Abstract
Yacon (Smallanthus sonchifolius), a perennial plant of the family Asteraceae native to the Andean regions of South America, is an abundant source of fructooligosaccharides (FOS). This comprehensive review of the literature addressed the role of yacon supplementation in promoting health and reducing the risk of chronic diseases. According to several preclinical and clinical trials, FOS intake favors the growth of health-promoting bacteria while reducing pathogenic bacteria populations. Moreover, the endproducts of FOS fermentation by the intestinal microbiota, short chain fatty acids (SCFA), act as substrates or signaling molecules in the regulation of the immune response, glucose homeostasis and lipid metabolism. As a result, glycemic levels, body weight and colon cancer risk can be reduced. Based on these findings, most studies reviewed concluded that due to their functional properties, yacon roots may be effectively used as a dietary supplement to prevent and treat chronic diseases.
Keywords: yacon, prebiotics, fructooligosacharides, functional food, chronic diseases
1. Introduction
Yacon (Smallanthus sonchifolius) is a perennial herbaceous plant of the family Asteraceae, native to the Andean regions of South America [1,2]. This plant has a branching system that gives rise to aerial stems about 2 to 2.5 m high. Yacon yields starchy, fruit-like roots of different shapes and sizes that are usually consumed raw and taste sweet. Their crunchy texture very much resembles that of an apple. One plant is estimated to produce more than 10 kilos of roots [3,4]. The fact that the yacon plant adapts to different climatic regions, altitudes and soils explains its expansion outside the Andean region. Yacon is currently cultivated in Argentina, Bolívia, Brazil, the Czech Republic, Ecuador, Italy, Japan, Korea, New Zealand, Peru and the United States [4].
There is a variety of common names for yacon around the world. These include aricoma and aricuma in Bolivia, jicama, chicama and shicama in Ecuador, and arboloco in Colombia. However, the Spanish term yacon, derived from the Quéchua word “yaku” which means “watery”, is the most used worldwide. Interestingly, water is the most abundant component of the yacon root [2,4].
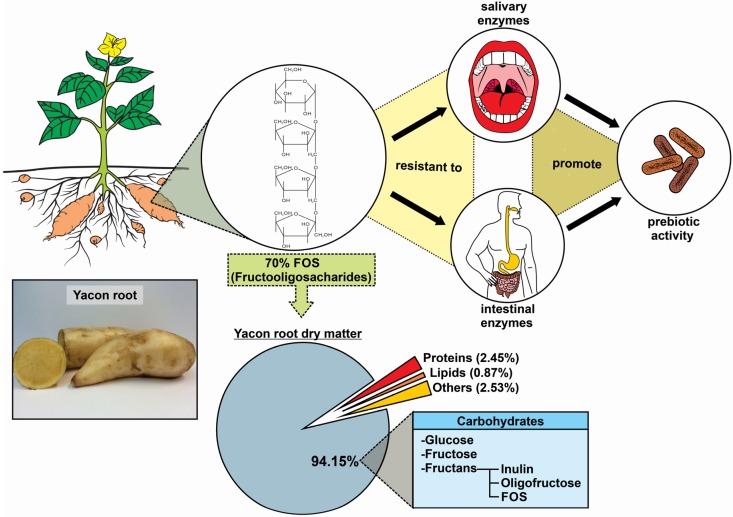
Yacon roots’ water content usually exceeds 70% of the fresh weight while the major portion of the dry matter consists of fructooligosacharides (FOS) [5]. FOS content ranges from 6.4% to 70% of the dry matter (0.7% to 13.2% of the fresh weight) depending upon the specific crop and location. In yacon roots, the antioxidant capacity varies between 23 and 136 µmol/g trolox equivalent of the dry matter, and total phenolic compounds represent 0.79% to 3.08% of the dry matter [6,7,8]. Figure 1 summarizes the physicochemical and functional characteristics of yacon roots.
Figure 1.
Chemical composition and functional properties of yacon roots.
The high content of FOS in yacon roots is considered to offer health benefits, as it can reduce glycemic index, body weight and the risk of colon cancer [9]. Yacon functional properties, long recognized by folk medicine, have been the subject of a number of research projects and clinical trials [10]. Thus, the nutraceutical potential of yacon roots has garnered great public interest as a dietary supplement. In this comprehensive review, we focused on yacon FOS health-promoting benefits regarding human chronic diseases.
2. Fructooligosacharides: Bioactivity and Potential Health Benefits
Fructooligosacharides (FOS) are fructans consisting of linear short chains of fructose molecules. Fructans are synthesized from sucrose in the cell vacuoles of plant leaves, stems and roots. They help protect against drying out and are carbohydrate reserves in a wide number of plant families [11,12]. FOS are natural food components that can be found in garlic, onion, asparagus, artichoke, banana, wheat and yacon. However, the highest concentrations of FOS are found in yacon [13].
