Abstract
Objectives
To quantitatively assess time-series studies of daily nitrogen dioxide (NO2) and mortality and hospital admissions which also controlled for particulate matter (PM) to determine whether or to what extent the NO2 associations are independent of PM.
Design
A systematic review and meta-analysis.
Methods
Time-series studies—published in peer-reviewed journals worldwide, up to May 2011—that reported both single-pollutant and two-pollutant model estimates for NO2 and PM were ascertained from bibliographic databases (PubMed, EMBASE and Web of Science) and reviews. Random-effects summary estimates were calculated globally and stratified by different geographical regions, and effect modification was investigated.
Outcome measures
Mortality and hospital admissions for various cardiovascular or respiratory diseases in different age groups in the general population.
Results
60 eligible studies were identified, and meta-analysis was conducted on 23 outcomes. Two-pollutant model study estimates generally showed that the NO2 associations were independent of PM mass. For all-cause mortality, a 10 µg/m3 increase in 24-hour NO2 was associated with a 0.78% (95% CI 0.47% to 1.09%) increase in the risk of death, which reduced to 0.60% (0.33% to 0.87%) after control for PM. Heterogeneity between geographical region-specific estimates was removed by control for PM (I2 from 66.9% to 0%). Estimates of PM and daily mortality assembled from the same studies were greatly attenuated after control for NO2: from 0.51% (0.29% to 0.74%) to 0.18% (−0.11% to 0.47%) per 10 µg/m3 PM10 and 0.74% (0.34% to 1.14%) to 0.54% (−0.25% to 1.34%) for PM2.5.
Conclusions
The association between short-term exposure to NO2 and adverse health outcomes is largely independent of PM mass. Further studies should attempt to investigate whether this is a generic PM effect or whether it is modified by the source and physicochemical characteristics of PM. This finding strengthens the argument for NO2 having a causal role in health effects.
Keywords: nitrogen dioxide, time series, mortality, hospital admissions, systematic review, meta-analysis
Strengths and limitations of this study.
This is, to date, the most comprehensive quantitative systematic review of the time-series literature on nitrogen dioxide (NO2) published worldwide to evaluate the two-pollutant model estimates of mortality or hospital admissions and short-term exposure to NO2 adjusted for particulate air pollution.
It reports meta-analytical estimates both globally and for different geographical regions, as well as provides an assessment of heterogeneity between the region-specific estimates.
The protocol-led approach to the identification of studies and estimates for use in meta-analysis minimised selection bias at each stage of the review.
Meta-analysis was limited to studies that provided effect estimates in numerical—rather than graphical—form, along with sufficient quantitative data to enable standardisation of estimates.
Further work is needed to understand reasons for the heterogeneity observed and to quantitatively assess the extent to which PM may be associated with health independently of NO2.
Introduction
Outdoor air pollution has long been established as a hazard to human health, with particulate matter (PM) regarded as the most plausible toxicant in the mixture of ambient air pollutants.1–5 The epidemiological evidence has consistently shown adverse associations between chronic and short-term exposure to PM and mortality and morbidity from cardiovascular and respiratory disease, and this is supported by experimental evidence.6 While the epidemiological evidence also shows relationships between nitrogen dioxide (NO2) and adverse health effects, concerns have been expressed repeatedly about the causal nature of these associations.7–11 It has been asserted that the NO2 associations do not reflect adverse effects of NO2 itself but, rather, reflect the health effects of other air pollutants, mainly PM or other components of the complex mixture of traffic-related air pollutants. Primarily, this is due to the strong correlations between NO2 and other combustion-derived air pollutants, especially PM. The extent of these correlations varies from city-to-city and over time, due to variations in emission sources. Scepticism also exists because of limited experimental evidence (controlled human exposure and animal toxicology studies) for NO2, which, to date, has focused largely on respiratory endpoints and has generally employed concentrations of NO2 well above current ambient levels.7–9 In light of the uncertainties regarding NO2 and the stronger evidence for associations between PM and health, many researchers and policymakers have adopted a view that the epidemiological associations of NO2 reflect adverse health effects of PM.
In an earlier paper, we reviewed the time-series evidence associating daily concentrations of NO2 with daily mortality and emergency hospital admissions.12 In this study, we assess the subset of time-series studies, reporting all-year estimates of NO2 from both single-pollutant and two-pollutant models adjusted for PM to determine whether the NO2 associations are attenuated after adjustment for PM.
Methods
The full method and a priori protocols governing the identification of studies and effect estimates for the systematic review have been described previously,12–14 but a synopsis, along with aspects unique to this review, is provided below.
Identification of studies for review
Three bibliographic databases were searched to identify peer-reviewed time-series studies of NO2 and daily mortality or hospital admissions indexed up to May 2011. No restriction on language was applied. The literature search strategy is described in the online supplementary material, and the following inclusion criteria were used: papers must (1) have had a minimum of 1 year of data; (2) been based on the general population; (3) have controlled for important confounding factors, including season and meteorological factors; and (4) have reported sufficient quantitative information, in numeric format, to enable the calculation of standardised effect estimates and standard errors for use in quantitative analysis. Two authors of the review—ICM and RWA—undertook the literature search.
bmjopen-2015-010751supp.pdf (6MB, pdf)
Data extraction and coding
Data from each relevant study were entered into a Microsoft Access database (Microsoft Office 2010, Microsoft Corporation). These included:
Citation details of each paper;
All-year single-pollutant and two-pollutant model estimates of NO2 adjusted for PM;
Single-pollutant and two-pollutant model estimates of PM adjusted for NO2 reported in studies providing data for NO2;
Season-specific estimates of NO2, including those adjusted for PM, from studies reporting all-year estimates;
Descriptive (outcome, diagnosis (International Classification of Diseases codes), age, etc) and quantitative data (pollution increment and averaging time etc) associated with each estimate, and needed for calculating standardised estimates expressed as the percentage change (and 95% CI) in the mean number of daily events associated with a 10 µg/m3 increase in NO2 (or PM);
Correlations between concentrations of NO2 and PM;
Effect modifiers for investigating of sources of heterogeneity in all-year estimates.
