Abstract
High-temperature requirement protease A (HtrA) represents a family of serine proteases that play important roles in microbial biology. Unlike the genomes of most organisms, that of Borrelia burgdorferi notably encodes a single HtrA gene product, termed BbHtrA. Previous studies identified a few substrates of BbHtrA; however, their physiological relevance could not be ascertained, as targeted deletion of the gene has not been successful. Here we show that BbhtrA transcripts are induced during spirochete growth either in the stationary phase or at elevated temperature. Successful generation of a BbhtrA deletion mutant and restoration by genetic complementation suggest a nonessential role for this protease in microbial viability; however, its remarkable growth, morphological, and structural defects during cultivation at 37°C confirm a high-temperature requirement for protease activation and function. The BbhtrA-deficient spirochetes were unable to establish infection of mice, as evidenced by assessment of culture, PCR, and serology. We show that transcript abundance as well as proteolytic processing of a borrelial protein required for cell fission and infectivity, BB0323, is impaired in BbhtrA mutants grown at 37°C, which likely contributed to their inability to survive in a mammalian host. Together, these results demonstrate the physiological relevance of a unique temperature-regulated borrelial protease, BbHtrA, which further enlightens our knowledge of intriguing aspects of spirochete biology and infectivity.
INTRODUCTION
Lyme disease is a common vector-borne infectious disease in the Northern Hemisphere, posing a serious health threat to humans and animals (1–3). Newly revised estimates from the Centers for Disease Control and Prevention suggest that there are likely to be over 300,000 new cases per year in the United States alone, and a vaccine to prevent human infection is currently unavailable. The disease can be transmitted via the bite of the infected arthropod vector, the Ixodes scapularis tick, harboring the spirochete pathogen Borrelia burgdorferi, often resulting in serious illness in susceptible hosts (2, 3). While most Lyme disease cases can be treated with an antibiotic, a minority of patients will have persistent or relapsing nonobjective symptoms that vary in intensity, are nonresponsive to further antibiotic therapy, and are collectively termed chronic Lyme disease or posttreatment Lyme disease syndrome, the underlying mechanism and pathogenesis of which remain highly controversial (4). Detection of early Lyme disease, when antibiotic treatment is most effective, also remains challenging, as the infection reflects many nonspecific symptoms that are shared by many other febrile or influenza-like diseases (5, 6). Thus, the development of efficient diagnostics, vaccines, or new effective drugs is a high-priority goal that requires a thorough understanding of the unusual biology and infection process of this pathogen.
The pathogen of Lyme disease exhibits evolutionary divergence from other bacteria, even related pathogenic spirochetes, and persists in a complex enzootic infection cycle. Thus, it is perhaps not surprising that a great majority of the proteins encoded by the B. burgdorferi genome have unknown functions relevant only to the special biology of spirochetes (7–10). The genome also shows notable structural and functional redundancy, reflected by the presence of a significant number of paralogous gene clusters, as well as many genes that are not required for infectivity in mammals and/or ticks (2, 7–11). Although various aspects of spirochete infectivity are well studied, many questions remain unanswered, particularly regarding mechanisms that dictate the remarkable ability of the pathogen to adapt to and persist in diverse hosts, including the multifactorial processes that influence the inflammatory outcome in susceptible hosts. Maintenance of B. burgdorferi in the enzootic cycle requires successful persistence in the arthropod and reservoir hosts, as well as efficient transmission between the tick and its host (11). Therefore, in order to survive transitions between diverse vector and host environments, B. burgdorferi must be able not only to detect changes in its environment but also to generate suitable response to these changes. As a result, gene products playing roles in adaptation to cellular stress, including temperature, oxidative stress, pH, etc., must be critical for the life cycle of the pathogen.
The high-temperature requirement A (HtrA) family of serine proteases can be found in all forms of life, from prokaryotes to mammals (12–14). HtrA proteases function primarily in protein homeostasis and quality control, acting as proteases and chaperones that stabilize specific proteins and modulate signaling pathways (12). Similarly, HtrA proteases have been reported to play an essential role in the virulence of a number of pathogens, such as Mycobacterium tuberculosis (15), Salmonella enterica (16, 17), Helicobacter pylori (18), Streptococcus pneumoniae (19), Bacillus anthracis (20), and Staphylococcus aureus (21), and have also been associated with several human diseases (21–23). While the predominant mechanism of loss of virulence lies in the substrate proteins that are stabilized or processed by HtrA, a direct role for HtrA in pathogen invasiveness has also been reported (18, 24). The unique feature of HtrA is the presence of a protease domain containing a highly conserved active-site catalytic triad (Ser-His-Asp) and one or two PDZ domains toward the carboxyl terminus (12, 13). The overall structures of HtrA proteases are similar; however, their functions and substrates are remarkably quite diverse. While the common structural building unit is a trimer, upon recognizing substrates, HtrA monomers form higher-order oligomeric structures ranging from 6- to 24-mers (13, 25). These oligomers form a cage with the proteolytically active site facing the interior and the PDZ domains protruding outside. The involvement of HtrA proteases in the processing of critical substrates supporting microbial virulence, as well as their pathogen-specific diversification, makes them an attractive target for therapeutic intervention (23, 26).
Most of the metazoan or bacterial genomes encode multiple HtrA homologs that are implicated in diverse physiological functions (27). Notably, the genome of B. burgdorferi contains a single htrA gene (locus bb0104) (28) that encodes BbHtrA, which has been biochemically characterized and shown to possess conserved, catalytic, serine-dependent proteolytic activity (28–32). BbHtrA possesses an N-terminal signal peptide sequence, and apart from its periplasmic and cytosolic fractions, it also localizes in outer membrane vesicles and is secreted into growth media (29, 32). Few interacting partners and substrates of BbHtrA have also been identified (28–32). For instance, BbHtrA has been shown to cleave basic membrane protein D (BmpD) and chemotaxis signal transduction phosphatase (CheX) (29). In addition, previous in vitro studies have suggested that BbHtrA also cleaves BB0323 (28), a borrelial protein important for cell growth, fission, and infection (33, 34). Also, it has been shown that BbHtrA degrades the extracellular matrix proteoglycan aggrecan, fibronectin, and numerous other proteoglycans (32), and recently, the inhibition of this protease by zinc has been reported (35). However, despite these seminal studies, the precise biological significance of BbHtrA remains enigmatic, as efforts aimed at targeted deletion of the gene have thus far not been successful (28–30). Here we report the successful generation and characterization of a BbhtrA deletion mutant and complemented isolates of an infectious isolate of B. burgdorferi, which highlight a critical role for this protease in spirochete biology and infectivity.
