Abstract
Preeclampsia is a pregnancy-related disorder characterized by hypertension, and could lead to maternal and fetal morbidity and mortality. Although the causative factors and pathophysiological mechanisms are unclear, endothelial dysfunction is a major hallmark of preeclampsia. Clinical tests and experimental research have suggested that generalized endotheliosis in the systemic, renal, cerebral and hepatic circulation could decrease endothelium-derived vasodilators such as nitric oxide, prostacyclin and hyperpolarization factor and increase vasoconstrictors such as endothelin-1 and thromboxane A2, leading to increased vasoconstriction, hypertension and other manifestation of preeclampsia. In search for the upstream mechanisms that could cause endothelial dysfunction, certain genetic, demographic and environmental risk factors have been suggested to cause abnormal expression of uteroplacental integrins, cytokines and matrix metalloproteinases, leading to decreased maternal tolerance, apoptosis of invasive trophoblast cells, inadequate spiral arteries remodeling, reduced uterine perfusion pressure (RUPP), and placental ischemia/hypoxia. RUPP may cause imbalance between the anti-angiogenic factors soluble fms-like tyrosine kinase-1 and soluble endoglin and the pro-angiogenic factors vascular endothelial growth factor and placental growth factor, or stimulate the release of other circulating bioactive factors such as inflammatory cytokines, hypoxia-inducible factor-1, reactive oxygen species, and angiotensin AT1 receptor agonistic autoantibodies. These circulating factors could then target endothelial cells and cause generalized endothelial dysfunction. Therapeutic options are currently limited, but understanding the factors involved in endothelial dysfunction could help design new approaches for prediction and management of preeclampsia.
Keywords: Endothelium, Nitric Oxide, Endothelin, Placental Ischemia, Growth Factors, Cytokines, Hypoxia, Oxidative Stress, Hypertension
1. INTRODUCTION
Normal pregnancy is associated with several maternal hemodynamic and cardiovascular changes in order to meet the oxygen and nutrient demands of the growing fetus including increased blood volume and cardiac output, systemic vasodilation, decreased vascular resistance and slight decrease in blood pressure (BP) (Poston et al., 1995; Thornburg et al., 2000). These hemodynamic and vascular changes involve increases in various endothelium-derived vasodilator substances and redistribution of blood flow in different maternal tissues and organs (Valdes et al., 2009; Tanbe and Khalil, 2010). In 5 to 8% of pregnancies, women may have hypertension in pregnancy (HTN-Preg). HTN-Preg could be manifested in one of four forms: chronic HTN that predates pregnancy, preeclampsia (PE)-eclampsia, chronic HTN with superimposed PE, and nonproteinuric gestational HTN (Ali and Khalil, 2015).
PE is diagnosed after the 20th week of pregnancy by new onset HTN (systolic pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg), often proteinuria and may be associated with edema and increased platelet aggregation (Brennan et al., 2014). PE may also be a part of HELLP syndrome, manifested as hemolysis, elevated liver enzymes and low platelet count. If untreated, PE may progress to eclampsia, characterized by severe HTN and convulsions, which could culminate into coma and death, causing an estimated 14% of pregnancy-related maternal deaths (Say et al., 2014). PE may also be associated with intrauterine growth restriction (IUGR) and preterm birth, causing ~13% of premature births in the United States (McBride et al., 2015). The complications associated with PE are particularly important in developing countries where the incidence of HTN-Preg is greater and the rates of maternal mortality and preterm births are higher than those in developed countries (Lain and Roberts, 2002).
Although PE is a major cause of maternal and fetal morbidity and a significant burden on the healthcare system, its etiology and pathophysiology remain unclear. The ambiguity of the cause of PE has made it difficult to develop efficient approaches for prevention or management, and delivery of the infant and placenta remains the only definitive treatment for PE and prevention of its progression to more serious maternal and fetal complications.
Clinical and experimental studies have suggested endothelial dysfunction as a major mechanism in HTN-Preg (Crews et al., 2000; Maynard et al., 2008; Sanchez-Aranguren et al., 2014; Tuzcu et al., 2015). PE could develop at early gestational age <34 weeks or late gestational age ≥34 weeks (Jardim et al., 2015). Early PE is generally linked to compromised trophoblast invasion, placental hypoxia and release of bioactive factors that could target the endothelium, while late PE has been linked to preexisting maternal conditions that could affect endothelial integrity (Brandao et al., 2014). Nevertheless, endothelial dysfunction is present in both early and late PE (Brandao et al., 2014). Interestingly, PE remits after delivery, pointing to the placenta as the main culprit. Clinical observations have implicated certain predisposing genetic, demographic and environmental factors that could cause placental maladaptations. Other studies have shown changes in the levels of various bioactive factors in the placenta and maternal circulation. However, because of the difficulty to perform mechanistic studies in pregnant women, it has been difficult to draw a definitive association between the predisposing factors, placental ischemia/hypoxia, circulating bioactive factors and endothelial dysfunction in humans. In order to further understand the pathophysiological mechanisms of PE, animal models of HTN-Preg have been developed. Both clinical observations and experimental studies have led to the suggestion that certain genetic and environmental risk factors may trigger reduction in uteroplacental perfusion pressure (RUPP) and the resulting placental ischemia/hypoxia could cause the release of bioactive factors that target endothelial cells (ECs) and cause endothelial dysfunction and HTN (Fig. 1). In support of this paradigm, studies have shown changes in the local and circulating levels of multiple factors including the pro-angiogenic vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), the anti-angiogenic factors soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng), cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), hypoxia-inducible factor (HIF), reactive oxygen species (ROS) and angiotensin II (AngII) type 1 receptor (AT1R) agonistic autoantibodies (AT1-AA). These bioactive factors could in turn cause generalized EC dysfunction and HTN, renal glomerular endotheliosis and increased glomerular permeability leading to proteinuria, and cerebral endotheliosis leading to cerebral edema and seizures (Ali and Khalil, 2015; Shah and Khalil, 2015).
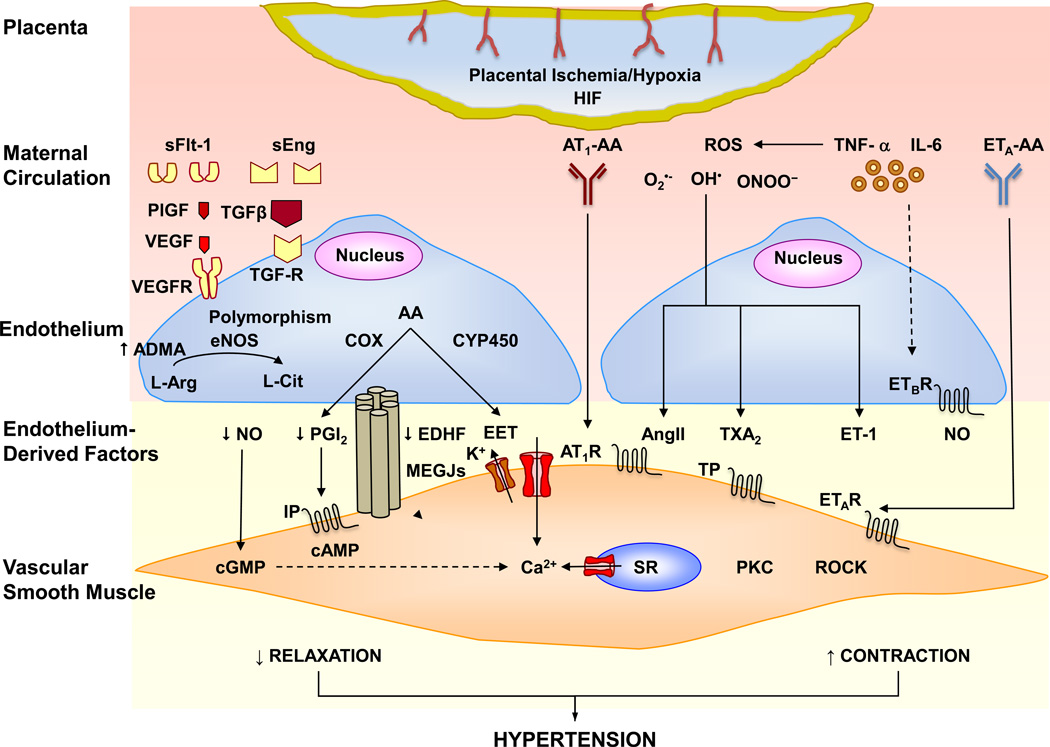
Fig. 1.
Circulating bioactive factors cause endothelial dysfunction and hypertension in preeclampsia. Placental ischemia/hypoxia cause an increase in HIF-1 and the release of circulating bioactive factors such as sFlt-1, sEng, AT1-AA, ROS, TNF-α, IL-6 and ETA-AA. Excessive sFlt-1 binds VEGF and PlGF and prevents their angiogenic effects mediated by VEGFR binding. sEng binds TGFβ and prevents its angiogenic effects mediated by activation of TGFβ receptor. sFlt-1 and sEng may also lead to endothelial dysfunction and decreased release of the endothelium-derived relaxing factors NO, PGI2, EDHF and EET. Polymorphisms in eNOS and elevated ADMA also decrease NO production. AT1-AA acts on AT1R and increases the mechanisms of VSM contraction. Cytokines such as TNF-α and IL-6 may increase the production of ROS, which decrease the bioavailability of NO and stimulate the release of vasoconstrictor substances as AngII, ET-1 and TXA2 which increase VSM [Ca2+]i, PKC, ROCK and stimulate VSM contraction. Endothelial ETBR mediates the release of NO, and downregulation of ETBR in PE decreases ETBR-mediated relaxation. ETA-AA stimulates ETAR and further stimulates VSM contraction. Decreased vascular relaxation and increased contraction cause HTN in PE. Solid arrows indicate stimulation. Dashed arrows indicate inhibition. AA, arachidonic acid; ADMA, asymmetric dimethylarginine; AngII, angiotensin II; AT1R, angiotensin II type 1 receptor; AT1-AA, AngII AT1R agonistic autoantibodies; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; COX, cyclooxygenase; CYP450, cytochrome 450; EC, endothelial cell; EDHF, endothelium-derived hyperpolarizing factor; EET, epoxyeicosatrienoic acid; eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; ETAR, endothelin receptor type A; ETA-AA, ETAR agonistic autoantibodies; ETBR, endothelin receptor type B; H2O2, hydrogen peroxide; HIF, hypoxia-inducible factor; IL-6, interleukin-6; MEGJs, myoendothelial gap junctions; IP, PGI2 receptor; NO, nitric oxide; O2•−, superoxide anion; OH−, hydroxyl ion; PGI2, prostacyclin; PKC, protein kinase C; PlGF, placental growth factor; ROCK, Rho-kinase; ROS, reactive oxygen species; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; SR, sarcoplasmic reticulum; TGFβ, transforming growth factor-β; TGF-R, TGFβ receptor; TNF-α, tumor necrosis factor-α; TXA2, thromboxane A2; TP, TXA2 receptor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; VSMC, vascular smooth muscle cell
In this chapter, we will discuss evidence of endothelial dysfunction in human PE and animal models of HTN-Preg. We will describe the potential upstream mechanisms that could cause endothelial dysfunction starting with the genetic, demographic and environmental risk factors, the changes in uteroplacental perfusion and placental ischemia, and the release of bioactive factors that could target ECs in the systemic circulation and various tissues and organs. We will also discuss how the advances in our knowledge of the potential predisposing factors and circulating bioactive factors involved in endothelial dysfunction could help design new biomarkers for diagnosis and novel approaches for management of PE.
2. ENDOTHELIAL DYSFUNCTION IN PREECLAMPSIA
Since the initial observations that ECs play a role in modulation of vascular tonus (Furchgott and Zawadzki, 1980), changes in ECs and the factors released by them have been observed in numerous physiological and pathological conditions. An increase in endothelial function during pregnancy is important for healthy gestation, ensuring a favorable prognosis for the mother and fetus (Brandao et al., 2014). In humans, endothelial function is often assessed using flow-mediated dilation (FMD) of the brachial artery, an ultrasonography test that evaluates EC response to temporary ischemia and reactive hyperemia (Brandao et al., 2014). During normal pregnancy, there is an increase in brachial artery diameter and FMD as gestation progresses (Sierra-Laguado et al., 2006). Also, endothelium-dependent bradykinin-induced relaxation is increased in human small subcutaneous arteries from pregnant compared with non-pregnant women (Knock and Poston, 1996). Experimental studies corroborated EC adaptations during pregnancy. ATP causes periodic bursts in intracellular free Ca2+ concentration ([Ca2+]i) that are more frequent in uterine artery ECs from pregnant compared with non-pregnant ewes (Yi et al., 2010), which could influence the vasodilator response and in turn attenuate the uterine artery myogenic tone and ensure adequate uterine blood flow during pregnancy (Hu et al., 2011).
In contrast with normal pregnancy, women with PE may show systemic EC dysfunction and HTN, glomerular endotheliosis causing renal injury and proteinuria, and cerebral endotheliosis leading to cerebral edema and seizures (Ali and Khalil, 2015). FMD of brachial artery is less in PE than normal pregnant (Norm-Preg) women (Brandao et al., 2014; Guimaraes et al., 2014) (Table 1). PE women also show less vasodilation in the radial artery (~7.9%) when compared to Norm-Preg women (~17.4%) (Yoshida et al., 2000).
Table 1.
Endothelium-derived factors and markers of endothelial function in human normal pregnancy and preeclampsia
| Factor | Specimen (Units) | Normal pregnancy | Preeclampsia | Reference |
|---|---|---|---|---|
| FMD | Brachial Artery (mm) | 17.55±8.35 9.00±5.00 |
5.36±4.61 4.00±6.00 Early PE 3.00±3.00 Late PE |
(Guimaraes et al., 2014) (Brandao et al., 2014) |
| Nitrite | Serum (µmol/L) Serum (µmol/L) Plasma (µmol/L) Plasma (µmol/L) Plasma (mM/mg pr) Plasma (nM) Urine (µmol/L) Placental Supernatant (µmol/L) |
~40 26.45±3.03 1st trimester 25.18±3.92 3rd trimester 7.26±9.65SD 58.1±40.3SD 8.68±2.27SD 177.2±151.3 361.41±423.12SD 24.9±6.9SD |
~48 16.83±0.48 1st trimester 31.28±2.58 3rd trimester 6.84±7.54SD 36.5±20.9SD 5.16±2.66SD 104.5±84.3 223.88±294.96SD 16.4±5.5SD |
(Noorbakhsh et al., 2013) (Matsubara et al., 2010) (Schiessl et al., 2006) (Ehsanipoor et al., 2013) (Pimentel et al., 2013) (Eleuterio et al., 2013) (Schiessl et al., 2006) (Ehsanipoor et al., 2013) |
| eNOS | Serum (pg/mL) Serum (pg/mL) |
0.86±0.64 1.46±0.31 |
0.67±0.55 1.12±0.16 Mild PE 0.88±0.23 Severe PE |
(Zawiejska et al., 2014) (Wang et al., 2015) |
| L-Arginine | Plasma (µM) | 62.00±31.06 | 19.23±10.54 | (Pimentel et al., 2013) |
| ADMA | Serum (µM) Plasma (µmol/L) |
0.68±0.20 2.14±0.33 |
0.86±0.16 1.88±0.19 |
(Bian et al., 2015) (Ehsanipoor et al., 2013) |
| cGMP | Plasma (pmol/mL) Urine (nmol/mL) |
6.82±2.57SD 612.95±440.63SD |
9.02±4.47SD 518.13±418.68SD |
(Schiessl et al., 2006) |
| 6-keto-PGF1α | Plasma (pg/mL) | 254.51±27.31 | 158.01±15.20 | (Lewis et al., 2010) |
| ET-1 | Serum (pg/mL) Serum (ng/mL) Placental Perfusate (pg/mL) - Maternal side - Fetal side |
17.15±6.14SD 45.9±50.1SD ~7 ~10 |
18.48±5.69SD 62.9±67.3SD(HELLP) ~11 ~22 |
(Celik et al., 2013) (Karakus et al., 2016) (Jain et al., 2014) |
| ETA-AA | Serum | Present | Absent | (Velloso et al., 2015) |
| TXB2 | Plasma (pg/mL) | 46.36±4.23 | 41.28±8.42 | (Lewis et al., 2010) |
|
Endothelial Progenitor Cells |
Whole blood (cells/mL) |
1918±563 | 620±105 | (Sakashita et al., 2014) |
| CEC | Whole blood (cells/mL) |
3.0±4.1SD 5.2±1.4 |
9.9±7.9SD 13.2±5.2 |
(Tuzcu et al., 2015) (Canbakan et al., 2007) |
| sVCAM-1 | Serum (ng/mL) | 404±147SD | 825±328SD | (Tuzcu et al., 2015) |
| E-Selectin | Serum (ng/mL) | 37.5±24.1SD | 69.5±38.3SD | (Tuzcu et al., 2015) |
| Endocan | Serum (ng/mL) Serum (ng/mL) Plasma (ng/mL) |
15.5±6.19 10.3±3.2 22.5 (13.8–44.4) |
20.04±12.26 10.7±4.5 18.2 (10.6–28.0) |
(Cakmak et al., 2016) (Yuksel et al., 2015) (Adekola et al., 2015) |
Values represent means±standard error of the mean.
indicates standard deviation.
Numbers in parenthesis indicate range.
6-keto-PGF1α, 6-keto-prostaglandin F1α; ADMA, asymmetric dimethylarginine; CEC, circulating endothelial cells; cGMP, cyclic guanosine monophosphate; eNOS, endotelial nitric oxide synthase; ET-1, endothelin-1; ETA-AA, ETA receptor agonistic autoantibodies; FMD, flow mediated dilation; HELLP, hemolysis elevated liver enzymes low platelets; PE, preeclampsia; sVCAM-1, soluble vascular cell adhesion molecule-1; TXB2, thromboxane B2
Studies in isolated human vessels have shown decreased bradykinin-induced relaxation in small subcutaneous arteries of PE compared with Norm-Preg women (Knock and Poston, 1996). However, no differences in the magnitude of relaxation or sensitivity to bradykinin were observed in the small myometrial arteries of PE versus Norm-Preg women (Kenny et al., 2002). While the endothelial dysfunction in the systemic vessels may explain the elevated BP during PE, the lack of change in the myometrial vessels may be a compensatory rescue mechanism to maintain blood supply to the fetus.
Circulating ECs (CEC) and other EC factors are often used as markers of endothelial activation/injury. Studies have shown an increase in CEC number and other markers of EC damage such as soluble vascular cell adhesion molecule-1 (sVCAM-1), E-selectin and endocan in PE compared with Norm-Preg women (Canbakan et al., 2007; Adekola et al., 2015; Tuzcu et al., 2015; Cakmak et al., 2016), although some studies reported no difference in serum endocan levels (Yuksel et al., 2015). Other studies have shown a decrease in circulating endothelial progenitor cells as a marker of endothelial damage in PE women (Sakashita et al., 2014) (Table 1).
Studies in animal models of HTN-Preg have supported endothelial dysfunction as an important mechanism in PE. The rat model of surgically-induced RUPP in late pregnancy has some of the characteristics of PE including high BP, proteinuria, decreased glomerular filtration rate (GFR) and renal plasma flow (RPF), and IUGR, and therefore has been used extensively to understand the molecular mechanisms of PE (Alexander et al., 2001a; Mazzuca et al., 2014; Amaral et al., 2015). Acetylcholine (ACh), a known stimulant of ECs, is less potent in inducing relaxation in the aorta and mesenteric microvessels of RUPP than Norm-Preg rats, suggesting endothelial damage in RUPP rats (Crews et al., 2000; Mazzuca et al., 2014). ECs release various factors that promote vascular relaxation such as nitric oxide (NO), prostacyclin (PGI2) and endothelium-derived hyperpolarizing factor (EDHF) as well as factors that cause vascular contraction such as endothelin-1 (ET-1) and thromboxane A2 (TXA2) (Fig. 1). A balance between relaxing and contracting factors is essential to maintain vascular tone, and endothelial dysfunction is associated with an imbalance in the release of these endothelium-derived factors.
3. DECREASED ENDOTHELIUM-DERIVED RELAXING FACTORS IN PREECLAMPSIA
Nitric Oxide (NO)
Nitric oxide (NO) is a potent vasodilator and relaxant of vascular smooth muscle (VSM). NO is a gaseous molecule generated from the conversion of L-arginine to L-citrulline by NO synthase (NOS). NO is produced by endothelial eNOS, inducible iNOS, and neuronal nNOS. NO diffuses into VSM and activates cyclic guanosine monophosphate (cGMP), which promotes Ca2+ efflux and thereby decreases VSM [Ca2+]i and causes VSM relaxation.
Studies using venous occlusion plethysmography showed that infusion of norepinephrine produced a smaller contractile response and that inhibition of NO production using Nω-monomethyl-L-arginine (L-NMMA) caused a greater reduction in hand blood flow in Norm-Preg than non-pregnant women, suggesting increased generation of NO in peripheral vascular beds in healthy pregnancies (Williams et al., 1997). Also, nitrites are important metabolites of NO used to indirectly determine NO production, and have been shown to be increased in serum of Norm-Preg compared with non-pregnant women (Shaamash et al., 2000). The plasma concentration and urinary excretion of cGMP, a second messenger of NO, are also increased in normal pregnancy. NOS expression and activity are also increased in human uterine artery (Nelson et al., 2000) and in the placenta along with gestational age (Dotsch et al., 2001), supporting increased NO production during normal pregnancy in humans.
