Abstract
The worldwide prevalence of metabolic diseases is increasing, and there are global recommendations to limit consumption of certain nutrients, especially saturated lipids. Insulin resistance, a common trait occurring in obesity and type 2 diabetes, is associated with intestinal lipoprotein overproduction. However, the mechanisms by which the intestine develops insulin resistance in response to lipid overload remain unknown. Here, we show that insulin inhibits triglyceride secretion and intestinal microsomal triglyceride transfer protein expression in vivo in healthy mice force-fed monounsaturated fatty acid-rich olive oil but not in mice force-fed saturated fatty acid-rich palm oil. Moreover, when mouse intestine and human Caco-2/TC7 enterocytes were treated with the saturated fatty acid, palmitic acid, the insulin-signaling pathway was impaired. We show that palmitic acid or palm oil increases ceramide production in intestinal cells and that treatment with a ceramide analogue partially reproduces the effects of palmitic acid on insulin signaling. In Caco-2/TC7 enterocytes, ceramide effects on insulin-dependent AKT phosphorylation are mediated by protein kinase C but not by protein phosphatase 2A. Finally, inhibiting de novo ceramide synthesis improves the response of palmitic acid-treated Caco-2/TC7 enterocytes to insulin. These results demonstrate that a palmitic acid-ceramide pathway accounts for impaired intestinal insulin sensitivity, which occurs within several hours following initial lipid exposure.
Keywords: Akt PKB, ceramide, fatty acid, insulin, intestine, lipid, signaling, palmitic acid
Introduction
The worldwide obesity epidemic has stimulated numerous research efforts to identify factors that affect energy balance. As compared with the liver, pancreas, muscle, or adipose tissues, the intestine has received little attention with regard to its potential role in the onset of metabolic disorders. Nevertheless, the intestine could also contribute to the development of metabolic disease, especially through its role in postprandial lipemia.
The increased amplitude and duration of the postprandial peak of circulating triglyceride-rich lipoproteins (TRL)5 are known risk factors for atherosclerosis and cardiovascular diseases (1, 2). Postprandial hypertriglyceridemia can be due to impaired TRL catabolism, and/or lipoprotein remnant uptake, and may also result from intestinal TRL overproduction (3). Thus, investigating intestinal lipoprotein secretion might help to understand aberrant postprandial lipemia (4).
The intestine ensures the transport of alimentary fat, which is the most calorie-dense nutrient. Enterocytes produce intestinal TRLs (chylomicrons) along a multistep pathway, which includes long chain fatty acid uptake, triglyceride synthesis in the endoplasmic reticulum, and assembly with apolipoproteins (apo) such as apoB48. Chylomicrons are then secreted into the lymph and ultimately into the blood. Overproduction of intestinal TRL may be an important contributor of both fasting and postprandial dyslipidemia (3, 5). Microsomal triglyceride transfer protein (MTP) transports neutral lipids (6) and plays a central role in the efficiency of lipid absorption by modulating chylomicron size. Alterations in MTP protein expression can impact intestinal fat transport and lipoprotein metabolism (7). However, in metabolic syndrome, which encompasses dyslipidemia, hypertension, and insulin resistance, the mechanisms underlying perturbed chylomicron assembly and secretion remain poorly explored.
Insulin also plays a central role in regulating energy metabolism. In the liver, the other TRL-secreting organ, insulin inhibits the production of very low density lipoproteins via the inhibition of MTP expression and apoB secretion (8–10). In the human intestine, insulin inhibits the production of apoB48-containing lipoproteins by both direct and indirect mechanisms (11). In healthy chow-fed hamsters, a decrease in circulating levels of triglyceride-rich apoB48-containing lipoproteins was observed 60 min after insulin administration (12). The mechanisms involved in the inhibitory effect of insulin on chylomicron production remain unclear.
In hamsters, intestinal insulin resistance can be induced by a high fructose diet (12, 13), a high fat diet (15), or by TNF-α infusion (14). In these conditions, an overproduction of intestinal lipoproteins and/or an induction of MTP expression are observed. Moreover, several groups have reported that the intestine plays a role in the onset of insulin resistance in humans. Indeed, hyperinsulinemic insulin-resistant human subjects display increased production rates of intestinal apoB48-containing lipoproteins (16), and in individuals with type 2 diabetes, intestinal chylomicron production is resistant to insulin's acute suppressive effects (17).
Dyslipidemia is often reported during the progression from obesity to type 2 diabetes, thus exposing tissues to a variety of elevated lipids. Among these lipid species, ceramides have recently gained attention as important in the development of insulin resistance and impaired glycemic control (18). With excess saturated fat intake, ceramides accumulate in insulin-sensitive tissues, either as a consequence of de novo synthesis or through mobilization from complex sphingolipids (18). Insulin-resistant rodents and humans often display elevated ceramide concentrations in the liver, muscle, or serum, as compared with lean or untreated control subjects (19). At the cellular level, ceramide accumulation impairs insulin signaling and intracellular handling of glucose and lipids, resulting in deleterious effects on cell metabolism in liver, muscle, and adipose tissue (20, 21). However, in these studies the intestine was not investigated.
The cellular mechanisms that link lipid overload, the intestine, and insulin resistance remain to be clarified. Moreover, most of the above reported results were obtained under established states of insulin resistance or after long term high fat or fructose diets. Considering the rapid renewal of intestinal epithelium, local insulin resistance may occur rather quickly. In this work, we aimed to determine whether an acute supply of the saturated fatty acid palmitic acid would be sufficient to interfere with insulin action in intestinal cells and, if so, by which mechanisms.
Results
In Mouse Intestine, a Single Oral Gavage with Palm Oil Impairs Insulin Effects on Lipid Absorption
Following a single administration of those oils that are enriched in monounsaturated fatty acids (olive oil) or saturated fatty acids (palm oil) (Table 1), the effects of insulin on lipid absorption were analyzed in mice. In the absence of insulin treatment and 1 h after an olive or palm oil bolus, the postprandial plasma triglyceride content was increased as compared with control (water), as expected (Fig. 1A). Insulin injection reduced the plasma triglyceride level rise in mice that received an olive oil bolus, although no effect was observed in mice that received a palm oil bolus (Fig. 1A). Modifications of MTP activity (Fig. 1B) and expression (Fig. 1C) were analyzed in intestinal cells of mice force-fed palm oil, olive oil, or water and treated with or without insulin. Gavage with olive or palm oil both increased MTP activity when compared with water. However, insulin exerted an inhibitory effect on MTP activity in mice force-fed water or olive oil but not in mice force-fed palm oil (Fig. 1B). Concerning changes of Mttp gene expression in intestinal epithelial cells, in the absence of insulin treatment, MTP mRNA levels were similar in mice that received either water or palm oil boluses (Fig. 1C), although they increased after an olive oil bolus. Insulin treatment reduced the rise of MTP mRNA in intestinal epithelial cells of mice force-fed olive oil (Fig. 1C) but led to increased MTP expression in mice force-fed palm oil (Fig. 1C). Differences between MTP mRNA levels and MTP activity following treatment may be explained by the complexity of the mechanisms responsible for MTP regulation, which has been shown to occur at transcriptional, translational, and/or post-translational levels (6).
TABLE 1.
Fatty acid composition of olive and palm oils, according to the manufacturer
| Component | Olive oil (100 g) | Palm oil (100 g) |
|---|---|---|
| Palmitic acid | 11.3 | 43.5 |
| Oleic acid | 71.3 | 36.6 |
| Linoleic acid | 12.5 | 9.1 |
| Others | 4.9 | 10.8 |
| Total saturated fatty acids | 13.8 | 49.3 |
| Total monounsaturated fatty acids | 72.9 | 37 |
| Total polyunsaturated fatty acids | 10.5 | 9.3 |
FIGURE 1.