MATERIALS AND METHODS
B. burgdorferi strains and mice.
Low-passage-number B. burgdorferi strain 297 clone AH130 (36) was used in this study. Bacteria were cultivated in Barbour-Stoenner-Kelly II (BSK-II) medium at 34°C or 37°C. BbhtrA mutant strains were maintained under antibiotic selection with gentamicin (40 mg/ml). Four- to 6-week-old C3H/HeN mice were purchased from the National Institutes of Health. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee and Institutional Biosafety Committee of the University of Maryland, College Park.
Generation of BbhtrA deletion mutant and complemented strains.
BbhtrA-deficient B. burgdorferi clones were generated by homologous recombination, replacing the bb0104 gene with a gentamicin resistance cassette. Briefly, the 1.5-kb upstream region and the 1.5-kb downstream region of bb0104 (BbhtrA) were PCR amplified from the B. burgdorferi genomic DNA with the primers indicated in Table S1 in the supplemental material. The DNA amplicons were then cloned upstream and downstream of a gentamicin resistance cassette in a pUC18-derived plasmid (pMP016) at the SacI/MluI and SacII/PstI restriction sites, respectively. The resulting suicide vector was confirmed by sequencing and designated pMP007. The pMP007 plasmid DNA was then transformed into an infectious clone of B. burgdorferi strain 297, AH130 (37). Gentamicin-resistant transformants were analyzed by PCR, reverse transcription (RT)-PCR, and immunoblotting to confirm the loss of bb0104. Endogenous plasmid profiles of the bb0104 mutant clones were determined by PCR on the basis of recently published sequences (10). Two clones with a plasmid profile identical to that of AH130 were chosen for further study.
For genetic complementation of the BbhtrA mutant, we used B. burgdorferi shuttle plasmid pBSV2-derived shuttle vector pYY003 (38) to deliver the wild-type (WT) copy of the BbhtrA gene. Since the intergenic region upstream region of the BbhtrA open reading frame (ORF) does not seem to harbor a discernible promoter region, we fused the BbhtrA ORF with the B. burgdorferi flaB promoter. The DNA fragment encompassing the BbhtrA gene downstream of the flaB promoter was cloned into the NdeI and BglII sites of pYY003. About 10 μg of the recombinant plasmid, designated pXW003, was electroporated into the BbhtrA-deficient B. burgdorferi strain, and complemented clones were selected with 350 μg/ml kanamycin in the growth medium. PCR analyses were further performed to confirm that the BbhtrA-complemented clones retained the same plasmid profile as the WT spirochetes.
Our repeated attempts to attain stable in cis complementation of BbhtrA isolates were unsuccessful. Therefore, the above-mentioned BbhtrA-complemented isolate, which contained a WT copy of the BbhtrA gene in the shuttle plasmid, was used in all in vitro studies in the presence of kanamycin. For phenotypic analysis in vivo, two isogenic and independent BbhtrA mutant clones (designated M1 and M2) were included in all of our experiments.
Western and far-Western blotting.
Immunoblotting assays were performed by standard procedures as detailed elsewhere (28). Briefly, whole-cell lysates of B. burgdorferi grown in BSK-II medium at 34°C and 37°C were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with anti-BB0323 (1:3,000), anti-BB0238 (1:3,000), anti-BB0365 (1:1,000), and anti-BbHtrA (1:3,000) antibodies (28, 39). Immunoblot assays were developed by the addition of horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000 to 10,000) and enhanced chemiluminescence as previously described (28). For far-Western analyses, the nitrocellulose membrane was incubated with His-BB0323 (2 μg) overnight at 4°C in blocking buffer (2% skim milk in Tris-buffered saline, pH 7.5, with 0.05% Tween 20) and washed with Tris-buffered saline with Tween 20, and bound proteins were detected by immunoblotting with an anti-BB0323 antibody and an HRP-conjugated secondary antibody. Quantitative densitometry was performed with ImageJ software (NIH).
PCR assays.
The primers used in PCR and quantitative RT-PCR (qRT-PCR) assays are shown in Table S1 in the supplemental material. Total RNA from in vitro-grown B. burgdorferi or from infected mouse tissues was isolated with TRIzol reagent (Invitrogen), treated with DNase I (NEB), reverse transcribed to cDNA (SuperScript VILO; Invitrogen), and analyzed by real-time PCR with iQ SYBR green Supermix (Roche Diagnostics) as detailed previously (40). For quantitative analysis of gene expression, the target transcripts were normalized to the number of flaB transcripts, whereas for quantitative measurement of B. burgdorferi burdens in infected tissues, flaB transcripts were normalized to mouse β-actin levels as previously detailed (40).
Growth analysis of spirochetes.
WT, BbhtrA mutant, and complemented spirochetes were assessed for growth in vitro and further analyzed by electron microscopy. Spirochetes were grown at 34°C in BSK-II medium containing 6% rabbit serum to early log phase and repassaged (105 spirochetes/ml) into new medium. The spirochetes were cultivated in triplicate at 34°C and 37°C and counted every 24 h with a Petroff-Hausser cell counter (Fisher Scientific) under a dark-field microscope as previously detailed (41).
Electron microscopy.
Electron microscopic analyses were performed as previously detailed (42), with minor modifications. The WT, BbhtrA deletion mutant, and complemented strains were grown at 34°C or 37°C to a density of 107 cells/ml. For transmission electron microscopy, spirochetes were washed four times with phosphate-buffered saline (PBS) and fixed in 2% glutaraldehyde–PBS for 60 min, followed by 1% osmium tetroxide for 30 min, at room temperature. The samples were dehydrated, embedded, sectioned, and then collected on copper grids. Samples were poststained with 2.5% uranyl acetate and lead citrate and finally analyzed with two transmission electron microscopes (Zeiss 10CA and JEOL 100CXII). Scanning electron microscopy was performed by a similar procedure, except that the samples were subjected to critical-point drying with liquid carbon dioxide after the dehydration step. Dried samples were mounted on stubs coated with a gold-palladium alloy and analyzed under a scanning electron microscope (Hitachi S-4700).
Infection studies.
Groups of three C3H/HeN mice were injected intradermally with the same number (105 cells/per mouse) of WT spirochetes or two independent clones of BbhtrA-deficient spirochetes (designated M1 and M2). Samples of skin, heart, and bladder were isolated at 14 days after infection, and B. burgdorferi levels were assessed by qRT-PCR analysis of flaB transcripts normalized to murine β-actin levels as described previously (40). Additionally, skin and spleen tissues were cultured in BSK-II medium and analyzed weekly for 4 weeks with a dark-field microscope (43).
Statistical analysis.
Results are expressed as the mean ± the standard error of the mean (SEM). The statistical significance of differences observed between experimental and control groups was analyzed with GraphPad Prism version 4.0 (GraphPad Software, CA). Student's t test was used to compare the mean values, and P < 0.05 was considered statistically significant.
