Abstract
Childhood lead (Pb) poisoning remains a global issue, especially in industrial areas. In this study, 115 children with average age 5.7 years were recruited as either patient diagnosed with Pb poisoning or controls at Xinhua Hospital in China. The subjects’ bone Pb was measured with a K-shell X-ray fluorescence (KXRF) and a portable X-ray fluorescence (XRF) system. A significant correlation between KXRF bone Pb and blood Pb and portable XRF and KXRF measurements were observed. The half-life of blood-lead was calculated to be 9.96 ± 3.92 d. Our results indicate that bone is a useful biomarker for Pb in children.
Keywords: In vivo, portable, X-ray fluorescence
Introduction
Despite strict standards for lead (Pb) use in many countries, childhood Pb poisoning remains a worldwide issue. This problem is more prevalent in heavily industrialized areas. China, in particular, presents unique exposure issues: pollution, alternative medicines with large amounts of Pb used to treat various diseases and ever-increasing consumer and industrial usage of Pb-containing products. The risk of Pb exposure to children has been well documented (Fadrowski et al., 2010; Wang et al., 2012). Recent studies show that Pb exposure results in significant decline in intellectual ability even at low-levels, and that the Pb associated intellectual decrement was steeper at low blood Pb levels than at higher blood Pb levels (Canfield et al., 2003; Jusko et al., 2008; Lanphear et al., 2005). These studies use blood Pb as a biomarker, which assesses recent exposure and has a half-life of about 1 month (Leggett, 1993). Bone Pb, which has a half-life of years to decades, serves as a cumulative biomarker (Rabinowitz, 1991). Cd-109 based K-shell X-ray fluorescence (KXRF) has been used to study bone Pb for over two decades, but not many studies have been performed on children using a bone Pb biomarker, and the studies that do exist have a relatively high uncertainty and were not able to detect bone Pb in most of the subjects due to the high uncertainty of the conventional bone Pb measurement system (Behinaein et al., 2014; Nie et al., 2011b). There is a significant gap in understanding children’s bone Pb measurements and their relationship to blood Pb, as well as the usefulness of bone Pb as a biomarker for Pb exposure and toxicity among children, which are addressed in this paper. The purposes of this study are: (1) to measure bone Pb concentration in a pediatric population; (2) to assess the usefulness of bone Pb as a biomarker for children; (3) to validate the use of portable XRF for bone Pb measurement among children and (4) to explore bone and blood Pb biokinetics in children.
Materials and methods
Study population
The study participants were Pb-exposed children and controls recruited through Xinhua Hospital, Shanghai Jiaotong University, China. The Pb-exposed group was recruited from children who were diagnosed as Pb poisoned with blood Pb levels >25 µg/dl. Some of these children had already undergone multiple treatments prior to our first measurements. The controls were recruited from children who visited the clinic for non-Pb-related diseases. The parents of the subjects completed a questionnaire, and the subjects had their blood samples taken, and their bone measured for Pb concentration using an advanced cloverleaf Cd-109 KXRF as well as a portable X-ray fluorescence (XRF) system. Blood Pb was also measured for these children. Some of the Pb-exposed children were treated using ethylenediaminetetraacetic acid (EDTA) chelation.
The study received Internal Review Board approval from Purdue University and Xinhua Hospital. When recruited, a trained research assistant would present the subjects and their parents with the details of the study and the consent forms. Signed consent forms were received from the parents of each subject, as well as an assent form from any child age 7 or older.
