Abstract
The multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) is known to participate in maintenance and switches of smooth muscle cell (SMC) phenotypes. However, which isoform of CaMKII is involved in differentiation of adult mesenchymal stem cells into contractile SMCs remains unclear. In the present study, we detected γ isoform of CaMKII in differentiation of human adipose derived stem cells (hASCs) into SMCs that resulted from treatment with TGF-β1 and BMP4 in combination for 7 days. The results showed that CaMKIIγ increased gradually during differentiation of hASCs as determined by real-time PCR and western blot analysis. The siRNA-mediated knockdown of CaMKIIγ decreased the protein levels and transcriptional levels of smooth muscle contractile markers (a-SMA, SM22a, calponin, and SM-MHC), while CaMKIIγ overexpression increases the transcriptional and protein levels of smooth muscle contractile markers. These results suggested that γ isoform of CaMKII plays a significant role in smooth muscle differentiation of hASCs.
1. Introduction
Adipose derived stem cell (ASCs) is self-renewing multipotent cells that have significant clinical potential in cellular therapy for tissue regeneration [1]. ASCs can be induced to differentiate along multiple lineages, including osteocytes [2], neural cells [3], and muscular cells [4]. Differentiation of ASCs into blood vessel smooth muscle cells (SMCs) under stimulation of transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein-4 (BMP4) has been fulfilled in our previous study [5]. The differentiated ASCs acquired SMC phenotype as evidenced by their expression of specific structural proteins and their contractility in response to contractile agonist. Furthermore, an elastic blood vessel wall was engineered under pulsatile conditions using smooth muscle cells differentiated from ASCs and polyglycolic acid scaffold, showing that hASCs can serve as an alternative cell source for SMCs in blood vessel engineering [6]. However, the mechanism underlying differentiation of ASCs into smooth muscle lineage remains unclear.
Homeostasis of intracellular Ca2+ maintains proliferation, extracellular matrix production, and phenotypic switch of differentiated SMCs. As a critical mediator of Ca2+ signals, the multifunctional serine/threonine Ca2+/calmodulin-dependent protein kinase II (CaMKII), which consists of 4 different isoforms with distinct expression patterns [7, 8], plays a critical role in modulating physiology and pathological process of SMCs [9, 10]. Among the four homologous CaMKII isoforms (α, β, δ, and γ), CaMKII isoform δ has been generally accepted as a major regulator in promoting SMCs synthetic phenotype functions [11, 12]. Recently, CaMKIIγ isoforms were shown to associate with acquirement of contractile activity of SMCs. Antisense knockdown of CaMKIIγ inhibited extracellular signal-related kinase (ERK) activation, myosin phosphorylation, and contractile force in differentiated SMCs [13]. These results indicated that γ isoform of CaMKII regulates smooth muscle differentiation. But there are no reports about whether CaMKIIγ regulates smooth muscle differentiation of ASCs.
In the present study, we hypothesized that γ isoform of CaMKII participates in smooth muscle differentiation of ASCs. We found that, in parallel to differentiation of ASCs, expression of CaMKIIγ was significantly upregulated. Inhibition of CaMKIIγ with si-RNA decreased smooth muscle differentiation of ASCs. This result indicted that, in addition to modulating phenotype switch of mature differentiated SMCs, CaMKIIγ showed fundamental function in regulation of smooth muscle differentiation of adult mesenchymal stem cells.
2. Materials and Methods
2.1. Cell Culture and Smooth Muscle Differentiation
Human adipose derived stem cells isolated from fresh human lipoaspirates (the process was approved by the Research Ethical Committee of the First Affiliated Hospital of Xinjiang Medical University), cultured in growth medium, comprised LG-DMEM (Gibco) supplemented with 10% FBS, 100 U/mL penicillin (Sigma-Aldrich), and 100 mg/mL streptomycin (Sigma-Aldrich) as previously described [5]. Cells from passages 3 to 5 were used in the following study. Smooth muscle cell differentiation of hASCs was induced using differentiation medium containing 1% FBS, 5 ng/mL TGF-β1, and 2.5 ng/mL BMP4. Smooth muscle differentiation of hASCs was evaluated by quantitative real-time PCR (qRT-PCR) and immunofluorescent staining.
