Abstract
The pregnane X receptor (PXR) (PXR/NR1I3) and constitutive androstane receptor (CAR) (CAR/NR1I2) members of the nuclear receptor (NR) superfamily of ligand-regulated transcription factors are well-characterized mediators of xenobiotic and endocrine-disrupting chemical signaling. The Nuclear Receptor Signaling Atlas maintains a growing library of transcriptomic datasets involving perturbations of NR signaling pathways, many of which involve perturbations relevant to PXR and CAR xenobiotic signaling. Here, we generated a reference transcriptome based on the frequency of differential expression of genes across 159 experiments compiled from 22 datasets involving perturbations of CAR and PXR signaling pathways. In addition to the anticipated overrepresentation in the reference transcriptome of genes encoding components of the xenobiotic stress response, the ranking of genes involved in carbohydrate metabolism and gonadotropin action sheds mechanistic light on the suspected role of xenobiotics in metabolic syndrome and reproductive disorders. Gene Set Enrichment Analysis showed that although acetaminophen, chlorpromazine, and phenobarbital impacted many similar gene sets, differences in direction of regulation were evident in a variety of processes. Strikingly, gene sets representing genes linked to Parkinson's, Huntington's, and Alzheimer's diseases were enriched in all 3 transcriptomes. The reference xenobiotic transcriptome will be supplemented with additional future datasets to provide the community with a continually updated reference transcriptomic dataset for CAR- and PXR-mediated xenobiotic signaling. Our study demonstrates how aggregating and annotating transcriptomic datasets, and making them available for routine data mining, facilitates research into the mechanisms by which xenobiotics and endocrine-disrupting chemicals subvert conventional NR signaling modalities.
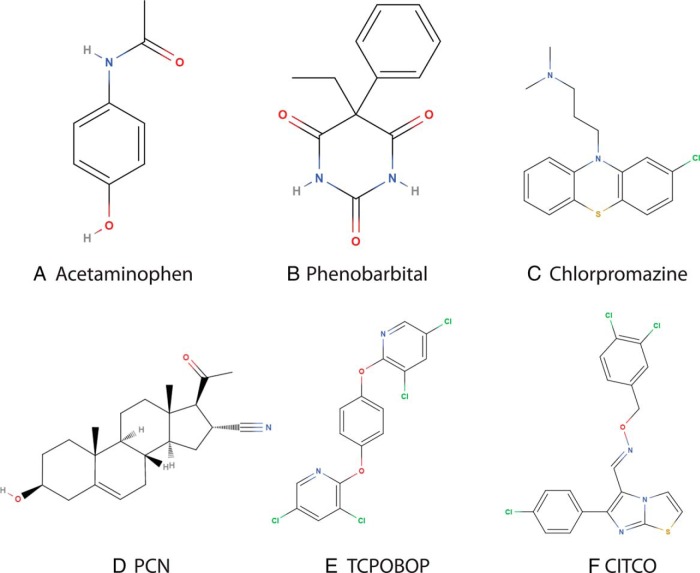
As transcription factors regulated in part by interactions with small lipophilic molecules, members of the nuclear receptor (NR) superfamily are well-characterized targets for a variety of xenobiotics and endocrine-disrupting chemicals (EDCs) (1). Two NRs that have been shown to be particularly frequent mediators of xenobiotic action are the constitutive androstane receptor (CAR) (CAR/NR1I2) and pregnane X receptor (PXR) (PXR/NR1I3). Numerous hypothesis-driven research studies have constructed a complex model of xenobiotic signaling, involving interactions with multiple signaling pathways with intricate tissue- and promoter-selective specificities. CAR and PXR have overlapping functions in the regulation of gene expression by the xenobiotics acetaminophen (Acmphn) (Figure 1A) (2, 3), phenobarbital (Phenob) (Figure 1B) (4, 5), chlorpromazine (Chlorprom) (Figure 1C) (6), and pregnenolone-16α-carbonitrile (PCN) (Figure 1D) (7), whereas the pesticide contaminant 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) (Figure 1E) and 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehydeO-(3,4-dichlorobenzyl)oxime (CITCO) (Figure 1F) are specific for CAR (8, 9).
Figure 1.
Chemical structures of CAR and PXR xenobiotic perturbants in transcriptomic datasets used to generate the reference transcriptome. CAR and PXR have overlapping specificity for Acmphn, Phenob, Chlorprom, and PCN, whereas TCPOBOP and CITCO are specific for CAR.
The universe of data points generated by the toxicotranscriptomics community has considerable collective value as a resource for identifying common transcriptional targets of CAR and PXR xenobiotic signaling pathways. Unfortunately, deficits in the curation and stewardship of these datasets have limited their reuse (10), to the extent that extracting a comprehensive list of xenotranscriptomic targets from the research literature is a far from straightforward task for the bench researcher. Reasoning that the xenobiotic research community might benefit from routine access to a reference CAR and PXR xenobiotic transcriptome based on unbiased, discovery-scale interrogation of xenobiotically perturbed model systems, we set out here to use the Nuclear Receptor Signaling Atlas (NURSA) Transcriptomine database (11) to generate this list. In addition to confirming experimentally documented components of the xenobiotic response, the list contains a number of prominent genes and cellular processes that conventional literature searches failed to associate with xenobiotic signaling, but that nevertheless shed light both on their known biology and suspected pathology of xenobiotics.
Materials and Methods
Dataset processing, annotation, and acquisition
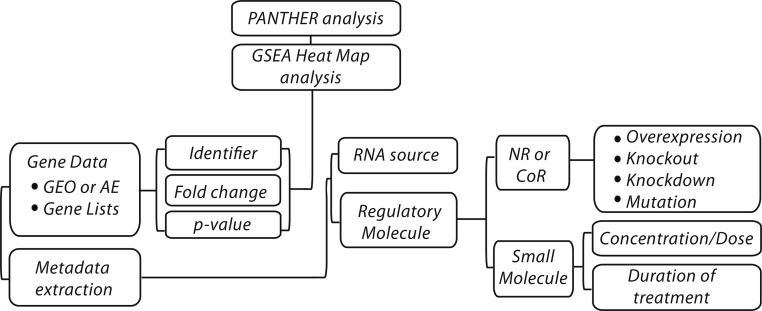
Datasets were processed and annotated for inclusion in the NURSA database using a previously described protocol (11, 12). Briefly, expression data obtained from Gene Expression Omnibus (13) and TG-GATES (14) as investigator-provided summarized and normalized array feature expression intensities were extracted and processed in the statistical program R. To calculate differential gene expression for investigator-defined experimental contrasts, we used the linear modeling functions from the Bioconductor limma analysis package (11). P values obtained from limma analysis were not corrected for multiple comparisons. In cases where a given gene was represented on an array by more than one probeset, data from individual probesets were generated separately and fold change values were not pooled across array features. Experimental contrasts were annotated for RNA Source and regulatory molecule with reference to the original publication accompanying the dataset, and fold changes were crosschecked to ensure faithful recapitulation of the data points reported in the original manuscript. Datasets were reviewed and 22 corresponding to perturbation of CAR/NR1I2 or PXR/NR1I3, or small molecules characterized as direct agonists or antagonists, were selected for generation of the reference transcriptomic signature (Table 1). Figure 2 summarizes the data analysis and metadata biocuration pipeline.
