Abstract
Autosomal recessive inheritance of NPC1 with loss-of-function mutations underlies Niemann–Pick disease, type C1 (NP-C1), a lysosomal storage disorder with progressive neurodegeneration. It is uncertain from limited biochemical studies and patient case reports whether NPC1 haploinsufficiency can cause a partial NP-C1 phenotype in carriers. In the present study, we examined this possibility in heterozygotes of a natural loss-of-function mutant Npc1 mouse model. We found partial motor dysfunction and increased anxiety-like behavior in Npc1 +/– mice by 9 weeks of age. Relative to Npc1 +/+ mice, Npc1 +/– mice failed to show neurodevelopmental improvements in motor coordination and balance on an accelerating Rotarod. In the open-field test, Npc1 +/– mice showed an intermediate phenotype in spontaneous locomotor activity compared with Npc1 +/+ and Npc1 –/– mice, as well as decreased center tendency. Together with increased stride length under anxiogenic conditions on the DigiGait treadmill, these findings are consistent with heightened anxiety. Our findings indicate that pathogenic NPC1 allele carriers, who represent about 0.66 % of humans, could be vulnerable to motor and anxiety disorders.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-016-0427-5) contains supplementary material, which is available to authorized users.
Keywords: NPC1, Niemann–Pick disease type C, haploinsufficiency, Carrier, Heterozygote disease, Neurological
Introduction
Niemann–Pick disease, type C1 (NP-C1; MIM 257220) is an autosomal recessive lysosomal storage disorder, caused by loss-of-function mutations in NPC1. NP-C1 cells have a distinct biochemical phenotype of lysosomal cholesterol and glycosphingolipids overload, which subsequently causes cellular damage and dysfunction. Clinically, visceral symptoms, such as hepatosplenomegaly, are most prominent in patients with NP-C1 presenting at an age younger than 6 years [1]. For patients aged ≥6 years, the main clinical presentations are neurological and psychiatric [1], which include cerebellar ataxia, loss of motor coordination, dysarthria, dysphagia, vertical supranuclear gaze palsy, seizures, psychosis, and dementia [1, 2]. The neurological and psychiatric symptoms are related to early loss of cerebellar Purkinje cells, later loss of hippocampal and cortical neurons, and impaired myelination [3–7]. Disease onset can occur at any age, with a predominant childhood manifestation that leads to premature death before adulthood [8]. The only approved drug treatment is with miglustat, a glucosylceramide synthase inhibitor. For patients with NP-C1 who were treated with miglustat continuously for an average of 2 years, the majority were categorized as improved/stable in their neurological disease progression [9]. Currently, NPC1 carriers are generally considered to be clinically unaffected, but it is uncertain whether NPC1 haploinsufficiency can predispose NPC1 carriers to moderate and potentially subclinical NP-C1 phenotypes.
The most recent estimate of the carrier frequency for pathogenic NPC1 alleles is 0.66 % [10]. Biochemically, NPC1 carriers have an intermediate phenotype in lipid regulation and metabolism. Cultured human and feline NPC1 obligate heterozygote skin fibroblast cells demonstrated an intermediate rate of cholesterol esterification, production of cholesteryl esters, and unesterified cholesterol storage level compared with healthy and homozygote NPC1 cells [11, 12]. Human fibroblasts also expressed an NPC1 dosage-dependent change in ABCA1 protein expression and apolipoprotein A-I-mediated lipid efflux [13]. In human NPC1 carriers, the concentrations of plasma oxysterols, 7-ketocholesterol, and cholestane-3β-5α-6β-triol are commensurately elevated between healthy and NP-C1 levels [14]. The liver in heterozygous Npc1 +/– mice compared with homozygous Npc1 +/+ and presymptomatic Npc1 –/– mice at 35 days of age has an intermediate level of sterol regulatory element-binding transcription factor 1 and 2 protein expression [15]. Subsequent mouse model studies revealed that a high-fat diet promoted greater weight gain, and impaired glucose metabolism and insulin resistance in Npc1 +/– mice [16, 17].
Despite harboring an intermediate biochemical phenotype, NPC1 carriers are generally considered subclinical. NPC1 haploinsufficiency has been found to be associated with early-onset and morbid adult obesity in a recent genome-wide association study of European populations [18], but little is known of other health risks. Two studies that report central nervous system abnormalities in NP-C1 animal models suggest potential neurological impairment in aging NPC1 carriers. Ten-year-old NPC1 heterozygous cats have ectopic dendrites in cortical pyramidal neurons, with increased GM2 ganglioside [12]. Npc1 +/– mice examined at advanced age (2 years) show neurodegeneration with a significant loss of Purkinje neurons, and increases in brain cholesterol and hyperphosphorylated tau [19]. Consistent with these animal studies, there are also some case reports of tremor, parkinsonism, and increased foamy storage cell in bone marrow manifesting in aging NPC1 carriers [20–22]. Also, an international genetic screen for the prevalence of NP-C1 in adults with neurological and psychiatric disorders revealed an apparent enrichment of NPC1 carriers within this patient population [23]. However, it is unclear in these cases whether pathogenic NPC1 allele carrier status alone can cause neurological and psychiatric symptoms, or whether a neuropsychiatric phenotype is the consequence of a vulnerability induced by NPC1 allele carrier status that emerges only after interaction with other genetic defects and/or environmental factors. Furthermore, the timing of the onset of the neuropsychiatric phenotype is not understood.
In this study, we interrogated the motor and behavioral phenotypes associated with NPC1 haploinsufficiency to early neurodevelopmental maturity, using the BALB/cJ Npc1 nih mouse model [24]. The use of a mouse model allows us to control genetic background and environment, examining the specific impact of NPC1 haploinsufficiency, which would not be feasible with human subjects. Our study revealed that Npc1 +/– mice have partially impaired locomotor activity and increased anxiety-like behavior in early maturity, intermediate to the phenotypes of Npc1 –/– mice. Therefore, pathogenic NPC1 carriers could be at risk of attenuated NP-C1 disease.
