Abstract
Maintenance of corneal hydration is dependent on the active transport properties of the corneal endothelium. We tested the hypothesis that lactic acid efflux, facilitated by buffering, is a component of the endothelial fluid pump. Rabbit corneas were perfused with bicarbonate-rich (BR) or bicarbonate-free (BF) Ringer of varying buffering power, while corneal thickness was measured. Perfusate was collected and analyzed for lactate efflux. In BF with no added HEPES, the maximal corneal swelling rate was 30.0 ± 4.1 μm/h compared with 5.2 ± 0.9 μm/h in BR. Corneal swelling decreased directly with [HEPES], such that with 60 mM HEPES corneas swelled at 7.5 ± 1.6 μm/h. Perfusate [lactate] increased directly with [HEPES]. Similarly, reducing the [HCO3−] increased corneal swelling and decreased lactate efflux. Corneal swelling was inversely related to Ringer buffering power (β), whereas lactate efflux was directly related to β. Ouabain (100 μM) produced maximal swelling and reduction in lactate efflux, whereas carbonic anhydrase inhibition and an monocarboxylic acid transporter 1 inhibitor produced intermediate swelling and decreases in lactate efflux. Conversely, 10 μM adenosine reduced the swelling rate to 4.2 ± 0.8 μm/h and increased lactate efflux by 25%. We found a strong inverse relation between corneal swelling and lactate efflux (r = 0.98, P < 0.0001). Introducing lactate in the Ringer transiently increased corneal thickness, reaching a steady state (0 ± 0.6 μm/h) within 90 min. We conclude that corneal endothelial function does not have an absolute requirement for bicarbonate; rather it requires a perfusing solution with high buffering power. This facilitates lactic acid efflux, which is directly linked to water efflux, indicating that lactate flux is a component of the corneal endothelial pump.
Keywords: corneal endothelium, water transport, buffering power, lactate flux, monocarboxylic acid transporters
the notion of a bicarbonate-carbonic anhydrase and monocarboxylic acid transporter (MCTs) metabolon (12) has been suggested as a means to facilitate lactic acid flux. Linkage among bicarbonate buffering, carbonic anhydrases, and lactate transport has been shown in several systems, including retinal pigment epithelium (1, 29, 37), muscle (20, 70), astrocytes (60, 64), tumors (18, 31), oocyte expression systems (4, 5), and cornea (8, 43, 63). Potential coupling of lactate flux or gradients with water has been suggested in a few cases such as retinal pigment epithelium (2, 21), jejunum (68), kidney (25), and cornea (47, 48). In this study, we examine the role of bicarbonate-carbonic anhydrase buffering in facilitating lactate efflux from the cornea and the potential coupling to water flux.
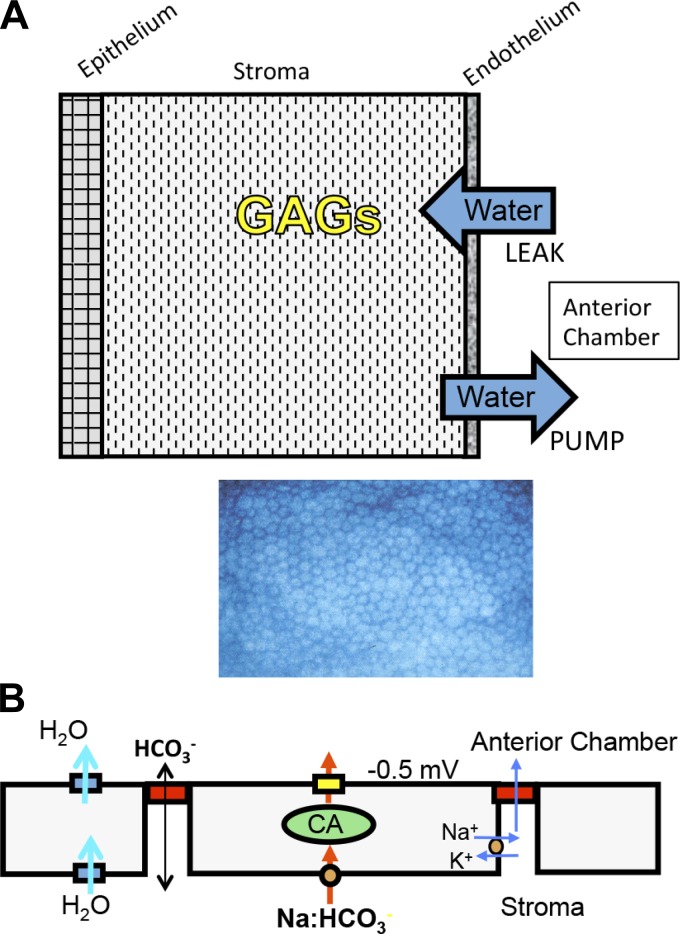
Optical transparency of the cornea is dependent on maintenance of corneal stromal hydration, which is controlled by the active transport properties of the corneal endothelium, often called the “endothelial pump.” The pump compensates for a passive “leak,” which is driven by highly charged stromal glycosaminoglycans that exert a tissue swelling pressure of about −60 mmHg at normal tissue hydration (3.5 mgH2O/mg dry wt) (22–24, 34). When the pump and leak are similar, corneal hydration remains within a small range (see Fig. 1A). Corneal endothelial dystrophies, which are characterized by cell degeneration, transport inhibition, and/or disruption of the endothelial monolayer, lead to corneal edema, light scatter, and loss of visual acuity.
Fig. 1.
Schematic diagrams illustrating the corneal endothelial pump. A: schematic of the anterior-posterior section of the cornea illustrating that stromal glycosaminoglycans (GAGs) exert a passive swelling pressure that drives water across the endothelium (also from the tears across the epithelium to a lesser extent), which is called the leak. The endothelial monolayer (en face view shown below) exerts an equal and opposite active transport-dependent driving force, called the pump, such that the hydration of the cornea is maintained at a level that minimizes light scatter. B: simplified model of the prevailing notion that the endothelial pump is a bicarbonate secretory mechanism where there is Na+-dependent HCO3− uptake at the basolateral membrane, facilitated by carbonic anhydrases (CA), and HCO3− secreted across the apical membrane (yellow box) by anion channels or transporters generating a small transendothelial voltage, driving Na+ across the tight junctions, yielding a small transendothelial NaHCO3− gradient that drives water osmotically and is facilitated by water channels.