Time-series studies often report results for different time lags (in days) between exposure and health events, and they vary in the lag for the reported results. We identified, for each outcome/disease/age/averaging time combination from each study, a pair of estimates of NO2, that is from a single-pollutant model and a corresponding estimate adjusted for PM for the same lag, to enable comparison of the NO2 association before and after adjustment for PM. To avoid selection bias, we developed an a priori protocol for identifying the principal lag for each outcome/disease/age/averaging time combination for use in our review. This was the lag highlighted by the author or stated a priori, and if this was not clear, because several lagged model estimates were reported, we chose (1) the lag with the highest statistical significance, regardless of the estimate being positive or negative, or (2) the lag with the largest estimate, again, irrespective of its direction. If only results from cumulative or distributed lag models—that is, lags averaged over several days—were reported in a study, these were used. In some instances, a different lag was investigated in two-pollutant models. In such cases, the lagged estimate from the two-pollutant model was coded according to the same algorithm and the (additional) corresponding single-pollutant estimate for the same lag was coded in our database.
Processing of data also included classifying each study into the geographical region, as the WHO region, in which the study was conducted, as well as categorising the various metrics of PM controlled for in two-pollutant models: see online supplementary material for details.
Statistical analyses
A similar procedure to that outlined in our earlier paper was used for meta-analysis,12 but with some modifications, in order to identify a pair of estimates of NO2 for each pollutant/outcome combination from each study. We applied an a priori protocol to select estimates for meta-analysis to avoid selection bias and duplication of studies from the same population. We gave priority to estimates from multicity studies over estimates from single-city studies and the results from any one city appeared only once in a meta-analysis. If results from more than one multicity study within a WHO region were available, we selected, in order of priority, the multicity estimate from the study: (1) with the most cities/greatest geographical coverage; (2) the most recently published; (3) the most recent study time period. If a multicity study did not report a summary estimate across the cities examined, for analysis, we treated estimates from these studies in the same manner as estimates from single-city studies. We selected estimates from single-city studies only if they did not appear in multicity studies. For cities not included in a multicity study summary result, we selected, in order of priority: (1) the most recently published, or (2) the most recent study time period.
Meta-analysis was conducted when ≥4 estimates were available for an outcome/disease/age/averaging time combination—including where a multicity estimate was available—and summary estimates were calculated using a random-effects model.15 We used a staged approach to meta-analysis, with single-city estimates pooled within WHO region prior to the pooled single-city and selected multicity estimates being pooled to produce a global estimate and WHO region-specific summary estimates. Heterogeneity between WHO region summary estimates was assessed using the I2 statistic,16 with I2 statistics >50% regarded as being evidence of high heterogeneity.17
Meta-analysis was undertaken for:
Single-pollutant NO2 estimates relating to two-pollutant models;
- Corresponding NO2 estimates adjusted for any PM metric:
- if within a study, several estimates of NO2 adjusted for different individual PM metrics were available, a NO2 estimate was selected according to the following order of priority of PM metric used in adjustment: PM10, PM2.5, Black Smoke, PM10−2.5;
- if, having applied the protocol, a NO2 estimate was not selected for a city because several were available due to different PM metrics used to adjust the NO2 effect in different studies, the NO2 estimate was chosen in the order of priority of the PM metrics listed above.
We conducted additional meta-analyses for NO2 adjusted for specific metrics of particles, for example, NO2 adjusted for PM10 and separately for PM2.5, and so on, to determine whether the NO2 associations showed different sensitivity to control for different PM metrics.
All analyses were conducted in STATA (STATA/SE V.11. StataCorp, Texas, USA).
Results
Sixty studies provided estimates of both (1) NO2, single-pollutant, and (2) NO2 adjusted for PM: a list of references is provided in the online supplementary material. Table 1 presents a summary of these 60 time-series studies stratified by the PM metric controlled for in regression models, broad disease categories, WHO regions in which the studies were conducted, single-city and multicity study designs, and by averaging time (24-hour and 1 hour).
Table 1.
Summary of time-series studies of daily mortality or hospital admissions and NO2 adjusted for PM
| Total |
Multicity study |
Single-city study |
||||
|---|---|---|---|---|---|---|
| Outcome | Mortality | Hospital admissions | Mortality | Hospital admissions | Mortality | Hospital admissions |
| Total | 36 | 24 | 9 | 4 | 27 | 20 |
| NO2+PM* | ||||||
| PM10 | 23 | 17 | 6 | 2 | 17 | 15 |
| PM2.5 | 7 | 1 | 3 | 1 | 4 | 0 |
| PM10–2.5 | 4 | 0 | 3 | 0 | 1 | 0 |
| BS | 5 | 4 | 3 | 2 | 2 | 2 |
| PNC | 3 | 1 | 0 | 0 | 3 | 1 |
| Carbon | 0 | 0 | 0 | 0 | 0 | 0 |
| TSP | 4 | 2 | 0 | 1 | 4 | 1 |
| Visibility | 2 | 1 | 2 | 1 | 0 | 0 |
| >1 PM metric | 0 | 1 | 0 | 0 | 0 | 1 |
| Disease† | ||||||
| All-cause | 27 | 1 | 7 | 0 | 20 | 1 |
| Cardiovascular | 17 | 11 | 4 | 2 | 13 | 9 |
| Respiratory | 7 | 17 | 3 | 3 | 4 | 14 |
| WHO region‡ | ||||||
| American A | 8 | 4 | 3 | 0 | 5 | 4 |
| European A | 9 | 12 | 3 | 2 | 6 | 10 |
| Western Pacific B | 14 | 5 | 2 | 0 | 12 | 5 |
| American B | 4 | 2 | 0 | 0 | 4 | 2 |
| Western Pacific A | 1 | 2 | 1 | 2 | 0 | 0 |
| South East Asia B | 2 | 0 | 2 | 0 | 0 | 0 |
| Averaging time | ||||||
| 24 hour | 29 | 21 | 6 | 3 | 23 | 18 |
| Maximum 1 hour | 7 | 5 | 3 | 2 | 4 | 3 |
*The eight categories of PM metrics listed in the table above have been generated by grouping different measures of particles. PM10 and PM2.5 refer to the mass per cubic metre of particles of generally <10 μm and 2.5 μm diameter, respectively, in the ambient air.