Methods
Animals
Breeding pairs of heterozygous BALB/cJ Npc1 nih (Npc1 +/–) mice were obtained as a kind gift from Dr. Steven Walkley (Albert Einstein College of Medicine, NY, USA) [24]. These mice were maintained at the Florey Core Animal Services Facility, on Barastoc standard rodent feed (Ridley Corporation, Victoria, Australia). Food and water were available ad libitum.
Timed mating of 14 female and 11 male Npc1 +/– mice produced Npc1 +/+ (n female = 11; n male = 7), Npc1 +/– (n female = 15; n male = 15), and Npc1 –/– (n female = 9; n male = 5) littermates used in this study. We studied all the mice that were generated to a maximum number of 15 per subgroup; as a result, only the Npc1 +/– subgroup was sex matched. Mouse genotypes were identified using real-time polymerase chain reaction with probes designed for Npc1 (Transnetyx, Cordova, TN, USA). After weaning at postnatal day (P) 21, male and female mice were housed separately, with a maximum of 6 mice of mixed genotype per cage, and maintained on a 12-h light–dark cycle. The mice were monitored daily, and from P24, weighed every 2–3 days, then daily on weekdays from P36 until the end of the experiment at P71.
Experimental procedures were approved by the Florey Animal Ethics Committee and were conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes, Eighth Edition (2013). The experimenter was blinded to the genotype of experimental animals, which was decoded at the completion of behavioral tests.
Behavioral Tests
Mice were subjected to motor coordination and balance tests at the age of 5, 7, and 9 weeks. The tests were performed separately on consecutively days at the respective age.
Rotarod Test
Balance and motor coordination of mice were assessed using an accelerating Rotarod (PanLab, Barcelona, Spain). The electronically controlled rotating rod (30 mm diameter) accelerates at a constant rate from 4–40 rpm over a 5-min period. When a mouse falls off the rotating rod onto the plastic platform below, the latency to fall (seconds) and the rotation speed (rpm) were recorded. The total distance travelled was calculated from these measures. The experiment consists of a training session and a testing session over 2 consecutive days. Each session consists of 3 trials that were at least 1 h apart. The average of 3 trials was used in the final analysis.
Open-field Test
Spontaneous locomotor and exploratory activities of mice were assessed in an open-field test. Each mouse was placed in the center of a Plexiglass locomotor cell (27.3 cm × 27.3 cm × 20.3 cm) connected to three 16-beam infrared arrays on the X, Y, and Z-axes (Med Associates Inc., St. Albans, VT, USA). Mouse spontaneous locomotor activity over 15 min was measured in 5 min time bins by a computer and analyzed using Activity Monitor v.6.02 (Med Associates Inc.). Locomotor activities measured include distance travelled, ambulatory counts, vertical counts, jump counts, and average velocity. For anxiety-like behavior assessment, the center of the open-field arena (15 cm × 15 cm) was defined with start and end (x, y) coordinates as (4, 4) and (12.5, 12.5), respectively.
DigiGait Analysis
Gait was assessed using a DigiGait Imaging System (Mouse Specifics Inc., Boston, MA, USA), which consists of a transparent treadmill belt encased within a Perspex chamber. To improve contrast for automated analysis, the paws of the mice were colored with red food dye. Mice were placed in the chamber, and the treadmill belt was accelerated rapidly to 20 cm/s. As the mouse walked on the motorized transparent treadmill, its gait was captured by a high-speed digital camera placed underneath the transparent treadmill. The footprints from a segment of 3–5 s of video were then analyzed using DigiGait v. 9.9 (Mouse Specifics Inc.), which quantified multiple gait indices, including stride length, stride time, swing time, braking time, stance width, stride frequency, gait symmetry, and ataxia coefficient. The treadmill belt and Perspex chamber were cleaned with 80 % (v/v) ethanol between each animal testing. As the Npc1 –/– mice were incapable of performing on the DigiGait at 9 weeks, owing to severe ataxia, this genotype was removed from the final analysis. The Npc1 +/+ and Npc1 +/– mice that failed to walk on DigiGait were also removed from final analysis.
Statistical Analysis
Mouse behavioral data analyses were performed using Stata v.13 IC (Stata Corp, College Station, TX, USA). Data were transformed as indicated to ensure satisfaction of distributional assumptions for statistical testing. Owing to the repeated-measure nature of the data, and based on the data distribution, data were analyzed using random effects generalized least squared regression or random effects negative binomial regression, with the animals as a random effect, or median regression with each animal as an individual cluster. All analyses were adjusted for age and sex, and for Rotarod analysis, data were also adjusted for training/testing day. The analyses also included examination of the interaction between genotype and age.
Results
Body Weight
Fed a standard diet, there was no body weight difference between mice of all genotypes up to P28 (Fig. S1). We observed from P29 onwards that male mice became significantly heavier. Over the course of the present study (71 days), the age-dependent gain in body weight was similar between sex-matched Npc1 +/+ and Npc1 +/– littermates, consistent with previous findings [25, 26]. Compared with Npc1 +/+ and Npc1 +/– littermates, the body weight of Npc1 –/– mice decreased dramatically, as expected [14, 27], from 7 weeks of age for males and from 8 weeks of age for females.
