Abstract
Patients with cortical blindness following a lesion to the primary visual cortex (V1) may retain nonconscious visual abilities (blindsight). One intriguing, though largely unexplored question, is whether nonconscious vision in the blind hemifield of hemianopic patients can be sensitive to higher-order perceptual organization, and which V1-independent structure underlies such effect. To answer this question, we tested two rare hemianopic patients who had undergone hemispherectomy, and in whom the only post-chiasmatic visual structure left intact in the same side of the otherwise damaged hemisphere was the superior colliculus (SC). By using a variant of the redundant target effect (RTE), we presented single dots, patterns composed by the same dots organized in quadruple gestalt-like configurations, or patterns of four dots arranged in random configurations, either singly to the intact visual hemifield or bilaterally to both hemifields. As reported in a number of prior studies on blindsight patients, we found that bilateral stimulation yielded faster reaction times (RTs) than single stimulation of the intact field for all conditions (i.e., there was an implicit RTE). In addition to this effect, both patients showed a further speeding up of RTs when the gestalt-like, but not the random shape, quadruple patterns were projected to their blind hemifield during bilateral stimulation. Because other retino-recipient subcortical and cortical structures in the damaged hemisphere are absent, the SC on the lesioned side seems solely responsible for such an effect. The present results provide initial support to the notion that nonconscious vision might be sensitive to perceptual organization and stimulus configuration through the pivotal contribution of the SC, which can enhance the processing of gestalt-like or structured stimuli over meaningless or randomly assembled ones and translate them into facilitatory motor outputs.
Keywords: Blindsight, Superior colliculus, Redundant target effect, Hemispherectomy, Gestalt configuration
1. Introduction
Following unilateral damage to the visual cortex, patients experience clinical blindness in both halves of each eye in their contralesional visual hemifield (homonymous hemianopia). Despite clinical blindness, some patients retain nonconscious visual abilities for processing unseen stimuli in the blind hemifield (Ajina et al., 2015, Barbur et al., 1993, Blythe et al., 1987, Bridge et al., 2008, Celeghin et al., 2015a, Corbetta et al., 1990, Kentridge et al., 2004, Kentridge et al., 2008, Marzi, 1986, Pizzamiglio et al., 1984, Pöppel et al., 1973, Rafal et al., 1990, Singer et al., 1977, Stoerig, 1987, Stoerig et al., 1985, Tinelli et al., 2013, Torjussen, 1976, Torjussen, 1978, Weiskrantz et al., 1974, Zihl and von Cramon, 1980). These residual nonconscious abilities, termed “blindsight” by Weiskrantz et al. (1974), have been described for different visual functions such as stimulus detection (Sahraie et al., 1997, Weiskrantz et al., 1995), shape or category-specific processing (Trevethan et al., 2007, Van den Stock et al., 2015, Van den Stock et al., 2014), color and motion discrimination (Hervais-Adelman et al., 2015, Kentridge et al., 2007, Morland et al., 1999), recognition of facial and bodily expressions (Bertini et al., 2013, Celeghin et al., 2015b, de Gelder et al., 2012, de Gelder et al., 1999, Tamietto et al., 2005) or gaze direction (Burra et al., 2013). Moreover, preserved processing of such visual properties has been described under a variety of task demands, such as visually guided or goal directed behaviour (Buetti et al., 2013, Celeghin et al., 2015c, Pöppel et al., 1973, Rafal et al., 1990, Spering and Carrasco, 2015), yes-no or alternative forced-choice tasks (Azzopardi and Cowey, 1997, Tamietto et al., 2009), and perceptual completion requirements (Torjussen, 1978) (see Tamietto & Morrone, 2016, for a recent review).
Two kinds of strategies have been typically employed to assess blindsight: direct and indirect methods. The former makes use of forced-choice paradigms where the subjects make an explicit decision regarding unseen attributes of the stimulus presented to their blind hemifield (Danckert and Rossetti, 2005, Stoerig and Cowey, 1989, Weiskrantz, 1990). Above chance performance, despite absence of awareness, is taken as indicative of blindsight. In contrast, the latter methods rely on the effect exerted by unseen stimuli presented to the blind hemifield on stimuli simultaneously presented to the intact counterpart. One of the indirect methods most often used for testing blindsight is the redundant target effect (RTE) (Marzi, Tassinari, Aglioti, & Lutzemberger, 1986). In healthy participants, the tachistoscopic presentation of two or more synchronous stimuli to both visual hemifields (BVF) across the vertical meridian results in faster reaction times (RTs) than a single stimulus presentation to one visual hemifield, either left (LVF) or right (RVF). This effect, also known as bilateral summation or redundancy gain, has been reported in many studies in healthy participants as well as in blindsight patients (Celeghin et al., 2015c, Corbetta et al., 1990, Leh et al., 2006b, Marzi, 1986, Marzi et al., 2009, Tamietto et al., 2010, Tomaiuolo et al., 1997). The main advantage of indirect methods is that patients make a choice about visual attributes they do not consciously acknowledge without being forced to do so, but are only required to respond as quickly as possible to the stimulus in their intact hemifield in a simple RT paradigm. Therefore, since patients have to respond to stimuli they can normally perceive, the range of visual operations that can potentially be investigated is wide and may include high-order visual operations.
Recently, Celeghin, Savazzi et al. (2015) introduced a variant of the RTE to obtain insights on the influence of stimulus numerosity and configuration on visuo-motor responses in blindsight patients. Participants were presented with either unilateral or bilateral black dots. For each of these two conditions, the stimuli could be a single dot or a pattern of four dots. The latter were presented in either a variable random spatial configuration or a fixed one wherein the four dots were arranged in a diamond-like shape. Notably, the two configurations subtended the same visual angle and had the same luminance. Orthogonal to the replication of the common RTE in the comparison between unilateral and bilateral conditions, the authors also found an additive effect of stimulus configuration with a speeding up of RT when the gestalt-like, but not the random shape, quadruple pattern was projected to the patients' blind field. These novel findings have allowed the establishment of a solid approach to study the influence of stimulus configuration on blindsight and its underlying neural structures, an issue that in the past has come under only desultory scrutiny. These results have provided initial support for the notion that nonconscious vision might be sensitive to perceptual organization, thereby enhancing the processing of gestalt-like or structured over meaningless or randomly assembled stimuli.
Concerning the neuro-functional mechanisms of RTE, several studies in patients with unilateral destruction of the primary visual cortex (V1) or with removal of the entire cortical mantle in one hemisphere (hemispherectomy) have provided convincing evidence that the superior colliculus (SC) is necessary and sufficient for the RTE to occur (Leh et al., 2006b, Leh et al., 2010, Marzi et al., 2009, Tamietto et al., 2010). This raises the interesting, entirely unexplored, possibility that the SC responds differentially to higher-order perceptual properties, such as those involved in stimulus configuration, even in the absence of the geniculo-striate pathway that deprives vision of its conscious component.