KXRF bone Pb measurement system
The KXRF bone Pb measurement system was used to measure tibia bone Pb as a metric of each individual’s cumulative lead exposure. The setup was the same as used in previous studies (Nie, 2005; Nie et al., 2004; Specht et al., 2014). The system uses four 16 mm diameter high-purified germanium detectors with 10 mm thickness, four feedback resistance pre-amplifiers, four digital signal processing systems and a computer. A 135 mCi Cd-109 source with 0.8 mm copper filter is used to irradiate tibia bone to produce the Pb K X-rays. Before measurement, the subjects’ legs were cleaned using alcohol and EDTA cotton swabs to remove any Pb contamination. For the KXRF measurement, the subject would sit on a wooden chair. The subject’s leg was immobilized by using two Velcro straps to attach their leg to the leg of the chair at the ankle and just below the knee. The measurement site was mid-tibia with the source at a distance to maintain ~30% dead time during the measurement. The measurement was taken for 30 min while the subject watched a movie. Finally, the spectra were analyzed using an in-house peak-fitting program, which gave results and error for each of the four detectors (Bevington & Robinson, 2003; Nie, 2005; Somervaille et al., 1989). This error and result was combined using inverse variance weighting (Todd, 2000). XRF provides a point estimate of Pb concentration, which can be negative if an individual’s bone Pb is close to zero. It is important to include these negative values, as with their associated uncertainties they are still a point estimate of that individual’s bone Pb.
The whole body effective dose delivered to the subject from this system was estimated to be less than 5 µSv for this population (Nie et al., 2007).
Portable XRF device
The portable XRF device used in this study was a customized device manufactured by Thermo Fisher (XL3t GOLDD+, Thermo Fisher Scientific Inc., Billerica, MA). The device specifications and optimization were discussed in a previous study (Specht et al., 2014). We used 50 kV and 40 µA settings with silver and iron filter combination for the X-ray tube. The subject’s measurement site was cleaned before each measurement the same as before the KXRF measurement. We again chose the measurement site to be mid-tibia. In placing the device on the subject’s leg, we used non-Pb-based ink to make a dot on the measurement site. This dot could then be found using a camera mounted in the head of the portable XRF, which ensured a consistent measurement location. The subject’s leg was held horizontally with their foot resting in the operator’s lap during the measurement. In this study, we used a measurement time of 2 min. The spectra were analyzed using traditional peak fitting as described in detail in our previous work (Specht et al., 2014). Based on our previous study, by adjusting values for increased measurement time and tube current, we estimated the entrance skin dose of the system was 21 mSv to a 1 cm2 area and the whole body effective dose was 2.4 µSv (Nie et al., 2011a). This can be compared to the whole body effective dose for a standard AP chest X-ray of about 100 µSv.
Blood Pb analysis
The blood of the subjects was collected in a Pb-free environment. We cleaned the subjects’ skin using alcohol swabs before sampling. The blood collection tubes along with the EDTA-K2 anticoagulants were measured to be Pb-free before sampling. All the samples were frozen and kept at −80 °C immediately after the collection. Blood Pb concentrations were measured and analyzed by an atomic absorption spectrometer (AAS) (AA900Z, PE) (Liu, 2005). The sensitivity of our device was 0.01 µg/dl, and inter and intra-assay variability was 5%.
Statistical methods
Linear regressions were used to determine correlation values and levels of significance of relationships between KXRF and portable XRF bone Pb measurements and KXRF bone Pb and blood Pb. The KXRF bone Pb data was excluded from our analysis if the final uncertainty was >10 µg/g. This would indicate an excessive amount of movement during the measurement or a measurement taken for less than 30 min. Portable XRF data was excluded if the measurement time was <2 min.
Results
The study had 115 participants (79 male and 36 female), ranging in age from 1 to 14.3 years old with a mean of 5.7± 3.3 years. Of these participants, 86 were Pb poisoned subjects and 29 were controls.
Bone and blood Pb concentrations in the study population
The bone and blood Pb concentrations for the exposed and control groups are listed in Table 1. The average uncertainty in this study for in vivo KXRF bone Pb measurement was 2.66 ± 1.05 and is shown for each group in Table 1 under Bone Pb Sigma. Blood Pb data shown in this table was taken at the time of the first bone Pb measurement, which for some exposed subjects was after multiple treatments.
Table 1.