Npc1+/– Mice Express Impaired Age-dependent Motor Coordination
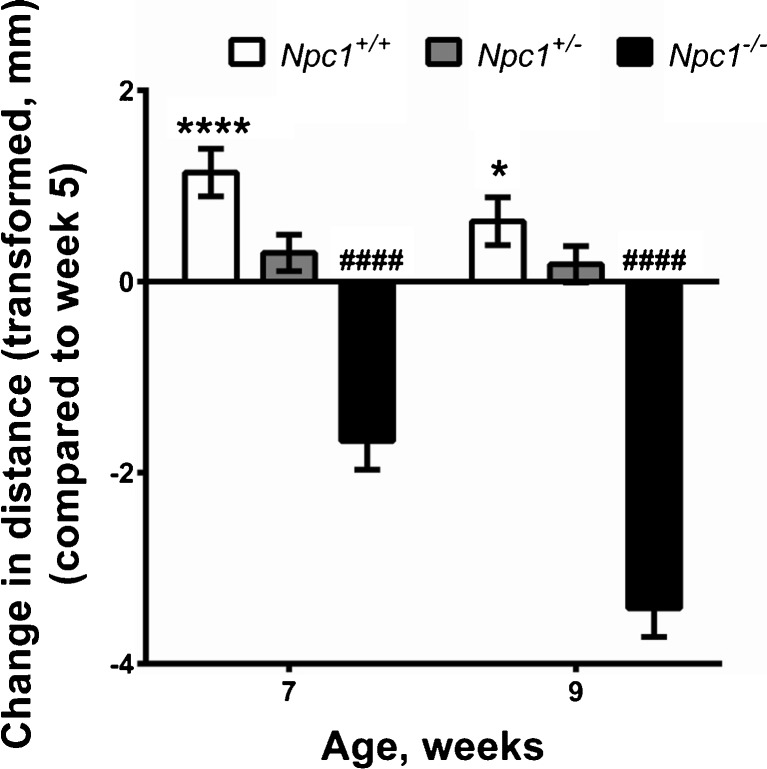
To assess the effect of Npc1 haploinsufficiency on motor coordination and balance, we tested Npc1 +/+, Npc1 +/–, and Npc1 –/– mice on an accelerating Rotarod at 5, 7, and 9 weeks of age. The data were analyzed as distance travelled on the accelerating Rotarod, which took into account the rotation speed and latency to fall (Fig. 1; Fig. S2 and Table S1). There was no significant difference in the distance travelled on the Rotarod by mice of all genotypes between training and testing days (p = 0.66). There was a significant interaction of genotype with age (p < 0.0001), with the main significant effect between Npc1 +/+ and Npc1 –/– mice, but not Npc1 +/+ and Np1 +/– mice, over age. As expected, the distance travelled by Npc1 –/– mice was significantly less than Npc1 +/+ mice (cube root transformed, b = –2.9; p < 0.0001), and markedly decreased progressively with age (Fig. 1). The distance travelled by Npc1 +/– mice on the Rotarod did not differ significantly from that of Npc1 +/+ mice (cube root transformed, b = 0.1; p = 0.7). However, examining the change in distance travelled at 7 and 9 weeks of age compared with 5 weeks of age, Npc1 +/+ mice showed improved motor coordination and balance with maturity (to 9 weeks), while Npc1 +/– mice failed to mature in these domains (Fig. 1). Male Npc1 +/– and Npc1 –/– mice were significantly less able to maintain their balance and coordination on the Rotarod compared with female Npc1 +/– and Npc1 –/– mice, but there was no sex difference in the Rotarod performance of Npc1 +/+ mice (Table S1).
Fig. 1.
Npc1 +/– mice express impaired age-dependent motor coordination. The change in distance travelled on an accelerating Rotarod by Npc1 +/+ (n female = 11; n male = 7), Npc1 +/– (n female = 15; n male = 15) and Npc1 –/– (n female = 9; n male = 5) at 7 and 9 weeks of age compared with 5 weeks of age revealed impaired motor development. Statistical analysis: cube root transformation was applied to ensure satisfaction of distributional assumptions prior to analysis by random effects generalized least squares regression with the animals as a random effect. All analyses were adjusted for age, sex, and training/test day. Data represent change in cube root of distance travelled (mm) ± SE compared with week 5. *p < 0.05, ****p < 0.0001 (Npc1 +/+); #### p < 0.0001 (Npc1 –/–)
Spontaneous Locomotion Impairment in Npc1+/– Mice
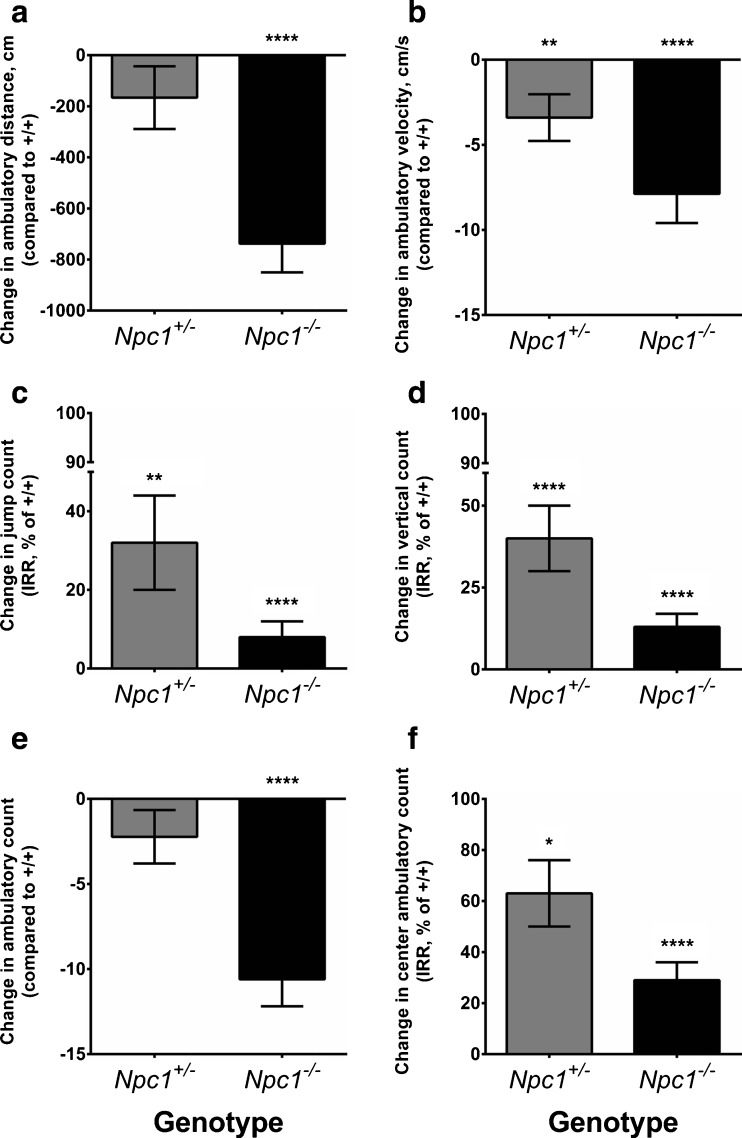
To appraise whether Npc1 haploinsufficiency affects spontaneous locomotor activity and novel environment exploratory behavior, we subjected Npc1 +/+, Npc1 +/–, and Npc1 –/– mice to an open-field test at 5, 7, and 9 weeks of age and analyzed their horizontal and vertical movements for 15 min. Horizontal movement indices in the total arena analyzed were total ambulatory distance travelled, total ambulatory count, and average velocity. Compared with Npc1 +/+ mice, the total ambulatory distance travelled in the entire arena by the Npc1 +/– mice was not different, whereas that of Npc1 –/– mice was significantly decreased by 737.6 ± 112.6 cm (Fig. 2A; Fig. S3A). There was a significant interaction of genotype with age (p = 0.01), with the main significant effect detected between Npc1 +/+ and Np1 –/– mice over age. By 9 weeks of age, the total ambulatory distance travelled by Npc1 +/+ mice increased significantly by 183.2 ± 93.5 cm compared with 5 weeks of age (p = 0.05), whereas there was no significant change detected in Npc1 +/– and Np1 –/– mice. The average velocity of Npc1 +/– mice movement was significantly slower than Npc1 +/+ mice, and intermediate between Npc1 +/+ and Npc1 –/– mice (Fig. 2B; Fig. S3B).
Fig. 2.