Early studies have shown that the corneal endothelial pump is dependent on Na+-K+-ATPase activity and the presence of bicarbonate in the perfusing solution (3, 14, 16, 26, 55). Partial inhibition of the pump could be achieved by inhibition of secondary active transport processes by amiloride (41), DIDS (35, 52), and by carbonic anhydrase inhibitors (16, 26, 51). These studies and others led to the hypothesis that the corneal endothelial pump uses a bicarbonate secretory mechanism (see Fig. 1B) that produces small transendothelial osmotic gradients to drive water efflux. Efforts to identify these bicarbonate transport components have found strong basolateral (stromal side) bicarbonate uptake [e.g., Na+-2HCO3− cotransporter (NBCe1)] (28, 62, 69). However, apical bicarbonate permeability did not show any Na or Cl dependency and overall was found to be three times smaller than basolateral (8, 39), although apical bicarbonate permeability can be temporarily doubled via activation of cystic fibrosis transmembrane conductance regulator (CFTR) or calcium-activated chloride channel 1 (38, 61, 72). Attempts to measure net bicarbonate fluxes by the corneal endothelium, however, have been equivocal (44, 45). More recently, an alternative electro-osmotic model of the endothelial pump incorporating bicarbonate transport has also been put forth (13, 57, 58).
Interestingly, one report indicated that the endothelial pump could be supported in the absence of bicarbonate if high concentrations of Goods buffers were included in the perfusing solution (15). The authors noted that buffering capacity was important but did not suggest a mechanism. Their report was followed by two studies that purported to refute the findings, suggesting that bicarbonate was essential (36, 51). However, neither of these studies focused on the buffering power (β) of the perfusing solution, which we show in the current study is a key factor in supporting the endothelial pump.
The cornea is very glycolytic. Eighty-five percent of glucose consumed is converted to lactate (50). This happens predominately in the surface epithelium and stromal keratocytes, which have few mitochondria. For every 100 glucose molecules taken up by the cornea, 170 lactate molecules are produced. This sets up a large diffusion gradient with ∼13 mM lactate in the cornea and ∼7 mM lactate in the aqueous humor (7, 33, 54, 59). Corneal hypoxia is long known to increase corneal hydration (i.e., thickness), and this was shown to be due to increasing corneal [lactate] (33), demonstrating that lactate is an important osmolyte in the cornea. Lactate does not cross the epithelium so all of it must diffuse across the endothelium (33). Previous studies from our laboratory have shown that the presence of bicarbonate and carbonic anhydrase activity facilitates basolateral to apical lactate flux across corneal endothelium (47, 48) and that MCTs are involved in regulating corneal hydration in vivo (40). In the current study we test the hypothesis that bicarbonate in the perfusing solution, which serves as an artificial aqueous humor, is not necessary for maintenance of the corneal endothelial pump, but rather that it provides the β that facilitates lactic acid efflux, which is coupled to water efflux.
MATERIALS AND METHODS
Ethical approval.
Animal procedures were approved by the Indiana University Bloomington Institutional Animal Care and Use Committee. New Zealand White rabbits (Oakwood Research Facility, Oxford, MI), both males and females, age 8–12 wk, weighing ∼2.5 kg, were used for these studies. Animals were fed standard rabbit chow ad libitum. To collect corneas, animals were killed by the following procedure: rabbits were anesthetized via intramuscular injection of ketamine hydrochloride (60 mg/kg) and xylazine (5 mg/kg); depth was checked by toe pinch. This was followed 3–5 min later by intracardiac injection of pentobarbital sodium (100 mg/kg). All animal procedures were conducted in accordance with the Guide to the Care and Use of Laboratory Animals at Indiana University and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cornea dissection and mounting.
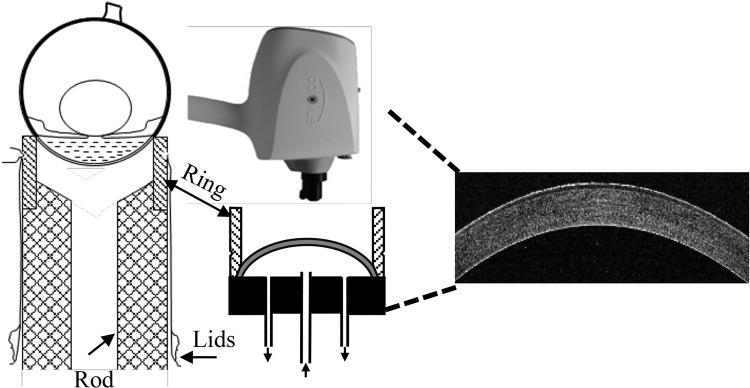
Isolation and atraumatic mounting of the cornea followed essentially the method of Dikstein and Maurice (14). Briefly, a circular incision was made in the lids around the margins of the orbit. Extraocular muscles and optic nerve were severed, and the eyeball was excised complete with conjunctiva and lids. The ocular surface with exposed cornea was placed face down on a plastic mounting ring that was pressed on a hollow methacrylate rod that was fixed to a stationary metal rod. The lids and conjunctiva were everted over the globe and pulled down tightly to the rod. The eye was then anchored in place by a suture tied over the everted conjunctiva and fit within a groove on the plastic mounting ring (Fig. 2). An incision was made with a scalpel blade a few millimeters posterior to the corneal-scleral junction, and the posterior sclera were removed with scissors. The vitreous, ciliary body-iris, and lens were carefully removed to reveal the corneal endothelial surface. The scleral rim remaining was reflected down over the ring. A metal platform was then placed from below over the plastic rod and pressed over the outside of the ring, which was against the anterior surface of the scleral rim. A plastic cap, to form an artificial anterior chamber, tightly sealed the posterior surface of the scleral rim. The plastic cap has three holes fitted with 23-gauge tubing. The central tube provided inflow, and the two side tubes provided outflow (see Fig. 2). The cornea chamber was fitted in a metal jacket with the epithelium facing up. The metal jacket had an embedded electric heater to maintain temperature at 37°C. A thermistor embedded in the posterior plastic cap indicated temperature equilibration with 10 min.
Fig. 2.
Left, initial arrangement of the eyeball on the mounting ring. Middle, after dissection the isolated cornea is perfused from the bottom and placed within a warming ring (not shown). An optical coherence tomography imager is placed above the cornea and produces cross-sectional images (right) at 5 μm resolution.
Perfusion and solutions.
The perfusion Ringer solution was driven in the corneal chamber by a micropump (P720 Peristaltic Pump, Plymouth Meeting, PA, www.instechlabs.com) at a constant speed of 50 μl/min. The two outflow tubes were joined behind the chamber, and the outflow tube was placed 20 cm above the epithelium to simulate an intraocular pressure of 15 mmHg. Each cornea was perfused for a period of 5 h. The perfusate was collected for 30-min intervals (1.5-ml aliquots), frozen, and assayed for lactate at a later time.
The corneal endothelial surface was perfused with glutathione bicarbonate Ringer (GBR), which contained (in mM): 110 NaCl, 2 KCl, 1 K2HPO4, 0.6 MgCl2, 1.4 calcium gluconate, 5 glucose, 0.3 reduced glutathione, 15 sodium gluconate, and 28.5 NaHCO3−, 5% CO2, pH 7.5. Ringer with varying [HCO3−] (5, 10, 18, 28.5, and 44 mM) was made by equimolar substitution with sodium gluconate. All bicarbonate solutions were equilibrated with 5% CO2, so pH was 6.75, 7.04, 7.30, 7.5, and 7.68, respectively. HCO3−-free Ringers (BF) were identical except NaHCO3− was substituted with equimolar sodium gluconate or Na-HEPES to achieve 0, 10, 25, 40, or 60 mM [HEPES], pH 7.5 and equilibrated with air. Osmolarity was measured with a vapor pressure osmometer (Wescor, Logan, UT) and adjusted to 295 mosmol/l with sucrose. GBR containing 28.5 mM HCO3− served as a standard control in all experiments. Perfusion Ringers solutions were maintained at 37°C in a water bath.