RESULTS
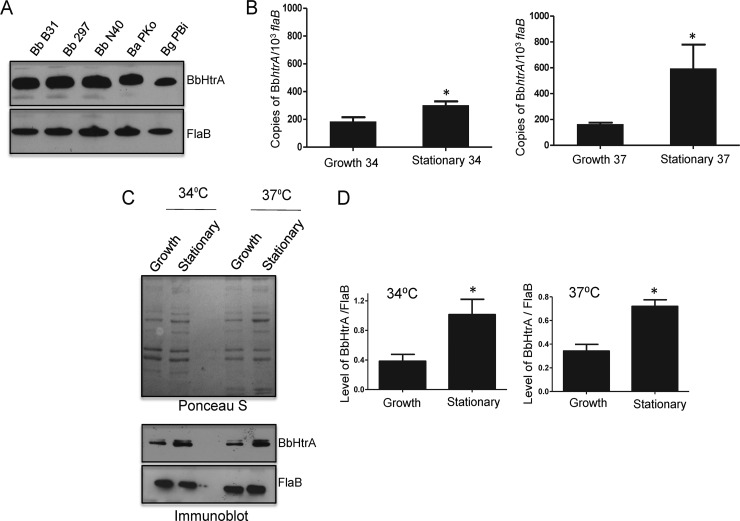
BbHtrA is a conserved spirochete protein induced in the stationary growth phase and at elevated temperatures.
The primary sequence of BbHtrA, which possesses typical serine protease activity (28–32), is highly conserved within diverse B. burgdorferi sensu lato strains. To further confirm the conservation of BbHtrA and to assess whether diverse isolates and strains of the species B. burgdorferi sensu lato produce HtrA orthologs, whole-cell lysates from different strains were probed with BbHtrA antibody raised against isolate B31. As shown in Fig. 1A (top), an antibody raised against isolate B31 recognized HtrA orthologs in all of the strains, which produced the antigen at comparable levels. As HtrA belongs to a family of proteases that are activated under various stress conditions, including high temperature, we examined the levels of BbhtrA expression at different temperatures and in different phases of growth. B. burgdorferi bacteria were cultured at the optimal laboratory growth temperature (34°C) or at an elevated, mammal-specific temperature (37°C). Samples were collected in the early (105 cells/ml) and late (108 cells/ml) growth phases and processed for assessment of BbhtrA transcript and protein levels by qRT-PCR and immunoblotting, respectively. Notably, as shown in Fig. 1B, BbhtrA transcripts were more abundant in the stationary phase than in the early growth phase, irrespective of the culture temperature. There was also an overall higher BbhtrA transcript level at 37°C than at 34°C, especially in the late phase of growth. To further confirm whether increased mRNA levels also resulted in an increase in corresponding protein expression levels, samples from the different growth phases were subjected to immunoblotting with anti-BbHtrA antiserum. In accordance with the transcript level, BbHtrA protein levels were higher in the stationary phase (Fig. 1C and D) than in the early growth phase, while the level of FlaB remained constant throughout the growth of spirochetes in vitro. Taken together, these data suggest that the expression of BbhtrA is induced under various stress conditions, such as higher temperatures, as mimicked by growth at 37°C and spirochete growth in the stationary phase.
FIG 1.
BbHtrA is conserved in infectious B. burgdorferi sensu lato isolates and highly expressed in the stationary phase or at an elevated temperature. (A) Production of BbHtrA in representative isolates of infectious B. burgdorferi sensu lato. Whole-cell extracts from B. burgdorferi isolates B31, 297, and N40; B. afzelii isolate PKo; and B. garinii isolate PBi were immunoblotted with BbHtrA antiserum generated against the recombinant HtrA protein of B. burgdorferi B31. The expression of FlaB in different isolates is shown at the bottom. (B) BbhtrA is induced in the stationary growth phase and at an elevated temperature. Spirochetes were grown at 34°C (left) and 37°C (right), and samples were harvested in the growth (105 cells/ml) and stationary (108 cells/ml) phases. The BbhtrA transcript level was measured by qRT-PCR and normalized to the number of copies of the flaB transcript. The bars represent the mean values of three independent experiments, and the error bars represent the SEM. The levels of BbhtrA mRNA are elevated in the stationary phase and at 37°C. *, P < 0.05. (C) BbHtrA protein expression levels in the log and stationary growth phases of spirochetes in culture. Bacterial lysates at the growth phases and temperatures indicated were either assessed by Ponceau S staining (top) or subjected to immunoblotting with anti-BbHtrA (middle) and anti-FlaB (bottom) antisera. (D) Levels of BbHtrA relative to FlaB from panel C were determined by quantitative densitometry. The levels of BbHtrA are increased in the stationary phase. *, P < 0.05.
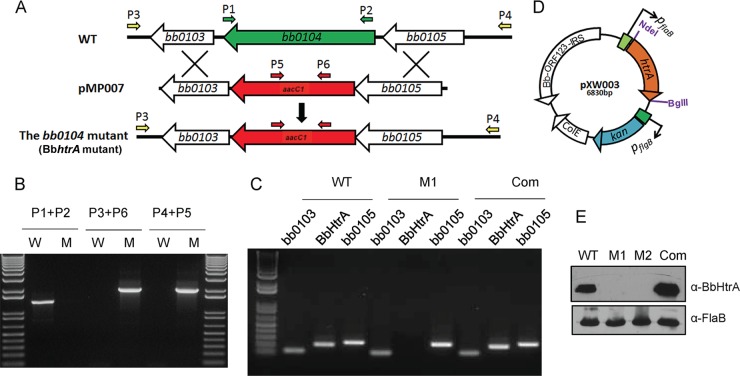
BbHtrA deficiency results in impairment of spirochete growth and cellular organization at a mammalian-phase-specific temperature.