Although suggestive in a number of aspects, the previous results by Celeghin, Savazzi, et al., 2015 are not conclusive for two reasons. Firstly, the patients had intact portions of extrastriate visual areas as well as spared retino-recipient subcortical structures besides the SC, such as the lateral geniculate nucleus (LGN) and the pulvinar (Pulv). All these subcortical structures have been shown to receive direct input from the retina and to send (mainly) ipsilateral efferents to several extrastriate visual areas bypassing V1 (Ajina et al., 2015, Bridge et al., 2008, Leh et al., 2008, Lyon et al., 2010, Schmid et al., 2010, Sincich et al., 2004, Tamietto and Morrone, 2016, Tamietto et al., 2012). Therefore, the relative contribution of the SC could not be disentangled from that of the other subcortical centers or their extrastriate targets, so that the SC specific role remains unresolved. Secondly, while a variety of random configurations were used in the original study, only one diamond-shape dot pattern was presented, thereby leaving the possibility that the effect found for the latter condition was due to familiarity and/or to spatially fixed versus variable stimulus configuration rather than to the presence of a gestalt-like dot pattern per se.
The aim of the present study is to tackle these questions by partially modifying the original experimental paradigm and by testing patients with hemispherectomy and blindsight. These patients had undergone removal of an entire cerebral hemisphere or of all the temporo-occipito-parietal cortices. Moreover, the LGN and Pulv in the affected hemisphere have been both removed leaving only the SC intact among retino-recipient subcortical structures. Therefore, testing the RTE in these patients has offered the unprecedented opportunity to examine the impact of perceptual configuration in nonconscious visually guided behaviour under the most stringent conditions in order to determine the putative crucial role of the SC.
2. Methods
2.1. Patients
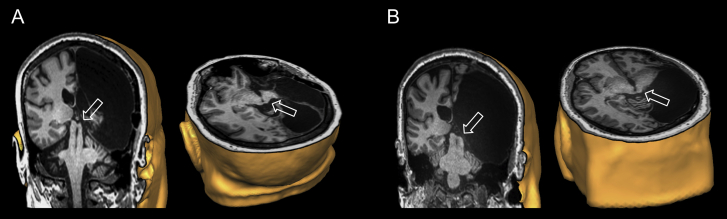
Patient DR is a right-handed woman (40 years old at the time of testing) who presented with left hemiparesis since birth and began suffering from epileptic seizures at the age of 5 years. CT and MRI scans performed at the age of 17 years revealed severe atrophy of the right cerebral hemisphere and EEG recording showed epileptiform activity over the right frontal-parietal-temporal regions. Cognitive testing indicated borderline intelligence scores: Full Scale Intelligence Quotient (FISQ), 77; verbal IQ, 92 and performance IQ, 65. At the same age, she underwent a functional hemispherectomy, which consisted of removing the temporal lobe including the mesial structures, the amygdala, the hippocampus, and a frontal-parietal corticectomy. The remaining cortical regions were left in situ but were disconnected from the rest of the brain by sectioning the white matter anteriorly and laterally, as well as posteriorly and laterally along the falx. Subsequent neuropathological investigation revealed an inflammatory process with diffuse gliosis characteristic of Rasmussen encephalitis. Follow-up assessments, at the age of 19 years, indicated that her level of intellectual function had increased to the low average range: FISQ, 83; verbal IQ, 87 and performance IQ, 83. MRI scans postoperatively, as well as further scans performed afterwards for research purposes and published elsewhere, showed the presence of intact left and right SC, whereas the presence of the Pulv was limited to the left (intact) hemisphere (Fig. 1A) (Leh et al., 2008, Leh et al., 2006a, Leh et al., 2010, Tomaiuolo et al., 1997). Complete contralateral (left) hemianopia without macular sparing has been confirmed by computerized perimetry (Allergan, Humphrey), and her visual acuity was 20/25.
Fig. 1.
A) Coronal (left) and transversal (right) 3-D anatomical reconstruction of patient DR's brain; B) Coronal (left) and transversal (right) 3-D anatomical reconstruction of patient SE's brain. The white transparent arrow indicates the intact superior colliculus in the otherwise damaged right hemisphere.
Patient SE is a right-handed man (49 years old at the time of testing) whose left hemiparesis was noted at birth. Seizure onset occurred at the age of 7 years. At the age of 23 years, CT and MRI scans showed a porencephalic cyst occupying the right temporal-parietal-occipital regions. EEG recordings detected epileptiform activity in the right occipital cortex alongside independent foci over the right temporo-parietal cortex. Neuropsychological testing revealed an FSIQ of 78; verbal IQ of 80 and performance IQ of 79. At the age of 25, he underwent a surgery to remove the congenital porencephalic cyst, and a temporal-parietal-occipital lobectomy was carried out to alleviate intractable seizures. The lobectomy included the hippocampus and the amygdala but spared the anterior portion of the frontal lobe. Postoperative neuropathological examination revealed a neuronal migration disorder (cortical dysplasia). MRI scans postoperatively, as well as further scans performed afterwards for research purposes and published elsewhere, showed the presence of intact left and right SC, but only presence of the Pulv on the left (intact) side (Fig. 1B) (Leh et al., 2008, Leh et al., 2006a, Leh et al., 2010, Tomaiuolo et al., 1997). Follow-up cognitive testing, at the age of 26 years, showed an increase in IQ to an average range: FSIQ, 93; verbal IQ, 90 and performance IQ, 99. Contralateral hemianopia without macular sparing was confirmed by computerized perimetry (Allergan, Humphrey), and his visual acuity was 20/30.
2.2. Stimuli and apparatus
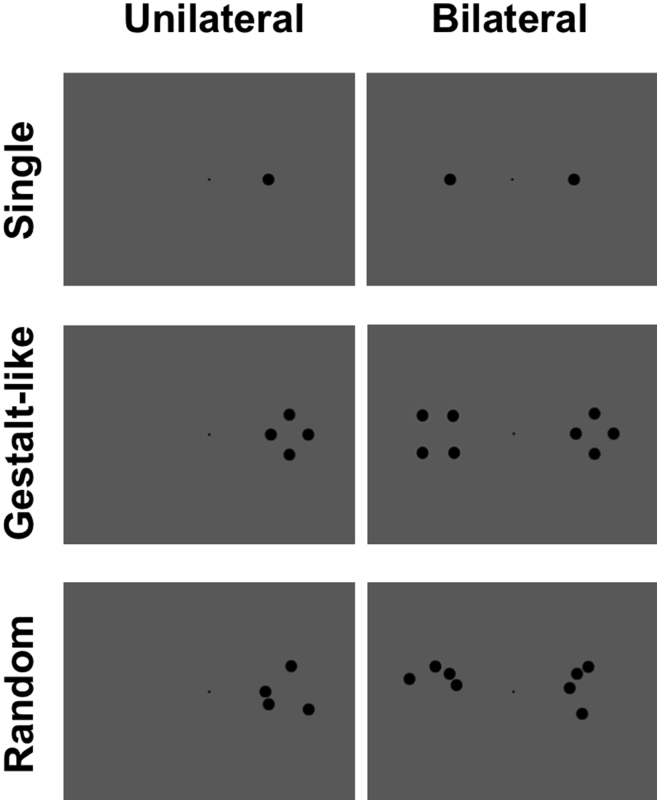
The stimuli were black dots presented against a uniform gray background of 11.42 cd/m2 luminance (RGB values = 126, 126, 126). The dots were presented either unilaterally, to the seeing RVF, or to BVF. For each of these two presentation conditions, there were three possible display types: a single dot, a quadruple pattern composed by the same dots organized in gestalt-like configurations, or a quadruple pattern of dots organized in random configurations. Quadruple arrays were displayed with the innermost dot at 6.5° of eccentricity with respect to the central fixation along the horizontal meridian (the same for single dot displays) and with the outermost dot at 8.5°. Gestalt-like configurations were of four different shapes: diamond, square, rectangles with longer vertical sides and rectangles with longer horizontal sides. Random configurations also consisted of the same dots organized in four different but equally meaningless combinations. In all BVF presentations with quadruple stimuli, the two patterns of stimuli projected to the two visual fields were of the same type, but not physically identical (e.g. a diamond shape in the LVF and a square shape in the RVF), in order to avoid any interpretation of the results in terms of bilateral symmetry (Fig. 2).
