Abstract
Background:
The diagnosis and treatment of bone nonunion have been studied extensively. Diagnosis and treatment of nonunion are mainly performed based on the interpretation of clinico-radiographic findings, which depend on the clinician's experience and the degree of bone callus formation during the fracture-healing process. However, resolution may be compromised when the bone mineral content is <25%. A feasible method of monitoring bone-healing is therefore needed. We monitored a rabbit model of bone nonunion by regular radiographic examinations, QCT detection, and biomarker concentrations.
Materials and Methods:
Twenty purebred New Zealand rabbits (10 male and 10 female, 5–6 months of age, 2.5–3.0 kg) were divided into bone defect Group (I) that 10 left radius bones underwent resection of 1.5 cm of mid-radius bone and bone fracture Group (II) that another 10 left radius bones underwent only mid-radius fracture. Quantitative computed tomography detection of bone mineral density (BMD) and serum markers of bone formation (osteocalcin [OC], bone-specific alkaline phosphatase) and bone resorption (C- and N-terminal telopeptides of type I collagen (NTX) and tartrate-resistant acid phosphatase 5b) were assayed. There are twenty rabbits (10 male and 10 females). The age was 5–6 months weighing 2.5–3.0 kg). The defect was created in middle 1/3 radius in 10 rabbits and fracture was created in middle 1/3 radius of 10 rabbits.
Results:
BMD and NTX concentrations were significantly lower at 5 weeks postoperatively compared to the preoperative values and were significantly different between the two groups. OC showed no significant difference before and after surgery.
Conclusions:
BMD and NTX concentrations may be useful for early detection of bone nonunion in rabbits.
Keywords: Biomarkers, bone mineral density, bone-specific alkaline phosphatase, nonunion, N-terminal telopeptide of type I collagen, tartrate-resistant acid phosphatase 5b
MeSh terms: Osteoporosis, biomarkers, bone mineral density, bone fractures
INTRODUCTION
The diagnosis and treatment of bone nonunion have been studied extensively. Diagnosis and treatment of nonunion are mainly performed based on the interpretation of clinico radiographic findings,1 which depend on the clinician's experience and the degree of bone callus formation during the fracture-healing process. However, resolution may be compromised when the bone mineral content is <25%. A feasible method of monitoring bone-healing is therefore needed. The role, however, of measuring serum biomarker concentrations and quantitative computed tomography (QCT) detection of bone mineral density (BMD) for monitoring fracture healing remains unclear. As changes in bone metabolism precede changes in morphology, changes in biomarker levels could be detected much earlier than changes in BMD.2 The balance between bone formation and bone resorption in fracture nonunion remains unclear.3
The present study measured serum concentrations of specific markers of bone turnover during the bone-healing process. The coupling of bone resorption and formation was closely related to collagen metabolism, growth factors, vascular mediators, and osteoblast activity. Serum biomarkers were chosen and their concentrations compared with morphological changes on radiographs and with the BMD determined via QCT to assess whether they could be indicators of bone nonunion. We monitored a rabbit model of bone nonunion by regular radiographic examinations, QCT detection, and biomarker concentrations. The serum biomarker concentrations and BMD were measured to investigate their usefulness for early diagnosis of bone nonunion and to monitor the changes in concentrations during early bone nonunion.
MATERIALS AND METHODS
Experimental materials
Surgical instruments included a scalpel handle, scalpel blades, mosquito forceps, bone knife, rongeur, tissue scissors, suture scissors, sterile surgical towels, no. 1 sutures, operating table, and cord to fix the rabbits on the operating table. Testing instruments included a Vernier caliper and radiography machine. Drugs used were amoxicillin, 3% pentobarbital sodium, ketamine, iodine, and lime. Animal housing and feeding equipment included rabbit cages and rabbit feed.
Animals and treatment
Twenty purebred New Zealand rabbits (10 male and 10 female, 5–6 months of age, 2.5–3.0 kg) were randomly divided into two groups of 10 rabbits each. (a) Group I - bone defect group, a 15-mm length of bone (including the periosteum) was removed from the mid-radius, and the medullary cavities of the bone stumps were closed with bone wax. (b) bone fracture group, the mid-radius was fractured. No bone wax was used. No fracture fixation was performed in either group. After surgery, the rabbits were housed in separate cages and allowed free activity. The “Principles of Laboratory Animal Care” (National Institutes of Health publication no. 85–23, revised 1985) were followed.