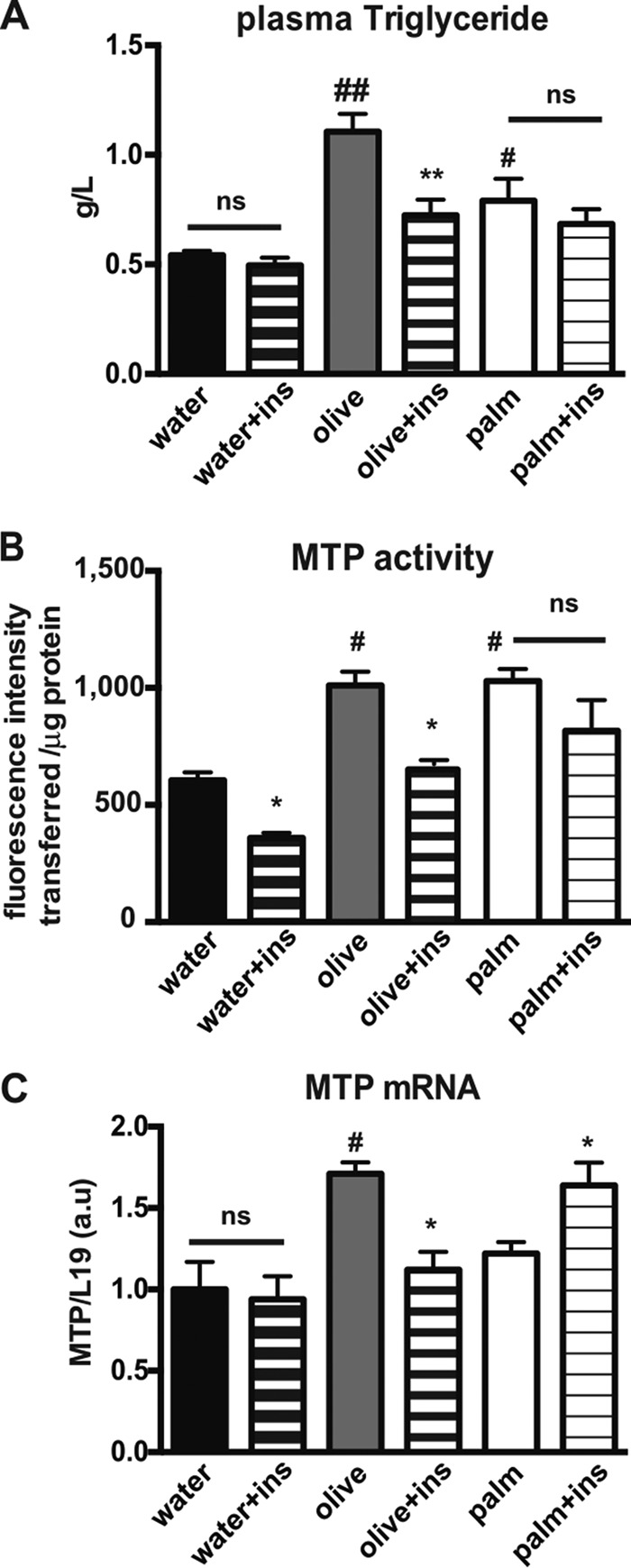
Effects of palm and olive oils on insulin-dependent intestinal lipid absorption in mice. A, fasted mice were force-fed water, olive oil, or palm oil and either subjected or not to an intraperitoneal injection of insulin (ins) 30 min later. Blood was collected 1 h after the bolus. Plasma triglyceride levels were quantified. #, p < 0.05, and ##, p < 0.01, as compared with water; **, p < 0.01 as compared with the same condition without insulin, ns, not significant, mean ± S.E., n = 8. B, fasted mice were force-fed either water, olive oil, or palm oil and were either subjected or not to an intraperitoneal insulin (ins) injection 4 h later. Mice were euthanized 30 min after insulin injection. MTP activity was assayed in cellular homogenates of intestinal epithelial cells. Results display mean ± S.E., n = 4, for each condition. #, p < 0.05 as compared with water; *, p < 0.05, as compared with the same condition without insulin; ns, not significant. C, fasted mice were force-fed water, olive oil, or palm oil and were either subjected or not to an intraperitoneal insulin (ins) injection at the same time as lipid bolus. Four hours later, mice were euthanized, and the jejunum was collected. MTP mRNA levels were quantified by RT-PCR in mouse intestinal epithelial cells, and by using L19 mRNA as a reference. Results display mean ± S.E., n = 4, for each condition. #, p < 0.05, as compared with water; *, p < 0.05, as compared with the same condition without insulin; ns, not significant.
These results indicate that palm oil prevents insulin exerting its inhibitory action on triglyceride secretion, Mttp gene expression, and MTP activity, suggesting that palm oil may interfere with the insulin-signaling pathway.
Palmitic Acid Impairs Insulin Signaling in Mouse Intestinal Epithelial Cells and in Human Enterocytes
To further understand the mechanisms by which saturated fatty acids alter intestinal insulin actions, we analyzed the effects of olive and palm oils on insulin signaling in mouse intestinal epithelial cells (Fig. 2). We observed that the increased AKT serine 473 phosphorylation in mouse intestinal epithelial cells, after insulin injection, was significantly impaired in mice that were force-fed palm oil but not olive oil. This suggests that a single bolus of palm oil is sufficient to alter insulin signaling in vivo.
FIGURE 2.
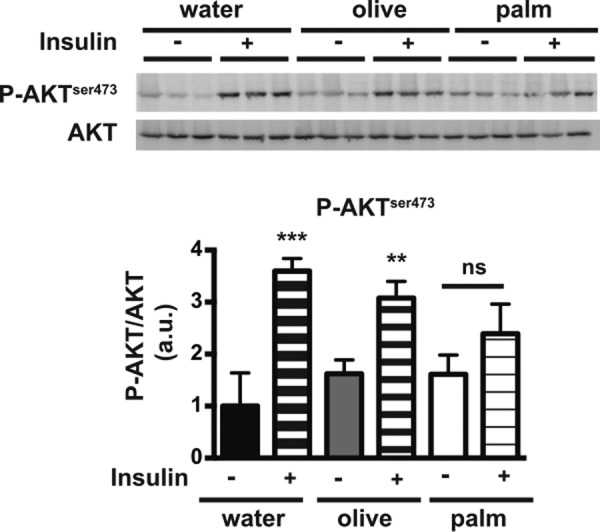
Effects of palm and olive oils on insulin signaling in the mouse intestine. Fasted mice were force-fed water, olive oil, or palm oil, and 4 h later were either subjected or not to an intraperitoneal insulin injection. Mice were euthanized 30 min after insulin injection, and cell lysates from intestinal epithelial cells were analyzed by Western blotting for AKT phosphorylation (P-AKT) at serine 473 (top panel). AKT phosphorylation was reported as the ratio of P-AKT to total AKT content (arbitrary units, a.u.) (bottom panel). The P-AKT/AKT ratio obtained in the water condition without insulin was set at 1. Results display mean ± S.E., n = 4, for each condition. **, p < 0.01, and ***, p < 0.001, compared with the same treatment without insulin; ns, not significant.
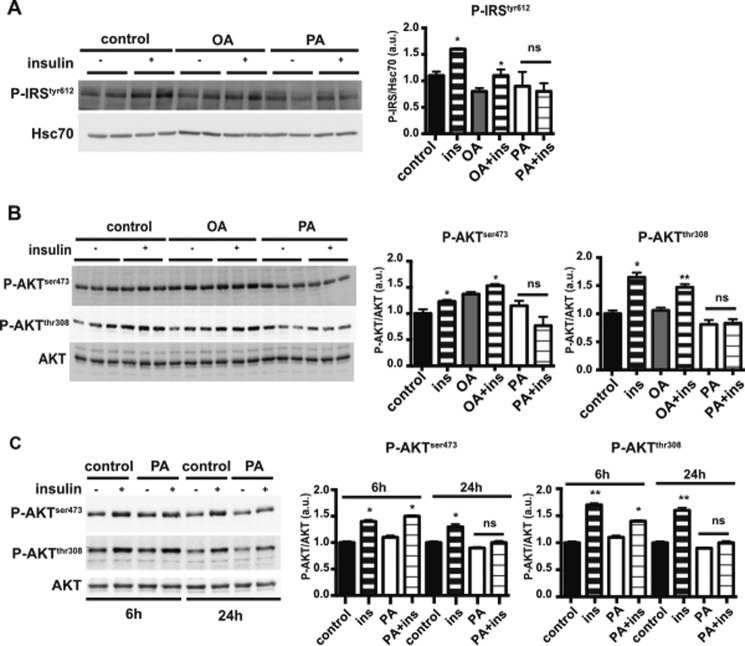
Because palmitic acid is the major fatty acid component of palm oil (Table 1), we tested the specific effect of this saturated fatty acid on insulin signaling in intestinal cells as compared with oleic acid (the main monounsaturated fatty acid in olive oil). For this experiment, we used cultured human Caco-2/TC7 enterocytes incubated for 24 h with lipid micelles containing either palmitic (PA) or oleic acid (OA) before an insulin challenge. As shown in Fig. 3, in control and oleic acid-treated cells, insulin treatment induced a significant increase in insulin receptor substrate (IRS) phosphorylation at tyrosine 612 (Fig. 3A) and in AKT phosphorylation at both serine 473 and threonine 308 (Fig. 3B). In contrast, no increase in IRS or AKT phosphorylation occurred in Caco-2/TC7 cells treated with palmitic acid-containing lipid micelles, indicating that this saturated fatty acid prevents insulin signaling in enterocytes. This inhibitory effect of PA-containing lipid micelles in Caco-2/TC7 cells was observed after 24 h of treatment but not after 6 h of treatment (Fig. 3C). This suggests that metabolic processing of palmitic acid is required for its effect on insulin signaling.