Table 1.
Datasets Used to Generate the Reference CAR and PXR Xenobiotic Transcriptome
| Dataset | DOI | Expts | Reference |
|---|---|---|---|
| Acmphn | |||
| Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat kidney | 10.1621/aYNzPYyURw | 9 | 77 |
| Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat primary hepatocytes | 10.1621/1vkREcPTii | 9 | 78 |
| Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat liver | 10.1621/dtsY1XDjLe | 12 | 79 |
| Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in human primary hepatocytes | 10.1621/p7F5N6o2kC | 9 | 80 |
| Chronic time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat kidney | 10.1621/ha2dfkibBe | 12 | 81 |
| Chronic time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat liver | 10.1621/ffXgOKigjs | 12 | 82 |
| Time course analysis of the acetaminophen (Acmphn)-regulated hepatic transcriptome in a variety of mouse strains | 10.1621/j7SRGHu1mv | 8 | 83 |
| Transcriptomic profiling of acetaminophen (Acmphn)-, clofibrate (Clofib)- and lithocholic acid (Litho)-treated primary rat hepatocytes | 10.1621/MIASPNjf4c | 2 | 84 |
| Chlorprom | |||
| Acute time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in rat primary hepatocytes | 10.1621/UMT1Pv11IH | 9 | 85 |
| Acute time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in human primary hepatocytes | 10.1621/RVje15d0l7 | 12 | 86 |
| Acute time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in rat liver | 10.1621/R8LBcqY0Kd | 8 | 61 |
| Chronic time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in rat liver | 10.1621/cq2ujHYtTF | 12 | 87 |
| PCN | |||
| Analysis of the pregnenolone carbonitrile (PCN)-regulated transcriptome in mouse liver | 10.1621/rVCNLZzcjj | 1 | 88 |
| Analysis of the pregnenolone carbonitrile (PCN)-regulated transcriptome in mouse duodenum | 10.1621/9nnuQmZqWd | 1 | 89 |
| Phenob | |||
| Analysis of the phenobarbital (Phenob)-regulated transcriptome in mouse liver | 10.1621/rVCNLZzcjj | 1 | 88 |
| Analysis of the phenobarbital (Phenob)-regulated transcriptome in HepaRG cells | 10.1621/RdJpfZvFp6 | 6 | 62 |
| Time- and dose-dependence analysis of phenobarbital (Phenob)-treated HepaRG cells and primary human hepatocytes | 10.1621/l9Xpov1ODL | 20 | 90 |
| Time course analysis of peroxisome proliferator activated receptor (PPARα/Ppara) agonist-dependent transcriptomes in rat liver | 10.1621/nvrYDqPZIP | 2 | 91 |
| Others | |||
| Analysis of constitutive androstane receptor (CAR/Nr1i3) ligand-dependent transcriptomes in wild type, CAR/Nr1i3 null mutant and human CAR/NR1I3 knockin mouse liver | 10.1621/datasets0.01003 | 9 | 92 |
| Analysis of the phenobarbital (Phenob)-regulated transcriptome in rat liver | 10.1621/EvWGyqgO1Y | 1 | 93 |
| Analysis of the pregnane X receptor (PXR/Nr1i2)-regulated, β-secretase-dependent transcriptome in mouse liver | 10.1621/gvx9xVfhUI | 2 | 94 |
| Time course transcriptomic analysis of the phenobarbital (Phenob)-regulated transcriptome in mouse liver | 10.1621/1lIAjWjBMh | 2 | 63 |
Digital object identifiers (DOIs) point to the dataset pages on the NURSA website.
Figure 2.
Transcriptomine data processing and metadata biocuration pipeline. GEO, Gene Expression Omnibus; AE, ArrayExpress. Datasets were processed and annotated for inclusion in the NURSA database using a previously described protocol (11, 12). Experimental contrasts were annotated for RNA Source and regulatory molecule with reference to the original publication accompanying the dataset, and fold changes were crosschecked to ensure faithful recapitulation of the data points reported in the original manuscript.
Generation of the reference xenobiotic transcriptome
To generate the reference transcriptome, we downloaded 22 Transcriptomine (11) expression profiling datasets that mapped to perturbations of the PXR or CAR xenobiotic pathways, corresponding to a total of 159 individual experiments and 25 801 significant (fold change ≥ ±2, P ≤ .05) fold changes (see Supplemental Dataset 1). Given that this list of genes represented human, mouse, and rat genes, we wished to map nonhuman genes to their human orthologs to provide for a unified, pan-species reference xenobiotic transcriptome. The NURSA data repository (12) maintains a collection of compilations of essential functions and properties of human, mouse, and rat genes, whose orthologous relationships are defined using NCBI's Homologene service (15). Human gene symbols corresponding to mouse and rat gene symbols in the list were replaced with their orthologous human gene symbols programmatically extracted from the NURSA database. Next, the pivot table function in Microsoft Excel was used to assign each human gene symbol a value corresponding to the frequency of its occurrence in the list, and symbols were then ranked in descending numerical order. Supplemental Dataset 2 contains the full reference xenobiotic transcriptome.
Protein annotation through evolutionary relationship (PANTHER) Gene Ontology (GO) biological process enrichment analysis
The PANTHER classification system (http://www.pantherdb.org/) combines gene function, ontology, and statistical analysis tools to support analysis of discovery-scale transcriptomic and proteomic datasets (16). The PANTHER Overrepresentation test (release 20160321) identifies statistically significant over- or underrepresentation of genes mapped to a specific biological process in a user's gene list with that of a reference gene list representing the universe of genes from the appropriate species. To identify biological processes that are selectively and consistently driven by exposure to xenobiotics, we carried out PANTHER Overrepresentation analysis on genes represented 10 or more times in the reference transcriptome (n = 575). Using the GO Hierarchy view from PANTHER, the most specific GO subclasses were selected for further analysis. These significantly overrepresented GO terms (Bonferroni corrected P ≤ .05) were ranked according to their fractional difference (number observed − number expected)/number expected). GO terms with a fractional difference more than or equal to 6.0 were used in Figure 1. Supplemental Dataset 3 contains all raw data pertaining to the PANTHER analysis.
Gene Set Enrichment Analysis (GSEA)
Using the Transcriptomine/NURSA API (17), we downloaded experiments for the endocrine disruptors groups Acmphn, Chlorprom, and Phenob. Due to the smaller numbers of experiments available for TCPOBOP, CITCO, and PCN, experiments involving these xenobiotics were not included in the analysis. For each experiment, we used the gene symbols and fold change values as inputs for the GSEA algorithm (18). Based on the compendium of biological pathways and processes compiled and curated by the Molecular Signature Database (MSigDB) (19, 20), we used the following gene set collections: Hallmark, KEGG, Reactome, Biocarta, and GO biological process, for a total of over 1900 gene sets. For each Transcriptomine experiment and each gene set, GSEA returned a Normalized Enrichment Score and an adjusted Q value. We considered gene set enrichments to be significant for Q < 0.25. Next, we collated enrichment profiles for all the experiment in a single matrix, and assigned a value of Normalized Enrichment Score = 0 for pathways and gene sets that did not reach significance. We then used ANOVA as implemented in the R software package (version 3.3.0) to determine pathways that have different enrichment profiles between distinct endocrine disruptor groups; significance was considered for P < .01. We used hierarchical agglomerative clustering with average linkage to identify cluster of pathways with similar characteristics, and used the Python (version 2.7.11) scipy (version 0.17.0) package to generate the heatmap. Supplemental Figure 1 contains a high resolution, fully annotated version of the heatmap.