2.2. Quantitative Real-Time Reverse Transcription PCR
RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) and PrimeScript™ RT Master Mix (Takara, Japan) was used for cDNA synthesis and the reactions were performed in a T3 thermocycler (Biometra). qRT-PCR was performed by using a 7500 Fast Real-Time PCR system (Applied Biosystems, USA) according to the manufacturer's protocol. Primers used in the PCR reactions were listed in Table 1. SYBER Green Premix Ex Taq (Takara, Japan) was used in each reaction. The relative expression of mRNA was normalized to β-actin. Fold change was calculated by using the ΔΔCt method of relative quantification. All experiments were repeated in triplicate.
Table 1.
qRT-PCR primer sequences.
| Name | Sequence (5′ → 3′) | |
|---|---|---|
| a-SMA | Forward | GGTGATGGTGGGAATGGG |
| Reverse | GCAGGGTGGGATGCTCTT | |
|
| ||
| Calponin | Forward | ATGTCCTCTGCTCACTTCA |
| Reverse | TTTCCGCTCCTGCTTCTCT | |
|
| ||
| SM22a | Forward | AACAGCCTGTACCCTGATGG |
| Reverse | CGGTAGTGCCCATCATTCTT | |
|
| ||
| SM-MHC | Forward | TGCTTTCGCTCGTCTTCC |
| Reverse | CGGCAACTCGTGTCCAAC | |
|
| ||
| Smoothelin 2 | Forward | CCCCTGAGATTGCCCAAAACT |
| Reverse | CATGGGTGATAGAGCCGCAG | |
|
| ||
| ACLP | Forward | ACCCACACTGGACTACAATGA |
| Reverse | GTTGGGGATCACGTAACCATC | |
|
| ||
| CaMKIIα | Forward | ACCACTACCTGATCTTCGACC |
| Reverse | CCGCCTCACTGTAATACTCCC | |
|
| ||
| CaMKIIβ | Forward | GCACACCAGGCTACCTGTC |
| Reverse | GGACGGGAAGTCATAGGCA | |
|
| ||
| CaMKIIγ | Forward | GTCTGTCAACGATCCACGGT |
| Reverse | TCTGCCTGCCAACTGAGAAG | |
|
| ||
| CaMKIIδ | Forward | AGGGCTTTCACTACTTGGTGT |
| Reverse | AGCCAAAGTCTGCCAATTTCA | |
2.3. Western Blotting
Western blot test was used to identify expression of SMC proteins. Cells were lysed in RIPA buffer with protease and phenylmethylsulfonyl fluoride (PMSF; Roche, Indianapolis, IN, USA). The protein concentration was assayed using the BCA method (Bio-Rad). Approximately 20 to 50 μg of total protein samples was loaded on a 10% SDS-PAGE after denaturation by boiling for 10 min. The separated proteins transferred to polyvinylidene difluoride membranes. Membranes were blocked by incubation in Tris-buffered saline containing 0.05% Tween 20 and 5% skimmed milk with constant shaking for 1 h. The membrane was probed with antibodies against a-SMA (1 : 1000, Abcam, Cambridge, UK), calponin (1 : 1000, Abcam, Cambridge, UK), SM22a (1 : 1000, Abcam, Cambridge, UK), SM-MHC (1 : 1000, Abcam, Cambridge, UK), smoothelin-like 2 (1 : 2000, Santa Cruz, Dallas, USA), ACLP (1 : 1000, Abcam, Cambridge, UK), CaMKIIα (1 : 5000, Abcam, Cambridge, UK), CaMKIIβ (1 : 1000, Abcam, Cambridge, UK), CaMKIIγ (1 : 500, Abcam, Cambridge, UK), and CaMKIIδ (1 : 1000, Santa Cruz, Dallas, USA) overnight at 4°C. GAPDH was used as an internal loading control. The membranes were washed three times with TBST and incubated with the appropriate secondary antibodies at room temperature for 1 h and detected using enhanced chemiluminescence.
2.4. Overexpression and Knockdown of CaMKIIγ
For the overexpression analysis, CaMKIIγ adenovirus vectors were constructed by using the AdEasy system (Agilent Technologies, Santa Clara, CA, USA). CaMKIIγ was cloned from pRC/CMV plasmid with the forward primer 5′-GTCTGTCAACGATCCACGGT-3′ and the reverse primer 5′-TCTGCCTGCCAACTGAGAAG-3′. The empty vector served as the green fluorescent protein (GFP) control. hASCs cell passages 3–5 were incubated with adenoviruses expressing GFP and CaMKIIγ at an MOI of 250 for 48 h [14]. CaMKIIγ mRNA and protein expressions were analyzed by qRT-PCR and western blotting. For knockdown of CaMKIIγ, siRNAs were synthesized by Usen Biotech Co. (Shanghai, China). The sequence of the CaMKIIγ siRNA wa: 5′-TACGATACAAGGCTGTTAGAGAG-3′. A random sequence was used as the negative control (NC). hASCs were seeded into 6-well plates and transfected with siRNA at 100 pmol/well using Lipofectamine 2000 (Invitrogen Corp.), according to the manufacturer's instructions. The medium was replaced 6 h later and the cells were collected 48 h after transfection for total RNA isolation and protein harvesting. Transfection efficiency was evaluated by qRT-PCR and western blotting.