†Respiratory includes all-respiratory diseases, asthma, COPD, COPD including asthma, lower respiratory infections and upper respiratory diseases; Cardiovascular includes all-cardiovascular diseases, cardiac disease, heart failure, ischaemic heart disease, dysrhythmia and stroke.
‡WHO regions: (A) very low child and adult mortality; (B) low child mortality and low adult mortality; (C) low child mortality and high adult mortality; (D) high child mortality and high adult mortality.
BS, Black Smoke; COPD, chronic obstructive pulmonary disease; PM, particulate matter; PNC, particle number concentration; TSP, total suspended particles.
There were 36 and 24 studies of daily mortality or hospital admissions, respectively, and 13 studies used a multicity design. The majority of the studies were conducted in the WHO regions European A and Western Pacific region B, and most used 24-hour NO2. Forty of the 60 studies controlled for the effects of daily PM10 in the regression models for NO2, and a much smaller number of studies used other particle size fractions or constituents of PM. Eight studies of mortality and two of hospital admissions reported estimates of NO2, each adjusted for a different PM metric. None of the studies investigated the influence of carbon on the NO2 associations, and four studies controlled for the effects of ultrafine particles.
NO2 and all-cause mortality
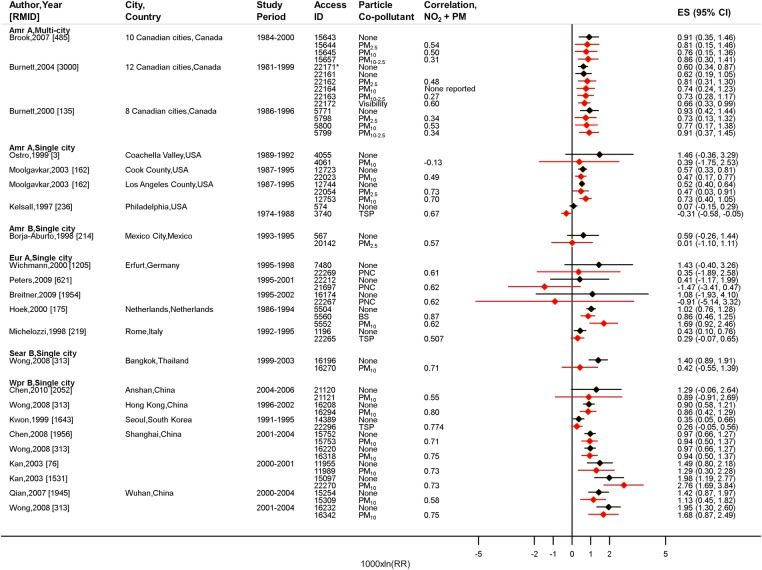
Figure 1 shows all available (32 pairs) single-pollutant and two-pollutant estimates for 24-hour NO2 and daily all-cause mortality in all ages. In the majority of studies, daily NO2 was positively and significantly associated with increases in the risk of death, including after controlling for daily PM. In many of the studies, the NO2 estimates were not greatly reduced in size, changed direction or lose statistical significance after adjustment for PM. In general, the NO2 estimates appeared robust to adjustment for PM at both high and low correlations between concentrations of NO2 and PM.
Figure 1.
All available studies providing two-pollutant model estimates for meta-analysis for all-cause mortality, all ages, 24-hour NO2. 1000×ln (RR) approximates to a percentage change per 10 µg/m3. *Single-pollutant model estimate for days with both NO2 and visibility (coefficient of haze, COH) data in Burnett et al,18 [RMID 3000].
 NO2, single-pollutant
NO2, single-pollutant  NO2 adjusted for PM.
NO2 adjusted for PM.
Fifteen (of 32) pairs of estimates for 24-hour NO2 and all-cause mortality, which represented 26 cities from five WHO regions, were selected for meta-analysis (see online supplementary figure S1). The random-effects single-pollutant summary estimate for all-cause mortality was 0.78% (95% CI 0.47% to 1.09%) per 10 µg/m3 increase in NO2. There was evidence of high heterogeneity (I2=66.9%) between the WHO region-specific estimates, which ranged from 0.48% for WHO region America A to 1.41% for South East Asia B (see online supplementary table S1). The overall estimate was comparable to the single-pollutant summary estimate of 0.71% (95% CI 0.43% to 1.00%) calculated from the larger body of time-series evidence analysed in our previous paper.12 After adjustment for daily PM, all-cause mortality remained positively and significantly associated with 24-hour NO2: 0.60% (95% CI 0.33% to 0.87%) per 10 µg/m3 increase in NO2, and there was no evidence of heterogeneity (I2=0%) between the region-specific estimates.
Control for specific PM metrics did not greatly alter the relationship of 24-hour NO2 with all-cause mortality (table 2). With the exception of NO2 adjusted for PM10, and to a lesser extent PM2.5, meta-analyses for NO2 adjusted for the remaining PM metrics were limited to findings from a multicity Canadian study by Burnett et al18—see figure 1.
Table 2.