Corneas were mounted in pairs to maximize utility of tissue and minimize animal use. After being mounted, the corneas were perfused with standard GBR for 90 min. Corneas that showed accelerated swelling due to endothelial or epithelial damage were not included. After 90 min equilibration in standard GBR the perfusing solution was switched to experimental Ringer, and perfusion continued for 210 min. The epithelial surface was covered with the same medium as that in the perfusion and replaced every 15 min.
Corneal thickness.
The central corneal thickness (CT) was measured by optical coherence tomography using an iVue instrument (Optovue, Fremont, CA) that was mounted above the cornea (Fig. 2). At the 15-min interval, the epithelial Ringer solution (0.6 ml) was carefully aspirated, three corneal thickness measurements were taken and averaged, and fresh Ringer was replaced on the epithelial surface.
Corneal lactate retention.
After each 5-h perfusion, the cornea was removed from the chamber and trephined to a 10-mm central button that was snap-frozen in liquid N2 and pulverized to powder using a ceramic mortar and pestle. The cornea powder was collected in a preweighed microcentrifuge tube, and 0.5 ml PBS was added, vortexed for 1 min, and centrifuged at 13,000 g for 15 min. The supernatant was collected and analyzed for lactate content. The remaining pellet was dried at 60°C within a vacuum centrifuge for 2 h and weighed. The lactate content (nmol) of the perfusate sample was collected every 30 min, and the corneal extracts (nmol/mg dry wt) were determined by an assay kit from BioVision Research Products (Milpitas, CA).
In some experiments, inhibitors were added to the standard medium as 0.1 mM ouabain (Sigma), 0.1 mM acetazolamide (Sigma), 0.01 mM MCT inhibitor (HY-13248; ChemExpress, Monmouth Junction, NJ; AR-C-155858, http://medchemexpress.com/AR-C155858.html), or 0.01 mM adenosine (Sigma).
Analysis of results.
Each experimental condition included a minimum of three corneas. The results are expressed as means ± SD and compared using t-test or regression analysis, as appropriate. Corneal thickness changes are reported as maximal rates (μm/h), which typically occurred between the 120- and 180-min perfusion period, or as total thickness change from 90 to 300 min.
RESULTS
HEPES buffer supports the endothelial pump.
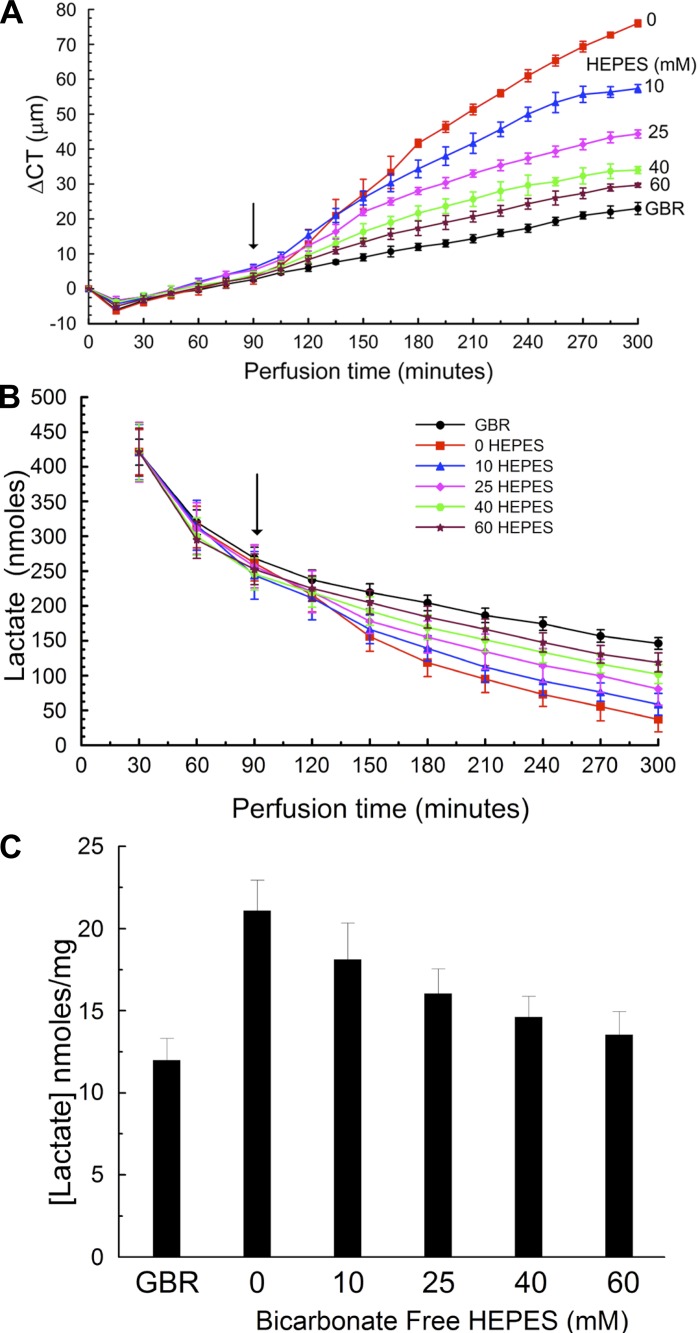
Figure 3A shows the general experimental sequence to test if BF Ringer could support corneal endothelial pumping by increasing the HEPES buffer concentration. After being mounted, corneas were perfused with the standard GBR Ringer for 90 min. During this equilibration time we observed a small decrease in CT followed by a slow increase. Decreases in CT indicate net water efflux, whereas increases in CT indicate net influx. When GBR perfusion was continued, CT increased at a fairly steady rate of 5.2 ± 0.9 μm/h, indicating that under the experimental conditions used the driving forces for water influx slightly exceeded those for water efflux. In contrast, when corneal perfusion was switched at 90 min to BF Ringer containing 0 HEPES, the CT swelling rate increased to 30.0 ± 4.1 μm/h. Interestingly, Fig. 3A also shows that the presence of HEPES in the perfusing Ringer (10, 25, 40, and 60 mM) decreased the maximum observed swelling rate to 22 ± 3.2, 13 ± 2.7, 10 ± 2.0, and 7.5 ± 1.6 μm/h, respectively. Fitting the maximal swelling rate vs. [HEPES] to an exponential model yielded a significant correlation (swelling rate = a × e−b[HEPES] + c, r = 0.985, P = 0.0005). These results demonstrate that the endothelial pump can be supported in the absence of HCO3− and in the presence of increasing HEPES buffer concentration.
Fig. 3.