Animal studies support a role of NO during normal pregnancy. Urinary levels of nitrites, eNOS, iNOS and nNOS mRNA expression. and the protein amount of activated phospho-eNOS are increased in Norm-Preg compared with virgin rats (Alexander et al., 1999). Also, BP is increased throughout pregnancy in eNOS knockout mice (Hefler et al., 2001). Other studies have reported an unexpected decrease in BP in eNOS knockout mice during pregnancy (Shesely et al., 2001), suggesting that other NOS isoforms could compensate for the loss of NO production via eNOS, or that NO may not be the only mediator of vascular homeostasis during pregnancy and other mediators could be triggered to maintain vascular function during eNOS deficiency. Of note, the diameter and medial cross sectional area of uterine artery are reduced, the number of viable pups and pup weight are less, and VSM cell (VSMC) differentiation and proliferation are impaired in pregnant eNOS knockout than Norm-Preg mice (van der Heijden et al., 2005), supporting a role of NO in vascular adaptations during pregnancy.
Endothelial dysfunction is often associated with decreased NO bioavailability due to either decreased synthesis or increased degradation, and changes in NO metabolism could be a factor in PE (Echeverri et al., 2015). Clinical studies have shown conflicting results, reporting increased (Noorbakhsh et al., 2013) or decreased (Schiessl et al., 2006; Ehsanipoor et al., 2013; Eleuterio et al., 2013; Pimentel et al., 2013) serum and plasma nitrite levels in PE compared with Norm-Preg women. Other studies have shown a decrease in serum levels of nitrites in PE during the first trimester that normalizes in the third trimester (Matsubara et al., 2010). Also, urinary nitrite levels may not differ in PE versus Norm-Preg women (Schiessl et al., 2006) (Table 1). The discrepancies in nitrite measurement could be related to the difficulty in controlling nitrate intake by diet. However, a study using carefully controlled dietary nitrate/nitrite intake did not show decreased NO production in PE women (Conrad et al., 1999b). Genetic polymorphisms of eNOS could also affect NO production. For instance, Norm-Preg women with the TT phenotype for the T-786C allele have lower plasma nitrite levels than those with the CC phenotype (Sandrim et al., 2010), and the TT phenotype has been proposed as a risk factor for PE in Tunisian women (Ben Ali Gannoun et al., 2015). Lower nitrite levels were also observed in women with the 4a4a versus 4b4b genotype for VNTR polymorphism (Sandrim et al., 2010).
Asymmetric dimethylarginine (ADMA) is a reversible competitive inhibitor of NOS that could also affect NO production (Ehsanipoor et al., 2013) (Fig. 1). The observations of decreased ADMA levels in normal pregnancy, and the association of abnormal uterine artery Doppler waveforms with high ADMA concentrations, suggest a role of endogenous NOS inhibitors in adversely affecting maternal vasodilation and BP (Myatt and Webster, 2009). Elevated circulating levels of ADMA are observed in PE and in women presenting with IUGR without PE (Myatt and Webster, 2009; Bian et al., 2015). However, other studies showed a decrease in plasma ADMA levels in PE versus Norm-Preg women (Ehsanipoor et al., 2013). NO production may also be affected by the availability of the NOS substrate L-arginine. While the plasma L-arginine levels do not differ in PE versus Norm-Preg women (Table 1), the transport of L-arginine to the platelets is reduced in PE, and could in turn affect NO production in PE women (Pimentel et al., 2013).
The lack of change in whole-body NO despite the increase in BP and the renal damage in PE suggest tissue-specific changes in NOS expression and NO bioavailability such that whole-body NO may not accurately reflect NO activity in the vasculature or the kidneys (Ali and Khalil, 2015). Studies have shown a decrease in nitrites in placentae from PE women (Ehsanipoor et al., 2013). Also, eNOS expression is decreased in umbilical cord of PE compared with Norm-Preg women (Bhavina et al., 2014), and the decrease is greater in women with severe PE (Zawiejska et al., 2014; Wang et al., 2015). However, some studies showed an increase in eNOS mRNA expression in placenta of PE women (Smith-Jackson et al., 2015). Also, while the levels of cGMP are increased during normal pregnancy, the plasma and urinary cGMP levels are not different in PE versus Norm-Preg women (Schiessl et al., 2006) (Table 1).
The role of NO has also been examined in animal models of HTN-Preg. In pregnant rats during mid to late gestation NOS blockade with Nω-nitro-L-arginine methyl ester (L-NAME) causes some of the changes observed in human PE including increases in BP and renal vasoconstriction, proteinuria, thrombocytopenia and IUGR (Khalil et al., 1998; Ramesar et al., 2011; Kemse et al., 2014), supporting a role of increased NO production during gestation. Similar to the observation in humans, measurements of NO production in HTN-Preg animals have not produced consistent results. Studies showed no difference in nitrite levels in rats treated with L-NAME compared with Norm-Preg (Ramesar et al., 2011). Also, while plasma nitrite levels were lower in RUPP than Norm-Preg rats (Amaral et al., 2015), no difference was observed in urinary nitrite levels (Alexander et al., 2001a; Javadian et al., 2013) (Table 2). Also, consistent with the studies in human, no changes were observed in the NOS substrate L-arginine in the circulation of RUPP versus Norm-Preg rats (Alexander et al., 2004) (Table 2).
Table 2.
Endothelium-derived factors and markers of endothelial function in Norm-Preg and RUPP rats
AU: arbitrary unit; DU: density unit
| Factor | Specimen (Units) | Norm-Preg | RUPP | Reference |
|---|---|---|---|---|
| Nitrite | Plasma (µmol/L) Urine (µmol/24 h) Urine (µmol/24 h) Aorta (pmol/mg) |
26.34±3.5 ~40 46.4±5.3 ~750 |
14.58±3.1 ~46 49.8±6.4 ~490 |
(Amaral et al., 2015) (Javadian et al., 2013) (Alexander et al., 2001a) (Mazzuca et al., 2014) |
| eNOS mRNA | Aorta (AU) | 0.65±0.11 | 0.33±0.01 | (Amaral et al., 2015) |
| L-arginine | Serum (nmol/mL) | 191±29 | 142±22 | (Alexander et al., 2004) |
| PreproET-1 | Renal Cortex (DU) Renal Medulla (DU) |
24.0±2.6 29.4±2.8 |
44.1±6.3 37.7±2.4 |
(Alexander et al., 2001b) |
| ET-1 | Plasma (pg/mL) | 0.46±0.06 | 0.99±0.25 | (Johnson et al., 2014) |
| TXB2 | Urine (pg/24 h) | 2646±257 | 3663±488 | (Llinas et al., 2002) |
Values represent means±standard error of the mean. eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; TXB2, thromboxane B2. AU: arbitrary unit of band intensity normalized to β-actin in Western blot analysis; DU: densitometry unit of band intensity normalized to β-actin in ribonuclease protection assay
Vascular function studies have shown an increase in aortic vascular reactivity to phenylephrine in pregnant rats treated with L-NAME (Khalil et al., 1998). Also, ACh-induced relaxation, eNOS expression, and NO production are reduced in mesenteric artery and aorta of RUPP versus Norm-Preg rats, supporting specific reduction in NO synthesis in the vasculature (Crews et al., 2000; Mazzuca et al., 2014; Amaral et al., 2015) (Table 2). In another rat model of HTN-Preg produced by infusion of deoxycorticosterone and replacement of drinking water with a 0.9% saline (DOCA-salt), NO-dependent relaxation was reduced in mesenteric vessels despite elevation of eNOS mRNA expression (Mitchell et al., 2007).
Thus while clinical observations and experimental studies point to the importance of NO during gestation and that its deficiency could be a possible mechanism in HTN-Preg, due to the difficulties in assessment of nitrite levels in humans and inconsistent data in animal models of HTN-Preg, whether NO production is decreased in PE needs to be further examined.
Prostacyclin (PGI2)
Prostacyclin (PGI2) is a prostanoid produced from the metabolism of arachidonic acid by cyclooxygenase-1 (COX-1) and COX-2. PGI2 promotes VSM relaxation and inhibits platelet aggregation acting via IP receptor, a cell surface G-protein coupled receptor. Stimulation of IP receptor in VSM causes an increase in cyclic adenosine monophosphate (cAMP) and leads to a decrease in [Ca2+]i and VSM relaxation (Fig. 1). PGI2 also promotes angiogenesis, decreases hypoxic injury by inducing neovascularization in ischemic tissues, and enhances the biological effects of proangiogenic factors in response to ischemia (Majed and Khalil, 2012).
Endothelium-derived PGI2 may contribute to the hemodynamic and vascular changes during pregnancy. Plasma and urinary levels of 6-keto-prostaglandin F1α (6-keto-PGF1α), a hydration product of PGI2, increase during pregnancy (Majed and Khalil, 2012). Also, PGI2 synthase mRNA and protein expression and 6-keto-PGF1α levels are elevated in pig endometrium during normal pregnancy (Morawska et al., 2012). However, vascular function studies showed little contribution of PGI2 to bradykinin-induced relaxation in small subcutaneous arteries from Norm-Preg women (Luksha et al., 2004).
Maternal plasma 6-keto-PGF1α levels are decreased in PE compared with Norm-Preg women (Lewis et al., 2010), and the decrease in PGI2 production may occur even before the onset of clinical symptoms of PE (Mills et al., 1999). As growth factors stimulate PGI2 production, changes in growth factors during PE may affect PGI2 levels. Also, hypoxia is a potential pathogenic mechanism in PE, which could cause downregulation of COX-1 with consequent reduction in endothelium-derived PGI2 (Shah and Khalil, 2015).
In pregnant rats, treatment with high doses of testosterone produces PE-like features, decreased placental IP receptor expression, and impaired PGI2-mediated relaxation in uterine artery (Chinnathambi et al., 2014), and the impaired relaxation may contribute to the increased vascular resistance and HTN-Preg. On the other hand, measurement of 6-keto-PGF1α concentrations showed no difference in human umbilical vein endothelial cells (HUVECs) from PE and Norm-Preg women (Parra et al., 2001). Interestingly, predominant basal directional release of constrictor prostanoids, but not PGI2, was observed in placental trophoblasts from PE pregnancies (Zhao et al., 2008). Thus, while PGI2 production may not be altered, the greater production of constrictor prostanoids and consequent imbalance between vasodilator and vasoconstrictor prostanoids may contribute to increased placental vasoconstriction in PE.
Endothelium-Derived Hyperpolarizing Factor (EDHF)
EDHF is a relaxing factor with particular importance in the control of small resistance vessels, local organ blood flow, peripheral vascular resistance and BP. EDHF’s role in vasodilation increases as arterial diameter decreases, causing greater dilation in the smaller distal than the bigger proximal uterine vessels (Gokina et al., 2010). Although the nature of EDHF is unclear, it often presents as K+ efflux from ECs through intermediate and small conductance Ca2+-activated K+ channels (IKCa and SKCa, respectively) causing hyperpolarization of ECs. EC hyperpolarization then spreads via myoendothelial gap junctions (MEGJs) and connexins to cause VSM hyperpolarization, reduction of Ca2+ influx via voltage-gated Ca2+ channels and suppression of the activity of phospolipase C, an enzyme involved in transduction pathways in VSM. The opening of EC IKCa and SKCa could also cause some accumulation of K+ ion in the myoendothelial interface which could induce VSM hyperpolarization by activating the inwardly rectifying K+ (KIR) channels and the Na+/K+-ATPase (Coleman et al., 2004). EDHF relaxation may also be caused by diffusible factors released from ECs. EDHF may be a product of cytochrome 450 (CYP450), such as epoxyeicosatrienoic acid (EET), which activate large conductance KCa (BKCa) and cause hyperpolarization in VSM (Fig. 1). In some vessels, hydrogen peroxide (H2O2) may mimic EDHF-mediated responses by mechanisms involving KCa activation (Shimokawa, 2010). Thus multiple EDHFs may exist and the identity of EDHF could vary depending on the vascular bed and animal species studied (Feletou and Vanhoutte, 2009).
In small subcutaneous and myometrial arteries of Norm-Preg women, EDHF is responsible for ~50% of bradykinin-induced relaxation, acting together with NO to maintain proper vascular tonus (Kenny et al., 2002; Luksha et al., 2004). The gap junction proteins connexins 37, 40 and 43 are partly involved in EDHF-mediated vascular response during normal pregnancy (Hammond et al., 2011). An increase in EC [Ca2+]i may activate IKCa and SKCa and promote EDHF-mediated dilation in uterine radial arteries of pregnant rats (Gokina et al., 2010). Also, PGI2 may activate endothelial SKCa in mesenteric arteries of Norm-Preg rats and cause EDHF-mediated activation of BKCa in VSM (Orie et al., 2006; Mandala et al., 2012). The delayed rectifier type of voltage-sensitive K+ channels (Kv) may also play a role in EDHF-mediated dilation in uterine artery of pregnant rats (Fulep et al., 2001).
Studies in subcutaneous arteries from Norm-Preg women have shown that MEGJs alone may be the main pathway in EDHF-mediated relaxation, while in women with PE MEGJs alone or in combination with H2O2 or CYP450 epoxygenase metabolites of arachidonic acid could mediate EDHF-induced vasodilation. The changes in the role of MEGJs may be caused by morphological changes within the vascular wall during PE (Luksha et al., 2008). Studies in mice have shown pregnancy-associated adaptations in the form of decreased sensitivity to phenylephrine and enhanced bradykinin-induced vasodilation in Norm-Preg wild-type mice but not in knockout mice lacking pregnane X receptor, a nuclear receptor that induces the expression of CYP450. Also, treatment with CYP450 inhibitor changed the vasodilatory response to bradykinin in wild-type but not the knockout mice, supporting that metabolites of CYP450 such as EET may play a role in the vascular adaptations during pregnancy (Hagedorn et al., 2007). As EET is one of the possible factors involved in EDHF-mediated relaxation, it is plausible to suggest that alterations in EDHF may lead to impaired vascular function and HTN-Preg. Although studies in mesenteric microvessels have suggested that the EDHF relaxation may not be compromised in RUPP versus Norm-Preg rats (Mazzuca et al., 2014), decreased EDHF-mediated relaxation is thought to contribute to the vasoconstriction observed in HTN and diabetes, and its role in PE needs further investigation.
4. INCREASED ENDOTHELIUM-DERIVED CONTRACTING FACTORS IN PREECLAMPSIA
Endothelin-1 (ET-1)
ET-1 is a major endothelium-derived vasoconstrictor that could play a role in PE (George and Granger, 2011). ET-1 synthesis is initiated by the production of the long 203 amino acid preproET, which is cleaved by furin-like protease to biologically inactive 37 to 41 amino acid big-ET. Big-ET is cleaved by ET converting enzymes (ECEs), members of the metalloprotease family, to produce active 21 amino acid ET-1.
Some of the circulating factors in PE including cytokines, hypoxia and AT1-AA may stimulate ECs to produce ET-1 (George and Granger, 2011). This is supported by reports that serum from PE women causes HUVECs to produce greater amounts of ET-1 than Norm-Preg serum (Scalera et al., 2001). Some studies suggest that plasma ET-1 levels are elevated in PE (Roberts, 1998). ET-1 levels are higher during later stages of PE and return to normal levels within 48 hours after delivery (Taylor et al., 1990), suggesting that ET-1 may be involved in the progression rather than the initiation of PE. However, in most studies serum ET-1 levels do not differ in PE versus Norm-Preg women, and higher levels of ET-1 are observed mainly in patients with HELLP syndrome (Naiker et al., 2001; Celik et al., 2013; Karakus et al., 2016). Of note, ET-1 is released in a paracrine fashion from EC directly on VSMCs, and the increases in ET-1 levels in PE may be localized in tissues. Studies have shown a 4- to 8-fold increase in ET-1 levels in umbilical cord cells (Kourembanas et al., 1993) and in renal tissues during later stages of PE (Taylor et al., 1990). In perfused placentas under hypoxia, both the maternal and fetal side produced increased levels of ET-1 (Jain et al., 2014). In RUPP rats, preproET levels show a 45% increase in renal cortex and 22% increase in the medulla (Alexander et al., 2001b). Thus, measurements of circulating ET-1 may not accurately reflect what happens locally in tissues. It is possible that in severe PE and in HELLP syndrome the production of ET-1 is so augmented that ET-1 release “loses” its paracrine directionality and consequently an increase in circulating ET-1 levels is detected. This is supported by reports that rat models that mimic severe PE and HELPP syndrome have increased plasma ET-1 levels (Johnson et al., 2014; Morris et al., 2015) (Table 2).
ET-1 may play a role in the pathogenesis of PE by inducing apoptosis of trophoblast cells (TCs) and increasing oxidant and anti-angiogenic substances (Fiore et al., 2005; George and Granger, 2011), but most studies have focused on the effects of ET-1 on the vasculature. ET-1 activates endothelin receptor type A (ETAR) and endothelin receptor type B (ETBR) (Mazzuca and Khalil, 2012). ET-1 activation of VSM ETAR stimulates Ca2+ release from the intracellular stores and Ca2+ entry through Ca2+ channels, and causes protein kinase C (PKC)-dependent inhibition of K+ channels leading to increased [Ca2+]i and VSM contraction (Mazzuca and Khalil, 2012) (Fig. 1). Of note, during hypoxia, up to 80% of ET-1 induced contraction is independent of [Ca2+]i or PKC (Weigand et al., 2006). ET-1-induced vasoconstriction is reduced in mesenteric vessels of Norm-Preg compared with non-pregnant rats (Mazzuca et al., 2013), and VSM ETAR and ETBR are reduced in aortic media and VSMCs of late-pregnant rat (Ou et al., 2014). Interestingly, among Brazilian women with PE, 52% of the patients diagnosed with severe PE exhibited increases in ETAR agonistic autoantibodies (ETA-AA) which could target ETAR and increase vasoconstriction (Velloso et al., 2015). Experimental studies have also suggested increases in ETAR activity in PE and have shown that treatment with ETAR antagonist reduces BP in RUPP and other models of HTN-Preg (Alexander et al., 2001b; LaMarca et al., 2005; Murphy et al., 2010)..
ET-1 also activates ETBR in ECs and stimulates the release of NO, PGI2, and EDHF which in turn reduce myogenic vascular tone, promote vasodilation of renal arteries and hyperfiltration in pregnant rats (Conrad et al., 1999a; Mazzuca and Khalil, 2012). ETBR may be reduced in ECs and renal cells of RUPP rats, and downregulation of ETBR may impair trophoblast invasion in PE, and decrease microvascular vasodilator activity in RUPP rats. Also, ETBR-mediated NO production is less in the aorta and mesenteric artery of RUPP than Norm-Preg rats, suggesting that downregulation of endothelial ETBR could play a role in HTN-Preg (Mazzuca et al., 2014).
Thromboxane A2 (TXA2)
TXA2 is one of the COX-1 prostanoid products in the platelets. TXA2 stimulates platelet aggregation, and VSMC proliferation and mitogenesis (Schramm and Clowse, 2014). TXA2 also causes vasoconstriction by activating prostanoid receptors, [Ca2+]i and protein kinases such as PKC, mitogen-activated protein kinase (MAPK) and Rho-kinase (ROCK) in VSM (Goulopoulou et al., 2012) (Fig. 1). Studies have shown decreased effects of TXA2 analogs in uterine artery of Norm-Preg guinea pigs (Weiner et al., 1992). Other studies have shown that the overall response to TXA2 analogs is not altered during pregnancy in rats, and any changes in vasoconstriction may be further down through PKC and ROCK signaling pathways (Goulopoulou et al., 2012).
Increased lipid peroxides may activate COX-1 and in turn increase TXA2 synthesis by the platelets in PE (Walsh, 2004). Some studies suggest that TXB2, a stable metabolite of TXA2, is increased in placentas of PE compared with Norm-Preg women (Wang et al., 1992). Other studies have shown no difference in plasma TXB2 levels, but rather a 25% reduction in 6-keto-PGF1α/TXA2 ratio in PE versus Norm-Preg women (Lewis et al., 2010). Also, TCs from PE women produce more TXA2 than Norm-Preg TCs (Zhao et al., 2008). A decrease in production of PGI2 and an increase in TXA2 may contribute to the vascular dysfunction in PE (Walsh, 2004).
In experimental studies, measurements of TXB2/6-keto-PGF1α ratio have shown greater levels in a transgenic activated renin-angiotensin system rat model of HTN-Preg compared with Norm-Preg rats (Verlohren et al., 2008). Also, uterine artery rings from the transgenic HTN-Preg rats showed relaxation at low ACh doses, but increased contraction at higher doses. This endothelium-dependent contraction was blocked by indomethacin or a TXA2 receptor blocker, suggesting that high ACh doses may facilitate the release of endothelial vasoconstrictor prostanoids, and this mechanism may contribute to the HTN during PE. Studies have shown that infusion of TXA2 analogs causes HTN in pregnant rats (Losonczy et al., 1995; Kriston et al., 1999). While some studies showed that urinary excretion of TXB2 was higher in RUPP than Norm-Preg rats, infusion of a TXA2 receptor antagonist did not reduce BP or change GFR or effective renal plasma flow (RPF) in RUPP rats, suggesting that increased TXA2 production may not play a major role in mediating HTN and renal vasoconstriction in RUPP rats (Llinas et al., 2002) (Table 2). These conflicting reports make it important to further test the role of TXA2 in HTN-Preg and PE.
5. RISK FACTORS FOR ENDOTHELIAL DYSFUNCTION IN PREECLAMPSIA
While several studies support a role of endothelial dysfunction in PE, the factors leading to endothelial damage remain the subject of extensive research. Several predisposing risk factors including genetic, demographic and environmental factors have been suggested to cause localized abnormalities in placental development, and these abnormalities would in turn lead to RUPP, placental ischemia/hypoxia, release of circulating bioactive factors and consequently endothelial dysfunction and HTN-Preg.