Previous attempts by several groups to generate a BbhtrA deletion mutant of B. burgdorferi were unsuccessful (28–32). This observation, together with the occurrence of BbhtrA as a single-copy gene in the spirochete genome, led to speculation that BbHtrA is likely involved in an essential housekeeping function and thus attempts to delete it would incur a lethal mutation. However, unlike our previous mutagenesis attempts involving the B. burgdorferi B31 isolate, we were successful in generating a BbhtrA deletion mutant of infectious and low-passage-number 297 isolate AH130 (37). The mutant was generated by replacing the ORF of BbhtrA (bb0104) with the gentamicin resistance cassette via homologous recombination as illustrated in Fig. 2A. Two independent gentamicin-resistant clones (designated M1 and M2) with the same endogenous plasmid profile as AH130 were used for subsequent analysis. The desired integration was confirmed by amplification of the product in mutants with primers specific to the antibiotic resistance gene, as opposed to the WT (Fig. 2B). qRT-PCR analysis confirmed the absence of a BbhtrA transcript in the mutants and its restoration in the complemented strain (Fig. 2C) and ruled out the polar effects of mutation on adjacent transcripts bb0103 and bb0105, which were expressed at levels similar to those of the WT. Finally, the loss of the BbHtrA protein was verified by immunoblotting whole-cell extracts from WT and mutant spirochetes with anti-BbHtrA antibody (Fig. 2E). To rule out the possibility of anomalous effects of genetic manipulation, we sought to complement the BbhtrA-deficient B. burgdorferi strain with a WT copy of the BbhtrA gene in cis. As the BbhtrA gene lacks an obvious upstream promoter and is probably part of an operon, we fused the ORF of BbhtrA with the B. burgdorferi flaB promoter. Although our exhaustive efforts to generate a stable and cis-complemented strain were unsuccessful, we were able to complement, in trans via a shuttle plasmid, the BbhtrA-deficient spirochetes with a WT copy of the gene under the control of the flaB promoter (Fig. 2D), which restored the production of BbHtrA in the complemented isolate (Fig. 2E).
FIG 2.
Construction and analysis of bb0104 (BbhtrA) mutant and complemented strains. (A) Schematic representation of WT and BbhtrA mutant isolates (M1 and M2) at the bb0104 (hrtA_Bb) locus of B. burgdorferi 297. The bb0103, bb0104, and bb0105 genes and the gentamicin cassette (aacC1) replacing the native bb0104 ORF are indicated. (B) Integration of the gentamicin resistance cassette at the intended locus in the genome. Primer pairs P1-P2, P4-P5, and P3-P6 were used to amplify the native bb0104 gene and the inserted gentamicin cassette at the bb0104 locus, respectively. PCR was performed with the genomic DNA of WT (lanes W) and mutant (lanes M) B. burgdorferi, and the amplified products were checked on a 1% agarose gel. The migration of a DNA molecular size ladder is shown in the leftmost and rightmost lanes. (C) qRT-PCR analysis of BbhtrA deletion and complementation and polar effects on cognate bb0103 and bb0105 transcripts. Total RNA was isolated from WT, mutant (M1), and complemented (Com) B. burgdorferi, converted to cDNA, and further used to amplify the regions with BbhtrA and the adjacent bb0103 and bb0105 genes. The amplified products were visualized on a 1% agarose gel. (D) Diagram of the shuttle vector pXW003 used for complementation. pXW003 is a pBSV2-derived shuttle vector carrying the bb0104 gene under the control of the flaB promoter. (E) Protein expression analyses of WT, BbhtrA mutant (M1 and M2), and complemented (Com) B. burgdorferi. Equal amounts of bacterial extracts were subjected to SDS-PAGE, transferred to a membrane, and immunoblotted with anti-BbHtrA (top) and anti-FlaB (bottom) antisera.
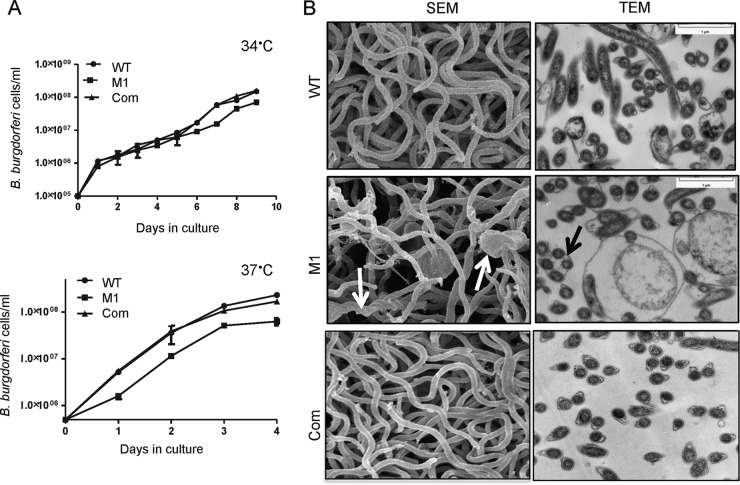
To assess the role of BbHtrA in the growth of B. burgdorferi, WT, BbhtrA mutant, and complemented spirochetes were cultured at laboratory (34°C) and elevated (37°C) temperatures. As shown in Fig. 3A, the BbhtrA mutant grew comparably to the WT at 34°C but exhibited a slow-growth phenotype at 37°C (Fig. 3A) and formed unusually large clumps at all cell densities. Notably, the mutant isolates regained their normal morphology when shifted back to 34°C either during early log phase or following inoculation into fresh medium (data not shown). Unlike the BbhtrA mutant, the complemented isolate displayed a growth pattern similar to that of WT spirochetes, indicating that the defect was specific to the deletion of BbhtrA (Fig. 3A). These data also suggest that BbHtrA might not be essential for spirochete growth in vitro at 34°C but is important for maintaining normal cell growth and morphology at elevated temperatures.
FIG 3.
Growth and morphological analysis of WT and genetically manipulated spirochetes in vitro. (A) WT, BbhtrA mutant (M1), and complemented (Com) spirochetes were diluted to a density of 105/ml, grown at either 34°C (top) or 37°C (bottom) in BSK-H medium, and counted under a dark-field microscope every 24 h with a Petroff-Hausser cell counter. Note that BbhtrA mutant isolates grew as single cells in the early growth phase but started forming clumps in the late growth phase at 37°C. (B) Morphological analysis of WT, BbhtrA mutant, and complemented B. burgdorferi isolates. On the left is a scanning electron microscopic (SEM) analysis of spirochetes grown at 37°C. Masses of intertwined spirochetes and spherical blebs (arrows) are visible. On the right are transmission electron micrographs (TEM) of Epon-embedded spirochetes. Note the presence of large, nearly round structures enclosing two or more cells (arrow) in the mutant.
Scanning and transmission electron microscopic studies revealed that the loss of BbhtrA results in striking alterations in cellular organization when spirochetes are grown at 37°C. In contrast to WT organisms that grew as single replicating cells, BbhtrA mutant organisms appeared as clumped cells or intertwined masses with spherical blebs (Fig. 3B). While the WT and complemented spirochetes showed distinct outer and inner membrane sandwiching the periplasmic space harboring flagella, BbhtrA mutant spirochetes exhibited several large, predominantly membranous, irregularly shaped, hollow structures containing round vesicles, protoplasmic cylinders revealing flagella beneath the outer membrane (Fig. 3B).