Bicarbonate-free HEPES buffer perfusion. A: corneal thickness (CT) change vs. time. All corneas were perfused with glutathione bicarbonate Ringer (GBR) for 90 min. At the arrow perfusion was switched to 0, 10, 25, 40, or 60 mM HEPES in the absence of bicarbonate. B: lactate content in 1.5-ml samples of perfusion effluent. C: lactate remaining in the cornea after 5 h of perfusion. Data from 18 corneas, n = 3 for each condition.
To test the hypothesis that lactic acid efflux across the endothelium is being buffered, we examined the [lactate] in the effluent solutions. Figure 3B shows that in general the [lactate] in the effluent decreased over the course of the experiment. However, the decrease was greatest with 0 HEPES perfusion and least with standard GBR. Total lactate efflux from 120 to 300 min was 751 ± 36, 856 ± 20, 983 ± 32, 1,084 ± 36, and 1,177 ± 21 nmol for 0, 10, 25, 40, and 60 mM HEPES, respectively. Fitting efflux vs. [HEPES] to an exponential model yielded a significant correlation [efflux = a × (1 − e−b[HEPES] + c), r = 0.976, P = 0.001]. Efflux was higher (1,325 ± 32 nmol) during GBR perfusion. These data indicate that the [lactate] of the cornea at the end of the experiment (lactate retention) should be lower in those with the greatest efflux. Figure 3C shows a progressive decrease in lactate retention from 0 to 60 mM HEPES with the least amount retained following perfusion with GBR. These data indicate that increasing HEPES buffer concentration facilitates lactate efflux across the endothelium.
Endothelial pump varies with bicarbonate concentration.
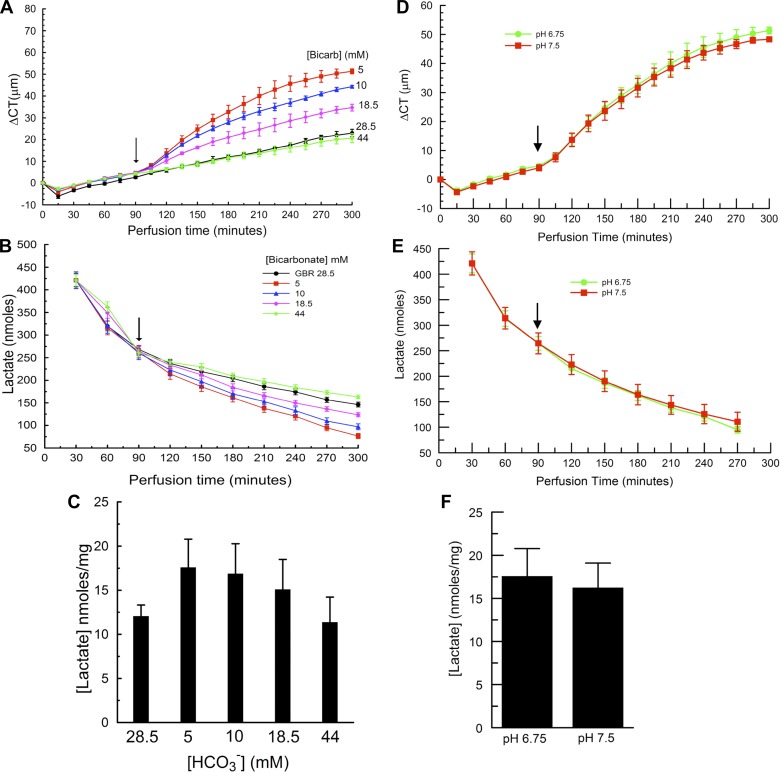
The perfusing Ringer β can also be varied by changing the [HCO3−]. Figure 4A shows that reducing [HCO3−] to 5, 10, and 18.5 mM produced maximal swelling rates of 20 ± 3.8, 13.7 ± 3.3, and 8.3 ± 2.8 μm/h, respectively, whereas increasing [HCO3−] to 44 mM yielded 4.3 ± 2.2 μm/h, relative to standard 28.5 mM GBR, 5.2 ± 0.9 μm/h. The swelling rate was significantly correlated with [HCO3−] (maximal swelling rate = a × e−b[HCO3−] + c, r = 0.967, P = 0.004). Figure 4B shows that lactate efflux was greatest at 44 mM and least at 5 mM [HCO3−]. Total lactate efflux from 120 to 300 min was 991 ± 14, 1,082 ± 21, 1,205 ± 36, 1,325 ± 32, and 1,397 ± 36 nmol for 5, 10, 18.5, 28.5, and 44 mM [HCO3−], respectively. Efflux was significantly correlated with [HCO3−] [efflux = a × (1 − e−b[HCO3−] + c), r = 0.956, P = 0.006]. Lactate retained in the cornea was greatest with 5 and least with 44 mM HCO3− (Fig. 4C). In the [HCO3−] experiments, the pH of the perfusing solutions varied because they were all equilibrated with 5% CO2. To test that the results were not simply a reflection of varying pH, we repeated the most acidic pH perfusion (5 mM [HCO3−], 5% CO2, pH 6.75) and paired it with perfusion of 5 mM [HCO3−], pH 7.5. We achieved pH 7.5 by bubbling with 5% CO2 while measuring the pH of the Ringer. When the pH of the bicarbonate solution decreased to 7.5, bubbling was stopped. The solution pH was then checked every 10 min, and bubbling recommenced and halted as needed to keep the pH at 7.5 ± 0.03. From the Henderson-Hasselbach equation, 5 mM HCO3−, pH 7.5, would contain 0.9% CO2. When the experiment reached the 90-min point, this solution was perfused. Figure 4, D–F, shows that the corneal swelling rates, lactate efflux, and lactate retention were not significantly different at pH 6.75 and 7.5 (paired t-tests, P > 0.05). The absence of a major effect of pH is in agreement with previous work (15). In summary, these data indicate that corneal swelling decreases, while lactate efflux increases, with increasing [HCO3−].
Fig. 4.
Bicarbonate-rich perfusion. A: CT change vs. time. All corneas were perfused with GBR for 90 min. At the arrow perfusion was switched to 5, 10, 18.5, or 44 mM bicarbonate. B: lactate content in 1.5-ml samples of perfusion effluent. C: lactate remaining in the cornea after 5 h of perfusion. D: CT change vs. time, comparing perfusion with 5 mM HCO3− at pH 7.5 and 6.75. E: lactate content in 1.5-ml samples of perfusion effluent from experiment in D. F: lactate retained in corneas from experiment in D. Data are from 21 corneas, n = 3 for each condition.
Corneal hydration and lactate efflux facilitated by solution β.