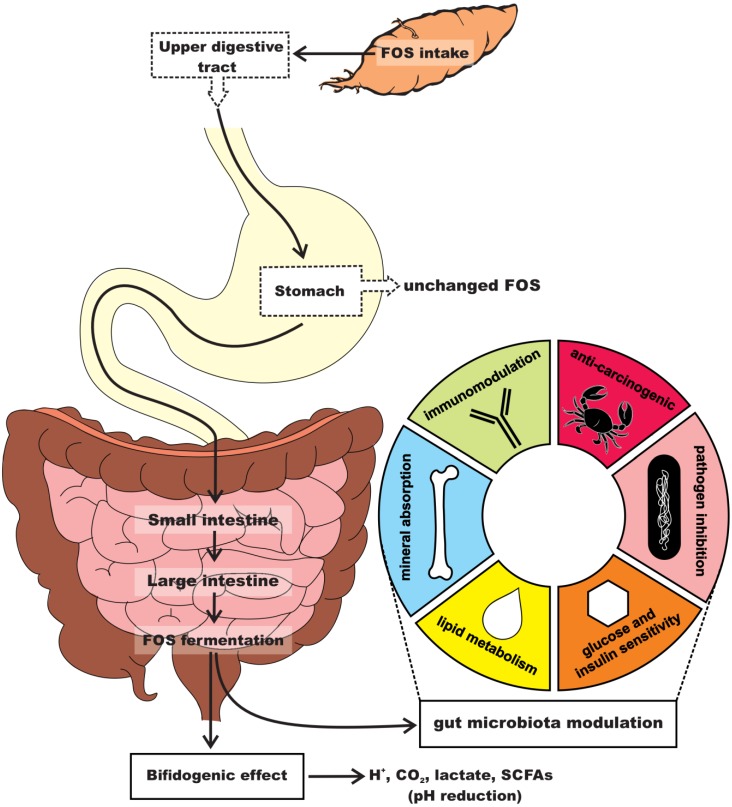
FOS are able to escape enzymatic digestion in the upper gastrointestinal tract, reaching the colon intact before undergoing microbial fermentation. FOS intake elicits a bifidogenic effect by selectively stimulating the proliferation of bifidobacteria, a group of beneficial bacteria naturally found in the human colon (Figure 2) [14,15,16]. Short chain fatty acids (SCFA), the endproducts of FOS fermentation by the intestinal microbiota, can also favor the growth of health-promoting bacteria such as Bifidobacterium spp. and Lactobacillus spp., while reducing or maintaining pathogenic populations (e.g., Clostridium spp. and Escherichia coli) at low levels [17,18,19]. Thus, FOS are small soluble dietary fibers that exhibit prebiotic activity.
Figure 2.
Yacon root consumption and health-promoting benefits of FOS.
The term prebiotic was coined by Gibson and Roberfroid in 1995 to describe a “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving host health” [20]. This concept was later revised by Roberfroid who redefined a prebiotic as “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health” [21].
Several other concepts have been proposed since then, but they all describe a prebiotic as a non-digestible compound able to selectively stimulate the growth of gut bacteria. According to the criteria proposed by FAO at the technical meeting on prebiotics [22], to be classified as a prebiotic, a compound must present the following qualifications: (a) component: a compound or substance that can be chemically characterized—not an organism or drug normally presented as a food-grade component; (b) health benefit: a compound or substance must resist digestion and absorption in the small intestine, over-riding any adverse effects; and (c) modulation: a compound or substance must promote health-related changes in the composition and/or activities of the colonic microbiota in the target host.
There is sufficient evidence to support the categorization of FOS as prebiotics. FOS offers physiological benefits that justify its use as a food supplement, particularly in cases of chronic diseases [14,23,24]. Since yacon has long been used in folk medicine for treating diabetes, constipation and various other human diseases, the present study aimed at reviewing the mechanisms underlying yacon FOS health benefits in colon cancer, diabetes, and obesity.
3. FOS Effects on Colorectal Cancer
Colorectal Cancer (CRC) is the third most commonly diagnosed type of cancer and a leading cause of death in the Western world. Although family history is an important risk factor for CRC development, only 15% of new cases have been linked to hereditary causes. In fact, the majority of CRCs (80%) occur sporadically and are associated with acquired risk factors, such as lifestyle and diet [25,26]. Dietary factors that potentially increase the risk of CRC include a high intake of red and processed meat, saturated fats and refined starches [27,28]. Diabetes and obesity are also associated with a higher risk of developing CRC [29].
Little is known about the feasibility, safety and efficacy of using dietary yacon to modulate or suppress CRC. Our research group was the first to report the chemopreventive effects of yacon root intake on dimethylhydrazine (DMH)-induced colon cancer in male rats. We showed a reduction in cell proliferation, number and multiplicity of preneoplastic lesions and invasive adenocarcinomas in a group receiving 1% of yacon powder [30]. In a more recent study evaluating the effects of yacon aqueous extract on the initiation step of CRC carcinogenesis, we found that yacon aqueous extract alone or that associated with Lactobacillus acidophilus (synbiotic formulation) reduced DMH-induced DNA damage in leukocytes. Moreover, we observed a reduction in cell proliferation indexes and a decrease in apoptosis levels in the group supplemented with the synbiotic formulation [31].
There is growing evidence that human intestinal microbiota plays an essential role in CRC carcinogenesis. The interplay between the intestinal microbiota, the intestinal epithelium and the host innate immune system is associated with several human diseases, including colitis and CRC [32,33]. Dysbiosis is a condition in which an imbalance in the microbial community favors the growth of specific pathogens that are potentially pro-carcinogenic. Intestinal microbiota disruption also exerts a great impact on colon metabolic profiles under the influence of the microbial community [34].
The influence of dietary habits on the composition of the microbiota has been widely accepted in the scientific community, supporting the hypothesis that diet patterns can induce dysbiosis [35]. Hence, the yacon root is thought to be a good dietary supplement, since its high content of FOS can selectively modulate the composition and function of the intestinal microbiota. FOS promote the growth of bifidobacteria, a genus of Gram-positive pleomorphic rods that play a regulatory role in the colon by inhibiting the growth of putrefactive bacteria. Bifidobacteria have been suggested to decrease the expression of xenobiotic-metabolizing enzymes and stimulate the immune system in the colonic mucosa [36,37,38].
FOS consumption also leads to increased SCFA production, primarily acetate, propionate and butyrate. Recent findings suggest that SCFA can suppress inflammation and cancer by increasing local immune response, decreasing colon pH and promoting ammonia and amine excretion [36,38]. During carcinogenesis, SCFA production in the colon by beneficial bacteria decreases cellular proliferation and induces apoptosis, especially in colon tumor cells. In fact, increasing butyrate production has also been shown to decrease the development of preneoplastic aberrant crypt foci lesions and delay tumor progression in rats [30,39,40].