Results and Discussion
CAR- and PXR-mediated xenobiotic signaling impacts myriad biological processes
We first ranked gene symbols based upon the frequency of their significant differential expression across 159 experiments involving perturbation with xenobiotics known to impact CAR- and PXR-mediated signaling pathways (Table 1 and Figure 1). Table 2 shows the top 50 ranked genes in the reference xenobiotic transcriptome and Figure 3 shows the top 10 most highly overrepresented GO Biological Process categories generated by the PANTHER analysis of genes with a reference transcriptome frequency (ƒ) of 10 or higher (Supplemental Dataset 2 and Supplemental Dataset 3). The robust overrepresentation of the ω-hydroxylase and epoxygenase P450 gene families, as well as components of the unfolded protein-response pathways are symptomatic of the cellular response to Acmphn and other sources of cellular stress (21, 22). Also prominent among highly ranked members of the gene list, and previously characterized targets of PXR and CAR in a variety of contexts (23), were 25 ABC transporters, 10 aldo-keto reductases, and 10 UDP-glucuronosyl-transferases, all of which have well-known roles in drug metabolism and detoxification (24, 25). In addition to these iconic cellular responses to xenobiotics are a number of transcriptional targets whose consistent and robust regulation by xenobiotic signaling is not visibly represented in the conventional research literature. The specific examples discussed here shed light upon several aspects of the known but mechanistically uncharacterized biology of xenobiotics in major physiological processes, and serve to illustrate the value of establishing a reference transcriptome for xenobiotic signaling.
Table 2.
Top 50 Genes in the NURSA CAR and PXR Xenobiotic Reference Transcriptome (Version 1)
| Rank | Gene | Name | Rank | Gene | Name |
|---|---|---|---|---|---|
| 1 | SRXN1 | Sulfiredoxin 1 | 25 | FABP4 | Fatty acid-binding protein 4 |
| 2 | KLF6 | Kruppel-like factor 6 | 25 | GADD45B | Growth arrest and DNA damage inducible-β |
| 3 | DUSP6 | Dual specificity phosphatase 6 | 28 | ABCB1 | ATP-binding cassette subfamily B member 1 |
| 4 | POR | Cytochrome p450 oxidoreductase porcupine homolog (Drosophila) | 28 | IL1B | IL-1β |
| 5 | CYP2B6 | Cytochrome P450 family 2 subfamily B member 6 | 28 | LOX | Lysyl oxidase |
| 5 | G6PC | Glucose-6-phosphatase catalytic subunit | 28 | TIPARP | TCDD-inducible poly (ADP-ribose) polymerase |
| 7 | EGR1 | Early growth response 1 | 28 | TSKU | Tsukushi, small leucine-rich proteoglycan |
| 7 | IGFBP1 | IGF-binding protein 1 | 28 | ZFAND2A | Zinc finger AN1-type containing 2A |
| 7 | THRSP | Thyroid hormone responsive | 34 | EDNRA | Endothelin receptor type A |
| 7 | TRIB3 | Tribbles pseudokinase 3 | 34 | ONECUT1 | One cut homeobox 1 |
| 11 | PRLR | Prolactin receptor | 34 | PER2 | Period circadian clock 2 |
| 12 | HMOX1 | Heme oxygenase 1 | 34 | SOCS2 | Suppressor of cytokine signaling 2 |
| 13 | CCND1 | Cyclin D1 | 34 | SOCS3 | Suppressor of cytokine signaling 3 |
| 14 | CTGF | Connective tissue growth factor | 39 | AKR7A3 | Aldo-keto reductase family 7 member A3 |
| 14 | JUN | Jun protooncogene, AP-1 transcription factor subunit | 39 | CYP1A1 | Cytochrome P450 family 1 subfamily A member 1 |
| 14 | NFKBIZ | NFKB inhibitor-ζ | 39 | GCLC | Glutamate-cysteine ligase catalytic subunit |
| 14 | SOX4 | SRY-box 4 | 39 | GSTM3 | Glutathione S-transferase μ 3 (brain) |
| 18 | ASNS | Asparagine synthetase (glutamine-hydrolyzing) | 39 | HSPA1B | Heat shock protein familyA (Hsp70) member 1B |
| 18 | FKBP5 | FK506-binding protein 5 | 39 | IDI1 | Isopentenyl-diphosphate-δ isomerase 1 |
| 20 | CHKA | Choline kinase-α | 39 | PDK4 | Pyruvate dehydrogenase kinase 4 |
| 20 | LPIN1 | Lipin 1 | 46 | ACOT2 | Acyl-CoA thioesterase 2 |
| 20 | SULT1E1 | Sulfotransferase family 1E member 1 | 46 | ATP1B1 | ATPase Na+/K+ transporting subunit β1 |
| 23 | CYP2C9 | Cytochrome P450 family 2 subfamily C member 9 | 46 | CCNB1 | Cyclin B1 |
| 23 | DUSP1 | Dual specificity phosphatase 1 | 46 | CISH | Cytokine inducible SH2 containing protein |
| 25 | CYR61 | Cysteine-rich angiogenic inducer 61 | 46 | CYP3A4 | Cytochrome P450 family 3 subfamily A member 4 |
The full list of genes is shown in Supplemental Dataset 2.
Figure 3.
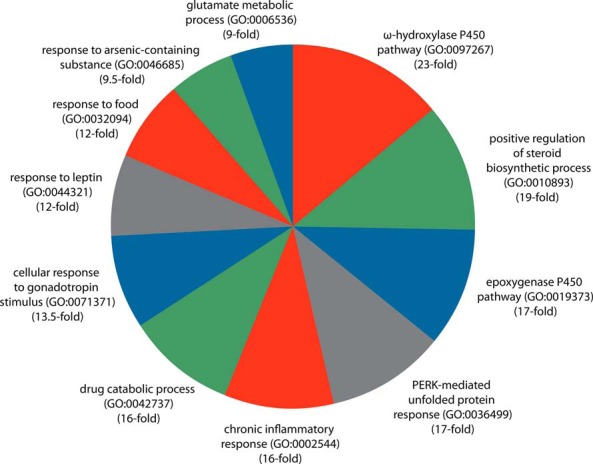
PANTHER GO biological process enrichment analysis identifies numerous cellular processes impacted by xenobiotic signaling. Biological processes enriched 9-fold or higher are shown (Supplemental Dataset 3 contains the full list of categories). The PANTHER Overrepresentation test identifies statistically significant over- or underrepresentation of genes mapped to a specific biological process in a user's gene list with that of a reference gene list representing the universe of genes from the appropriate species.