Fig. 2.
Examples of the stimuli and their spatial organization in different display types and a function of unilateral versus bilateral presentation, and for single, quadruple gestalt-like and random configurations. Note that in bilateral displays with gestalt-like or random configurations the two stimuli, while being of the same type, are not physically identical.
The stimuli were projected on a 17′ LCD monitor (refresh rate: 16.7 Hz) using a MacBook Pro Notebook with exposure duration of 80 ms (5 refresh rates). The observer's eyes were at a distance of 57 cm from the monitor. Stimulus presentation and response recording were controlled by means of the Presentation 16 software (NeuroBehavioral Systems, Albany, CA).
2.3. Procedure
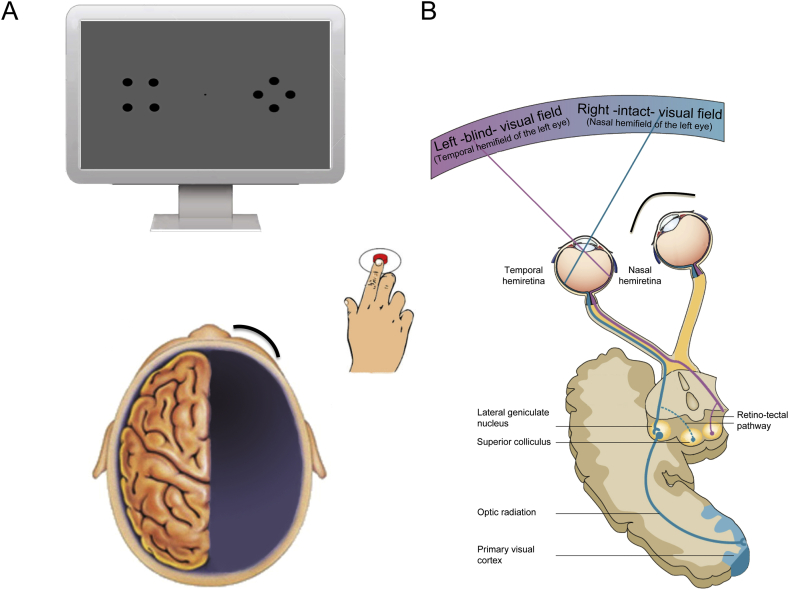
Participants' head movements were minimized through the use of a head and chin rest. They were required to maintain fixation on a small black circle (diameter .3°, luminance: .82 cd/m2) in the center of the screen and to refrain from moving their eyes. Eye movements were also monitored online by one experimenter through an infra-red camera connected with an eye-tracking system, and trials were removed in the event of unsteady fixation. In this rare case (<5%) an additional trial was added to the end of the block. Moreover, to ensure fixation during stimulus presentation, each trial started with a warning acoustic signal (duration: 150 ms; frequency: 1000 Hz) followed by the visual stimulus after a randomized temporal interval (varying between 300 and 700 ms). The patients were tested monocularly with the (left) contralesional eye while an eye patch covered the (right) ipsilesional eye, and response was performed by pressing the space bar of the notebook with the (right) ipsilesional hand. Monocular testing with the dominant (left) eye was used for two reasons. First, in both patients the non-dominant (right) eye ipsilateral to the damaged hemisphere tended to deviate independently from the gaze direction of the dominant eye. This had potentially undermined the correct lateralization of the stimuli during fixation, as two different locations could had been represented in the fovea of the two eyes and a stimulus assumed to be projected in the left blind hemifield might have actually fallen into the seeing field of the right eye. Second, naso-temporal asymmetries have been previously reported. For example, stimuli in the temporal hemifield (the left hemifield of the left eye) induce preferential gaze orienting or summon attention more readily than stimuli in the nasal hemifield (the right hemifield of the left eye) (Rafal et al., 1989, Rafal et al., 1991). These behavioural asymmetries have been proposed to indicate the contribution of the SC in such tasks. Anatomically, indeed, the superficial layers of the SC receive visual input predominantly from the nasal hemiretina, which samples the temporal hemifield, whereas the connections from the temporal hemiretina constitute a relatively weaker retino-tectal pathway (Hubel et al., 1975, Wilson and Toyne, 1970). This has been confirmed in an fMRI study showing higher activation of the SC following stimulation of the temporal rather than nasal hemifield (Sylvester, Josephs, Driver, & Rees, 2007). Therefore, testing the patients monocularly with the left eye ensured that the stimuli projected peripherally to the (left) blind temporal hemifield during bilateral conditions were processed uniquely by the nasal hemiretina, and thus relayed to the right SC, ipsilateral to the damaged hemisphere, through the stronger of the two retino-tectal pathways (Fig 3). In contrast, the stimuli projected to the (right) intact nasal hemifield reached the left SC, ipsilateral to the intact hemisphere, through the weaker connections involving the temporal hemiretina pathway. This was done to counterbalance the potentially weaker representation of the (left) unseen over (right) seen stimulus during bilateral stimulation, as well as the overall weaker response in the (right) ipsilesional SC compared to the (left) SC in the intact side, which might have compromised the RTE.
Fig. 3.
Schematic representation of the testing procedure. (A) Illustration of the experimental set-up; (B) Representation of the naso-temporal asymmetry in the retino-tectal pathway. The weaker pathway from the temporal hemiretina is represented by a blue dashed line, whereas the stronger pathway from the nasal hemiretina is represented by the purple continuous line.
The stimuli were presented in two blocks, each containing 84 randomized trials. Within each block, the six stimulation conditions and display types were equiprobable, and each was repeated for 14 trials (unilateral: single, quadruple gestalt-like, quadruple random; bilateral: single, quadruple gestalt-like, quadruple random). In total, each patient received 28 stimulus presentations per condition.
