ABSTRACT
Hepatitis B virus (HBV) produces large (L), middle (M), and small (S) envelope proteins, alternatively referred to as hepatitis B surface antigen (HBsAg). Currently, yeast-derived S protein serves as the preventive vaccine, while hepatitis B immune globulin (HBIG) concentrated from pooled plasma of vaccine recipients is employed for post-exposure prophylaxis. However, only a small proportion of the antibodies in HBIG are HBV specific. In the present study, a human monoclonal anti-S antibody (G12) was developed, produced under GLP conditions, and subjected to a panel of functional assays. In vitro results demonstrated high affinity of G12 for the S protein (KD = 7.56 nM). It reacted with envelope proteins of all 7 HBV genotypes tested (A-F, H) by immunofluorescent staining, and more than 97% of HBsAg-positive patient serum samples by enzyme-linked immunosorbent assay. G12 recognized a conformational epitope, although the exact sequence remains unknown. Strikingly, G12 was at least 1,000-fold more potent than HBIG in neutralizing HBV infectivity in both HepaRG cell line and HepG2 cells reconstituted with the HBV receptor. In a transgenic mouse model of HBV persistence, a single peritoneal injection of G12 markedly diminished serum HBsAg titers in all 7 mice, which was sustained for the observation period of 144 d in mice with low pre-treatment levels. While the therapeutic potential of G12 warrants further investigation using a large number of animals, G12 is a potent neutralizing human monoclonal antibody and a promising candidate to replace or supplement HBIG in the prevention of HBV infection.
KEYWORDS: Anti-S, hepatitis B immune globulin, hepatitis B virus, human monoclonal antibody, neutralization, small envelope protein, transgenic mice
Abbreviations
- anti-HBs
antibody against HBsAg
- anti-S
antibody against small envelope protein
- CDR
complementarity-determining region
- CHO cells
Chinese hamster ovary cells
- DAPI
4′,6-diamidino-2-phenylindole
- ELISA
enzyme-linked immunosorbent assay
- HBeAg
hepatitis B e antigen
- HBIG
hepatitis B immune globulin
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HRP
horseradish peroxidase
- HSPG
heparan sulfate proteoglycans
- IF staining
immunofluorescent staining
- mAb
monoclonal antibody
- NTCP
sodium taurocholate cotransporting polypeptide
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PEG
polyethylene glycol
- SDS-PAGE
sodium dodecyl sulfate - polyacrylamide gel electrophoresis
- S protein
small envelope protein
- SPR
surface plasmon resonance
- VH
variable gene segment of the heavy chain
- VL
variable gene segment of the light chain
Introduction
Approximately 350 million people worldwide are chronically infected with hepatitis B virus (HBV), and some may eventually develop liver cirrhosis and hepatocellular carcinoma (HCC). Due to universal immunization with HBV vaccine at birth,1 the hepatitis B surface antigen (HBsAg) carrier rate in China declined steadily from 10 to 7% in the past decade.2 HBsAg is the collective term for 3 co-terminal envelope proteins and serves as a sensitive marker of ongoing HBV infection. Loss of HBsAg is followed by the appearance of corresponding antibody (anti-HBs), and such a seroconversion event signals recovery from infection.
The large (L), middle (M), and small (S) envelope proteins contain preS1+preS2+S, preS2+S, and S domain alone, respectively. The S protein is the major envelope protein on HBV virions, which have internal capsids shielding the partially double-stranded DNA genome. In addition, the bulk of the S protein is secreted as empty subviral particles lacking internal capsids, which exceed virions by a factor of at least 1,000.3 During a new round of infection, the S domain mediates the first step of virion attachment to cell surface heparan sulfate proteoglycans (HSPG), the low-affinity receptor.4-6 This somehow exposes the preS1 domain on L protein for interaction with sodium taurocholate co-transporting polypeptide (NTCP), the high-affinity HBV receptor.7,8 Therefore, anti-S and anti-preS1 antibodies neutralize HBV infectivity9-11 by blocking virus binding to the low-affinity receptor and high-affinity receptor, respectively.
The current HBV vaccine consists of yeast-derived, recombinant S protein. For post-exposure prophylaxis, hepatitis B immune globulin (HBIG) with high anti-S titers provides immediate, although short-term, protection against infection. Furthermore, in babies born to hepatitis B e antigen (HBeAg) positive mothers who are characterized by high viremia titers, immediate injection of high-titer HBIG in addition to HBV vaccine is needed to prevent maternal transmission of HBV infection.12 As such a vertical mode of infection is very common in East Asian countries such as China, there is high demand for HBIG. In addition, HBV reactivation often occurs in patients undergoing organ transplantation due to immunosuppressive therapies, which can be prevented by administration of HBIG. Since HBIG is a product derived from blood of individuals hyperimmunized with HBV vaccine, it is not only expensive, but also rarely available in certain less developed countries and regions. Finally, there is always concern regarding the biosafety of a blood product.
Human monoclonal antibodies (mAbs) against the S protein with good protective efficacy would provide a solution to the high demand for HBIG and ease the biosafety concern. However, the paucity of animals susceptible to HBV infection other than chimpanzees has greatly handicapped evaluation of such mAbs.13,14 Tupaia belangeri (tree shrew) can be infected with HBV, but quite inefficiently.15 While uPA-SCID mice repopulated with human hepatocytes provide a much better system of in vivo infection,16 they are immune deficient and costly. HBV transgenic mice resembles an in vivo system of stable HBV DNA transfection, as the HBV genome integrated into mouse germline drives DNA replication and protein expression in the liver.17 Since this is not an infection system, anti-S antibodies can reduce HBsAg antigenemia only through complex formation with subviral particles, rather than the early step of virion entry (neutralization). On the other hand, differentiated HepaRG cells18 and HepG2 cells stably transfected with NTCP, the high-affinity HBV receptor,7 have been developed as in vitro systems of HBV infection. These systems provide an opportunity to evaluate the neutralization capacity of an anti-S antibody (ability to bind virions). Herein, we developed a human anti-S mAb and employed both cell lines of HBV infection and a transgenic mouse model of HBsAg persistence to evaluate its neutralizing and therapeutic effects.
Results
Screening phage display libraries to identify potent human anti-S mAbs
Phage display libraries VH-VL and VH-VK were constructed using antibody genes isolated from blood of 18 human donors immunized with hepatitis B vaccine. They contained 2.5 × 107 and 2.2 × 107 independent clones, respectively. After four rounds of panning against purified recombinant S protein, 400 colonies were randomly picked and 199 interacted with S protein by enzyme-linked immunosorbent assay (ELISA). Sequence analysis of the 199 Fab clones revealed the presence of 21 unique clones, namely HBFab 1–21. They were analyzed for variable immunoglobulin gene segments using VBASE2 software (www.vbase2.org). All the antibody light chains were derived from kappa gene family including VK1 and VK3 (Supplementary Table 1), while the antibody heavy chains were derived from VH1 (one clone), VH3 (one clone), or VH4 (19 clones). ELISA analysis of clarified supernatants from the bacterial lysate containing human Fab antibodies revealed variable Fab expression levels and anti-S titers (Fig. 1A). The two clones with higher anti-S titer relative to Fab expression level (HBFab12 and HBFab21) were converted into IgG1 molecules and renamed HBIgG12 (simplified as G12) and HBIgG21 (G21), respectively. The binding affinities (KD) and kinetic rates of G12 and G21 for immobilized S protein were 7.56 nM and 8.04 nM, respectively, according to surface plasmon resonance (SPR) analysis (Fig. 1B).