Spontaneous locomotion impairment and increased anxiety-like behavior in Npc1 +/– mice. Npc1 +/– mice (n female = 15; n male = 15) express intermediate spontaneous locomotor activity indices compared with normal Npc1 +/+ (n female = 11; n male = 7) and homozygous Npc1 –/– (n female = 9; n male = 5) mice in the open-field test performed at 5, 7, and 9 weeks of age. Data represent change ± SE compared with the Npc1 +/+ reference (0 for A, B, E, 100 % for C, D, F). A Differences in ambulatory distance travelled; (B) differences in average velocity; (C) differences in jump count incidence rate ratio (IRR); (D) differences in vertical count IRR; and (E) differences in ambulatory count (square root transformed). F Significantly reduced IRR for center ambulatory count in the Npc1 +/– mice compared with Npc1 +/+ mice (100 % reference) indicates increased anxiety. Statistical analysis: (A, B, E) random effects generalized least squares regression or (C, D, F) random effects negative binomial regression with the animals as a random effect. All analyses were adjusted for age and sex. There was a significant interaction effect between the genotypes and age for ambulatory distance travelled (p = 0.01), and vertical count (p = 0.03). *p < 0.05, **p < 0.01, ****p < 0.0001 (Npc1 +/+)
Vertical movements such as rearing and jumping are normal exploratory behavior of rodents in novel environments [28]. The incidence rate ratio (IRR) of jump counts showed that the expected jump counts in the total arena of both Npc1 +/– and Npc1 –/– mice were significantly less than Npc1 +/+ mice (Fig. 2C; Fig. S4A). Npc1 +/– and Npc1 –/– mice jumped with only about 30 % and about 10 %, respectively, of the frequency of Npc1 +/+ mice. Overall, male mice jumped with only about 40 % of the frequency of female mice. Further comparison of male mice with female mice within each genotype group showed that Npc1 +/+ and Npc1 +/– male mice jumped with only about 10 % and 40 %, respectively, of the frequency of their female counterparts. There was no significant difference in jump count IRR between male and female Npc1 –/– mice, which could be attributed to maximal impairment in both sexes.
Consistent with decreased jump count IRR, vertical count IRR also revealed significantly reduced vertical rearing activity in Npc1 +/– and Npc1 –/– mice (Fig. 2D; Fig. S4B). The IRRs for vertical rearing counts of Npc1 +/– and Npc1 –/– mice were only about 40 % and 10 %, respectively, that of Npc1 +/+ mice. There was a significant interaction of genotype with age for vertical count IRR (p = 0.03), with the main significant interaction between Npc1 +/+ and Npc1 +/– mice over age. Compared with their vertical rearing counts at 5 weeks of age, the Npc1 +/+ mice had significantly increased IRRs, by about 170 % and 190 %, at 7 and 9 weeks of age, respectively, but for the Npc1 +/– mice there was no developmental increase in vertical rearing performance at 7 weeks of age, and their performance took until 9 weeks of age to exhibit a significant improvement (about 180 %). As expected, there was no significant improvement in the vertical rearing count over age for Npc1 –/– mice, owing to their inherent motor impairment.
Increased Anxiety in Npc1+/– Mice
In addition to allowing us to examine unforced and voluntary locomotion, the open-field test is classically used to assess anxiety-like behavior in rodents [29]. Mice typically spend a greater amount of time exploring the periphery of an open-field arena than the unprotected center area; with the avoidance of the center area interpreted as anxiety. We found that Npc1 –/– mice exhibited significantly decreased ambulatory count in the total arena and center ambulatory count IRRs compared with Npc1 +/+ mice, consistent with motor impairment (Fig. 2E, F; Fig. S5). The ambulatory count in the total arena of the Npc1 +/– mice did not differ significantly from that of the Npc1 +/+ mice (Fig. 2E). However, the ambulatory count IRR of Npc1 +/– mice in the center of the arena was only about 60 % that of Npc1 +/+ mice (Fig. 2F), indicative of increased anxiety. Male mice entered the center of the arena at about 60 % of the incidence rate of female mice.