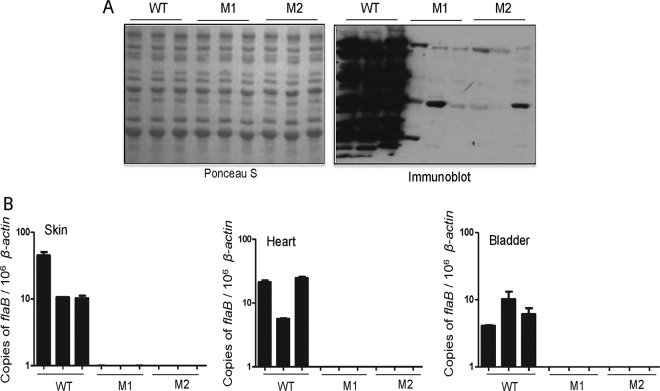
BbHtrA function is critical for infection of mice.
To examine the role of BbHtrA in infectivity, separate groups of C3H/HeN mice were inoculated intradermally with WT and genetically manipulated spirochetes (105 cells/mouse). As our complemented isolate was generated with a shuttle plasmid that uses the antibiotic kanamycin, which has a shorter half-life in mice, this isolate cannot be used for in vivo infection studies. In fact, our efforts to maintain the shuttle plasmid in mice via intermittent administration of various doses of the antibiotic remained unsuccessful, perhaps because of faster clearance or an insufficient concentration of kanamycin in vivo. Therefore, for the infection studies, we used two independent BbhtrA mutant clones, designated M1 and M2. Samples of skin, heart, bladder, and serum were collected at day 14 and tested for B. burgdorferi infection by serology, qRT-PCR, and culture. As shown in Fig. 4A, unlike the WT, which showed a strong serological response, both of the mutants had a severely attenuated ability to induce an antibody response to spirochete proteins, suggesting their clearance during early mammalian infection. The qRT-PCR analyses further revealed that BbhtrA mutant clones were undetectable in all of the tissues tested (Fig. 4B). Finally, culture analysis confirmed that the BbhtrA mutants remained undetectable in the samples collected from all six mice infected with either of the two mutant clones, whereas WT spirochetes could be readily detected in corresponding mice (see Table S2 in the supplemental material). Together, studies described above suggest an essential role for BbHtrA in the establishment of B. burgdorferi infection in murine hosts.
FIG 4.
BbhtrA is required for infection of mice. (A) Immunoreactive profiles of mice infected with WT and BbhtrA mutant spirochetes. Groups of three C3H/HeN mice were infected with WT and BbhtrA mutant isolates (M1 and M2). Serum samples collected 2 weeks postinoculation were used to screen an immunoblot of B. burgdorferi cell lysate. (B) Spirochete burdens in infected mice were assessed by qRT-PCR by measuring the number of copies of the B. burgdorferi flaB gene normalized to mouse β-actin levels in the skin, heart, and bladder. Error bars represent the SEM of three independent experiments. BbhtrA mutants M1 and M2 remained undetectable in the murine tissues tested.
The borrelial protein BB0323 is processed by BbHtrA.
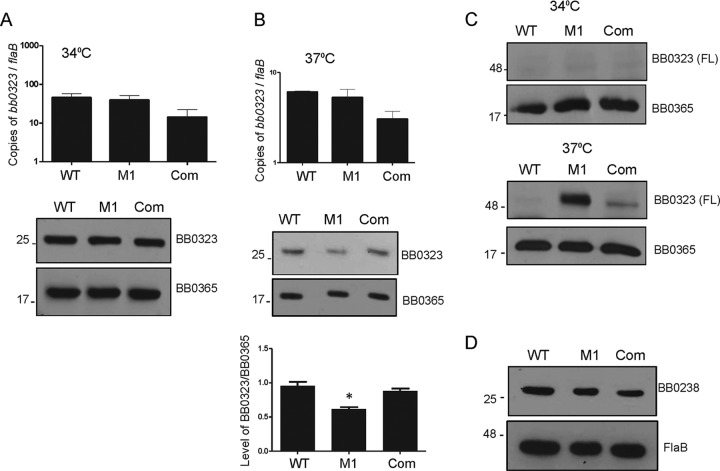
We previously demonstrated that BbHtrA cleaves BB0323, one of the proteins required for infectivity, in a manner dependent upon its catalytic serine residue (28). The BB0323 protein is required for normal organization of the outer membrane, cell fission, persistence in mice, and the transition between the host and the vector in vivo (34). Recent in vitro studies using recombinant BbHtrA have led to the identification of additional substrates that include borrelial virulence determinants like P66 and host proteins that are important for infectivity or pathogenesis (28–32). Specifically, our previous studies suggest that recombinant BbHtrA interacts with and specifically cleaves BB0323 into a larger N-terminal polypeptide and a shorter C-terminal polypeptide (28). As morphological abnormalities and the defect in virulence exhibited by BbHtrA are analogous to those of BB0323 deletion mutants (34), we sought to confirm whether maturation of BB0323 is dysregulated in the absence of BbHtrA. To test this, we measured the BB0323 mRNA and protein levels in the BbhtrA mutant and complemented isolates. As shown in Fig. 5A, no discernible difference in the BB0323 transcript or protein levels of the WT, BbhtrA mutant, and complemented strains were noted at 34°C. Interestingly, a less BB0323 protein expression was noticed in BbhtrA mutant clones than in the WT or complemented strain at 37°C (Fig. 5B). Our earlier studies suggested that the immature full-length BB0323 protein and its mature C-terminal polypeptide are difficult to detect in B. burgdorferi by conventional immunoblotting assays with anti-BB0323 antibodies, most likely because of their lower stability or rate of occurrence; however, they can be detected by a far-Western assay with the BB0323 N-terminal polypeptide that binds the C-terminal polypeptide (28). Using the latter approach, we detected full-length BB0323 in the BbhtrA mutant isolates, which was absent from the WT at 37°C (Fig. 5C). The expression of full-length BB0323 was significantly reduced in the complemented strain, further suggesting that the processing of full-length BB0323 is impaired in the absence of BbHtrA. It has previously been established that BB0323 interacts with another virulence determinant, BB0238, and regulates its expression in B. burgdorferi (44). In order to test whether BbHtrA regulates BB0323 via BB0238, we monitored the level of BB0238 expression in the BbhtrA mutant. As shown in Fig. 5D, BbHtrA-deficient spirochetes expressed BB0238 at a level similar to that of the WT or complemented strain at the elevated mammalian host-specific (37°C) temperature, further suggesting the specificity of BB0323 processing by BbHtrA.
FIG 5.