2.5. Immunofluorescent Staining
Cells were fixed with paraformaldehyde and incubated with the following primary antibodies: rabbit polyclonal anti-a-SMA, rabbit polyclonal anti-SM22a, and rabbit polyclonal anti-SM-MHC antibodies for 60 min at room temperature, and then they were washed with PBS for three times. Alexa Fluor 594-conjugated donkey anti-rabbit secondary antibody (R37119; Thermo Fisher, US) was used to detect the localization of anti-a-SMA, anti-SM22a, and anti-SM-MHC antibodies, respectively. Nuclear staining was done with 4′,6-diamidino-2-phenylindole (DAPI). The images were viewed by a confocal laser scanning microscope (TCS, SP8; Leica, Germany).
2.6. Statistical Analysis
The results presented are average of at least three experiments each performed in triplicate with mean ± SD. Statistical analysis was performed using Student's t-test. P < 0.05 was considered statistically significant.
3. Results
3.1. Smooth Muscle Differentiation of hASCs
As determined by qRT-PCR, expression of smooth muscle specific contractile genes including α-SMA, SM22a, calponin, and SM-MHC was detected with significant increase at 7 days in hASCs stimulated with combination of TGF-β1 and BMP4. But expressions of CArG-independent smooth muscle differentiation markers like smoothelin-like 2 and ACLP (also known as AE binding protein 1) were not different from noninduced group (Figures 1(a), 1(b), and 1(c)). To ascertain the results of qRT-PCR, expression of intracellular α-SMA, SM22a, and SM-MHC was analyzed by immunofluorescent staining. As shown in Figure 1(d), expressions of these markers were remarkably enhanced in hASCs stimulated with the combination of TGF-β1 and BMP4 for 7 days.
Figure 1.
Human ASCs were induced to differentiate into SMCs by treatment with TGF-β1 and BMP4 for 7 days. (a) Quantitative real-time PCR experiments were carried out to measure the expression level of smooth muscle specific markers. (b, c) Time course of the expression of SMC differentiation markers detected by qRT-PCR and western blot analysis, respectively. (d) Immunofluorescent staining of smooth muscle specific markers (red), respectively. Nuclear were counterstained with DAPI (blue). Scale bars: 100 μm. Data represent means ± SE, n = 3 (∗ P < 0.05 versus controls).
3.2. CaMKIIγ Was Highly Expressed during Smooth Muscle Differentiation
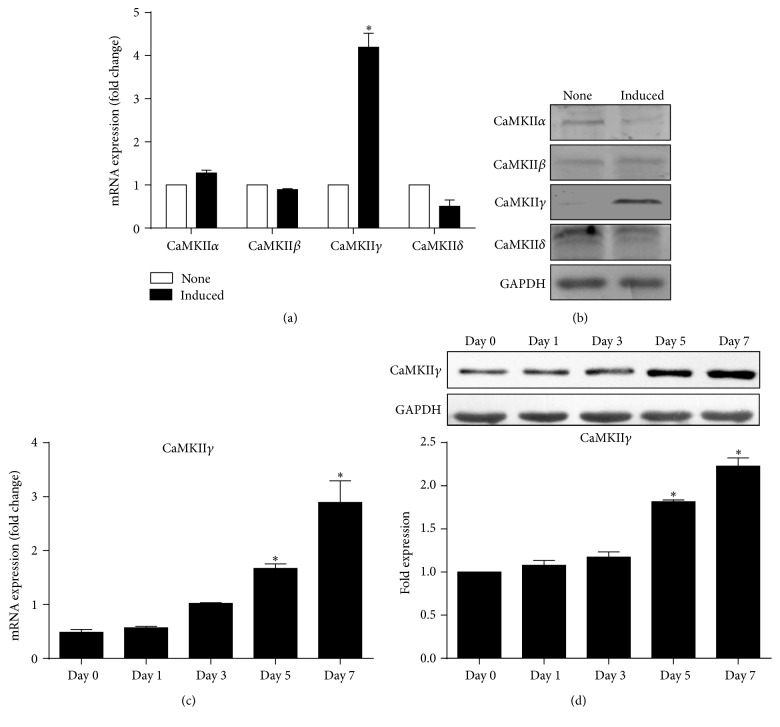
We examine expression of four isoforms of CaMKII on differentiation of hASCs into smooth muscle cells. As shown in Figures 2(a) and 2(b), among the four isoforms of the CaMKII, only the γ isoform has a significant change compared with the noninduced group. We also detected expressions of CaMKIIγ gene at 1, 3, 5, and 7 days after induction by qRT-PCR analysis. We found that expression of CaMKIIγ began to increase after 3 days and reached significant high level at 5 and 7 days, respectively (Figure 2(c)). By western blot analysis, it was found that, during treatment by TGF-β1 and BMP4 in combination, protein levels of CaMKIIγ gradually increased after 3 days in induced hASCs (Figure 2(d)).