FIGURE 3.
Effects of palmitic or oleic acid on AKT phosphorylation in cultured human Caco-2/TC7 enterocytes. A, Caco-2/TC7 cells were incubated with oleic acid- or palmitic acid-containing lipid micelles (OA and PA, respectively) for 24 h. As indicated, insulin (ins) was added to the culture 10 min prior to harvesting. Cell lysates were used for Western blotting analysis of IRS phosphorylation at tyrosine 612, with Hsc70 as loading control (left panel). Ratios of phosphorylated IRS to Hsc70 protein, expressed as arbitrary units (a.u.), are reported in the right panel. Results display mean ± S.E., n = 4. *, p < 0.05, as compared with the same condition without insulin; ns, not significant. B, Caco-2/TC7 cells were cultured in the presence or absence of OA- or PA-containing lipid micelles for 24 h. Cells were incubated with or without insulin (ins) for 10 min prior to harvest. AKT phosphorylation at serine 473 (P-AKTSer-473) or threonine 308 (P-AKTThr-308) was analyzed by Western blotting (left panel) using specific antibodies against the phosphorylated residues. Total AKT was used as control, and the ratios of the respective P-AKT to total AKT content are reported (right panels). The P-AKT/AKT ratio obtained under control conditions (without lipid micelles and insulin) was set at 1. *, p < 0.05; **, p < 0.01, as compared with the same conditions without insulin. ns, not significant, mean ± S.E., n = 4. C, Caco-2/TC7 cells were incubated in the presence or absence of palmitic acid-containing lipid micelles (PA) for 6 or 24 h and treated or not with insulin for 10 min prior to harvest. AKT phosphorylation in cell lysates was analyzed by Western blotting (left panel). Quantification of AKT phosphorylation was expressed as the ratio of P-AKT to total AKT (right panels) in arbitrary units (a.u.). Results display mean ± S.E., n = 4. *, p < 0.05, and **, p < 0.01, as compared with the same conditions without insulin. ns, not significant.
Palmitic Acid Increases Ceramide Production in Mouse Intestinal Cells
Palmitic acid has been described as a precursor of de novo ceramide synthesis, which contributes to insulin resistance (20, 27). We thus analyzed whether this saturated fatty acid increases ceramide production in intestinal cells.
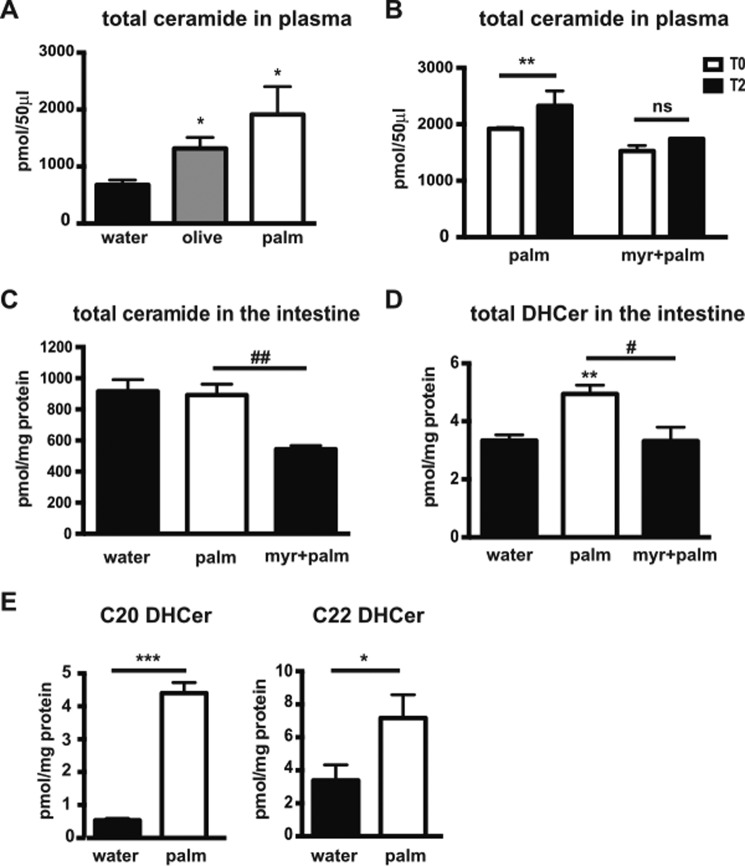
We first compared the amount of ceramide in mouse plasma 4 h after gavage with water, olive oil, or palm oil. An increase in plasma ceramide was observed following palm oil bolus and to a lesser extent after olive oil bolus (Fig. 4A). The effect of palm oil on ceramide production was further analyzed in mouse plasma and intestinal samples. Mice were treated or not with myriocin, an inhibitor of serine palmitoyltransferase (SPT) that is the first enzyme in de novo ceramide synthesis (28). Mice were then force-fed palm oil. Both treatments were administered daily for 4 consecutive days. Plasma ceramide levels were quantified in all mice before and 2 h after the last palm oil bolus. As shown in Fig. 4B, an increase (+21%) of total ceramide content was observed in mouse plasma 2 h after the palm oil bolus. This increase in plasma ceramide level was not observed in animals treated with myriocin, suggesting the occurrence of de novo ceramide synthesis in the small intestine during the 2-h period immediately following the palm oil bolus. The total ceramide content was also measured in the intestine of mice treated with palm oil in the presence or absence of myriocin (Fig. 4C). In epithelial intestinal cells, total ceramide remained unchanged after 2 h of palm oil treatment; however, an inhibitory myriocin effect was observed. According to the increase in total plasma ceramide after a palm oil bolus (Fig. 4B), we hypothesized that ceramides were rapidly secreted after synthesis. Thus, we assayed the intestinal content of dihydroceramides (DHCer), ceramide precursors that are specifically produced via de novo synthesis (20). We observed a +48% increase in total DHCer levels 2 h after palm oil bolus, which was prevented with myriocin treatment (Fig. 4D). DHCer species analysis revealed that palm oil mainly increased C20 and C22 DHCer in this tissue (8.3- and 2.1-fold, respectively) (Fig. 4E).
FIGURE 4.
Effects of palm oil on ceramide production in mouse intestinal epithelial cells. A, total ceramide was quantified in mouse plasma 4 h after a water, olive oil, or palm oil bolus. Results (mean ± S.E.) are expressed as pmol/50 μl plasma. *, p < 0.05 versus water, n = 4, for each condition. B–E, mice received or not myriocin (myr) by gavage and a bolus of water or palm oil 30 min later. These treatments were repeated for four consecutive days (n = 4, for each condition). On the 4th day of the experiment, ceramides and dihydroceramides were quantified in plasma and intestinal epithelial cells by mass spectrometry. B, total plasma ceramides were quantified before (T0, white boxes) and 2 h after the gavage (T2, black boxes). Results (mean ± S.E.) are expressed as pmol/50 μl plasma. **, p < 0.01, as compared with palm oil at T0. C, total ceramide content was quantified in intestinal epithelial cells 2 h after the final water or palm oil gavage, with or without myriocin treatment. Results (mean ± S.E.) are expressed as pmol/mg of protein. ##, p < 0.01, as compared with palm oil. D, total DHCers were quantified in mouse intestinal epithelial cells 2 h after the final gavage. Results (mean ± S.E.) are expressed as pmol/mg of protein. **, p < 0.01, as compared with control; #, p < 0.05, as compared with palm oil. E, quantification of C20- and C22-dihydroceramides (C20 DHCer and C22 DHCer, respectively) was carried out in mouse intestinal epithelial cells 2 h after the bolus. Results (mean ± S.E.) are expressed as pmol/mg of protein. *, p < 0.05, and ***, p < 0.001, as compared with water.
Because palm oil and olive oil contain a mixture of fatty acids (Table 1), we analyzed the specific effect of palmitic acid on ceramide production by comparing the intracellular content of ceramide in Caco-2/TC7 cells incubated with palmitic acid or oleic acid (Fig. 5). As compared with control cells, the intracellular content of C14-, C18-, C20-, C22-, and C24-ceramide species was increased in PA-treated cells but not in OA-treated cells (Fig. 5A). Interestingly, we observed a 46% increase in serine palmitoyltransferase 2 subunit (SPT2) expression in PA-treated cells as compared with control cells (Fig. 5B). SPT2 expression remained unchanged in OA-treated cells as compared with control cells.
FIGURE 5.