Random-effects summary estimates (as percentage change (95% CIs)) for mortality or hospital admissions associated with a 10 µg/m3 increase 24 hour average pollution
| All SC/MC* |
Selected SC/MC (cities)† |
24-hour NO2 |
24-hour PM |
|||
|---|---|---|---|---|---|---|
| Single-pollutant | Adjusted for PM | Single-pollutant | Adjusted for NO2 | |||
| All-cause mortality, all ages | ||||||
| PM10 | 13/3 | 4/1 (21) | 0.92 (0.58 to 1.72) | 0.85 (0.52 to 1.18) | 0.51 (0.29 to 0.74) | 0.18 (−0.11 to 0.47) |
| PM2.5 | 2/3 | 2/1 (14) | 0.53 (0.42 to 0.64) | 0.57 (0.24 to 0.89) | 0.74 (0.34 to 1.14) | 0.54 (−0.25 to 1.34) |
| PM10–2.5 | 0/3 | 0/1 (12) | 0.62 (0.19 to 1.06) | 0.73 (0.28 to 1.18) | 0.65 (−0.10 to 1.42) | 0.31 (−0.49 to 1.11) |
| Visibility | 0/1 | 0/1 (12) | 0.60 (0.34 to 0.87) | 0.66 (0.33 to 1.00) | 40.93 (23.39 to 60.97)‡ | 12.42 (−4.47 to 32.29)‡ |
| All cardiovascular mortality, all ages | ||||||
| PM10 | 10/0 | 4/0 (8) | 0.99 (0.49 to 1.49) | 0.87 (0.28 to 1.46) | 0.48 (0.18 to 0.78) | 0.19 (−0.21 to 0.59) |
| All respiratory mortality, all ages | ||||||
| PM10 | 7/0 | 2/0 (5) | 1.44 (0.63 to 2.27) | 1.15 (0.47 to 1.84) | 0.58 (0.22 to 0.93) | 0.13 (−0.18 to 0.44) |
| All respiratory hospital admissions, children (5–14 years) | ||||||
| PM10 | 0/1 | 0/1 (5) | 5.95 (1.74 to 10.33) | 6.56 (3.08 to 10.17) | – | – |
| Cardiac hospital admissions, all ages | ||||||
| PM10 | 2/1 | 2/1 (7) | 0.93 (0.46 to 1.40) | 0.75 (−0.13 to 1.64) | – | – |
| BS | 0/1 | 0/1 (4) | 0.68 (0.17 to 1.20) | 0.36 (−0.65 to 1.38) | – | – |
| TSP | 0/1 | 0/1 (6) | 1.03 (0.45 to 1.61) | 1.08 (0.43 to 1.72) | – | – |
*Numbers of available pairs of single-city (SC)/multi-city (MC) estimates from all studies.
†Numbers of pairs of pooled (from single-city estimates) and multicity estimates used to calculate the overall summary estimate across WHO regions. Estimates were selected for meta-analysis from all those available. The number of cities represented by the summary estimates is given in brackets.
‡The results for visibility (measured as coefficient of haze (COH units)) are not comparable to other PM results.
BS. Black Smoke; NO2, nitrogen dioxide; PM, particulate matter.
Six pairs of estimates were available for meta-analysis for all-cause mortality and 1 hour NO2 adjusted for PM (see online supplementary figure S2). Thirty of the 36 cities represented by these estimates were in Europe. Meta-analysis of four pairs of estimates resulted in an overall estimate of 0.32% (95% CI −0.02% to 0.66%) for a 10 µg/m3 increment in 1 hour NO2 and 0.20% (95% CI −0.24% to 0.65%) following adjustment for PM (see online supplementary table S2). High heterogeneity was observed between the WHO region-specific estimates. In contrast with findings for 24-hour measures, the summary estimate for 1 hour NO2 for WHO region European A was little affected by adjustment for PM10 (or Black Smoke)—see online supplementary table S2. Table 3 provides meta-analysis results for all-cause mortality and 1 hour NO2 adjusted for different PM metrics. Control for PM10 led to attenuation of the estimate and loss of statistical significance, while the association was robust to control for Black Smoke and visibility (measured as black suspended particles, BSP).
Table 3.
Random-effects summary estimates (as percentage change (95% CIs)) for mortality or hospital admissions associated with a 10 µg/m3 increase in air pollution
| All SC/MC* |
Selected SC/MC (cities)† |
1 hour NO2 |
24-hour PM |
|||
|---|---|---|---|---|---|---|
| Single-pollutant | Adjusted for PM | Single-pollutant | Adjusted for NO2 | |||
| All-cause mortality, all ages | ||||||
| PM10 | 2/1 | 2/1 (32) | 0.22 (−0.15 to 0.60) | 0.10 (−0.40 to 0.61) | 0.52 (0.29 to 0.75) | 0.48 (0.31 to 0.66) |
| BS | 0/2 | 0/1 (30) | 0.30 (0.22 to 0.38) | 0.33 (0.23 to 0.43) | 0.60 (0.30 to 0.90) | 0.26 (0.00 to 0.52) |
| Visibility | 0/1 | 0/1 (4) | 0.63 (0.21 to 1.05) | 0.52 (0.05 to 1.00) | 35.70 (3.97 to 77.12)‡ | 10.24 (−20.03 to 51.97)‡ |
| All cardiovascular mortality, all ages | ||||||
| PM10 | 1/1 | 0/1 (29) | 0.40 (0.29 to 0.51) | 0.35 (0.21 to 0.49) | 0.76 (0.47 to 1.05) | 0.32 (0.05 to 0.59) |
| BS | 1/1 | 0/1 (29) | 0.40 (0.29 to 0.51) | 0.44 (0.31 to 0.57) | 0.62 (0.35 to 0.90) | 0.17 (−0.10 to 0.44) |
| All respiratory mortality, all ages | ||||||
| PM10 | 0/1 | 0/1 (29) | 0.38 (0.17 to 0.59) | 0.37 (0.08 to 0.66) | 0.71 (0.22 to 1.20) | 0.20 (−0.29 to 0.69) |
| BS | 0/1 | 0/1 (29) | 0.38 (0.17 to 0.59) | 0.26 (−0.12 to 0.64) | 0.84 (0.11 to 1.58) | 0.57 (−0.34 to 1.48) |
| All respiratory hospital admissions, children (<5 years) | ||||||
| PM10 | 1/1 | 1/1 (6) | 0.77 (−0.59 to 2.15) | 0.13 (−0.09 to 0.35) | – | – |
| PM2.5 | 0/1 | 0/1 (4) | 1.62 (0.41 to 2.84) | 4.85 (0.41 to 9.50) | – | – |
| All respiratory hospital admissions, elderly (65+years) | ||||||
| Visibility | 0/1 | 0/1 (4) | 1.42 (0.79 to 2.06) | 1.21 (0.47 to 1.95) | – | – |
| Cardiac hospital admissions, elderly | ||||||
| Visibility | 0/1 | 0/1 (4) | 1.21 (0.84 to 1.58) | 0.73 (0.31 to 1.16) | – | – |
*Numbers of available pairs of single-city (SC)/multi-city (MC) estimates from all studies.