Figure 1.
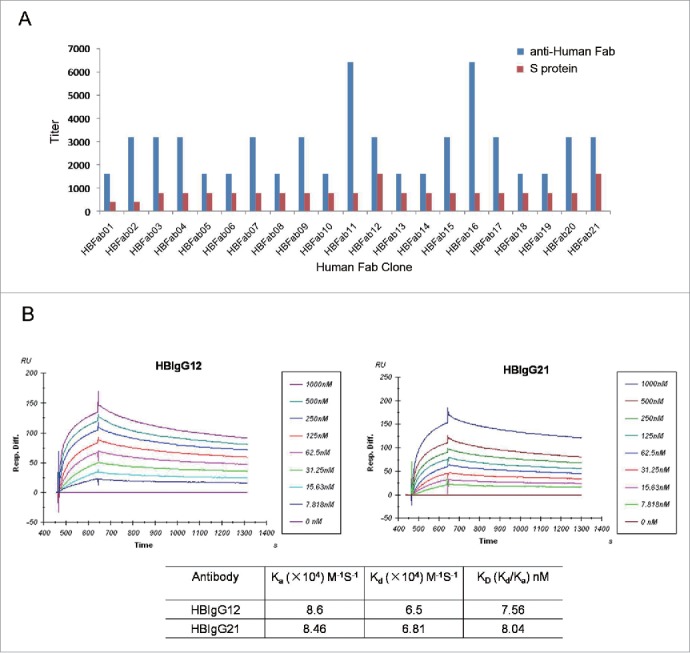
Characterization of 21 human anti-S mAbs to identify those with high affinity for the S protein. (A) ELISA method. Purified S protein (red) or anti-human Fab antibody (blue) were coated on 96-well plates, and incubated with human Fab antibodies. Bound antibodies were revealed by HRP-conjugated anti-human Fab. (B) SPR method. HBIgG12 and HBIgG21 at various concentrations were applied to BIAcore 3000 sensor chip CM5 immobilized with S protein. Each measurement represents an average of 3 independent assays.
G12 could detect HBsAg from major HBV genotypes and most clinical samples
To be useful, preventive or therapeutic antibodies should recognize most if not all HBV isolates. As HBV isolates worldwide are grouped into 8 genotypes (A-H),19,20 we transfected Huh7 cells with 7 full-length HBV genomes representing all HBV genotypes except for genotype G. G12 could detect intracellular envelope proteins from all 7 HBV genotypes by immunofluorescent (IF) staining revealing a cytoplasmic distribution (Fig. 2A). Moreover, HBsAg released to culture supernatant, irrespective of the viral genotype, could be detected by ELISA using G12-coated plates (Fig. 2B). These results suggest that G12 recognizes an epitope highly conserved among different HBV genotypes. In another assay, G12-based ELISA scored positive for 194 (97.98%) of 198 HBsAg-positive serum samples derived from chronic HBV carriers, and the HBsAg titer (ng/ml) correlated with S/CO value in G12-based ELISA (r2 = 0.4133, p < 0.0001) (Fig. 2C). The 4 negative samples had very low HBsAg titers (mean 19.37 ng/ml) in contrast to a mean titer of 441.24 ng/ml for all 198 samples. DNA was extracted from 2 negative samples and 4 samples with weak signals for amplification of the S region by polymerase chain reaction (PCR). Direct sequencing of the PCR products revealed no specific mutations, suggesting low HBsAg titers as the primary cause for detection failure.
Figure 2.
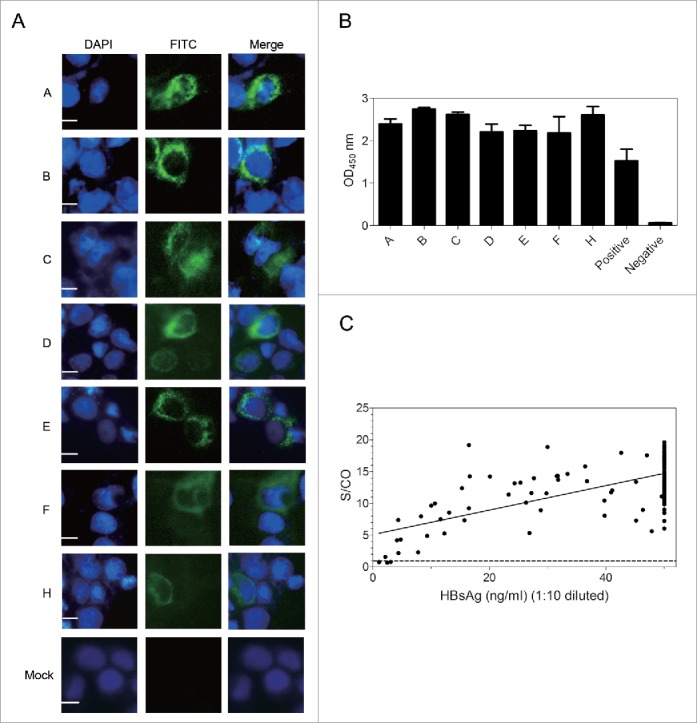
G12 can recognize HBsAg from different HBV genotypes and vast majority of clinical samples. ((A)& B) Huh7 cells were transfected with 1.3 copies of the HBV genome belonging to genotype A-F and H, followed by IF staining of intracellular envelope proteins by G12 (A) and ELISA detection of HBsAg released to culture supernatant using G12 coated plate (B). The three images for panel A are, from left to right, nuclear staining by DAPI (blue), HBsAg staining by G12 followed by FITC-conjugated anti-human antibody (green), and merged images. Each bar represents 10μm. Cells transfected with pUC18 DNA served as a negative control (Mock). (C) G12-based ELISA to detect HBsAg from 198 serum samples. The horizontal axis shows amounts of HBsAg (ng/ml) based on Light Initiated Chemiluminescence Analyzing System (Bo Yang), while vertical axis represents values (S/CO) from ELISA with G12-coated plates. For G12-based ELISA the cutoff value was 2.1 times the average of negative controls, and samples with S/CO >1 were scored positive (dotted line). S/CO: signal over cutoff.