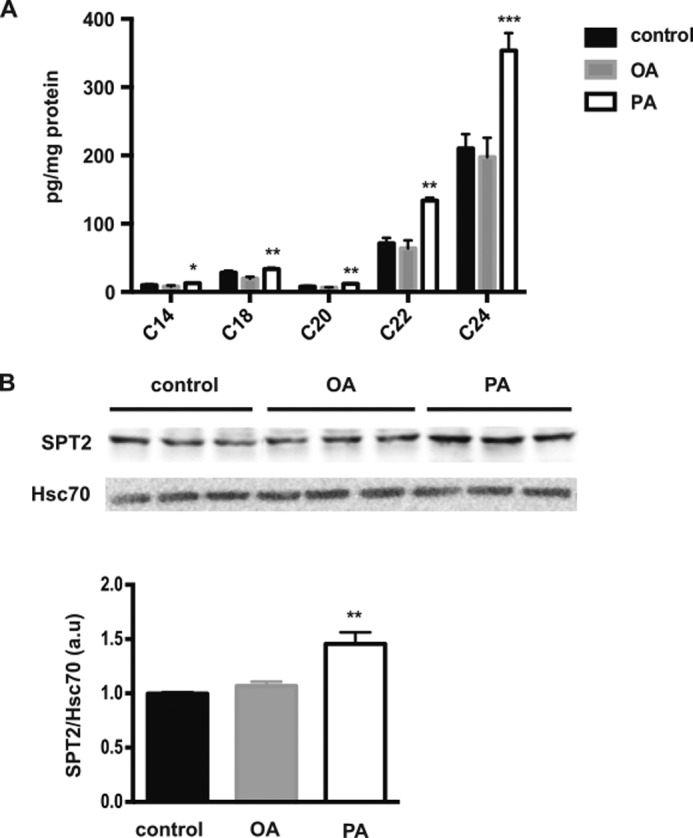
Effects of palmitic acid on ceramide production in Caco-2/TC7 enterocytes. A, cellular ceramide species content was quantified in control cells and in cells treated for 24 h with OA- or PA-containing lipid micelles. Results (mean ± S.E.) are expressed as pg/mg protein. *, p < 0.05; **, p < 0.01, and ***, p < 0.001 as compared with control cells, n = 4. B, representative Western blot of SPT2 (upper panel) in cell lysates from Caco-2/TC7 cells cultured under the same conditions as in A. Hsc70 protein was used as loading control. The lower panel represents the quantification of SPT2 protein levels expressed as the SPT2/Hsc70 ratio in arbitrary units (a.u.), the control value being set to 1. Results display mean ± S.E., n = 6; **, p < 0.01, as compared with control.
Altogether, these results demonstrate that palmitic acid induces de novo ceramide synthesis in intestinal cells. They indicate that the effects of palmitic acid on insulin signaling could occur through increased ceramide synthesis in enterocytes.
Alteration of Insulin Signaling in Intestinal Cells by Palmitic Acid Is Dependent on de Novo Ceramide Synthesis
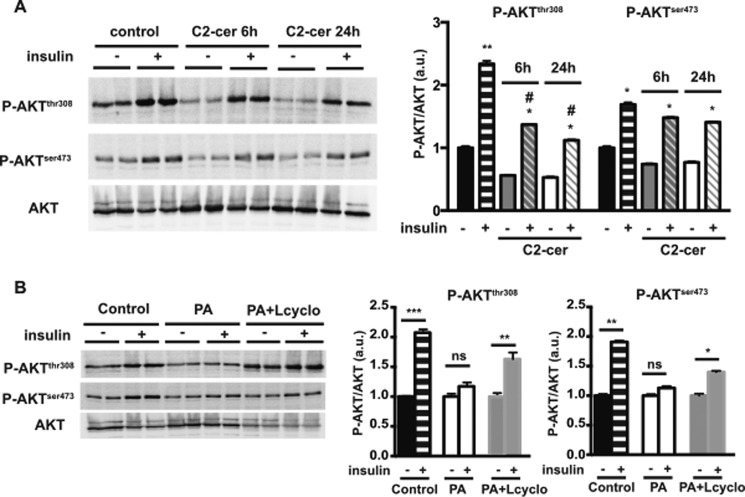
We then determined the direct impact of ceramide on insulin signaling in intestinal cells. Caco-2/TC7 cells were incubated with C2-ceramide, a short chain cell-permeable biologically active analogue of ceramide (Fig. 6A) (29, 30). Compared with control cells, the addition of C2-ceramide reduced the basal phosphorylation level of the AKT threonine 308 residue, and it significantly impaired the response to insulin, as early as 6 h after the beginning of the treatment. C2-ceramide addition also tended to decrease both basal and insulin-stimulated AKT phosphorylation at serine 473 (Fig. 6A), but it did not reach statistical significance when compared with untreated cells. These results demonstrate that ceramide addition in enterocytes partially reproduces the effects of palmitic acid on insulin signaling.
FIGURE 6.
Effects of C2-ceramide addition and de novo ceramide synthesis inhibition on insulin-dependent AKT phosphorylation. A, Caco-2/TC7 cells were incubated for 6 or 24 h with or without C2-ceramide. For some conditions, insulin was added 10 min prior to harvest. AKT phosphorylation in cell lysates was analyzed by Western blotting (left panel). Specific antibodies against phosphorylated serine 473 (P-AKTSer-473) and threonine 308 (P-AKTThr-308) AKT residues were used. Total AKT was used as control. The quantification of AKT phosphorylation is displayed in the right panel. Results are expressed as the P-AKT/total AKT ratio in arbitrary units (a.u.). Results represent mean ± S.E., n = 4. *, p < 0.05, and **, p < 0.01, as compared with the same condition without insulin. #, p < 0.05, as compared with control cells in the presence of insulin. B, Caco-2/TC7 cells were incubated 24 h with or without palmitic acid-containing lipid micelles (PA). For some conditions, cells were pre-treated with l-cycloserine for 1 h before incubation with PA-containing lipid micelles (PA+Lcyclo). When appropriate, insulin was added 10 min prior to harvest. AKT phosphorylation in cell lysates was analyzed by Western blotting (left panels). Specific antibodies against phosphorylated serine 473 (P-AKTSer-473) and threonine 308 (P-AKTThr-308) AKT residues were used. Total AKT was used as control. Quantification of AKT phosphorylation (right panels) was expressed as the P-AKT/total AKT ratio in arbitrary units (a.u.); the ratio value obtained without insulin for each condition was set at 1. *, p < 0.05; **, p < 0.01, and ***, p < 0.001, as compared with the same condition without insulin, ns, not significant, n = 4.
We then analyzed whether inhibiting de novo ceramide synthesis reversed the effect of palmitic acid on insulin signaling (Fig. 6B). As myriocin treatment is toxic to Caco-2/TC7 cells (data not shown), we used l-cycloserine, another potent inhibitor of de novo ceramide synthesis (31). l-Cycloserine treatment partially restored serine 473 and threonine 308 AKT phosphorylation upon insulin treatment of PA-treated cells (Fig. 6B).
Altogether, these results show that the effects of palmitic acid on insulin signaling are directly linked to de novo ceramide synthesis in enterocytes.
Inhibitory Effect of Ceramide on Insulin-stimulated AKT Phosphorylation Is Mediated by PKC Activity but Not by PP2A Activity
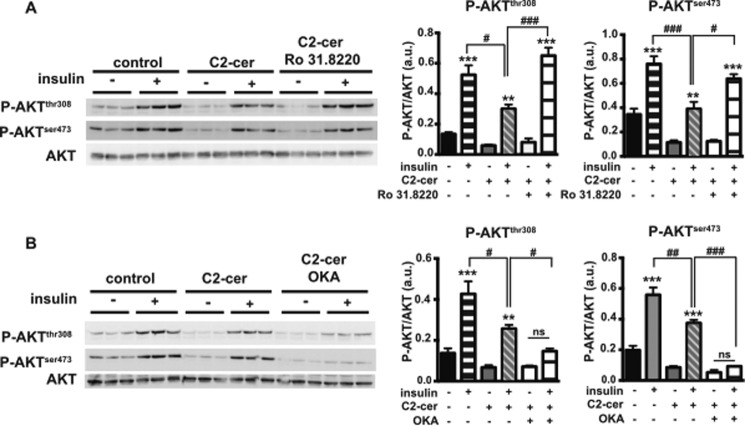
In 3T3-L1 adipocytes and L6 muscle cells, it has been reported that protein kinase C (PKC) and protein phosphatase 2A (PP2A) are involved in the inhibitory effect of ceramide on insulin signaling (31, 32). To determine whether PKC and/or PP2A pathways are also involved in enterocytes, we pre-treated Caco-2/TC7 cells with either a broad PKC inhibitor (Ro 31.8220) or a PP2A inhibitor (okadaic acid, OKA), before the addition of C2-ceramide and insulin (Fig. 7). When Caco-2/TC7 cells were incubated in the presence of ceramide and insulin, we observed that the ceramide-dependent low level of AKT phosphorylation was prevented by pre-treatment with Ro 31.8220 (Fig. 7A) but not with OKA (Fig. 7B). These results indicate that a ceramide-activated PKC pathway is likely to be involved in the inhibitory effect of ceramides on insulin signaling in Caco-2/TC7 cells.