†Numbers of pairs of pooled (from single-city estimates) and multicity estimates used to calculate the overall summary estimate across WHO regions. Estimates were selected for meta-analysis from all those available. The number of cities represented by the summary estimates is given in brackets.
‡The results for visibility (measured as black suspended particles (10−4/m)) are not comparable to other PM results.
BS, Black Smoke; NO2, nitrogen dioxide; PM, particulate matter.
NO2 and mortality from specific causes
NO2 estimates adjusted for PM were available for several specific causes of death in all ages: all cardiovascular (see online supplementary figures S3 and S4), all respiratory (see online supplementary figure S5), stroke (see online supplementary figure S6), cardiac (see online supplementary figure S7), ischaemic heart disease, dysrhythmia, chronic obstructive pulmonary disease including asthma and lower respiratory infections (see online supplementary figure S8). Sufficient numbers of estimates for meta-analysis were available for all cardiovascular (see online supplementary table S3), all respiratory (see online supplementary table S4) and stroke (see online supplementary table S5) mortality.
Eight studies providing 14 pairs of estimates showed positive associations between all cardiovascular deaths and 24-hour NO2, including after adjustment mainly for PM10 (see online supplementary figure S3). However, attenuation of estimates and loss of statistical significance was observed in the few studies with control for PM2.5 or Black Smoke. Meta-analysis of 10 pairs of estimates found a 1.07% (95% CI 0.43% to 1.72%) increase in the risk of death from all cardiovascular diseases per 10 µg/m3 increase in 24-hour NO2 (see online supplementary table S3 and figure S9). This was attenuated (0.82% (95% CI 0.22% to 1.42%))—see online supplementary table S3—following adjustment for PM, but comparable to our earlier result (0.88% (95% CI 0.63% to 1.13%)).12 Control of the NO2 association with all cardiovascular mortality for specific PM metrics showed an association that was robust to adjustment for PM10 (table 2). There were too few estimates to permit meta-analysis for other PM metrics controlled for in the studies. The available data for 1 hour NO2 and all cardiovascular mortality were sparse and limited to two studies representing 29 European cities that showed positive NO2 associations that were robust to adjustment for both PM10 and Black Smoke (see table 3 and online supplementary figure S4).
Evidence for all respiratory mortality and 24-hour NO2 adjusted for PM came from six cities (see online supplementary figure S5). Meta-analysis produced a 1.42% (95% CI 0.64% to 2.21%) increased risk of all respiratory deaths per 10 µg/m3 increase in 24-hour NO2 (see online supplementary table S4 and figure S10). The corresponding estimate adjusted for particles was attenuated (1.13% (95% CI 0.46% to 1.81%)) but was comparable to the single-pollutant estimate (1.09% (95% CI 0.75% to 1.42%)) derived from the larger body of time-series evidence examined in our previous paper.12 There was no evidence of heterogeneity (I2=0%) between the geographic specific estimates either before or after adjustment for PM (see online supplementary table S4). Evidence for associations between all respiratory mortality and 1 hour NO2 came solely from the multicity APHEA II study of 29 European cities,19 which showed a positive association that was robust to adjustment for PM10 but not Black Smoke (table 3).
PM and mortality
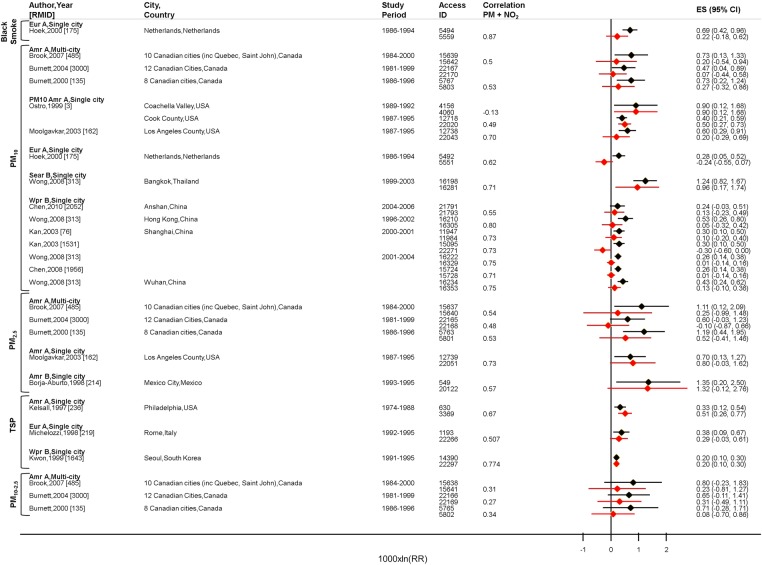
Meta-analyses were undertaken separately for PM adjusted for the different averaging times of NO2 to allow comparison with the relevant meta-analyses for NO2, using data from the same studies, cities and time periods. Figure 2 shows positive, single-pollutant associations between various mass metrics of PM and all-cause mortality. In the majority of studies, attenuation of estimates was observed following control for 24-hour NO2. Estimates for ultrafine particles and all-cause mortality were robust to adjustment for 24-hour NO2 (see online supplementary figure S11), but the data came from three studies conducted in the same city—Erfurt, Germany. Results of meta-analysis for all-cause mortality and PM metrics are shown in tables 2 and 3 for adjustment for 24-hour and 1 hour NO2, respectively. In contrast to the results for NO2, the summary estimates for PM were attenuated, in most cases by more than half, and CIs overlapped zero. Evidence of high heterogeneity between region-specific summary estimates for PM10 and all-cause mortality was identified (see online supplementary table S6). Summary estimates for deaths from all cardiovascular or all respiratory diseases and PM were also sensitive to control for NO2 (see tables 2 and 3; study estimates in online supplementary figures S12, S13, tables S7 and S8 for region-specific results).
Figure 2.
All studies providing two-pollutant model estimates for all-cause mortality, all ages, PM adjusted for 24-hour NO2. PM, particulate matter.  PM, single-pollutant
PM, single-pollutant  PM adjusted for NO2.
PM adjusted for NO2.