Genetic Risk Factors for Preeclampsia
Placental gene mutations are associated with the incidence of PE, and 31 out of 36 placental genes are downregulated in PE (Founds et al., 2009). Mutations in placental mitochondrial genes could cause incomplete reduction of O2 and in turn increase production of ROS and lead to oxidative stress in the placenta and maternal vessels (Trifonova et al., 2014). ACVR2A on chromosome 2q22 and STOX1 on chromosome 10q22 are two of the first PE susceptibility genes identified and both involve normal variations single nucleotide polymorphism. STOX1 Y153H common polymorphism was observed in families where several generations of women exhibited severe early PE, and has been linked to trophoblast dysfunction and IUGR (van Dijk and Oudejans, 2011). Also, wild-type female mice crossed with transgenic male mice overexpressing human STOX1 gene show characteristics of PE including HTN and proteinuria (Doridot et al., 2013). Other genes such as FOXP3 are important in activation of regulatory T cell (Tregs) and the control of immune response and maternal tolerance during normal pregnancy, and downregulation of FOXP3 and its polymorphism may predispose women to PE (Sasaki et al., 2007; Rahimzadeh et al., 2016). Polymorphisms of eNOS gene could also be a risk factor for PE. The VNTRa and 894T alleles of eNOS gene are associated with early and late severe PE, respectively. For the eNOS VNTRb/a polymorphism, plasma NO metabolites are lower in subjects homozygous for the “a” allele. Also, the eNOS 894T allele is subject to selective proteolytic cleavage in ECs and vascular tissues, and this could account for the reduced vascular NO generation in homozygous subjects for this variant (Alpoim et al., 2014). The T−786C allele is also increased in PE compared with Norm-Preg women (Ben Ali Gannoun et al., 2015; Leonardo et al., 2015). Paternal genes may also play a role, and a paternal history of PE was associated with a 2.7% risk of PE when compared to men whose mothers had normal pregnancy (Esplin et al., 2001). However, other studies suggest a limited role of paternal genes in the development of PE (Boyd et al., 2013).
Demographic, Environmental, and Other Risk Factors for Preeclampsia
Ethnic background, extreme maternal age <16 or >40 years old, personal and family history of PE, primiparity, multiple pregnancy and environmental factors have been suggested as risk factors for PE (Tanbe and Khalil, 2010; Jardim et al., 2015; Shah and Khalil, 2015).
Ethnic background may influence the incidence of PE, with African-American women having the highest rate (5.2%) while Asian women have the lowest rate (3.5%) (Rosenberg et al., 2005). Age could also be a factor, and studies from Finland and India showed that women with advanced age may be at higher risk of developing PE than young women (Lamminpaa et al., 2012; Kanagal et al., 2014). The maternal lifestyle, diet and pre-pregnancy overweight or obesity could increase the risk of PE (Wei et al., 2015). While the incidence of PE is ~3% in normal weight women (body mass index BMI=18.5–24.9), the incidence increases to 7% in women with class I obesity (BMI=30–34.9) and to 13% in super-obese women (BMI=50) (Spradley et al., 2015). Preexisting medical condition such as diabetes, renal or cardiac disease, systemic lupus erythematosus, mental stress, previous neonatal macrosomia, history of reproductive tract surgery and antepartum hemorrhage, and chronic respiratory conditions may also be associated with PE (Tanbe and Khalil, 2010). Of note, some risk factors such as advanced age and obesity or medical conditions such as diabetes are often associated with endothelial damage and the preexisting endothelial dysfunction could be an important mechanism in the development of PE.
6. PLACENTAL ISCHEMIA AS AN INITIATING EVENT IN PREECLAMPSIA
The placenta is a maternal-fetal interface developed in early-pregnancy. Vasculogenesis, angiogenesis and trophoblast-mediated remodeling are required to ensure proper placental and vascular development. Vasculogenesis is the development of de novo vessels from pluripotent mesenchymal stem cells ~18–35 days after conception in humans. Angiogenesis is the formation of new blood vessels by branching from preexisting ones and is regulated by the actions of pro-angiogenic factors and the invasive capacity of TCs (Pereira et al., 2015).
Adequate placental vascularization is essential for healthy pregnancy. During the first trimester, placental extravillous trophoblasts (EVTs) invade the maternal decidua up to one-third of the myometrium and change the spiral arteries from low-capacity high-resistance into high-capacity low-resistance vessels, thus ensuring sufficient blood supply to the growing fetus (Fig. 2). This also involves substitution of arterial VSM and elastic tissue with fibrinoid material (VanWijk et al., 2000).
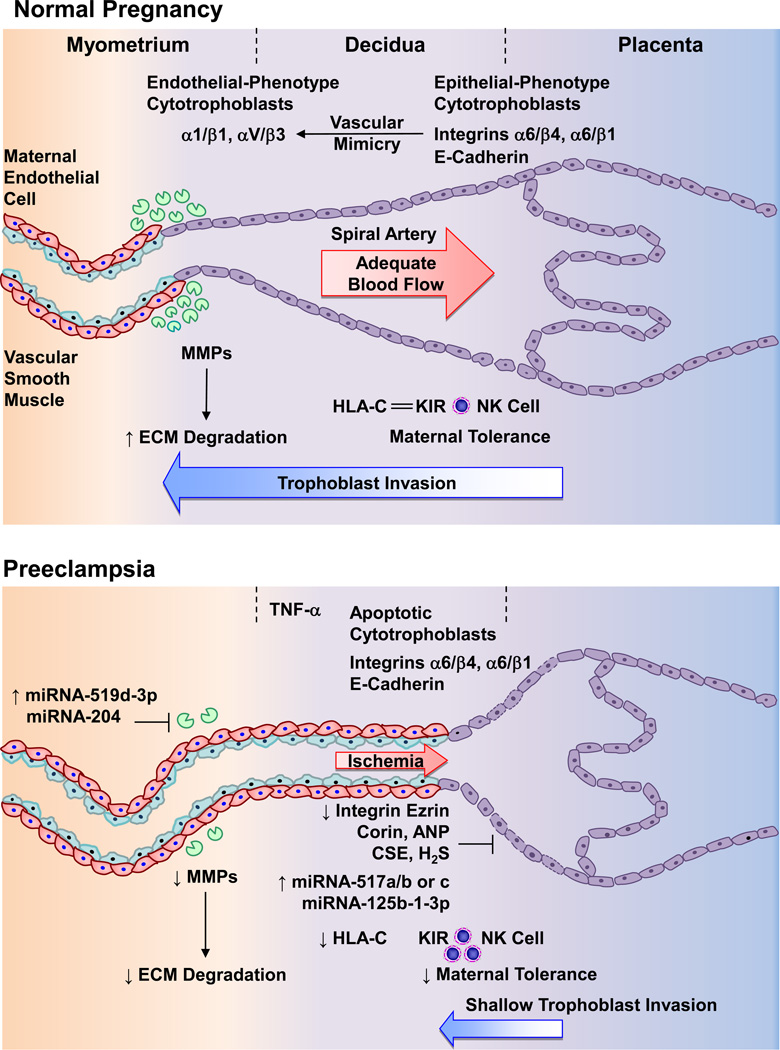
Fig. 2.
Deficient placentation in preeclampsia. During normal pregnancy, cytotrophoblasts initially express adhesion molecules characteristic of epithelial cells such as integrins α6/β4 and α6/β1, and E-cadherin. As cytotrophoblasts take the invasive pathway, they express endothelial-phenotype integrins α1/γ1 and αV/β3 (“vascular mimicry”). MMPs also cause degradation of ECM and allow vascular remodeling. Cytotrophoblasts overexpress HLA-C that interact with the inhibitory KIR receptor and decrease NK cells, leading to maternal tolerance. As a result, cytotrophoblasts invade the decidua to one-third of the myometrium, causing remodeling of the spiral arteries from small-caliber resistance to high-caliber capacitance vessels, and adequate placental blood flow. In PE, increased immune response cause release of cytokines such as TNF-α, apoptosis of cytotrophoblasts and continued expression of integrins α6/β4 and α6/β1, and E-cadherin. Decreased expression of the integrin ezrin, corin/ANP, and CSE/H2S pathway and increased miRNA-517a/b, -517c, and -125b-1-3p, prevent cytotrophoblasts from changing into an invasive endothelial phenotype. Increased miRNA-519d-3p and -204 also decrease MMPs leading to decreased ECM degradation and vascular remodeling. Decreased HLA-C interaction with the inhibitory KIR receptor increases NK cells and further decreases maternal tolerance. The decrease in trophoblast invasion of spiral arteries and vascular remodeling leads to shallow placentation to only superficial layers of the decidua, leading to decreased blood flow and placental ischemia. ANP, atrial natriuretic peptide; CSE, cystathionine gamma-lyase; ECM, extracellular matrix; H2S, hydrogen sulfide; MMP, matrix metalloproteinase; NK, natural killer cell
The remittance of symptoms of PE after delivery has pointed to the placenta as a central culprit in this disorder. Abnormal placental development, RUPP and consequent placental ischemia/hypoxia are important initial events in PE (Alexander et al., 2001a; Khalil and Granger, 2002; Gilbert et al., 2007). Inadequate placentation could be initiated by abnormal expression of cytokines, major histocompatibility complex (MHC) molecules, natural killer (NK) cells, and macrophages leading to abnormal inflammatory and immune responses and apoptosis of TCs. Also, abnormal expression of integrins and matrix metalloproteinases (MMPs) could lead to decreased extracellular matrix (ECM) remodeling, shallow trophoblastic invasion and poor remodeling of spiral arteries (Fig. 2).
Immune Responses and Inadequate Placentation in Preeclampsia
Human pregnancy poses a challenge to maternal immune tolerance. Mother must tolerate the semi-allogenic fetus throughout gestation and the fetus must be protected from rejection by the maternal immune system (Zarate et al., 2014). In PE pro-inflammatory cytokines such as TNF-α and IL-6 are increased and the inflammatory responses are enhanced. Interestingly, in conditions associated with suppressed immune response such as HIV, the incidence of HTN disorders and PE seems to be lower (Hall et al., 2014).
In normal pregnancy, TCs overexpress the MHC-I molecules HLA-C, HLA-E and HLA-G which interact, respectively, with the inhibitory KIR, CD 94/NKGs and ILT-2 receptors on NK cells and thereby prevent maternal NK cells from killing placental and fetal tissues (Trowsdale and Moffett, 2008). Decreased HLA-C/KIR interaction increases the risk of PE (Hiby et al., 2004). The decidual NK (dNK) cells are the most abundant immune cells at the maternal-fetal interface constituting 50–70% of the host defense cells. dNK cells may be stimulated by IL-15 to release interferon-γ (INF-γ) which is important for remodeling of the spiral arteries, and a decrease in placental IL-15 mRNA expression and protein levels may be associated with PE (Agarwal et al., 2001), although other studies link an increase in IL-15 and release of INF-γ to higher incidence of miscarriages and PE (Sones et al., 2014).
Activation of complement system is important to the development of healthy pregnancy; however, excess activation of complement can lead to PE. In PE, increased levels of complement activation products Bb, C3a and C5a are observed (Lillegard et al., 2013). Also, studies have found more neutrophils adhered to the endothelium of resistance-sized vessels in the subcutaneous fat of PE compared with Norm-Preg women. As neutrophils may produce toxic substances, the higher neutrophil infiltration may explain the endothelial dysfunction in PE (Leik and Walsh, 2004). In RUPP rats, inhibition of complement activation causes a decrease in BP independent of alterations in pro-angiogenic levels, suggesting complement activation in HTN-Preg (Lillegard et al., 2013). Also, depletion of neutrophils causes a decrease in BP in RUPP rats, supporting a role for innate immune response in HTN-Preg (Regal et al., 2015).
Integrins and Reduced TC Invasion and Spiral Arteries Remodeling in Preeclampsia
Integrins, adhesion molecules and other factors could affect trophoblast invasion and remodeling of the spiral arteries. Cytotrophoblasts initially express adhesion molecules characteristic of epithelial cells such as integrins α6/β4 and α6/β1, and E-cadherin. In normal pregnancy as cytotrophoblasts take the invasive pathway, epithelial cell-like adhesion molecules cease to be expressed and expression of endothelial-phenotype integrins α1/β1 and αV/β3 is observed; a process referred to as vascular mimicry or pseudovasculogenesis (McMaster et al., 2004) (Fig. 2). During hypoxia, expression of fibronectin and integrin α5 are high while expression of integrin α1 is low, suggesting that decreased oxygenation could alter the synthesis of integrins and fibronectin in the placenta (Iwaki et al., 2004). In PE, abnormal expression of epithelial cell-like adhesion molecules and apoptosis of TCs lead to limited invasion of spiral arteries, RUPP and placental ischemia (McMaster et al., 2004; Roberts and Escudero, 2012; van Dijk and Oudejans, 2013). Ezrin, an integrin involved in cell surface adhesion, migration and organization, is downregulated in PE syncytiotrophoblast microvesicles, leading to shallow trophoblast invasion and defective placental vascularization (Baig et al., 2014). Also, retention of smooth muscle in the spiral arteries increases the vessels constriction (Perez-Sepulveda et al., 2014), further decreasing uteroplacental blood flow and promoting placental ischemia (Fig. 2).
Other molecules could influence trophoblast invasion and vascular remodeling during pregnancy. During normal pregnancy, downregulation of EC adhesion molecules minimizes leukocyte adhesion to ECs and thereby ensures patency and maintained blood flow in the spiral arteries. In contrast, in PE, plasma levels of soluble intracellular adhesion molecule-1 (sICAM-1) and, sVCAM-1 are increased, which may facilitate leukocyte adhesion and decrease blood flow in spiral arteries (Fei et al., 2012; Rios et al., 2015).
Corin is a membrane-bound protease that activates atrial natriuretic peptide (ANP), an important factor for trophoblast invasion. Corin expression is decreased in uterus of PE versus Norm-Preg women. Also, corin knockout mice have high BP and proteinuria in late pregnancy and show impaired trophoblast invasion and spiral artery remodeling (Cui et al., 2012).
Hydrogen sulfide (H2S), a gaseous molecule endogenously produced by the enzyme cystathionine gamma-lyase (CSE), may also play a role in PE. Inhibition of CSE abolishes the invasion of first-trimester EVTs, suggesting that dysregulation of the CSE/H2S pathway may dysregulate maternal spiral artery remodeling and placental development. In support, low levels of H2S were reported in PE plasma (Wang et al., 2013).
MicroRNA (miRNA) may also play a role in impaired TC invasion in PE. miRNA-125b-1-3p is overexpressed in PE placentas and inhibits the expression of S1PR1, a G-coupled receptor in human EVTs that facilitates TC invasion, and the decrease in S1PR1 expression may decrease TC invasion (Li et al., 2014a). Also, miRNA-517a/b and miRNA-517c are overexpressed in PE placentas, and in vitro studies of EVTs have shown that under hypoxic conditions these miRNAs are expressed and cause a decrease in TC invasion (Anton et al., 2015).
Due to defective trophoblast invasion, arterial blood flow becomes intermittent, resulting in periods of ischemia/reperfusion and a hypoxic environment which favors oxidative stress, oxidative damage and inflammation, and lead to endothelial dysfunction in PE (Sanchez-Aranguren et al., 2014).
Role of MMPs in Abnormal Placentation
MMPs are zinc-dependent degradative enzymes which include collagenases, gelatinases, stromelysins and other subtypes, and are involved in tissue remodeling and other cellular functions (Montagnana et al., 2009; Li et al., 2014b). MMPs are produced as pro-MMPs which are cleaved into active MMPs and play a role in endometrial tissue remodeling during the estrous cycle, menstrual cycle and pregnancy in human and animals (Li et al., 2014b).
MMP-2 and -9 may be involved in ECM remodeling and trophoblast invasion of the spiral arteries during pregnancy. MMP-2 is the main collagenolytic enzyme in umbilical cord artery (UCA) (Ali and Khalil, 2015) and serum MMP-9 levels are elevated in Norm-Preg women (Montagnana et al., 2009) (Table 3). MMP-2 and -9 expression and gelatinase activity are upregulated in the myometrium and aorta of Norm-Preg rats (Li et al., 2014b). Genetic polymorphisms may alter MMP-2 and -9 transcription in PE (Palei et al., 2013a). Also, miRNA-519d-3p and miRNA-204 are overexpressed in PE patients (Choi et al., 2013; Li et al., 2013) and could target MMP-2 and MMP-9, respectively and in turn decrease trophoblast invasiveness (Yu et al., 2015) (Fig. 2). Some studies showed an increase in circulating MMP-2 and -9 in PE versus Norm-Preg women (Eleuterio et al., 2015) (Table 3). Other studies showed a decrease in serum MMP-9 in PE (Montagnana et al., 2009). MMP-2 and MMP-9 levels are also reduced in uterus, placenta and aorta of RUPP rats (Table 4), and low MMP levels may cause excessive collagen deposition, affect smooth muscle growth and decrease spiral arteries remodeling (Li et al., 2014b). Also, in an in vitro model of first trimester trophoblasts, suppression of MMP-9 expression inhibited TC invasive capability, supporting a role of MMP-9 in modulating trophoblast invasion (Yu et al., 2015). MMP-1 is also expressed in cytotrophoblasts and syncytiotrophoblasts of the placenta and decidua and may play a role in trophoblast invasion. MMP-1 levels in the umbilical cord blood, placenta and decidua are lower in PE than Norm-Preg women, and the low levels of MMP-1 are in a positive correlation with the severity of the disease (Deng et al., 2015).
Table 3.
Bioactive factors in human normal pregnancy and preeclampsia
| Factor | Specimen (Units) | Normal pregnancy | Preeclampsia | Reference |
|---|---|---|---|---|
| VEGF | Serum (ng/mL) Serum (pg/mL) Serum (pg/mL) Plasma (ng/mL) Villous explants (ng/mL/mg protein) |
13.9 14.20±14.54SD 90.55 (90–521) 6.83 (5.32–8.94) 55±64 |
51.7 314.45±260.74SD 90 (90–211) 25.24 (21.22–42.92) 117±3.7 |
(Hunter et al., 2000) (Celik et al., 2013) (Masoura et al., 2014) (Tsatsaris et al., 2003) (Ahmad and Ahmed, 2004) |
| PlGF | Serum (pg/mL) Serum (pg/mL) Serum (pg/mL) Plasma (pg/mL) Plasma (pg/mL) |
432.9 (359.6–815.2) 217.30±74.48 183 (126–307) 585.9 (356–1101) 324.6 (101.8–881.4) ≤34 weeks 206.8 (60.9–395.7) >34 weeks |
65.5 (33.0–101.2) 115.72±32.55 98.0 (63.7–146) 67.41 (57.1–118.5) 18.3 (9.9–22.5) Early PE 82.6 (57.1–215.6) Late-PE |
(Ramma et al., 2012) (Bian et al., 2015) (Molvarec et al., 2015) (Tsatsaris et al., 2003) (March et al., 2015) |
| sFlt-1 | Serum (ng/mL) Serum (ng/mL) Serum (ng/mL) Serum (pg/mL) Plasma (ng/mL) Plasma (pg/mL) Villous explants (ng/mL/mg protein) |
1.5 (1.1–1.8) 1.8±1.6SD 0.308±0.019 3252 (2509–4751) 0.12 (0–0.29) 3391 (2412–4918) ≤34 weeks 4378 (2618–5731) >34 weeks 28±1.7 |
21.5 (15.2–30.5) 6.6±5.5SD 0.321±0.023 6814 (3736–12.720) 2.69 (2.31–2.97) 12895 (8303–17417) Early PE 6304 (3127–10638) Late PE 128±9.8 |
(Ramma et al., 2012) (Tuzcu et al., 2015) (Bian et al., 2015) (Molvarec et al., 2015) (Tsatsaris et al., 2003) (March et al., 2015) (Ahmad and Ahmed, 2004) |
| sFlt-1 e15a | Serum (pg/mL) | ~10000 | ~80000 | (Palmer et al., 2015) |
|
sFlt-1/PlGF ratio |
Serum Plasma |
15.6 (8.52–36.6) 9.6 (3.5–58.6) ≤34 weeks 22.4 (10.2–58.7) >34 weeks |
70.5 (31.8–144) 703.1 (146.6–1614.9) Early PE 77.0 (18.3–145.1) Late PE |
(Molvarec et al., 2015) (March et al., 2015) |
| sEng | Serum (ng/mL) Serum (ng/mL) Serum (ng/mL) Serum (ng/mL) |
~10 4.3 (3.5–6.1) 9.8 13.3 |
~38 Mild PE ~50 Severe PE ~100 HELLP 70.1 (41.3–109.4) 46.4 Early PE 31.0 Late PE |
(Venkatesha et al., 2006) (Ramma et al., 2012) (Levine et al., 2006) (Levine et al., 2006) |
| TNF-α | Serum (pg/mL) Monocytes supernatant (pg/mL) |
14.62±5.61 ~25 |
26.49±12.14 ~130 |
(Cakmak et al., 2016) (Matias et al., 2015) |
| LIGHT | Plasma (pg/mL) | ~2 | ~46 | (Wang et al., 2014) |
| IL-6 | Serum (pg/mL) | 0.6 (0.4–1.0) | 1.1 (0.6–7.9) | (Ramma et al., 2012) |
| IL-1β | Serum (pg/mL) Monocytes supernatant (pg/mL) |
0.10 ~260 |
0.16 ~600 |
(Siljee et al., 2013) (Matias et al., 2015) |
| IL-17 | Serum (pg/mL) | 0 | 0.47 (0–0.53) | (Molvarec et al., 2015) |
| IL-18 | Monocytes supernatant (pg/mL) |
~14 | ~22 | (Matias et al., 2015) |
| HIF-1α | Blood (µL) | ~0.8 | ~2.4 | (Akhilesh et al., 2013) |
| O2•− | Neutrophills Monocytes |
3.63±0.91 nmol/106 cellsSD ~1.7 nmol/105 cells |
6.20±0.92 nmol/106 cellsSD ~2.2 nmol/105 cells |
(Tsukimori et al., 2005) (Peracoli et al., 2011) |
| H2O2 | Monocytes | ~1.4 nmol/105 cells | ~1.7 nmol/105 cells | (Peracoli et al., 2011) |
| HO-1 mRNA | Blood (RC) | 9.87 (8.61–10.53) | 9.13 (5.42–10.19) | (Nakamura et al., 2009) |
| HO-2 mRNA | Blood (RC) | 7.05 (3.19–7.47) | 6.81 (4.73–7.34) | (Nakamura et al., 2009) |
| SOD mRNA | Blood (RC) | 5.91 (4.95–6.44) | 5.40 (3.90–6.23) | (Nakamura et al., 2009) |
| GPx mRNA | Blood (RC) | 7.56 (7.03–8.10) | 6.90 (4.54–7.52) | (Nakamura et al., 2009) |
| CAT mRNA | Blood (RC) | 7.38 (4.39–7.77) | 7.07 (4.90–7.63) | (Nakamura et al., 2009) |
| TAOC | Serum (mmol/L) | 1.1 (1.0–1.2) | 0.5 (0.2–0.6) | (Turpin et al., 2015) |
| Ang II | Chorionic villi | 15±2 fmol/mg protein | 26±6 fmol/mg protein | (Anton et al., 2008) |
| AT1R mRNA | Chorionic villi (RGE) |
1.0±0.1 | 3.0±0.7 | (Anton et al., 2008) |
| AT1-AA | Serum (OD) Serum (OD) Serum (RLU) % Over baseline |
0.27±0.12 0.315±0.093 ~12 |
0.54±0.13 OD 0.703±0.132 Early PE 0.567±0.111 Late PE ~75 |
(Bai et al., 2013) (Yang et al., 2015) (Siddiqui et al., 2013) |
| MMP-1 | Umbilical serum (pg/mL) |
294.33±11.53 | 177.67±12.63 | (Deng et al., 2015) |
| MMP-2 | Serum (ng/mL) Plasma (ng/mL) |
669 (560–760) 241.1±35.3SD |
834 (656–1002) 290.5±48.4SD |
(Montagnana et al., 2009) (Eleuterio et al., 2015) |
| MMP-9 | Serum (ng/mL) Plasma (ng/mL) |
390 (277–569) 240.0±197.7SD |
290 (280–470) 262.4±153.8SD |
(Montagnana et al., 2009) (Eleuterio et al., 2015) |
| TIMP-1 | Serum (ng/mL) Plasma (ng/mL) Umbilical serum (pg/mL) |
148 (121–188) 142.8±39.2SD 1304.20±69.66 |
213 (212–220) 187.1±35.4SD 1363.00±71.50 |
(Montagnana et al., 2009) (Eleuterio et al., 2015) (Deng et al., 2015) |
| TIMP-2 | Serum (ng/mL) Plasma (ng/mL) |
228 (207–267) 158.3±32.3SD |
232 (225–245) 194.3±49.3SD |
(Montagnana et al., 2009) (Eleuterio et al., 2015) |
| Uric acid | Serum (µM) | 185 (28.7–642) 1st trimester 217 (102–428) 2nd trimester 278 (68.4–535) 3rd trimester |
191 (120–457) 1st trimester 215(130–248) 2nd trimester 350 (157–720) 3rd trimester |
(Chen et al., 2016) |
Values represent means±standard error of the mean.
indicates standard deviation.