G12 recognizes a conformational epitope in the immunodominant loop
G12 originated from recipients of HBV vaccine, the yeast-derived S protein. The S protein has 226 residues and traverses the membrane 4 times. Residues 101–163 form the major hydrophilic (immunodominant) loop, which is exposed on the surface of viral and subviral particles. Most anti-S antibodies recognize the “a” determinant (residues 124–147) within the immunodominant loop. To map the G12 epitope, we generated 38 consecutive deletion mutants of 6 amino acids to cover the entire S protein. These deletion constructs of the S protein were transfected to Huh7 cells, and S protein was measured from both cell lysate and culture supernatant by ELISA using either a commercial HBsAg detection kit (KHB) or G12-coated plates followed by other reagents from the KHB kit. Deleting residues 2–6 did not prevent S protein detection by G12. Deletions involving residues 7–72 abolished S protein secretion (Fig. 3B), but intracellular S protein was still detectable by both KHB kit and G12-based ELISA (Fig. 3A). Therefore, the G12 epitope lies outside residues 2–72. By the same token, residues 163–180, and 205–226 can be excluded as G12 epitope. Surprisingly, within the immunodominant loop only one mutant with residues 127–132 deleted remained detectable by G12. Since deletion mutants 109–114, 115–120, 121–126, 133–138, and 139–144 could still be detected by the KHB kit (Fig. 3B), negative results by the G12 antibody was not simply a consequence of poor protein expression from the deletion mutants. Rather, failure to precisely map the G12 epitope using deletion mutants is consistent with the involvement of a conformational epitope.
Figure 3.
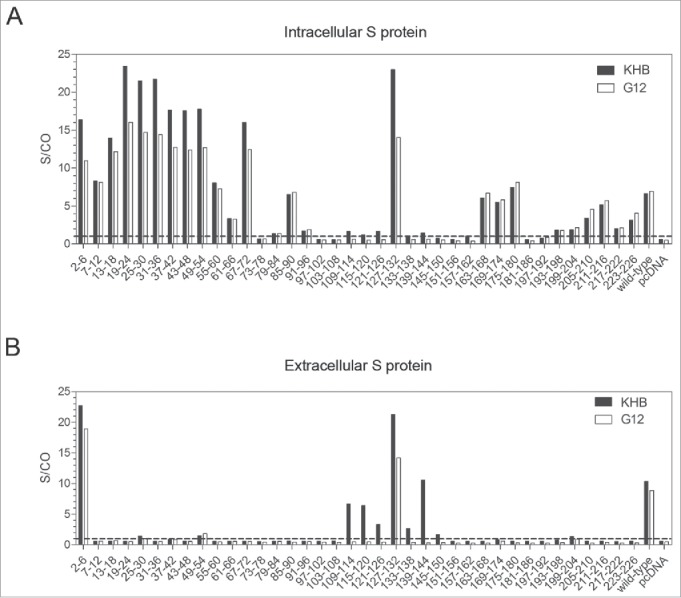
Mapping the epitope of G12 using 38 deletion mutants of the S gene. The S gene was cloned to pcDNA3.1-flag vector and 38 consecutive deletion mutants with every 6 residues removed (with the exceptions of mutant 2–6 and mutant 223–226) were generated and transfected to Huh7 cells. (A) Cells were harvested 48 hours later and lysed with RIPA buffer for S protein detection by both G12-based ELISA (white bars) and KHB ELISA (black bars) without dilution. (B) Corresponding culture supernatant was diluted 1:5 for S protein detection by G12 based ELISA (white bars) and commercial KHB ELISA (black bars). Signals below the dotted line are considered as negative. S/CO: signal over cutoff.
Indeed, G12 failed to detect by conventional Western blot the S protein from lysate of Huh7 cells transiently transfected with the HBV genome or S protein expression construct (data not shown). On the other hand, if unheated proteins in the cell lysate were separated in native agarose gel followed by transfer to nitrocellulose filter, G12 could detect 2 major bands from S protein transfected cells, but not from cells transfected with pcDNA vector (Supplementary Fig. 1A). In another experiment, G12 failed to detect even 2 μg of yeast-derived S protein per se, but reacted with a protein band about twice the size (Supplementary Fig. 1B). Thus, G12 might have much higher affinity for S protein dimer than monomer.
G12 was over 1,000-fold more potent than HBIG in neutralizing HBV infectivity in cell culture
Not all monoclonal anti-S antibodies are neutralizing. To verify the neutralization capacity of G12, we employed differentiated HepaRG cells18 and NTCP stably transfected HepG2 cells (HepG2/NTCP cells)7 as susceptible cell lines of HBV infection. These cells were infected with viral particles concentrated from culture supernatant of a cell line stably transfected with HBV,21 in the absence or presence of serially diluted G12 or HBIG. HBeAg released from infected cells, which was detected by a commercial ELISA kit (KHB), served as a sensitive and quantifiable marker of infectivity.
G12 could neutralize HBV infectivity in both HepaRG cells and HepG2/NTCP cells. In HepaRG cells, G12 at 0.005 μg/ml was more effective than HBIG at 0.0056 mg/ml in neutralizing HBV infectivity (Fig. 4A). Therefore, G12 was at least 1,000-fold more potent than HBIG in blocking HBV infectivity in this cell line. In HepG2/NTCP cells, G12 at 0.013 μg/ml achieved a similar neutralization effect as HBIG at 0.023 mg/ml, and G12 at 0.003 μg/ml was as effective as HBIG at 0.0056 mg/ml (Fig. 4B). Thus, G12 was more than 1,000-fold more effective than HBIG in neutralizing HBV infectivity in HepG2/NTCP cells.
Figure 4.
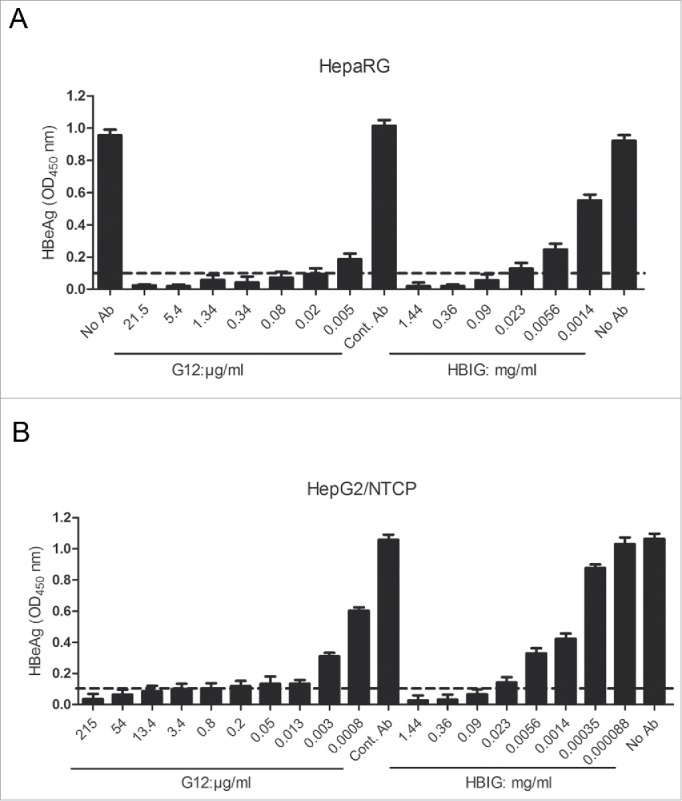
Neutralization of HBV infectivity in HepaRG cells (A) and HepG2/NTCP cells (B) by G12 or HBIG. Cells seeded in 96-well plates were incubated overnight with cell culture derived HBV particles in the absence or presence of serial 4-fold dilutions of G12 or HBIG, or control antibody (Cont. Ab). Cells were washed and further cultured with medium change every 2 or 3 d HBeAg values at one week post-infection were measured using an ELISA kit (KHB). The cutoff value (2.1 times the negative control) is shown as dotted line. Please note that the concentration of G12 shown is μg/ml, whereas that of HBIG is mg/ml.