Bone and blood Pb statistics for exposed and control subjects.
| N | Minimum | Maximum | Mean | Std. deviation | |
|---|---|---|---|---|---|
| Exposed | |||||
| Blood Pb (µg/dl) | 86 | 1.9 | 62.4 | 18.54 | 12.30 |
| Bone Pb (µg/g) | 86 | −10.52 | 129.59 | 19.75 | 23.33 |
| Bone Pb Sigma | 86 | 1.49 | 8.52 | 2.74 | 1.14 |
| Control | |||||
| Blood Pb (µg/dl) | 23 | 1.0 | 12.4 | 2.80 | 2.48 |
| Bone Pb (µg/g) | 29 | −9.59 | 14.94 | −1.13 | 4.23 |
| Bone Pb Sigma | 29 | 1.68 | 5.62 | 2.42 | 0.75 |
Correlation between bone and blood Pb concentrations
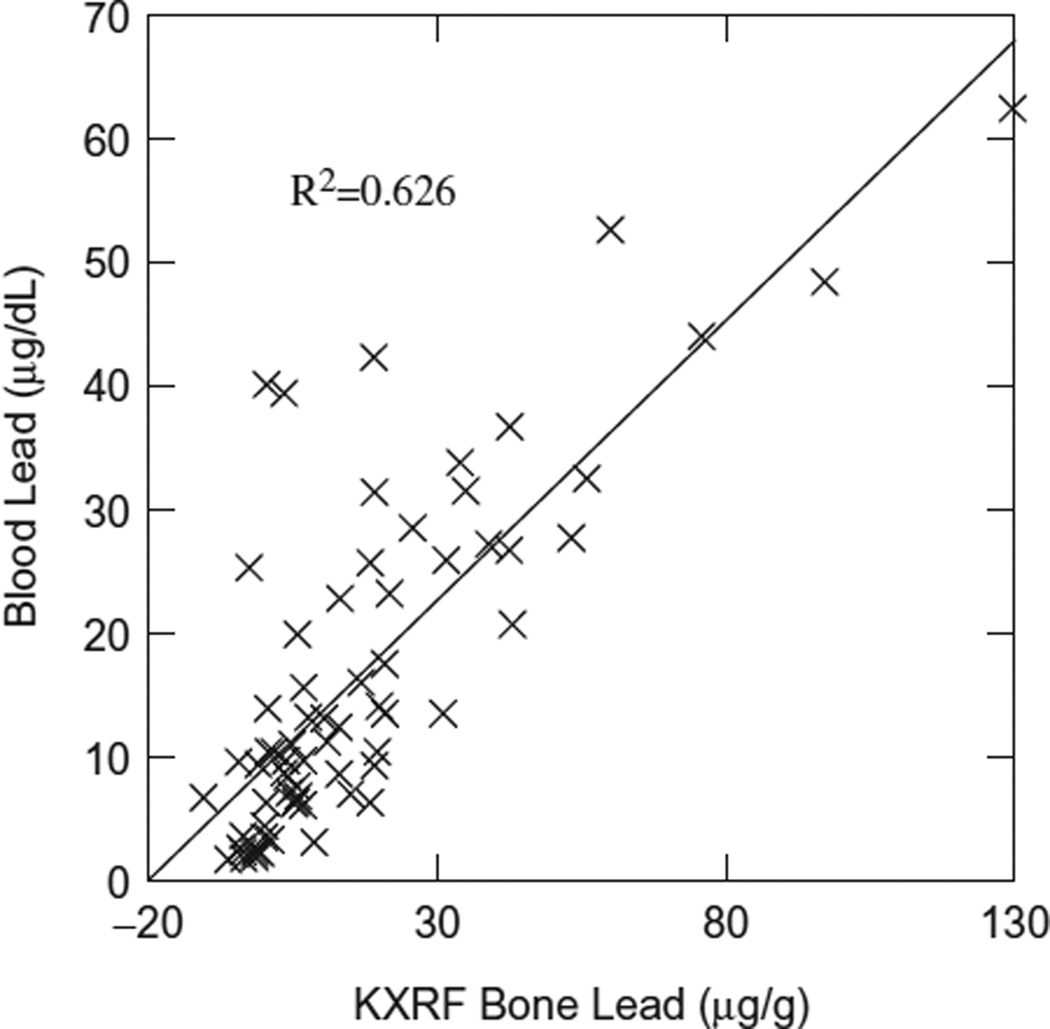
Figure 1 shows the correlation between bone Pb concentrations obtained from KXRF and blood Pb concentrations using only exposed subjects. No correlation was found in this relationship when using only control subjects, and only a slight negative change in correlation value when using both control and exposed subjects (R2 = 0.624). The p value of this regression was <0.001.