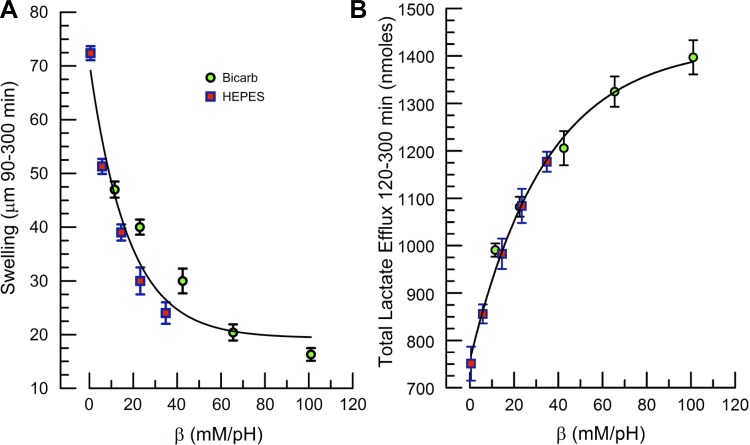
The data presented in Figs. 3 and 4 indicate that bicarbonate perfusion is not necessary to maintain the endothelial pump but that β is important. Figure 5, A and B, plots total corneal swelling between 90 and 300 min and lactate efflux over the 120- to 300-min period vs. β. β (β mM/pH) of HEPES at pH 7.5 (pK 7.5) was assumed to be 0.58 × [HEPES] and bicarbonate β = 2.3 × [HCO3−] (30, 56). Corneal swelling decreased exponentially with increasing β (swelling = a × e−b[β] + c, r = 0.969, P = 0.0005). The HEPES and bicarbonate data partially overlapped and were relatively similar. Conversely, total lactate efflux from 120 to 300 min increased exponentially with β, and there was good overlap between HEPES and bicarbonate [efflux = a × (1 − e−b[β] + c), r = 0.99, P = 0.0001]. These data indicate that the β of the perfusing solution has a strong influence on corneal hydration and lactate efflux. Facilitating lactate efflux reduces corneal hydration.
Fig. 5.
Effect of solution buffering power (β) on CT and total lactate efflux. A: CT change (swelling) from 90 to 300 min vs. β for bicarbonate-rich and HEPES-buffered perfusion. Line is fit to exponential model (swelling = a × e−b[β] + c, r = 0.969, P = 0.0005). B: total lactate flux over 120–300 min vs. β. Line is fit to exponential model [efflux = a × (1 − e−b[β] + c), r = 0.99, P = 0.0001].
Lactate flux and corneal thickness linked during endothelial pump modulation.
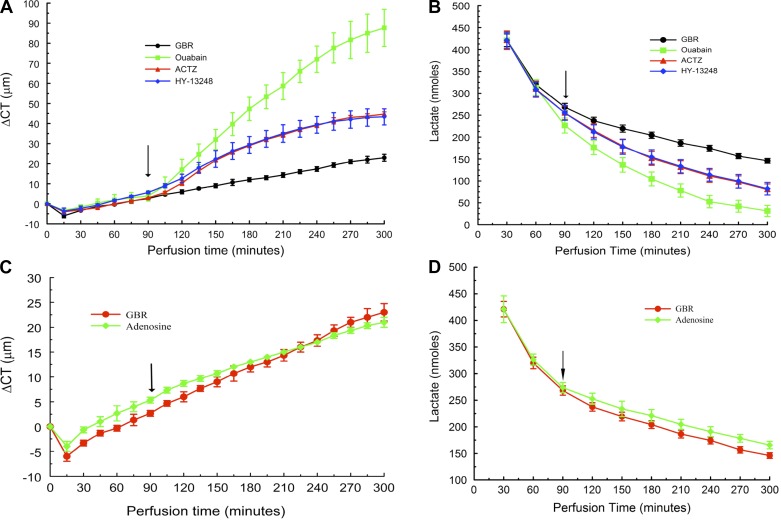
Next, we examined other conditions that affect the corneal endothelial pump and measured concomitant lactate fluxes. The Na+-K+-ATPase inhibitor ouabain causes the maximum amount of corneal swelling of all the transport inhibitors that have been tested (55). Figure 6A shows that 0.1 mM ouabain induced 30.1 ± 4.1 μm/h maximal swelling and 88 ± 9.2 μm total swelling at 300 min compared with 5.2 ± 0.9 μm/h and 23 ± 1.7 μm for the GBR control. Figure 6B shows that lactate efflux decreased significantly with ouabain. Total lactate flux from 120 to 300 min was 621 ± 53 for ouabain compared with 1,324 ± 33 nmol for GBR over the 120- to 300-min time period. Lactate retention was 24.0 ± 4.4 nmol/mg with ouabain, two times that of the control. In the presence of ouabain, lactate efflux over the last 3 h decreased by a factor of 2.13 relative to control, and lactate retention, measured at the fifth hour, increased by a factor of 2.0 relative to the GBR control, indicating that lactate production by the cornea is not significantly altered by the presence of ouabain.
Fig. 6.
Effect of transport modulators on CT and total lactate efflux. A: CT change vs. time showing effects of 100 μM ouabain, 100 μM acetazolamide, and 10 μM HY-13248 (AR-C155858). B: lactate content of effluent samples. C: effect of 10 μM adenosine on CT. D: effect of adenosine on lactate efflux.
Carbonic anhydrase inhibitors (CAIs) are also known to produce moderate inhibition of the endothelial pump (16, 26, 51). CAIs also reduce bicarbonate buffering capacity (67) and thereby slow lactate-H+ cotransport (47, 64, 70). Figure 6A shows that 0.1 mM acetazolamide produced 16.8 μm/h maximal swelling and 47 ± 1.5 μm total swelling. Total lactate efflux (Fig. 6B) was 967 ± 47 nmol and lactate retention 17.6 ± 47 nmol/mg, commensurate with the intermediate level of pump inhibition.
Facilitated transport of lactate is via monocarboxylate lactate-H+ cotransporters (MCTs) (19). MCT1, -2, and -4 are expressed in corneal endothelium (40, 48). We used HY-13248 (AR-C155858, 10 μM) a relatively new and specific lactate-H+ cotransport inhibitor of MCT1 and -2 activity, but not MCT4 (49). Figure 6A shows that partial block of MCTs by HY-13248 produced 16.3 ± 2.0 μm/h maximal swelling and 43.3 ± 4.0 μm total swelling. Total lactate efflux (Fig. 6B) was reduced to 973 ± 46 nmol and lactate retention (Fig. 7C) was 16.3 ± 3.0 nmol/mg, indicating that lactate efflux occurs through MCTs and inhibition of MCTs slows water efflux.
Fig. 7.