Preliminary studies suggested that G12 might promote HBsAg elimination in HBV transgenic mice with low pre-treatment levels
A transgenic mouse model of HBsAg expression established in our laboratory22,23 was employed to assess the potential therapeutic effect of G12 in vivo. Previous work revealed variability in serum HBsAg levels ranging from 100 to 2,000 IU/ml.22 Twelve mice with descending HBsAg titers at week 7 (Fig. 5A & B; Supplementary Table 2) were divided into 6 groups of 2 mice each, and 2 mice of the first 5 groups were randomly assigned to phosphate-buffered saline (PBS) group (#9, #11, #13, #16, and #7) or G12 group (#3, #12, #8, #20, and #19). The last 2 mice (#22, #24) were also given G12 to examine whether G12 could clear HBsAg when the starting level was already low. At week 11, the mice were injected intraperitoneally with 0.5 ml of PBS (5 mice) or 600 IU (214 μg) of G12 diluted in PBS (5+2 mice), and sera were collected 5 hours (0.2 day) and 1, 2, 5, 8, 17, 47, 87, and 144 d post-injection. Injection of G12 led to marked drop of free HBsAg titers in all 7 mice 5 hours later (Fig. 5B; supplementary Table 2), from a mean value of 909 IU/ml to 15 IU/ml. In contrast, the mean value for PBS-treated mice changed only moderately from 1,005 IU/ml to 655 IU/ml (Fig. 5A; supplementary Table 2). In three G12-injected mice with high pretreatment levels (#3, #12, #8), HBsAg titer rebounded at day 5 or 8, although at day 144 post-injection the mean HBsAg titer (294 IU/ml) was much lower than the pretreatment level (1,675 IU/ml) (Fig. 5B). Since a similar decline of HBsAg titer was observed in the 3 mice from the PBS group (#9, #11, #13) with high pretreatment levels (from 1,313 IU/ml to 217 IU/ml) (Fig. 5A), this most likely reflects a time-dependent decline unrelated to therapeutic effect. Strikingly, in the 4 mice with low pretreatment HBsAg levels (#20, #19, #22, #24), the extremely low HBsAg titer maintained throughout the observation period of 144 d without significant rebound (Fig. 5B). The mean values were 334 IU/ml before antibody injection and only 6 IU/ml at day 144 post-injection.
Figure 5.
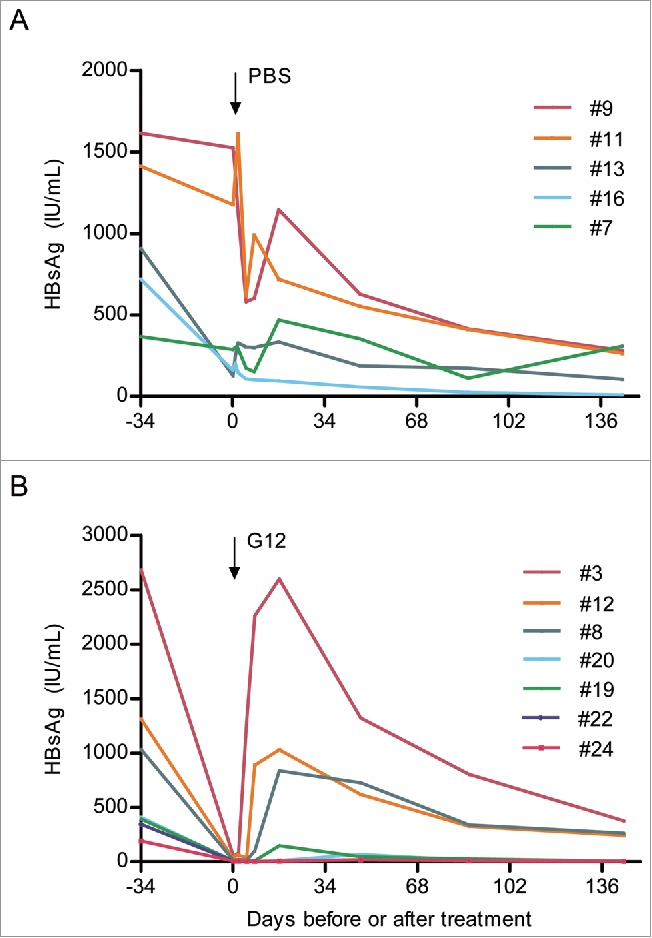
Effect of PBS and G12 treatment on serum HBsAg titers in lineage 59 HBV transgenic mice. (A) Sequential serum HBsAg titers in 5 mice receiving intraperitoneal injection of PBS. (B) Sequential serum HBsAg titers in 7 mice receiving intraperitoneal injection of 600 IU of G12.
We also measured free anti-S titers in the mouse sera. Anti-S was undetectable in PBS-treated mice at any time point (data not shown). In the G12 group, highest anti-S titer was observed at 5 hr post-injection (Fig. 6; Supplementary Table 3). The titer was highest in mouse #20, followed by #19, #22, #24, #8, #3, and #12. Consequently, anti-S persisted for 17 d in #20 mouse but only 2 d in #3 mouse (Fig. 6). The four mice with the highest initial anti-S titers (#20, #19, #22, #24) happened to be those with sustained HBsAg elimination. They had much lower pretreatment HBsAg titers (mean 334 IU/ml) than the 3 mice (#3, #12, #8) with HBsAg rebound (mean 1,675 IU/ml), which had lower anti-S titers at 5 hr post-injection. Therefore, from the limited number of mice studied it appeared that a low pretreatment HBsAg titer correlated with higher serum anti-S concentration after antibody injection, and sustained HBsAg elimination.
Figure 6.