Oxidative stress
KLF6 (ƒ = 55, rank = 2) encodes a member of the Krüppel-like family of transcription factors, a total of 5 of which are represented in the reference xenotranscriptome. In addition to an implied tumor suppressive role in a variety of cancers (26, 27), it is induced in the livers of mouse models of nonalcoholic steatosis (28) and oxidative stress (29). Moreover, glomerular podocyte-specific depletion of KLF6 has been shown to result in mitochondrial dysfunction and apoptosis (30). Consistent with this, it has been previously identified as a DNA-binding partner of the arylhydrocarbon receptor (31). Its prominent position in the xenobiotic transcriptome suggests that it plays a key early role in marshalling of the transcriptional response to xenobiotic signaling.
Although most reactions involving CYP450 enzymes result in detoxification of xenobiotics, some reactions, such as the conversion of Acmphn to N-acetyl-p-benzoquinone imine, are thought to contribute to xenobiotic-induced oxidative stress and liver injury. The inhibition by N-acetyl-p-benzoquinone imine of thioredoxin reductase 1 (TXNRD1) (ƒ = 25, rank = 58) (32), a central component of cellular disulfide bond homeostasis, has been suggested to contribute to the development of oxidative stress in xenobiotic-exposed cells. It can be speculated therefore that the elevated ranking of TXNRD1 in the reference transcriptome reflects a compensatory mechanism to supplement depleted functional TXNRD1 levels to maintain appropriate redox homeostasis. Induction of NRF2/NFE2L2 has been identified as a potential compensatory factor in maintaining cellular redox homeostasis in hepatic-specific knockout mice (33), although its low ranking in the reference transcriptome suggests that this is a less than frequent event.
Carbohydrate metabolism
Reflecting the roles of PXR and CAR in homeostatic energy metabolism (34, 35) and previous reports of its regulation by PXR and/or CAR (36, 37), the gene encoding the last and rate-limiting enzyme of gluconeogenesis (catalytic α-subunit of glucose-6-phosphatase [G6PC]; ƒ = 40, rank = 5) was prominent among the reference xenobiotic targets. Also highly ranked was PGC-1/PPARGC1A (ƒ = 19, rank = 125), which encodes a transcriptional coregulator originally characterized as a cold-inducible coactivator of PPARγ/PPARG and subsequently implicated as a central component of cellular carbohydrate energy metabolism (38). Although the protein product has a well-established role in supporting NR-mediated transcriptional programs (39), the PPARGC1A gene itself has not been previously characterized as a transcriptional target of xenobiotic signaling. Its presence in the xenotranscriptomic signature is not entirely surprising given its regulation in response to other forms of stress, including cold and fasting (40). A plausible rationale for its consistent regulation by xenobiotic stimuli, and one lent credence by its reciprocal regulation of PXR/NR1I3 expression (41) and coactivation of genes encoding xenobiotic-metabolizing enzymes (42), is as part of a feed-forward loop supporting efficient clearance of elevated cellular xenobiotic concentrations. Interestingly, another reference xenobiotic target, SIRT1 (ƒ =10, rank = 474), encodes a FOXO1 deacetylase, and FOXO1 itself has been shown to contribute to transcriptional regulation of PCK and PGC1 (43). In concert these data point to the existence of a previously uncharacterized regulatory loop supporting a cellular shift towards energy conservation in response to xenobiotic stress.
Reproduction
A number of genes encoding key players in mammalian male and female reproduction are highly ranked in the reference xenotranscriptome. INHBA (ƒ = 19, rank = 125) and INHBB (ƒ = 11, rank = 396) encode the α- and β-subunits of the peptide hormone inhibin, which effects down-regulation of the synthesis and secretion of the gonadotropin FSH. In the case of the FGF pathway, which has been implicated in regulation of sperm development and maturation (44), genes encoding both ligand (FGF1: ƒ = 12, rank = 350; and FGF21: ƒ = 20, rank = 111) and receptor (FGFR2: ƒ = 10, rank = 474) components of the pathway were represented in the xenotranscriptome. Another highly ranked gene in the reference signature was that encoding the prolactin receptor (PRLR) (ƒ = 38, rank = 11), which mediates pleotropic cellular responses to prolactin. Consonant with the profound link between energy metabolism and reproduction, PRLR has recently been shown to potentiate insulin action (45). Interestingly, a search for PRLR in the Transcriptomine database identified 2 independent datasets establishing positive regulation of PRLR in response to SIRT1 overexpression (46). These data points are consistent with the insulin-sensitizing role of SIRT1 (47) and suggest that the coordination of energy metabolism with key reproductive processes might be critically exposed to the disruptive effects of xenobiotics and EDCs.
Inflammation
NFKBIZ (ƒ = 35, rank = 14) encodes a toll-like receptor signaling-inducible member of the ankyrin-repeat family (48) that has been recently identified as a key player in the development of inflammatory conditions, including psoriasis (49, 50) and asthma (51). Consistent with this, Transcriptomine displays data points documenting its induction in lipopolysaccharide (52)- and oxidative stress-derived extracellular vesicles (52)-treated bone marrow macrophages, and in colitic colonic mucosa (53). Given its role in the inflammatory response, there is a striking resonance between the prominent position of NFKBIZ in the xenobiotic reference transcriptome and emerging, albeit epidemiologically underdeveloped, evidence linking Acmphn to psoriasis (54) and asthma (55).
The dual specificity phosphatase family member DUSP6 (ƒ = 53, rank = 3) is a negative regulator of ERK1/2 in the context of FGF and ETS-1 signaling (56, 57) and has a well-characterized role in suppression of the immune response (58). DUSP6 has been shown to be robustly induced in response to treatment of nonsmall cell lung cancer cell lines with the epidermal growth factor receptor inhibitor CL-387785 (57) and in gastric cancer cell lines by the HGF inhibitory therapeutic PHA-665752 (59), indicating a potential role in cancer cell survival mechanism. Consistent with such a notion, siRNA depletion of DUSP6 has been shown to potentiate the cytotoxicity in cancer cell lines of CL-387785 and other cytotoxic agents (60). Collectively these results indicate a prominent role of DUSP6 in mediating the cellular response to exogenous chemicals and suggest that the CAR/PXR/xenobiotic pathway represents a potential therapeutic leverage point in a variety of cancers. Regulation of DUSP6 by xenobiotics is dynamic and a function of the duration of treatment (Supplemental Dataset 1), indicating the potential involvement of feedback mechanism in fine tuning and maintaining its cellular concentration at homeostatically appropriate levels.
GSEA of Acmphn, Chlorprom, and Phenob transcriptomes
Having established a reference transcriptome across all CAR and PXR xenobiotics, we next used GSEA to visualize commonalities and similarities in biological processes regulated by individual xenobiotics. Using the Transcriptomine API, we retrieved significantly differentially expressed genes for experiments in which Acmphn, Chlorprom, and Phenob were perturbants. To suppress differences between each of the treatments at the level of individual genes, we first represented each experiment as a collection of enriched pathways, based on GSEA (18) and a collection of over 1900 MSigDB pathways (19, 20), then used ANOVA parametric testing (P < .01) to determine significant pathways.