Operative procedure
Rabbits were anesthetized with an intravenous injection of 3% pentobarbital sodium (20 mg/kg) via an ear vein and an intramuscular injection of ketamine (50 mg/kg). After anesthesia, the rabbit was positioned on the operating table with a cord. The right forelimb was shaved and disinfected with iodine. A 2.5–3.0 cm skin incision was made at the mid-radial level, exposing the radius. In the bone defect group, a 15 mm length of bone was removed from the mid-radius. The length was controlled to ≤0.1 mm using Vernier calipers. The bone stumps were trimmed using rongeurs, and the defect was closed with bone wax. In the bone fracture group, the mid-radius was fractured vertically using scalpel blades. No fracture fixation was performed on either group. The skin wounds were closed using no. 1 silk sutures and were disinfected with iodine twice daily after surgery. The sutures were removed after 12 days. After surgery, all rabbits were given oral amoxicillin for 1 week.
Radiographic and biomarker examinations
The affected forelegs of all rabbits underwent radiographic examination and QCT detection preoperatively and at 2–8, 10, and 12 weeks postoperatively to observe the healing of bone defects. Additionally, 2-mL blood samples were harvested and stored at −80°C to measure the serum concentrations of biomarkers.
Quantitative computed tomography evaluation of bone mineral density
Computed tomography (CT) scans were performed with GE HiSpeed NX/1 PROCT and an ADW4.2 postprocessing workstation with parameters of 120 kv, 80 MA, 512 × 512 matrix and 64 mm × 0.625 mm, 10 mm thickness, and 10 mm space.
Image postprocessing
The contents of mineral substance per cubic centimeter (X) on the bone defect side and contralateral side were measured with the GE ADW4.2 workstation. The peak of normal tissue (T) and the control normal tissue (Z) were calculated, depending on the mineral substance content per cubic centimeter (expressed as mg/cm3).
Main outcome index
The serum biomarker concentrations measured were osteocalcin (OC) (USCN Life Science, Wuhan, China) and bone-specific alkaline phosphatase (BSAP) as markers of bone formation, and C-terminal telopeptide of type I collagen (CTX), N-terminal telopeptide of type I collagen (NTX), and tartrate-resistant acid phosphatase 5b (TRACP 5b) (Shanghai Langdun Shengwu, Shanghai, China) as markers of bone resorption.
All biomarker concentrations were measured by biotin double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). For NTX, the ELISA plate was embedded with NTX monoclonal antibody, and NTX was then added. After incubation, biotin-labeled anti-NTX antibody was added to the mixture, thus binding to streptavidin-horseradish peroxidase and forming immune complexes. The complexes were subsequently incubated and rinsed to eliminate unbound enzyme. They were then developed in a blue stain with substrates A and B and finally developed in a yellow stain with acid. The degree of staining positively correlated with the NTX concentration in the specimen.
Statistical analysis
All data are expressed as the mean ± standard deviation and were analyzed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). One way analysis of variance was used to estimate average values, followed by effect comparisons between groups, the spherical test, intragroup (repeated) effect comparisons, and interaction effect comparisons. P < 0.05 was considered to indicate a statistically significant difference. The sample sizes were determined based on the preselected power level of 0.8.
RESULTS
General observations after surgery
None of the involved 20 rabbits developed infection of the surgical incision, and none died during the experimental period. All of the rabbits were included in all of the analyses.
Radiographic findings
In the bone defect group, bone callus formation was observed in three rabbits at 2 weeks. The calluses stabilized at 5 weeks, but none of the bones had healed at 8 weeks. There was no callus formation in the other seven rabbits in the group. In all of the rabbits in the bone fracture group, the fracture line was blurry at 2 weeks, and a large number of bone calluses had formed in all of the rabbits by 6–8 weeks [Figures 1 and 2].
Figure 1.