Figure 2.
Expression of CaMKII isoforms in hASCs subjected to combined treatment with TGF-β1 and BMP4 for 7 days. mRNA (a) and protein (b) expressions of CaMKII isoforms after smooth muscle differentiation of hASCs. (c) qRT-PCR analysis of CaMKIIγ expression at 1, 3, 5, and 7 days, respectively. (d) Expression of CaMKIIγ detected by western blot at 1, 3, 5, and 7 days of stimulation. ∗ P < 0.05 when (a) versus none, (b) versus day 0, and (d) versus day 0.
3.3. CaMKIIγ Has Positive Effect on Smooth Muscle Differentiation of hASCs
To further understand the role of CaMKIIγ in the control of contractile gene expression, we constructed overexpression vector of CaMKIIγ and transfected hASCs. Figures 3(a) and 3(b) show that CaMKIIγ overexpressed in the transfected cell and ad-CaMKIIγ is specific to γ isoform of CaMKII only. For knockdown of CaMKIIγ, siRNA against CaMKIIγ or si-control transfected into hASCs for 48 hours. The qRT-PCR results showed that mRNA levels of CaMKIIγ, but not other isoforms of CaMKII, were downregulated by si-CaMKIIγ more than 70% compared to si-control (Figure 3(c)). In addition, CaMKIIγ protein level decreased by 90% with si-CaMKIIγ transfection as determined by western blot analysis (Figure 3(d)). These results indicated that transfection of siRNA effectively suppressed expression of CaMKIIγ and si-CaMKIIγ is specific to γ isoform of CaMKII only.
Figure 3.
Cell transfection efficiency. CaMKII mRNA (a) and protein (b) expression levels in hASCs transfected with CaMKIIγ and control. (c) qRT-PCR analysis of CaMKIIα, CaMKIIβ, CaMKIIγ, and CaMKIIδ in hASCs transfected with siRNA targeting CaMKIIγ (si-CaMKIIγ) and nontargeting siRNA (si-control) for 48 hours, respectively. (d) Detection of CaMKII isoforms by western blot in hASCs transfected with siRNA targeting CaMKIIγ (si-CaMKIIγ) and nontargeting siRNA (si-control). ∗ P < 0.05 when (a) versus ad-GFP and (c) si-control.
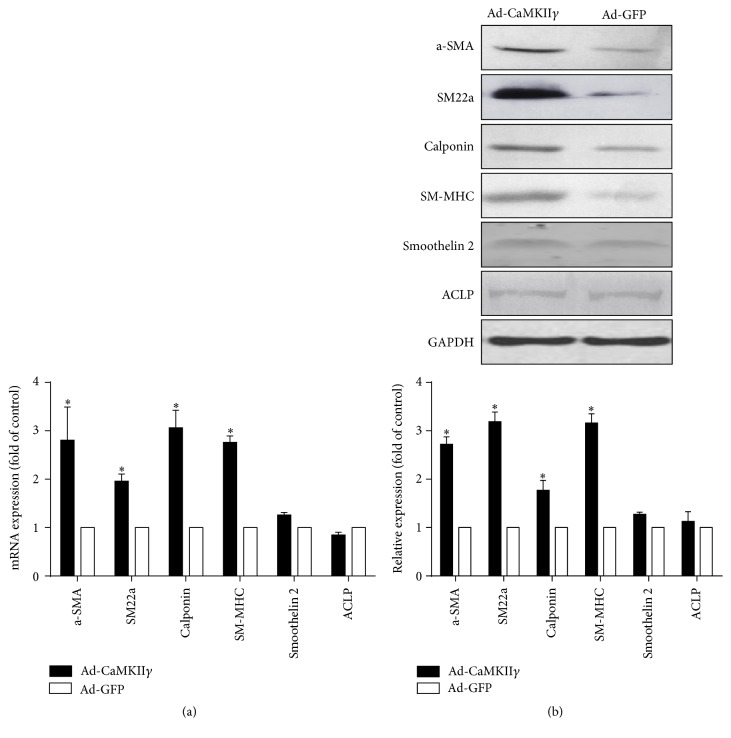
We also detected expression levels of smooth muscle contractile markers after transfection of CaMKIIγ. mRNA expression levels of CArG-dependent smooth muscle differentiation markers was upregulated in the CaMKIIγ group compared to control group analyzed by qRT-PCR (Figure 4(a)). Western blot analysis also confirms these kinds of expressions (Figure 4(b)). But CArG-independent smooth muscle differentiation markers (smoothelin-like 2 and ACLP) were not changed by CaMKIIγ.