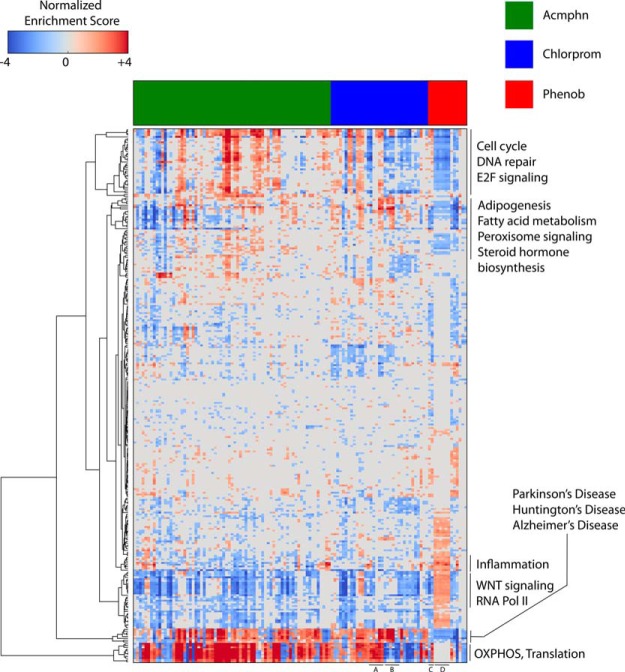
Figure 4 shows a GSEA-based heatmap of the Acmphn, Chlorprom, and Phenob transcriptomes. Although reasserting the existence of commonalities between the different xenobiotics with respect to regulation of gene expression, the results of this analysis also highlighted interesting differences in their transcriptional endpoints, both between individual xenobiotics as well as across different variables for a given xenobiotic. Interestingly, gene sets related to cell cycle, DNA repair, and E2F signaling were induced in Acmphn and suppressed by Chlorprom and Phenob, whereas oxidative phosphorylation and protein translation were induced by both Acmphn and Chlorprom and suppressed by Phenob. Conversely, Wnt Signaling and RNA Polymerase II/Transcription pathways were induced by Phenob, but suppressed by Acmphn and Chlorprom. Furthermore, Adipogenesis, Fatty acid metabolism, Peroxisome signaling, and Steroid Hormone Biosynthesis gene sets were induced in small subsets of both Acmphn and Chlorprom experiments and suppressed in the Phenob group, whereas Inflammation appeared to be induced by Phenob experiments but was not significantly altered by the other 2 xenobiotics. Although it is beyond the compass of this study to identify which of these effects are specifically CAR and/or PXR dependent, and not due to some non-NR-related mechanism of action, they do invite speculation as to the nature of this discrepancy in the context of NR signaling. They may reflect, for example, functional differences in the PXR and CAR transcriptional complexes assembled by Phenob and the other xenobiotics, or the relative contributions of PXR and CAR to Phenob-responsive transcriptional programs compared with those regulated by Acmphn and Chlorprom.
Figure 4.
GSEA heat map identifies convergence and divergence in the Acmphn, Phenob, and Chlorprom transcriptomes. Using the Transcriptomine API, we retrieved significantly differentially expressed genes for experiments in which Acmphn, Chlorprom, and Phenob were perturbants. To suppress differences between each of the treatments at the level of individual genes, we first represented each experiment as a collection of enriched pathways, based on GSEA and a collection of over 1900 MSigDB pathways, then used ANOVA parametric testing (P < .01) to determine significant pathways.
GSEA analysis also identified duration of exposure and species as important variables in determining the transcriptional impact of a given xenobiotic. A striking exemplar was the reversal in polarity of oxidative phosphorylation and protein translation gene set regulation effected by acute (Figure 4, bottom, A, and Supplemental Dataset 1 and Supplemental Figure 1, dataset 442, experiments 100084–88) (61) or extended (Figure 4, bottom, A, and Supplemental Dataset 1 and Supplemental Figure 1, dataset 442, experiments 100089–94) (61) exposure to Chlorprom. Similar discrepancies were observed in the Phenob datasets between experiments performed in human HepG2 cells (Figure 4, bottom, C, and Supplemental Dataset 1 and Supplemental Figure 1, dataset 215, experiments 854–859) (62) and mouse liver (Figure 4, bottom, D, and Supplemental Figure 1, dataset 172, experiments 679 and 680) (63). It bears pointing out that for the most part, the effects of these variables were less on-off in nature, and more yin-yang, in other words, the same sets of genes were regulated but in opposite directions.
A compelling trend across all 3 xenobiotics was the enrichment of KEGG gene sets relating to diseases of the central nervous system, including Parkinson's, Huntington's, and Alzheimer's diseases (Figure 4 and Supplemental Figure 1). This observation resonates strongly with previous research inferring connections between deficiencies in xenobiotic clearance exposure and central nervous system diseases (64–67) and is particularly interesting given the documented ability of Acmphn (68), Chlorprom (69), and Phenob (70) to traverse the blood brain barrier.
Conclusion
Despite the existence of numerous excellent reviews in the area of NR toxicotranscriptomics, identifying a validated list of transcriptional targets of xenobiotic signaling pathways can be a challenge for a researcher unfamiliar with the field, or even for those active in it. The goal of this study was to provide research communities in endocrinology, pharmacology, and related disciplines with a convenient reference of common and frequent transcriptional targets of xenobiotic signaling. Our reference xenobiotic transcriptome will have a variety of practical uses, for example, in the design of a panel of reporter genes to provide for screening of potential xenobiotics and EDCs, or to assess the impact of a given genetic perturbation on the integrity of PXR- and CAR-mediated xenobiotic signaling.
NURSA is continually growing its curated dataset holdings, and as a consequence, the reference xenobiotic transcriptome presented here is dynamic and will be reversioned as additional future relevant datasets are incorporated into the resource. At any given point in time therefore, it will represent the xenobiotic research community's “best guess” as to a reference xenobiotic transcriptomic signature. It should be borne in mind of course that the scope of our resource is currently largely restricted to NR signaling pathways, and transcriptomic datasets relevant to other signal transduction pathways are not currently represented in significant quantities. Future expansion of the resource to a broader cross-section of signaling modalities will most likely identify other signaling pathways that are significantly impacted by xenobiotic signaling.
A number of caveats and assumptions apply to this reference transcriptome. Our approach was predicated upon ranking genes according to the frequency with which their expression was significantly perturbed in transcriptome-wide experiments involving perturbants known to impact CAR/NR1I2 and PXR/NR1I3 signaling. Given the well-characterized context specificity of cellular signaling, which is influenced by diverse factors, including promoter epigenetic status and cell type, the reference transcriptome deemphasizes the direction or magnitude of individual fold changes, and focuses more on identifying those genes that are most consistently and reproducibly impacted across all species by perturbation of the CAR/PXR/xenobiotic network. Consequently, no specific mechanism, such as CAR or PXR dependence, direct or indirect regulation, or induction or repression is implied in the list. The GSEA analysis makes evident that although broad convergence exists across xenobiotics with respect to regulation of gene expression, factors such as small molecule structure, tissue context and others exert a considerable effect on the biological endpoints of exposure to xenobiotics. Such parameters are highly contextual, in that expression of a gene might be receptor dependent under one set of conditions but not another, for example, and their definition requires detailed bench characterization of individual promoter-cell-receptor-signal relationships that is beyond the scope of this study.