3. Results
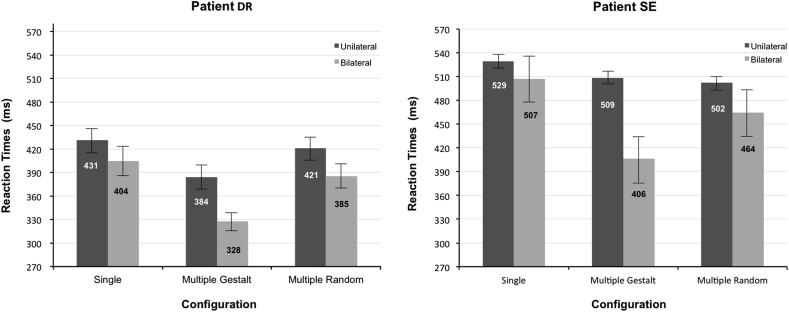
Based on previous reports on the same patients, only RTs in the time-range 200–1000 ms were considered (Leh et al., 2006b, Leh et al., 2010, Tomaiuolo et al., 1997). Patient DR responded to all 168 trials within the accepted range, whereas SE did not respond to 3 trials (1.8%), and had one anticipation (.6%), while the responses to the remaining trials (97.6%) were within the accepted range. Mean RTs as a function of the six stimulus conditions are shown separately for patient DR and SE in Fig. 4. Visual inspection reveals that RTs decreased in bilateral compared to unilateral presentations irrespective of the different display types and for both patients, although this decrease of RTs was particularly pronounced for gestalt-like configurations. Initially, a 2 × 3 repeated-measures ANOVA was carried out on RTs data with the within-subjects factors of Presentation Condition (Unilateral vs Bilateral) and Configuration (Single, Gestalt-like, Random). The same ANOVA was computed on the two patients separately.
Fig. 4.
Mean RTs for unilateral and bilateral conditions and as a function of the single, gestalt-like and random quadruple configurations for the two patients separately. Error bars represent SEM.
Patient DR showed a significant main effect of Presentation Condition [F(1, 27) = 47.507, p < .0001] indicating that a RTE occurred for all display patterns. The main effect of Configuration was also significant [F(2, 54) = 38.133, p < .0001), but there was no significant Presentation Condition × Configuration interaction [F(2, 54) = 1.777, p < .178]. A post-hoc Bonferroni comparison performed on the Configuration factor revealed that RTs were significantly faster for gestalt-like patterns than for either single or quadruple random dot shapes [t(55) ≥ 6.31 p ≤ .0001], which in turn did not differ from each other significantly [t(55) = 1.92, p = .149].
Two additional ANOVAs were computed separately for the unilateral and bilateral presentation conditions, each with the factor Configuration and the usual three levels (Single, Gestalt-like, Random). The aim of this additional analysis was to determine whether the presentation of gestalt-like configurations in the blind hemifield was pivotal for the effect to occur. This is because in unilateral trials the stimuli were projected in the patients' intact hemifield and any effect potentially observed thus reflects sensitivity for consciously seen stimuli. Conversely, in bilateral displays a stimulus was added in the blind hemifield, and any significant difference arising among conditions in this case can only be accounted for by the nature of the unseen stimulus. On unilateral trials there was a significant difference between display conditions [F(2, 54) = 12.47, p < .0001]. Post-hoc tests showed that RTs for gestalt-like configurations in the intact hemifield were significantly faster than for single or quadruple random patterns [t(27) ≥ 3.22 p ≤ .002], while the latter two configurations did not differ from each other (p = .864). The ANOVA performed on bilateral trials was also highly significant [F(2, 54) = 22.93, p < .0001]. Post-hoc comparisons revealed a significant speeding up of RTs with bilateral gestalt-like patterns with respect to single or bilateral random configurations [t(27) ≥ 6.08 p < .0001], while there was no significant difference between single and random patterns (p = .334).
Patient SE displayed a significant main effect of Presentation Condition [F(1, 25) = 12.41, p = .002] indicative of an RTE. The Configuration factor was also significant [F(2, 50) = 41.26, p < .0001], as well as the Presentation Condition × Configuration interaction [F(2, 50) = 40.48, p < .0001]. Post-hoc comparisons on the interaction showed that the RTs for gestalt-like configurations were significantly faster than for either single or random patterns, but only in bilateral trials [t(25) ≥ 7.73 p ≤ .0001]. This significant interaction made it unnecessary to compute the additional ANOVAs separately for unilateral and bilateral trials. Indeed, the interaction already indicates unambiguously that, unlike DR who was sensitive to gestalt-like patterns in both her intact hemifield (during unilateral presentation) and blind hemifield (during bilateral presentation), patient SE was differentially responsive to gestalt-like configurations only when the stimuli were projected bilaterally, and hence to his blind hemifield as well.
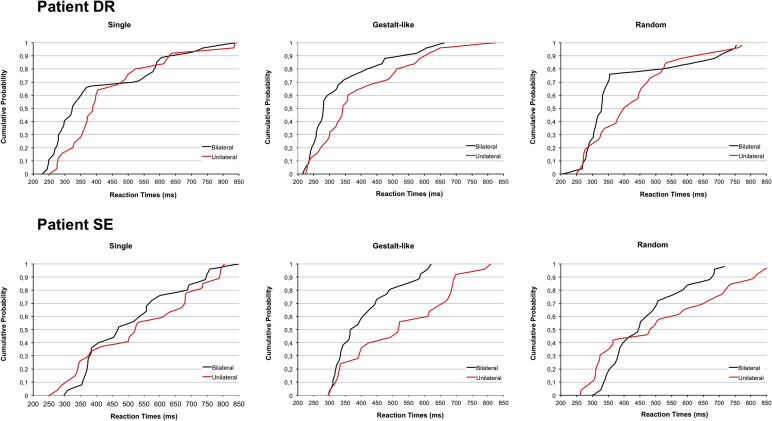
Additionally, we plotted the cumulative distribution functions (CDFs) of RTs for all six stimulation conditions and for both patients separately. This detailed graphical description enabled us to check whether the bilateral gain observed on mean values occurred throughout the whole RTs distribution. Furthermore, we carried out a Kolmogorov–Smirnov test of the CDFs, which represents a nonparametric version suitable for carrying out a single-subject analysis of Miller's inequality test (Miller, 1982), a mathematical tool to assess whether the RTE is more likely to be related to probability or neural summation. This further analysis is important, because only the latter type of bilateral gain postulates the existence of a neural centre where the visual input from the two hemifields is summed. In fact, observation of RTE on mean values is not per se conclusive of neural summation. Separate-activation or race models account for a bilateral gain by simply relying on the fact that the probability of a fast detection increases with the number of stimuli (Raab, 1962, Townsend and Ashby, 1983). Since speed of processing is a random variable, multiple stimuli are on average more likely to yield a faster RT than single stimuli for purely probabilistic reasons. In contrast, co-activation models assume the presence of a functional interaction or neural summation between perceptual channels that result in a reduction of RTs larger than that predicted by probability summation alone (Colonius, 1986, Colonius and Diederich, 2006, Miller, 1982). Note that violation of the inequality test unambiguously supports neural summation, whereas no conclusion can be reached if the inequality is not violated, owing to the conservative nature of the test (Miller, 1982).