FIGURE 7.
Effects of PKC and PP2A inhibitors on insulin-dependent AKT phosphorylation. Caco-2/TC7 cells were incubated with or without 100 μm C2-ceramide (C2-cer) for 6 h. For some conditions, insulin was added 10 min prior to harvest. When indicated, cells were treated for 24 h with 5 μm Ro 31.8220 (A) or 100 nm OKA (B). AKT phosphorylation in cell lysates was analyzed by Western blotting (left panels). Specific antibodies against phosphorylated serine 473 (P-AKTSer-473) and threonine 308 (P-AKTThr-308) AKT residues were used. Total AKT was used as control. Quantifications of AKT phosphorylation are displayed in the right panels. Results are expressed as the P-AKT/total AKT ratio in arbitrary units (a.u.). Results represent mean ± S.E., n = 3. **, p < 0.01, and ***, p < 0.001, as compared with the same condition without insulin; #, p < 0.05, ##, p < 0.01, and ###, p < 0.001, as compared with the indicated condition.
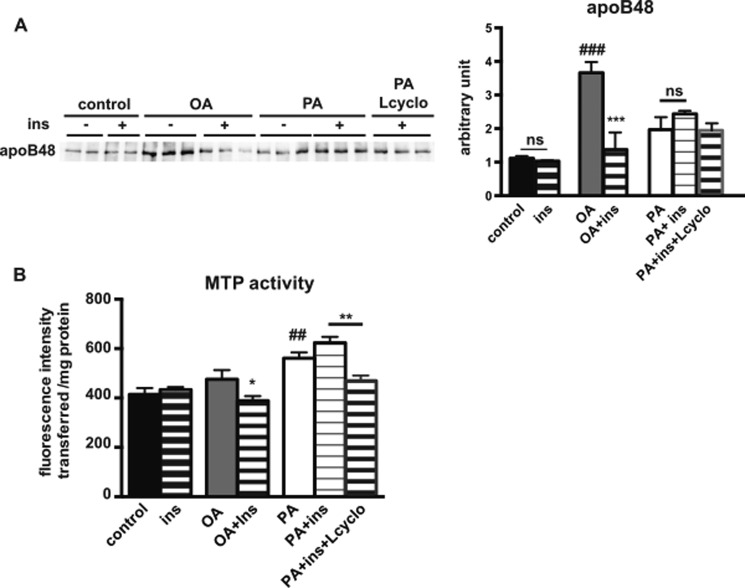
Inhibiting de Novo Ceramide Synthesis Partially Restores Insulin Effects on ApoB48 Secretion and MTP Activity in PA-treated Caco-2/TC7 Cells
We next determined the impact of palmitic acid supply and inhibiting de novo ceramide synthesis on apoB48 secretion and MTP activity in Caco-2/TC7 cells. As observed previously (33), palmitic acid-containing micelles were less potent than oleic acid-containing micelles in inducing the secretion of apoB48 (Fig. 8A). Insulin decreased apoB48 secretion in OA-treated cells but not in PA-treated cells. Upon insulin stimulation, apoB48 secretion tended to be lower in PA-treated cells that were pre-treated with l-cycloserine as compared with the observed apoB48 level in the absence of the de novo ceramide synthesis inhibitor.
FIGURE 8.
Effects of de novo ceramide synthesis inhibition on MTP activity and apoB48 secretion in Caco-2/TC7 cells. Caco-2/TC7 cells were incubated with or without OA- or PA-containing lipid micelles (PA) for 24 h. When indicated, l-cycloserine was added 1 h before incubation with PA-containing lipid micelles (PA+Lcyclo). Insulin (ins) was added 30 min prior to harvest. A, apoB48 secretion in basal culture medium was determined by Western blotting (left panel). Quantification of the Western blotting is displayed in the right panel. Results are expressed as arbitrary units (mean ± S.E., n = 6). ***, p < 0.001, as compared with the same condition without insulin; ###, p < 0.001, as compared with control; ns, not significant. B, MTP activity was assayed in cell homogenates. Results display mean ± S.E., n = 6, for each condition. *, p < 0.05, as compared with the same condition without insulin; **, p < 0.01, as compared with the indicated condition; ##, p < 0.01, as compared with control.
Furthermore, MTP activity was determined in Caco-2/TC7 cells (Fig. 8B). As observed previously in these cells (23, 34), MTP activity was not modified by the supply of oleic acid-containing micelles. However, we observed an increase of MTP activity in PA-treated cells as compared with control cells. Insulin decreased MTP activity in OA-treated cells but not in PA-treated cells. Upon insulin stimulation, the pre-treatment of PA-treated cells with l-cycloserine decreased MTP activity as compared with the same conditions but without l-cycloserine pre-treatment.
Altogether, these results indicate that the inhibitory effects of insulin on apoB48 secretion and MTP activity observed in OA-treated cells do not occur in PA-treated cells and that an inhibition of the de novo ceramide synthesis pathway can restore insulin action.
Discussion
It has been established that enterocytes express all of the receptors and mediators involved in the insulin-signaling pathway (35). However, few data exist on the intestinal role of insulin during fat absorption under healthy conditions. The inhibitory effect of insulin on intestinal lipoprotein secretion has previously been observed (11, 36, 37), but the mechanisms implicated remain unknown. Here, we report for the first time that in enterocytes (in vivo as well as in Caco-2/TC7 cells) insulin modulates the expression and/or activity of MTP, which has a crucial role in the assembly of intestinal apoB-containing lipoproteins (6).
Most of the knowledge concerning the effects of insulin on intestinal lipoprotein secretion has been obtained in pathological situations with an overt insulin-resistant state, such as in obesity or type 2 diabetes. In such pathologies, the increased production of chylomicrons and their reduced clearance contribute to the postprandial dyslipidemia observed in insulin-resistant individuals (38). The physiological suppressive effect of insulin on chylomicron production is reportedly absent in type 2 diabetes subjects (17). Duodenal explants from insulin-resistant obese subjects undergoing bariatric surgery were shown to express higher mRNA levels of free fatty acid-binding proteins and MTP and to secrete more intestinal triglyceride-rich lipoproteins, i.e. chylomicrons (39). Interestingly, it has also been recently shown that bariatric surgery improves triglyceride-rich lipoprotein metabolism and, in particular, decreases the levels of intestinal lipoproteins in obese subjects (40). These changes were associated with decreases in HOMA-IR (homeostasis model assessment-insulin resistance) index after surgery, indicating an improvement in insulin sensitivity (40). Specific intestinal perturbations in insulin signaling have been observed in enterocytes from the fructose-fed hamster model of insulin resistance (41). In this model, reduced levels of IRS-1 phosphorylation were observed (12). Although chronic administration of fructose led to increased intestinal lipoprotein secretion, such an effect was not observed after short term treatment (2 days) (12), suggesting that the perturbation of intestinal lipoprotein secretion in this model could be a consequence of intestinal adaptation to an overt insulin-resistant state. In this study, we demonstrate that an acute supply of palm oil in mice, or of palmitic acid in Caco-2/TC7 enterocytes, is sufficient to impair insulin signaling as well as insulin effects on lipid-induced MTP expression and/or activity. This indicates that intestinal insulin resistance rapidly occurs within a time frame compatible with an enterocyte's short life span in the intestinal epithelium (42). It is thus possible that repeated consumption of saturated fat containing large amounts of palmitic acid might maintain this intestinal insulin-resistant state, leading to altered intestinal function, which in turn could affect the function of peripheral tissues.