Numbers in parenthesis indicates range.
AngII, angiotensin II; AT1-AA, AngII AT1R agonistic autoantibodies; AT1R, angiotensin II type 1 receptor; CAT, catalase; GPx, glutathione peroxidase; H2O2, hydrogen peroxide; HIF-1α, hypoxia-inducible factor-1α; HO, hemeoxygenase; IL, interleukin; MMP, matrix metalloproteinase; O2•−, superoxide anion; PE, preeclampsia; PlGF, placental growth factor; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; SOD, superoxide dismutase; TAOC, total antioxidant capacity; TIMP, tissue inhibitor of metalloproteinase; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; OD, optical density measured by spectrophotometry; RC, relative concentration to total mRNA extracted from Norm-Preg placenta; RGE, relative gene expression of target gene compared to expression in Norm-Preg; RLU, relative light unit from CHO.AT1A cells encoding rat AT1R and luciferase reporter
Table 4.
Bioactive factors in normal pregnant and RUPP rats
| Factor | Specimen (Units) | Norm-Preg | RUPP | Reference |
|---|---|---|---|---|
| VEGF | Plasma (pg/mL) Plasma (pg/mL) Placenta (pg/mg) |
1017±95 830±33 30±2 |
670±68 594±34 43±5 |
(George et al., 2013) (Gilbert et al., 2007) (George et al., 2013) |
| PlGF | Plasma (pg/mL) | 1.7±0.5 | 0.28±0.05 | (Gilbert et al., 2007) |
| TGF-β | Serum (pg/mL) | 1036±82 pg/mL | 567±88 pg/mL | (Cornelius et al., 2015) |
| sFlt-1 | Plasma (pg/mL) Plasma (pg/mL) Placenta (pg/mg) CD4+T supernatant (pg/mL) |
82±26 1432±255 643±44 1046±280 |
660±270 3431±454 809±81 2500±650 |
(Gilbert et al., 2007) (Murphy and Cockrell, 2015) (George et al., 2013) (Wallace et al., 2011) |
| sFlt-1/PlGF Ratio | Plasma | 8.9±1.6 | 37.2±7.8 | (Gilbert et al., 2007) |
| sEng | Serum (APU) Placenta (APU) |
~0.05 ~1.5 |
~0.10 ~4.8 |
(Gilbert et al., 2009) (Gilbert et al., 2009) |
| TNF-α | Plasma (pg/mL) CD4+T (pg/mL) |
16.0±6.4 133±23 |
61.4±12.2 250±50 |
(Cornelius et al., 2015) (Wallace et al., 2011) |
| IL-6 | Plasma (pg/mL) CD4+T supernatant (pg/mL) |
30±7 287±12 |
74±15 778±29 |
(Cornelius et al., 2015) (Wallace et al., 2011) |
| IL-10 | Plasma (pg/mL) | 77.3±22.2 | 19.6±4.8 | (Cornelius et al., 2015) |
| HIF-1α | Placenta (APU) | 0.68±0.09 | 1.42±0.25 | (Gilbert et al., 2009) |
| Total ROS | Placenta (RLU) Aorta (AU) |
240.9±24.1 5.2±0.4 |
339.3±58.7 7.7±0.3 |
(Cornelius et al., 2015) (Amaral et al., 2013) |
| HO-1 | Placenta (APU) | 2.5±0.1 | 1.4±0.3 | (Gilbert et al., 2009) |
| 8-Isoprostane | Plasma (ng/mL) | 305±85 | 689±8 | (Amaral et al., 2013) |
| AT1-AA | Serum (bpm) Plasma (bpm) Serum (bpm) |
0.6±0.3 0.08±0.25 1.1 |
15.3±1.6 17.81±1.1 14.8 |
(LaMarca et al., 2008b) (Cornelius et al., 2015) (Novotny et al., 2012) |
| MMP-2 | Uterus (OD) Placenta (OD) Aorta (OD) |
~1.0 ~0.9 ~0.9 |
~0.7 ~0.5 ~0.6 |
(Li et al., 2014b) |
| MMP-9 | Uterus (OD) Placenta (OD) Aorta (OD) |
~0.4 ~0.3 ~0.4 |
~0.2 ~0.2 ~0.2 |
(Li et al., 2014b) |
Values represent means±standard error of the mean.
AT1AA, angiotensin II type 1 receptor agonistic autoantibodies; HIF-1α, hypoxia-inducible factor-1α; HO, hemeoxygenase; IL, interleukin; MMP, matrix metalloproteinase; PlGF, placental growth factor; ROS, reactive oxygen species; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor. APU: arbitrary pixel unit relative to the 67-kDa band corresponding to albumin in Ponceau-stained membranes; AU, arbitrary unit of fluorescence generated by dihydroethidium oxidation; bpm, beats/min measured in spontaneously beating neonatal rat cardiomyocytes exposed to sera conatining AT1-AA and antagonized specifically by AT1R antagonist; OD: optical densitometry of Western blot bands normalized to β-actin; RLU, relative light unit measured by lucigenin luminescence
In addition to their proteolytic activities, MMPs may increase cytokines and ROS in PE (Palei et al., 2013b), MMPs may also modulate vascular tonus. MMP-2 induces vasodilation in rat vena cava via hyperpolarization and activation of K+ channels (Raffetto et al., 2007). Other studies have shown that MMP-2 and -9 may increase the production of ET-1 and related peptides and decrease vasodilator peptides such as adrenomedullin (Fernandez-Patron et al., 2001; Nascimento et al., 2015), leading to an imbalance between dilator and constrictor factors and causing endothelial dysfunction. MMP-2 may also enhance big-ET-1 induced constriction in mesenteric vessels of RUPP rats (Abdalvand et al., 2013). Also, in omental vessels of Norm-Preg women, MMP-1 causes vasoconstriction and enhances reactivity to AngII via an endothelium-dependent protease-activated receptor and ET-1 pathway (Nugent et al., 2016).
MMP activity is modulated by tissue inhibitors of metalloproteinases (TIMPs) (Montagnana et al., 2009). Measurements of TIMP-1 and -2 in the circulation and umbilical serum have shown increases or no change in PE versus Norm-Preg women (Montagnana et al., 2009; Deng et al., 2015; Eleuterio et al., 2015), while other studies have shown increases in TIMP-1 and -3 in PE patients (Zhu et al., 2014). The role of MMPs and TIMPs in modulating vascular function and the endothelial dysfunction associated with PE should be further examined in future studies.
7. CIRCULATING BIOACTIVE FACTORS IN PREECLAMPSIA
Placental hypoxia/ischemia is believed to trigger the release of several bioactive factors including the antiangiogenic factors sFlt-1 and sEng, pro-inflammatory cytokines such as TNF-α and IL-6, HIF, ROS and AT1-AA (Fig. 3). These factors could cause EC dysfunction, severe vasoconstriction and the increases in BP observed in PE women and in animal models of HTN-Preg (Jardim et al., 2015; Shah and Khalil, 2015).
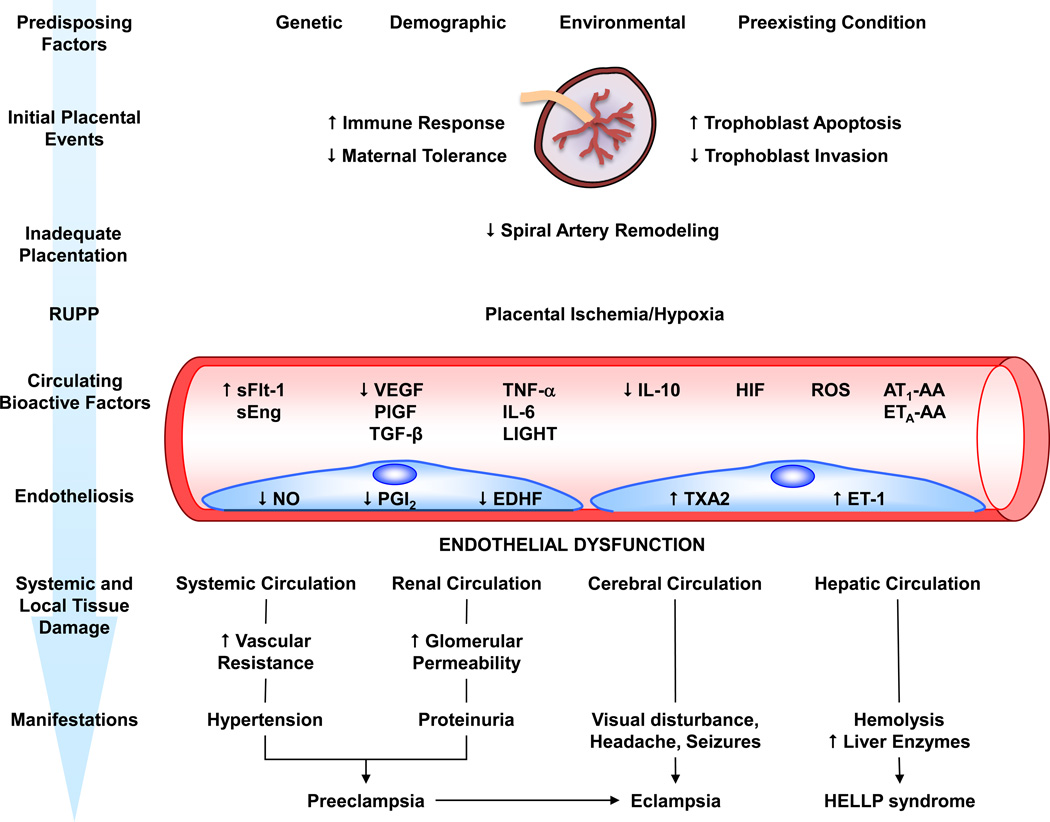
Fig. 3.
Predisposing risk factors, intermediary bioactive factors, and endothelial damage in preeclampsia. Genetic, demographic, environmental and other risk factors cause abnormal placentation. Increased immune response, trophoblast cell apoptosis and decreased trophoblast invasion cause poor remodeling of spiral arteries and reduced uteroplacental perfusion pressure (RUPP). RUPP triggers the release of several circulating bioactive factors. Bioactive factors target endothelial cells in the systemic circulation causing generalized vasoconstriction, increased vascular resistance and HTN, renal circulation causing increased glomerular permeability and proteinuria, cerebral circulation causing visual disturbance, headaches, seizures, and eclampsia, and the hepatic circulation causing hemolysis, elevated liver enzymes and low platelets (HELLP syndrome). AT1-AA, angiotensin II type 1 receptor agonistic autoantibodies; EDHF, endothelium-derived hyperpolarizing factor; ET-1, endothelin-1; ETA-AA, endothelin receptor type A agonistic autoantibodies; HELLP, hemolysis elevated liver enzymes low platelets; HIF, hypoxia-inducible factor; IL, interleukin; NO, nitric oxide; PGI2, prostacyclin; PlGF, placental growth factor; ROS, reactive oxygen species; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; TXA2, thromboxane A2; VEGF, vascular endothelial growth factor
Pro-angiogenic and Anti-angiogenic Factors in Preeclampsia
Vascular Endothelial Growth Factor (VEGF)
VEGF gene is located on chromosome 6 (6p21.3), which consists of 8 exons involved in the expression of a family of proteins including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF (Jardim et al., 2015). VEGF-A, VEGF-B and PlGF bind to tyrosine kinase receptor flt-1 (Flt-1 or VEGFR-1) and only VEGF-A binds to VEGFR-2 (Flk-1 or KDR) to induce the development of the placental vasculature (Jardim et al., 2015). VEGF regulates EC proliferation, angiogenesis and vascular permeability (Jardim et al., 2015; Shah and Khalil, 2015). In ECs, VEGF increases [Ca2+]i, Ca2+/calmodulin, eNOS activity, and PGI2 leading to decreased vessel tonicity and BP (He et al., 1999; Shen et al., 1999; Cindrova-Davies et al., 2011). VEGF could also induce Akt activation and eNOS Ser1177 phosphorylation in ECs, leading to Ca2+-independent generation of NO. In HUVECs, blockade of VEGFR leads to decreased Akt activity and eNOS phosphorylation and impaired endothelial function, supporting a link between changes in VEGF activity and endothelial damage in PE (Cindrova-Davies et al., 2011).
Circulating levels of VEGF vary in PE, with some studies showing an increase in circulating VEGF (Hunter et al., 2000; Tsatsaris et al., 2003; Celik et al., 2013), while other reports show decreased or unchanged levels of serum VEGF (Maynard et al., 2003; Masoura et al., 2014). VEGF production is higher in villous explants from PE than Norm-Preg women (Ahmad and Ahmed, 2004). It is likely that during the vasoconstrictive and latent phase of PE the increase in vascular shear-stress could increase circulating VEGF (Tanbe and Khalil, 2010). Also, women with the T allele of VEGF 936C/T produce lower levels of VEGF and have a higher risk of developing PE than women with VEGF 936C/C (Papazoglou et al., 2004). While VEGF plasma levels are decreased in RUPP rats (Gilbert et al., 2007), in agreement with the findings in human villous explants, placenta from RUPP rats show greater production of VEGF (George et al., 2013) (Table 4). The differences in the results may be related to differences in the methods of VEGF measurement (Maynard et al., 2003). In PE, there is an increase in circulating anti-angiogenic factors that may bind to VEGF. Thus, total (bound and unbound) VEGF measured using radioimmunoassay or competitive enzyme immunoassay could be higher while free VEGF measured using ELISAs may be lower in PE than Norm-Preg women (Bates, 2011).
Changes in VEGF activity could also influence protein excretion, and a decrease in VEGF may play a role in the glomerular endotheliosis in PE. VEGF is synthesized constitutively in the glomerulus by podocytes where it maintains endothelial health and induces the formation of fenestrae. Interestingly, endotheliosis and loss of fenestrae is observed in genetic glomerular VEGF deficiency (Stillman and Karumanchi, 2007). Also, in clinical cancer trials, the use of VEGF-neutralizing antibodies therapy is associated with proteinuria (Zhu et al., 2007). In non-pregnant mice, downregulation of VEGF by infusion of VEGF antibodies leads to glomerular endotheliosis and proteinuria similar to what is seen in PE (Eremina et al., 2008). Also, mice lacking one VEGF allele in renal podocytes develop the typical renal pathology found in PE, and infusion of VEGF ameliorates the renal lesions, glomerulonephritis and thrombotic microangiopathy in RUPP rats (Kim et al., 2000; Masuda et al., 2001). Thus, a decrease in VEGF activity in PE could cause reduction in GFR and leads to proteinuria.
Placental Growth Factor (PlGF)
PlGF is a pro-angiogenic factor that binds to VEGFR-1 and enhances the angiogenic effects of VEGF (Romero et al., 2008). PlGF has only 1/10th the affinity for VEGFR-1 compared to VEGF, but its level is ~40 times higher than VEGF during normal pregnancy. PIGF dilates uterine vessels, and promotes EC growth, vasculogenesis, and placental development (Shah and Khalil, 2015).
Plasma PlGF levels are low in non-pregnant women (~44 pg/mL), but are substantially higher in Norm-Preg women (Romero et al., 2008). PlGF levels during 21 and 22 weeks of gestation are ~353 pg/mL, rising steadily during gestation to reach a median level of ~574 pg/mL after 29 and 30 weeks of gestation (Krauss et al., 2004). During PE, circulating PlGF levels decrease (Tsatsaris et al., 2003; Ramma et al., 2012; Bian et al., 2015; Molvarec et al., 2015) and the decrease is more apparent in early than late PE (March et al., 2015). PlGF has four alternatively spliced mRNA species (PIGF 1–4), and its predominant isoform PIGF-1 is downregulated in PE (Bates, 2011). RUPP and DOCA-salt rats also show a decrease in circulating levels of PlGF (Gilbert et al., 2007; Agunanne et al., 2010).
In addition to its growth promoting effects, PlGF could exert vasodilator effects via VEGFR-1 and an EDHF-dependent pathway involving activation of SKCa (Mandala et al., 2012; Morton and Davidge, 2013). Also, in mesenteric resistance arteries from pregnant rats treated with L-NAME, L-NNA and indomethacin, a second application of PlGF could produce a greater reduction in VSM [Ca2+]i and greater vasodilation. Since VEGF and PlGF signaling is thought to involve receptor dimerization, initial exposure to PlGF may facilitate subsequent responses via stimulated formation of receptor homodimers and their associated submembrane signaling pathways leading to increases in both the speed and the amplitude of the vasodilatory response to repeated stimulation (Mandala et al., 2012). As the levels of PlGF are likely reduced in PE, the vasodilatory responses of PlGF are expected to be decreased.
Soluble fms-like Tyrosine Kinase-1 (sFlt-1)
sFlt-1 (sVEGFR-1) is an anti-angiogenic factor expressed as an alternatively spliced variant of VEGFR-1 and lacks both the transmembrane and cytoplasmic domains. sFlt-1 binds to VEGF and PlGF in the circulation and inhibits their action on cell surface receptors. sFlt-1 may also form a heterodimer with the surface receptors and inhibit any signaling action they might have (Charnock-Jones, 2016). TCs express sFlt-1 mRNA, and sFlt-1 level is ~1.5 ng/mL in Norm-Preg compared to ~0.15 ng/mL in non-pregnant women (Shah and Khalil, 2015). sFlt-1 levels are stable in Norm-Preg women, showing an increase after the 36th week of gestation. Throughout the third trimester, the increase in sFlt1 persists and there is a reduction in VEGF and PlGF. In PE, the increase in sFlt-1 and decrease PlGF occur at more pronounced levels than those seen in Norm-Preg women (Jardim et al., 2015). sFlt-1 gene is localized on chromossome 13q12, and an extra copy of this gene in women with trisomy 13 may lead to excess circulating sFlt-1, reduced PlGF and increased risk of PE (Kakigano et al., 2013). Several reports have shown higher circulating levels of sFlt-1 in early and late PE (Tsatsaris et al., 2003; Ramma et al., 2012; Bian et al., 2015; March et al., 2015; Molvarec et al., 2015; Tuzcu et al., 2015). Serum sFlt-1 is also higher in women with previous PE (~0.5 ng/mL) than in women with previous normal pregnancy (~0.3 ng/mL), even 6 months or more after delivery (Tuzcu et al., 2015). sFlt-1 levels are also increased in villous explants from PE versus Norm-Preg women (Ahmad and Ahmed, 2004).
During placental hypoxia, HIF-1 may bind to the promoter region of flt-1 gene leading to up-regulation of sFlt-1 (Maynard et al., 2003; Ahmad and Ahmed, 2004). In vitro studies in EVTs have shown that overexpression of miR-517a/b and miR-517c increase the expression of TNFSF15, a cytokine involved in Flt-1 splicing, leading to increases in sFlt-1 release (Anton et al., 2015). sFlt-1 e15a, a splice variant of sFlt-1, is the most abundant form released by the placenta. sFlt-1 e15a binds and inhibits VEGF and in turn decreases EC migration, invasion, and tube formation. sFlt-1 e15a is expressed in syncytiotrophoblasts and shows a 10-fold increase in serum levels in PE versus Norm-Preg women (Palmer et al., 2015).