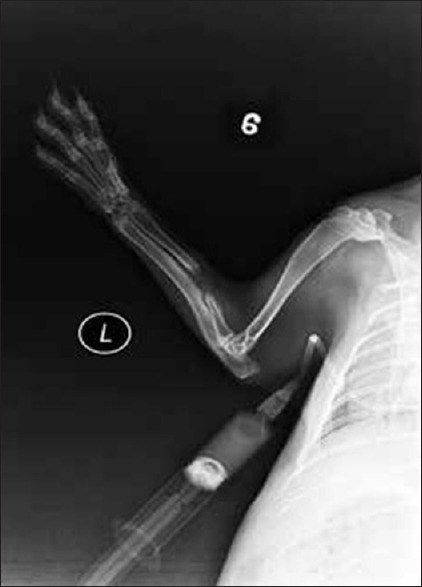
X-ray of the rabbit's forearm anteroposterior view showing none of the bones had healed at 8 weeks in the bone defect group
Figure 2.
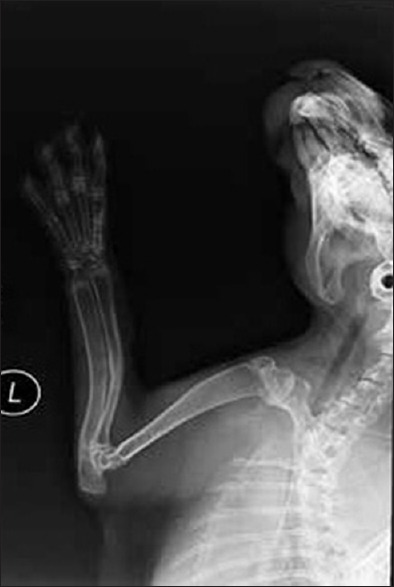
X-ray of the rabbits forearm anteroposterior view showing a large number of bone calluses had formed in the rabbits by 6–8 weeks in the bone fracture group
Detection of serum biomarker concentrations and bone mineral density
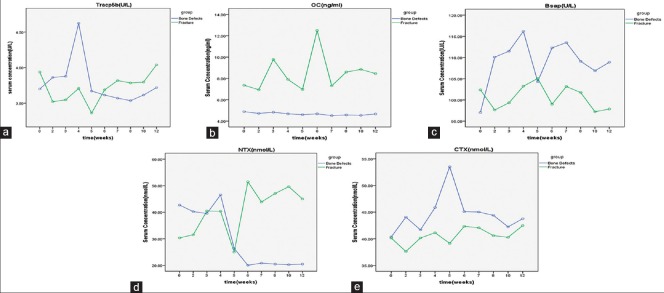
The serum OC, BSAP, CTX, and NTX concentrations were significantly different between the two groups (OC: F =22.989, P < 0.001; BSAP: F =16.051, P = 0.001; CTX: F =27.737, P < 0.001; NTX: F =187.512, P < 0.001). There were no significant differences in the serum OC, BSAP, CTX, or TRACP 5b concentrations within each group at different time points. In the bone defect group, the serum NTX concentration was significantly lower at 5 weeks after surgery. There were significant differences between the serum NTX concentrations at 7, 8, 10, and 12 weeks (combined) and at 3–5 weeks (combined). The serum NTX concentration stabilized after 6 weeks [Figure 3].
Figure 3.
Postoperative time course of the serum concentrations of (a) tartrate-resistant acid phosphatase 5b, (b) osteocalcin, (c) bone-specific alkaline phosphatase, (d) N-terminal telopeptide of type I collagen, and (e) C-terminal telopeptide of type I collagen at 12 weeks after surgery. The data are shown as absolute values. Each data point is a mean
In the bone defect group, the BMD value was significantly decreased at 5 weeks postoperatively compared with that of the bone fracture group and the preoperative control values. There were significant differences between the BMD value at 7, 8, 10, and 12 weeks (combined) and at 3–5 weeks (combined). The BMD value stabilized after 6 weeks [Figure 4].
Figure 4.