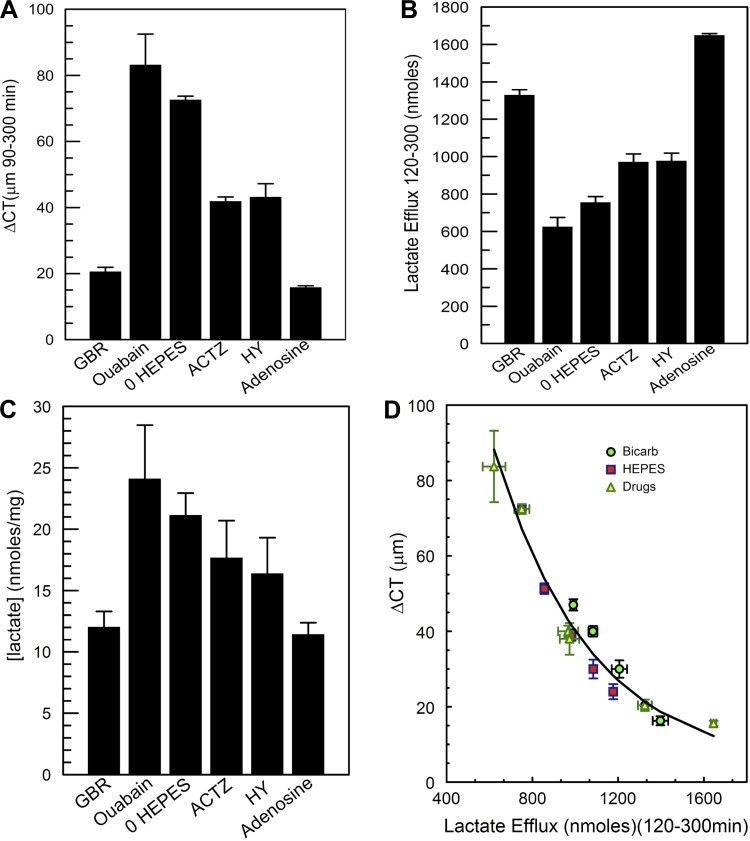
Summary of CT, total lactate efflux, and retention. A: comparison of GBR, bicarbonate-free (BF, 0 HEPES), acetazolamide-CA inhibition (ACTZ), monocarboxylic acid transporter (MCT) inhibition (HY), and adenosine perfusion on CT change between 90 and 300 min of perfusion. B: comparison of total lactate efflux between 120 and 300 min of perfusion. C: comparison of lactate retention in the cornea at the end of each experiment. D: regression analysis for all experiments [BF and bicarbonate-rich (BR) perfusion, ouabain, acetazolamide, HY-13248, and adenosine; ΔCT vs. lactate efflux, ΔCT = ae−b × efflux + c, r = 0.98, P < 0.0001]. Symbols show means ± SD of each experiment.
Conversely, increasing endothelial [cAMP] is known to produce a modest stimulation of the endothelial pump (17, 52). Figure 6C shows that 10 μM adenosine reduced the maximal swelling rate in GBR from 5.2 ± 0.9 to 4.2 ± 0.8 μm/h (t-test, P = 0.025). Moreover, Fig. 6D shows that total lactate efflux was 1,648 ± 13 nmol with adenosine, which was significantly greater than with GBR (1,324 ± 33 nmol, independent t-test, P = 0.001).
Figure 7 summarizes the ΔCT, total lactate efflux, and lactate retention data due to these transport modifiers and compares them with GBR control and 0 HEPES perfusion. The data clearly indicate that blocking primary active transport causes: 1) maximal corneal swelling (Fig. 7A), 2) the least lactate efflux (Fig. 7B), and 3) the most lactate retention (Fig. 7C). This compares closely with the effects of 0 HEPES perfusion, which has the lowest buffering capacity (<1 mM/pH due to phosphate). Intermediate levels of swelling show intermediate inhibition of lactate flux and lactate retention, whereas stimulation of the pump with adenosine reduces swelling with maximal lactate efflux and the least lactate retention. Figure 7D plots the ΔCT vs. lactate efflux for all experiments (BF and bicarbonate-rich perfusion, ouabain, acetazolamide, HY-13248, and adenosine) and also shows a fit of the data to an exponential model (ΔCT = ae−b × efflux + c, r = 0.98, P < 0.0001), indicating a strong association between water flux and lactate flux.
Lactate in perfusing Ringer stabilizes corneal thickness.
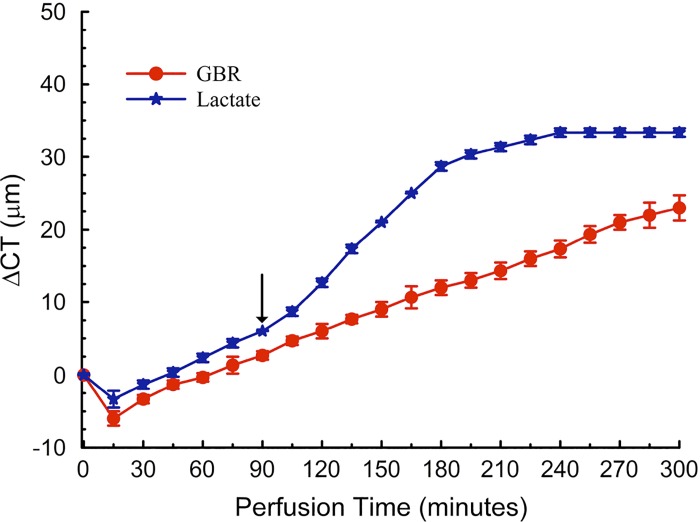
In all cases, the [lactate] gradient from cornea to perfusate was infinite since the perfusing Ringer has zero lactate. Although this has been the customary perfusion setup, the [lactate] of the aqueous humor in vivo is about 5–10 mM (7, 54, 59). If lactate efflux is linked to water efflux, then adding lactate to the perfusing Ringer will reduce the gradient, slow lactate efflux, and increase corneal thickness. To test this we switched GBR perfusion to include 5 mM sodium lactate (substituted for sodium gluconate). The osmolality of the solutions was the same. Figure 8 shows that, when lactate was introduced at 90 min, the corneas swelled. Within 90 min, however, corneal thickness reached a steady state with no swelling (0 ± 0.6 μm/h), whereas the corneas perfused without lactate have not reached a steady state. Because of the large [lactate] in the perfusing solution, efflux was not measured. Not surprisingly, lactate retention in the cornea was almost two times (22.2 ± 2.1 nmol/mg) that of the GBR control. These data confirm that lactate gradients affect corneal hydration and suggest that fixing the lactate concentration at the endothelial surface may stabilize the transendothelial lactate gradient, thereby slowing background swelling.
Fig. 8.
Effect of lactate in the perfusion Ringer. CT change vs. time for 5 mM lactate and GBR control. Switched to lactate containing Ringer at arrow.
DISCUSSION
The earlier study of Doughty and Maurice had suggested that the corneal endothelial pump can be supported by high concentrations of Good's buffers in the absence of bicarbonate (15). Although several studies appeared to refute this notion (36, 51), none focused on β as a potential perfusing solution property that could be the basis for the observations. The data presented here now show that endothelial pump activity can be supported in the absence of bicarbonate if sufficient solution β is present. A close examination of the study by Riley et al. (51) showed maximal swelling in a bicarbonate-free phosphate (9.6 mM)-buffered Ringer (β ∼5 mM/pH) and 25% less swelling with a 25 mM HEPES Ringer (β ∼12.5 mM/pH), consistent with our hypothesis that endothelial pump function varies directly with β. Endothelial pump function in vivo is maximally supported due to the presence of very robust β provided by the HCO3−-CO2-carbonic anhydrase buffering system.