Npc1+/– Mice have Altered Stride
To appraise gait parameters sensitively, we examined the mice with the DigiGait motorized treadmill system [30–32]. This is a forced locomotor test, which can be used to highlight gait abnormalities not detectable in a voluntary locomotor test. Npc1 +/– mice were evaluated on the DigiGait treadmill walking at a constant speed of 20 cm/s. Owing to severe motor impairment, the Npc1 –/– mice failed to perform on the DigiGait and were excluded from final data analysis for this experiment. A stride is defined as a complete step cycle composed of stance and swing phases involving all 4 paws. Npc1 +/– mice expressed significantly altered stride measures compared with Npc1 +/+ mice. The stride length and duration of Npc1 +/– mice were both significantly longer than Npc1 +/+ mice, with a concomitant decrease in stride frequency (Table 1; Figs S6–S9). Male mice had greater stride length, longer stride duration, and reduced stride frequency compared with their female counterparts, which was exaggerated in male Npc1 +/– mice (Figs S6–S8).
Table 1.
DigiGait indices with significant difference between Npc1 +/+ (n female = 10; n male = 5–6) and Npc1 +/– (n female = 14; n male = 14–15) mice, measured at 5, 7, and 9 weeks of age
| Change in Npc1 +/– (compared with Npc1 +/+) | SE | 95 % CI | p-Value | |
|---|---|---|---|---|
| Stride length (cm) | 0.20 | 0.100 | 0.01–0.30 | 0.04 |
| Stride duration (ms) | 0.01 | 0.004 | 0.0004–0.0200 | 0.04 |
| Stride frequency (strides/ms) | –0.11 | 0.050 | –0.22 to –0.01 | 0.04 |
| Ataxia coefficient (forelimbs) | –0.13 | 0.070 | –0.260 to –0.004 | 0.04 |
Random effects generalized least squares regression with animals as a random effect, adjusted for age and sex
CI Confidence interval
The ataxia coefficient is an index of step-to-step variability. This index was significantly decreased in the forelimbs of Npc1 +/– mice, indicating an improved consistency in the gait changes observed, compared with Npc1 +/+ mice (Table 1; Fig. S9). However, at 9 weeks of age, there was a significant increase in Npc1 +/– mice forelimbs ataxia coefficient compared with 5 weeks of age, while there was no change in Npc1 +/+ mice forelimb ataxia coefficient with age.
There was no significant difference in the stance and swing durations between Npc1 +/+ and Npc1 +/– mice (Table S2). The stance width, an indication of postural adjustment for stability, was similar between Npc1 +/+ and Npc1 +/– mice. Gait symmetry was similar between both genotypes.
Discussion
To our knowledge, these are the first data to reveal motor and behavioral deficits in early maturity of Npc1 +/– mice. Previous studies have reported that NPC1 haploinsufficiency results in an intermediate biochemical deficit in obligate heterozygous human plasma samples, human and feline skin fibroblast cells, and mouse liver [11–15], cortical pyramidal neuron abnormalities in heterozygous NPC1 cats [12], and neurodegeneration in aged Npc1 +/– mice [19]. These findings suggest NPC1 carriers could be predisposed to moderate and potentially subclinical neurological impairments, and may be the basis of motor syndromes reported in limited studies of NPC1 carriers [20, 21]. The motor disturbance in these few case studies could not be attributed definitively to these individuals’ NPC1 heterozygosity, as humans are inherently heterogeneous with respect to their genetic background and environments. By studying an Npc1 mouse model under controlled laboratory conditions, we demonstrated that partial motor dysfunction and increased anxiety-like behavior are, indeed, attributable to Npc1 haploinsufficiency in young mice up to 9 weeks of age. Therefore, subclinical NPC1 carriers may represent a previously unrecognized disease burden, deserving more consideration in research and medical practice.
Cerebellar ataxia is a prominent feature of NP-C1. As previously reported [27], our Npc1 –/– mice demonstrated severely impaired motor coordination and balance, which deteriorates with age, as determined on an accelerating Rotarod and in an open-field test, to a degree of severity that precluded DigiGait analysis. By comparison, Npc1 +/– mice showed comparable motor coordination and balance to that of Npc1 +/+ mice on the Rotarod. However, while Npc1 +/+ mice improved motor coordination and balance with maturity (to 9 weeks), Npc1 +/– mice failed to mature in these domains. Therefore, Npc1 +/– mice have impaired motor development.
Evaluation of voluntary movements using an open-field test showed that Npc1 +/– mice have a partial loss of both voluntary horizontal and vertical movements. In the horizontal movement, Npc1 +/– mice showed an intermediate decrease in average velocity compared with Npc1 +/+. Despite their lower average velocity, the ambulatory count and ambulatory distance travelled by the Npc1 +/– mice in the total arena was not different to Npc1 +/+ mice. Taken together, these results suggest that the voluntary horizontal movement of Npc1 +/– mice is slower and continuous, whereas Npc1 +/+ mice move quickly and intermittently. The Npc1 +/– mice also exhibited an intermediate phenotype in their voluntary vertical movement. The IRR indicated that Npc1 +/– mice are less likely to jump or make vertical rearing movements than Npc1 +/+ mice, but are not as severely disabled as Npc1 –/– mice. Loss of vertical movement can indicate postural and gait abnormalities [33]. These differences were not immediately discernible in the Npc1 +/– mice by visual inspection, but were detected using a sensitive computer-assisted locomotor array in the open-field test. Therefore, NPC1 carriers, who are generally regarded as clinically unaffected, may be predisposed to subtle locomotor impairments that may become apparent as neurodevelopmental delay, and as yet have not been assessed in clinical research studies. They may also present in later life with neurodegeneration, such as those exhibiting parkinsonism in 2 case studies [20, 21].