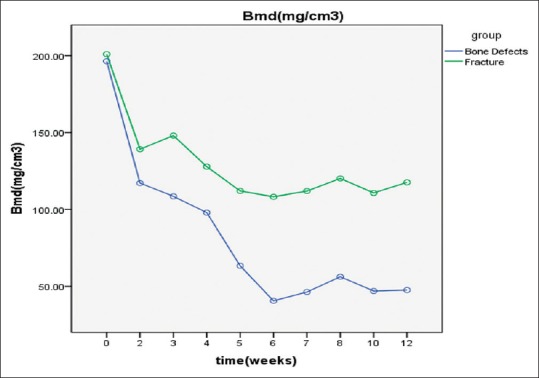
Postoperative time course for bone mineral density shown by quantitative computed tomography at 12 weeks after surgery. The data are shown as absolute values. Each data point is a mean
DISCUSSION
The present study found that BMD values began to decrease immediately after surgery in the bone defect group. They stabilized at 2–4 weeks but had significantly decreased at 5 weeks postoperatively. There were significant differences from findings in the bone fracture group at the same time and from the preoperative control values. After 6 weeks, the BMD values remained stable. The decreased BMD indicated absorption and decomposition of bone tissue. This evidence does not imply poor calcification of bone tissue or slow bone metabolism. It is possible that when fracture healing enters the bone remodeling process bone structure becomes more orderly and more efficient.
At present, the commonly used method to detect BMD is dual-energy X-ray absorptiometry, which is prone to be influenced by bone morphology and size.4 Plain radiography can visualize cortical thickness and trabecular bone morphology, but it obtains only quantitative analysis results and fails to achieve accuracy. The double-photon method can quantitatively analyze BMD using photoelectrons at varying energy, but the detection range includes both the bone and adjacent tissue. CT can improve the sensitivity and accuracy of BMD measurements as it excludes the impact of adjacent tissue and bone cortex on the measurement results.
In this study, we adopted high-resolution QCT to measure BMD values, with adjacent muscle and adipose tissue as the reference. This technology requires no external model and eliminates the impact of non uniformity and position variations of the external model, thus improving the accuracy and precision of the measurement results.5 QCT can visualize bone microstructure, including the bone volume ratio, bone surface ratio, trabecular thickness, trabecular spacing, length of the trabecular bone, connection density, and structural model parameters.6,7,8 QCT allows a separate determination of BMD among bone tissues at high bone turnover rates and sensitive bone metabolism in a three-dimensional space (mg/cm3) – that is, the unit volume of bone minerals. QCT provides a cross-sectional image of the bone and selectively measures BMD, which reflects early changes in the bone mass in vivo.
Bone loss in both groups may partly be attributed to immobilization atrophy. Rabbits in the bone defect group may be less mobile than those in the fracture group. This study still found postoperative activity of rabbits in both groups was similar and no obvious difference of disuse atrophy-related bone loss. The same pain caused by incision, fracture and bone defect, and forearm which wasn’t the main load-bearing limb could be the ideal interpretation for these.
The bone metabolic index has been widely used to study bone nonunion because it measures changes noninvasively and so can be performed repeatedly. Bone formation, bone resorption, and static conditions contribute to the process of bone remodeling. Bone turnover involves continuous removal of old bone by osteoclasts and simultaneous formation of osteoid and mineralization by osteoblasts. These two processes are tightly coupled. The bone mass is determined by the balance of bone formation and bone resorption in the same bone remodeling unit. When this balance is disrupted and the rate of bone resorption is higher than the rate of bone formation, nonunion occurs.9 Serum biomarker concentrations can reflect the status of the bone turnover process by indirectly measuring osteoblast and osteoclast activity.
A number of sensitive and specific biomarkers have been studied with regard to monitoring bone loss, predicting fracture risk, and differential diagnosis of metabolic bone diseases. The main biomarkers are BSAP, CTX, NTX, and TRACP 5b. It is of increasing interest to establish whether measuring serum concentrations of these biomarkers more adequately enables earlier assessment of treatment effects than measuring bone density.
Southwood et al.10 (2003) studied a rabbit model for femoral defects and found that the serum BSAP concentration was low during the first 4 weeks after surgery, increased to a peak at 8 weeks, and then decreased. Moghaddam et al.11 compared 15 patients with atrophic nonunion after a long bone shaft fracture and 15 matched patients with normal fracture healing selected from a pool of 248 patients who underwent orthopedic surgery. They measured the serum biomarker concentrations at 1, 2, 4, 8, 12, and 52 weeks after surgery and found that the serum BSAP concentrations initially increased and then decreased during the 1st week after surgery. There were no significant differences between the groups regarding the absolute or relative concentrations of these biomarkers during the healing process. In the bone defect group in the present study, the serum BSAP concentration increased after surgery, peaking at 4 weeks. It began to decrease at 5 weeks and stabilized after 6 weeks. The serum BSAP concentration was significantly different between the two groups but was not significantly different among the various time points within each group. These results show that the bone defect stimulated bone formation, resulting in an increased serum BSAP concentration. This finding was consistent with those of Southwood et al.10 and Moghaddam et al. (2011).11 Dynamic changes in the serum BSAP concentration could thus contribute to monitoring bone healing in experimental rabbits.