As displayed in Fig. 5, when gestalt-like configurations were presented, RTs for the bilateral condition were faster than for the unilateral condition throughout the entire distribution and in both patients DR and SE (p < .001 by Kolmogorov–Smirnov test), thereby providing convincing evidence for an interpretation of the RTE in terms of neural summation. Conversely, the CDFs for unilateral and bilateral presentations when a single or random dot configurations were displayed overlapped substantially and crossed, thus failing to support an interpretation of the RTE in terms of neural summation (p ≥ .1 by Kolmogorov–Smirnov test). This latter finding confirms a previous study showing that it is not always possible to attribute the RTE for single dots to neural summation (Turatto, Mazza, Savazzi, & Marzi, 2004), but that its nature depends on stimulus and task factors. Nevertheless, the present results using CDFs indicate that, under identical conditions, neural summation for gestalt-like configurations was significantly more likely to occur than that for single or random dot configurations in both patients.
Fig. 5.
Cumulative distribution functions (CDF) of RTs for unilateral and bilateral conditions as a function of single, quadruple gestalt-like and random configurations showing a significant bilateral gain throughout the entire distribution only for gestalt-like patterns.
4. Discussion
In the present study, we investigated the sensitivity of two blindsight patients with hemispherectomy to stimulus perceptual organization when the display is presented to the blind hemifield. We found that, in addition to the overall RTE often reported in previous studies in hemianopic patients, there was a RTE specific for gestalt-like stimulus configurations but not for spatially random patterns. These findings confirm and extend previous observations in patients with blindsight following lesions restricted to portions of the visual cortex (Celeghin, Savazzi, et al., 2015), and also rule out extant alternative interpretations not related to stimulus configuration. A difference in stimulus familiarity or variability between gestalt-like and random configurations cannot account for the present results, since there were four different patterns counterbalanced for each of the two display types. Moreover, gestalt-like and random patterns were randomly intermingled and presented within the same block of trials, whereas in the previous study by Celeghin, Savazzi et al. (2015), trials with these different configurations were administered in separate blocks always starting with gestalt-like configurations. Hence, this new procedure also rules out the possibility that the original findings were partly due to fatigue or habituation determining the lack of effect for random patterns. While both patients exhibited similar overall results, they differed in that DR showed a speeding up of RTs also when gestalt-like patterns were presented unilaterally in her intact hemifield, whereas patient SE did not. This is possibly related to individual differences in sensitivity to consciously perceived gestalt-like configurations also present in the healthy population (Wagemans et al., 2012).
Our present study provides the first causal evidence of the sensitivity of the SC for overall stimulus configuration, as witnessed by the facilitation exerted when a structured perceptual organization is translated into motor output. Since the SC in our patients is the only intact visual structure remaining in the ipsilateral side of the otherwise damaged hemisphere, the contribution of other subcortical structures such as the LGN and the Pulv can be ruled out. This does not necessarily imply that the ipsilesional SC is solely responsible for the observed effect (i.e., does not support sufficiency of the SC for the effect to occur). In fact, visual information might well have been transferred from the (right) SC, ipsilateral to the damaged hemisphere, to the corresponding contralateral SC in the (left) intact side via the inter-collicular commissure or through other inter-hemispheric tracts, and from there to other subcortical structures such as the Pulv as well as extrastriate visual areas in the (left) intact hemisphere. Prior Positron Emission Tomography (PET) (Ptito, Johannsen, Faubert, & Gjedde, 1999) and fMRI (Bittar, Ptito, Faubert, Dumoulin, & Ptito, 1999) studies on the two patients tested here demonstrated activation in extrastriate visual areas of the (left) intact hemisphere following stimulation of the ipsilateral (left) hemifield, thereby documenting substantial neuronal plasticity and reorganization. Importantly, however, this activation does not seem to originate from cortical reorganization, e.g. owing to the expansion of the visual receptive fields in cortical areas of the intact hemisphere, but rather from the development of aberrant fibre tracts that connect the SC in the (right) damaged hemisphere to cortical areas in the opposite intact hemisphere (Leh, Johansen-Berg, et al., 2006). Hence, the critical point here is that the visual information concerning stimulus configuration must have been initially processed by the right SC ipsilateral to the damaged hemisphere, thus indicating its necessity in processing stimulus configuration and in visuo-motor integration, as other alternatives are not possible. In keeping with this notion, the crucial involvement of the SC in the RTE has been convincingly demonstrated behaviourally (Leh et al., 2006b, Tomaiuolo et al., 1997), and with combined behavioural and brain imaging studies in hemispherectomized patients (Leh et al., 2010) as well as in an hemianopic patient with lesion confined to V1 (patient GY) (Tamietto et al., 2010). However, it should be stressed that all prior investigations used simple stimuli, whereas the present study used different perceptual configurations matched for stimulus intensity and position.
According to a traditional view, it may appear surprising that the SC is able to represent complex stimulus configurations and respond differentially to a gestalt-like perceptual organization. The SC is indeed a phylogenetically ancient visual structure considered to have coarse retinotopy, which receives visual information only from Magnocellular (M) and Koniocellular (K), but not from Parvocellular (P) ganglion cells, and has a relative differential sensitivity to low spatial frequencies (Merigan and Maunsell, 1993, Stone, 1984). However, recent neurophysiological evidence as well as previously somewhat overlooked findings from single-cell recordings in monkeys and rats clearly indicate otherwise. For example, several types of neurons in the superficial (i.e., retino-recipient) layers of macaque monkeys' SC respond very poorly to simple visual stimuli and their activation requires real objects or certain two-dimensional patterns (Rizzolatti, Buchtel, Camarda, & Scandolara, 1980). Likewise, neurons in the monkey SC can separately encode faces or face-like patterns (Nguyen et al., 2014). Furthermore, neurons in the most superficial lamina of the rat's SC perform sophisticated analysis of visual information and exhibit complex properties previously thought to be characteristic of visual cortical neurons only (Girman & Lund, 2007). Therefore, the SC seems to participate in early stages of figure-ground segmentation and the combined interaction of M and K channels can enable encoding of complex and high-level properties of the visual input. Moreover, early evidence of visuo-spatial localization and discrimination surviving hemidecortication has been provided by the seminal neuropsychological work of Perenin and Jeannerod (Perenin, 1978, Perenin and Jeannerod, 1978). These studies clearly underline the possibility that some degree of perceptual functions can be carried out by subcortical centers in the absence of the visual cortical mantle. Lastly, one interesting issue concerns possible inter-hemispheric specialization for global versus local processing, which can contribute to the encoding of gestalt-like configurations. Deficits in global processing following right hemisphere damage (Hugdahl & Davidson, 2004), or due to unbalancing of the complementary functions of the two hemispheres (Negro et al., 2015), have been repeatedly reported. In principle, the preserved sensitivity for gestalt-like configuration observed here despite right hemispherectomy could arise from the SC mirroring lateralized functions thus far reported primarily at the cortical level, or from lack of hemispheric specialization at the level of the SC. In the present study, both patients had undergone right hemispherectomy and it was thus not possible to compare the effects of right versus left hemispherectomy; an issue that awaits further investigation.