Our results demonstrate that the effects of palmitic acid on insulin signaling in enterocytes depend on the production of ceramides. Ceramides are generated through three different pathways as follows: 1) de novo synthesis, in which the condensation of serine and palmitoyl-CoA by SPT constitutes the rate-limiting step; 2) from sphingomyelin through the activation of sphingomyelinases; and 3) the salvage pathway, through the breakdown of complex sphingolipids (43). In palm oil-treated mice and in palmitic acid-treated Caco-2/TC7 cells, our results show that the observed increase in ceramide production mainly occurs via the de novo synthesis pathway. Indeed, in intestinal cells, we observed an increase in dihydroceramides (Figs. 4 and 5), which are intermediate metabolites specifically generated during the de novo ceramide synthesis pathway (43). Moreover, an increase of SPT2 expression, one of the SPT subunits, is observed in palmitic acid-treated Caco-2/TC7 cells (Fig. 5). Finally, the inhibition of the de novo ceramide synthesis pathway partially reversed the effects of palmitic acid in these cells (Figs. 6B and 8). We delved deeper into the mechanism and showed that in enterocytes the effects of C2-ceramide on AKT phosphorylation involved the activation of the PKC pathway. This ceramide-activated pathway has previously been observed to mediate deleterious ceramide effects on insulin signaling in different insulin-sensitive cell types (31, 32).
Interestingly, we show that both C2-ceramide addition and inhibition of the de novo ceramide synthesis pathway mainly exert their effects on the AKT threonine 308 residue and to a lesser extent on its serine 473 residue (Fig. 6). These AKT serine and threonine residues are also known to be phosphorylated upon insulin stimulation by distinct protein kinases, PDK-1 and mTORC2, respectively (44). Further studies will be needed to determine whether palmitic acid and ceramides could also act negatively on these kinases in intestinal cells.
Our present results must be taken into account in the context of the augmented saturated fat consumption and the concomitant increased incidence of obesity and type 2 diabetes in humans. Indeed, elevated ceramide levels associated with metabolic disease have been observed in the plasma of subjects (45–47). Interestingly, gastric bypass surgery, which improves insulin sensitivity in severely obese patients, also reduces levels of plasma ceramide subspecies (48). Moreover, inhibiting de novo ceramide synthesis by myriocin treatment, which decreased ceramide levels in tissues and/or plasma (49–51), also diminishes atherosclerotic lesions in rodents (51) and reverses diet-induced insulin resistance (49, 50, 52). Altogether, these data suggest that elevated plasma ceramide levels could contribute to the onset of metabolic diseases. In our study, we observed that a single oral administration of palmitic acid is sufficient to augment plasma ceramide levels in mice. Increased plasma ceramide levels resulting from intestinal metabolism after consumption of saturated fatty acid-rich diet, may thus contribute to the altered insulin sensitivity in peripheral tissues.
In summary, our study has demonstrated for the first time that the palmitic acid-ceramide-AKT pathway modifies intestinal insulin sensitivity. We show that the small intestine is able to rapidly and locally produce ceramides from palmitic acid, which then alter intestinal insulin responses. An elevation of intestine-derived plasma ceramides due to a saturated fat-containing diet may thus contribute to the onset of metabolic diseases.
Experimental Procedures
Animals
Male 10–12-week-old C57BL/6 mice were obtained from Janvier (St Berthevin, France), housed in standard cages, and supplied with food and drinking water ad libitum. Animals were kept in a 12-h light/dark cycle and at a controlled temperature (22 °C). All animal care and experimental procedures were conducted in accordance with the French Law of April 6, 2010, and they were approved by the local ethics committee (Charles Darwin, Ce5/2012/052).
Cell Culture
Cell culture media and supplements were obtained from Invitrogen (Cergy-Pontoise, France) and FCS (fetal calf serum) from AbCys Biowest (Paris, France). Microporous polyethylene terephthalate membrane inserts were from Corning (Avon, France) (23.1 mm diameter, 3-μm pore size high density). C2-ceramide was purchased from Cayman Chemical Co. (Montigny-le-Bretonneux, France), and insulin, myriocin, or l-cycloserine was from Sigma. Ro 31.8820 and OKA inhibitors were purchased from Merck Millipore (Molsheim, France).
Caco-2/TC7 cells were plated at a density of 0.25 × 106 cells on each insert in 6-well plates and grown and differentiated as described previously (22) in Dulbecco's modified Eagle's medium (DMEM) GlutaMAX I containing 25 mm glucose, supplemented with penicillin (100 IU/ml) and streptomycin (100 μg/ml), 1% non-essential amino acids, and 20% (v/v) heat-inactivated (56 °C, 30 min) fetal calf serum. Cells were cultured for 1 week in media containing FCS in the upper and lower compartments and then for 1 week with FCS in the lower compartment only. Media were changed daily. The day before and during treatment with insulin, cells were cultured in media devoid of FCS. After incubation, media and cells were immediately collected and processed for Western blotting as described below. In some experiments, cells were treated with insulin (100 nm), l-cycloserine (20 mm), C2-ceramide (100 μm), OKA (100 nm), or Ro 31.8220 (5 μm), and the duration of treatment is indicated in figure legends.
Preparation of Lipid Micelles
Lipid micelles were prepared as described (23). Briefly, stock solutions (100 mm) of PA, OA, l-α-lysophosphatidylcholine, 2-mono-oleoylglycerol, and 25 mm cholesterol (all from Sigma) were prepared in chloroform/methanol (2:1, v/v). To prepare 1 ml of lipid micelles, 6 μl PA or OA was mixed with 2 μl of other lipids in a sterile glass tube. The mixture was dried under a stream of nitrogen gas; the residue was dissolved in 83 μl of a sterile solution of 24 mm sodium taurocholate in serum-free medium, and the volume was brought to 1 ml with serum-free medium. The final lipid micelle preparation therefore consisted of serum-free medium containing sodium taurocholate (2 mm), PA or OA (0.6 mm), l-α-lysophosphatidylcholine (0.2 mm), and cholesterol (0.05 mm) with 2-mono-oleoylglycerol (0.2 mm).
Mouse Treatments
Mice were fasted overnight and force-fed either 0.2 ml of olive or palm oil. The lipid composition of olive and palm oils is described in Table 1. Insulin (0.5 units/25 g of mouse weight) was injected intraperitoneally. Treatment duration and biological sample collection time points are indicated in the figure legends. For myriocin treatment, mice were subjected to a daily oral gavage for 4 days with vehicle (100 μl of 5% w/v carboxymethylcellulose) or myriocin (0.3 mg/kg/day) 30 min before a palm oil bolus (0.2 ml). On day 5, mice received the same gavage, 2 h before euthanasia. Five minutes before treatment or euthanasia, blood samples were collected into EDTA tubes from the tail vein of conscious animals by gentle massage following tail snip and kept chilled on ice. Following centrifugation of blood samples, plasma was used for triglyceride measurements using a kit from DiaSys (DiaSys, Condom, France) according to the manufacturer's instructions. Intestinal epithelial cells were prepared as described previously (24). Briefly, the jejunum was cut into small pieces and incubated at 4 °C for 2 h in 3 ml of cell recovery solution (BD Biosciences, Le Pont de Claix, France) containing protease and phosphatase inhibitor mixtures (Roche Diagnostics, Meylan, France). Epithelial cell homogenates were filtered, washed with PBS, centrifuged to obtain villus epithelial cells, and then homogenized for protein extraction.
Protein Extraction and Measurement
Proteins were solubilized from isolated intestinal epithelial cells or Caco-2/TC7 cells in high sucrose buffer containing protease and phosphatase inhibitors (25). Protein concentration was determined using the Bio-Rad DC protein assay (Bio-Rad, Marnes-La-Coquette, France) with BSA standards.
Western Blotting
Cell lysates (30 μg of protein) were fractionated by SDS-PAGE, and proteins were transferred to a nitrocellulose membrane. After an overnight incubation at 4 °C in TBS-T (20 mm Tris/HCl, pH 7.6, 137 mm NaCl, and 0.1% Tween 20) supplemented with 5% (w/v) BSA, blots were probed with primary antibodies, i.e. anti-AKT, anti-P-AKTThr-308, anti-P-AKTSer-473 (Cell Signaling Technology, Ozyme, Saint Quentin en Yvelines, France), anti-P-IRS1Tyr-612 (Millipore, Guyancourt, France), anti-HSC70 (Santa Cruz Biotechnology, CliniSciences, Nanterre, France), anti-apoB (Chemicon, Millipore, Guyancourt, France), and anti-SPT2 (a gift from Hornemann Thorsten, Institute of Clinical Chemistry, Zurich, Switzerland), followed by peroxidase-conjugated secondary antibodies. Blots were developed with enhanced chemiluminescence (ECL®) reagents according to the manufacturer's instructions (Amersham Biosciences, Orsay, France).
Quantitative Real Time PCR
Total RNA was extracted from intestinal epithelial cells using Tri-Reagent (Invitrogen) according to the manufacturer's recommendations. Quantitative real time PCR was performed with the SYBR Green PCR kit (Life Technologies, Inc.) using a Stratagene system according to instructions. Relative quantification was determined using the 2-ΔΔCt method. Oligonucleotide sequences were as follows: forward, 5′-GGCAGTGCTTTTTCTCTGCT-3′, and reverse, 5′-TGAGAGGCCAGTTGTGTGTGAC-3′ for murine Mtp mRNA and forward, 5′-ATGTATCACAGCCTGTACCTG-3′, and reverse, 5′-CGTGCTTCCTTGGTCTTAGAC-3′ for murine L19 mRNA (reference gene).