Figure 1.
KXRF bone Pb versus blood Pb.
Correlation between bone Pb concentrations obtained by KXRF and portable XRF
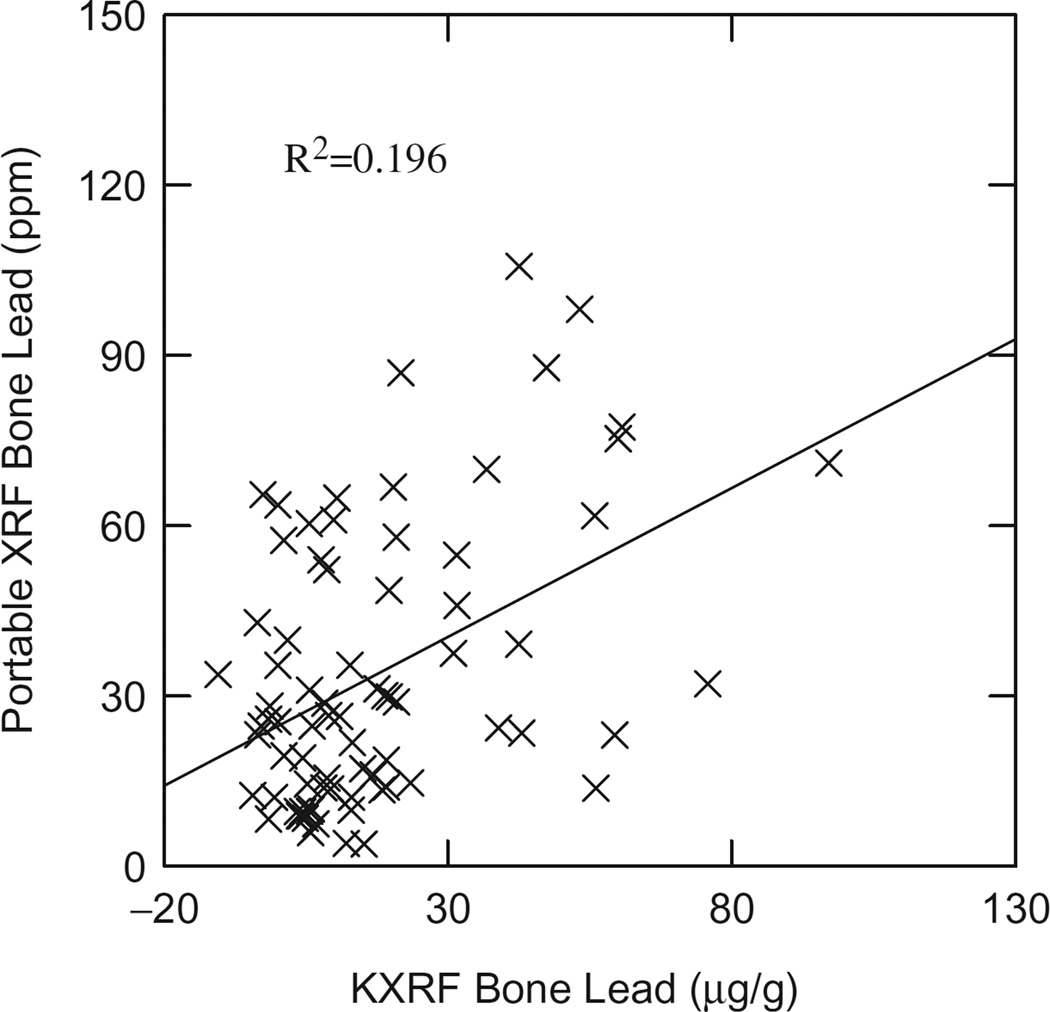
Figure 2 shows the correlation of bone Pb obtained by KXRF versus that obtained by portable XRF using only exposed subjects. Again with this relationship, no correlation was found when using only control subjects, and there was a slight negative change in correlation values when using both control and exposed subjects (R2 = 0.185). The p value of this regression was <0.001.