Figure 4.
Transfection of CaMKIIγ upregulated expression of smooth muscle contractile markers. (a) qRT-PCR analysis of smooth muscle specific markers. (b) Western blot analysis of smooth muscle contractile proteins in CaMKIIγ and control group, respectively. Data represent means ± SE, n = 3 (∗ P < 0.05 versus si-control).
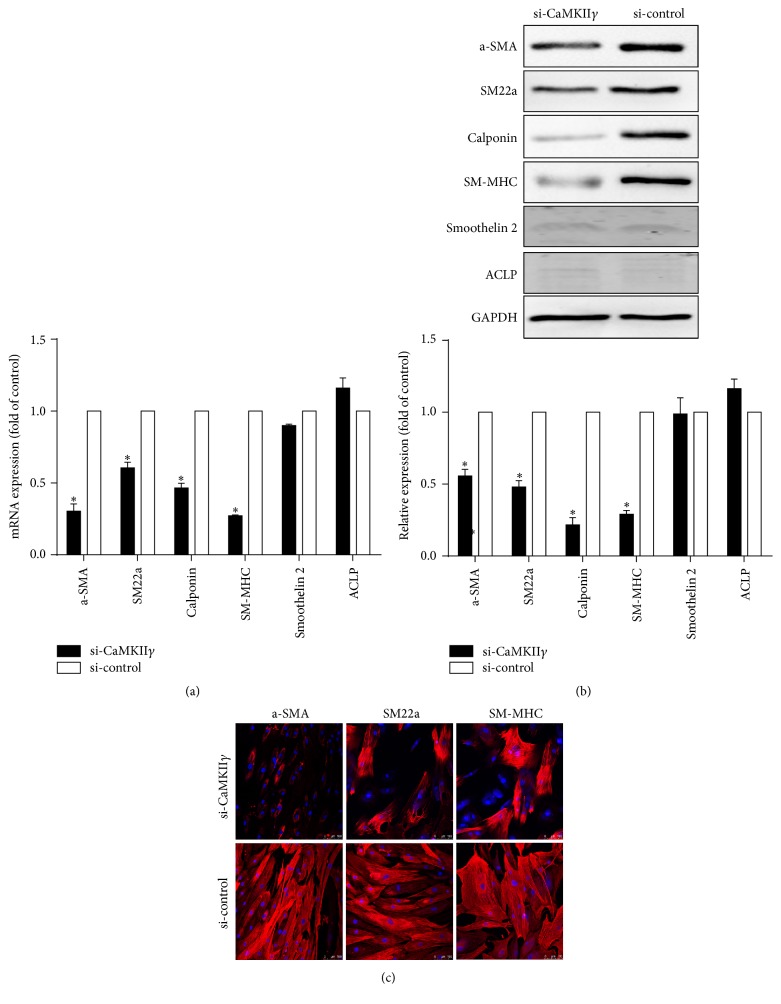
After being transfected with si-CaMKIIγ, hASCs were induced with exposure to TGF-β1 and BMP4 in combination for seven days to differentiate into smooth muscle cells. Compared with si-control group, mRNA expressions of a-SMA, SM22a, calponin, and SM-MHC, but not smoothelin-like 2 and ACLP, in induced hASCs were significantly reduced by transfection of si-CaMKIIγ, among which decrease of a-SMA and SM-MHC was more evident (Figure 5(a)). As determined by western blot analysis, it was shown that CArG-dependent smooth muscle contractile proteins were downregulated by si-CaMKIIγ (Figure 5(b)). Furthermore, immunofluorescent staining exhibited reduced distribution of α-SMA, SM22a, and SM-MHC in si-CaMKIIγ transfected hASCs (Figure 5(c)).