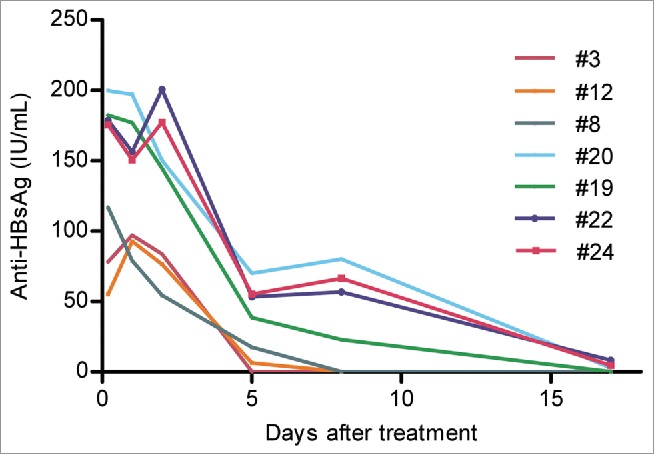
Sequential serum anti-HBs titers in 7 lineage 59 HBV transgenic mice receiving intraperitoneal injection of 600 IU of G12. Shown are anti-HBs titers from 0.2 day (5 hours) to 17 d post-injection.
Discussion
Preventing HBV infection and treating those already infected are the 2 main fronts in our battle against HBV-related health burdens. HBIG is needed to block HBV vertical transmission, transmission through accidental needle stick, sexual contact, or re-infection of transplanted liver. However, HBIG suffers from high cost, limited availability, and a low percentage of antibodies specific for HBV. Recent advances in antibody engineering have allowed humanized or fully human antibodies to be made,24 and human mAbs with broad reactivity toward different viral genotypes have been used for prevention or treatment of infectious diseases and cancer therapy.25-27 Therefore, high titer neutralizing human monoclonal anti-S antibodies could overcome the problems associated with HBIG.
In this study, a clone with high affinity for the S protein was identified and converted into an IgG1 molecule (Fig. 1). Subsequent studies demonstrated that this clone, G12, could recognize HBsAg from all 7 genotypes tested and all HBsAg-positive blood samples except for those with low titers (Fig. 2). G12 was found to recognize a highly conserved epitope and can bind its target as assessed through IF staining, ELISA, as well as immunoprecipitation (unpublished data). Its inability to detect denatured S protein by Western blot, but ability to detect S protein under nondenaturing conditions (supplementary Fig. 1A), is consistent with a conformational rather than linear epitope. Indeed, many 6-aa deletions inside the immunodominant loop abolished G12 binding (Fig. 3), suggesting that the epitope targeted by G12 is formed by discontinuous amino acids across a long distance. Alternatively, the G12 epitope is formed by higher structure such as a dimer (Supplementary Fig. 1B). A precise mapping of the G12 contact sites will require the construction of site-directed mutants covering the entire immunodominant loop.
G12 was compared head-to-head with HBIG for neutralization of HBV infectivity in cell culture. G12 could block HBV infection not only in HepG2 cells reconstituted with NTCP, a recently identified HBV receptor,7 but also in differentiated HepaRG cells (Fig. 4). G12 could no longer neutralize HBV infectivity if added immediately after removal of the viral inoculum, which is consistent with the fact that the S domain mediates the early step of virus attachment to cell surface.4-6 Over 1,000-fold higher protein concentration of HBIG was needed to achieve comparable neutralization effect. Similar to our findings, others found an anti-S mAb had 1,000-fold higher neutralizing capacity than HBIG in primary human hepatocytes.28 The much greater neutralization capacity of G12 than HBIG is most likely attributable to the low abundance of anti-S antibodies in HBIG preparation, as well as the much higher affinity of G12 for the S domain (KD = 7.56 nM) than most anti-S molecules in the HBIG pool. Considering that neutralization is mediated by antibody binding to virions rather than the large excess of subviral particles, an additional possibility is that G12 has higher affinity for virions than subviral particles. At any rate, such high neutralizing titer, coupled with its relatively simple production and purification procedures, makes G12 a promising candidate to replace HBIG in blocking HBV mother-to-infant transmission, re-infection of transplanted liver, and reactivation of HBV in immune compromised patients. As nucleos(t)ide analogs (NAs) could markedly reduce viremia titer (see below), combination therapy between G12 and potent NAs could achieve synergistic effect in preventing infection or re-infection.
In addition to G12, others have produced human or non-human mAbs against HBsAg and tested their neutralization capacity.10,11,13,14,28 However, differences in the system of infection (chimpanzees,10,13,14 primary human28 or Tupaia hepatocytes,11 HepaRG cells, NTCP-reconstituted HepG2 cells), method of neutralization (preincubation with the virus or co-administration to cell culture), and multiplicity of infection make these diverse reports difficult to compare. It will be crucial to compare different antibodies under the identical experimental conditions so as to identify the most potent human mAb against HBV infection.
Currently, pegylated interferons and NAs have been approved for therapy of HBV infection. As immune modulators, interferons are effective in only about one-third of patients and may display severe side effects. NAs target a late step of the intracellular HBV lifecycle, namely DNA synthesis. They can markedly reduce viremia titer to undetectable levels, but fail to affect viral mRNA transcription or protein translation. Consequently, they do not reduce HBsAg expression and are ineffective at promoting HBsAg seroconversion. In this regard, anti-S antibodies have the potential to reduce HBsAg titer by immune complex formation with subviral particles followed by clearance. To study whether there is a potential use for G12 in reducing HBsAg titer, its therapeutic effect was examined in transgenic mouse lineage 59 established in our laboratory.22,23 A single injection with 600 IU of G12 resulted in a significant short-term decrease in serum HBsAg titers for at least 2 d (Fig. 5B). Interestingly, 4 of the 7 mice injected with G12 continued to have only trace amount of HBsAg 144 d later, and all 4 had lower HBsAg levels before injection (191–409 IU/ml) than the 3 mice with HBsAg rebound (1,032–2,683 IU/ml). The mechanism for sustained HBsAg elimination in the 4 mice remains unclear at present, but could involve phagocytosis of the immune complex via the Fc receptor. Curiously, HBsAg also declined to some extent in the 5 mice receiving PBS within the first few days post-injection. In mouse #16 HBsAg eventually became nearly negative (9 IU/ml at day 144). Nevertheless, compared with HBsAg loss in only 1 of the 5 mice from the PBS group, 4 of the 7 mice from the G12 group showed sustained HBsAg elimination. Therefore, our results suggest that G12 does contribute to HBsAg elimination in this HBsAg transgenic mouse line despite simultaneous age-dependent decline in HBsAg titer as manifested in the PBS group. Certainly, our initial experimental design was flawed in that the 2 mice with the lowest HBsAg titers were both assigned to the G12 group, and the total number of mice was too small to reach statistical significance. Future experiments using a much larger number of mice with low pre-treatment HBsAg levels will be needed to validate the therapeutic effect of G12.