We tested the hypothesis that lactic acid efflux was being buffered and that lactate efflux was linked to water efflux. Increasing β increased lactate efflux under bicarbonate or bicarbonate-free conditions (Figs. 3 and 4). Lactate efflux was directly related to β (Fig. 5B), whereas corneal swelling (leak > pump) was inversely related to β (Fig. 5A), indicating that lactic acid efflux was being buffered, and this was directly associated with water efflux. A similar relation between corneal swelling and lactate efflux was observed by inhibiting primary active transport with the Na+-K+-ATPase inhibitor ouabain, even though solution β was high, indicating that transendothelial lactate flux is dependent on active transport. We also show that the carbonic anhydrase inhibitor acetazolamide, which produces moderate corneal swelling, led to moderate decreases in lactate efflux. For the corneal endothelium, carbonic anhydrase enzymes are located on both basolateral (CA12) and apical (CA4) membranes and in the cytoplasm (CA2). Acetazolamide is cell permeable, so all isozymes were inhibited. Carbonic anhydrase inhibitors slow the hydration and dehydration of CO2, which in turn slows the consumption or production of H+ and lowers effective β. While buffering is facilitating lactic acid flux, MCTs provide the transporter route for transcellular flux. Partial inhibition of corneal endothelial MCTs by HY-13248, directly inhibiting MCT1 and MCT2 lactate-H+ cotransport (but not MCT4), slows lactate and water efflux concomitantly. Conversely, stimulating endothelial function by increasing cAMP decreased corneal swelling and increased lactate efflux. The excellent correlation between water flux (ΔCT) and lactate flux under all conditions tested (Fig. 7D) is strong evidence that lactate efflux is directly linked to water efflux.
Previous studies from our laboratory are consistent with this hypothesis. We have shown that bicarbonate and carbonic anhydrase activity supports lactate flux across cultured bovine corneal endothelial cells (43, 47). Lactate fluxes were inhibited by the carbonic anhydrase inhibitor acetazolamide, the anion transport inhibitor DIDS, and NBCe1-specific small-interfering RNA knockdown. Moreover, ouabain, DIDS, or carbonic anhydrase inhibition in the in vivo rabbit cornea produced corneal edema with a concomitant increase in corneal [lactate] (48). NBCe1 short-hairpin RNA (shRNA) in vivo produced variable knockdown, which overall showed no increase in CT (43). However, if topical 1% brinzolamide (a carbonic anhydrase inhibitor) was added, increased corneal edema was seen in those eyes that received the NBCe1 shRNA (43). When a subset of corneas that showed high levels of NBCe1 knockdown were analyzed, the ΔCT measured was associated with a concomitant increase in corneal [lactate] (48). These observations point out the robustness of the in vivo cellular bicarbonate buffering system. Last, shRNA silencing of CD147, the MCT1 and -4 chaperone, resulted in stromal lactate accumulation and corneal edema in the in vivo rabbit cornea (40), consistent with the actions of HY-13248 in the current study.
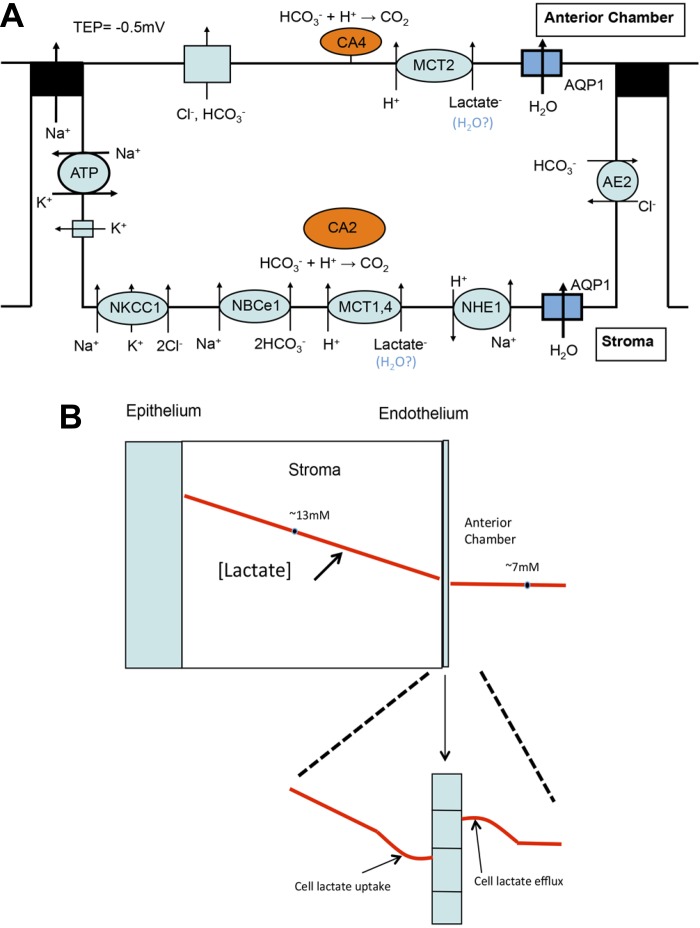
Whereas solution β supports endothelial pumping, active transport is also necessary. The Na+-K+-ATPase provides the Na+ gradient that drives Na+-2HCO3− cotransport and Na+/H+ exchange, two transporters that are essential for pHi regulation. Dysregulation of pHi will likely perturb basolateral to cellular as well as cellular to apical surface pH gradients, which in turn will inhibit lactate-H+ cotransporter fluxes at each membrane. In Fig. 9A, we show a transport model for transendothelial lactate flux across the corneal endothelium. At the basolateral (stromal side) membrane, MCT1 and -4 provide lactate-H+ cotransport influx. The influx of protons is buffered by bicarbonate and facilitated by CA2. NBCe1 provides for continual bicarbonate influx. At the apical membrane MCT2 provides lactate-H+ efflux. The efflux of protons is buffered by aqueous humor bicarbonate and facilitated by CA4. Apical anion channel activation, e.g., CFTR, by A2b receptor agonist adenosine induced increases in cAMP (66), can provide a modest boost in [HCO3−] at the apical surface (61, 63), enhancing lactic acid buffering.
Fig. 9.
Model for in vivo corneal endothelial lactate and water flux. A: cellular transport model: basolateral MCT1 and -4 provide lactate-H+ cotransport uptake facilitated by bicarbonate buffering [Na+-2HCO3− cotransporter (NBCe1) and CA enzymes in the cytoplasm (CA2)] and supported by the Na+/H+ exchanger (NHE1). Lactate efflux at the apical membrane is facilitated by carbonic anhydrase enzymes located on the apical membrane (CA4)-bicarbonate buffering. Transcellular water flux is facilitated by aquaporin 1 (AQP1). B: possible model for a transendothelial lactate standing gradient. Schematic cornea cross section showing lactate concentration gradient (red line), mean stromal lactate concentration ([lactate]) of 13 mM, and anterior chamber [lactate] of 7 mM. Expanded area below shows perturbation of lactate gradient in the basolateral space due to robust lactate-H+ uptake and in the apical unstirred layer due to lactate-H+ efflux, creating a transendothelial lactate gradient that drives water flux.