Figure 5.
Expression of smooth muscle contractile markers was downregulated in induced hASCs transfected with siRNA against CaMKIIγ. (a) Expression of smooth muscle contractile markers in si-CaMKIIγ and si-control group determined by qRT-PCR. Data represent means ± SE, n = 3 (∗ P < 0.05 versus si-control). (b) Western blot analysis of smooth muscle contractile proteins in si-CaMKIIγ and si-control group, respectively. Data represent means ± SE, n = 3 (∗ P < 0.05 versus si-control). (c) Immunofluorescent staining of a-SMA, SM22a, and SM-MHC (red) in si-CaMKII and si-control group, respectively. Nuclear were stained with DAPI (blue). Scale bars: 100 μm.
4. Discussion
Smooth muscle cells (SMCs) play an important role in angiogenesis and regulate blood pressure by contracting and relaxing in response to a variety of stimulus [15]. Thus, any defect or damage in smooth muscle tissue may result in severe dysfunctions of cardiovascular system. Up to now, a variety of cell sources including mature SMCs and adult mesenchymal stem cells have been used in blood vessel tissue engineering to repair vascular defects. Because of their limited ability to proliferate and usually loss of their contractile phenotype in expansion, mature differentiated SMCs have great limitation in tissue engineering. Adipose derived stem cells (ASCs) have advantages in that they are easy to be harvested, have relatively lower donor site morbidity, and can expand more rapidly in vitro [16]. ASCs have the potential to differentiate into functional smooth muscle cells and, therefore, adipose tissue can be a useful source of cells for treatment of injured tissues where smooth muscle plays an important role [17]. Thus, ASCs could be a preferred novel cell source for blood vessel engineering. A better understanding of the molecular mechanisms involved in regulating SMC differentiation is critical for facilitating the development of blood vessel tissue engineering. In previous studies, several transcriptional factors have been reported to be involved in differentiation of ASCs along smooth muscle cell pathway, which includes serum response factor (SRF), myocardin (Myocd), Myocd-related transcription factors (MRTFs) [18–20], and Krüppel-like factor-4 (KLF-4) [21].
CaMKII is a multimeric enzyme and its activity is regulated by the binding of Ca2+/calmodulin (CaM), which activates its protein kinase activity and promotes intrasubunit autophosphorylation. The autophosphorylated enzyme retains its kinase activity after the release of Ca2+/CaM, a phenomenon called autonomous activity [22]. Although CaMKII is multifunctional and all isoforms appear to have similar peptide substrate specificities and kinetics [23], it remains possible that specific isoforms may be discriminated at the level of protein substrate specificity. Among the four different isoforms of CaMKII, the α and β isoforms are predominantly expressed in neural system [24] and involved in synaptic plasticity, memory, and learning process [25]. In vitro, signaling through CaMKIIδ was demonstrated in upregulation of the proliferation and migration of vascular SMCs [26]. Moreover, in vivo molecular/genetic loss-of-function approaches indicate an important function of CaMKIIδ in promoting injury-induced vascular wall remodeling [27, 28], flow dependent remodeling [29], and angiotensin II-induced vascular wall hypertrophy. Expression of endogenous CaMKIIγ in VSM after vascular injury is permissive of coupling CaMKIIδ-enriched holoenzymes to promotion of VSM synthetic phenotype functions [14].
CaMKII has been implicated as a regulator of smooth muscle contraction for more than a decade [11–13, 30, 31]. Early reports indicated that γ isoform of CaMKII was highly expressed in differentiated smooth muscle cells that acquired contractile activity [9, 12]. However, whether CaMKIIγ plays a role in differentiation of mesenchymal stem cells into contractile smooth muscle cells remains unclear. To our knowledge, this study is the first to demonstrate that CaMKIIγ isoforms are involved in modulation of smooth muscle cell differentiation of adult mesenchymal stem cells.