In conclusion, the present findings offer a clear demonstration that hemianopic patients as a result of hemispherectomy can be selectively sensitive to complex stimulus configuration within the context of the RTE task. The SC can act as an interface between structured perceptual organization and motor processing, thereby providing an essential contribution to visually guided behavior despite being functionally and anatomically segregated from the geniculo-striate or extrastriate pathway, and therefore entirely outside conscious visual experience. An important avenue for future research is to try to examine other higher-order visual functions that can be carried out in the absence of striate and extrastriate cortical areas and whether such sensitivity can be proficiently exploited to foster rehabilitation of cortical blindness (Chokron et al., 2008, Perez and Chokron, 2014).
Conflict of interest
The authors declare no competing financial interests.
Acknowledgements
L.G. and A.P. are supported by the Natural Science and Engineering Research Council of Canada (NSERC: 37354-2011). M.T. and A.C are supported by a “Vidi” grant from the Netherlands Organization for Scientific Research (NWO) (grant 452-11-015), and by a FIRB – Futuro in Ricerca 2012 – grant from the Italian Ministry of Education University and Research (MIUR) (grant RBFR12F0BD). C.A.M is supported by a European Research Council (ERC) (grant 339939) Advanced Grant “Perceptual Awareness” under the European Union's Seventh Framework Programme.
Thanks to Matteo Diano for his help in the preparation of Fig. 1.
Reviewed 31 January 2016
Action editor Gus Buchtel
Contributor Information
Alessia Celeghin, Email: A.Celeghin@uvt.nl, alessia.celeghin@unito.it.
Marco Tamietto, Email: marco.tamietto@unito.it, M.Tamietto@uvt.nl, marco.tamietto@psy.ox.ac.uk.
References
- Ajina S., Pestilli F., Rokem A., Kennard C., Bridge H. Human blindsight is mediated by an intact geniculo-extrastriate pathway. Elife. 2015;4 doi: 10.7554/eLife.08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi P., Cowey A. Is blindsight like normal, near-threshold vision? Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):14190–14194. doi: 10.1073/pnas.94.25.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbur J.L., Watson J.D., Frackowiak R.S., Zeki S. Conscious visual perception without V1. Brain. 1993;116:1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- Bertini C., Cecere R., Ladavas E. I am blind, but I “see” fear. Cortex. 2013;49(4):985–993. doi: 10.1016/j.cortex.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Bittar R.G., Ptito M., Faubert J., Dumoulin S.O., Ptito A. Activation of the remaining hemisphere following stimulation of the blind hemifield in hemispherectomized subjects. NeuroImage. 1999;10(3 Pt 1):339–346. doi: 10.1006/nimg.1999.0474. [DOI] [PubMed] [Google Scholar]
- Blythe I.M., Kennard C., Ruddock K.H. Residual vision in patients with retrogeniculate lesions of the visual pathways. Brain. 1987;110(Pt 4):887–905. doi: 10.1093/brain/110.4.887. [DOI] [PubMed] [Google Scholar]
- Bridge H., Thomas O., Jbabdi S., Cowey A. Changes in connectivity after visual cortical brain damage underlie altered visual function. Brain. 2008;131(Pt 6):1433–1444. doi: 10.1093/brain/awn063. [DOI] [PubMed] [Google Scholar]
- Buetti S., Tamietto M., Hervais-Adelman A., Kerzel D., de Gelder B., Pegna A.J. Dissociation between goal-directed and discrete response localization in a patient with bilateral cortical blindness. Journal of Cognitive Neuroscience. 2013;25(10):1769–1775. doi: 10.1162/jocn_a_00404. [DOI] [PubMed] [Google Scholar]
- Burra N., Hervais-Adelman A., Kerzel D., Tamietto M., de Gelder B., Pegna A.J. Amygdala activation for eye contact despite complete cortical blindness. Journal of Neuroscience. 2013;33(25):10483–10489. doi: 10.1523/JNEUROSCI.3994-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeghin A., Barabas M., Mancini F., Bendini M., Pedrotti E., Prior M. Speeded manual responses to unseen visual stimuli in hemianopic patients: what kind of blindsight? Consciousness and Cognition. 2015;32:6–14. doi: 10.1016/j.concog.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeghin A., de Gelder B., Tamietto M. From affective blindsight to emotional consciousness. Consciousness and Cognition. 2015;36:414–425. doi: 10.1016/j.concog.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Celeghin A., Savazzi S., Barabas M., Bendini M., Marzi C.A. Blindsight is sensitive to stimulus numerosity and configuration: evidence from the redundant signal effect. Experimental Brain Research. 2015;233(5):1617–1623. doi: 10.1007/s00221-015-4236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokron S., Perez C., Obadia M., Gaudry I., Laloum L., Gout O. From blindsight to sight: cognitive rehabilitation of visual field defects. Restorative Neurology and Neuroscience. 2008;26(4–5):305–320. [PubMed] [Google Scholar]
- Colonius H. Measuring channel dependence in separate activation models. Perception & Psychophysics. 1986;40(4):251–255. doi: 10.3758/bf03211504. [DOI] [PubMed] [Google Scholar]
- Colonius H., Diederich A. The race model inequality: interpreting a geometric measure of the amount of violation. Psychological Review. 2006;113(1):148–154. doi: 10.1037/0033-295X.113.1.148. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Marzi C.A., Tassinari G., Aglioti S. Effectiveness of different task paradigms in revealing blindsight. Brain. 1990;113(Pt 3):603–616. doi: 10.1093/brain/113.3.603. [DOI] [PubMed] [Google Scholar]
- Danckert J., Rossetti Y. Blindsight in action: what can the different sub-types of blindsight tell us about the control of visually guided actions? Neuroscience and Biobehavioral Reviews. 2005;29(7):1035–1046. doi: 10.1016/j.neubiorev.2005.02.001. [DOI] [PubMed] [Google Scholar]
- de Gelder B., Hortensius R., Tamietto M. Attention and awareness each influence amygdala activity for dynamic bodily expressions-a short review. Frontiers in Integrative Neuroscience. 2012;6:54. doi: 10.3389/fnint.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B., Vroomen J., Pourtois G., Weiskrantz L. Non-conscious recognition of affect in the absence of striate cortex. NeuroReport. 1999;10(18):3759–3763. doi: 10.1097/00001756-199912160-00007. [DOI] [PubMed] [Google Scholar]
- Girman S.V., Lund R.D. Most superficial Sublamina of rat superior colliculus: neuronal response properties and correlates with perceptual figure – ground segregation. Journal of Neurophysiology. 2007;98:161–177. doi: 10.1152/jn.00059.2007. [DOI] [PubMed] [Google Scholar]