FOS can indirectly influence immune activity via SCFA production that modifies the intestinal microbiota composition. SCFA promote a state of immune tolerance and modulate interleukin (IL) production and natural killer (NK) cell activity [41]. Vaz-Tostes et al. [42] reported that the consumption of yacon flour (0.14 g FOS kg body weight) over 18 weeks increased serum IL-4 and fecal secretory IgA in overweight preschool children with an inadequate dietary intake of zinc and fiber [42]. However, the role of prebiotic-induced immunomodulation in CRC is still unclear.
Increasing evidence suggests that FOS can also directly modulate the immune system through the gut-associated lymphoid tissue (GALT) rather than the gut microbiota [43]. Natural plant compounds such as fructans and polysaccharides may activate specialized immune cells (macrophages, dendritic cells, lymphocytes and neutrophils) by mimicking pathogen-associated molecular patterns (PAMPs) that bind to toll-like receptors (TLR), causing immunomodulatory effects [44]. For instance, TLR-mediated activation of NK cells can promote IFN-γ production and thus increase anti-tumor cytotoxicity. Furthermore, direct and indirect immunomodulation mechanisms can synergistically induce robust regulatory cellular immune responses [45]. Indeed, yacon treatment increased cytokine production (i.e., IL-10, IFN-α and IL-4) and the expression of toll-like receptor 4 (TLR4) and CD206 in cells in infant mice [46,47]. The increase in the expression of these receptors in gut-associated immune cells results in an enhanced status of the innate immune response with remarkable macrophage activity. The increased phagocytic activity of macrophages, mediated by the CD206 receptor and TLR4, is able to maintain colonic homeostasis without inducing inflammatory responses, reinforcing the intestinal barrier against pathogens and improving anti-tumor defense [47,48].
Table 1 shows that the effects of yacon consumption on colorectal cancer include: (a) suppressed cell proliferation; (b) reduced preneoplastic lesions; (c) significantly changed composition of the colonic microbiota; and (d) modulated immune response in CRC.
Table 1.
Effects of yacon consumption on colorectal cancer.
| Yacon Source | Research, Subject Randomized, Dose and Duration | Health Properties | References |
|---|---|---|---|
| Dried extract of yacon root | Mouse (BALB/c) Dose: 340 mg/kg day in diet, for 75 days | Growth of Bifidobacteria and Lactobacilli | Bonet et al. [16] |
| Dried extract of yacon root | Rats (Wistar) Dose: 0.5%, 1.0% (20.4% FOS) in diet for 13 weeks | Reduce tumor multiplicity, preneoplastic lesions and cell proliferation | De Moura et al. [27] |
| Aqueous extract of yacon root | Rats (Wistar) Dose: 2.2 mL (1% FOS) for 8 months | Reduce DNA damage and cell proliferation | Almeida et al. [28] |
| Dried extract of yacon root | Mouse (BALB/c) Dose: 3.0%, 5% FOS in diet for 30 days | Improves the immune parameters | Delgado et al. [46] |
4. FOS Effects on Diabetes
Diabetes is the most common chronic disorder in developed countries, and a leading cause of death worldwide, with the global prevalence being 8.4% among adults (>18 years) in 2014 [49]. Obesity and physical inactivity have been related to increased risk of developing diabetes. Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion and/or insulin action. Untreated chronic hyperglycemia can cause long-term tissue damage and dysfunction that might lead to adverse outcomes such as skin ulcers and amputations. Type 2 diabetes mellitus, characterized by insulin resistance and pancreatic β-cell dysfunction, is the most common form of diabetes [50,51].
The current standard care for diabetes type 2 prevention and management is dietary intervention [52]. Hence, antidiabetic nutraceuticals, such as yacon, with reduced or no side effects have been high in demand. Due to their hypoglycemic properties, yacon roots have long been recognized by folk medicine as an effective alternative for diabetes treatment. Moreover, yacon roots, either crude or refined, can be used as low-calorie sweeteners by dieters as well as people suffering from diabetes [53].
Several preclinical and clinical trials have shown that yacon root FOS have a notable hypoglycemic effect. In an experiment using streptozotocin-induced diabetic rats, the number of insulin-positive pancreatic cells and glucagon-like peptide-1 (GLP-1) significantly increased, while visceral abdominal fat was reduced and fasting insulin serum levels were slightly increased in diabetic rats supplemented with yacon flour (340 or 6800 mg FOS/kg body weight (bw).) for 90 days [54]. In another study using Zucker fa/fa male rats, yacon at 6.5% in chow reduced blood glucose levels and improved hepatic insulin sensitivity. In this case, dietary yacon significantly reduced Trb3 hepatic expression and increased Akt expression, improving insulin sensitivity in the liver [55].
In a trial evaluating the daily intake of freeze-dried yacon among elderly individuals, FOS content (7.4 g) was positively correlated with decreasing serum glucose levels [56]. Among obese and slightly dyslipidemic pre-menopausal women, Genta et al. observed that yacon syrup at 0.14 g/kg bw reduced fasting serum insulin and was significantly associated with decreased beta-cell function and insulin resistance in a homeostasis model assessment (HOMA), suggesting that yacon syrup FOS promote glucose absorption in peripheral tissues and improve insulin sensitivity via SCFA production [57].
Plasma glucose homeostasis is achieved through a tightly controlled balance between glucose input (food intake and liver production) and glucose uptake by multiple organs [58]. FOS putative effects on glucose disposal and insulin tolerance are mediated via multiple mechanisms. These mechanisms are part of the milieu of interactions that take place between the intestinal microflora and the host metabolism, and converge to a similar outcome—the production of SCFA by FOS fermentation. SCFA produced by the intestinal microbiota are promptly absorbed in the colon and conveyed into blood, where they play their physiological roles as substrates or signaling molecules [59,60,61].