Eastell et al.12 (1988) proposed that the serum OC concentration is a sensitive and specific biomarker of bone turnover and bone formation. OC is currently a major focus of bone metabolism studies. Delmas13 found that the serum OC concentration reflects bone turnover when bone formation is coupled with bone resorption but reflects only bone formation (not turnover) when it is not coupled with bone resorption. Chinese studies found that a high serum OC concentration was associated with high BMD, suggesting that bone formation and bone mass increase in the presence of a high serum OC concentration. The serum OC concentration therefore reflects BMD and can be used to predict bone nonunion. In the present study, the serum OC concentration did not change before and after surgery in the bone defect group despite changes in the BMD. Hence, the serum OC concentration is not a sensitive indicator of bone nonunion in rabbits.
Moghaddam et al.11 measured serum biomarker concentrations in 15 patients with atrophic nonunion. The results showed that at 1 week after surgery the serum CTX concentration was significantly lower in the nonunion group than in the normal healing group. At 4 and 8 weeks, the serum TRACP 5b concentrations were significantly lower, although the absolute and relative TRACP 5b concentrations were not significantly different between the two groups at any of the time points. In the bone defect group in the present study, the serum TRACP 5b concentration increased after surgery, peaked at 4 weeks, and then decreased and remained at a low level. The serum CTX concentrations changed in a parallel pattern in both groups but with significant differences in concentration between the two groups. The serum CTX concentration peaked at 5 weeks after surgery in the bone defect group, but there were no significant differences in CTX concentration among different time points within each group. The absolute values of serum TRACP 5b and CTX concentrations increased after surgery in the bone defect group, indicating increasing bone resorption after the bone defect occurred. Monitoring the serum TRACP 5b and CTX concentrations may reflect the early healing process and may be useful for clinical monitoring.
NTX is a stable peptide excreted in the urine after bone resorption.14 Theoretically, a high serum NTX concentration is highly specific for bone resorption. García-Pérez et al.9 (2006) found that the serum NTX concentration was a sensitive indicator of bone resorption. Rosen et al.15 (1998) found that NTX concentration was negatively correlated with BMD and that a high baseline serum NTX concentration indicated a more rapid decline in bone density. In the bone defect group in the present study, the serum NTX concentration was significantly lower 5 weeks after surgery than before surgery, and it stabilized after 6 weeks. Serum NTX concentrations were significantly different between the bone defect and bone fracture groups.
In this study, the serum OC concentration did not change significantly before and after surgery in the bone defect group, whereas the serum BSAP, CTX, and TRACP 5b concentrations fluctuated after surgery. Although the serum OC, BSAP, CTX, and TRACP 5b concentrations showed no significant differences within the bone defect group, this group had a high bone turnover rate. Measurement of serum biomarker concentrations is important to clinical evaluation of the early healing process. In the bone defect group, the serum NTX concentration was significantly lower at 5 weeks after surgery than before surgery, suggesting that serum NTX provides an accurate reflection of bone turnover in vivo and changes quickly after changes in bone resorption. Similar to NTX, the BMD values were significantly lower at 5 weeks in the bone defect group. We speculate that bone absorption, as reflected in the NTX levels, was obviously reduced at 5 weeks, and then remained stable until bone absorption and bone formation achieved a balance. This process is indicated by the sharp decrease and rapid stabilization of the BMD values.
The limitations of study are as follows. First, NTX may be useful to early diagnose bone nonunion. The histological molecular changes of serum NTX in bone defect - nonunion are little known. How can we analyse NTX activity in granulation tissue of bone defects? Second, how can we use BMD to predict clinical bone nonunion, or just use it as an experimental monitoring tool?