Using HBV transgenic mice to evaluate the therapeutic efficacy of human anti-HBs mAbs has its limitations because human antibodies are foreign proteins for mice and can be rapidly cleared from the circulation through induction of anti-human antibodies. Such a limitation makes it impossible to examine the effect of repeated antibody injection. In the future, Fc and Fab fragments of G12 can be remodeled for higher dendritic cell-stimulation efficacy29,30 and for higher antibody-mediated cytotoxicity.29,31,32 Alternatively, construction of divalent anti-preS1/anti-S antibodies could efficiently prevent mother-to-infant HBV transmission by blocking virus attachment to both HBV receptors.
Materials and methods
Derivation of G12 mAb from a phage display library
The phage display Fab libraries were constructed following established methods.33,34 Briefly, lymphocytes were isolated from the peripheral blood of 18 healthy Chinese volunteers who responded well to an HBV vaccine (Beijing Tiantan Biological Products Co., Ltd., China, licensed from Merck Co.). The collection and use of human samples were reviewed and approved by the Ethics Committee of China CDC, which uses international guidelines to ensure confidentiality, anonymity, and informed consent. Written informed consent form was signed by the donors. Total cellular mRNAs were extracted using RNeasy Mini Kit (Qiagen) and cDNA synthesis was primed with oligo (dT) using Transcriptor High Fidelity cDNA Synthesis Kit (Roche). The light and heavy chain genes were amplified from the cDNA by PCR and sequentially cloned into the pComb 3 H vector using a standard protocol.34,35 The final antibody libraries were panned against S protein purified from Chinese hamster ovary (CHO) cell culture (Beijing WantaiBioPharm) following the standard panning procedure.36 After 4 rounds of panning, crude Fab antibody preparations were tested by indirect ELISA using 96-well plates coated with 0.5–1 μg of purified S protein, with horseradish peroxidase (HRP)-conjugated anti-human Fab serving as the secondary antibody. Twenty-one unique clones reactive to the S protein were identified, and 2 of them (HBFab12 and HBFab21) were converted to human IgG by cloning the Fab genes into IgG expression cassette vector pAc-K-Fc.37 These 2 human mAbs (HBIgG12 and HBIgG21, simplified as G12 and G21, respectively) were separately expressed in SF9 insect cells (ATCC CRL-1711) and purified on Protein A columns. They were dissolved in PBS (pH7.4) for further characterization and functional analysis. The purity of the mAbs was confirmed using SDS-PAGE.
GLP level production of the G12 mAb
GLP production of G12 was carried out under contract by Wuxi Pharma (Wuxi Apptec) with a well-established protocol. Briefly, the G12 gene was subcloned to pOptiVEC-TOPO and pcDNA 3.3-TOPO (Freedom DG44 Kit, Invitrogen) and expressed in CHO/DHFR G44 cells. Pools and several cell lines were screened for their ability to produce high levels of G12. Candidate cells were used for further analysis to meet industrial standards for mAb drugs. Two of such cell lines were established and subjected to a small scale production in a 5-liter bio-tractor. With a yield over 0.5 g/l during the dish culture system, G12 was purified through a G protein chromatography and polishing protocol. The final product passed all the established evaluation tests, including color, purity, Tm, stickiness and glycosylation. A stock solution of 21.5 mg/ml (61,511 IU/ml) in 20 mM Tris buffer (pH 6.0) was used in this study.
Determination of the affinity constants of G12 and G21 for the S protein
Affinity of G12 and G21 for the S protein was measured by SPR, using BIAcore biosensor CM5 (BIAcore 3000, GE). Purified S protein (Beijing WantaiBioPharm) was immobilized on the sensor chip in 10 mM sodium acetate buffer, pH5.0, and exposed to G12 or G21 at 7.82, 15.63, 31.25, 62.5, 125, 250, 500, and 1000 nM concentration in HBS-Ep Buffer (GE). The binding on (Ka) and off (Kd) rates were calculated using the bivalent analysis model included in the BIA evaluation software.
G12-based ELISA for HBsAg detection from serum samples
Serum samples from 198 HBsAg-positive carriers were collected from Putuo District Center Hospital, Shanghai. HBV infection markers (HBsAg, anti-HBs, HBeAg, anti-HBe and anti-HBc) were quantified with Light Initiated Chemiluminescence Analyzing system (Bo Yang), while the HBV DNA viral load was determined by StepOnePlus system (KHB). For HBsAg detection by G12-based ELISA, 96-well plates (Thermo Fisher) were coated at 4°C for 16 hours with G12 diluted 1:10,000 in carbonate sodium buffer (pH9.6). The plates were washed with PBST, blocked at 37°C for 2 hours with 5% skimmed milk dissolved in PBST. Serum samples at 1:10 dilution as well as reference positive and negative controls were added to the plates, which were incubated at 37°C for 1 hour. After another wash, HRP-conjugated anti-HBs antibody (KHB) was added and incubation continued at 37°C for 1 hour. Tetramethylbenzidine was added followed by a 15-minute incubation. The reaction was stopped by H2SO4 and the absorbance values at 450 nm and 630 nm were measured.
Two serum samples that failed to be detected by G12-based ELISA and 4 samples with weak signals were analyzed further. DNA was extracted from 200 μl of serum samples using the QIAmp DNA Mini kit, and dissolved in 50 μl TE buffer. DNA (10 μl) was subjected to 35 cycles of PCR amplification using PrimeSTAR DNA polymerase from Takara, sense primer ATTGGATCCATGGAGAACATCGC (S region start codon in bold) and antisense primer CGCGAATTCTTAAATGTATACCC (S region stop codon in bold). The PCR product was purified and sequenced directly.
G12 detection of HBsAg from different HBV genotypes
One.3 copies of the HBV genome (1.3mer) of 7 HBV genotypes (A-F, H) were chemically synthesized based on sequences from GenBank (accession numbers AP007263, AB540582, AB540583, AB267090, AP007262, AB214516, AB298362) and inserted to pUC18 vector. Huh7 cells were cultured at 37°C with 5% carbon dioxide in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin, 100μg/ml streptomycin and 0.03% L-glutamine. Cells seeded in a 24-well plate were co-transfected with 0.4μg of the 1.3 mer plasmids and 0.1μg of luciferase reporter plasmid using TurboFect Transfection Reagent (Thermo Fisher Scientific). Cells were fixed 48 hours later with 4% formaldehyde and permeabilized with PBST buffer. After incubation at room temperature for 2 hours with 1:500 diluted G12 in 1% BSA/PBS, cells were further incubated with 1:1000 dilution of FITC-labeled goat anti-human IgG. Cells were stained with DAPI reagent for 5 min, and viewed under the microscope. Cells transfected with pUC18 DNA served as a negative control. HBsAg released to culture supernatant was detected by ELISA using G12-coated plates, similar to the detection of HBsAg in serum samples described above. Culture supernatant was diluted 1:5 with PBS before testing.