Figure 2.
KXRF versus portable XRF bone lead.
Bone and blood Pb biokinetics
With the bone Pb and blood Pb concentration data obtained from the study, we can estimate the percentage of bone Pb over the total Pb amount in blood and bone, which are two of the largest Pb storage sites in the human body, as well as the Pb transfer rate between bone and blood. Total bone Pb and blood Pb amount were calculated by multiplying bone and blood concentrations and the bone mineral amount and blood volume. The total bone mineral was calculated from the data shown in Specker et al.’s paper (Specker et al., 2001). Specifically, it was calculated using a combination of age, height, weight and other variables as shown in the paper. The age, height and weight of the subjects were collected in our study. The other variables were estimated from the average values corresponding to our age group. The total blood volume for each child was calculated from the data shown in Linderkamp et al.’s paper (Linderkamp et al., 1977). Specifically, it was calculated using an age-dependent logarithmic model, which relates blood volume to weight, height and a few other variables. Again, the age, height and weight were collected in our study. The other variables were estimated from average values corresponding to our age group. Then, using the 49 subjects that met the previous criteria and had sufficient data to calculate the total body bone mineral content and blood volume levels, we found that total bone Pb on average accounted for 96 ± 5% of total blood and bone Pb burden. The maximum and minimum values calculated for each individual exposed subject were 99.5% and 69.7% total body Pb burden from bone Pb, respectively. Separating the subjects by age, we see larger variation in this value with exposed subjects of 1–3 years old with 92.3±11% bone Pb and exposed subjects 3+ years with 96.5 ± 4% bone Pb.
Throughout the course of the study subjects returned for multiple treatments, there was a time gap of 41.5 ± 30.0 d (minimum: 7; maximum: 97) between these treatments. We can assume that bone and blood Pb had reached equilibrium by the next visit, which then allowed us to calculate an estimated half-life of blood Pb. Possible sources of Pb exposure were removed from these children after their first treatment, so the calculated half-life is expected to be the biological half-life without significant external exposure sources. The bone-remodeling rate for children was calculated from the data shown in ICRP 70 (ICRP, 1995). We plotted the bone-remodeling rate versus age for children 0–15 years old, fitted the data with second-degree polynomial function and calculated the bone-remodeling rate for each child from the fitted line. Assuming that bone and blood Pb were in equilibrium, that the Pb from other organs to blood is negligible compared to the Pb from bone to blood, and that there was negligible exposure from external sources, the Pb transferred from bone to blood would be equal to the Pb transferred from blood to urine, feces and other organs per unit time. This can be expressed as:
where TBonePb is total body Pb in bone (total bone mineral × bone Pb concentration obtained from KXRF in unit of µg/g bone mineral), TBloodPb is total body blood Pb (blood volume × blood Pb concentrations obtained from AAS), λBloodPb is the percentage of Pb in blood which would be transferred to urine, feces and other organs per day and λBone→Blood is the percentage of Pb in bone which would be transferred to blood per day. Because bone resorption rate represents the percentage of bone that would be resorbed to blood, λBone→Blood is equivalent to bone resorption rate, which can be calculated as described before. From these, we can calculate λBloodPb:
Given that:
where T1/2,blood is the biological half-life of Pb in the blood. The half-life of Pb in blood can be calculated as Ln(2)/λBlood Pb. The half-life was calculated for all the exposed subjects that had all the necessary measurements to perform the calculation, which resulted in 13 calculations of the half-life. The data and results for those subjects are shown below in Tables 2 and 3.
Table 2.