In addition to changes in the levels of sFlt-1, a decline in VEGF/sFlt-1 and PlGF/sFlt-1 ratio by 53% and 70%, respectively is observed in PE placenta (Ahmad and Ahmed, 2004). The sFlt-1/PlGF ratio in the circulation is higher in PE than Norm-Preg women from second trimester onwards and can be a good predictor of the onset of PE (March et al., 2015; Molvarec et al., 2015), although some reports suggest that it is lower in late compared to early PE (Noori et al., 2010; March et al., 2015). Circulating sFlt-1 levels and sFlt-1/PlGF ratio are higher in twin than singleton pregnancies possibly due to greater placental mass (Bdolah et al., 2008; Faupel-Badger et al., 2015), supporting that the placenta is a likely source of these factors. Studies have suggested a direct association between the degree of angiogenic imbalance and ET-1 levels. Patients with sFlt-1/PlGF ratio >85 have higher levels of ET-1 than patients with sFlt-1/PlGF ratio <85 (Verdonk et al., 2015). Also, extracorporeal removal of circulating sFlt-1 in PE patients decreases sFlt-1/PlGF ratio and improves symptoms and prolongs pregnancy (Thadhani et al., 2016).
Of note, the decrease in FMD and elevation of BP in women who subsequently develop late PE may occur before the rise in plasma sFlt-1 levels (26 to 36 weeks). These findings suggest the involvement of other bioactive factors and that the impaired endothelial function may represent a baseline risk factor for PE rather than a consequence of elevated sFlt-1 levels in cases of late PE (Noori et al., 2010).
RUPP rats show increases in plasma and placental levels of sFlt-1 and plasma sFlt/PlGF ratio (Gilbert et al., 2007; George et al., 2013; Murphy and Cockrell, 2015) (Table 4). Other animal models of HTN-Preg show increased or unchanged circulating levels of sFlt-1 (Agunanne et al., 2010; Ramesar et al., 2011; Sunderland et al., 2011; Siddiqui et al., 2013; Bobek et al., 2015; Gillis et al., 2015). Importantly, pregnant rats treated with exogenous sFlt-1 develop HTN, proteinuria, marked glomerular endotheliosis with occlusion of renal capillaries and focal fibrin deposition in glomerular cells (Maynard et al., 2003; Holwerda et al., 2014; Jiang et al., 2015). Also, mice treated with sFlt-1 show an increase in the vascular response to ET-1 (Amraoui et al., 2014). Exposure of ECs to PE plasma results in reduced angiogenesis, and removal of sFlt-1 or treatment with VEGF or a sFlt-1 antibody reverses the anti-angiogenic effects of sFlt-1 and restores EC function and angiogenesis (Ahmad and Ahmed, 2004).
Of note, VEGF has been shown to stimulate sFlt-1 production in human placental explants through an action on VEGFR-2 (Fan et al., 2014). It appears that VEGF levels are tightly controlled at the maternal-fetal interface, partly through feedback modulation by sFlt-1, which may play a critical local role in preventing any damage to the placenta or fetus that would otherwise be caused by excess VEGF even during normal pregnancy (Fan et al., 2014). Dysregulation of this feedback mechanism may play a role in the pathogenesis of PE.
Soluble Endoglin (sEng)
Endoglin (Eng) is a co-receptor for transforming growth factor (TGF)-β1 and TGF-β3 that is highly expressed on cell membranes of ECs and syncytiotrophoblasts. TGF-β1 induces the migration and proliferation of ECs by binding to TGF receptors (Jardim et al., 2015). Eng is a proliferation-associated and hypoxia-inducible protein abundantly expressed in angiogenic ECs (Luft, 2006). In contrast, sEng acts as an anti-angiogenic protein that inhibits TGF-β1 signaling and TGF-β1-induced eNOS activation and vasodilation (Jardim et al., 2015). Mutations in Eng gene result in loss of capillaries, arteriovenous malformations, and hereditary hemorrhagic telangiectasia (Maynard et al., 2008).
sEng is barely detectable in non-pregnant women and has much lower levels in the serum of Norm-Preg women (Venkatesha et al., 2006). sEng levels are 3-, 5- and 10-fold higher in mild PE, severe PE and HELLP syndrome, respectively, compared to gestational age–matched preterm controls (Venkatesha et al., 2006). Serum sEng levels may be increased in both early and late PE (Levine et al., 2006; Ramma et al., 2012), although one study showed that sEng levels increase at 10–17 gestational weeks in women who developed early PE, but not in those that developed late PE (Noori et al., 2010). sEng expression also increases in placental extracts exposed to a hypoxic environment (Yinon et al., 2008).
In RUPP rats, the levels of sEng increase in both serum and placenta (Gilbert et al., 2009) while serum TGF-β levels decrease (Cornelius et al., 2015) (Table 4). However, no changes in sEng levels were observed in rat models of HTN-Preg produced by DOCA-salt or L-NAME (Agunanne et al., 2010; Ramesar et al., 2011) (Table 5). sEng may act in concert with sFlt-1 to induce vascular permeability, proteinuria, IUGR and severe HTN in pregnant rats (Venkatesha et al., 2006). Pregnant rats infused with both sFlt-1 and sEng via mini-osmotic pump show HELLP syndrome-like features (Morris et al., 2015). Also, sEng impairs the formation of endothelial tubes in cultured HUVECs (Venkatesha et al., 2006).
Table 5.
Characteristics, specific EC changes and specific biomarkers in other animal models of HTN-Preg
| Model | Procedure | Characteristics | Norm-Preg | HTN-Preg | Reference |
|---|---|---|---|---|---|
| L-NAME | Rats received L-NAME 0.3g/L in drinking water on gestation days 1–19 Rats received 50 mg/kg/day of L-NAME by oral gavage on gestation days 14–19 |
= plasma nitrite (µM) = plasma L-arginine (µM) = plasma sEng (ng/mL) ↑ plasma sFlt-1 (pg/mL) ↑ BP (mmHg) = litter size (n. of pups) Pup weight (g) Plasma MDA (µM/mL) Placental lysate TNF-α (pg/mL) |
11.20±1.05 10.47±2.31 178.52±5.33 774.91±26.81 ~119 ~11 ~3.5 ~46 ~300 |
9.87±0.59 5.08±0.81 183.44±8.294 1228.80±116.29 ~136 ~12 ~3.0 ~125 ~500 |
(Ramesar et al., 2011) (Kemse et al., 2014) |
| DOCA-Salt | Female rats received 12.5 mg of DOCA (i.p.) and 0.9% saline to drink, followed by weekly i.p. injections of DOCA (6.25 mg) during pregnancy |
↑ Systolic BP (mmHg) Proteinuria (mg/24h) ↓ Litter weight (g) ↓ # of pups ↓ Vascular ACh relaxation (%) ↑ Aortic superoxide (RLU) ↑ Aortic Peroxynitrite (RLU) ↑ Aortic eNOS (AU) ↑ BP (mmHg) = Plasma sFlt-1 (pg/mL) ↓ Plasma VEGF (pg/mL) ↓ Plasma PlGF (pg/mL) = Plasma sEng (pg/mL) Plasma sFlt-1/PlGF ratio |
90±4 5.7±0.6 73±5 16±2 102±4 ~15000 ~20000 ~0.6 102±4 2778.8±52.6 29.2±1.8 26.5±1.9 821.5±22.4 105±3 |
138±4 9.8±0.3 36±6 10±1 68±2 ~28000 ~38000 ~1.0 140±8 2781.8±30.9 19.2±1.3 18.2±1.6 830.7±25.3 154±13 |
(Mitchell et al., 2007) (Agunanne et al., 2010) |
|
Dahl Salt- Sensitive (Superimpose d PE) |
Dahl S rats are a genetic model of hypertension and kidney disease |
↑ BP (mmHg) Early Mid Late Proteinuria (mg/24h) Early Mid Late ↑ UARI ↑ Placental HIF-1α (RDU) ↑ Placental TNF-α (pg/g) ↑ Plasma sFlt-1 (RDU) ↓ Number of pups ↓ Pup weight (g) ↓ Pup length (mm) |
~105 ~98 ~96 <10 <10 <10 ~0.5 1.0±0.1 92±5 1.00±0.09 ~13 ~2.5 ~34 |
~158 ~160 ~162 ~98 ~100 ~250 ~0.7 1.4±0.1 152±8 1.83±0.16 ~9 ~1.8 ~27 |
(Gillis et al., 2015) |
|
Recombinant sFlt-1-Fc |
Mice were injected (i.p.) with recombinant mouse sFlt-1-Fc (12 µg sFlt-1 in saline) on gestation days 9–16 |
↑ Systolic BP (mmHg) ↑ Urine albumin/creatinine ratio (µg/mg) ↑ Serum sFlt-1 levels (ng/mL) = Number of pups = Pup weight (g) |
112.2±3.8 53.4±2.6 0.9±0.03 11.4±0.5 1.3±0.1 |
160.8±3.3 332.3±29.7 2.5±0.13 10.6±0.9 1.2±0.1 |
(Jiang et al., 2015) |
|
sFlt-1 and sEng Infused |
Sprague-Dawley rats were infused with sFlt-1 (4.7 µg/kg) and sEng (7 µg/kg) via mini-osmotic pumps on gestation days 12–20 |
↑ BP (mmHg) ↑ Plasma Lactate dehydrogenase (IU/L) ↑ Plasma Aspartate Aminotransferase (IU/L) ↓ Platelets (platelets/µL) ↑ Urinary protein:creatinine (mg/g) ↑ Plasma endothelin-1 (ng/mL) |
99.5±2.32 408.5±52.6 89.29±9.03 597,250±34,775 ~1.4 ~110 |
121.5±2.44 1137±124.2 140.5±12.66 347,125±59,821 ~2.0 ~175 |
(Morris et al., 2015) |
| TNF-α Infused | Mice were infused with TNF-α (500 ng/Kg/day) via subcutaneous mini-osmotic pump on gestation days 13–17 |
↑ BP (mmHg) Proteinuria (g/L) = Number of pups = Pup weight (g) = sFlt-1 serum levels (ng/mL) ↑ Expression of placental HIF- 1α (RE) |
- ~0.2 ~7 ~0.9 99±18 ~1.0 |
- ~0.7 ~8 ~0.9 86±25 ~1.5 |
(Bobek et al., 2015) |
|
AT1-AA Treated |
Pregnant C57BL/6 mice received on gestation days 13.5 and 14.5 intra-orbital injections of IgG obtained from Norm-Preg or PE women |
↑ BP (mmHg) Proteinuria (µg albumin/mg creatinine) ↑ Serum sFlt-1 (ng/mL) |
124±2 132±17 ~21 |
142±8 242±27 ~50 |
(Siddiqui et al., 2013) |
Values represent means±standard error of the mean.
ACh, acetylcholine; BP, blood pressure; DOCA, deoxycorticosterone acetate; eNOS, endothelial nitric oxide synthase; HIF, hypoxia-inducible factor; L-NAME, Nω-nitro-L-arginine methyl ester; MDA, malondialdehyde; PlGF, placental growth factor; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; TNF-α, tumor necrosis factor-α; UARI, uterine artery resistance index; VEGF, vascular endothelial growth factor; AU, arbitrary unit of bands intensity in Western blot analysis; IU, international units; RDU, relative densitometry units of Western blot bands normalized to Norm-Preg bands; RE, relative expression normalized to β-actin; RLU, relative light units of chemiluminescence from lucigenin-superoxide and luminol-peroxynitrite reaction
Cytokines: TNF-α and Interleukins
Placental ischemia may enhance the synthesis of inflammatory cytokines. Immediately after placental reperfusion injury, reestablished blood flow releases cytokines and inflammatory factors like TNF-α and ILs (Zarate et al., 2014). TNF-α is released from the hypoxic placenta and is a primary modifier of the immune reaction. TNF-α increases vascular permeability, fibroblast proliferation and lymphocyte activation, and induces production of IL-6 and IL-8. TNF-α downregulates eNOS and mitochondrial biogenesis, leading to mitochondrial dysfunction, oxidative stress and increased ROS (Sanchez-Aranguren et al., 2014). TNF-α can also modify the expression of adhesion molecules in placental vessels (LaMarca et al., 2008a) and induce abnormal production of MMPs in PE (Palei et al., 2013a).
Circulating TNF-α levels are increased in PE compared with Norm-Preg women (Moreno-Eutimio et al., 2014; Pinheiro et al., 2015; Cakmak et al., 2016), although the placental levels of TNF-α may not be different in PE versus normal pregnancy (Peixoto et al., 2016). LIGHT, a TNF superfamily member, is also increased in PE and may contribute to placental damage and ischemia (Wang et al., 2014). Plasma levels and CD4+T cell production of TNF-α are increased in RUPP compared with Norm-Preg rats (LaMarca et al., 2008a; Wallace et al., 2011; Cornelius et al., 2015). Infusion of TNF-α causes HTN and proteinuria during late pregnancy in baboons (Sunderland et al., 2011), rats (Alexander et al., 2002) and mice (Bobek et al., 2015) (Table 5). Similarly, infusion of LIGHT, increases BP, induces the expression of ET-1 and sFlt-1, and causes proteinuria in pregnant rats (Wang et al., 2014). TNF-α may also work in concert with other inflammatory cytokines to increase ET-1 levels and cause HTN in RUPP rats (LaMarca et al., 2008a). TNF-α may also act in synergy with sFlt-1 to promote a pro-inflammatory state and endothelial dysfunction. In HUVECs, treatment with TNF-α and sFlt-1 causes an increase in the adhesion molecules ICAM and VCAM and markers of endothelial dysfunction such as von Willebrand factor and ET-1 (Cindrova-Davies et al., 2011). In support of a role of TNF-α in HTN-Preg, blockade of TNF-α with Etanercept reduced HTN in RUPP rats. Also, in HUVECs, treatment with serum from RUPP rats treated with the TNF-α blocker produced less ET-1 than serum from untreated RUPP rats (LaMarca et al., 2008a).
IL-6 is a pro-inflammatory cytokine that may also be elevated in PE (Ramma et al., 2012; Moreno-Eutimio et al., 2014). RUPP rats show increased plasma levels and higher CD4+T cell production of IL-6 (Wallace et al., 2011; Cornelius et al., 2015), and infusion of IL-6 leads to HTN and proteinuria in pregnant rats (Lamarca et al., 2011). IL-6 may stimulate dimerization of surface receptor GP-130 on ECs leading to abnormal intracellular signaling and vascular dysfunction. IL-6 also disrupts the tight junctions in ECs and increases vascular permeability (Lockwood et al., 2008). Chronic infusion of TNF-α or IL-6 in late pregnant rats leads to increased BP, enhanced vascular contraction and reduced endothelium-dependent relaxation via the NO-cGMP pathway (Davis et al., 2002; Orshal and Khalil, 2004). Also, IL-1β is a pro-inflammatory cytokine that could contribute to the inflammatory response and disrupts ECs in PE. Monocytes of PE women produce more IL-1β than those of Norm-Preg women (Matias et al., 2015).
IL-10 is an anti-inflammatory cytokine whose levels are reduced in the plasma and placenta of PE women (Pinheiro et al., 2015; Peixoto et al., 2016) and in plasma of RUPP rats (Cornelius et al., 2015). Also, hypoxic placental trophoblasts show decreased IL-10 and increased pro-inflammatory cytokines (Royle et al., 2009).
The source of pro-inflammatory cytokine in PE is mostly in the maternal circulation. Monocytes/macrophages are the main reservoir of pro-inflammatory cytokines and are the firstly activated cells in nonspecific immune response (Rahardjo et al., 2014). Monocytes treated with PE plasma produce more TNF-α and IL-6 than cells treated with Norm-Preg plasma (Rahardjo et al., 2014). This is likely due to the regulatory effects of IL-10 on monocytes regulating the inflammatory response that occurs during normal pregnancy by controlling TNF-α and IL-1β gene expression (Matias et al., 2015), and this IL-10-mediated regulatory activity could be lost in PE. Interestingly, uric acid is an important stimulator of monocytes to release cytokines, and PE patients often have hyperuricemia. Also, monocytes from PE patients exhibiting high levels of uric acid produce more TNF-α and IL-1β than those from Norm-Preg women (Matias et al., 2015).
Hypoxia-Inducible Factor (HIF)
HIF is a transcriptional factor that plays a role in the physiologic responses to hypoxia and may be involved in PE. HIF-1 is a heterodimer consisting of an oxygen-regulated HIF-1α and HIF-2α subunits and a constitutively expressed HIF-1β subunit. Of note, de novo synthesis of HIF-1α may largely occur in response to non-hypoxic stimuli such as inflammatory factors, and TNF-α causes upregulation of HIF-1α mRNA (Bobek et al., 2015). More than 100 genes are regulated downstream from HIF-1 including VEGF, leptin, TGF-β3, and NOS. Also, DNA microarrays have shown that more than 2% of all human genes are regulated directly or indirectly by HIF-1 in arterial ECs (Ali and Khalil, 2015).
Circulating HIF-1α levels are increased in PE women (Akhilesh et al., 2013), although the molecular mechanisms underlying the changes in HIF expression and the genes affected are unclear. HIF-1α has been suggested to upregulate sFlt-1 and sEng, bind to ET-1 gene and regulate ET-1 expression, contribute to shallow trophoblast invasion, and induce AngII-converting enzyme (ACE) expression in the lungs and kidney during PE (Tal, 2012; Ali and Khalil, 2015).
Experimental models support a role of HIF-1α in HTN-Preg. RUPP rats show elevated placental levels of HIF-1α (Gilbert et al., 2009). In mice models of HTN-Preg, knockdown of HIF-1α mRNA using specific siRNA, attenuates hallmark features of PE such as high BP, proteinuria, renal damage and elevated sFlt-1 levels (Iriyama et al., 2015). Also, HIF-1α may increase sFlt-1 production in human villous trophoblast (Iriyama et al., 2015).
Reactive Oxygen Species (ROS)
ROS such as superoxide anion (O2•−), H2O2 and hydroxyl ion (OH−) contain highly reactive O2. During normal pregnancy, ROS generation is increased and may be necessary for proper physiology (Sanchez-Aranguren et al., 2014), and therefore pregnancy may represent a state of oxidative stress due to increased maternal metabolism and metabolic activity of the placenta (Myatt and Webster, 2009). However, in normal pregnancy, placental production of ROS is counterbalanced by the presence of abundant antioxidants (Ali and Khalil, 2015).
During PE, the mRNA expression of antioxidants such as hemeoxygenase-1 (HO-1), HO-2, copper/zinc superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase is decreased and antioxidants may fail to counterbalance the increased ROS production (Nakamura et al., 2009). This leads to lipid peroxidation, increased TXA2 and loss of GPx activity in placenta (Reslan and Khalil, 2010). The mRNA levels of HO-1, HO-2, SOD, GPx and catalase are decreased in the cellular component of the maternal blood of PE women (Nakamura et al., 2009). Also, the total antioxidant capacity (TAOC) of serum from PE is reduced when compared with that of Norm-Preg women (Turpin et al., 2015). Interestingly, antioxidant levels were found to be decreased in women who were subsequently diagnosed with early-PE (Cohen et al., 2015), suggesting a role of oxidative stress in the pathogenesis of early-PE. In PE, reduced brachial artery FMD is associated with decreased plasma levels of the antioxidant ascorbate and is ameliorated with ascorbic acid administration (Chambers et al., 2001). Also, placental HO-1 is reduced in RUPP versus Norm-Preg rats (Gilbert et al., 2009).
Neutrophils and monocytes represent an important source of ROS and EC damage in PE. Monocytes from PE women release more H2O2 (Peracoli et al., 2011) and stimulated cells from women with PE produce more O2•− and cause more EC damage compared to those from Norm-Preg women (Tsukimori et al., 2005; Peracoli et al., 2011). Of note, neutrophils also produce NO, which can protect cells from O2•−-induced damage during normal pregnancy. However, in PE, excess O2•− scavenge the NO produced by neutrophils to form peroxynitrite (ONOO–), effectively reducing NO bioavailability and causing EC injury (Tsukimori et al., 2005). Another source of O2•− formation is NADPH oxidase, a membrane-bound enzyme complex that catalyzes the one-electron reduction of oxygen to O2•− via NADPH. NADPH oxidase isoform NOX1 is overexpressed in PE placentas (Cui et al., 2006). HUVECs treated with serum from PE women showed an increase in mRNA expression of the NADPH oxidase subunit gp91phox, leading to high O2•− production (Matsubara et al., 2010). Also, in HUVECs treatment with PE serum induces overexpression of iNOS (Matsubara et al., 2010), which could produce excessive amounts of NO and in turn increases ROS production and EC injury and leads to HTN. This is supported by the observation that RUPP rats treated with specific inhibitors of iNOS show a decrease in BP, aortic ROS levels and NADPH-dependent ROS production (Amaral et al., 2013). Biopterin (BH4) promotes eNOS dimerization and activity. During hypoxia, a reduction in BH4 may induce an uncoupling reaction leading to ROS production and decreased NO formation (Mitchell et al., 2007). In DOCA-salt rats, supplementation with BH4 such as sepiapterin increases NO levels, and reduces O2•− and ONOO− production (Mitchell et al., 2007).
Other oxidative markers such as malondialdehyde, a marker of lipid peroxidation, and prostaglandin F2α in serum from women at 10–14 gestation weeks were found to be increased in PE. This correlates with the gradual oxidative damage of the placenta, even before the onset of clinical symptoms of PE (Genc et al., 2011). Also, plasma 8-isoprostane, marker of oxidative stress, and total aortic and placental ROS are elevated in RUPP versus Norm-Preg rats (Amaral et al., 2013; Cornelius et al., 2015). Oxidative stress can also modulate miRNA expression in placenta and this could be a potential mechanism in the pathogenesis of PE. Studies in first-trimester villous trophoblasts showed that excessive oxidative stress could affect the expression of several miRNAs including those involved in angiogenesis, apoptosis, immune response and inflammation (Cross et al., 2015).