The breadth of biological processes impacted in the reference transcriptome reflects the numerous emergent human health indices in which xenobiotics and EDCs have been implicated (71). It should be borne in mind however that due to the currently limited number of studies in the database, the reference list was performed across different tissues and species. As a result, to gain an appreciation for the suppression or potentiation of human biological processes by xenobiotics, we inferred comparable regulation of human orthologs of mouse and rat genes. Although a well-established convergence exists between human and rodent metabolism (72, 73), the reliability of such extrapolation is not universally endorsed (74). As the resource grows with the incorporation of additional datasets, whether from large scale initiatives such as the excellent TG-GATES (14), or from individual investigators, the opportunity will arise for users to compare and contrast species-specific xenotranscriptomic responses.
The reference xenobiotic transcriptomic signature list is currently static and available as a Supplemental Dataset 2 accompanying this publication or for download from the NURSA website. Future work will provide for the 1-click availability of an interactive list that will be regularly updated by the addition of new CAR- and PXR-relevant xenobiotic datasets to the NURSA resource; these updated lists will be versioned and time-stamped so that the full complement of underlying datasets underlying any given list can be clearly identified. In addition to PXR- and CAR-cognate xenobiotics, NURSA curates datasets relevant to EDCs that target other signaling pathways, including the ER/estrogen signaling pathway. Future work will generate similar reference transcriptomes and provide for users of the site to readily identify commonalities and contrasts between the transcriptomic impact of diverse NR signaling pathways.
The reference transcriptome is perhaps best thought of as an informatic review, representing a convenient, high-level survey of the relative responsiveness to toxic exposure of the regulatory elements governing expression of mammalian genes. It bears emphasizing that despite the fact that all of the datasets that contributed to the reference transcriptome have been archived in public repositories such as Gene Expression Omnibus (13) and TG-GATES (14), they were largely opaque to conventional literature searches through PubMed and Google. The value in our study, and the wider NURSA biocurative and web tool development initiative, is in removing the barriers to their discovery, aggregation, annotation, reuse and citation by researchers (75). The National Institutes of Health's Big Data To Knowledge initiative (76) represents an expansive and coordinated effort to enhance its investment in discovery-scale datasets by investing in the infrastructure supporting their stewardship and accessibility to the community. We anticipate that as the ideals articulated in these principles find more widespread adoption, the assembly and maintenance of reference transcriptomes for cellular signaling pathways will accelerate the development of human therapeutics towards which the biomedical research community ultimately aspires.
Acknowledgments
We thank all the investigators who publically archived their datasets, without whom this resource would not have been possible. We also thank the input of the Nuclear Receptor Signaling Atlas Program Officers, Corinne Silva (National Institute of Diabetes Digestive and Kidney Diseases) and Dr Koji Yoshinaga (Eunice Kennedy Shriver National Institute of Child Health and Development).
The Nuclear Receptor Signaling Atlas Consortium was funded for this research by awards from National Institute of Diabetes Digestive and Kidney Diseases and National Institute of Child Health and Development (DK097748 and DK097748-S3).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Acmphn
- acetaminophen
- CAR
- constitutive androstane receptor
- Chlorprom
- chlorpromazine
- CITCO
- 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehydeO-(3,4-dichlorobenzyl)oxime
- EDC
- endocrine-disrupting chemical
- GO
- Gene Ontology
- GSEA
- Gene Set Enrichment Analysis
- MSigDB
- Molecular Signature Database
- NR
- nuclear receptor
- NURSA
- Nuclear Receptor Signaling Atlas
- PANTHER
- protein annotation through evolutionary relationship
- PCN
- pregnenolone-16α-carbonitrile
- Phenob
- phenobarbital
- PRLR
- prolactin receptor
- PXR
- pregnane X receptor
- TCPOBOP
- 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene
- TXNRD1
- thioredoxin reductase 1.
References
- 1. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. [DOI] [PubMed] [Google Scholar]
- 3. Guo GL, Moffit JS, Nicol CJ, et al. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol Sci. 2004;82:374–380. [DOI] [PubMed] [Google Scholar]
- 4. Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie W, Barwick JL, Simon CM, Pierce AM, et al. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117–126. [DOI] [PubMed] [Google Scholar]
- 7. Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. [DOI] [PubMed] [Google Scholar]
- 8. Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maglich JM, Parks DJ, Moore LB, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–17283. [DOI] [PubMed] [Google Scholar]
- 10. Ochsner SA, Steffen DL, Stoeckert CJ, Jr, McKenna NJ. Much room for improvement in deposition rates of expression microarray datasets. Nat Methods. 2008;5:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ochsner SA, Watkins CM, McOwiti A, et al. Transcriptomine, a web resource for nuclear receptor signaling transcriptomes. Physiol Genomics. 2012;44:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becnel LB, Darlington YF, Ochsner SA, et al. Nuclear Receptor Signaling Atlas: opening access to the biology of nuclear receptor signaling pathways. PLoS One. 2015;10:e0135615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Igarashi Y, Nakatsu N, Yamashita T, et al. Open TG-GATEs: a large-scale toxicogenomics database. Nucleic Acids Res. 2015;43:D921–D927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44(database issue):D7–D19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nuclear Receptor Signaling Atlas. Transcriptomine 3.1 REST API URLs and contracted request/response objects. 2016. Available from https://www.nursa.org/nursa/rs/index.jsf Accessed July 18th, 2016.