Several studies have been conducted to elucidate the underlying mechanisms of SCFA on glucose homeostasis. For instance, acetate has been shown to reduce free fatty acids (FFA) plasma levels, which are known to cause peripheral insulin resistance in obese individuals, inhibiting glucose uptake and glycogen synthesis [62]. The oral administration of propionate to both diabetic hyperglycemic and normal rats has been shown to decrease gluconeogenesis by increasing AMPK expression in the liver [63]. SCFA have also been reported to affect glycemic levels through the gut hormones peptide YY (PYY) and GLP-1 by directly activating colonic free fatty acid receptors 2 and 3 (Ffar2 and Ffar3). PYY and GLP-1 have also been proposed to improve plasma glucose levels after a meal in a dependent manner, stimulating insulin and inhibiting glucagon secretion in the pancreas [64,65].
Table 2 shows that the effects of yacon consumption on diabetes include: (a) increased glucose absorption in peripheral tissues; (b) decreased gluconeogenesis; (c) improved insulin tolerance in the liver; and (d) increased insulin secretion in the pancreas.
Table 2.
Effects of yacon consumption on diabetes.
| Yacon Source | Research, Subject Randomized, Dose and Duration | Health Properties | References |
|---|---|---|---|
| Yacon flour | Rats (Wistar) | Increase insulin-positive pancreatic cell | Habib et al. [54] |
| Dose: Yacon flour (340 mg FOS/kg/day) for 90 days | |||
| Dried extract of yacon root | Rats (Zucker fa/fa) | Improve insulin sensitivity in the insulin-resistant state | Satoh et al. [55] |
| Dose: 6.5% yacon for 5 weeks | |||
| Dried extract of yacon root | Elderly man and woman | Decrease in serum glucose levels | Scheid et al. [56] |
| Dose: Yacon powder (7.4 g of FOS) for 9 weeks | |||
| Yacon syrup | Obese and slightly dyslipidemic pre-menopausal women | Improve insulin-resistance state | Genta et al. [57] |
| Dose: Yacon syrup (0.29 g and 0.14 g FOS/kg/day), for 120 days |
5. FOS Effects on Obesity
Overweight and obesity comprises one of the main public health challenges worldwide because of the associated increased risk of developing type 2 diabetes, heart disease, hypertension, cancer and a number of other diseases [66]. Over the past few decades, the increasing number of overweight and obese people has been claimed as a pandemic. According to the World Health Organization (WHO), the prevalence of overweight was estimated to be 39% among adults aged 18 years and over, while obesity represented 13% of the overall world’s adult population in 2014 [67]. Overweight and obesity are defined as a condition of abnormal or excessive accumulation of adipose tissue in the body. This condition may impair health and lead, for instance, to the development of chronic inflammation and metabolic syndrome [68]. The main causes of overweight and obesity are related to energy imbalance (i.e., energy intake exceeds energy expenditure) modulated by metabolic factors, diet and physical activity. Hence, there has been a global trend to an increasing intake of energy-dense foods that are rich in saturated fat and refined starches, as well as increasing rates of physical inactivity and a sedentary lifestyle [69].
Metabolic syndrome is a cluster of cardiometabolic risk factors that arises from insulin resistance accompanying abnormal visceral adiposity, glucose intolerance, dyslipidemia and hypertension [70]. As a consequence, metabolic syndrome leads to a state of chronic inflammation produced by a complex interaction between genetic and environmental factors. At the moment, there is no consensus on what is the most appropriate nutritional intervention for treating metabolic syndrome related to obesity [71]. However, certain dietary bioactive compounds found in over 800 plants can help to prevent or ameliorate multiple facets of metabolic syndrome. In this regard, yacon has been hypothesized to exert anti-obesity and hypolipidemic effects by improving biochemical parameters and satiety [72]. Though there is a popular claim that yacon syrup can aid in weight loss, scientific evidence is nevertheless scarce. These properties, however, are thought to be directly related to the high content of FOS found in yacon root.
In a sub-chronic four-month oral toxicity study, dried yacon root (340 mg and 6800 mg FOS/kg bw) was given as a diet supplement to healthy, non-obese Wistar rats. During the feeding trial, yacon administration was well tolerated and did not produce any toxic effect. Furthermore, yacon consumption at both doses significantly reduced post-prandial serum triacylglycerol (TAG) levels [73]. Similar findings were reported when yacon flour (340 or 6800 mg FOS/kg bw) was administered to streptozotocin-induced diabetic rats. The oral consumption of yacon flour decreased fasting plasma TAG, very low-density lipoprotein (VLDL) and the postprandial peak of plasma TAG [54]. In another study using synbiotic formulations, a positive effect on TAG and high-density lipoprotein (HDL) cholesterol levels was reported in diabetic rats that received an aqueous extract of yacon roots and soybean, in association or not with Enterococcus faecium CRL 183 and Lactobacillus helveticus ssp jugurti [74].
Although the hypolipidemic effects of yacon roots have been demonstrated in pre-clinical studies, evidence from well-designed human trials is still scarce. As cited before, in a study with premenopausal, obese and slightly dyslipidemic women, yacon syrup intake (0.14 g FOS/kg bw) over 120 days showed improvements in fasting low density lipoproteins (LDL) and visceral fat [57]. Otherwise, no such effect was reported in a study conducted in elderly who consumed a daily intake of freeze-dried powdered yacon [56]. Moreover, yacon administered to healthy individuals (6.4 g FOS/day) over two weeks markedly accelerated colonic transit in a placebo-controlled, double-blind study design [75].
The beneficial effects of FOS on lipid metabolism are well recognized, although the underlying mechanisms are still unclear. FOS exert hypolipidemic effects through SCFA production by the intestinal microbiota, resulting in the modulation of biochemical and cellular pathways related to lipid metabolism, satiety and intestinal transit [76]. Indeed, SCFA have been shown to positively regulate the lipid homeostasis by inhibiting lipolysis, increasing triglyceride mobilization and adipogenic differentiation [60,77]. In vitro studies also reported that SCFA were able to reduce cholesterol synthesis by decreasing hepatic activity of the 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) enzymes [68]. AMPK activation by SFCAs has also been suggested to inhibit HMGCS and HMGCR activation in an independent manner [60].