Mapping of the G12 epitope
The S gene of HBV isolate C8 (GenBank accession number AF461363) was cloned to pcDNA3.1-flag vector to express the full-length S protein of 226 residues. From this parental construct 38 deletion mutants with consecutive 6-aa deletions extending from the N-terminus were made using a mutagenesis kit (Toyobo). The parental construct and deletion mutants (1 μg) were transfected to Huh7 cells grown in 24-well plates. Culture supernatant harvested 48 hours later was diluted 1:5 for S protein detection by G12 based ELISA in comparison with the commercial ELISA kit (KHB). In addition, cells were lysed with 100 μl of RIPA buffer for S protein detection by ELISA without dilution.
Cell culture systems of HBV infection and neutralization by G12
HepaRG cells were cultured according to the established protocol.18 Cells were grown in 96-well plates in William's E medium supplemented with cortisone and insulin. Once the cells reached confluency, they were further cultured in above medium supplemented with 2% DMSO for a minimum of 2 weeks to stimulate cell differentiation. The establishment of HepG2 cell line stably transfected with human NTCP will be described elsewhere (Li et al., manuscript in preparation). HBV inoculum was concentrated from culture supernatant of HepDE19 cell line21 by incubation with PEG (5% final concentration) at 4°C overnight followed by centrifugation at 14,000 rpm for 30 min. For infection experiments, cells were incubated overnight with the viral inoculum (M.O.I. of 500) alone or together with various dilutions of G12 or HBIG (from Chengdu Rongsheng Bioproduct Company, with a protein concentration of 144 mg/ml), with 4% PEG present during virus infection. Medium was changed every 2 or 3 days, and HBeAg was measured at 1 week post infection using Diagnostic Kit for Hepatitis B e antigen (ELISA) (KHB).
G12 treatment of HBV transgenic mice
The experiment was performed on HBV transgenic mouse line 59 previously established in our lab.22,23 Blood was collected from retinal venous plexus of the 7-week old mice and serum HBsAg levels were measured by the ELISA kit from KHB. Serum samples were diluted 1:100 with PBS and tested in triplicate. The HBsAg titers in IU were calculated by charting the OD 450 nm values against a curve generated by serial dilutions of an HBsAg standard (Beijing Jinhao Pharmaceutics; stock concentration at 2 IU/ml). When the absorbance value fell outside the upper limit of the standard curve, the 1:100 diluted sample was further diluted 1:10 for another measurement. Twelve mice with descending HBsAg titers were divided into the PBS group (5 mice) and G12 group (7 mice) as detailed in the Results section. The mice were injected intraperitoneally with 0.5 ml of PBS (5 mice) or 600 IU (214 μg) of G12 in PBS (7 mice), respectively, 34 d later. Blood samples were collected at specified time points from retinal venous plexus and subjected to ELISA detection of HBsAg using the KHB kit. Finally, all samples (including the pretreatment samples) at 1:50 or 1:100 dilution were further quantified for HBsAg levels by a chemiluminescent microparticle enzyme immunoassay (CMIA, Abbott) at the Clinical Virology Laboratory, Ruijin Hospital, School of Medicine, Shanghai Jiaotong University. The values calculated for the undiluted samples were very similar from the 2 methods and only values from the Abbott assay were presented.
Anti-HBs in mouse sera was detected by the Diagnostic Kit for Antibody to Hepatitis B Surface Antigen (ELISA) (KHB), using 1:100 or 1:1,000-fold diluted samples for samples collected at 5hr, 1, 2, 5, 8, 17, and 47 d post-injection with G12. A standard curve was generated using HBIG diluted to 0.2 IU/ml, as well as 6 2-fold serial dilutions from that concentration. No anti-HBs could be detected at the pre-exposure (−34 day) samples as well as samples collected at day 87 and 144, even at 1:10 dilution.
Statistical analysis
Pearson's correlation coefficient was used to measure the linear correlation between variables. Independent-sample t test was used to compare the difference between samples. Kruskal-Wallis test was used to analyze ordinal categorical data.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest were disclosed.
Acknowledgments
We would like to thank Dr. Haitao Guo, Indiana University, for kindly providing the HepDE19 cell line. This work was supported by China National Science and Technology Major Project for Infectious Diseases (2012 ZX10002006 and 2014 ZX09101046-004), National Natural Science Foundation of China (81371822), and by the National Institutes of Health, USA (R21AI107618).
References
- 1.Gerlich WH. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Med Microbiol Immun 2015; 204:39-55; http://dx.doi.org/ 10.1007/s00430-014-0373-y [DOI] [PubMed] [Google Scholar]
- 2.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al.. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis 2009; 200:39-47; PMID:19469708; http://dx.doi.org/ 10.1086/599332 [DOI] [PubMed] [Google Scholar]
- 3.Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 2004; 350:1118-29; PMID:15014185; http://dx.doi.org/ 10.1056/NEJMra031087 [DOI] [PubMed] [Google Scholar]
- 4.Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 2007; 46:1759-68; PMID:18046710; http://dx.doi.org/ 10.1002/hep.21896 [DOI] [PubMed] [Google Scholar]
- 5.Leistner CM, Gruen-Bernhard S, Glebe D. Role of glycosaminoglycans for binding and infection of hepatitis B virus. Cell Microbiol 2008; 10:122-33; PMID:18086046; http://dx.doi.org/10.1111/j.1462-5822.2007.01023.x [DOI] [PubMed] [Google Scholar]
- 6.Sureau C, Salisse J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus a-determinant. Hepatology 2013; 57:985-94; PMID:23161433; http://dx.doi.org/ 10.1002/hep.26125 [DOI] [PubMed] [Google Scholar]
- 7.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, et al.. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012; 1:e49; http://dx.doi.org/ 10.7554/eLife.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, Stindt J, Koniger C, Nassal M, Kubitz R, et al.. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014; 146:1070-83; PMID:24361467; http://dx.doi.org/ 10.1053/j.gastro.2013.12.024 [DOI] [PubMed] [Google Scholar]
- 9.Neurath AR, Seto B, Strick N. Antibodies to synthetic peptides from the preS1 region of the hepatitis B virus (HBV) envelope (env) protein are virus-neutralizing and protective. Vaccine 1989; 7:234-6; PMID:2476893; http://dx.doi.org/ 10.1016/0264-410X(89)90235-1 [DOI] [PubMed] [Google Scholar]
- 10.Eren R, Ilan E, Nussbaum O, Lubin I, Terkieltaub D, Arazi Y, Ben-Moshe O, Kitchinzky A, Berr S, Gopher J, et al.. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology 2000; 32:588-96; PMID:10960454; http://dx.doi.org/ 10.1053/jhep.2000.9632 [DOI] [PubMed] [Google Scholar]