NO2 and hospital admissions
Few cause-specific and age-specific combinations of hospital admissions for 24-hour or 1 hour NO2 with control for PM had sufficient numbers of estimates for meta-analysis—all respiratory diseases in children and the elderly, asthma in children, and cardiac disease in all ages and the elderly—and half were based solely on a multicity estimate from a single study.
Positive associations were identified between all respiratory hospital admissions in different age groups and 24-hour or 1 hour NO2, which remained after control for PM (see tables 2 and 3; online supplementary figures S14 and S15 for available study estimates).
Evidence for the association between hospitalisation for asthma in different ages and daily NO2 adjusted for PM came from seven studies (see online supplementary figures S16 and S17), six of which were conducted in Europe. Sufficient estimates for meta-analysis were only available for asthma admissions in children and 24-hour NO2 adjusted for any particles (measured as Black Smoke, PM10 and PNC): a 2.81% (95% CI −1.28% to 7.06%) increase in risk per 10 µg/m3 24-hour NO2 was attenuated following adjustment for particles (2.24% (95% CI −1.12% to 5.71%)).
Five studies provided evidence for the relationship between 24-hour NO2 adjusted for PM and hospitalisation for cardiac disease in all ages (see online supplementary figure S18) and the elderly (see online supplementary figure S19). Meta-analysis for the all age category (table 2) identified positive estimates that were attenuated and CIs overlapped zero after controlling for PM10 and Black Smoke. One multicity study of four Australian cities provided evidence for the relationship between 1 hour NO2 and cardiac admissions in the elderly. The association (1.21% (95% CI 0.84% to 1.58%)) was weakened by control for BSP (an indicator of fine particles), but remained statistically significant (0.73% (95% CI 0.31% to 1.16%)).
Sources of variation in NO2 estimates
We examined season-specific NO2 estimates of mortality from studies that reported all-year estimates to explore possible effect modification by season. Some studies, mainly from Western Europe, Canada and the USA, reported stronger associations between daily mortality and NO2 in the summer months (see online supplementary figures S20–S22). The extent of the correlations between concentrations of NO2 and PM in the different seasons is unclear because very few studies reported these data, and only one study reported season-specific estimates adjusted for PM. Similarly, limited evidence is available on which to base an assessment of seasonal variation of associations between hospitalisation for cardiovascular and respiratory diseases and 24-hour NO2 (see online supplementary figure S23).
We explored reasons for the observed high heterogeneity by ranking study estimates for all-cause mortality and 24-hour NO2 (from the full data set)12 by different potential effect modifiers (see online supplementary figures S24–S27). None of the variables used to represent the pollution and meteorological environments in the cities examined accounted for the observed between-study variability.
Discussion
Sixty time-series studies of NO2 were used to determine whether NO2 is associated with daily mortality or hospital admissions independently of daily PM. In general, our results demonstrate that after controlling for PM, daily NO2 remained significantly associated with increases in the risk of adverse health outcomes. The evidence appears clearest for daily deaths from all causes and from all cardiovascular and all respiratory diseases, and for all respiratory hospital admissions, outcomes for which more co-pollutant estimates were available. Robustness of the NO2 associations to control for PM was observed at both high and low correlations between NO2 and PM, and no clear relationship could be discerned between the correlations and changes in the size of the adjusted NO2 estimates. In contrast to the results for NO2, the associations between daily PM and the main mortality outcomes (all cause, all cardiovascular, all respiratory) were very sensitive to the inclusion of NO2 in two-pollutant models.
Two/multipollutant models are increasingly being used to draw conclusions about whether or not NO2 is independently associated with adverse health outcomes. This comprehensive review provides systematic evaluation and formal meta-analysis of the full body of two-pollutant estimates of NO2 adjusted for PM, across several cause-specific and age-specific health outcomes, both globally and by different geographical regions. While earlier reviews7–8 13 20–23 included some assessment of these data, they were either limited in scope to specific health outcomes, and/or examined two-pollutant and multipollutant model NO2 estimates together, or did not undertake meta-analysis whatsoever. Another key strength of this review is the protocol-led approach to identifying and assembling studies and estimates, which aimed to minimise selection bias in the different stages of the review.
The subset of studies of NO2 analysed in this paper were generally comparable to the studies examined in our earlier paper in terms of the magnitudes of summary estimates and overlap in CIs.12 For example, the single-pollutant summary estimates for all-cause mortality, the outcome with the most data, were similar across both data sets, suggesting that the studies reporting two-pollutant model estimates were typical of the wider body of time-series evidence of NO2.
While evidence of NO2 associations which are robust to control for PM mass has been identified, it is possible that there may be some residual confounding by PM. The components of PM—primary combustion particles, for example, ultrafine particles or Black Carbon—which have been proposed as the real causal agents of the NO2 associations, were not included in co-pollutant models of NO2 because concentration data for these pollutants were either unavailable or sparse, reflecting the fact that these PM metrics are not routinely measured. PM10 was by far the most used metric— in 67% of the studies. Summary estimates of NO2 were generally robust to adjustment for PM10. However, PM10 may not adequately reflect the toxic component of PM because it reflects a number of sources that do not include combustion/traffic and that, are not shared with NO2. Where the data permitted meta-analysis, robustness of the NO2 associations to adjustment for PM2.5 and Black Smoke was observed. Few data were available to permit an assessment of the extent to which the NO2 associations are sensitive to control for combustion-derived particles such as Black Carbon or ultrafine particles. This has also been noted by others.7–8 24
Given that the sources and composition of PM vary by location, and hence its toxicity, it cannot be assumed that PM represents the same thing in each study (city/country). In view of the differential toxicity of PM, it is preferable to examine individual studies that used more than one particle metric to investigate possible confounding of the NO2 associations by PM when answering the research question, because they ‘tested’ the robustness of the NO2 associations to different fractions/components of the ambient aerosol in the same location. Unfortunately, such studies were few in number (8), but their findings support the view that the associations of NO2 with major health outcomes are robust to adjustment for PM measured in different ways.