Quantification of Ceramides and Dihydroceramides by LC-MS/MS
Ceramide standards (d18:1–14:0 Cer, d18:1–16:0 Cer, d18:1–17:0 Cer, d18:1–20:0 Cer, d18:1–22:0 Cer, d18:1–24:0 Cer, and d18:1–24:1 Cer) were obtained from Avanti Polar Lipids (Coger SAS, Paris, France). Chemicals of the highest available grade were purchased from Sigma. LC-MS/MS quality grade solvents were purchased from Fisher (Illkirch, France).
Ceramides and dihydroceramides were extracted according to the Bligh and Dyer method as described by Reis et al. (26). Briefly, plasma aliquots (50 μl of plasma + 150 μl of saline) or cell lysates (200 μl) were mixed with d18:1–17:0 Cer, used as an internal standard, and extracted with 750 μl of 2:1 chloroform/methanol for 10 min. Chloroform (250 μl) was then added and extraction followed for 10 min. Distilled water (250 μl) was added and extraction continued for 10 more min. After centrifugation (10,000 × g, 10 min, 4 °C), the organic phase was collected. The aqueous phase was acidified with hydrochloric acid (8 μl, 3 mol/liter) and further extracted with 600 μl of chloroform for 10 min. After centrifugation (10,000 × g, 10 min, 4 °C) the organic phase was collected and combined with the previous sample. Pooled organic phases were washed with 800 μl of the upper phase from a chloroform/methanol/water (96.7:93.3:90) mixture. The organic phase was evaporated under vacuum. Extracts were finally dissolved with 200 μl of 60:30:4.5 chloroform/methanol/distilled water, and 3 μl were injected on a 1200 6460-QqQ LC-MS/MS system equipped with an ESI source (Agilent Technologies, Les Ulis, France). Separation was achieved on a Poroshell C8 2.1 × 100 mm, 2.7-μm column (Agilent Technologies) at a flow rate of 0.3 ml/min, 30 °C, with a linear gradient (solvent A) of formic acid/ammonium formate (0.2%/1 mm final concentration) and (solvent B) methanol containing formic acid/1 mm ammonium formate as follows: 70% B for 1 min, up to 100% B in 4 min, and maintained at 100% for 5 min. Acquisition was performed in positive multiple reaction monitoring mode (source temperature, 300 °C, nebulizer gas flow rate 10 liters/min, sheath gas flow 11 liters/min, temperature 325 °C, capillary 3500 V, nozzle 1000 V, fragmentor 180 V, collision energy 27 V). Transitions, [M − 18]+ → 262.2, [M − 18]+ → 264.2, and [M − 18]+ → 266.2, were used for quantitation of d18:2-ceramide, d18:1-ceramide, and d18:0-ceramide, respectively.
Calibration curves were obtained for each molecule using authentic standards extracted by identical methods as used for plasma samples and cell lysates. Quadratic regression and linear regression were applied to calculate plasma and cell lysate ceramide concentrations, respectively.
MTP Activity
MTP activity was assayed using 100–200 μg of cell homogenate according to the manufacturer's instructions (MAK110 kit, Sigma, Saint-Quentin Fallavier, France).
Statistics
Comparisons between the two groups were performed with Student's t tests. Comparisons involving multiple groups were done using one-way analysis of variance. A level of p < 0.05 was considered as significant.
Author Contributions
T. T. T. T. designed and conducted the experiments and analyzed the results. B. G. P. carried out in vitro experiments. V. C. designed experiments and performed in vivo and some in vitro experiments. E. H. designed experiments on ceramides. S. D. contributed to the design of cell culture treatments and experiments. C. O. performed some in vitro experiments. J.-P. B. and A. B.-Z. performed quantification of ceramides and analyzed the results. A. R. conducted experiments on insulin signaling in the intestine with T. T. T. T., P. F., M. R., and E. H. conceived the project and obtained financial support from Institute of Cardiometabolism and Nutrition and Fondation pour la Recherche Médicale. V. C. and T. T. T. T. wrote the paper. P. F., E. H., A. L., S. D., and M. R. revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
The SPT2 antibody was a generous gift from Hornemann Thorsten (Institute of Clinical Chemistry, Zurich, Switzerland). We thank all the staff in charge of animal housing and care at the animal core facility of the Centre d'Explorations Fonctionnelles at the Centre de Recherche des Cordeliers, Paris, France. Experimentation on animals was approved by the French Ethical Committee (Ce5/2012/052 from Comité Charles Darwin, Paris, France). We thank Béatrice Riveau for the maintenance of Caco-2/TC7 cell culture. This article was revised by a professional editing service (Froogh Hajduch). We are indebted to Rachel Peat (from ICAN) for revising the manuscript.
This work was supported in part by the Institute of Cardiometabolism and Nutrition and by Fondation pour la Recherche Médicale (Équipe FRM DEQ20140329504). The authors declare that they have no conflicts of interest with the contents of this article.
- TRL
- triglyceride-rich lipoprotein
- apo
- apolipoprotein
- DHCer
- dihydroceramide
- IRS
- insulin receptor substrate
- MTP
- microsomal triglyceride transfer protein
- OA
- oleic acid
- OKA
- okadaic acid
- PA
- palmitic acid
- PP2A
- protein phosphatase 2A
- SPT
- serine palmitoyltransferase subunit.
References
- 1. Bansal S., Buring J. E., Rifai N., Mora S., Sacks F. M., and Ridker P. M. (2007) Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298, 309–316 [DOI] [PubMed] [Google Scholar]
- 2. Nordestgaard B. G., Benn M., Schnohr P., and Tybjaerg-Hansen A. (2007) Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298, 299–308 [DOI] [PubMed] [Google Scholar]
- 3. Duez H., Pavlic M., and Lewis G. F. (2008) Mechanism of intestinal lipoprotein overproduction in insulin resistant humans. Atherosclerosis 9, 33–38 [DOI] [PubMed] [Google Scholar]
- 4. Phillips C., Madigan C., Owens D., Collins P., and Tomkin G. H. (2002) Defective chylomicron synthesis as a cause of delayed particle clearance in diabetes? Int. J. Exp. Diabetes Res. 3, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adeli K., and Lewis G. F. (2008) Intestinal lipoprotein overproduction in insulin-resistant states. Curr. Opin. Lipidol. 19, 221–228 [DOI] [PubMed] [Google Scholar]
- 6. Hussain M. M., Nijstad N., and Franceschini L. (2011) Regulation of microsomal triglyceride transfer protein. Clin. Lipidol. 6, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hooper A. J., Burnett J. R., and Watts G. F. (2015) Contemporary aspects of the biology and therapeutic regulation of the microsomal triglyceride transfer protein. Circ. Res. 116, 193–205 [DOI] [PubMed] [Google Scholar]
- 8. Allister E. M., Borradaile N. M., Edwards J. Y., and Huff M. W. (2005) Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes 54, 1676–1683 [DOI] [PubMed] [Google Scholar]
- 9. Haas M. E., Attie A. D., and Biddinger S. B. (2013) The regulation of ApoB metabolism by insulin. Trends Endocrinol. Metab. 24, 391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewis G. F., Uffelman K. D., Szeto L. W., Weller B., and Steiner G. (1995) Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J. Clin. Invest. 95, 158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pavlic M., Xiao C., Szeto L., Patterson B. W., and Lewis G. F. (2010) Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes 59, 580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Federico L. M., Naples M., Taylor D., and Adeli K. (2006) Intestinal insulin resistance and aberrant production of apolipoprotein B48 lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes 55, 1316–1326 [DOI] [PubMed] [Google Scholar]
- 13. Haidari M., Leung N., Mahbub F., Uffelman K. D., Kohen-Avramoglu R., Lewis G. F., and Adeli K. (2002) Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 277, 31646–31655 [DOI] [PubMed] [Google Scholar]
- 14. Qin B., Qiu W., Avramoglu R. K., and Adeli K. (2007) Tumor necrosis factor-α induces intestinal insulin resistance and stimulates the overproduction of intestinal apolipoprotein B48-containing lipoproteins. Diabetes 56, 450–461 [DOI] [PubMed] [Google Scholar]
- 15. Leung N., Naples M., Uffelman K., Szeto L., Adeli K., and Lewis G. F. (2004) Rosiglitazone improves intestinal lipoprotein overproduction in the fat-fed Syrian Golden hamster, an animal model of nutritionally-induced insulin resistance. Atherosclerosis 174, 235–241 [DOI] [PubMed] [Google Scholar]