- 18. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. [DOI] [PubMed] [Google Scholar]
- 22. Uzi D, Barda L, Scaiewicz V, et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495–503. [DOI] [PubMed] [Google Scholar]
- 23. Timsit YE, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev. 2008;40:553–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou J, Zhang J, Xie W. Xenobiotic nuclear receptor-mediated regulation of UDP-glucuronosyl-transferases. Curr Drug Metab. 2005;6:289–298. [DOI] [PubMed] [Google Scholar]
- 26. Simmen RC, Pabona JM, Velarde MC, Simmons C, Rahal O, Simmen FA. The emerging role of Krüppel-like factors in endocrine-responsive cancers of female reproductive tissues. J Endocrinol. 2010;204:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Resende C, Ristimaki A, Machado JC. Genetic and epigenetic alteration in gastric carcinogenesis. Helicobacter. 2010;15(suppl 1):34–39. [DOI] [PubMed] [Google Scholar]
- 28. Stärkel P, Sempoux C, Leclercq I, et al. Oxidative stress, KLF6 and transforming growth factor-β up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J Hepatol. 2003;39:538–546. [DOI] [PubMed] [Google Scholar]
- 29. Urtasun R, Cubero FJ, Nieto N. Oxidative stress modulates KLF6Full and its splice variants. Alcohol Clin Exp Res. 2012;36:1851–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kopp JB. Loss of Krüppel-like factor 6 cripples podocyte mitochondrial function. J Clin Invest. 2015;125:968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilson SR, Joshi AD, Elferink CJ. The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J Pharmacol Exp Ther. 2013;345:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jan YH, Heck DE, Dragomir AC, Gardner CR, Laskin DL, Laskin JD. Acetaminophen reactive intermediates target hepatic thioredoxin reductase. Chem Res Toxicol. 2014;27:882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patterson AD, Carlson BA, Li F, et al. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem Res Toxicol. 2013;26:1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wada T, Gao J, Xie W. PXR and CAR in energy metabolism. Trends Endocrinol Metab. 2009;20:273–279. [DOI] [PubMed] [Google Scholar]
- 35. Konno Y, Negishi M, Kodama S. The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet. 2008;23:8–13. [DOI] [PubMed] [Google Scholar]
- 36. Kodama S, Moore R, Yamamoto Y, Negishi M. Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J. 2007;407:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ling Z, Shu N, Xu P, et al. Involvement of pregnane X receptor in the impaired glucose utilization induced by atorvastatin in hepatocytes. Biochem Pharmacol. 2016;100:98–111. [DOI] [PubMed] [Google Scholar]
- 38. Yoon JC, Puigserver P, Chen G, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. [DOI] [PubMed] [Google Scholar]
- 39. Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1 α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. [DOI] [PubMed] [Google Scholar]
- 40. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buler M, Aatsinki SM, Skoumal R, Hakkola J. Energy sensing factors PGC-1α and SIRT1 modulate PXR expression and function. Biochem Pharmacol. 2011;82:2008–2015. [DOI] [PubMed] [Google Scholar]
- 42. Arpiainen S, Järvenpää SM, Manninen A, et al. Coactivator PGC-1α regulates the fasting inducible xenobiotic-metabolizing enzyme CYP2A5 in mouse primary hepatocytes. Toxicol Appl Pharmacol. 2008;232:135–141. [DOI] [PubMed] [Google Scholar]
- 43. Schilling MM, Oeser JK, Boustead JN, Flemming BP, O'Brien RM. Gluconeogenesis: re-evaluating the FOXO1-PGC-1α connection. Nature. 2006;443:E10–E11. [DOI] [PubMed] [Google Scholar]
- 44. Cotton LM, O'Bryan MK, Hinton BT. Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr Rev. 2008;29:193–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu J, Xiao F, Zhang Q, et al. PRLR regulates hepatic insulin sensitivity in mice via STAT5. Diabetes. 2013;62:3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park J, Shinichi O, Park JY, et al. Analysis of the silent mating type information regulation 1 (Sirt1) and peroxisome proliferator-activated receptor α (PPARα/Ppara)-regulated transcriptomes in mouse heart. Nuclear Receptor Signaling Atlas Datasets. 2011; 10.1621/ZYLtb2Vvan. Jun 13, 2016. [Google Scholar]
- 47. Cao Y, Jiang X, Ma H, Wang Y, Xue P, Liu Y. SIRT1 and insulin resistance. J Diabetes Complications. 2016;30:178–183. [DOI] [PubMed] [Google Scholar]
- 48. Yamamoto M, Yamazaki S, Uematsu S, et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature. 2004;430:218–222. [DOI] [PubMed] [Google Scholar]
- 49. Sundaram K, Mitra S, Gavrilin MA, Wewers MD. House dust mite allergens and the induction of monocyte interleukin 1β production that triggers an IκBζ-dependent granulocyte macrophage colony-stimulating factor release from human lung epithelial cells. Am J Respir Cell Mol Biol. 2015;53:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsoi LC, Spain SL, Ellinghaus E. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat Commun. 2015;6:7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johansen C, Mose M, Ommen P, et al. IκBζ is a key driver in the development of psoriasis. Proc Natl Acad Sci USA. 2015;112:E5825–E5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Horvat S, Manček-Keber M, Frank M, et al. Analysis of the oxidative stress-dependent transcriptome in mouse bone marrow-derived macrophages. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/pbcLTrujND. Jun 10, 2016. [Google Scholar]
- 53. Mohapatra SK, Guri AJ, Horne WT, et al. Analysis of the peroxisome proliferator-activated receptor-γ (PPARγ/Pparg)-dependent colonic mucosal transcriptome in a mouse model of colitis. Nuclear Receptor Signaling Atlas Datasets. 2010; 10.1621/aZAIwFEImT. Jun 10, 2016. [Google Scholar]
- 54. Wu S, Han J, Qureshi AA. Use of aspirin, non-steroidal anti-inflammatory drugs, and acetaminophen (paracetamol), and risk of psoriasis and psoriatic arthritis: a cohort study. Acta Derm Venereol. 2015;95:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, Fitzgerald JM. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest. 2009;136:1316–1323. [DOI] [PubMed] [Google Scholar]
- 56. Ekerot M, Stavridis MP, Delavaine L, et al. Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem J. 2008;412:287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Z, Kobayashi S, Borczuk AC, et al. Dual specificity phosphatase 6 (DUSP6) is an ETS-regulated negative feedback mediator of oncogenic ERK signaling in lung cancer cells. Carcinogenesis. 2010;31:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bertin S, Lozano-Ruiz B, Bachiller V, et al. Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice. Mucosal Immunol. 2015;8:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cory S, Lai A, Park M, et al. Time-course transcriptomic analysis of a panel of MET receptor tyrosine kinase-inhibited gastric cancer cell lines. Nuclear Receptor Signaling Atlas Datasets. 2014; 10.1621/fl1TWZZjRA. Jun 08, 2016. [Google Scholar]
- 60. Bagnyukova TV, Restifo D, Beeharry N, et al. DUSP6 regulates drug sensitivity by modulating DNA damage response. Br J Cancer. 2013;109:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Igarashi Y, Nakatsu N, Yamashita T, et al. Acute time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in rat liver. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/R8LBcqY0Kd. Jul 01, 2016. [Google Scholar]