We observed confounding of the associations between daily PM and mortality outcomes by NO2. This suggests that NO2, rather than the PM metrics examined, is a better predictor of the observed mortality effects in the cities examined. An alternative interpretation may be that daily variation in NO2 in the cities better represents the mortality effects of daily variations in the complex urban air pollution mixture or an unknown toxic entity than the metrics of PM used in the analyses. Some caution is, however, needed in drawing conclusions about the analysis of PM estimates because it only reflects a subset of the available studies on PM. Whether the results are a feature of the subset of studies examined is unclear, and formal meta-analysis of the full body of PM estimates, similar to the current review, is warranted. This may provide further insights into whether the different fractions/components of PM might show different sensitivity to adjustment for NO2.
Our results for PM are in contrast with the predominant views in the literature: although confounding of the PM-mortality associations by NO2 has been observed in some time-series studies25–27 and noted in reviews,6 the general consensus is that the PM-mortality estimates are robust to adjustment for co-pollutants.6 The associations have been regarded as reflecting a causal relationship, and experimental evidence has been used to support this. There is a lack of experimental evidence for NO2 at current ambient concentrations and for cardiovascular endpoints, and this has contributed to uncertainty regarding whether NO2 is causally related to health.
We also found evidence of high heterogeneity between the geographic specific summary estimates of NO2, which suggests that it cannot be assumed that the results for one city (region) represent the results for all cities (regions). For all-cause mortality and 24-hour NO2, the high heterogeneity between WHO region-specific estimates was completely removed after control for PM (I2 from 66.9% to 0%), suggesting that some study estimates were a bit extreme in comparison with others in the meta-analysis, but were less so after adjustment for PM. Geographical variation in effect estimates may be due to variations in population characteristics and in pollution sources, mixtures and ambient concentrations. However, none of the variables used to represent the pollution and meteorological environments in the cities examined accounted for the high between-study variability we observed. Further work is therefore required to investigate potential explanations for the heterogeneity.
Results from the studies published since our literature search cut-off are summarised and discussed in the online supplementary appendix 1. The studies indicate that, in general, the associations between NO2 and mortality and hospital admissions remain after control for PM. This is in keeping with the findings set out in this paper.
In addition to the issue of confounding, studies have examined the potential for factors (eg, season, socioeconomic status, age, etc) to modify the relationship between daily NO2 and mortality or hospital admissions. Few studies have, however, examined modification of the associations of NO2 with health by particulate air pollution. The available evidence suggests that the size of an NO2 association may be dependent on concentrations of PM10.25 However, studies have also observed the potential for daily NO2 to modify the relationship between PM and mortality.28 The few available data on this issue come largely from the USA and Europe, but interaction between NO2 and PM (on cardiac hospitalisation) has also been observed in Hong Kong.29 Further research on this aspect of the NO2–PM issue is needed.
Our review supports the conclusions of recent narrative reviews,7 8 but also provides meta-analytical estimates based on two-pollutant model estimates of NO2 from the worldwide data. Taken together with the recent quantitative reviews of cohort studies on long-term exposure to NO2 and mortality,30 31 and of short-term exposure to NO2 and respiratory symptoms in children with asthma from panel studies,8 32 the evidence suggests a need for re-evaluation of the approach to health risk assessment (hazard identification and health impact assessment) for air pollution, an activity that has long been dominated by PM.33 The current review suggests that the relationship between temporal variations in PM and mortality may not be as robust to control for NO2 as previously thought. We note also that attenuation of PM–mortality estimates following control for NO2 has been observed in long-term exposure studies.34 35 These findings could have implications for the calculation of health impacts attributable to these pollutants and for possible double counting of effects.
In summary, we identified evidence of associations between NO2 and adverse health outcomes that are independent of PM mass. However, there was limited evidence on adjustment of the NO2 associations for primary combustion particles that are thought to be responsible for the NO2 associations. Therefore, some uncertainty remains regarding possible confounding and health impact assessments should reflect this.
Acknowledgments
We wish to thank the authors of peer-reviewed papers who responded to our requests for study specific results.
Footnotes
Contributors: ICM, RWA, HRA, RLM and DPS contributed to the design of the study and to the drafting of the paper, and have seen and approved the final version. ICM and RWA undertook the literature search. ICM read all papers, checked data prior to meta-analysis and carried out all analyses. RWA and ICM produced the statistical code in STATA, used by ICM in the analyses. ICM is responsible for the overall content as lead author of the paper.
Funding: This work was supported by Public Health England (formerly Health Protection Agency).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Schwartz J. Particulate air pollution and daily mortality: a synthesis. Public Health Rev 1991. –1992;19:39–60. [PubMed] [Google Scholar]
- 2.Schwartz J. Air pollution and daily mortality: a review and meta-analysis. Environ Res 1994;64:36–52. 10.1006/enrs.1994.1005 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manage Assoc 1996;46:927–39. 10.1080/10473289.1996.10467528 [DOI] [PubMed] [Google Scholar]
- 4.Lippmann M. Human health risks of airborne particles: historical perspective. In: Schneider T, ed. Air pollution in the 21st century—priority issues and policy. Studies in environmental science. Vol 72 The Netherlands: Elsevier, 1998:49–85. [Google Scholar]
- 5.Anderson HR. Air pollution and mortality: a history. Atmos Environ 2009;43:142–52. 10.1016/j.atmosenv.2008.09.026 [DOI] [Google Scholar]
- 6.U.S. EPA. Final report: Integrated Science Assessment for Particulate Matter. Washington DC: U.S. Environmental Protection Agency, EPA/600/R-08/139F, 2009. http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=216546&CFID=39659091&CFTOKEN=38401757 (accessed Dec 2015). [PubMed] [Google Scholar]
- 7.U.S. EPA. Integrated Science Assessment for Oxides of Nitrogen—Health Criteria (Second External Review Draft, 2015). Washington DC: U.S. Environmental Protection Agency, EPA/600/R-14/006. http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=288043 (accessed Dec 2015). [Google Scholar]
- 8.World Health Organization (WHO) Regional Office for Europe. Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project: final technical Report. 2013. http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2013/review-of-evidence-on-health-aspects-of-air-pollution-revihaap-project-final-technical-report, (accessed Dec 2015). [PubMed]
- 9.Health Protection Agency (HPA). Report of a Workshop to Identify Needs for Research on the Health Effects of Nitrogen Dioxide—London, 2–3 March 2011. HPA-CRCE-026 2011. http://www.hpa.org.uk/Publications/Radiation/CRCEScientificAndTechnicalReportSeries/HPACRCE026/ (accessed Dec 2015).