Forced locomotor tests such as DigiGait are used to highlight gait abnormalities not detectable in a voluntary locomotor test. DigiGait analysis revealed that Npc1 +/– mice have altered strides compared with Npc1 +/+ mice, demonstrating an increase in stride length and duration concomitant with decreased stride frequency. This is a surprising result, as animals with cerebellar ataxia usually present with decreased stride length and duration accompanied by an increase in stride frequency [31, 34, 35]. Given that Npc1 +/– mice presented with an intermediate voluntary movement phenotype, we expected these mice to also have moderate gait abnormalities. A possible explanation may be that Npc1 +/– mice failed to adjust their gait to the forced locomotion imposed by the treadmill, whereas Npc1 +/+ mice adapted their walking cadence to the treadmill speed by shortening their stride length with increased stride frequency. This is comparable to the slower and continuous movement of the Npc1 +/– mice observed in the open-field test. An alternative explanation based on affective state is also discussed below. Additionally, the forelimbs of Npc1 +/– mice have a decreased ataxia coefficient. This result suggested that forelimb control by Npc1 +/– mice becomes ataxic with age after a period of compensation for the underlying gait abnormality. It would be of interest to investigate locomotor deficiency in NPC1 carriers using a sensitive locomotor test such as the GAITRite electronic walkway system [36].
Psychiatric illness is a common clinical presentation often seen in patients with NP-C1 who are > 6 years of age [1, 2, 37, 38]. While our phenotypic assessment focused on motor performance, an anxiety-like phenotype was apparent in Npc1 +/– mice. As Npc1 +/– mice have comparable ambulatory counts in the total arena of an open-field test to that of Npc1 +/+ mice, their decreased center tendency is consistent with increased anxiety. Anxiety-like behavior in Npc1 +/– mice is also suggested by their longer stride length measured on the DigiGait, a system where an anxious mouse increases its stride length under exposed and anxiogenic conditions, such as that presented by the transparent DigiGait enclosure [32]. The anxiety-like phenotype of Npc1 +/– mice thus warrants assessment in additional paradigms, such as elevated plus maze, light–dark exploration, and fear conditioning, to further characterize the nature of their anxiety-like behavior. Nevertheless, our findings suggest that NPC1 carriers may be more prone to anxiety, which would be consistent with the report of an apparent enrichment of NPC1 carriers in adult patients with neurological and psychiatric symptoms [23].
The sexual dimorphism we observed in Npc1 +/– mice recapitulates previous findings in Npc1 –/– mice and patients with NP-C1 [27, 37]. Overall, motor dysfunction and anxiety-like behavior were less severe in female Npc1 +/– mice. Cholesterol is the precursor of steroid hormones, including sex hormones. Therefore, sexual dimorphism in Npc1 +/– mice may be the result of partial loss of cholesterol regulation and metabolism affecting sex hormone production. In particular, decreased testosterone production has been proposed to underlie the greater loss in motor function in common between male Npc1 +/– and Npc1 –/– mice, as well as patients with NP-C1 [27, 37, 39].
We conclude that Npc1 haploinsufficiency causes neurodevelopmental delay with motor and anxiety phenotypes manifest in early-maturity in mice. This raises the suspicion that NPC1 carriers, who are generally regarded as clinically unaffected, may be predisposed to subtle locomotor impairments that may become apparent as neurodevelopmental delay, and as yet have not been assessed in clinical research studies. They may also present in later life with neurodegeneration, such as those exhibiting parkinsonism in 2 case studies [20, 21]. It is noteworthy that haploinsufficiency of GBA1, the gene implicated in another lysosomal storage disorder, Gaucher disease, is a major genetic risk factor for Parkinson’s disease [40–42]. Similarly, NPC1 haploinsufficiency may be a genetic risk factor for Alzheimer’s disease, where NPC1 polymorphisms are associated with risk [43], and Npc1 haploinsufficiency accelerated formation of neuropathology in an Alzheimer’s disease mouse model [44].
Conclusions
Our study has provided proof of principle that NPC1 haploinsufficiency may cause an attenuated heterozygous disease phenotype. Therefore, NPC1 haploinsufficiency has the potential to add to the disease burden of about 0.66 % of the population who are pathogenic NPC1 allele carriers. Further studies will determine whether carriers could benefit from increased medical vigilance and secondary prevention with a drug treatment such as miglustat. Npc1 +/– mice may be useful for testing such potential therapeutics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1225 kb)
Rotarod analysis of Npc1 +/+ mice, measured at 5, 7, and 9 weeks of age (PDF 44 kb)
DigiGait indices of Npc1 +/+ mice, measured at 5, 7, and 9 weeks of age (PDF 53 kb)
Bodyweight of Npc +/+ and Npc1 –/– mice from 3 to 10 weeks of age (PDF 54 kb)
Distance travelled on an accelerating Rotarod by Npc1 +/+, Npc1 +/–, and Npc1 –/– mice, measured at 5, 7, and 9 weeks of age (PDF 73 kb)
Spontaneous horizontal locomotor activity of Npc1 +/+, Npc1 +/–, and Npc1 –/– mice in open-field test performed at 5, 7, and 9 weeks of age (PDF 83 kb)
Spontaneous vertical locomotor activity of Npc1 +/+, Npc1 +/–, and Npc1 –/– mice in open-field test performed at 5, 7, and 9 weeks of age (PDF 69 kb)
Npc1 +/– mice may have increased anxiety compared with Npc1 +/+ mice (PDF 74 kb)
Stride length of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 49 kb)
Stride duration of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 49 kb)
Stride frequency of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 50 kb)
Ataxia coefficient of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 49 kb)
Acknowledgments
We thank Dr Steven Walkley for kindly providing us with the breeding pairs of heterozygous BALB/cJ Npc1 nih mice. This work was supported, in part, by a research grant from the University of Pennsylvania Orphan Disease Center, and by grants from the Australian Niemann–Pick Type C Disease Foundation to Y.H.H., A.I.B., and M.W.; the Addi and Cassi Fund to Y.H.H. and A.I.B.; the National Health and Medical Research Council of Australia (AF79) to A.I.B.; and Cooperative Research Centre for Mental Health to A.I.B. The Florey Institute of Neuroscience and Mental Health acknowledges the strong support of the Victorian Government and, in particular, the funding from the Operational Infrastructure Support Grant.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Disclosures
A.I.B. is a shareholder in Prana Biotechnology Ltd, Cogstate Ltd, NextVet Ltd, and Mesoblast Ltd, and a paid consultant for Collaborative Medicinal Development Pty Ltd.