Exposed subject data for blood Pb half-life calculations.
| Age (years) |
Weight (kg) |
Height (cm) |
Total blood volume (l) |
Total bone mineral (g) |
Bone lead (µg/g) |
Second blood Pb (µg/l) |
Total bone Pb (mg) |
Blood Pb half-life (d) |
|---|---|---|---|---|---|---|---|---|
| 1.02 | 11.2 | 75.5 | 0.83 | 316.37 | 9.30 | 246 | 0.203 | 16.90 |
| 1.31 | 9 | 75 | 0.72 | 265.48 | 19.96 | 141 | 0.101 | 5.31 |
| 1.42 | 10 | 82 | 0.80 | 311.91 | 42.51 | 288 | 0.230 | 5.07 |
| 2.06 | 16 | 90 | 1.14 | 492.45 | 15.18 | 205 | 0.234 | 11.13 |
| 2.27 | 13 | 91 | 1.00 | 424.95 | 25.68 | 215 | 0.216 | 7.40 |
| 3.23 | 11 | 92 | 0.91 | 394.10 | 19.65 | 167 | 0.151 | 8.85 |
| 3.38 | 17 | 102 | 1.28 | 572.47 | 7.62 | 94 | 0.120 | 12.77 |
| 3.50 | 20.5 | 108 | 1.50 | 677.60 | 34.89 | 245 | 0.367 | 7.34 |
| 4.85 | 22.5 | 116 | 1.67 | 771.26 | 33.94 | 236 | 0.395 | 8.50 |
| 5.03 | 16 | 103 | 1.24 | 577.34 | 18.98 | 220 | 0.272 | 14.28 |
| 5.27 | 16 | 110 | 1.29 | 601.25 | 59.96 | 413 | 0.534 | 8.73 |
| 6.00 | 31 | 125 | 2.18 | 1024.27 | 5.20 | 62 | 0.135 | 16.03 |
| 6.93 | 35 | 125 | 2.36 | 1137.36 | 53.21 | 270 | 0.637 | 7.17 |
Table 3.
Blood Pb half-life split by age group for exposed subjects.
| Group | N | Average blood Pb half-life |
Standard deviation |
|---|---|---|---|
| Age 1–3 | 5 | 9.16 | 4.96 |
| Age 3+ | 8 | 10.46 | 3.40 |
| Total | 13 | 9.96 | 3.92 |
Discussion
The study demonstrated the capabilities of in vivo bone Pb measurement on children. The average uncertainty for the KXRF bone Pb measurement for this population is 2.66 ± 1.05, which is slightly higher than that for an adult population. This is expected because there is an average more fatty tissue in a child’s bone and the bone density is lower for a child’s bone. The portable XRF has a higher detection limit than the KXRF device used in this study. The detection limit for the portable XRF heavily depends on the soft tissue of the subject and thus varies more throughout the study (Specht et al., 2014). An issue associated with the anatomical structure of the bone in children, which is illustrated below, further deteriorated the detection limit for portable XRF.
The correlation between KXRF bone Pb and blood shows a higher slope and stronger r-squared than similar studies with adults (Bleecker et al., 1995; Fleming et al., 1997; Gerhardsson et al., 1993; Nie et al., 2009). One of the reasons for both these differences could be that children have a higher bone resorption rate, which indicates a more frequent transition of Pb between bone and blood, and hence their bone Pb is more significantly correlated with their blood Pb. The more significant correlation also indicates that for this population the majority of Pb in blood comes from the bone. This is expected since there is a significant store of Pb in these children’s bones and children’s bones are well known to have turnover rates higher than that of adults.
The portable XRF in comparison to the KXRF results gave a correlation less than what we would expect based on previous observations in adults and lab samples, as well as what we would have expected given the high Pb concentrations in this study. Since KXRF is a gold standard for in vivo bone Pb measurement, the correlation between KXRF and portable XRF demonstrates the limited ability of the portable XRF with current calibration methods to quantify bone Pb in children. In a previous paper, we detailed calibration methods and found the best calibration method as determined by in-lab samples (Specht et al., 2014). In that study, we found that the best calibration utilized the Compton scattering peak in relation to the soft tissue thickness over bone. This technique, although valid for adults, has complications with children. The Compton scattering peak is much larger than anticipated with children and thus overestimates soft tissue thickness and background from our spectrum. We attribute this to differences in composition and structure of the bone in children. Although our phantoms are designed to give accurate calibration for bone in adults, children’s bones are different in terms of density and composition which may affect our XRF spectra (ICRP, 1995). Given this issue, we applied an alternative calibration using traditional peak fitting of the beta Pb peak, which is also detailed in the previous study of the device (Specht et al., 2014). We chose to do this because when looking at the individual spectrum there was a visible Pb peak in this region with no surrounding elemental peaks for distortion of the signal. Calibration could be improved with proper calibration phantoms to reflect the appropriate anatomy of children’s bone. Given the simple calibration of the portable XRF used in this study, future measurements of children using this technology may be feasible, but further investigation on the effect of bone composition and an improved calibration method would be necessary to obtain more accurate results.