AngII and AT1 Receptor Agonistic Autoantibodies (AT1-AA)
AngII is an important circulating hormone in the control of BP and electrolyte homeostasis. AngII acts via AT1R to promotes vasoconstriction, vascular growth, inflammation, and increases [Ca2+]i and Rho/Rho-kinase activity in VSM. AngII can also acts via endothelial AT2R to activate eNOS, and increase production of NO and PGI2, counteracting AngII-induced vasoconstriction.
It was first demonstrated that the dose of AngII required to elicit a pressor response of 20 mmHg in diastolic BP in 23–26 weeks Norm-Preg women was lower in women who subsequently developed PE (Gant et al., 1973). This was a significant study as it showed that the increased pressor response to AngII in PE women occurred long before the manifestation of clinical symptoms. Although normal pregnancy is associated with increased plasma levels of renin and AngII, the pressor response to AngII is decreased due to decreased expression of AT1R or increased expression of AT2R.
Angiotensinogen levels may be similar in PE and Norm-Preg women, but PE patients have lower levels of the free thiol form of angiotensinogen and higher levels of the oxidized sulfhydryl-bridge form, which preferentially interacts with renin resulting in increased generation of angiotensin (Rahgozar et al., 2015). AngII levels and AT1R gene expression are increased in chorionic villi and placenta of PE women (Anton et al., 2008; Mistry et al., 2013). Increased plasma hemopexin activity downregulates AT1R in human monocytes and ECs in pregnant rats, and inhibition of hemopexin during PE enhances AT1R expression and vasoconstriction (Bakker et al., 2009).
AT1-AA is a bioactive factor that mediates its responses via AT1R. AT1-AA levels are elevated in serum of PE versus Norm-Preg women (Bai et al., 2013; Siddiqui et al., 2013) and further elevated in severe PE and in early than late PE (Yang et al., 2015). AT1-AA may enhance collagen-induced platelet aggregation, thus contributing to the hypercoagulability observed in PE (Bai et al., 2013). Circulating AT1-AA is increased in RUPP versus Norm-Preg rats (LaMarca et al., 2008b; Novotny et al., 2012; Cornelius et al., 2013; Cornelius et al., 2015) and AT1-AA infusion in pregnant mice induces several features of PE such as elevated BP, proteinuria and increased sFlt-1 levels (Siddiqui et al., 2013) (Table 5). Also, AT1-AA infusion in pregnant rats causes increases in ET-1 levels, 11-fold in renal cortex and 4-fold in the placenta (LaMarca et al., 2009). Also, the vasodilatory response to the endothelial-dependent agonist ACh is reduced in renal interlobar arteries of pregnant rats infused with AT1-AA, suggesting renal endothelial damage as an underlying mechanism in HTN-Preg. Interestingly, the impaired vasodilator response to ACh was abolished by ETAR blockade, suggesting cross-talk between the bioactive factors in promoting endothelial dysfunction in HTN-Preg (Parrish et al., 2011). AT1-AA reduces trophoblast invasion, increases ROS and [Ca2+]i, activates the tissue factor causing thrombosis, increases BP, causes vascular damage to the adrenal glands, and reduces aldosterone secretion in sFlt-1 treated pregnant mice (Xia et al., 2007; Siddiqui et al., 2013). In cultured trophoblasts, stimulation of AT1R with IgG isolated from PE women causes an increase in sFlt-1 levels (Zhou et al., 2008). Also, HUVECs treated with AT1-AA isolated from PE patients induces the release of lactate dehydrogenase (LDH) (Yang et al., 2014), a marker of both cellular death and necrosis, suggesting that AT1-AA may directly cause EC necrosis via AT1R activation. Also in HUVECs, AT1-AA induces the activity of caspase-3 and caspase-8, suggesting that it may promote EC apoptosis (Yang et al., 2014). While the mechanisms of the generation of AT1-AA in PE are not clearly understood, studies have shown that the plasma levels of AT1-AA are increased in pregnant rats infused with TNF-α, suggesting the involvement of cytokine-induced pathways (LaMarca et al., 2008b).
8. IMPLICATIONS FOR PREDICTION AND MANAGEMENT OF PREECLAMPSIA
Because PE has a relatively long preclinical phase before clinically manifesting in late gestation, the identification of women at risk, early diagnosis using various biomarkers, and prompt management could improve the maternal and perinatal outcome (Ali and Khalil, 2015).
A decrease in FMD could be an early indicator of compromised EC function between the 24th and 28th gestational weeks and before the clinical diagnosis of PE. The sensitivity of FMD is 87.5% and 95.5% for the prediction of early and late PE, respectively (Brandao et al., 2014).
Measurements of plasma VEGF, PlGF, sFlt-1 and sEng may help early detection in asymptomatic pregnant women, especially those at high risk for PE (Ali and Khalil, 2015). Angiogenic/anti-angiogenic imbalance is an important feature in PE. In PE, circulating levels of sFlt-1 are increased more than one month before the onset of clinical symptoms, and PlGF is decreased in women who subsequently develop PE from the end of the first trimester (Herraiz et al., 2015). The sFlt-1/PlGF ratio is increased in PE versus Norm-Preg women and in both early and late PE (March et al., 2015). A meta-analysis of 20 different studies revealed that the overall diagnostic accuracy of sFlt-1/PlGF ratio for PE is relatively high, but suggested that the sFlt-1/PlGF has higher diagnostic efficiency in early than late PE, which might be due to differences in pathogenesis of different forms of PE (Liu et al., 2015).
Assessment of the immune system activity could be useful in predicting PE in early pregnancy. An increase in inflammatory factors may represent an abnormal maternal immunological response, with a change in the role of monocytes and NK cells, altered release of cytokines, and increased AT1-AA and activation of pro-inflammatory AT1R (Ali and Khalil, 2015). TNF-α levels could be an early predictor of PE. Plasma obtained at gestational weeks 11–13 had higher TNF-α levels in women who developed PE in the future (Hamai et al., 1997). However, other reports suggest that plasma TNF-α levels may not represent a PE predictive marker in the first and second trimesters, but might be useful in the early third trimester (Serin et al., 2002). A more recent study suggested that an association of elevated plasma TNF-α levels and changes in uterine artery Doppler at 11–13th gestational weeks had a 100% sensitivity in the prediction of PE (Gomaa et al., 2015).
Other markers could help in the early detection of PE. Vasopressin, a hormone with vasoconstrictor activities, was found to be elevated around week 6 of pregnancy in women who then developed PE (Santillan et al., 2014). Also, miRNAs have been proposed as early biomarkers for PE. At 28 weeks of pregnancy, miRNA-206, which interacts with several genes known to be directly involved in the pathology of PE, is elevated in plasma and placenta from women who subsequently develop PE (Akehurst et al., 2015), although other studies show little predictive value of miRNAs for PE (Luque et al., 2014). Thus, despite the existence of several potential markers, their reliability in predicting PE has not been consistent and their predictive value needs to be further assessed in order to identify the best marker combinations for use in clinical settings (Ali and Khalil, 2015).
Currently, inducing labor remains as the only effective treatment for PE and few pharmacological options are available. International guidelines recommend the use of one of the following antihypertensive agents in PE: methyldopa, labetalol, other beta-blockers (acebutolol, metoprolol, pindolol, and propranolol), and Ca2+ channel blockers (nifedipine) (Magee et al., 2014). ACE inhibitors should not be used due to their teratogenic effects and atenolol and prazosin are not recommended prior to delivery. If PE evolves to eclampsia, magnesium sulfate is often used to prevent eclamptic seizures (McDonald et al., 2012).
Sildenafil therapy has been proposed for women with severe early-onset IUGR, and was associated with increased fetal growth and no maternal side effects (von Dadelszen et al., 2011). Sildenafil citrate vasodilates myometrial arteries isolated from women with IUGR-complicated pregnancies. Also, treatment of an L-NAME mouse model of HTN-Preg with sildenafil citrate restored EC integrity in the placental vessels (Motta et al., 2015).
Eculizumab, an anti-C5 antibody, was found to normalize lab values and prolong pregnancy by 17 days in a woman with PE/HELLP syndrome, suggesting the benefits of manipulating the complement system during PE. However, complement system inhibitors have the potential of increasing susceptibility to infection and their use for long term therapy would require close monitoring (Burwick and Feinberg, 2013).
MMPs could be an important target in the management of PE. Doxycycline, a non-specific MMP inhibitor, was thought to alleviate HTN and vascular dysfunction in PE, but was found to decrease placenta weight and cause IUGR in both Norm-Preg and HTN-Preg rats, and to reduce trophoblast invasion and placental perfusion in HTN-Preg rats (Palei et al., 2013a).
Studies suggest potential benefits of correcting the pro-angiogenic/anti-angiogenic imbalance in PE. Edaravone is a free radical scavenger that inhibits sFlt-1 expression and reduces placental vessels abnormalities in hypoxic human TCs (Zhao et al., 2014). H2S donors attenuate HTN, reduce sFlt-1-mediated damage and upregulates VEGF in animal models of HTN-Preg (Wang et al., 2013; Holwerda et al., 2014). VEGF could improve the proangiogenic/antiangiogenic imbalance, but may impair bradykinin-induced vascular relaxation, and enhance basal tone and vascular permeability in PE (VanWijk et al., 2000). Modulators of PlGF could be more promising in PE, and low molecular weight heparin therapy was associated with increased circulating PlGF levels during third trimester pregnancy (Yinon et al., 2015).
TNF-α antagonists such as Etanercept decrease BP, increase eNOS expression, decrease ET-1 levels and prevent cardiac changes in RUPP rats (LaMarca et al., 2008a; Gutkowska et al., 2011). Also, IL-17 soluble receptor C could inhibit IL-17 and prevent the recruitment of host defense cells, suppress the inflammatory response, decrease AT1-AA and ROS, and ameliorate HTN and improve pup and placental weight in RUPP rats (Cornelius et al., 2013).
9. CONCLUSIONS AND FUTURE DIRECTIONS
Clinical and experimental studies have helped in understanding the mechanisms of PE. Endothelial dysfunction is a major hallmark of PE and identifying the factors that lead to endothelial damage would be key to further understand the pathogenesis of PE and help in developing appropriate therapies. Genetic, environmental and dietary factors may cause localized abnormal placentation and altered maternal immune response, and abnormal expression of integrins, inflammatory cytokines and MMPs leading to increased apoptosis of TCs, shallow trophoblastic invasion and poor spiral artery remodeling, causing RUPP and placental ischemia/hypoxia. Ischemic/hypoxic placenta causes the release of bioactive factors such as sFlt-1, sEng, TNF-α, IL-6, HIF, ROS and AT1-AA that target ECs, cause endothelial dysfunction, decrease vasodilators and increase vasoconstrictors and lead to HTN. However, whether the changes in vascular mediators and bioactive factors are the cause or the consequence of PE remains unclear. Further understanding of the vascular mediators, cellular mechanisms and bioactive factors should help design more specific and efficient measures for prevention, early detection, and management of PE.
Acknowledgments
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-111775).
ABBREVIATIONS
- ACh
acetylcholine
- ADMA
asymmetric dimethylarginine
- AngII
angiotensin II
- AT1R
angiotensin II type 1 receptor
- AT1-AA
AngII AT1R agonistic autoantibodies
- BP
blood pressure
- [Ca2+]i
intracellular free Ca2+ concentration
- CEC
circulating endothelial cells
- cGMP
cyclic guanosine monophosphate
- COX
cyclooxygenase
- CSE
cystathionine gamma-lyase
- EC
endothelial cell
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- EVT
extravillous trophoblast
- FMD
flow-mediated dilation
- GFR
glomerular filtration rate
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- HIF
hypoxia-inducible factor
- HELLP
hemolysis elevated liver enzymes low platelets
- HTN
hypertension
- HTN-Preg
hypertension in pregnancy
- HUVEC
human umbilical vein endothelial cell
- ICAM-1
intercellular adhesion molecule-1
- IL
interleukin
- IUGR
intrauterine growth restriction
- L-NAME
Nω-nitro-L-arginine methyl ester
- MEGJ
myoendothelial gap junction
- MMP
matrix metalloproteinase
- NO
nitric oxide
- Norm-Preg
normal pregnant
- O2•−
superoxide anion
- PE
preeclampsia
- PGI2
prostacyclin
- PlGF
placental growth factor
- ROS
reactive oxygen species
- RPF
renal plasma flow
- RUPP
reduced uterine perfusion pressure
- sEng
soluble endoglin
- sFlt-1
soluble fms-like tyrosine kinase-1
- TC
trophoblast cell
- TNF-α
tumor necrosis factor-α
- TXA2
thromboxane A2
- VEGF
vascular endothelial growth factor
- VCAM-1
vascular cell adhesion molecule-1
- VSM
vascular smooth muscle
- VSMC
VSM cell
Footnotes
CONFLICT OF INTEREST
None
REFERENCES
- Abdalvand A, Morton JS, Bourque SL, Quon AL, Davidge ST. Matrix metalloproteinase enhances big-endothelin-1 constriction in mesenteric vessels of pregnant rats with reduced uterine blood flow. Hypertension. 2013;61:488–493. doi: 10.1161/HYPERTENSIONAHA.111.00055. [DOI] [PubMed] [Google Scholar]
- Adekola H, Romero R, Chaemsaithong P, Korzeniewski SJ, Dong Z, Yeo L, Hassan SS, Chaiworapongsa T. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. J Matern Fetal Neonatal Med. 2015;28:1621–1632. doi: 10.3109/14767058.2014.964676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Loganath A, Roy AC, Wong YC, Ng SC. Expression profiles of interleukin-15 in early and late gestational human placenta and in pre-eclamptic placenta. Mol Hum Reprod. 2001;7:97–101. doi: 10.1093/molehr/7.1.97. [DOI] [PubMed] [Google Scholar]
- Agunanne EE, Uddin MN, Horvat D, Puschett JB. Contribution of angiogenic factors in a rat model of pre-eclampsia. Am J Nephrol. 2010;32:332–339. doi: 10.1159/000319463. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- Akehurst C, Small HY, Sharafetdinova L, Forrest R, Beattie W, Brown CE, Robinson SW, McClure JD, Work LM, Carty DM, McBride MW, Freeman DJ, Delles C. Differential expression of microRNA-206 and its target genes in preeclampsia. J Hypertens. 2015;33:2068–2074. doi: 10.1097/HJH.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhilesh M, Mahalingam V, Nalliah S, Ali RM, Ganesalingam M, Haleagrahara N. Hypoxia-inducible factor-1alpha as a predictive marker in pre-eclampsia. Biomed Rep. 2013;1:257–258. doi: 10.3892/br.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15:170–175. doi: 10.1016/s0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001a;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43:832–836. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension. 1999;33:435–439. doi: 10.1161/01.hyp.33.1.435. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001b;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- Ali SM, Khalil RA. Genetic, immune and vasoactive factors in the vascular dysfunction associated with hypertension in pregnancy. Expert Opin Ther Targets. 2015;19:1495–1515. doi: 10.1517/14728222.2015.1067684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpoim PN, Gomes KB, Pinheiro Mde B, Godoi LC, Jardim LL, Muniz LG, Sandrim VC, Fernandes AP, Dusse LM. Polymorphisms in endothelial nitric oxide synthase gene in early and late severe preeclampsia. Nitric Oxide. 2014;42:19–23. doi: 10.1016/j.niox.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN, Jr, LaMarca B. 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension. 2015;65:225–231. doi: 10.1161/HYPERTENSIONAHA.114.04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral LM, Pinheiro LC, Guimaraes DA, Palei AC, Sertorio JT, Portella RL, Tanus-Santos JE. Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J Cell Mol Med. 2013;17:1300–1307. doi: 10.1111/jcmm.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amraoui F, Spijkers L, Hassani Lahsinoui H, Vogt L, van der Post J, Peters S, Afink G, Ris-Stalpers C, van den Born BJ. SFlt-1 elevates blood pressure by augmenting endothelin-1-mediated vasoconstriction in mice. PLoS One. 2014;9:e91897. doi: 10.1371/journal.pone.0091897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. Activation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsia. Hypertension. 2008;51:1066–1072. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton L, Olarerin-George AO, Hogenesch JB, Elovitz MA. Placental expression of miR-517a/b and miR-517c contributes to trophoblast dysfunction and preeclampsia. PLoS One. 2015;10:e0122707. doi: 10.1371/journal.pone.0122707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K, Wang K, Li X, Wang J, Zhang J, Song L, Zhang S, Lau WB, Ma X, Liu H. Autoantibody against angiotensin AT1 receptor from preeclamptic patients enhances collagen-induced human platelet aggregation. Acta Biochim Biophys Sin (Shanghai) 2013;45:749–755. doi: 10.1093/abbs/gmt059. [DOI] [PubMed] [Google Scholar]
- Baig S, Kothandaraman N, Manikandan J, Rong L, Ee KH, Hill J, Lai CW, Tan WY, Yeoh F, Kale A, Su LL, Biswas A, Vasoo S, Choolani M. Proteomic analysis of human placental syncytiotrophoblast microvesicles in preeclampsia. Clin Proteomics. 2014;11:40. doi: 10.1186/1559-0275-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker WW, Henning RH, van Son WJ, van Pampus MG, Aarnoudse JG, Niezen-Koning KE, Borghuis T, Jongman RM, van Goor H, Poelstra K, Navis G, Faas MM. Vascular contraction and preeclampsia: downregulation of the Angiotensin receptor 1 by hemopexin in vitro. Hypertension. 2009;53:959–964. doi: 10.1161/HYPERTENSIONAHA.108.127951. [DOI] [PubMed] [Google Scholar]
- Bates DO. An unexpected tail of VEGF and PlGF in pre-eclampsia. Biochem Soc Trans. 2011;39:1576–1582. doi: 10.1042/BST20110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bdolah Y, Lam C, Rajakumar A, Shivalingappa V, Mutter W, Sachs BP, Lim KH, Bdolah-Abram T, Epstein FH, Karumanchi SA. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:428 e421–428 e426. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- Ben Ali Gannoun M, Zitouni H, Raguema N, Maleh W, Gris JC, Almawi W, Mahjoub T. Association of common eNOS/NOS3 polymorphisms with preeclampsia in Tunisian Arabs. Gene. 2015;569:303–307. doi: 10.1016/j.gene.2015.05.072. [DOI] [PubMed] [Google Scholar]
- Bhavina K, Radhika J, Pandian SS. VEGF and eNOS expression in umbilical cord from pregnancy complicated by hypertensive disorder with different severity. Biomed Res Int. 2014;2014:982159. doi: 10.1155/2014/982159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z, Shixia C, Duan T. First-Trimester Maternal Serum Levels of sFLT1, PGF and ADMA Predict Preeclampsia. PLoS One. 2015;10:e0124684. doi: 10.1371/journal.pone.0124684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobek G, Surmon L, Mirabito KM, Makris A, Hennessy A. Placental Regulation of Inflammation and Hypoxia after TNF-alpha Infusion in Mice. Am J Reprod Immunol. 2015;74:407–418. doi: 10.1111/aji.12417. [DOI] [PubMed] [Google Scholar]
- Boyd HA, Tahir H, Wohlfahrt J, Melbye M. Associations of personal and family preeclampsia history with the risk of early-, intermediate- and late-onset preeclampsia. Am J Epidemiol. 2013;178:1611–1619. doi: 10.1093/aje/kwt189. [DOI] [PubMed] [Google Scholar]
- Brandao AH, Felix LR, Patricio Edo C, Leite HV, Cabral AC. Difference of endothelial function during pregnancies as a method to predict preeclampsia. Arch Gynecol Obstet. 2014;290:471–477. doi: 10.1007/s00404-014-3243-3. [DOI] [PubMed] [Google Scholar]
- Brennan LJ, Morton JS, Davidge ST. Vascular dysfunction in preeclampsia. Microcirculation. 2014;21:4–14. doi: 10.1111/micc.12079. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34:201–203. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Cakmak M, Yilmaz H, Baglar E, Darcin T, Inan O, Aktas A, Celik HT, Ozdemir O, Atalay CR, Akcay A. Serum levels of endocan correlate with the presence and severity of pre-eclampsia. Clin Exp Hypertens. 2016;38:137–142. doi: 10.3109/10641963.2015.1060993. [DOI] [PubMed] [Google Scholar]
- Canbakan B, Keven K, Tutkak H, Danisman N, Ergun I, Nergizoglu G. Circulating endothelial cells in preeclampsia. J Hum Hypertens. 2007;21:558–563. doi: 10.1038/sj.jhh.1002199. [DOI] [PubMed] [Google Scholar]
- Celik H, Avci B, Isik Y. Vascular endothelial growth factor and endothelin-1 levels in normal pregnant women and pregnant women with pre-eclampsia. J Obstet Gynaecol. 2013;33:355–358. doi: 10.3109/01443615.2013.769944. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS. Placental hypoxia, endoplasmic reticulum stress and maternal endothelial sensitisation by sFLT1 in pre-eclampsia. J Reprod Immunol. 2016;114:81–85. doi: 10.1016/j.jri.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lau S, Tong M, Wei J, Shen F, Zhao J, Zhao M. Serum uric acid may not be involved in the development of preeclampsia. J Hum Hypertens. 2016;30:136–140. doi: 10.1038/jhh.2015.47. [DOI] [PubMed] [Google Scholar]