It has also been shown that dietary FOS are able to increase the secretion of peptides by the gastrointestinal diffuse neuroendocrine system via SCFA production, acting as modulators of appetite and increasing satiety [78]. The physiological control of satiety is partly regulated by intestinal peptide secretion including cholecystokinin (CCK), PYY and GLP-1. It is noteworthy that this regulation is complex and involves a range of mechanisms and multiple control systems [79]. Nevertheless, SCFA can directly increase PYY and GLP-1 secretion by Ffar1 and Ffar2 activation in the colon [80]. Conversely, long-term studies have suggested that a long exposure time is needed for the intestinal microbiota to adapt and produce the amounts of SCFA to elicit the physiological effect of satiety. Increased gut motility may also be affected by intestinal peptide secretion [81]. However, SCFA such as butyrate are able to exert direct effects on myenteric neurons and increase the intestinal motility, supporting the hypothesis by which a high fiber intake accelerates the colonic transit [82].
Although there have been several studies reporting the beneficial effects of yacon intake on obesity, much needs to be understood about the mechanisms and processes that underlie such effects. Table 3 shows that the effects of yacon consumption on obesity include: (a) modulated biochemical and cellular pathways related to lipid homeostasis; (b) increased satiety; and (c) increased gut motility.
Table 3.
Effects of yacon consumption on obesity.
| Yacon Source | Research, Subject Randomized, Dose and Duration | Health properties | References |
|---|---|---|---|
| Yacon flour | Rats (Wistar) Dose: Yacon flour (340 mg FOS/kg/day) for 90 days | Hypolipidemic effect | Habib et al. [54] |
| Yacon syrup | Obese and slightly dyslipidemic pre-menopausal women Dose: Yacon syrup (0.29/g and 0.14/g FOS/kg/day) for 120 days. | Increased defecation frequency and satiety sensation | Genta et al. [57] |
| Dried extract of yacon root | Rats (wistar) Dose: Dried yacon root (340 mg and 6800 mg FOS/bw) for 4 months | Reduced post-prandial serum TAG levels | Genta et al. [73] |
| Aqueous extract of yacon root | Rats (wistar) Dose: 1 mL/kg body weight/day, 4.30 g/100 g of frutans, for 7 weeks | Positive effect on TAG and HDL | Roselino et al. [74] |
| Yacon syrup | Healthy individuals Dose: 6.4 g FOS/day | Accelerates the colonic transit | Geyer et al. [75] |
6. Yacon Consumption Adverse Effects
Although yacon consumption is safe at recommended dosages, overdosing may be uncomfortable, but not life-threatening. Symptoms of yacon overdose include abdominal pain, bloating, flatulence and diarrhea [57]. In addition, yacon consumption markedly accelerates colonic transit, increasing stool frequency [75]. The only report of adverse effects found in the literature describes the case of a 55-year-old woman who developed anaphylaxis after yacon ingestion [83].
A side effect that should be taken into account when evaluating the proportion of oligofructans/fructose within yacon roots is the partial hydrolysis of yacon oligofructans to fructose that starts shortly after harvest and may accelerate during food processing [84]. This can seriously affect yacon’s health-promoting benefits because high-fructose administration correlates with the induction of insulin resistance by modifying the early steps of insulin signal transduction [85]. Therefore, cold storage and temperature-controlled environments are highly recommended to keep the functional properties of the yacon roots [84].
7. Conclusions
Experimental and clinical studies have reported that yacon consumption is important to regulate several pathways related to colon cancer, diabetes, and obesity. The FOS content found in yacon roots can modulate the human intestinal microbiota, increase glucose absorption in peripheral tissues, stimulate insulin secretion in the pancreas and modulate cellular pathways related to lipid homeostasis. Therefore, based on these findings, most studies reviewed concluded that due to their functional properties, yacon roots may be effectively used as a dietary supplement to prevent and treat chronic diseases.
Acknowledgments
This review is based upon research projects supported by the Sao Paulo Research Foundation (FAPESP) under grants 09/12239-2, 11/01126-2 and 16/12800-0. The authors acknowledge the support of PROPe-PROINTER/UNESP.