References
- 1.Mengel E, Klunemann HH, Lourenco CM, et al. Niemann–Pick disease type C symptomatology: an expert-based clinical description. Orphanet J Rare Dis 2013;8:166. [DOI] [PMC free article] [PubMed]
- 2.Patterson MC, Hendriksz CJ, Walterfang M, et al. Recommendations for the diagnosis and management of Niemann–Pick disease type C: an update. Mol Genet Metab 2012;106:330-344. [DOI] [PubMed]
- 3.Patterson MC, Mengel E, Wijburg FA, et al. Disease and patient characteristics in NP-C patients: findings from an international disease registry. Orphanet J Rare Dis 2013;8:12. [DOI] [PMC free article] [PubMed]
- 4.Love S, Bridges LR, Case CP. Neurofibrillary tangles in Niemann–Pick disease type C. Brain 1995;118:119-129. [DOI] [PubMed]
- 5.Ong WY, Kumar U, Switzer RC, et al. Neurodegeneration in Niemann–Pick type C disease mice. Exp Brain Res 2001;141:218-231. [DOI] [PubMed]
- 6.Yamada A, Saji M, Ukita Y, et al. Progressive neuronal loss in the ventral posterior lateral and medial nuclei of thalamus in Niemann–Pick disease type C mouse brain. Brain Dev 2001;23:288-297. [DOI] [PubMed]
- 7.Yu T, Lieberman AP. Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin. PLoS Genet 2013;9:e1003462. [DOI] [PMC free article] [PubMed]
- 8.Vanier MT. Niemann–Pick disease type C. Orphanet J Rare Dis 2010;5:16. [DOI] [PMC free article] [PubMed]
- 9.Patterson MC, Mengel E, Vanier MT, et al. Stable or improved neurological manifestations during miglustat therapy in patients from the international disease registry for Niemann–Pick disease type C: an observational cohort study. Orphanet J Rare Dis 2015;10:65. [DOI] [PMC free article] [PubMed]
- 10.Wassif CA, Cross JL, Iben J, et al. High incidence of unrecognized visceral/neurological late-onset Niemann–Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet Med 2016;18:41-48. [DOI] [PMC free article] [PubMed]
- 11.Kruth HS, Comly ME, Butler JD, et al. Type C Niemann–Pick disease. Abnormal metabolism of low density lipoprotein in homozygous and heterozygous fibroblasts. J Biol Chem 1986;261:16769-16774. [PubMed]
- 12.Brown DE, Thrall MA, Walkley SU, et al. Metabolic abnormalities in feline Niemann–Pick type C heterozygotes. J Inherit Metab Dis 1996;19:319-330. [DOI] [PubMed]
- 13.Choi HY, Karten B, Chan T, et al. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann–Pick type C disease. J Biol Chem 2003;278:32569-32577. [DOI] [PubMed]
- 14.Porter FD, Scherrer DE, Lanier MH, et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann–Pick C1 disease. Sci Transl Med 2010;2:56ra81. [DOI] [PMC free article] [PubMed]
- 15.Garver WS, Jelinek D, Oyarzo JN, et al. Characterization of liver disease and lipid metabolism in the Niemann–Pick C1 mouse. J Cell Biochem 2007;101:498-516. [DOI] [PubMed]
- 16.Jelinek D, Castillo JJ, Garver WS. The C57BL/6J Niemann-Pick C1 mouse model with decreased gene dosage has impaired glucose tolerance independent of body weight. Gene 2013;527:65-70. [DOI] [PMC free article] [PubMed]
- 17.Jelinek D, Millward V, Birdi A, Trouard TP, Heidenreich RA, Garver WS. Npc1 haploinsufficiency promotes weight gain and metabolic features associated with insulin resistance. Hum Mol Genet 2011;20:312-321. [DOI] [PMC free article] [PubMed]
- 18.Meyre D, Delplanque J, Chevre JC, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 2009;41:157-159. [DOI] [PubMed]
- 19.Yu W, Ko M, Yanagisawa K, Michikawa M. Neurodegeneration in heterozygous Niemann–Pick type C1 (NPC1) mouse: implication of heterozygous NPC1 mutations being a risk for tauopathy. J Biol Chem 2005;280:27296-27302. [DOI] [PubMed]
- 20.Josephs KA, Matsumoto JY, Lindor NM. Heterozygous Niemann–Pick disease type C presenting with tremor. Neurology 2004;63:2189-2190. [DOI] [PubMed]
- 21.Kluenemann HH, Nutt JG, Davis MY, Bird TD. Parkinsonism syndrome in heterozygotes for Niemann-Pick C1. J Neurol Sci 2013;335:219-220. [DOI] [PMC free article] [PubMed]
- 22.Harzer K, Beck-Wodl S, Bauer P. Niemann-pick disease type C: new aspects in a long published family—partial manifestations in heterozygotes. JIMD Rep 2014;12:25-29. [DOI] [PMC free article] [PubMed]
- 23.Bauer P, Balding DJ, Klunemann HH, et al. Genetic screening for Niemann–Pick disease type C in adults with neurological and psychiatric symptoms: findings from the ZOOM study. Hum Mol Genet 2013;22:4349-4356. [DOI] [PMC free article] [PubMed]
- 24.Loftus SK, Morris JA, Carstea ED, et al. Murine model of Niemann–Pick C disease: mutation in a cholesterol homeostasis gene. Science 1997;277:232-235. [DOI] [PubMed]
- 25.Jelinek D, Heidenreich RA, Erickson RP, Garver WS. Decreased Npc1 gene dosage in mice is associated with weight gain. Obesity (Silver Spring) 2010;18:1457-1459. [DOI] [PMC free article] [PubMed]
- 26.Jelinek DA, Maghsoodi B, Borbon IA, Hardwick RN, Cherrington NJ, Erickson RP. Genetic variation in the mouse model of Niemann Pick C1 affects female, as well as male, adiposity, and hepatic bile transporters but has indeterminate effects on caveolae. Gene 2012;491:128-134. [DOI] [PMC free article] [PubMed]
- 27.Voikar V, Rauvala H, Ikonen E. Cognitive deficit and development of motor impairment in a mouse model of Niemann–Pick type C disease. Behav Brain Res 2002;132:1-10. [DOI] [PubMed]
- 28.Belzung C. Measuring rodent exploratory behavior. In: Crusio WE, Gerlai RT (eds) Handbook of molecular-genetic techniques for brain and behavior research. 1st ed. Techniques in the behavioral and neural sciences, Vol. 13. Amsterdam. New York: Elsevier; 1999. p. xxvii, 965 p.