Firstly, according to our previous study [5], we treated hASCs with TGF-β1 and BMP4 in combination and observed smooth muscle contractile proteins were highly expressed with induction, indicating a successful differentiation to SMCs. The highly expressed smooth muscle contractile proteins were CArG-dependent smooth muscle differentiation markers, because TGF-β1 and BMP4 induce CArG-dependent smooth muscle differentiation. We observed a paralleled upregulation of CaMKIIγ, but not other isoforms of CaMKII, during smooth muscle differentiation of hASCs. To examine the role of CaMKIIγ in the control of contractile gene expression, we overexpressed CaMKIIγ in the hASCs; this overexpression caused upregulation of smooth muscle contractile markers. We also used siRNA to knockdown CaMKIIγ expression. We found that inhibiting CaMKIIγ resulted in suppression of smooth muscle contractile relevant proteins expression as determined by western blot analysis and immunofluorescent staining. These results indicated that, among the four isoforms of the CaMKII, only CaMKIIγ promotes smooth muscle differentiation of hASCs. Saddouk et al. [14] indicated that decrease of CaMKIIγ coincided with decrease of contractile smooth muscle phenotype markers in the medial wall of carotid arteries. These results are also consistent with Kim et al.'s reports [12], in which they treated aorta tissue with non-variant-specific-CaMKIIγ antisense oligonucleotides and found that contractility of aorta was decreased significantly when it was stimulated with KCI. Taken together, these results including ours indicate that CaMKIIγ is one of the fundamental players that improves differentiation of hASCs into contractile smooth muscle phenotype. In addition, CaMKIIγ participates in CArG-dependent smooth muscle differentiation.
In conclusion, results of this study demonstrated that γ isoform of CaMKII is upregulated in the differentiation of hASCs into smooth muscle cells. Interfering expression of CaMKIIγ with siRNA significantly decreased contractile proteins expression. Further work needs to be done to explore molecular mechanism responsible for regulatory effect of CaMKIIγ in differentiation of contractile SMC phenotype.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (nos. 81271724 and 81471798), the Yangfan Project from Beijing Municipal Administration of Hospitals (XMLX201611), and the National Natural Science Foundation of China (nos. 81160088).
Competing Interests
The authors declare no conflict of interests.
Authors' Contributions
Kaisaier Aji and Lei Cui performed study design. Kaisaier Aji, Munila Maimaijiang, Abudusaimi Aimaiti, and Mulati Rexiati performed experiments. Baihetiya Azhati and Hamulati Tusong analyzed the data. Kaisaier Aji and Lei Cui wrote the paper.
References
- 1.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 2.Zuk P. A., Zhu M., Ashjian P., et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashjian P. H., Elbarbary A. S., Edmonds B., et al. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plastic and Reconstructive Surgery. 2003;111(6):1922–1931. doi: 10.1097/01.PRS.0000055043.62589.05. [DOI] [PubMed] [Google Scholar]
- 4.Deslex S., Negrel R., Vannier E. J., Etienne J., Ailhaud G. Differentiation of human adipocyte precursors in a chemically defined serum-free medium. Experimental Cell Research. 2007;313:2875–2886. [Google Scholar]
- 5.Wang C., Yin S., Cen L., et al. Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-β1 and bone morphogenetic protein-4. Tissue Engineering—Part A. 2010;16(4):1201–1213. doi: 10.1089/ten.tea.2009.0303. [DOI] [PubMed] [Google Scholar]
- 6.Wang C., Cen L., Yin S., et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials. 2010;31(4):621–630. doi: 10.1016/j.biomaterials.2009.09.086. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg O. S., Deindl S., Comolli L. R., et al. Oligomerization states of the association domain and the holoenyzme of Ca2+/CaM kinase II. The FEBS Journal. 2006;273(4):682–694. doi: 10.1111/j.1742-4658.2005.05088.x. [DOI] [PubMed] [Google Scholar]
- 8.Hudmon A., Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annual Review of Biochemistry. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z. L., Ikebe M. New isoforms of Ca2+/calmodulin-dependent protein kinase II in smooth muscle. Biochemical Journal. 1994;299(2):489–495. doi: 10.1042/bj2990489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schworer C. M., Rothblum L. I., Thekkumkara T. J., Singer H. A. Identification of novel isoforms of the delta subunit of Ca2+/calmodulin-dependent protein kinase II. Differential expression in rat brain and aorta. The Journal of Biological Chemistry. 1993;268(19):14443–14449. [PubMed] [Google Scholar]
