbidities that are known to affect nutrition behavior and outcomes.
Previous clinical and epidemiologic studies have been generally consistent in reporting that sleep deprivation or poor sleep quality is associated with greater caloric intake (10–12, 25–29). However, most of these studies were limited by one or more factors, which included the use of small sample sizes, an investigation of only one characteristic of sleep, and inducing sleep deprivation or poor sleep quality in a controlled clinical setting rather than studying individuals who were already affected by sleep problems in the community setting. Thus, to address these gaps in literature, we conducted a community-based analysis on the association between probable insomnia and future nutrition outcomes in a large population-based cohort.
Of epidemiologic studies, participants with poor sleep quality or short-duration sleep consumed an average of between 7 and 314 kcal/d more than did individuals with normal sleep duration or quality (25–29). Our result falls in the lower spectrum of that range and is apparently modest. Because we excluded individuals with a physician diagnosis of cancer, cardiovascular disease, and diabetes and adjusted for a variety of lifestyle factors and comorbidities, the result could potentially reflect the pure effect of probable insomnia on caloric intake.
In our current study, we observed that men with probable insomnia had significantly lower scores for 3 individual diet quality items (i.e., trans fat, vegetables, and sodium), which was consistent with previous findings. Stern et al. (26) conducted a cross-sectional analysis (n = 769) and showed that women in the lowest category of sleep quality (very restless or restless) had the lowest AHEI scores. An inferior diet behavior was also noted with male smokers between the ages of 50–69 y in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (30), which showed that subjects with insomnia had lower vegetable consumption than did other smokers with no insomnia (30). Similarly, in the Three-generation Study of Women on Diets and Health in 3129 Japanese women (mean age: 47 y), subjects with poor sleep quality consumed less vegetables and more confectionary items (13). In general, insomnia symptoms have been linked with less-desirable nutrient intake (14–16).
There are several potential mechanisms to explain the observed associations in insomnia, energy intake, and diet quality (31). Some researchers have proposed that poor sleep quality or sleep insufficiency could alter appetite hormones such as ghrelin and leptin and increase the hunger sensation (“homeostatic drive to eat”) (31), but evidence behind this hypothesis has remained inconsistent. Some (26, 32) but not all (33, 34) studied have shown that sleep curtailment or restriction was associated with a reduction in leptin concentrations and increases in ghrelin, hunger, and appetite. The hedonic motivation to eat has been suggested as another promising explanation in recent studies. Moubarac et al. (35) showed that the relation between daytime sleepiness and sweetened-food consumption was mediated by psychological distress, possibly suggesting that individuals with psychological distress are selecting energy-dense food items as a way to alleviate their stresses and improve their moods or increase their energy levels (31). Moreover, several studies have reported an increase in brain sensitivity toward a food-related reward (e.g., unhealthy food options) after sleep restriction (36, 37). Alternate underlying mechanisms also include an increased opportunity for eating (31, 38) and a higher disinhibition eating pattern (39). However, note that most of the supporting evidence has been derived from sleep-restriction studies, and sleep restriction or duration and poor sleep quality may have different etiologies. Thus, future studies are warranted to investigate whether the aforementioned mechanisms could be extended to explain the observed relations in insomnia, energy intake, and diet quality.
Our study further confirmed the differential effect of individual insomnia symptoms on nutrition and health outcomes. We observed that a difficulty maintaining sleep, nonrestorative sleep, and a difficulty initiating sleep (trend) were associated with greater energy intake, whereas a difficulty initiating sleep was related to a lower overall diet-quality score. Consistently, in our previous prospective study and a subsequent meta-analysis that included 10 other relevant prospective studies, only the difficulty initiating sleep and nonrestorative sleep were associated with higher adjusted risks of cardiovascular mortality and all-cause mortality (7).
Major strengths of our study include the relatively large sample size, high follow-up rate, and use of a validated FFQ. Moreover, we purposely excluded individuals with certain health conditions and adjusted for a variety of lifestyle factors and medical comorbidities to minimize reverse causation. Second, the homogeneous nature of our cohort regarding the socioeconomic background could have helped to lower potential confounding that was attributable to social, educational, and health care disparities. Third, to unravel the complex associations of insomnia with both depression and daytime sleepiness, we conducted joint-effects models, which showed probable insomnia was an independent risk factor for an inferior nutrition outcome.
Several limitations should be considered when interpreting our outcomes. First, this cohort included mostly Caucasian men who were US health professionals. Thus, our results might not be generalizable to other populations. However, the estimated 25% prevalence of probable insomnia in this cohort was comparable to that in the general population (10–30% Americans) (1). Furthermore, similar results have been noted in other studies (29, 40) with different populations such as in young female students and postmenopausal women. Second, although current models suggested that men with probable insomnia had higher intakes of total energy, trans fat, and sodium and lower intake of vegetables than did subjects without probable insomnia, the alternate direction could also have been possible. For instance, high sodium intake or having certain underlying diseases could impair sleep quality. To address this issue, we controlled for baseline dietary components, excluded individuals with cancer, cardiovascular disease, or diabetes, and adjusted for a variety of lifestyle factors and medical comorbidities. However, we could not totally exclude the possibility of reverse causality. Third, the accuracy of an FFQ in the estimation of total energy and nutrient intakes has been of concern. In a recent validation study that was based on 632 participants in the Nurses’ Health Study, Yuan et al. (41) compared intakes of energy and nutrients that were estimated with the FFQ that was used in the current study with intakes that were measured with two 7-d dietary records (7DDRs) and concluded that the FFQ provided “reasonably valid estimates for intakes of a wide variety of dietary variables.” However, compared with 7DDRs, the FFQ underestimated sodium intake (2061 mg/d on the basis of the FFQ and 2647 mg/d on the basis of the average of two 7DDRs) and overestimated energy intake (1857 kcal/d on the basis of the FFQ and 1741 kcal/d on the basis of the two 7DDRs)]. Future studies are needed to replicate these associations with the use of other dietary assessment tools such as 7DDRs. Last, we did not have information on the timing of food intake (i.e., eating closer to bedtime), which could have affected sleep patterns.