- Hervais-Adelman A., Legrand L.B., Zhan M., Tamietto M., de Gelder B., Pegna A.J. Looming sensitive cortical regions without V1 input: evidence from a patient with bilateral cortical blindness. Frontiers in Integrative Neuroscience. 2015;9:51. doi: 10.3389/fnint.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., LeVay S., Wiesel T.N. Mode of termination of retinotectal fibers in macaque monkey: an autoradiographic study. Brain Research. 1975;96(1):25–40. doi: 10.1016/0006-8993(75)90567-3. [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Davidson R.J., editors. The Asymmetrical Brain. The MIT Press; Cambridge, MA: 2004. [Google Scholar]
- Kentridge R.W., Heywood C.A., Weiskrantz L. Spatial attention speeds discrimination without awareness in blindsight. Neuropsychologia. 2004;42(6):831–835. doi: 10.1016/j.neuropsychologia.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Kentridge R.W., Heywood C.A., Weiskrantz L. Color contrast processing in human striate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15129–15131. doi: 10.1073/pnas.0706603104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentridge R.W., Nijboer T.C., Heywood C.A. Attended but unseen: visual attention is not sufficient for visual awareness. Neuropsychologia. 2008;46(3):864–869. doi: 10.1016/j.neuropsychologia.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Leh S.E., Chakravarty M.M., Ptito A. The connectivity of the human pulvinar: a diffusion tensor imaging tractography study. International Journal of Biomedical Imaging. 2008;2008:789539. doi: 10.1155/2008/789539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh S.E., Johansen-Berg H., Ptito A. Unconscious vision: new insights into the neuronal correlate of blindsight using diffusion tractography. Brain. 2006;129(Pt 7):1822–1832. doi: 10.1093/brain/awl111. [DOI] [PubMed] [Google Scholar]
- Leh S.E., Mullen K.T., Ptito A. Absence of S-cone input in human blindsight following hemispherectomy. The European Journal of Neuroscience. 2006;24(10):2954–2960. doi: 10.1111/j.1460-9568.2006.05178.x. [DOI] [PubMed] [Google Scholar]
- Leh S.E., Ptito A., Schonwiesner M., Chakravarty M.M., Mullen K.T. Blindsight mediated by an S-cone-independent collicular pathway: an fMRI study in hemispherectomized subjects. Journal of Cognitive Neuroscience. 2010;22(4):670–682. doi: 10.1162/jocn.2009.21217. [DOI] [PubMed] [Google Scholar]
- Lyon D.C., Nassi J.J., Callaway E.M. A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron. 2010;65(2):270–279. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi C.A. Transfer of visual information after unilateral input to the brain. Brain and Cognition. 1986;5(2):163–173. doi: 10.1016/0278-2626(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Marzi C.A., Mancini F., Metitieri T., Savazzi S. Blindsight following visual cortex deafferentation disappears with purple and red stimuli: a case study. Neuropsychologia. 2009;47(5):1382–1385. doi: 10.1016/j.neuropsychologia.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Marzi C.A., Tassinari G., Aglioti S., Lutzemberger L. Spatial summation across the vertical meridian in hemianopics: a test of blindsight. Neuropsychologia. 1986;24(6):749–758. doi: 10.1016/0028-3932(86)90074-6. [DOI] [PubMed] [Google Scholar]
- Merigan W.H., Maunsell J.H. How parallel are the primate visual pathways? Annual Review of Neuroscience. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Miller J. Divided attention: evidence for coactivation with redundant signals. Cognitive Psychology. 1982;14(2):247–279. doi: 10.1016/0010-0285(82)90010-x. [DOI] [PubMed] [Google Scholar]
- Morland A.B., Jones S.R., Finlay A.L., Deyzac E., Le S., Kemp S. Visual perception of motion, luminance and colour in a human hemianope. Brain. 1999;122(Pt 6):1183–1198. doi: 10.1093/brain/122.6.1183. [DOI] [PubMed] [Google Scholar]
- Negro E., D'Agata F., Caroppo P., Coriasco M., Ferrio F., Celeghin A. Neurofunctional signature of hyperfamiliarity for unknown faces. Plos One. 2015;10(7):e0129970. doi: 10.1371/journal.pone.0129970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M.N., Matsumoto J., Hori E., Maior R.S., Tomaz C., Tran A.H. Neuronal responses to face-like and facial stimuli in the monkey superior colliculus. Frontiers in Behavioral Neuroscience. 2014;8:85. doi: 10.3389/fnbeh.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perenin M.T. Visual function within the hemianopic field following early cerebral hemidecortication in man–II. Pattern discrimination. Neuropsychologia. 1978;16(6):697–708. doi: 10.1016/0028-3932(78)90004-0. [DOI] [PubMed] [Google Scholar]
- Perenin M.T., Jeannerod M. Visual function within the hemianopic field following early cerebral hemidecortication in man–I. Spatial localization. Neuropsychologia. 1978;16(1):1–13. doi: 10.1016/0028-3932(78)90037-4. [DOI] [PubMed] [Google Scholar]
- Perez C., Chokron S. Rehabilitation of homonymous hemianopia: insight into blindsight. Frontiers in Integrative Neuroscience. 2014;8:1–12. doi: 10.3389/fnint.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzamiglio L., Antonucci G., Francia A. Response of the cortically blind hemifields to a moving visual scene. Cortex. 1984;20(1):89–99. doi: 10.1016/s0010-9452(84)80026-x. [DOI] [PubMed] [Google Scholar]
- Pöppel E., Held R., Frost D. Residual visual function after brain wounds involving the central visual pathways in man. Nature. 1973;243:295–296. doi: 10.1038/243295a0. [DOI] [PubMed] [Google Scholar]
- Ptito M., Johannsen P., Faubert J., Gjedde A. Activation of human extrageniculostriate pathways after damage to area V1. NeuroImage. 1999;9(1):97–107. doi: 10.1006/nimg.1998.0390. [DOI] [PubMed] [Google Scholar]
- Raab D.H. Statistical facilitation of simple reaction times. Transactions of the New York Academy of Sciences. 1962;24:574–590. doi: 10.1111/j.2164-0947.1962.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Rafal R., Calabresi P.A., Brennan C.W., Sciolto T.K. Saccade preparation inhibits reorienting to recently attended locations. Journal of Experimental Psychology. Human Perception and Performance. 1989;15(4):673–685. doi: 10.1037//0096-1523.15.4.673. [DOI] [PubMed] [Google Scholar]
- Rafal R., Henik A., Smith J. Extrageniculate contributions to reflex visual orienting in normal humans: a temporal hemifield advantage. Journal of Cognitive Neuroscience. 1991;3(4):322–328. doi: 10.1162/jocn.1991.3.4.322. [DOI] [PubMed] [Google Scholar]
- Rafal R., Smith J., Krantz J., Cohen A., Brennan C. Extrageniculate vision in hemianopic humans: saccade inhibition by signals in the blind field. Science. 1990;250(4977):118–121. doi: 10.1126/science.2218503. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Buchtel H.A., Camarda R., Scandolara C. Neurons with complex visual properties in the superior colliculus of the macaque monkey. Experimental Brain Research. 1980;38(1):37–42. doi: 10.1007/BF00237928. [DOI] [PubMed] [Google Scholar]