Abbreviations
The following abbreviations are used in this manuscript:
| BW | body weight |
| CRC | colorectal cancer |
| CCK | cholecystokinin |
| DMH | 1,2-dimethylhydrazine |
| GLP-1 | glucagon-like peptide 1 |
| SCFA | short chain fatty acids |
| FFA | free fatty acids |
| FFAR | free fatty acid receptor |
| FOS | fructooligosacharides |
| HDL | high density lipoprotein |
| LDL | low density lipoprotein |
| PAMPs | pathogen-associated molecular patterns |
| PYY | peptide YY |
| TAG | tryacylglycerol |
| TLR | toll-like receptors |
| VLDL | very low density lipoprotein |
Author Contributions
All authors contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lachman J., Fernández E.C., Orsák M. Yacon (Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson) chemical composition and use-a review. Plant Soil Environ. 2003;49:283–290. [Google Scholar]
- 2.Zardini E. Ethnobotanical notes of yacon, Polymnia sonchifolia (Asteraceae) Econ Bot. 1991;45:72–85. doi: 10.1007/BF02860051. [DOI] [Google Scholar]
- 3.Grau A., Rea J. Andean Roots and Tuberous Roots: Ahipa, Arracacha, Maca and Yacon. Gatersleben/IPGRI; Rome, Italy: 1997. Yacon. Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson; pp. 199–256. [Google Scholar]
- 4.Ojansivua I., Ferreira C.L., Salminena S. Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends Food Sci. Tech. 2011;22:40–46. doi: 10.1016/j.tifs.2010.11.005. [DOI] [Google Scholar]
- 5.Campos D., Betalleluz-Pallardel I., Chirinos R., Aguilar-Galvez A., Noratto G. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012;135:1592–1599. doi: 10.1016/j.foodchem.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 6.Castro A., Céspedes G., Carballo S., Bergenståhl B., Tornberg E. Dietary fiber, fructooligosaccharides, and physicochemical properties of homogenized aqueous suspensions of yacon (Smallanthus sonchifolius) Food Res. Int. 2013;50:392–400. doi: 10.1016/j.foodres.2012.10.048. [DOI] [Google Scholar]
- 7.Jiménez M.E., Sammán N. Chemical characterization and quantification of fructooligosaccharides, phenolic compounds and antiradical activity of Andean roots and tubers grown in Northwest of Argentina. Arch. Latinoam Nutr. 2014;64:131–138. [PubMed] [Google Scholar]
- 8.Pereira J.A.R., Barcelos M.F.P., Pereira M.C.A., Ferreira E.B. Studies of chemical and enzymatic characteristics of Yacon (Smallanthus sonchifolius) and its flour. Food Sci. Technol. 2013;33 doi: 10.1590/S0101-20612013005000020. [DOI] [Google Scholar]
- 9.Delgado G.T., Tamashiro W.M., Maróstica-Junior M.R., Pastore G.M. Yacon (Smallanthus sonchifolius): A functional food. Plant Foods Hum. Nutr. 2013;68:222–228. doi: 10.1007/s11130-013-0362-0. [DOI] [PubMed] [Google Scholar]
- 10.De Almeida P.H.A., Abranches M.V., de Luces Fortes Ferreira C.L. Yacon (Smallanthus sonchifolius): A food with multiple functions. Crit. Rev. Food Sci. Nutr. 2015;55:32–40. doi: 10.1080/10408398.2011.645259. [DOI] [PubMed] [Google Scholar]
- 11.Apolinário A.C., de Lima Damasceno B.P., de Macêdo Beltrão N.E, Pessoa A., Converti A., da Silva J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr Polym. 2014;30:368–378. doi: 10.1016/j.carbpol.2013.09.081. [DOI] [PubMed] [Google Scholar]
- 12.Fujishima M., Furuyama K., Ishihiro Y., Onodera S., Fukushi E., Benkeblia N., Shiomi N. Isolation and Structural Analysis In Vivo of Newly Synthesized Fructooligosaccharides in Onion Bulbs Tissues (Allium cepa L.) during storage. Int. J. Carbohydr. 2009 doi: 10.1155/2009/493737. [DOI] [Google Scholar]
- 13.Santana I., Cardoso M.H. Yacon tuberous root (Smallanthus sonchifolius): Cultivation potentialities, technological and nutritional aspects. Ciência Rural. 2008;38:898–905. doi: 10.1590/S0103-84782008000300050. [DOI] [Google Scholar]
- 14.Sabater-Molina M., Larqué E., Torrella F., Zamora S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009;65:315–328. doi: 10.1007/BF03180584. [DOI] [PubMed] [Google Scholar]
- 15.Sivieri K., Morales M.V., Saad S.M.I., Adorno M.A., Sakamoto I.K., Rossi E.A. Prebiotic effect of fructooligosaccharide in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME Model) J. Med. Food. 2014;17:1–8. doi: 10.1089/jmf.2013.0092. [DOI] [PubMed] [Google Scholar]
- 16.Bibas Bonet M.E., Meson O., de Moreno de LeBlanc A., Dogib C.A., Chaves S., Kortsarz A., Grau A., Perdigón G. Food Agric. Immunol. 2010. Prebiotic effect of yacon (Smallanthus sonchifolius) on intestinal mucosa using a mouse model. [DOI] [Google Scholar]
- 17.Gibson G.R. Dietary modulation of the human gut microflora using prebiotics. Br. J. Nutr. 1998;4:209–212. [PubMed] [Google Scholar]
- 18.Whelan K. Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proc. Nutr. Soc. 2013;72:288–298. doi: 10.1017/S0029665113001262. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y., O’Riordan M.X.D. Regulation of Bacterial Pathogenesis by Intestinal Short-Chain Fatty Acids. Adv. Appl. Microbiol. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 21.Roberfroid M. Prebiotics: The concept revisited. J. Nutr. 2007;137:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 22.Pineiro M., Asp N.G., Reid G., Macfarlane S., Morelli L., Brunser O., Tuohy K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008 doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 23.Valentová K., Ulrichová J. Smallanthus sonchifolius and Lepidium meyenii—Prospective andean crops for the prevention of chronic diseases. Biomed. Papers. 2003;147:119–130. doi: 10.5507/bp.2003.017. [DOI] [PubMed] [Google Scholar]
- 24.Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013;22:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66 doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 26.Jasperson K.W., Tuohy T.M., Neklason D.W., Burt R.W. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baena R., Show S. Diet and colorectal cancer. Maturitas. 2015;80:258–264. doi: 10.1016/j.maturitas.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Steck S.E., Guinter M., Zheng J., Thomson C.A. Index-based dietary patterns and colorectal cancer risk: A systematic review. Adv. Nutr. 2015;6:763–773. doi: 10.3945/an.115.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters P.J., Bazelier M.T., Leufkens H.G., de Vries F., De Bruin M.L. The risk of colorectal cancer in patients with type 2 diabetes: Associations with treatment stage and obesity. Diabetes Care. 2015;38:495–502. doi: 10.2337/dc14-1175. [DOI] [PubMed] [Google Scholar]