- 11.Rokolya A., Singer H. A. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. American Journal of Physiology—Cell Physiology. 2000;278(3):C537–C545. doi: 10.1152/ajpcell.2000.278.3.C537. [DOI] [PubMed] [Google Scholar]
- 12.Kim I., Je H.-D., Gallant C., et al. Ca2+-calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. The Journal of Physiology. 2000;526(2):367–374. doi: 10.1111/j.1469-7793.2000.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marganski W. A., Gangopadhyay S. S., Je H.-D., Gallant C., Morgan K. G. Targeting of a novel Ca2+/calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase-mediated signaling in differentiated smooth muscle cells. Circulation Research. 2005;97(6):541–549. doi: 10.1161/01.res.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- 14.Saddouk F. Z., Sun L.-Y., Liu Y. F., et al. Ca2+/calmodulin-dependent protein kinase II-γ (CaMKIIγ) negatively regulates vascular smooth muscle cell proliferation and vascular remodeling. The FASEB Journal. 2016;30(3):1051–1064. doi: 10.1096/fj.15-279158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens G. K., Kumar M. S., Wamhoff B. R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 16.Zuk P. A., Zhu M., Mizuno H., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez L. V., Alfonso Z., Zhang R., Leung J., Wu B., Ignarro L. J. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(32):12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida T., Sinha S., Dandré F., et al. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circulation Research. 2003;92(8):856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Wang D.-Z., Hockemeyer D., McAnally J., Nordheim A., Olson E. N. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428(6979):185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 20.Miano J. M. Channeling to myocardin. Circulation Research. 2004;95(4):340–342. doi: 10.1161/01.RES.0000140893.16465.2d. [DOI] [PubMed] [Google Scholar]
- 21.Davis-Dusenbery B. N., Chan M. C., Reno K. E., et al. Down-regulation of Krüppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. The Journal of Biological Chemistry. 2011;286(32):28097–28110. doi: 10.1074/jbc.m111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujisawa H. Regulation of the activities of multifunctional Ca2+/calmodulin-dependent protein kinases. Journal of Biochemistry. 2001;129(2):193–199. doi: 10.1093/oxfordjournals.jbchem.a002843. [DOI] [PubMed] [Google Scholar]
- 23.Gaertner T. R., Kolodziej S. J., Wang D., et al. Comparative analyses of the three-dimensional structures and enzymatic properties of α, β, γ, and δ isoforms of Ca 2+-calmodulin-dependent protein kinase II. Journal of Biological Chemistry. 2004;279(13):12484–12494. doi: 10.1074/jbc.m313597200. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T., Fujisawa H. Evidence for three distinct forms of calmodulin-dependent protein kinases from rat brain. FEBS Letters. 1980;116(2):141–144. doi: 10.1016/0014-5793(80)80628-4. [DOI] [PubMed] [Google Scholar]
- 25.Shen K., Meyer T. Dynamic control of caMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284(5411):162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 26.House S. J., Ginnan R. G., Armstrong S. E., Singer H. A. Calcium/calmodulin-dependent protein kinase II-δ isoform regulation of vascular smooth muscle cell proliferation. American Journal of Physiology—Cell Physiology. 2007;292(6):C2276–C2287. doi: 10.1152/ajpcell.00606.2006. [DOI] [PubMed] [Google Scholar]
- 27.House S. J., Singer H. A. CaMKII-δ isoform regulation of neointima formation after vascular injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(3):441–447. doi: 10.1161/ATVBAHA.107.156810. [DOI] [PubMed] [Google Scholar]
- 28.Li W., Li H., Sanders P. N., et al. The multifunctional Ca2+/calmodulin-dependent Kinase II δ (CaMKIIδ) controls neointima formation after carotid ligation and vascular smooth muscle cell proliferation through cell cycle regulation by p21. Journal of Biological Chemistry. 2011;286(10):7990–7999. doi: 10.1074/jbc.m110.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott J. A., Xie L., Li H., et al. The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. American Journal of Physiology—Heart and Circulatory Physiology. 2012;302(10):H1953–H1964. doi: 10.1152/ajpheart.00978.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tansey M. G., Luby-Phelps K., Kamm K. E., Stull J. T. Ca2+-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. Journal of Biological Chemistry. 1994;269(13):9912–9920. [PubMed] [Google Scholar]
- 31.Kim M., Han I. S., Koh S. D., Perrino B. A. Roles of CaM kinase II and phospholamban in SNP-induced relaxation of murine gastric fundus smooth muscles. American Journal of Physiology—Cell Physiology. 2006;291(2):C337–C347. doi: 10.1152/ajpcell.00397.2005. [DOI] [PubMed] [Google Scholar]