CONCLUSIONS
Bone biochemical markers and BMD are better tools for the study on bone metabolism. Bone nonunion followup by biochemical markers and BMD detection is a combination of basic and clinical applications. The existing statistical data is not very satisfactory yet. However, BMD and NTX concentrations may be useful for early detection of bone nonunion in rabbits. Further studies are needed to definitively determine whether both serum NTX concentration and BMD are sensitive and specific indicators of early bone turnover and can predict bone nonunion. Deeper molecular and genetic areas of fracture healing should be further explored and a better standardization of study is needed to determine the problem.
Financial support and sponsorship
Natural Science Foundation of Hainan Province, China (Grant No. 808212).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–8. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 2.Lin JP, Song SF, Yao LL. Feasibility of predicting fracture risk with bone turnover markers and bone mineral density. Zhongguo Zuzhi Gongcheng Yanjiu Yu Linchuang Kangfu. 2010;14:317–20. [Google Scholar]
- 3.Liao EY, Wu XP, Deng XG, Huang G, Zhu XP, Long ZF, et al. Age-related bone mineral density, accumulated bone loss rate and prevalence of osteoporosis at multiple skeletal sites in Chinese women. Osteoporos Int. 2002;13:669–76. doi: 10.1007/s001980200091. [DOI] [PubMed] [Google Scholar]
- 4.Wu SY, Yang L, Qi J, Wang B, Wen LQ, Li JX. Study on bone mineral density and bone structure of lumbar vertebrae in osteoporotic elderly women with multi-slice CT. Chin J Radiol. 2005;39:1165–70. [Google Scholar]
- 5.Boden SD, Goodenough DJ, Stockham CD, Jacobs E, Dina T, Allman RM. Precise measurement of vertebral bone density using computed tomography without the use of an external reference phantom. J Digit Imaging. 1989;2:31–8. doi: 10.1007/BF03168013. [DOI] [PubMed] [Google Scholar]
- 6.Cortet B, Dubois P, Boutry N, Varlet E, Cotten A, Marchandise X. Does high-resolution computed tomography image analysis of the distal radius provide information independent of bone mass? J Clin Densitom. 2000;3:339–51. doi: 10.1385/jcd:3:4:339. [DOI] [PubMed] [Google Scholar]
- 7.Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah B. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;73:423–32. doi: 10.1007/s00223-002-2104-4. [DOI] [PubMed] [Google Scholar]
- 8.Boyd SK, Davison P, Müller R, Gasser JA. Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone. 2006;39:854–62. doi: 10.1016/j.bone.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 9.García-Pérez MA, Moreno-Mercer J, Tarín JJ, Cano A. Similar efficacy of low and standard doses of transdermal estradiol in controlling bone turnover in postmenopausal women. Gynecol Endocrinol. 2006;22:179–84. doi: 10.1080/09513590600624291. [DOI] [PubMed] [Google Scholar]
- 10.Southwood LL, Frisbie DD, Kawcak CE, McIlwraith CW. Evaluation of serum biochemical markers of bone metabolism for early diagnosis of nonunion and infected nonunion fractures in rabbits. Am J Vet Res. 2003;64:727–35. doi: 10.2460/ajvr.2003.64.727. [DOI] [PubMed] [Google Scholar]
- 11.Moghaddam A, Müller U, Roth HJ, Wentzensen A, Grützner PA, Zimmermann G. TRACP 5b and CTX as osteological markers of delayed fracture healing. Injury. 2011;42:758–64. doi: 10.1016/j.injury.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Eastell R, Yergey AL, Vieira NE, Cedel SL, Kumar R, Riggs BL. Interrelationship among Vitamin D metabolism, true calcium absorption, parathyroid function, and age in women: Evidence of an age-related intestinal resistance to 1,25-dihydroxyvitamin D action. J Bone Miner Res. 1991;6:125–32. doi: 10.1002/jbmr.5650060205. [DOI] [PubMed] [Google Scholar]
- 13.Delmas PD. Biochemical markers of bone turnover for the clinical assessment of metabolic bone disease. Endocrinol Metab Clin North Am. 1990;19:1–18. [PubMed] [Google Scholar]
- 14.Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7:1251–8. doi: 10.1002/jbmr.5650071119. [DOI] [PubMed] [Google Scholar]
- 15.Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the followup of patients treated with bisphosphonates. Calcif Tissue Int. 1998;63:363–8. doi: 10.1007/s002239900541. [DOI] [PubMed] [Google Scholar]