The relationships between KXRF bone Pb and blood Pb and KXRF bone Pb and portable XRF bone Pb measurements became slightly more positive when isolating based with only the exposed group. This is because the controls in the study all had values of nearly zero for both blood and bone Pb. The same relationships with only controls show no correlation, which can again be caused by the values being close to zero with uncertainties higher than measured bone Pb values.
The Pb body burden of the children assessed in our study is much higher than that suggested in other studies, which is expected because these children were Pb poisoned (Nie et al., 2011b). Our proportion of grams bone Pb to grams bone and blood Pb results are in agreement with previous studies of Pb body burden (Barry, 1975).
Only a few studies in literature investigated half-life of Pb in blood in children (Duggan, 1983; Manton et al., 2000). The half-life of Pb in blood calculated in our study lies in between results from these previous studies. Manton et al. reported a half-life of Pb in blood ranging from 8–11 months to 2–3 years (Manton et al., 2000). This study did not isolate bone Pb from blood Pb and thus, may reflect the long half-life of lead in bone since Pb stored in bone would gradually be released to blood endogenously. In addition, because the blood lead levels in those children were relatively low, a continuous external exposure, even at low level, could contribute to a prolonged half-life. Duggan’s study reported a much shorter half-life of Pb in the blood of 3.35 ± 1.34 d using blood and urine-Pb data (Duggan, 1983). However, the data are based on only two subjects. The half-life of blood Pb in our study is calculated from bone Pb and blood Pb data using the best bone and blood parameters we could find from the literature. The data used are the ones taken as far from chelation as possible (41.5 ± 30.0 d after chelation) to reduce the effect of chelation. However, chelation may still affect the equilibrium of Pb in bone and blood at that point of time and hence affect the half-life of blood Pb calculated.
Conclusion
Bone is a major storage site for Pb in children and serves as an indicator of total body Pb burden. KXRF is a viable measure of bone Pb in children with detection limits capable of measuring even environmental exposures. Portable XRF has limitations for bone Pb measurements in children based on calibration, which need to be addressed before being used further in pediatric populations. Bone Pb contributes significantly to blood Pb levels in Pb poisoned children and the blood Pb half-life in children is calculated to be 9.96 ± 3.92 d. The results from the study indicate that bone Pb may be a better marker for determination of chelation efficacy.
Acknowledgments
This work was supported by the National Institute for Occupational Safety and Health (NIOSH) R21 grant 1R21OH010044, National Natural Science Foundation of China (81373016, 30901205), National Basic Research Program of China (“973” Program, 2012CB525001), Shanghai Science and Technology Committee [124119a1400], Purdue Ross Fellowship and Purdue US-China visiting scholar network travel grant program.