BbHtrA plays a role in the proteolytic processing of the borrelial virulence determinant BB0323. (A) Expression of BB0323 in the WT, BbhtrA mutant, and complemented strains at 34°C. WT, BbhtrA mutant (M1), and complemented (Com) isolates were grown at 34°C, and samples were harvested at stationary (108 cells/ml) phase. Expression of bb0323 was monitored either at the transcription level by qRT-PCR and normalized to copies of the flaB transcript (top) or at the protein level after immunoblotting (bottom) with anti-BB0323 or anti-BB0365 antiserum. The bars represent the mean values from three independent experiments, and the error bars represent the SEM. The levels of BbhtrA mRNA are similar in all of the isolates (P > 0.05). (B) Production of BB0323 in the WT, BbhtrA mutant, and complemented strains at 37°C. Expression of bb0323 mRNA (top) or protein (middle and bottom) was determined in WT, BbhtrA mutant, and complemented spirochetes at 37°C. Extracts from WT, BbhtrA mutant, and complemented isolates were resolved by 12% SDS-PAGE and immunoblotted with anti-BB0323 and anti-BB0365 antisera. Levels of BbHtrA relative to those of BB0365 (bottom) were determined by quantitative densitometry. The levels of BB0323 are reduced in the BbhtrA mutant. *, P < 0.05. The bars represent the mean values from three independent experiments, and the error bars represent the SEM. The levels of bb0323 mRNA are similar in all of the isolates (P > 0.05). (C) Detection of full-length (FL) BB0323 in the BbhtrA mutant by far-Western blotting. Extracts from WT, BbhtrA mutant, and complemented isolates grown at either 34°C (top) or 37°C (bottom) were separated by 12% SDS-PAGE, transferred to a nitrocellulose membrane, incubated overnight with recombinant His-BB0323 purified from E. coli, and immunoblotted with anti-BB0323 or anti-BB0365 antisera. (D) Expression of BB0238 in WT, BbhtrA mutant, and complemented spirochetes at 37°C. Extracts from WT, BbhtrA mutant, and complemented isolates were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with anti-BB0238 antiserum (top) or anti-FlaB antiserum (bottom) as a loading control. The values to the left of the blots are molecular sizes in kilodaltons.
DISCUSSION
HtrA proteins are generally involved in cellular adaptation to various stress response events via their protease and chaperone functions, maintaining tight protein quality control in organisms, including microbes (12). These proteases-chaperones function in an ATP-independent manner; therefore, many prokaryotic and eukaryotic genomes encode multiple homologs of these highly efficient proteins that are involved in diverse cellular functions (12, 13). Interestingly, HtrA has been shown to play an important role in the pathogenesis of several Gram-positive, as well as Gram-negative, bacteria (27, 45). Owing to their important roles in cellular physiology, several pathogens harbor multiple homologs of HtrA. However, B. burgdorferi encodes a single HtrA, termed BbHtrA, which is pivotal in the establishment of infection in the mammalian host. Recent studies have suggested that BbHtrA is responsible for the proteolytic processing of a number of key virulence determinants, such as BB0323, P66, BmpD, and CheX (31), indicating its role in diverse cellular processes. For instance, the cleavage of the phosphatase CheX by BbHtrA suggests that it has a role in regulating bacterial motility and chemotaxis, the key cellular processes that contribute to the invasiveness of B. burgdorferi and play important roles in the progression of the disease (29). On the other hand, the identification of outer membrane proteins such as BB0323, P66, and BmpD as the substrates of BbHtrA implies that it has a role in the regulation of key virulence determinants that are important for the biogenesis or organization of the membrane, transport across the membrane, or host-pathogen interaction (28–30). Although studies using B. burgdorferi stains overexpressing BbhtrA have shed new light on the function of the protease it encodes and its potential roles in virulence, previous efforts to generate BbhtrA deletion mutants have been unsuccessful (28–30), which has also limited our understanding of its role in spirochetal biology and infection. The study described here shows that the successful deletion of BbhtrA in low-passage-number strain 297 of B. burgdorferi results in a severely distorted morphology of the spirochetes at elevated temperatures, thereby attenuating their ability to infect mice. The aberrant cell morphology of BbhtrA mutants grown at the higher, mammalian host-specific temperature included striking alterations in their cellular structures and occasional fission defects, as well as the formation of blebs within a nearly round sac-like structure. The restoration of normal morphology upon the reintroduction of the gene in trans further reinforced the idea that the effect was specifically mediated by BbHtrA. Similar structurally heterogeneous entities have previously been recorded and are known to occur in spirochete populations that encounter stress, such as in unfed ticks or upon exposure to borreliacidal agents (46–49). Here, we propose a critical role for BbHtrA, a high-temperature-induced protease, in the cellular regulation and stress tolerance of Lyme disease spirochetes. A similar high-temperature sensitivity of HtrA deletion mutants has also been observed in other organisms such as Escherichia coli and Saccharomyces cerevisiae (50, 51). Interestingly, despite the presence of BbhtrA as a single gene, the generation of deletion mutants with growth kinetics similar to those of WT spirochetes, especially at 34°C, suggests that its role is nonessential, at least in the in vitro growth of the organism.
While determination of the precise cellular function of BbHtrA awaits future studies, genetic manipulation of its expression in B. burgdorferi affected the levels of several spirochete proteins, such as P66 (30) and BB0323, but not those of several other proteins tested, suggesting that the protease is involved in the regulation of a specific set of borrelial proteins performing diverse functions in spirochete biology. As both the transcript levels and posttranslational maturation of target proteins are affected by BbhtrA deficiency or overexpression, as shown for BB0323 and P66 (30), it is likely that this protein could affect the processing of an unknown gene regulator, as speculated earlier (30). Additionally, BbHtrA could also act as a chaperone by providing an extended protein conformation in the periplasm before folding and insertion into the membrane or may promote the degradation of misfolded proteins prior to translocation in the membrane (12, 13). These observations are supported by the localization of BbHtrA in the various cellular compartments, including the periplasmic fraction, of B. burgdorferi (28). Apart from its role in the intracellular regulation of spirochete proteins, the secreted form of BbHtrA, which is potentially released into the mammalian host during infection and degrades the host extracellular matrix and proteoglycan (32), is directly involved in host infection and pathogenesis. One caveat regarding our study is that we did not perform infection experiments with the trans-complemented clone. However, two independent mutants that have all of the plasmids described here showed the same phenotype, strongly indicating that the BbHtrA-deficient spirochete is not infectious. While the precise reason why BbhtrA mutants lost virulence in mammalian hosts remains enigmatic, we speculate that multiple mechanisms could be responsible. To persist in a higher-temperature environment such as a mammalian host, spirochetes would require the critical functional support of BbHtrA, the absence of which renders mutants impaired for optimal growth and successful dissemination through host tissues.