- Chinnathambi V, Blesson CS, Vincent KL, Saade GR, Hankins GD, Yallampalli C, Sathishkumar K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. 2014;64:405–414. doi: 10.1161/HYPERTENSIONAHA.114.03283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Yun J, Lee OJ, Han HS, Yeo MK, Lee MA, Suh KS. MicroRNA expression profiles in placenta with severe preeclampsia using a PNA-based microarray. Placenta. 2013;34:799–804. doi: 10.1016/j.placenta.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Sanders DA, Burton GJ, Charnock-Jones DS. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc Res. 2011;89:671–679. doi: 10.1093/cvr/cvq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JM, Kramer MS, Platt RW, Basso O, Evans RW, Kahn SR. The association between maternal antioxidant levels in midpregnancy and preeclampsia. Am J Obstet Gynecol. 2015;213:695 e691–695 e613. doi: 10.1016/j.ajog.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol. 2004;31:641–649. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Gandley RE, Ogawa T, Nakanishi S, Danielson LA. Endothelin mediates renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. Am J Physiol. 1999a;276:F767–F776. doi: 10.1152/ajprenal.1999.276.5.F767. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NO(x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO(x) diet. Am J Physiol. 1999b;277:F48–F57. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, Scott J, Herse F, Haase N, Moseley J, Wallukat G, Dechend R, LaMarca B. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309:R884–R891. doi: 10.1152/ajpregu.00154.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013;62:1068–1073. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35:367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- Cross CE, Tolba MF, Rondelli CM, Xu M, Abdel-Rahman SZ. Oxidative Stress Alters miRNA and Gene Expression Profiles in Villous First Trimester Trophoblasts. Biomed Res Int. 2015;2015:257090. doi: 10.1155/2015/257090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XL, Brockman D, Campos B, Myatt L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta. 2006;27:422–431. doi: 10.1016/j.placenta.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, Liu Z, Dong L, Zhou Y, Wu Q. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R390–R399. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- Deng CL, Ling ST, Liu XQ, Zhao YJ, Lv YF. Decreased expression of matrix metalloproteinase-1 in the maternal umbilical serum, trophoblasts and decidua leads to preeclampsia. Exp Ther Med. 2015;9:992–998. doi: 10.3892/etm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doridot L, Passet B, Mehats C, Rigourd V, Barbaux S, Ducat A, Mondon F, Vilotte M, Castille J, Breuiller-Fouche M, Daniel N, le Provost F, Bauchet AL, Baudrie V, Hertig A, Buffat C, Simeoni U, Germain G, Vilotte JL, Vaiman D. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension. 2013;61:662–668. doi: 10.1161/HYPERTENSIONAHA.111.202994. [DOI] [PubMed] [Google Scholar]
- Dotsch J, Hogen N, Nyul Z, Hanze J, Knerr I, Kirschbaum M, Rascher W. Increase of endothelial nitric oxide synthase and endothelin-1 mRNA expression in human placenta during gestation. Eur J Obstet Gynecol Reprod Biol. 2001;97:163–167. doi: 10.1016/s0301-2115(00)00532-7. [DOI] [PubMed] [Google Scholar]
- Echeverri I, Ortega-Avila JG, Mosquera M, Castillo A, Jimenez E, Suarez-Ortegon MF, Mateus JC, Aguilar-de Plata C. Relationship between maternal and newborn endothelial function and oxidative stress. Am J Hum Biol. 2015;27:822–831. doi: 10.1002/ajhb.22733. [DOI] [PubMed] [Google Scholar]
- Ehsanipoor RM, Fortson W, Fitzmaurice LE, Liao WX, Wing DA, Chen DB, Chan K. Nitric oxide and carbon monoxide production and metabolism in preeclampsia. Reprod Sci. 2013;20:542–548. doi: 10.1177/1933719112459231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleuterio NM, Palei AC, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, Sandrim VC. Relationship between adiponectin and nitrite in healthy and preeclampsia pregnancies. Clin Chim Acta. 2013;423:112–115. doi: 10.1016/j.cca.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Eleuterio NM, Palei AC, Rangel Machado JS, Tanus-Santos JE, Cavalli RC, Sandrim VC. Positive correlations between circulating adiponectin and MMP2 in preeclampsia pregnant. Pregnancy Hypertens. 2015;5:205–208. doi: 10.1016/j.preghy.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplin MS, Fausett MB, Fraser A, Kerber R, Mineau G, Carrillo J, Varner MW. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867–872. doi: 10.1056/NEJM200103223441201. [DOI] [PubMed] [Google Scholar]
- Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest. 2014;124:4941–4952. doi: 10.1172/JCI76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faupel-Badger JM, McElrath TF, Lauria M, Houghton LC, Lim KH, Parry S, Cantonwine D, Lai G, Karumanchi SA, Hoover RN, Troisi R. Maternal circulating angiogenic factors in twin and singleton pregnancies. Am J Obstet Gynecol. 2015;212:636 e631–636 e638. doi: 10.1016/j.ajog.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei X, Hongxiang Z, Qi C, Daozhen C. Maternal plasma levels of endothelial dysfunction mediators including AM, CGRP, sICAM-1 and tHcy in pre-eclampsia. Adv Clin Exp Med. 2012;21:573–579. [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C, Zouki C, Whittal R, Chan JS, Davidge ST, Filep JG. Matrix metalloproteinases regulate neutrophil-endothelial cell adhesion through generation of endothelin-1[1–32] FASEB J. 2001;15:2230–2240. doi: 10.1096/fj.01-0178com. [DOI] [PubMed] [Google Scholar]
- Fiore G, Florio P, Micheli L, Nencini C, Rossi M, Cerretani D, Ambrosini G, Giorgi G, Petraglia F. Endothelin-1 triggers placental oxidative stress pathways: putative role in preeclampsia. J Clin Endocrinol Metab. 2005;90:4205–4210. doi: 10.1210/jc.2004-1632. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulep EE, Vedernikov YP, Saade GR, Garfield RE. The role of endothelium-derived hyperpolarizing factor in the regulation of the uterine circulation in pregnant rats. Am J Obstet Gynecol. 2001;185:638–642. doi: 10.1067/mob.2001.117665. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R, Guralp O. Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk. Arch Gynecol Obstet. 2011;284:1367–1373. doi: 10.1007/s00404-011-1865-2. [DOI] [PubMed] [Google Scholar]
- George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24:964–969. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2013;305:R397–R403. doi: 10.1152/ajpregu.00216.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension. 2009;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309:R62–R70. doi: 10.1152/ajpregu.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol. 2010;299:H1642–H1652. doi: 10.1152/ajpheart.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa MF, Naguib AH, Swedan KH, Abdellatif SS. Serum tumor necrosis factor-alpha level and uterine artery Doppler indices at 11–13 weeks' gestation for preeclampsia screening in low-risk pregnancies: a prospective observational study. J Reprod Immunol. 2015;109:31–35. doi: 10.1016/j.jri.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Goulopoulou S, Hannan JL, Matsumoto T, Webb RC. Pregnancy reduces RhoA/Rho kinase and protein kinase C signaling pathways downstream of thromboxane receptor activation in the rat uterine artery. Am J Physiol Heart Circ Physiol. 2012;302:H2477–H2488. doi: 10.1152/ajpheart.00900.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes MF, Brandao AH, Rezende CA, Cabral AC, Brum AP, Leite HV, Capuruco CA. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch Gynecol Obstet. 2014;290:441–447. doi: 10.1007/s00404-014-3220-x. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: effect of tumor necrosis factor blockade. J Hypertens. 2011;29:1203–1212. doi: 10.1097/HJH.0b013e3283468392. [DOI] [PubMed] [Google Scholar]
- Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension. 2007;49:328–333. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- Hall D, Gebhardt S, Theron G, Grove D. Pre-eclampsia and gestational hypertension are less common in HIV infected women. Pregnancy Hypertens. 2014;4:91–96. doi: 10.1016/j.preghy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Hamai Y, Fujii T, Yamashita T, Nishina H, Kozuma S, Mikami Y, Taketani Y. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38:89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Hammond S, Mathewson AM, Baker PN, Mayhew TM, Dunn WR. Gap junctions and hydrogen peroxide are involved in endothelium-derived hyperpolarising responses to bradykinin in omental arteries and veins isolated from pregnant women. Eur J Pharmacol. 2011;668:225–232. doi: 10.1016/j.ejphar.2011.06.050. [DOI] [PubMed] [Google Scholar]
- He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- Hefler LA, Reyes CA, O'Brien WE, Gregg AR. Perinatal development of endothelial nitric oxide synthase-deficient mice. Biol Reprod. 2001;64:666–673. doi: 10.1095/biolreprod64.2.666. [DOI] [PubMed] [Google Scholar]
- Herraiz I, Simon E, Gomez-Arriaga PI, Martinez-Moratalla JM, Garcia-Burguillo A, Jimenez EA, Galindo A. Angiogenesis-Related Biomarkers (sFlt-1/PLGF) in the Prediction and Diagnosis of Placental Dysfunction: An Approach for Clinical Integration. Int J Mol Sci. 2015;16:19009–19026. doi: 10.3390/ijms160819009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ, Karumanchi SA, Lely AT. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol. 2014;25:717–725. doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–1139. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter A, Aitkenhead M, Caldwell C, McCracken G, Wilson D, McClure N. Serum levels of vascular endothelial growth factor in preeclamptic and normotensive pregnancy. Hypertension. 2000;36:965–969. doi: 10.1161/01.hyp.36.6.965. [DOI] [PubMed] [Google Scholar]
- Iriyama T, Wang W, Parchim NF, Song A, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Hypoxia-independent upregulation of placental hypoxia inducible factor-1alpha gene expression contributes to the pathogenesis of preeclampsia. Hypertension. 2015;65:1307–1315. doi: 10.1161/HYPERTENSIONAHA.115.05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T, Yamamoto K, Matsuura T, Sugimura M, Kobayashi T, Kanayama N. Alteration of integrins under hypoxic stress in early placenta and choriocarcinoma cell line BeWo. Gynecol Obstet Invest. 2004;57:196–203. doi: 10.1159/000076688. [DOI] [PubMed] [Google Scholar]
- Jain A, Schneider H, Aliyev E, Soydemir F, Baumann M, Surbek D, Hediger M, Brownbill P, Albrecht C. Hypoxic treatment of human dual placental perfusion induces a preeclampsia-like inflammatory response. Lab Invest. 2014;94:873–880. doi: 10.1038/labinvest.2014.76. [DOI] [PubMed] [Google Scholar]
- Jardim LL, Rios DR, Perucci LO, de Sousa LP, Gomes KB, Dusse LM. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clin Chim Acta. 2015;447:34–38. doi: 10.1016/j.cca.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Javadian P, Salmanian B, Javadi-Paydar M, Shamshirsaz AA, Ejtemaei Mehr S, Gharedaghi MH, Dehpour AR. Effect of morphine on the reduced uteroplacental perfusion model of pre-eclampsia in rats. Eur J Obstet Gynecol Reprod Biol. 2013;168:161–166. doi: 10.1016/j.ejogrb.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Zou Y, Ge Z, Zuo Q, Huang SY, Sun L. A Role of sFlt-1 in Oxidative Stress and Apoptosis in Human and Mouse Pre-Eclamptic Trophoblasts. Biol Reprod. 2015;93:73. doi: 10.1095/biolreprod.114.126227. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Tremble SM, Chan SL, Moseley J, LaMarca B, Nagle KJ, Cipolla MJ. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLoS One. 2014;9:e113670. doi: 10.1371/journal.pone.0113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakigano A, Mimura K, Kanagawa T, Nakayama M, Kanayama T, Fujita S, Kinugasa-Taniguchi Y, Endo M, Tomimatsu T, Kimura T. Imbalance of angiogenic factors and avascular edematous cystic villi in a trisomy 13 pregnancy: a case report. Placenta. 2013;34:628–630. doi: 10.1016/j.placenta.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Kanagal DV, Rajesh A, Rao K, Devi UH, Shetty H, Kumari S, Shetty PK. Levels of Serum Calcium and Magnesium in Pre-eclamptic and Normal Pregnancy: A Study from Coastal India. J Clin Diagn Res. 2014;8:OC01–OC04. doi: 10.7860/JCDR/2014/8872.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakus S, Bozoklu Akkar O, Yildiz C, Sancakdar E, Cetin M, Cetin A. Serum levels of ET-1, M30, and angiopoietins-1 and-2 in HELLP syndrome and preeclampsia compared to controls. Arch Gynecol Obstet. 2016;293:351–359. doi: 10.1007/s00404-015-3803-1. [DOI] [PubMed] [Google Scholar]
- Kemse NG, Kale AA, Joshi SR. A combined supplementation of omega-3 fatty acids and micronutrients (folic acid, vitamin B12) reduces oxidative stress markers in a rat model of pregnancy induced hypertension. PLoS One. 2014;9:e111902. doi: 10.1371/journal.pone.0111902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. Differential mechanisms of endothelium-dependent vasodilator responses in human myometrial small arteries in normal pregnancy and pre-eclampsia. Clin Sci (Lond) 2002;103:67–73. doi: 10.1042/cs1030067. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension. 1998;31:1065–1069. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–R45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- Kim YG, Suga SI, Kang DH, Jefferson JA, Mazzali M, Gordon KL, Matsui K, Breiteneder-Geleff S, Shankland SJ, Hughes J, Kerjaschki D, Schreiner GF, Johnson RJ. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int. 2000;58:2390–2399. doi: 10.1046/j.1523-1755.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Knock GA, Poston L. Bradykinin-mediated relaxation of isolated maternal resistance arteries in normal pregnancy and preeclampsia. Am J Obstet Gynecol. 1996;175:1668–1674. doi: 10.1016/s0002-9378(96)70123-0. [DOI] [PubMed] [Google Scholar]
- Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993;92:99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004;23:101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- Kriston T, Venuto RC, Baylis C, Losonczy G. Hemodynamic and renal effects of U-46619, a TXA2/PGH2 analog, in late-pregnant rats. Am J Physiol. 1999;276:R831–R837. doi: 10.1152/ajpregu.1999.276.3.R831. [DOI] [PubMed] [Google Scholar]
- Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002;287:3183–3186. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- Lamarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate? Int J Infereron Cytokine Mediator Res. 2011;2011:59–64. doi: 10.2147/IJICMR.S16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008a;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008b;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997–2008. BMC Pregnancy Childbirth. 2012;12:47. doi: 10.1186/1471-2393-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- Leonardo DP, Albuquerque DM, Lanaro C, Baptista LC, Cecatti JG, Surita FG, Parpinelli MA, Costa FF, Franco-Penteado CF, Fertrin KY, Costa ML. Association of Nitric Oxide Synthase and Matrix Metalloprotease Single Nucleotide Polymorphisms with Preeclampsia and Its Complications. PLoS One. 2015;10:e0136693. doi: 10.1371/journal.pone.0136693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Canzoneri BJ, Gu Y, Zhao S, Wang Y. Maternal levels of prostacyclin, thromboxane, ICAM, and VCAM in normal and preeclamptic pregnancies. Am J Reprod Immunol. 2010;64:376–383. doi: 10.1111/j.1600-0897.2010.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ge Q, Guo L, Lu Z. Maternal plasma miRNAs expression in preeclamptic pregnancies. Biomed Res Int. 2013;2013:970265. doi: 10.1155/2013/970265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Pan Z, Wang X, Gao Z, Ren C, Yang W. miR-125b-1-3p inhibits trophoblast cell invasion by targeting sphingosine-1-phosphate receptor 1 in preeclampsia. Biochem Biophys Res Commun. 2014a;453:57–63. doi: 10.1016/j.bbrc.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and-9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem Pharmacol. 2014b;89:370–385. doi: 10.1016/j.bcp.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol. 2013;56:91–97. doi: 10.1016/j.molimm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhao Y, Yu A, Zhao B, Gao Y, Niu H. Diagnostic accuracy of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio for preeclampsia: a meta-analysis based on 20 studies. Arch Gynecol Obstet. 2015;292:507–518. doi: 10.1007/s00404-015-3671-8. [DOI] [PubMed] [Google Scholar]
- Llinas MT, Alexander BT, Seedek M, Abram SR, Crell A, Granger JP. Enhanced thromboxane synthesis during chronic reductions in uterine perfusion pressure in pregnant rats. Am J Hypertens. 2002;15:793–797. doi: 10.1016/s0895-7061(02)02975-8. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172:1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy G, Singh JP, Schoenl M, Mucha I, Venuto RC. Pregnancy enhances the pressor response to thromboxane analogues in rabbits. Am J Physiol. 1995;269:R720–R725. doi: 10.1152/ajpregu.1995.269.3.R720. [DOI] [PubMed] [Google Scholar]
- Luft FC. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant. 2006;21:3052–3054. doi: 10.1093/ndt/gfl439. [DOI] [PubMed] [Google Scholar]
- Luksha L, Nisell H, Kublickiene K. The mechanism of EDHF-mediated responses in subcutaneous small arteries from healthy pregnant women. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1102–R1109. doi: 10.1152/ajpregu.00550.2003. [DOI] [PubMed] [Google Scholar]
- Luksha L, Nisell H, Luksha N, Kublickas M, Hultenby K, Kublickiene K. Endothelium-derived hyperpolarizing factor in preeclampsia: heterogeneous contribution, mechanisms, and morphological prerequisites. Am J Physiol Regul Integr Comp Physiol. 2008;294:R510–R519. doi: 10.1152/ajpregu.00458.2007. [DOI] [PubMed] [Google Scholar]
- Luque A, Farwati A, Crovetto F, Crispi F, Figueras F, Gratacos E, Aran JM. Usefulness of circulating microRNAs for the prediction of early preeclampsia at first-trimester of pregnancy. Sci Rep. 2014;4:4882. doi: 10.1038/srep04882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014;4:105–145. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. 2012;64:540–582. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala M, Gokina N, Barron C, Osol G. Endothelial-derived hyperpolarization factor (EDHF) contributes to PlGF-induced dilation of mesenteric resistance arteries from pregnant rats. J Vasc Res. 2012;49:43–49. doi: 10.1159/000329821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March MI, Geahchan C, Wenger J, Raghuraman N, Berg A, Haddow H, McKeon BA, Narcisse R, David JL, Scott J, Thadhani R, Karumanchi SA, Rana S. Circulating Angiogenic Factors and the Risk of Adverse Outcomes among Haitian Women with Preeclampsia. PLoS One. 2015;10:e0126815. doi: 10.1371/journal.pone.0126815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoura S, Kalogiannidis I, Makedou K, Theodoridis T, Koiou K, Gerou S, Athanasiadis A, Agorastos T. Biomarkers of endothelial dysfunction in preeclampsia and neonatal morbidity: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2014;175:119–123. doi: 10.1016/j.ejogrb.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y, Yamanaka N. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias ML, Romao M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, Araujo JP, Jr, Peracoli JC, de Oliveira L, Peracoli MT. Endogenous and Uric Acid-Induced Activation of NLRP3 Inflammasome in Pregnant Women with Preeclampsia. PLoS One. 2015;10:e0129095. doi: 10.1371/journal.pone.0129095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J Obstet Gynaecol Res. 2010;36:239–247. doi: 10.1111/j.1447-0756.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca MQ, Dang Y, Khalil RA. Enhanced endothelin receptor type B-mediated vasodilation and underlying [Ca(2)(+)]i in mesenteric microvessels of pregnant rats. Br J Pharmacol. 2013;169:1335–1351. doi: 10.1111/bph.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol. 2012;84:147–162. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca MQ, Li W, Reslan OM, Yu P, Mata KM, Khalil RA. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension. 2014;64:632–643. doi: 10.1161/HYPERTENSIONAHA.114.03315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CA, Bernstein IM, Badger GJ, Horbar JD, Soll RF. The effect of maternal hypertension on mortality in infants 22, 29 weeks gestation. Pregnancy Hypertens. 2015;5:362–366. doi: 10.1016/j.preghy.2015.10.002. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Lutsiv O, Dzaja N, Duley L. A systematic review of maternal and infant outcomes following magnesium sulfate for pre-eclampsia/eclampsia in real-world use. Int J Gynaecol Obstet. 2012;118:90–96. doi: 10.1016/j.ijgo.2012.01.028. [DOI] [PubMed] [Google Scholar]
- McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol. 2004;24:540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- Mills JL, DerSimonian R, Raymond E, Morrow JD, Roberts LJ, 2nd, Clemens JD, Hauth JC, Catalano P, Sibai B, Curet LB, Levine RJ. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA. 1999;282:356–362. doi: 10.1001/jama.282.4.356. [DOI] [PubMed] [Google Scholar]
- Mistry HD, Kurlak LO, Broughton Pipkin F. The placental renin-angiotensin system and oxidative stress in pre-eclampsia. Placenta. 2013;34:182–186. doi: 10.1016/j.placenta.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Mitchell BM, Cook LG, Danchuk S, Puschett JB. Uncoupled endothelial nitric oxide synthase and oxidative stress in a rat model of pregnancy-induced hypertension. Am J Hypertens. 2007;20:1297–1304. doi: 10.1016/j.amjhyper.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Czegle I, Szijarto J, Rigo J., Jr Increased circulating interleukin-17 levels in preeclampsia. J Reprod Immunol. 2015;112:53–57. doi: 10.1016/j.jri.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno GL, Franchi M, Guidi GC. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal. 2009;23:88–92. doi: 10.1002/jcla.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska E, Kaczmarek MM, Blitek A. Regulation of prostacyclin synthase expression and prostacyclin content in the pig endometrium. Theriogenology. 2012;78:2071–2086. doi: 10.1016/j.theriogenology.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Moreno-Eutimio MA, Tovar-Rodriguez JM, Vargas-Avila K, Nieto-Velazquez NG, Frias-De-Leon MG, Sierra-Martinez M, Acosta-Altamirano G. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. Biomed Res Int. 2014;2014:413249. doi: 10.1155/2014/413249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R, Spencer SK, Kyle PB, Williams JM, Harris A, Owens MY, Wallace K. Hypertension in an Animal Model of HELLP Syndrome is Associated With Activation of Endothelin 1. Reprod Sci. 2015;23:42–50. doi: 10.1177/1933719115592707. [DOI] [PubMed] [Google Scholar]
- Morton JS, Davidge ST. Arterial endothelium-derived hyperpolarization: potential role in pregnancy adaptations and complications. J Cardiovasc Pharmacol. 2013;61:197–203. doi: 10.1097/FJC.0b013e31827b6367. [DOI] [PubMed] [Google Scholar]