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- Barry PS. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32:119–139. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behinaein S, Chettle DR, Marro L, et al. Factors influencing uncertainties of in vivo bone lead measurement using a (109)Cd K X-ray fluorescence clover leaf geometry detector system. Environ Sci Process Impacts. 2014;16:2742–2751. doi: 10.1039/c4em00446a. [DOI] [PubMed] [Google Scholar]
- Bevington P, Robinson D. Data reduction and error analysis for the physical sciences. New York, NY: McGraw-Hill; 2003. [Google Scholar]
- Bleecker ML, McNeill F, Lindgren KN, et al. Relationship between bone lead and other indices of lead exposure in smelter workers. Toxicol Lett. 1995;77:241–248. doi: 10.1016/0378-4274(95)03303-3. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Cory-Slechta DA, et al. Intellectual impairment in children with blood lead concentrations below 10 µg per deciliter. N Engl J Med. 2003;348:26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan MJ. The uptake and excretion of lead by young children. Arch Environ Health. 1983;38:246–247. doi: 10.1080/00039896.1983.10545810. [DOI] [PubMed] [Google Scholar]
- Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, et al. Blood lead level and kidney function in US adolescents: the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2010;170:75–82. doi: 10.1001/archinternmed.2009.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming DE, Boulay D, Richard NS, et al. Accumulated body burden and endogenous release of lead in employees of a lead smelter. Environ Health Perspect. 1997;105:224–233. doi: 10.1289/ehp.97105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardsson L, Attewell R, Chettle DR, et al. In vivo measurements of lead in bone in long-term exposed lead smelter workers. Arch Environ Health. 1993;48:147–156. doi: 10.1080/00039896.1993.9940813. [DOI] [PubMed] [Google Scholar]
- ICRP. Publication 70, Basic anatomical and physiological data for use in radiological protection – the skeleton. Ann ICRP. 1995;25:1–80. [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, et al. Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993;101:18. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderkamp O, Versmold HT, Riegel KP, Betke K. Estimation and prediction of blood volume in infants and children. Eur J Pediatr. 1977;125:227–234. doi: 10.1007/BF00493567. [DOI] [PubMed] [Google Scholar]
- Liu KL. Review of atomic spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi. 2005;25:95–103. [PubMed] [Google Scholar]
- Manton WI, Angle CR, Stanek KL, et al. Acquisition and retention of lead by young children. Environ Res. 2000;82:60–80. doi: 10.1006/enrs.1999.4003. [DOI] [PubMed] [Google Scholar]
- Nie H. Interpreting low concentration data [dissertation] Hamilton, ON, Canada: McMaster University; 2005. Studies in bone lead: a new cadmium-109 XRF measurement system. Modeling bone lead metabolism. [Google Scholar]
- Nie H, Chettle DR, Luo L, O’Meara J. Dosimetry study for a new in vivo X-ray fluorescence (XRF) bone lead measurement system. Nucl Instrum Meth B. 2007;263:225–230. [Google Scholar]
- Nie H, Chettle DR, Stronach IM, et al. A study of MDL improvements for the in vivo measurement of lead in bone. Nucl Instrum Meth B. 2004;213:4. [Google Scholar]
- Nie H, Sanchez BN, Wilker E, et al. Bone lead and endogenous exposure in an environmentally exposed elderly population: the normative aging study. J Occup Environ Med. 2009;51:848–857. doi: 10.1097/JOM.0b013e3181aa0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Sanchez S, Newton K, et al. In vivo quantification of lead in bone with a portable x-ray fluorescence system – methodology and feasibility. Phys Med Biol. 2011a;56:N39–N51. doi: 10.1088/0031-9155/56/3/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie LH, Wright RO, Bellinger DC, et al. Blood lead levels and cumulative blood lead index (CBLI) as predictors of late neurodevelopment in lead poisoned children. Biomarkers. 2011b;16:517–524. doi: 10.3109/1354750X.2011.604133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:4. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille LJ, Nilsson U, Chettle DR, et al. In vivo measurements of bone lead – a comparison of two X-ray fluorescence techniques used at three different bone sites. Phys Med Biol. 1989;34:1833–1845. doi: 10.1088/0031-9155/34/12/007. [DOI] [PubMed] [Google Scholar]
- Specht AJ, Weisskopf M, Nie LH. Portable XRF technology to quantify Pb in bone in vivo. J Biomarkers. 2014;2014:398032. doi: 10.1155/2014/398032. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specker BL, Johannsen N, Binkley T, Finn K. Total body bone mineral content and tibial cortical bone measures in preschool children. J Bone Miner Res. 2001;16:2298–2305. doi: 10.1359/jbmr.2001.16.12.2298. [DOI] [PubMed] [Google Scholar]
- Todd AC. Calculating bone-lead measurement variance. Environ Health Perspect. 2000;108:383–386. doi: 10.1289/ehp.00108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Miller G, Ding G, et al. Health risk assessment of lead for children in tinfoil manufacturing and e-waste recycling areas of Zhejiang Province, China. Sci Total Environ. 2012;426:106–112. doi: 10.1016/j.scitotenv.2012.04.002. [DOI] [PubMed] [Google Scholar]