In conclusion, this large cohort study of men suggests that probable insomnia and certain insomnia symptoms may have a potential unfavorable impact on diet. Future studies are warranted to replicate these associations in other populations and with other objective measures of insomnia.
Acknowledgments
The authors’ responsibilities were as follows—FWC, YL, and XG: analyzed or contributed to the interpretation of the data; FWC and XG: wrote the manuscript; XG: had primary responsibility for the final content of the manuscript; and all authors: designed and conducted the research and read and approved the final manuscript. JWW reported receiving fees for serving on advisory boards from Merck, UCB Pharma, XenoPort, and Flex Pharma; fees for providing expert testimony in a patent suit between a potential generic manufacturer and patent holders (Purdue Pharma and Transcept Pharmaceuticals); and grant support from UCB Pharma, XenoPort, Purdue Pharma, and NeuroMetrix as well as holding stock options in Flex Pharma. The remaining authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: AHEI, Alternate Healthy Eating Index; FFQ, food-frequency questionnaire; HPFS, Health Professionals Follow-Up Study; 7DDR, 7-d dietary record.
REFERENCES
- 1.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 2.NHLBI, NIH. What is insomnia [Internet]? [updated 2011 Dec 13; cited 2016 Apr 22]. Available from: http://www.nhlbi.nih.gov/health/health-topics/topics/inso.
- 3.Troxel WM, Buysse DJ, Matthews KA, Kip KE, Strollo PJ, Hall M, Drumheller O, Reis SE. Sleep symptoms predict the development of the metabolic syndrome. Sleep 2010;33:1633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009;32:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation 2011;124:2073–81. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care 2009;32:1980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, Ma J, Gao X. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation 2014;129:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Research Council (US) Committee on Diet and Health. Diet and health: implications for reducing chronic disease risk. Washington (DC): National Academy Press; 1989. [PubMed] [Google Scholar]
- 9.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9. [DOI] [PubMed] [Google Scholar]
- 11.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011;94:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J Occup Health 2014;56:359–68. [DOI] [PubMed] [Google Scholar]
- 14.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res 2014;23:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka E, Yatsuya H, Uemura M, Murata C, Otsuka R, Toyoshima H, Tamakoshi K, Sasaki S, Kawaguchi L, Aoyama A. Associations of protein, fat, and carbohydrate intakes with insomnia symptoms among middle-aged Japanese workers. J Epidemiol 2013;23:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan X, Alén M, Cheng SM, Mikkola TM, Tenhunen J, Lyytikäinen A, Wiklund P, Cong F, Saarinen A, Tarkka I, et al. . Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J Sleep Res 2015;24:414–24. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services, USDA. 2015–2020 Dietary Guidelines for Americans. 8th ed. 2015. [Internet]. [cited 2016 Apr 22]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 18.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 19.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 20.George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014;180:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, Riemann D. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 2011;135:10–9. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet F, Irving K, Terra J-L, Nony P, Berthezène F, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis 2005;178:339–44. [DOI] [PubMed] [Google Scholar]
- 24.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 25.Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005-2010. Am J Clin Nutr 2014;100:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, Woods NF, Chen Z. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring) 2014;22:E55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zadeh SS, Begum K. Comparison of nutrient intake by sleep status in selected adults in Mysore, India. Nutr Res Pract 2011;5:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013;64:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haghighatdoost F, Karimi G, Esmaillzadeh A, Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition 2012;28:1146–50. [DOI] [PubMed] [Google Scholar]
- 30.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lönnqvist J. Food and nutrient intake in relation to mental wellbeing. Nutr J 2004;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaput J-P. Sleep patterns, diet quality and energy balance. Physiol Behav 2014;134:86–91. [DOI] [PubMed] [Google Scholar]
- 32.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50. [DOI] [PubMed] [Google Scholar]
- 33.Littman AJ, Vitiello MV, Foster-Schubert K, Ulrich CM, Tworoger SS, Potter JD, Weigle DS, McTiernan A. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31:466–75. [DOI] [PubMed] [Google Scholar]
- 34.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moubarac J-C, Cargo M, Receveur O, Daniel M. Psychological distress mediates the association between daytime sleepiness and consumption of sweetened products: cross-sectional findings in a Catholic Middle-Eastern Canadian community. BMJ Open 2013;3:e002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benedict C, Brooks SJ, O’Daly OG, Almèn MS, Morell A, Åberg K, Gingnell M, Schultes B, Hallschmid M, Broman J-E, et al. . Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab 2012;97:E443–7. [DOI] [PubMed] [Google Scholar]
- 37.St-Onge M-P, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond) 2014;38:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013;36:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaput J-P, Després J-P, Bouchard C, Tremblay A. The association between short sleep duration and weight gain is dependent on disinhibited eating behavior in adults. Sleep 2011;34:1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med 2010;11:180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Nutrient validation utilizing self-reported dietary assessment methods in Women’s Lifestyle Validation Study. Am J Epidemiol. In press. [Google Scholar]