- 11.Glebe D, Aliakbari M, Krass P, Knoop EV, Valerius KP, Gerlich WH. Pre-s1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J Virol 2003; 77:9511-21; PMID:12915565; http://dx.doi.org/ 10.1128/JVI.77.17.9511-9521.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andre FE, Zuckerman AJ. Review: protective efficacy of hepatitis B vaccines in neonates. J Med Virol 1994; 44:144-51; PMID:7852954; http://dx.doi.org/ 10.1002/jmv.1890440206 [DOI] [PubMed] [Google Scholar]
- 13.Ogata N, Ostberg L, Ehrlich PH, Wong DC, Miller RH, Purcell RH. Markedly prolonged incubation period of hepatitis B in a chimpanzee passively immunized with a human monoclonal antibody to the a determinant of hepatitis B surface antigen. Proc Natl Acad Sci U S A 1993; 90:3014-8; PMID:8464917; http://dx.doi.org/ 10.1073/pnas.90.7.3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijtink R, Paulij W, van Bergen P, van Roosmalen M, Rohm D, Eichentopf B, Muchmore E, de Man R, Osterhaus A. In vivo activity of a mixture of two human monoclonal antibodies (anti-HBs) in a chronic hepatitis B virus carrier chimpanzee. J Gen Virol 1999; 80 (Pt 6):1529-35; PMID:10374972; http://dx.doi.org/ 10.1099/0022-1317-80-6-1529 [DOI] [PubMed] [Google Scholar]
- 15.Walter E, Keist R, Niederost B, Pult I, Blum HE. Hepatitis B virus infection of tupaia hepatocytes in vitro and in vivo. Hepatology 1996; 24:1-5; PMID:8707245; http://dx.doi.org/10.1002/hep.510240101 [DOI] [PubMed] [Google Scholar]
- 16.Dandri M, Burda MR, Torok E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, et al.. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology 2001; 33:981-8; PMID:11283864; http://dx.doi.org/ 10.1053/jhep.2001.23314 [DOI] [PubMed] [Google Scholar]
- 17.Chisari FV, Pinkert CA, Milich DR, Filippi P, McLachlan A, Palmiter RD, Brinster RL. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science 1985; 230:1157-60; PMID:3865369; http://dx.doi.org/ 10.1126/science.3865369 [DOI] [PubMed] [Google Scholar]
- 18.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A 2002; 99:15655-60; PMID:12432097; http://dx.doi.org/ 10.1073/pnas.232137699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu CJ, Lok AS. Clinical significance of hepatitis B virus genotypes. Hepatology 2002; 35:1274-6; PMID:11981779; http://dx.doi.org/ 10.1053/jhep.2002.33161 [DOI] [PubMed] [Google Scholar]
- 20.Norder H, Courouce AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, Robertson BH, Locarnini S, Magnius LO. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology 2004; 47:289-309; PMID:15564741; http://dx.doi.org/ 10.1159/000080872 [DOI] [PubMed] [Google Scholar]
- 21.Guo H, Jiang D, Zhou T, Cuconati A, Block TM, Guo JT. Characterization of the intracellular deproteinized relaxed circular DNA of hepatitis B virus: an intermediate of covalently closed circular DNA formation. J Virol 2007; 81:12472-84; PMID:17804499; http://dx.doi.org/ 10.1128/JVI.01123-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren J, Wang L, Chen Z, Ma ZM, Zhu HG, Yang DL, Li XY, Wang BI, Fei J, Wang ZG, et al.. Gene expression profile of transgenic mouse kidney reveals pathogenesis of hepatitis B virus associated nephropathy. J Med Virol 2006; 78:551-60; PMID:16555286; http://dx.doi.org/ 10.1002/jmv.20575 [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, Fang CY, Tian XC, Wang L, Yang PY, Wen YM. Proteomic analysis of hepatitis B surface antigen positive transgenic mouse liver and decrease of cyclophilin A. J Med Virol 2007; 79:1478-84; PMID:17705187; http://dx.doi.org/ 10.1002/jmv.20945 [DOI] [PubMed] [Google Scholar]
- 24.Dimitrov DS, Marks JD. Therapeutic antibodies: current state and future trends–is a paradigm change coming soon? Methods Mol Biol 2009; 525:1-27; PMID:19252861; http://dx.doi.org/ 10.1007/978-1-59745-554-1_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2004; 2:695-703; PMID:15372080; http://dx.doi.org/ 10.1038/nrmicro974 [DOI] [PubMed] [Google Scholar]
- 26.Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol 2007; 25:1421-34; PMID:18066039; http://dx.doi.org/ 10.1038/nbt1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov 2006; 5:147-59; PMID:16424916; http://dx.doi.org/ 10.1038/nrd1957 [DOI] [PubMed] [Google Scholar]
- 28.Ryu CJ, Gripon P, Park HR, Park SS, Kim YK, Guguen-Guillouzo C, Yoo OJ, Hong HJ. In vitro neutralization of hepatitis B virus by monoclonal antibodies against the viral surface antigen. J Med Virol 1997; 52:226-33; PMID:9179773; http://dx.doi.org/ 10.1002/(SICI)1096-9071(199706)52:2%3c226::AID-JMV18%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 29.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8:34-47; PMID:18064051; http://dx.doi.org/ 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 30.Vogelpoel LT, Baeten DL, De Jong EC, Den Dunnen J. Control of cytokine production by human fc gamma receptors: implications for pathogen defense and autoimmunity. Front Immunol 2015; 6:79; PMID:25759693; http://dx.doi.org/ 10.3389/fimmu.2015.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Curr Opin Biotechnol 2009; 20:685-91; PMID:19896358; http://dx.doi.org/ 10.1016/j.copbio.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 32.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol 2006; 6:343-57; PMID:16622479; http://dx.doi.org/ 10.1038/nri1837 [DOI] [PubMed] [Google Scholar]
- 33.Kashyap AK, Steel J, Oner AF, Dillon MA, Swale RE, Wall KM, Perry KJ, Faynboym A, Ilhan M, Horowitz M, et al.. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci U S A 2008; 105:5986-91; PMID:18413603; http://dx.doi.org/ 10.1073/pnas.0801367105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L, Lu X, Li C, Wang M, Liu Q, Li Z, Hu X, Li J, Liu F, Li Q, et al.. Generation, characterization and epitope mapping of two neutralizing and protective human recombinant antibodies against influenza A H5N1 viruses. PLoS One 2009; 4:e5476; PMID:19421326; http://dx.doi.org/ 10.1371/journal.pone.0005476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbas CR, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A 1991; 88:7978-82; PMID:1896445; http://dx.doi.org/ 10.1073/pnas.88.18.7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbas CR, Burton DR. Selection and evolution of high-affinity human anti-viral antibodies. Trends Biotechnol 1996; 14:230-4; PMID:8771795; http://dx.doi.org/ 10.1016/0167-7799(96)10029-9 [DOI] [PubMed] [Google Scholar]
- 37.Liang M, Dubel S, Li D, Queitsch I, Li W, Bautz EK. Baculovirus expression cassette vectors for rapid production of complete human IgG from phage display selected antibody fragments. J Immunol Methods 2001; 247:119-30; PMID:11150543; http://dx.doi.org/ 10.1016/S0022-1759(00)00322-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