- 30.De Moura N.A., Caetano B.F.R., Sivieri K., Urbano L.H., Cabello C., Rodrigues M.A., Barbisan L.F. Protective effects of yacon (Smallanthus sonchifolius) intake on experimental colon carcinogenesis. Food Chem. Toxicol. 2012;50:2902–2910. doi: 10.1016/j.fct.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Almeida A.P.S., Avia C.M., Barbisan L.F., de Moura N.A., Caetano B.F.R., Romualdo G.R., Sivieri K. Yacon (Smallanthus sonchifolius) and Lactobacillus acidophilus CRL 1014 reduce the early phases of colon carcinogenesis in male Wistar rats. Food Res. Int. 2015;74:48–54. doi: 10.1016/j.foodres.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Vipperla K., O’Keefe S. Diet, microbiota, and dysbiosis: A “recipe” for colorectal cancer. Food Funct. 2016;20:1731–1740. doi: 10.1039/C5FO01276G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2009;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 34.Mondot S., Lepage P. The human gut microbiome and its dysfunctions through the meta-omics prism. Ann NY Acad. Sci. 2016 doi: 10.1111/nyas.13033. [DOI] [PubMed] [Google Scholar]
- 35.Brown K., DeCoffe D., Molcan E., Gibson D.L. Diet-Induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease. Nutrients. 2012;4:1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolim P.M. Development of prebiotic food products and health benefits. Food Sci. Technol. 2015;35 doi: 10.1590/1678-457X.6546. [DOI] [Google Scholar]
- 37.Respondek F., Gerard P., Bossis M., Boschat L., Bruneau A., Rabot S., Wagner A., Martin J.C. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS ONE. 2013;8:436. doi: 10.1371/journal.pone.0071026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raman M., Ambalam P., Kondepudi K.K, Pithva S., Kothari C., Patel A.T., Purama R.K., Dave J.M., Vyas B.R. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut. Microbes. 2013;4:181–192. doi: 10.4161/gmic.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong J.M., de Souza R., Kendall C.W., Emam A., Jenkins D. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Tang Y., Chen Y., Jiang H., Nie D. The role of short-chain fatty acids in orchestrating two types of programmed cell death in colon cancer. Autophagy. 2011;7:235–237. doi: 10.4161/auto.7.2.14277. [DOI] [PubMed] [Google Scholar]
- 41.Kim H.C., Park J., Kim M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014;14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaz-Tostes M., Viana M.L., Grancieri M., Luz T.C., Paula H., Pedrosa R.G., Costa N.M. Yacon effects in immune response and nutritional status of iron and zinc in preschool children. Nutrition. 2014;30:666–672. doi: 10.1016/j.nut.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Peshev D., Van den Ende W. Fructans: Prebiotics and immunomodulators. J. Funct. Foods. 2014;8:348–357. doi: 10.1016/j.jff.2014.04.005. [DOI] [Google Scholar]
- 44.Liu X., Zheng J., Zhou H. TLRs as pharmacological targets for plant-derived compounds in infectious and inflammatory diseases. Int. Immunopharmacol. 2011;10:1451–1456. doi: 10.1016/j.intimp.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 45.Vogt L., Ramasamy U., Meyer D., Pullens G., Venema K., Faas M.M., Schols H.A., de Vos P. Immune modulation by different types of β2→1-fructans is toll-like receptor dependent. PLoS ONE. 2013;5:436. doi: 10.1371/journal.pone.0068367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delgado G.T., Thomé R., Gabriel D.L., Tamashiro W.M., Pastore G.M. Yacon (Smallanthus sonchifolius)-derived fructooligosaccharides improves the immune parameters in the mouse. Nutr. Res. 2012;32:884–892. doi: 10.1016/j.nutres.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Velez E., Castillo N., Mesón O., Grau A., Bonet M.E.B., Perdigón G. Study of the effect exerted by fructo-oligosaccharides from yacon (Smallanthus sonchifolius) root flour in an intestinal infection model with Salmonella Typhimurium. Br. J. Nutr. 2013;109:1971–1979. doi: 10.1017/S0007114512004230. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura Y., Nosaka S., Suzuki M., Nagafuchi S., Takahashi T., Yajima T., Takenouchi-Ohkubo N., Iwase T., Moro I. Dietary fructooligosaccharides up-regulate immunoglobulin A res-ponse and polymeric immunoglobulin receptor expression in intestines of infant mice. Clin. Exp. Immunol. 2004;137:52–58. doi: 10.1111/j.1365-2249.2004.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization (WHO)—Diabetes Fact Sheet. [(accessed on 9 May 2016)]. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/
- 50.Qin L., Mirjam J., Knol S., Corpeleijn E., Ronald P. Does physical activity modify the risk of obesity for type 2 diabetes: A review of epidemiological data. Eur. J. Epidemiol. 2010;25:5–12. doi: 10.1007/s10654-009-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tunaiji H.A., Davis J.C., Mackey D.C., Khan K.M. Population attributable fraction of type 2 diabetes due to physical inactivity in adults: A systematic review. BMC Public Health. 2014;14:469. doi: 10.1186/1471-2458-14-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franz M.J., Boucher J.L., Evert A.B. Evidence-based diabetes nutrition therapy recommendations are effective: The key is individualization. Diabetes Metab. Syndr. Obes. 2014;7:65–72. doi: 10.2147/DMSO.S45140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo D., Valentão P., Andrade P.B., Fernandez E.C., Milellal L. Evaluation of Antioxidant, Antidiabetic and Anticholinesterase Activities of Smallanthus sonchifolius Landraces and Correlation with Their Phytochemical Profiles. Int. J. Mol. Sci. 2015;16:17696–17718. doi: 10.3390/ijms160817696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Habib N.C., Honoré S.M., Genta S.B., Sánchez S.S. Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: Biochemical approach. Chem. Biol. Interact. 2011;194:31–39. doi: 10.1016/j.cbi.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Satoh H., Nguyen M.T.A., Kudoh A., Watanabe T. Yacon diet (Smallanthus sonchifolius, Asteraceae) improves hepatic insulin resistance via reducing Trb3 expression in Zucker fa/fa rats. Nutr. Diabetes. 2013 doi: 10.1038/nutd.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheid M.M., Genaro P.S., Moreno Y.M., Pastore G.M. Freeze-dried powdered yacon: Effects of FOS on serum glucose, lipids and intestinal transit in the elderly. Eur. J. Nutr. 2014;53:1457–1464. doi: 10.1007/s00394-013-0648-x. [DOI] [PubMed] [Google Scholar]