- Sahraie A., Weiskrantz L., Barbur J.L., Simmons A., Williams S.C., Brammer M.J. Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(17):9406–9411. doi: 10.1073/pnas.94.17.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M.C., Mrowka S.W., Turchi J., Saunders R.C., Wilke M., Peters A.J. Blindsight depends on the lateral geniculate nucleus. Nature. 2010;466(7304):373–377. doi: 10.1038/nature09179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich L.C., Park K.F., Wohlgemuth M.J., Horton J.C. Bypassing V1: a direct geniculate input to area MT. Nature Neuroscience. 2004;7(10):1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Singer W., Zihl J., Poppel E. Subcortical control of visual thresholds in humans: evidence for modality specific and retinotopically organized mechanisms of selective attention. Experimental Brain Research. 1977;29(2):173–190. doi: 10.1007/BF00237040. [DOI] [PubMed] [Google Scholar]
- Spering M., Carrasco M. Acting without seeing: eye movements reveal visual processing without awareness. Trends in Neurosciences. 2015;38:247–258. doi: 10.1016/j.tins.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerig P. Chromaticity and achromaticity. Evidence for a functional differentiation in visual field defects. Brain. 1987;110(Pt 4):869–886. doi: 10.1093/brain/110.4.869. [DOI] [PubMed] [Google Scholar]
- Stoerig P., Cowey A. Wavelength sensitivity in blindsight. Nature. 1989;342(6252):916–918. doi: 10.1038/342916a0. [DOI] [PubMed] [Google Scholar]
- Stoerig P., Hubner M., Poppel E. Signal detection analysis of residual vision in a field defect due to a post-geniculate lesion. Neuropsychologia. 1985;23(5):589–599. doi: 10.1016/0028-3932(85)90061-2. [DOI] [PubMed] [Google Scholar]
- Stone J. Parallel processing in the visual system. The Quarterly Review of Biology. 1984;59:502. [Google Scholar]
- Sylvester R., Josephs O., Driver J., Rees G. Visual FMRI responses in human superior colliculus show a temporal-nasal asymmetry that is absent in lateral geniculate and visual cortex. Journal of Neurophysiology. 2007;97(2):1495–1502. doi: 10.1152/jn.00835.2006. [DOI] [PubMed] [Google Scholar]
- Tamietto M., Castelli L., Vighetti S., Perozzo P., Geminiani G., Weiskrantz L. Unseen facial and bodily expressions trigger fast emotional reactions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(42):17661–17666. doi: 10.1073/pnas.0908994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M., Cauda F., Corazzini L.L., Savazzi S., Marzi C.A., Goebel R. Collicular vision guides nonconscious behavior. Journal of Cognitive Neuroscience. 2010;22(5):888–902. doi: 10.1162/jocn.2009.21225. [DOI] [PubMed] [Google Scholar]
- Tamietto M., Latini Corazzini L., Pia L., Zettin M., Gionco M., Geminiani G. Effects of emotional face cueing on line bisection in neglect: a single case study. Neurocase. 2005;11(6):399–404. doi: 10.1080/13554790500259717. [DOI] [PubMed] [Google Scholar]
- Tamietto M., Morrone M.C. Visual plasticity: blindsight bridges anatomy and function in the visual system. Current Biology: CB. 2016;26(2):R70–R73. doi: 10.1016/j.cub.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M., Pullens P., de Gelder B., Weiskrantz L., Goebel R. Subcortical connections to human amygdala and changes following destruction of the visual cortex. Current Biology: CB. 2012;22(15):1449–1455. doi: 10.1016/j.cub.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Tinelli F., Cicchini G.M., Arrighi R., Tosetti M., Cioni G., Morrone M.C. Blindsight in children with congenital and acquired cerebral lesions. Cortex. 2013;49(6):1636–1647. doi: 10.1016/j.cortex.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F., Ptito M., Marzi C.A., Paus T., Ptito A. Blindsight in hemispherectomized patients as revealed by spatial summation across the vertical meridian. Brain. 1997;120:795–803. doi: 10.1093/brain/120.5.795. [DOI] [PubMed] [Google Scholar]
- Torjussen T. Residual function in cortically blind hemifields. Scandinavian Journal of Psychology. 1976;17(4):320–323. doi: 10.1111/j.1467-9450.1976.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Torjussen T. Visual processing in cortically blind hemifields. Neuropsychologia. 1978;16(1):15–21. doi: 10.1016/0028-3932(78)90038-6. [DOI] [PubMed] [Google Scholar]
- Townsend J.T., Ashby F.G. Cambridge University Press; Cambridge, MA: 1983. Stochastic modelling of elementary psychological processes. [Google Scholar]
- Trevethan C.T., Sahraie A., Weiskrantz L. Form discrimination in a case of blindsight. Neuropsychologia. 2007;45(9):2092–2103. doi: 10.1016/j.neuropsychologia.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Turatto M., Mazza V., Savazzi S., Marzi C.A. The role of the magnocellular and parvocellular systems in the redundant target effect. Experimental Brain Research. 2004;158(2):141–150. doi: 10.1007/s00221-004-1884-3. [DOI] [PubMed] [Google Scholar]
- Van den Stock J., Tamietto M., Hervais-Adelman A., Pegna A.J., de Gelder B. Body recognition in a patient with bilateral primary visual cortex lesions. Biological Psychiatry. 2015;77(7):e31–33. doi: 10.1016/j.biopsych.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Van den Stock J., Tamietto M., Zhan M., Heinecke A., Hervais-Adelman A., Legrand L.B. Neural correlates of body and face perception following bilateral destruction of the primary visual cortices. Frontiers in Behavioral Neuroscience. 2014;8:30. doi: 10.3389/fnbeh.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagemans J., Elder J.H., Kubovy M., Palmer S.E., Peterson M.A., Singh M. A century of Gestalt psychology in visual perception: I. Perceptual grouping and figure-ground organization. Psychological Bulletin. 2012;138(6):1172–1217. doi: 10.1037/a0029333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L. The Ferrier lecture, 1989. Outlooks for blindsight: explicit methodologies for implicit processes. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1990;239(1296):247–278. doi: 10.1098/rspb.1990.0016. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L., Barbur J.L., Sahraie A. Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (V1) Proceedings of the National Academy of Sciences of the United States of America. 1995;92(13):6122–6126. doi: 10.1073/pnas.92.13.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L., Warrington E.K., Sanders M.D., Marshall J. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain. 1974;97(4):709–728. doi: 10.1093/brain/97.1.709. [DOI] [PubMed] [Google Scholar]
- Wilson M.E., Toyne M.J. Retino-tectal and cortico-tectal projections in Macaca mulatta. Brain Research. 1970;24(3):395–406. doi: 10.1016/0006-8993(70)90181-2. [DOI] [PubMed] [Google Scholar]
- Zihl J., von Cramon D. Registration of light stimuli in the cortically blind hemifield and its effect on localization. Behavioural Brain Research. 1980;1(4):287–298. doi: 10.1016/0166-4328(80)90022-4. [DOI] [PubMed] [Google Scholar]