- 62. Lambert CB, Spire C, Renaud MP, Claude N, Guillouzo A. Analysis of the phenobarbital (Phenb)-regulated transcriptome in HepaRG cells. Nuclear Receptor Signaling Atlas Datasets. 2009; 10.1621/RdJpfZvFp6. Jun 08, 2016. [Google Scholar]
- 63. Ward W, Nesnow S, Moore T, et al. Time course transcriptomic analysis of the phenobarbital (Phenb)-regulated transcriptome in mouse liver. Nuclear Receptor Signaling Atlas Datasets. 2009; 10.1621/1lIAjWjBMh. Jul 01, 2016. [Google Scholar]
- 64. Heafield MT, Fearn S, Steventon GB, Waring RH, Williams AC, Sturman SG. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci Lett. 1990;110:216–220. [DOI] [PubMed] [Google Scholar]
- 65. Steventon GB, Heafield MT, Sturman S, Waring RH, Williams AC. Xenobiotic metabolism in Alzheimer's disease. Neurology. 1990;40:1095–1098. [DOI] [PubMed] [Google Scholar]
- 66. Ravindranath V. Metabolism of xenobiotics in the central nervous system: implications and challenges. Biochem Pharmacol. 1998;56:547–551. [DOI] [PubMed] [Google Scholar]
- 67. Dutheil F, Beaune P, Loriot MA. Xenobiotic metabolizing enzymes in the central nervous system: contribution of cytochrome P450 enzymes in normal and pathological human brain. Biochimie. 2008;90:426–436. [DOI] [PubMed] [Google Scholar]
- 68. Kumpulainen E, Kokki H, Halonen T, Heikkinen M, Savolainen J, Laisalmi M. Paracetamol (acetaminophen) penetrates readily into the cerebrospinal fluid of children after intravenous administration. Pediatrics. 2007;119:766–771. [DOI] [PubMed] [Google Scholar]
- 69. Changeux JP, Pinset C, Ribera AB. Effects of chlorpromazine and phencyclidine on mouse C2 acetylcholine receptor kinetics. J Physiol. 1986;378:497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Paulson OB, Györy A, Hertz MM. Blood-brain barrier transfer and cerebral uptake of antiepileptic drugs. Clin Pharmacol Ther. 1982;32:466–477. [DOI] [PubMed] [Google Scholar]
- 71. Tabb MM, Blumberg B. New modes of action for endocrine-disrupting chemicals. Mol Endocrinol. 2006;20:475–482. [DOI] [PubMed] [Google Scholar]
- 72. Liu Y, Meyer C, Xu C, et al. Animal models of chronic liver diseases. Am J Physiol Gastrointest Liver Physiol. 2013;304:G449–G468. [DOI] [PubMed] [Google Scholar]
- 73. Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol Sin. 2012;33:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Darlington YF, Naumov A, McOwiti A, Kankanamge WH, Becnel LB, McKenna NJ. Improving the discoverability, accessibility and citability of 'omics datasets: a case report [published online ahead of print July 12, 2016]. J Am Med Inform Assoc. 10.1093/jamia/ocw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bourne PE, Bonazzi V, Dunn M, et al. The NIH Big Data to Knowledge (BD2K) initiative. J Am Med Inform Assoc. 2015;22:1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Igarashi Y, Nakatsu N, Yamashita T, et al. Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat kidney. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/aYNzPYyURw. Jul 04, 2016. [Google Scholar]
- 78. Igarashi Y, Nakatsu N, Yamashita T, et al. Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat primary hepatocytes. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/1vkREcPTii. Jul 04, 2016. [Google Scholar]
- 79. Igarashi Y, Nakatsu N, Yamashita T, et al. Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat liver. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/dtsY1XDjLe. Jul 04, 2016. [Google Scholar]
- 80. Igarashi Y, Nakatsu N, Yamashita T, et al. Acute time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in human primary hepatocytes. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/p7F5N6o2kC. Jul 04, 2016. [Google Scholar]
- 81. Igarashi Y, Nakatsu N, Yamashita T, et al. Chronic time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat kidney. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/ha2dfkibBe. Jul 04, 2016. [Google Scholar]
- 82. Igarashi Y, Nakatsu N, Yamashita T, et al. Chronic time course and dose-dependence analysis of the acetaminophen (Acmphn)-regulated transcriptome in rat liver. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/ffXgOKigjs. Jul 04, 2016. [Google Scholar]
- 83. Liu H, Lu P, Farrell E, et al. Time course analysis of the acetaminophen (Acmphn)-regulated hepatic transcriptome in a variety of mouse strains. Nuclear Receptor Signaling Atlas Datasets. 2010; 10.1621/j7SRGHu1mv. Jun 08, 2016. [Google Scholar]
- 84. Suzuki H, Inoue T, Matsushita T, et al. Transcriptomic profiling of acetominophen (Acmphn)-, clofibrate (Clofib)- and lithocholic acid (Litho)-treated primary rat hepatocytes. Nuclear Receptor Signaling Atlas Datasets. 2008; 10.1621/MIASPNjf4c. Jun 08, 2016. [Google Scholar]
- 85. Igarashi Y, Nakatsu N, Yamashita T, et al. Acute time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in rat primary hepatocytes. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/UMT1Pv11IH. Jul 04, 2016. [Google Scholar]
- 86. Igarashi Y, Nakatsu N, Yamashita T, et al. Acute time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in human primary hepatocytes. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/RVje15d0l7. Jul 04, 2016. [Google Scholar]
- 87. Igarashi Y, Nakatsu N, Yamashita T, et al. Chronic time course and dose-dependence analysis of the chlorpromazine (Chlorprom)-regulated transcriptome in rat liver. Nuclear Receptor Signaling Atlas Datasets. 2015; 10.1621/cq2ujHYtTF. Jul 04, 2016. [Google Scholar]
- 88. Cui JY, Gunewardena SS, Rockwell CE, Klaassen CD. Analysis of the pregnenolone carbonitrile (PCN)-regulated transcriptome in mouse liver. Nuclear Receptor Signaling Atlas Datasets. 2010; 10.1621/rVCNLZzcjj. Jun 08, 2016. [Google Scholar]
- 89. Xu C, Wang X, Staudinger JL. Analysis of the pregnenolone carbonitrile (PCN)-regulated transcriptome in mouse duodenum. Nuclear Receptor Signaling Atlas Datasets. 2009; 10.1621/9nnuQmZqWd. Jun 08, 2016. [Google Scholar]
- 90. Lambert CB, Spire C, Claude N, Guillouzo A. Time- and dose-dependence analysis of phenobarbital (Phenb)-treated HepaRG cells and primary human hepatocytes. Nuclear Receptor Signaling Atlas Datasets. 2009; 10.1621/l9Xpov1ODL. Jun 08, 2016. [Google Scholar]
- 91. Hamadeh HK, Hamadeh HK, Bushel PR, et al. Time course analysis of peroxisome proliferator activated receptor (PPARα/Ppara) agonist-dependent transcriptomes in rat liver. Nuclear Receptor Signaling Atlas Datasets. 2002; 10.1621/nvrYDqPZIP. Jun 08, 2016. [Google Scholar]
- 92. Ochsner SA, Chua SS, Moore DD. Analysis of constitutive androstane receptor (CAR/Nr1i3) ligand-dependent transcriptomes in wild type, CAR/Nr1i3 null mutant and human CAR/NR1I3 knockin mouse liver. Nuclear Receptor Signaling Atlas Datasets. 2012; 10.1621/datasets.01003. Jun 08, 2016. [Google Scholar]
- 93. Lubet RA, Yao R, Grubbs CJ, You M, Wang Y. Analysis of the phenobarbital (Phenb)-regulated transcriptome in rat liver. Nuclear Receptor Signaling Atlas Datasets. 2009; 10.1621/EvWGyqgO1Y. Jun 08, 2016. [Google Scholar]
- 94. Tran S, Mongan MA, Dunn RT, et al. Analysis of the pregnane X receptor (PXR/Nr1i2)-regulated, β-secretase-dependent transcriptome in mouse liver. Nuclear Receptor Signaling Atlas Datasets. 2010; 10.1621/gvx9xVfhUI. Jun 08, 2016. [Google Scholar]