- 10.Committee on the Medical Effects of Air Pollutants (COMEAP). Statement and supporting papers on Quantification of the Effects of Long-term Exposure to Nitrogen Dioxide on Respiratory Morbidity in Children. 2009. http://webarchive.nationalarchives.gov.uk/20140505104658/http://www.comeap.org.uk/documents/statements/39-page/linking/86-quantification-of-the-effects-of-long-term-exposure-to-nitrogen-dioxide (accessed Dec 2015).
- 11.Seaton A, Dennekamp M. Hypothesis: ill health associated with low concentrations of nitrogen dioxide—an effect of ultrafine particles? Thorax 2003;58:1012–15. 10.1136/thorax.58.12.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills IC, Atkinson RW, Kang S et al. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open 2015;5:e006946 10.1136/bmjopen-2014-006946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson HR, Atkinson RW, Bremner SA et al. Quantitative Systematic Review of Short Term Associations Between Ambient Air Pollution (Particulate Matter, Ozone, Nitrogen Dioxide, Sulphur Dioxide and Carbon Monoxide), and Mortality and Morbidity. Report to the United Kingdom Department of Health, 2007. https://www.gov.uk/government/publications/quantitative-systematic-review-of-short-term-associations-between-ambient-air-pollution-particulate-matter-ozone-nitrogen-dioxide-sulphur-dioxide-and-carbon-monoxide-and-mortality-and-morbidity (accessed Jun 2015). [Google Scholar]
- 14.Atkinson RW, Kang S, Anderson HR et al. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 2014;69:660–5. 10.1136/thoraxjnl-2013-204492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSmionian R, Liard N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 16.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F et al. Assessing heterogeneity in meta-analysis: Q Statistic or I2 Index? Psychol Methods 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2015. http://handbook.cochrane.org/ (accessed Apr 2015). [Google Scholar]
- 18.Burnett RT, Stieb D, Brook JR et al. Associations between short-term changes in nitrogen dioxide and mortality in Canadian cities. Arch Environ Health 2004;59:228–36. 10.3200/AEOH.59.5.228-236 [DOI] [PubMed] [Google Scholar]
- 19.Samoli E, Aga E, Touloumi G et al. Short-term effects of nitrogen dioxide on mortality: an analysis within the APHEA project. Eur Respir J 2006;27:1129–38. 10.1183/09031936.06.00143905 [DOI] [PubMed] [Google Scholar]
- 20.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc 2002;52:470–84. 10.1080/10473289.2002.10470794 [DOI] [PubMed] [Google Scholar]
- 21.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: update in relation to the use of generalized additive models. J Air Waste Manag Assoc 2003;53:258–61. 10.1080/10473289.2003.10466149 [DOI] [PubMed] [Google Scholar]
- 22.Committee on the Medical Effects of Air Pollutants (COMEAP). Cardiovascular Disease and Air Pollution. 2006. http://www.gov.uk/government/collections/comeap-reports (accessed Nov 2015).
- 23.U.S. EPA. Integrated Science Assessment for Oxides of Nitrogen—Health Criteria (Final Report). Washington DC: U.S. Environmental Protection Agency, EPA/600/R-08/071, 2008. http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=194645, (accessed Dec 2015). [Google Scholar]
- 24.Clean Air Scientific Advisory Committee (CASAC). Review of the EPA's Integrated Science Assessment for Oxides of Nitrogen—Health Criteria (First External Review Draft—November 2013). http://yosemite.epa.gov/sab/sabproduct.nsf/15E4619D3CD3409A85257CF30069387D/$File/EPA-CASAC-14-002+unsigned.pdf, (accessed Apr 2015).
- 25.Katsouyanni K, Touloumi G, Samoli E et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology 2001;12:521–31. [DOI] [PubMed] [Google Scholar]
- 26.Wong CM, Vichit-Vadakan N, Kan H et al. Public health and air pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect 2008;116: 1195–202. 10.1289/ehp.11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brook JR, Burnett RT, Dann TF et al. Further interpretation of the acute effect of nitrogen dioxide observed in Canadian time-series studies. J Expo Sci Environ Epidemiol 2007;17(Suppl 2):S36–44. 10.1038/sj.jes.7500626 [DOI] [PubMed] [Google Scholar]
- 28.Katsouyanni K, Samet J, Anderson HR et al. Air Pollution and Health: a European and North American Approach (APHENA). HEI Research Report 142 Boston, MA: Health Effects Institute, 2009. [PubMed] [Google Scholar]
- 29.Yu IT, Qiu H, Wang X et al. Synergy between particles and nitrogen dioxide on emergency hospital admissions for cardiac diseases in Hong Kong. Int J Cardiol 2013;168:2831–6. 10.1016/j.ijcard.2013.03.082 [DOI] [PubMed] [Google Scholar]
- 30.Faustini A, Stafoggia M, Colais P et al. Air pollution and multiple acute respiratory outcomes. Eur Respir J 2013;42:304–13. 10.1183/09031936.00128712 [DOI] [PubMed] [Google Scholar]
- 31.Hoek G, Krishnan RM, Beelen R et al. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health 2013;12:43 10.1186/1476-069X-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinmayr G, Romeo E, De Sario M et al. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect 2010;118:449–57. 10.1289/ehp.0900844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maynard RL. The effects on health of ambient particles: time for an agonizing reappraisal? Cell Biol Toxicl 2015;31:131–47. 10.1007/s10565-015-9296-7 [DOI] [PubMed] [Google Scholar]
- 34.Cesaroni G, Badaloni C, Gariazzo C et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect 2013;121:324–31. 10.1289/ehp.1205862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerrett M, Burnett RT, Beckerman BS et al. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med 2013;188:593–9. 10.1164/rccm.201303-0609OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2015-010751supp.pdf (6MB, pdf)