- 57.Genta S., Cabrera W., Habib N., Pons J., Carillo I.M., Grau A., Sara S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 2009;28:182–187. doi: 10.1016/j.clnu.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Triplitt C.L. Examining the mechanisms of glucose regulation. Am. J. Manag. Care. 2012;18:S4–10. [PubMed] [Google Scholar]
- 59.López V.L., Medina J.A.L., Gutiérrez M.V., Soto M.L.F. Carbohydrate: Current role in diabetes mellitus and metabolic disease. Nutr. Hosp. 2014;30:1020–1031. doi: 10.3305/nh.2014.30.5.7475. [DOI] [PubMed] [Google Scholar]
- 60.Den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2014;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 62.Fernandes J., Vogt J., Wolever T.M. Intravenous acetate elicits a greater free fatty acid rebound in normal than hyperinsulinaemic humans. Eur. J. Clin. Nutr. 2012;66:1029–1034. doi: 10.1038/ejcn.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boillot J., Alamowitch C., Berger A.M., Luo J., Bruzzo F., Bornet F.R., Slama G. Effects of dietary propionate on hepatic glucose production, whole-body glucose utilization, carbohydrate and lipid metabolism in normal rats. Br. J. Nutr. 1995;73:241–251. doi: 10.1079/BJN19950026. [DOI] [PubMed] [Google Scholar]
- 64.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M, Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Psichas A., Sleeth M.L., Murphy K.G., Brooks L., Bewick G.A., Hanyaloglu A.C., Ghatei M.A., Bloom S.R., Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahima R.S., Lazar M.A. The Health Risk of Obesity—Better Metrics Imperative. Science. 2013;341:6148. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization (WHO)—Obesity and overweight Fact Sheet. [(accessed on 9 May 2016)]. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/
- 68.Lehnert T., Sonntag D., Konnopka A., Riedel-Heller S., König H.H. Economic costs of overweight and obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:105–115. doi: 10.1016/j.beem.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 69.Lifshitz F., Lifshitz J.Z. Globesity: The root causes of the obesity epidemic in the USA and now worldwide. Pediatr. Endocrinol. Rev. 2014;12:17–34. [PubMed] [Google Scholar]
- 70.Kaur J. A Comprehensive Review on Metabolic Syndrome. Cardiol. Res. Pract. 2014 doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Leão L.S., de Moraes M.M., de Carvalho G.X., Koifman R.J. Nutritional interventions in metabolic syndrome: A systematic review. Arq. Bras. Cardiol. 2011;97:260–265. doi: 10.1590/S0066-782X2011001200012. [DOI] [PubMed] [Google Scholar]
- 72.Mohamed S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardio vascular disease. Trends Food Sci. Technol. 2014;35:114–128. doi: 10.1016/j.tifs.2013.11.001. [DOI] [Google Scholar]
- 73.Genta S.B., Cabrera W.M., Grau A., Sánchez S.S. Subchronic 4-month oral toxicity study of dried Smallanthus sonchifolius (yacon) roots as a diet supplement in rats. Food Chem. Toxicol. 2005;43:1657–1665. doi: 10.1016/j.fct.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Roselino M.N., Pauly-Silveira N.D., Cavallini D.C., Celiberto L.S., Pinto R.A., Vendramini R.C., Rossi E.A. A potential synbiotic product improves the lipid profile of diabetic rats. Lipids Health Dis. 2012 doi: 10.1186/1476-511X-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geyer M., Manrique I., Degen L., Beglinger C. Effect of yacon (Smallanthus sonchifolius) on colonic transit time in healthy volunteers. Digestion. 2008;78:30–33. doi: 10.1159/000155214. [DOI] [PubMed] [Google Scholar]
- 76.Mora S., Fullerton R. Effects of Short Chain Fatty Acids on Glucose and Lipid metabolism in Adipocytes. FASEB J. 2015;29:627–625. [Google Scholar]
- 77.Hara H., Haga S., Aoyama Y., Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999;129:942–948. doi: 10.1093/jn/129.5.942. [DOI] [PubMed] [Google Scholar]
- 78.Byrne S., Chambers E.S., Morrison D.J., Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015;39:1331–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Alessio D. Intestinal hormones and regulation of satiety: The case for CCK, GLP-1, PYY, and Apo A-IV. JPEN J. Parenter Enteral Nutr. 2008;32:567–568. doi: 10.1177/0148607108322401. [DOI] [PubMed] [Google Scholar]
- 80.Ichimura A., Hasegawa S., Kasubuchi M., Kimura I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol. 2014;5:236. doi: 10.3389/fphar.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isken F., Klaus S., Osterhoff M., Pfeiffer A.F., Weickert M.O. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J. Nutr. Biochem. 2010;21:278–284. doi: 10.1016/j.jnutbio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 82.Eswaran S., Muir J., Chey W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013;108:718–727. doi: 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- 83.Yun E.Y., Kim H.S., Kim Y.E., Kang M.K., Ma J.E., Lee G.D., Cho Y.J., Kim H.C., Lee J.D., Hwang Y.S., et al. A case of anaphylaxis after the ingestion of yacon. Allergy Asthma. Immunol. Res. 2010;2:149–152. doi: 10.4168/aair.2010.2.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graefea S., Hermann M., Manrique I., Golombeka S., Buerkerta A. Effects of post-harvest treatments on the carbohydrate composition of yacon roots in the Peruvian Andes. F. Cr. Res. 2004;86:157–165. doi: 10.1016/j.fcr.2003.08.003. [DOI] [Google Scholar]
- 85.Di Bartolomeo F., Van den Ende W. Fructose and Fructans: Opposite Effects on Health. Plant Foods Hum. Nutr. 2015;70:227–237. doi: 10.1007/s11130-015-0485-6. [DOI] [PubMed] [Google Scholar]