- Motta C, Grosso C, Zanuzzi C, Molinero D, Picco N, Bellingeri R, Alustiza F, Barbeito C, Vivas A, Romanini MC. Effect of Sildenafil on Pre-Eclampsia-Like Mouse Model Induced By L-Name. Reprod Domest Anim. 2015;50:611–616. doi: 10.1111/rda.12536. [DOI] [PubMed] [Google Scholar]
- Murphy SR, Cockrell K. Regulation of soluble fms-like tyrosine kinase-1 production in response to placental ischemia/hypoxia: role of angiotensin II. Physiol Rep. 2015;3 doi: 10.14814/phy2.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55:394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7:375–384. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- Naiker S, Naidoo S, Naicker T, Gathiram P, Nadar A, Moodley J. Immunolocalisation and endothelin-1 values In pre-eclampsia: an immunocytochemical study. J Obstet Gynaecol. 2001;21:39–45. doi: 10.1080/01443610020022104. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sekizawa A, Purwosunu Y, Okazaki S, Farina A, Wibowo N, Shimizu H, Okai T. Cellular mRNA expressions of anti-oxidant factors in the blood of preeclamptic women. Prenat Diagn. 2009;29:691–696. doi: 10.1002/pd.2278. [DOI] [PubMed] [Google Scholar]
- Nascimento RA, Mendes G, Possomato-Vieira JS, Goncalves-Rizzi VH, Kushima H, Delella FK, Dias-Junior CA. Metalloproteinase Inhibition Protects against Reductions in Circulating Adrenomedullin during Lead-induced Acute Hypertension. Basic Clin Pharmacol Toxicol. 2015;116:508–515. doi: 10.1111/bcpt.12337. [DOI] [PubMed] [Google Scholar]
- Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res. 2000;87:406–411. doi: 10.1161/01.res.87.5.406. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh M, Kianpour M, Nematbakhsh M. Serum levels of asymmetric dimethylarginine, vascular endothelial growth factor, and nitric oxide metabolite levels in preeclampsia patients. ISRN Obstet Gynecol. 2013;2013:104213. doi: 10.1155/2013/104213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–487. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1197–R1201. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent WH, Mishra N, Strauss JF, 3rd, Walsh SW. Matrix Metalloproteinase 1 Causes Vasoconstriction and Enhances Vessel Reactivity to Angiotensin II via Protease-Activated Receptor 1. Reprod Sci. 2016;23:542–548. doi: 10.1177/1933719115607998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orie NN, Fry CH, Clapp LH. Evidence that inward rectifier K+ channels mediate relaxation by the PGI2 receptor agonist cicaprost via a cyclic AMP-independent mechanism. Cardiovasc Res. 2006;69:107–115. doi: 10.1016/j.cardiores.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension. 2004;43:434–444. doi: 10.1161/01.HYP.0000113044.46326.98. [DOI] [PubMed] [Google Scholar]
- Ou M, Dang Y, Mazzuca MQ, Basile R, Khalil RA. Adaptive regulation of endothelin receptor type-A and type-B in vascular smooth muscle cells during pregnancy in rats. J Cell Physiol. 2014;229:489–501. doi: 10.1002/jcp.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Curr Drug Targets. 2013a;14:325–334. doi: 10.2174/1389450111314030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 2013b;208:224–233. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KR, Kaitu'u-Lino TJ, Hastie R, Hannan NJ, Ye L, Binder N, Cannon P, Tuohey L, Johns TG, Shub A, Tong S. Placental-Specific sFLT-1 e15a Protein Is Increased in Preeclampsia, Antagonizes Vascular Endothelial Growth Factor Signaling, and Has Antiangiogenic Activity. Hypertension. 2015;66:1251–1259. doi: 10.1161/HYPERTENSIONAHA.115.05883. [DOI] [PubMed] [Google Scholar]
- Papazoglou D, Galazios G, Koukourakis MI, Kontomanolis EN, Maltezos E. Association of-634G/C and 936C/T polymorphisms of the vascular endothelial growth factor with spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2004;83:461–465. doi: 10.1111/j.0001-6349.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- Parra MC, Lees C, Mann GE, Pearson JD, Nicolaides KH. Vasoactive mediator release by fetal endothelial cells in intrauterine growth restriction and preeclampsia. Am J Obstet Gynecol. 2001;184:497–502. doi: 10.1067/mob.2001.110311. [DOI] [PubMed] [Google Scholar]
- Parrish MR, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN, Jr, Lamarca BB. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gend Med. 2011;8:184–188. doi: 10.1016/j.genm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto AB, Araujo Junior E, Ribeiro JU, Rodrigues DB, Castro EC, Caldas TM, Rodrigues Junior V. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. J Matern Fetal Neonatal Med. 2016;29:75–79. doi: 10.3109/14767058.2014.987117. [DOI] [PubMed] [Google Scholar]
- Peracoli MT, Bannwart CF, Cristofalo R, Borges VT, Costa RA, Witkin SS, Peracoli JC. Increased reactive oxygen species and tumor necrosis factor-alpha production by monocytes are associated with elevated levels of uric acid in pre-eclamptic women. Am J Reprod Immunol. 2011;66:460–467. doi: 10.1111/j.1600-0897.2011.01016.x. [DOI] [PubMed] [Google Scholar]
- Pereira RD, De Long NE, Wang RC, Yazdi FT, Holloway AC, Raha S. Angiogenesis in the placenta: the role of reactive oxygen species signaling. Biomed Res Int. 2015;2015:814543. doi: 10.1155/2015/814543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sepulveda A, Torres MJ, Khoury M, Illanes SE. Innate immune system and preeclampsia. Front Immunol. 2014;5:244. doi: 10.3389/fimmu.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel AM, Pereira NR, Costa CA, Mann GE, Cordeiro VS, de Moura RS, Brunini TM, Mendes-Ribeiro AC, Resende AC. L-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertens Res. 2013;36:783–788. doi: 10.1038/hr.2013.34. [DOI] [PubMed] [Google Scholar]
- Pinheiro MB, Gomes KB, Ronda CR, Guimaraes GG, Freitas LG, Teixeira-Carvalho A, Martins-Filho OA, Dusse LM. Severe preeclampsia: association of genes polymorphisms and maternal cytokines production in Brazilian population. Cytokine. 2015;71:232–237. doi: 10.1016/j.cyto.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacol Ther. 1995;65:215–239. doi: 10.1016/0163-7258(94)00064-a. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45:373–380. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahardjo B, Widjajanto E, Sujuti H, Keman K. Different levels of IL-1alpha, IL-6, TNF-alpha, NF-kappaB and PPAR-gamma in monocyte cultures exposed by plasma preeclampsia and normotensive pregnancy. Pregnancy Hypertens. 2014;4:187–193. doi: 10.1016/j.preghy.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Rahgozar S, Amirian T, Qi M, Shahshahan Z, Entezar EGM, Ghasemi Tehrani H, Miroliaei M, Krilis SA, Giannakopoulos B. Improved Assay for Quantifying a Redox Form of Angiotensinogen as a Biomarker for Pre-Eclampsia: A Case-Control Study. PLoS One. 2015;10:e0135905. doi: 10.1371/journal.pone.0135905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol. 2016;12:209–227. doi: 10.1586/1744666X.2016.1105740. [DOI] [PubMed] [Google Scholar]
- Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol. 2011;157:136–140. doi: 10.1016/j.ejogrb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Ramma W, Buhimschi IA, Zhao G, Dulay AT, Nayeri UA, Buhimschi CS, Ahmed A. The elevation in circulating anti-angiogenic factors is independent of markers of neutrophil activation in preeclampsia. Angiogenesis. 2012;15:333–340. doi: 10.1007/s10456-012-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal JF, Lillegard KE, Bauer AJ, Elmquist BJ, Loeks-Johnson AC, Gilbert JS. Neutrophil Depletion Attenuates Placental Ischemia-Induced Hypertension in the Rat. PLoS One. 2015;10:e0132063. doi: 10.1371/journal.pone.0132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem. 2010;8:204–226. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios DR, Alpoim PN, Godoi LC, Perucci LO, de Sousa LP, Gomes KB, Dusse LM. Increased Levels of sENG and sVCAM-1 and Decreased Levels of VEGF in Severe Preeclampsia. Am J Hypertens. 2015 doi: 10.1093/ajh/hpv170. [DOI] [PubMed] [Google Scholar]
- Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens. 2012;2:72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health. 2005;95:1545–1551. doi: 10.2105/AJPH.2005.065680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle C, Lim S, Xu B, Tooher J, Ogle R, Hennessy A. Effect of hypoxia and exogenous IL-10 on the pro-inflammatory cytokine TNF-alpha and the anti-angiogenic molecule soluble Flt-1 in placental villous explants. Cytokine. 2009;47:56–60. doi: 10.1016/j.cyto.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Sakashita T, Higashi Y, Soga J, Miyoshi H, Kudo Y. Circulating endothelial progenitor cells and placental abruption in women with preeclampsia. Pregnancy Hypertens. 2014;4:203–208. doi: 10.1016/j.preghy.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Sanchez-Aranguren LC, Prada CE, Riano-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. 2014;5:372. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrim VC, Palei AC, Sertorio JT, Cavalli RC, Duarte G, Tanus-Santos JE. Effects of eNOS polymorphisms on nitric oxide formation in healthy pregnancy and in pre-eclampsia. Mol Hum Reprod. 2010;16:506–510. doi: 10.1093/molehr/gaq030. [DOI] [PubMed] [Google Scholar]
- Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension. 2014;64:852–859. doi: 10.1161/HYPERTENSIONAHA.114.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- Scalera F, Schlembach D, Beinder E. Production of vasoactive substances by human umbilical vein endothelial cells after incubation with serum from preeclamptic patients. Eur J Obstet Gynecol Reprod Biol. 2001;99:172–178. doi: 10.1016/s0301-2115(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Schiessl B, Strasburger C, Bidlingmaier M, Mylonas I, Jeschke U, Kainer F, Friese K. Plasma- and urine concentrations of nitrite/nitrate and cyclic Guanosinemonophosphate in intrauterine growth restricted and preeclamptic pregnancies. Arch Gynecol Obstet. 2006;274:150–154. doi: 10.1007/s00404-006-0149-8. [DOI] [PubMed] [Google Scholar]
- Schramm AM, Clowse ME. Aspirin for prevention of preeclampsia in lupus pregnancy. Autoimmune Dis. 2014;2014:920467. doi: 10.1155/2014/920467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serin IS, Ozcelik B, Basbug M, Kilic H, Okur D, Erez R. Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2002;100:143–145. doi: 10.1016/s0301-2115(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Shaamash AH, Elsnosy ED, Makhlouf AM, Zakhari MM, Ibrahim OA, HM EL-d. Maternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsia. Int J Gynaecol Obstet. 2000;68:207–214. doi: 10.1016/s0020-7292(99)00213-1. [DOI] [PubMed] [Google Scholar]
- Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol. 2015;95:211–226. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. J Biol Chem. 1999;274:33057–33063. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Gilbert C, Granderson G, Carretero CD, Carretero OA, Beierwaltes WH. Nitric oxide synthase gene knockout mice do not become hypertensive during pregnancy. Am J Obstet Gynecol. 2001;185:1198–1203. doi: 10.1067/mob.2001.118142. [DOI] [PubMed] [Google Scholar]
- Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch. 2010;459:915–922. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- Siddiqui AH, Irani RA, Zhang W, Wang W, Blackwell SC, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated soluble fms-like tyrosine kinase-1 induction contributes to impaired adrenal vasculature and decreased aldosterone production in preeclampsia. Hypertension. 2013;61:472–479. doi: 10.1161/HYPERTENSIONAHA.111.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Laguado J, Garcia RG, Lopez-Jaramillo P. Flow-mediated dilatation of the brachial artery in pregnancy. Int J Gynaecol Obstet. 2006;93:60–61. doi: 10.1016/j.ijgo.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Siljee JE, Wortelboer EJ, Koster MP, Imholz S, Rodenburg W, Visser GH, de Vries A, Schielen PC, Pennings JL. Identification of interleukin-1 beta, but no other inflammatory proteins, as an early onset pre-eclampsia biomarker in first trimester serum by bead-based multiplexed immunoassays. Prenat Diagn. 2013;33:1183–1188. doi: 10.1002/pd.4219. [DOI] [PubMed] [Google Scholar]
- Smith-Jackson K, Hentschke MR, Poli-de-Figueiredo CE, Pinheiro da Costa BE, Kurlak LO, Broughton Pipkin F, Czajka A, Mistry HD. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta. 2015;36:607–610. doi: 10.1016/j.placenta.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Sones JL, Lob HE, Isroff CE, Davisson RL. Role of decidual natural killer cells, interleukin-15, and interferon-gamma in placental development and preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2014;307:R490–R492. doi: 10.1152/ajpregu.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradley FT, Palei AC, Granger JP. Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1326–R1343. doi: 10.1152/ajpregu.00178.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas) Cytokine. 2011;56:192–199. doi: 10.1016/j.cyto.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod. 2012;87:134. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- Tanbe AF, Khalil RA. Circulating and Vascular Bioactive Factors during Hypertension in Pregnancy. Curr Bioact Compd. 2010;6:60–75. doi: 10.2174/157340710790711737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71:1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U, Kribs A, Danner M, Hamacher S, Mallmann P, Stepan H, Benzing T. Removal of Soluble Fms-Like Tyrosine Kinase-1 by Dextran Sulfate Apheresis in Preeclampsia. J Am Soc Nephrol. 2016;27:903–913. doi: 10.1681/ASN.2015020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24:11–14. doi: 10.1016/s0146-0005(00)80047-6. [DOI] [PubMed] [Google Scholar]
- Trifonova EA, Gabidulina TV, Ershov NI, Serebrova VN, Vorozhishcheva AY, Stepanov VA. Analysis of the placental tissue transcriptome of normal and preeclampsia complicated pregnancies. Acta Naturae. 2014;6:71–83. [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J, Moffett A. NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol. 2008;20:317–320. doi: 10.1016/j.smim.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- Tsukimori K, Fukushima K, Tsushima A, Nakano H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension. 2005;46:696–700. doi: 10.1161/01.HYP.0000184197.11226.71. [DOI] [PubMed] [Google Scholar]
- Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzcu ZB, Asicioglu E, Sunbul M, Ozben B, Arikan H, Koc M. Circulating endothelial cell number and markers of endothelial dysfunction in previously preeclamptic women. Am J Obstet Gynecol. 2015;213:533, e531–e537. doi: 10.1016/j.ajog.2015.06.043. [DOI] [PubMed] [Google Scholar]
- Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol. 2009;7:79. doi: 10.1186/1477-7827-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden OW, Essers YP, Fazzi G, Peeters LL, De Mey JG, van Eys GJ. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod. 2005;72:1161–1168. doi: 10.1095/biolreprod.104.033985. [DOI] [PubMed] [Google Scholar]
- van Dijk M, Oudejans C. (Epi)genetics of pregnancy-associated diseases. Front Genet. 2013;4:180. doi: 10.3389/fgene.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk M, Oudejans CB. STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J Pregnancy. 2011;2011:521826. doi: 10.1155/2011/521826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWijk MJ, Kublickiene K, Boer K, VanBavel E. Vascular function in preeclampsia. Cardiovasc Res. 2000;47:38–48. doi: 10.1016/s0008-6363(00)00087-0. [DOI] [PubMed] [Google Scholar]
- Velloso EP, Pimentel RL, Braga JF, Cabral AC, Reis ZS, Bader M, Santos RA, Wallukat G. Identification of a Novel Agonist-Like Autoantibody in Preeclamptic Patients. Am J Hypertens. 2015 doi: 10.1093/ajh/hpv099. [DOI] [PubMed] [Google Scholar]
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- Verdonk K, Saleh L, Lankhorst S, Smilde JE, van Ingen MM, Garrelds IM, Friesema EC, Russcher H, van den Meiracker AH, Visser W, Danser AH. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension. 2015;65:1316–1323. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Niehoff M, Hering L, Geusens N, Herse F, Tintu AN, Plagemann A, LeNoble F, Pijnenborg R, Muller DN, Luft FC, Dudenhausen JW, Gollasch M, Dechend R. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension. 2008;51:547–553. doi: 10.1161/HYPERTENSIONAHA.107.103176. [DOI] [PubMed] [Google Scholar]
- von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D, Sherlock RL, Skoll MA, Wareing MM, Baker PN. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG. 2011;118:624–628. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
- Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN, Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57:949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2004;70:223–232. doi: 10.1016/j.plefa.2003.04.010. [DOI] [PubMed] [Google Scholar]
- Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, Baily J, Miller MR, Cudmore M, Hadoke PW, Wang R, Gratacos E, Buhimschi IA, Buhimschi CS, Ahmed A. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127:2514–2522. doi: 10.1161/CIRCULATIONAHA.113.001631. [DOI] [PubMed] [Google Scholar]
- Wang L, Yang T, Ding Y, Zhong Y, Yu L, Peng M. Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine. 2015;48:299–308. doi: 10.1007/s12020-014-0286-y. [DOI] [PubMed] [Google Scholar]
- Wang W, Parchim NF, Iriyama T, Luo R, Zhao C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L, Tao L, Hicks MJ, Kellems RE, Xia Y. Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension. 2014;63:595–606. doi: 10.1161/HYPERTENSIONAHA.113.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Walsh SW, Kay HH. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. Am J Obstet Gynecol. 1992;167:946–949. doi: 10.1016/s0002-9378(12)80017-2. [DOI] [PubMed] [Google Scholar]
- Wei YM, Yang HX, Zhu WW, Liu XY, Meng WY, Wang YQ, Shang LX, Cai ZY, Ji LP, Wang YF, Sun Y, Liu JX, Wei L, Sun YF, Zhang XY, Luo TX, Chen HX, Yu LJ. Risk of adverse pregnancy outcomes stratified for pre-pregnancy body mass index. J Matern Fetal Neonatal Med. 2015:1–5. doi: 10.3109/14767058.2015.1081167. [DOI] [PubMed] [Google Scholar]
- Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2006;290:L284–L290. doi: 10.1152/ajplung.00449.2004. [DOI] [PubMed] [Google Scholar]
- Weiner CP, Thompson LP, Liu KZ, Herrig JE. Endothelium-derived relaxing factor and indomethacin-sensitive contracting factor alter arterial contractile responses to thromboxane during pregnancy. Am J Obstet Gynecol. 1992;166:1171–1178. doi: 10.1016/s0002-9378(11)90603-6. discussion 1179–1181. [DOI] [PubMed] [Google Scholar]
- Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol. 1997;272:H748–H752. doi: 10.1152/ajpheart.1997.272.2.H748. [DOI] [PubMed] [Google Scholar]
- Xia Y, Ramin SM, Kellems RE. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li L, Shang JY, Cai L, Song L, Zhang SL, Li H, Li X, Lau WB, Ma XL, Liu HR. Angiotensin II type 1 receptor autoantibody as a novel regulator of aldosterone independent of preeclampsia. J Hypertens. 2015;33:1046–1056. doi: 10.1097/HJH.0000000000000521. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang F, Lau WB, Zhang S, Liu H, Ma XL. Autoantibodies isolated from preeclamptic patients induce endothelial dysfunction via interaction with the angiotensin II AT1 receptor. Cardiovasc Toxicol. 2014;14:21–29. doi: 10.1007/s12012-013-9229-8. [DOI] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod. 2010;82:66–75. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinon Y, Ben Meir E, Margolis L, Lipitz S, Schiff E, Mazaki-Tovi S, Simchen MJ. Low molecular weight heparin therapy during pregnancy is associated with elevated circulatory levels of placental growth factor. Placenta. 2015;36:121–124. doi: 10.1016/j.placenta.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Yinon Y, Nevo O, Xu J, Many A, Rolfo A, Todros T, Post M, Caniggia I. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am J Pathol. 2008;172:77–85. doi: 10.2353/ajpath.2008.070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Nakao S, Kobayashi M, Kobayashi H. Flow-mediated vasodilation and plasma fibronectin levels in preeclampsia. Hypertension. 2000;36:400–404. doi: 10.1161/01.hyp.36.3.400. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang L, Liu T, Guan H. MicroRNA-204 suppresses trophoblast-like cell invasion by targeting matrix metalloproteinase-9. Biochem Biophys Res Commun. 2015;463:285–291. doi: 10.1016/j.bbrc.2015.05.052. [DOI] [PubMed] [Google Scholar]
- Yuksel MA, Tuten A, Oncul M, Acikgoz AS, Temel Yuksel I, Toprak MS, Ekmekci H, Balci Ekmekci O, Madazli R. Serum endocan concentration in women with pre-eclampsia. Arch Gynecol Obstet. 2015;292:69–73. doi: 10.1007/s00404-014-3605-x. [DOI] [PubMed] [Google Scholar]
- Zarate A, Saucedo R, Valencia J, Manuel L, Hernandez M. Early disturbed placental ischemia and hypoxia creates immune alteration and vascular disorder causing preeclampsia. Arch Med Res. 2014;45:519–524. doi: 10.1016/j.arcmed.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Zawiejska A, Wender-Ozegowska E, Iciek R, Brazert J. Concentrations of endothelial nitric oxide synthase, angiotensin-converting enzyme, vascular endothelial growth factor and placental growth factor in maternal blood and maternal metabolic status in pregnancy complicated by hypertensive disorders. J Hum Hypertens. 2014;28:670–676. doi: 10.1038/jhh.2014.42. [DOI] [PubMed] [Google Scholar]
- Zhao S, Gu Y, Lewis DF, Wang Y. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta. 2008;29:81–88. doi: 10.1016/j.placenta.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zheng YF, Luo QQ, Yan T, Liu XX, Han L, Zou L. Edaravone inhibits hypoxia-induced trophoblast-soluble Fms-like tyrosine kinase 1 expression: a possible therapeutic approach to preeclampsia. Placenta. 2014;35:476–482. doi: 10.1016/j.placenta.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhong M, Pang Z, Yu Y. Dysregulated expression of matrix metalloproteinases and their inhibitors may participate in the pathogenesis of pre-eclampsia and fetal growth restriction. Early Hum Dev. 2014;90:657–664. doi: 10.1016/j.earlhumdev.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]