- 29.Hall C, Ballachey EL. A study of the rat's behavior in a field. A contribution to method in comparative psychology. Univ Calif Publ Psychol 1932;6:1-12.
- 30.Sashindranath M, Daglas M, Medcalf RL. Evaluation of gait impairment in mice subjected to craniotomy and traumatic brain injury. Behav Brain Res 2015;286:33-38. [DOI] [PubMed]
- 31.Pallier PN, Drew CJ, Morton AJ. The detection and measurement of locomotor deficits in a transgenic mouse model of Huntington's disease are task- and protocol-dependent: influence of non-motor factors on locomotor function. Brain Res Bull 2009;78:347-355. [DOI] [PubMed]
- 32.Lepicard EM, Venault P, Abourachid A, Pelle E, Chapouthier G, Gasc JP. Spatio-temporal analysis of locomotion in BALB/cByJ and C57BL/6J mice in different environmental conditions. Behav Brain Res 2006;167:365-372. [DOI] [PubMed]
- 33.Crawley JN. What's Wrong With My Mouse?: Behavioral phenotyping of transgenic and knockout mice, 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2007.
- 34.Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J Neuroeng Rehab 2005;2:20. [DOI] [PMC free article] [PubMed]
- 35.Hurlock EC, Bose M, Pierce G, Joho RH. Rescue of motor coordination by Purkinje cell-targeted restoration of Kv3.3 channels in Kcnc3-null mice requires Kcnc1. J Neurosci 2009;29:15735-15744. [DOI] [PMC free article] [PubMed]
- 36.Bridenbaugh SA, Kressig RW. Laboratory review: the role of gait analysis in seniors' mobility and fall prevention. Gerontology 2011;57:256-264. [DOI] [PubMed]
- 37.Walterfang M, Fietz M, Abel L, Bowman E, Mocellin R, Velakoulis D. Gender dimorphism in siblings with schizophrenia-like psychosis due to Niemann–Pick disease type C. J Inherit Metab Dis 2009;32(Suppl. 1):S221-S226. [DOI] [PubMed]
- 38.Walterfang M, Fietz M, Fahey M, et al. The neuropsychiatry of Niemann–Pick type C disease in adulthood. J Neuropsychiatry Clin Neurosci 2006;18:158-170. [DOI] [PubMed]
- 39.Roff CF, Strauss JF, 3rd, Goldin E, et al. The murine Niemann-Pick type C lesion affects testosterone production. Endocrinology 1993;133:2913-2923. [DOI] [PubMed]
- 40.Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. J Med Genet 2004;41:937-940. [DOI] [PMC free article] [PubMed]
- 41.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med 2009;361:1651-1661. [DOI] [PMC free article] [PubMed]
- 42.Tayebi N, Callahan M, Madike V, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab 2001;73:313-321. [DOI] [PubMed]
- 43.Erickson RP, Larson-Thome K, Weberg L, et al. Variation in NPC1, the gene encoding Niemann–Pick C1, a protein involved in intracellular cholesterol transport, is associated with Alzheimer disease and/or aging in the Polish population. Neurosci Lett 2008;447:153-157. [DOI] [PMC free article] [PubMed]
- 44.Borbon IA, Erickson RP. Interactions of Npc1 and amyloid accumulation/deposition in the APP/PS1 mouse model of Alzheimer's. J Appl Genet 2011;52:213-218. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)
Rotarod analysis of Npc1 +/+ mice, measured at 5, 7, and 9 weeks of age (PDF 44 kb)
DigiGait indices of Npc1 +/+ mice, measured at 5, 7, and 9 weeks of age (PDF 53 kb)
Bodyweight of Npc +/+ and Npc1 –/– mice from 3 to 10 weeks of age (PDF 54 kb)
Distance travelled on an accelerating Rotarod by Npc1 +/+, Npc1 +/–, and Npc1 –/– mice, measured at 5, 7, and 9 weeks of age (PDF 73 kb)
Spontaneous horizontal locomotor activity of Npc1 +/+, Npc1 +/–, and Npc1 –/– mice in open-field test performed at 5, 7, and 9 weeks of age (PDF 83 kb)
Spontaneous vertical locomotor activity of Npc1 +/+, Npc1 +/–, and Npc1 –/– mice in open-field test performed at 5, 7, and 9 weeks of age (PDF 69 kb)
Npc1 +/– mice may have increased anxiety compared with Npc1 +/+ mice (PDF 74 kb)
Stride length of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 49 kb)
Stride duration of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 49 kb)
Stride frequency of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 50 kb)
Ataxia coefficient of Npc1 +/+ and Npc1 +/– mice, measured using DigiGait at 5, 7, and 9 weeks of age (PDF 49 kb)