- 16. Duez H., Lamarche B., Uffelman K. D., Valero R., Cohn J. S., and Lewis G. F. (2006) Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 26, 1357–1363 [DOI] [PubMed] [Google Scholar]
- 17. Nogueira J.-P., Maraninchi M., Béliard S., Padilla N., Duvillard L., Mancini J., Nicolay A., Xiao C., Vialettes B., Lewis G. F., and Valéro R. (2012) Absence of acute inhibitory effect of insulin on chylomicron production in type 2 diabetes. Arterioscler. Thromb. Vasc. Biol. 32, 1039–1044 [DOI] [PubMed] [Google Scholar]
- 18. Larsen P. J., and Tennagels N. (2014) On ceramides, other sphingolipids and impaired glucose homeostasis. Mol. Metab. 3, 252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holland W. L., and Summers S. A. (2008) Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr. Rev. 29, 381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chavez J. A., and Summers S. A. (2012) A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 [DOI] [PubMed] [Google Scholar]
- 21. Hage Hassan R., Bourron O., and Hajduch E. (2014) Defect of insulin signal in peripheral tissues: important role of ceramide. World J. Diabetes 5, 244–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chateau D., Pauquai T., Delers F., Rousset M., Chambaz J., and Demignot S. (2005) Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells. J. Cell. Physiol. 202, 767–776 [DOI] [PubMed] [Google Scholar]
- 23. Pauquai T., Bouchoux J., Chateau D., Vidal R., Rousset M., Chambaz J., and Demignot S. (2006) Adaptation of enterocytic Caco-2 cells to glucose modulates triacylglycerol-rich lipoprotein secretion through triacylglycerol targeting into the endoplasmic reticulum lumen. Biochem. J. 395, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frochot V., Alqub M., Cattin A. L., Carrière V., Houllier A., Baraille F., Barbot L., Saint-Just S., Ribeiro A., Lacasa M., Cardot P., Chambaz J., Rousset M., and Lacorte J. M. (2012) The transcription factor HNF-4α: a key factor of the intestinal uptake of fatty acids in mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1253–G1263 [DOI] [PubMed] [Google Scholar]
- 25. Hajduch E., Alessi D. R., Hemmings B. A., and Hundal H. S. (1998) Constitutive activation of protein kinase Bα by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 47, 1006–1013 [DOI] [PubMed] [Google Scholar]
- 26. Reis A., Rudnitskaya A., Blackburn G. J., Mohd Fauzi N., Pitt A. R., and Spickett C. M. (2013) A comparison of five lipid extraction solvent systems for lipidomic studies of human LDL. J. Lipid Res. 54, 1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimabukuro M., Higa M., Zhou Y. T., Wang M. Y., Newgard C. B., and Unger R. H. (1998) Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J. Biol. Chem. 273, 32487–32490 [DOI] [PubMed] [Google Scholar]
- 28. Hanada K. (2003) Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632, 16–30 [DOI] [PubMed] [Google Scholar]
- 29. Uchida Y. (2014) Ceramide signaling in mammalian epidermis. Biochim. Biophys. Acta 1841, 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hajduch E., Balendran A., Batty I. H., Litherland G. J., Blair A. S., Downes C. P., and Hundal H. S. (2001) Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia 44, 173–183 [DOI] [PubMed] [Google Scholar]
- 31. Powell D. J., Turban S., Gray A., Hajduch E., and Hundal H. S. (2004) Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem. J. 382, 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blouin C. M., Prado C., Takane K. K., Lasnier F., Garcia-Ocana A., Ferré P., Dugail I., and Hajduch E. (2010) Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59, 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Greevenbroek M. M., van Meer G., Erkelens D. W., and de Bruin T. W. (1996) Effects of saturated, mono-, and polyunsaturated fatty acids on the secretion of apo B containing lipoproteins by Caco-2 cells. Atherosclerosis 121, 139–150 [DOI] [PubMed] [Google Scholar]
- 34. Mathur S. N., Born E., Murthy S., and Field F. J. (1997) Microsomal triglyceride transfer protein in CaCo-2 cells: characterization and regulation. J. Lipid Res. 38, 61–67 [PubMed] [Google Scholar]
- 35. Marandi S., De Keyser N., Saliez A., Maernoudt A. S., Sokal E. M., Stilmant C., Rider M. H., and Buts J. P. (2001) Insulin signal transduction in rat small intestine: role of MAP kinases in expression of mucosal hydrolases. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G229–G240 [DOI] [PubMed] [Google Scholar]
- 36. Loirdighi N., Ménard D., and Levy E. (1992) Insulin decreases chylomicron production in human fetal small intestine. Biochim. Biophys. Acta 1175, 100–106 [DOI] [PubMed] [Google Scholar]
- 37. Levy E., Sinnett D., Thibault L., Nguyen T. D., Delvin E., and Ménard D. (1996) Insulin modulation of newly synthesized apolipoproteins B-100 and B-48 in human fetal intestine: gene expression and mRNA editing are not involved. FEBS Lett. 393, 253–258 [DOI] [PubMed] [Google Scholar]
- 38. Dash S., Xiao C., Morgantini C., and Lewis G. F. (2015) New insights into the regulation of chylomicron production. Annu. Rev. Nutr. 35, 265–294 [DOI] [PubMed] [Google Scholar]
- 39. Veilleux A., Grenier E., Marceau P., Carpentier A. C., Richard D., and Levy E. (2014) Intestinal lipid handling: evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler. Thromb. Vasc. Biol. 34, 644–653 [DOI] [PubMed] [Google Scholar]
- 40. Padilla N., Maraninchi M., Béliard S., Berthet B., Nogueira J.-P., Wolff E., Nicolay A., Bégu A., Dubois N., Grangeot R., Mattei C., Vialettes B., Xiao C., Lewis G. F., and Valéro R. (2014) Effects of bariatric surgery on hepatic and intestinal lipoprotein particle metabolism in obese, nondiabetic humans. Arterioscler. Thromb. Vasc. Biol. 34, 2330–2337 [DOI] [PubMed] [Google Scholar]
- 41. Hsieh J., Hayashi A. A., Webb J., and Adeli K. (2008) Postprandial dyslipidemia in insulin resistance: mechanisms and role of intestinal insulin sensitivity. Atherosclerosis 9, 7–13 [DOI] [PubMed] [Google Scholar]
- 42. Potten C. S. (1998) Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castro B. M., Prieto M., and Silva L. C. (2014) Ceramide: a simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 54, 53–67 [DOI] [PubMed] [Google Scholar]
- 44. Guo S. (2014) Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J. Endocrinol. 220, T1–T23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haus J. M., Kashyap S. R., Kasumov T., Zhang R., Kelly K. R., Defronzo R. A., and Kirwan J. P. (2009) Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58, 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Mello V. D., Lankinen M., Schwab U., Kolehmainen M., Lehto S., Seppänen-Laakso T., Oresic M., Pulkkinen L., Uusitupa M., and Erkkilä A. T. (2009) Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 52, 2612–2615 [DOI] [PubMed] [Google Scholar]
- 47. Boon J., Hoy A. J., Stark R., Brown R. D., Meex R. C., Henstridge D. C., Schenk S., Meikle P. J., Horowitz J. F., Kingwell B. A., Bruce C. R., and Watt M. J. (2013) Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang H., Kasumov T., Gatmaitan P., Heneghan H. M., Kashyap S. R., Schauer P. R., Brethauer S. A., and Kirwan J. P. (2011) Gastric bypass surgery reduces plasma ceramide subspecies and improves insulin sensitivity in severely obese patients. Obesity 19:2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ussher J. R., Koves T. R., Cadete V. J., Zhang L., Jaswal J. S., Swyrd S. J., Lopaschuk D. G., Proctor S. D., Keung W., Muoio D. M., and Lopaschuk G. D. (2010) Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59, 2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holland W. L., Brozinick J. T., Wang L.-P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., and Summers S. A. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 [DOI] [PubMed] [Google Scholar]
- 51. Hojjati M. R., Li Z., Zhou H., Tang S., Huan C., Ooi E., Lu S., and Jiang X.-C. (2005) Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 280, 10284–10289 [DOI] [PubMed] [Google Scholar]
- 52. Ussher J. R., Folmes C. D., Keung W., Fillmore N., Jaswal J. S., Cadete V. J., Beker D. L., Lam V. H., Zhang L., and Lopaschuk G. D. (2012) Inhibition of serine palmitoyltransferase I reduces cardiac ceramide levels and increases glycolysis rates following diet-induced insulin resistance. PLoS One 7, e37703. [DOI] [PMC free article] [PubMed] [Google Scholar]