Previously, we identified a virulence determinant, BB0323, that plays an important role in infectivity (34), possibly because of its critical involvement in spirochete fission and outer membrane integrity (33, 34), including other unknown functions. The recombinant form of BbHtrA specifically cleaved BB0323 into N- and C-terminal polypeptides with sizes that roughly corresponded to the mature polypeptides in spirochetes, suggesting that BB0323 is the specific substrate of BbHtrA (28). These initial results and the similarity of the morphological aberrations associated with the deletion of both BB0323 (34) and BbHtrA in the present study underscore the important role of the protease in the processing of BB0323 in B. burgdorferi. Interestingly, we not only noticed a reduction in cleaved and mature BB0323 but also detected uncleaved BB0323 in the BbhtrA deletion mutant at 37°C, which was further reduced upon complementation of the gene in trans. The processing of BB0323 remains unaffected at the normal growth temperature (34°C), raising the possibility that another cleavage mechanism or an unknown protease with a function similar to that of BbHtrA exists in spirochetes. It is noteworthy that the C-terminal protease CtpA also plays a role in the final processing of BB0323 polypeptides (52), and therefore, it might be possible that multiple proteases cleave BB0323 at the various temperatures and environments the spirochetes encounter in mammals and in the arthropod vector. At least two mechanisms could be attributed to the role of BbHtrA in the processing of BB0323 at the mammal-specific temperature. First, BbHtrA directly processes BB0323, and precise cleavage of BB0323 might be essential for cell fission or infectivity; therefore, absence of BbHtrA results in accumulation of the uncleaved polypeptide, leading to altered cell morphology, as seen in BbhtrA deletion mutants, and subsequent loss of virulence. Second, BbHtrA might act as a chaperone for the cleaved N terminus, and the loss of BbHtrA increased the susceptibility of the N terminus to degradation, thereby resulting in a defect in fission and hence infectivity. At this point, it cannot be confirmed that the reduction in the processed or cleaved BB0323 polypeptide was due to its increased susceptibility to degradation in the absence of BbHtrA at the elevated temperature or to impaired cleavage of the full-length protein. Nevertheless, our data strongly argue that BbHtrA is involved in the processing and regulation of BB0323 in B. burgdorferi, especially at an elevated, mammalian host-specific temperature.
In summary, we demonstrated that targeted deletion of BbhtrA results in specific spirochete phenotypes, preferentially at an elevated temperature, including slower growth, altered morphology and cellular architecture, and attenuated infection of mice. Although the loss of virulence could not be reversed in the complemented isolate because of technical constraints, most of the defects exhibited by BbhtrA mutants in vitro can be cured by the introduction of a copy of the gene in trans. The transcription and posttranslational processing of at least one key virulence determinant, BB0323, are also affected. These studies open a new line of investigation in understanding the regulation of proteins involved in key cellular processes and infection by BbHtrA. Further studies are warranted to investigate the role of BbHtrA in the tick phase of the cycle of B. burgdorferi and further elucidate its involvement in the global regulation of important cellular proteins.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by funding from the National Institute of Allergy and Infectious Diseases (AI080615 and AI116620 to U.P.; AI083640 to X.F.Y.) and from the National Science Foundation of China (81501772 to M.Y.; 81171611 to Y.L.). O.B. is supported by faculty research funding from Ondokuz Mayis University, Turkey.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00360-16.
REFERENCES
- 1.Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129(Suppl):S191–S220. doi: 10.1017/S0031182003004694. [DOI] [PubMed] [Google Scholar]
- 2.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steere AC, Coburn J, Glickstein L. 2004. The emergence of Lyme disease. J Clin Invest 113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marques A. 2008. Chronic Lyme disease: a review. Infect Dis Clin North Am 22:341–360. doi: 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schriefer ME. 2015. Lyme disease diagnosis: serology. Clin Lab Med 35:797–814. doi: 10.1016/j.cll.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 35:490–516. [DOI] [PubMed] [Google Scholar]
- 8.Casjens SR, Mongodin EF, Qiu WG, Dunn JJ, Luft BJ, Fraser-Liggett CM, Schutzer SE. 2011. Whole-genome sequences of two Borrelia afzelii and two Borrelia garinii Lyme disease agent isolates. J Bacteriol 193:6995–6996. doi: 10.1128/JB.05951-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 10.Schutzer SE, Fraser-Liggett CM, Casjens SR, Qiu WG, Dunn JJ, Mongodin EF, Luft BJ. 2011. Whole-genome sequences of thirteen isolates of Borrelia burgdorferi. J Bacteriol 193:1018–1020. doi: 10.1128/JB.01158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kung F, Anguita J, Pal U. 2013. Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector. Future Microbiol 8:41–56. doi: 10.2217/fmb.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clausen T, Kaiser M, Huber R, Ehrmann M. 2011. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol 12:152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- 13.Clausen T, Southan C, Ehrmann M. 2002. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell 10:443–455. doi: 10.1016/S1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- 14.Merdanovic M, Clausen T, Kaiser M, Huber R, Ehrmann M. 2011. Protein quality control in the bacterial periplasm. Annu Rev Microbiol 65:149–168. doi: 10.1146/annurev-micro-090110-102925. [DOI] [PubMed] [Google Scholar]
- 15.Roberts DM, Personne Y, Ollinger J, Parish T. 2013. Proteases in Mycobacterium tuberculosis pathogenesis: potential as drug targets. Future Microbiol 8:621–631. doi: 10.2217/fmb.13.25. [DOI] [PubMed] [Google Scholar]
- 16.Johnson K, Charles I, Dougan G, Pickard D, O'Gaora P, Costa G, Ali T, Miller I, Hormaeche C. 1991. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol 5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis C, Skovierova H, Rowley G, Rezuchova B, Homerova D, Stevenson A, Spencer J, Farn J, Kormanec J, Roberts M. 2009. Salmonella enterica serovar Typhimurium HtrA: regulation of expression and role of the chaperone and protease activities during infection. Microbiology 155:873–881. doi: 10.1099/mic.0.023754-0. [DOI] [PubMed] [Google Scholar]
- 18.Hoy B, Lower M, Weydig C, Carra G, Tegtmeyer N, Geppert T, Schroder P, Sewald N, Backert S, Schneider G, Wessler S. 2010. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep 11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim YM, Kerr AR, McCluskey J, Mitchell TJ. 2004. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect Immun 72:3584–3591. doi: 10.1128/IAI.72.6.3584-3591.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitlaru T, Zaide G, Ehrlich S, Inbar I, Cohen O, Shafferman A. 2011. HtrA is a major virulence determinant of Bacillus anthracis. Mol Microbiol 81:1542–1559. doi: 10.1111/j.1365-2958.2011.07790.x. [DOI] [PubMed] [Google Scholar]
- 21.Rigoulay C, Entenza JM, Halpern D, Widmer E, Moreillon P, Poquet I, Gruss A. 2005. Comparative analysis of the roles of HtrA-like surface proteases in two virulent Staphylococcus aureus strains. Infect Immun 73:563–572. doi: 10.1128/IAI.73.1.563-572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiaden AN, Richards PJ. 2013. The emerging roles of HTRA1 in musculoskeletal disease. Am J Pathol 182:1482–1488. doi: 10.1016/j.ajpath.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Zurawa-Janicka D, Skorko-Glonek J, Lipinska B. 2010. HtrA proteins as targets in therapy of cancer and other diseases. Expert Opin Ther Targets 14:665–679. doi: 10.1517/14728222.2010.487867. [DOI] [PubMed] [Google Scholar]
- 24.Hoy B, Geppert T, Boehm M, Reisen F, Plattner P, Gadermaier G, Sewald N, Ferreira F, Briza P, Schneider G, Backert S, Wessler S. 2012. Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. J Biol Chem 287:10115–10120. doi: 10.1074/jbc.C111.333419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krojer T, Sawa J, Huber R, Clausen T. 2010. HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat Struct Mol Biol 17:844–852. doi: 10.1038/nsmb.1840. [DOI] [PubMed] [Google Scholar]
- 26.Skorko-Glonek J, Zurawa-Janicka D, Koper T, Jarzab M, Figaj D, Glaza P, Lipinska B. 2013. HtrA protease family as therapeutic targets. Curr Pharm Des 19:977–1009. [DOI] [PubMed] [Google Scholar]
- 27.Frees D, Brondsted L, Ingmer H. 2013. Bacterial proteases and virulence. Subcell Biochem 66:161–192. doi: 10.1007/978-94-007-5940-4_7. [DOI] [PubMed] [Google Scholar]
- 28.Kariu T, Yang X, Marks CB, Zhang X, Pal U. 2013. Proteolysis of BB0323 results in two polypeptides that impact physiologic and infectious phenotypes in Borrelia burgdorferi. Mol Microbiol 88:510–522. doi: 10.1111/mmi.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman JL, Crowley JT, Toledo AM, Benach JL. 2013. The HtrA protease of Borrelia burgdorferi degrades outer membrane protein BmpD and chemotaxis phosphatase CheX. Mol Microbiol 88:619–633. doi: 10.1111/mmi.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman JL, Toledo A, Benach JL. 2016. Borrelia burgdorferi HtrA: evidence for twofold proteolysis of outer membrane protein p66. Mol Microbiol 99:135–150. doi: 10.1111/mmi.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gherardini FC. 2013. Borrelia burgdorferi HtrA may promote dissemination and irritation. Mol Microbiol 90:209–213. doi: 10.1111/mmi.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell TM, Johnson BJ. 2013. Lyme disease spirochaetes possess an aggrecan-binding protease with aggrecanase activity. Mol Microbiol 90:228–240. doi: 10.1111/mmi.12276. [DOI] [PubMed] [Google Scholar]
- 33.Stewart PE, Hoff J, Fischer E, Krum JG, Rosa PA. 2004. Genome-wide transposon mutagenesis of Borrelia burgdorferi for identification of phenotypic mutants. Appl Environ Microbiol 70:5973–5979. doi: 10.1128/AEM.70.10.5973-5979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Yang X, Kumar M, Pal U. 2009. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. J Infect Dis 200:1318–1330. doi: 10.1086/605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell TM, Tang X, Goldstein JM, Bagarozzi D, Johnson BJ. 2016. The salt-sensitive structure and zinc inhibition of Borrelia burgdorferi protease BbHtrA. Mol Microbiol 99:586–596. doi: 10.1111/mmi.13251. [DOI] [PubMed] [Google Scholar]
- 36.Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, Norgard MV, Fikrig E. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest 113:220–230. doi: 10.1172/JCI200419894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med 199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrasco SE, Yang Y, Troxell B, Yang X, Pal U, Yang XF. 2015. Borrelia burgdorferi elongation factor EF-Tu is an immunogenic protein during Lyme borreliosis. Emerg Microbes Infect 4:e54. doi: 10.1038/emi.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Hegde S, Shroder DY, Smith AA, Promnares K, Neelakanta G, Anderson JF, Fikrig E, Pal U. 2013. The lipoprotein La7 contributes to Borrelia burgdorferi persistence in ticks and their transmission to naive hosts. Microbes Infect 15:729–737. doi: 10.1016/j.micinf.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Coleman AS, Anguita J, Pal U. 2009. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog 5:e1000326. doi: 10.1371/journal.ppat.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Lenhart TR, Kariu T, Anguita J, Akins DR, Pal U. 2010. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect Immun 78:4477–4487. doi: 10.1128/IAI.00501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, Bao F, Yang X, Pypaert M, Pradhan D, Kantor FS, Telford S, Anderson JF, Fikrig E. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Kumar M, Yang X, Coleman AS, Pal U. 2010. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis 201:1084–1095. doi: 10.1086/651172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kariu T, Sharma K, Singh P, Smith AA, Backstedt B, Buyuktanir O, Pal U. 2015. BB0323 and novel virulence determinant BB0238: Borrelia burgdorferi proteins that interact with and stabilize each other and are critical for infectivity. J Infect Dis 211:462–471. doi: 10.1093/infdis/jiu460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingmer H, Brondsted L. 2009. Proteases in bacterial pathogenesis. Res Microbiol 160:704–710. doi: 10.1016/j.resmic.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Brorson O, Brorson SH, Scythes J, MacAllister J, Wier A, Margulis L. 2009. Destruction of spirochete Borrelia burgdorferi round-body propagules (RBs) by the antibiotic tigecycline. Proc Natl Acad Sci U S A 106:18656–18661. doi: 10.1073/pnas.0908236106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunham-Ems SM, Caimano MJ, Eggers CH, Radolf JD. 2012. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog 8:e1002532. doi: 10.1371/journal.ppat.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merilainen L, Herranen A, Schwarzbach A, Gilbert L. 2015. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology 161:516–527. doi: 10.1099/mic.0.000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, Samuels DS. 2015. The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog 11:e1005160. doi: 10.1371/journal.ppat.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padmanabhan N, Fichtner L, Dickmanns A, Ficner R, Schulz JB, Braus GH. 2009. The yeast HtrA orthologue Ynm3 is a protease with chaperone activity that aids survival under heat stress. Mol Biol Cell 20:68–77. doi: 10.1091/mbc.E08-02-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipinska B, Zylicz M, Georgopoulos C. 1990. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol 172:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostberg Y, Carroll JA, Pinne M, Krum JG, Rosa P, Bergstrom S. 2004. Pleiotropic effects of inactivating a carboxyl-terminal protease, CtpA, in Borrelia burgdorferi. J Bacteriol 186:2074–2084. doi: 10.1128/JB.186.7.2074-2084.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.