Our data show that other buffers at the apical surface can also facilitate lactate efflux. In the absence of bicarbonate, however, the basolateral buffering system, NBCe1-CA2, will be weakened. Because of the leakiness of the endothelial cell tight junctions (25 Ω·cm2), it is likely that HEPES can diffuse into the basolateral space to provide buffering. Nevertheless, cytosolic buffering would have to rely on Na+/H+ exchanger (NHE) 1 activity or other unidentified proton transporters where the ejected protons are buffered by HEPES. Previous studies have shown that the absence of bicarbonate lowers pHi in endothelial cells (10) and this will activate NHE1 (9, 65), providing additional cytosolic buffering. Moreover, it is possible that nonenzymatic H+ shuttling via carbonic anhydrases could facilitate transport activity of MCT1 and -4 (6) at the basolateral membrane and by CA4-MCT2 (32) at the apical membrane in the absence of bicarbonate.
Model for linking water flux to lactate.
In addition to lactate flux through transporters, there is potentially a small nonionic lactate flux and a larger paracellular flux, as illustrated by the continual efflux of lactate in the presence of ouabain. Also, our data show large decreases in lactate efflux concomitant with very little change in corneal thickness during the first 90 min of perfusion. This is most likely due to paracellular diffusion of stromal lactate driven by the infinite gradient for lactate across the endothelium. Presumably, it is the flux through the transporters that has the greatest potential for linkage to water flux. One possibility is direct coupling of water molecules with lactate by the MCTs, as has been suggested for the retinal pigment epithelium (21). Another possibility is the presence of a standing lactate gradient that drives water by osmosis across the cells and is facilitated by aquaporin 1. The cornea is a source of lactate, and the steady-state [lactate] is higher in the stroma than in the aqueous humor. To achieve the needed gradient to drive water osmotically, the apical surface [lactate] must be a little higher than the basolateral [lactate]. This can be realized if the basolateral influx of lactate is greater than the rate of diffusion in the stroma, creating a slight drop in [lactate] in the basolateral space. In addition, lactate efflux via MCT2 in the apical unstirred layer provides a slight increase in the local [lactate] such that the transendothelial lactate gradient is sufficient to drive water flux. This is shown in Fig. 9B. Adding lactate to the perfusing solution, at a level approximating that in vivo, had an interesting effect. As expected, it produced a transient increase in CT since this will slow lactate efflux from the cornea. However, within 90 min, CT stabilized such that CT did not change during the last hour of perfusion, whereas the control continued to show small increases in CT. This is consistent with the model in that lactate-free perfusion could reduce or destabilize apical unstirred layer lactate accumulation that helps drive water osmotically, whereas having a fixed amount of lactate in the bulk solution could stabilize the lactate gradient. The notion that production of lactate can produce a physiological osmotic gradient is not limited to the cornea. Lactate gradients have been suggested as a urine-concentrating mechanism within the renal inner medulla (25). Moreover, pathophysiological water flux induced by lactate production in brain edema has also been documented (11, 42, 46).
Of the three major anions, Cl−, HCO3−, and lactate, our data indicate that lactate is a component of the endothelial fluid pump mechanism. Whereas Cl− has been shown to accumulate within endothelial cells above electrochemical equilibrium through the action of Na+-K+-2Cl− cotransporter (NKCC1) (27), the lack of constitutively active apical Cl− channels or transporters and the fact that the NKCC1 inhibitor bumetanide had no effect on corneal hydration (53) ruled out a direct role for a Cl− secretory mechanism in the endothelial pump. A direct role for a HCO3− secretory mechanism is also ruled out since we show that HCO3− is not an absolute necessity for pump maintenance. While there is robust basolateral HCO3− influx via NBCe1 (62), the apical membrane HCO3− permeability is three times smaller and no Na- or Cl−-dependent HCO3− transport could be found (39). However, under conditions where apical anion channels are activated, a small apical HCO3− flux can be measured (71, 72). We show that apical anion channel activation by adenosine increases lactate efflux, suggesting that the apical HCO3− flux is buffering apical lactic acid. As for lactate, there are several remaining questions: 1) is lactate flux effective in de-epithelialized corneal preparations? Previous studies have shown that the corneal epithelium can be removed and replaced with silicone oil, and the stromal-endothelial preparation can maintain hydration (55, 58). This removes a major source of lactate production. Of course, lactate is still being produced by the stromal keratocytes and endothelial cells, but is that sufficient to form the needed concentration gradient or MCT-linked water flux? 2) is there a link between lactate and water flux directly via MCTs?; and 3) do the modeled basolateral to apical lactate gradients, as shown in Fig. 9B, exist?
In summary, this study has shown that perfusing solutions of high β can support the active transport properties of the corneal endothelial fluid pump. Lactic acid efflux across the endothelium is being buffered, and the lactate flux via MCTs is directly related to water flux. The system works well in vivo due to the presence of high aqueous humor bicarbonate concentrations, robust basolateral bicarbonate transport, and multi-isozyme carbonic anhydrase activity, which impart a high β. The data suggest that enhancing lactate efflux in patients with corneal endothelial dystrophies could improve corneal clarity and vision as long as an intact endothelial monolayer is present. Last, the results indicate that efforts to engineer an endothelial cell replacement that could substitute for traditional donor transplant tissue must have the requisite lactate-H+ cotransport activity to be effective.
GRANTS
This work was funded by National Eye Institute Grant RO1-EY-008834 to J. A. Bonanno.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L. and E.K. performed experiments; S.L., E.K., and J.A.B. analyzed data; S.L. and J.A.B. interpreted results of experiments; S.L. and J.A.B. prepared figures; S.L., E.K., and J.A.B. edited and revised manuscript; S.L., E.K., and J.A.B. approved final version of manuscript; J.A.B. conception and design of research; J.A.B. drafted manuscript.
REFERENCES
- 1.Adijanto J, Banzon T, Jalickee S, Wang NS, Miller SS. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol 133: 603–622, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adijanto J, Philp NJ. The SLC16A family of monocarboxylate transporters (MCTs)–physiology and function in cellular metabolism, pH homeostasis, and fluid transport. Curr Top Membr 70: 275–311, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Baum J, Maurice D, McCarey B. The active and passive transport of water across the corneal endothelium. Exp Eye Res 39: 335–342, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Becker HM, Broer S, Deitmer JW. Facilitated lactate transport by MCT1 when coexpressed with the sodium bicarbonate cotransporter (NBC) in Xenopus oocytes. Biophys J 86: 235–247, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker HM, Deitmer JW. Nonenzymatic proton handling by carbonic anhydrase II during H+-lactate cotransport via monocarboxylate transporter 1. J Biol Chem 283: 21655–21667, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Becker HM, Klier M, Schuler C, McKenna R, Deitmer JW. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc Natl Acad Sci USA 108: 3071–3076, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmanson JP, Johnsson J, Soderberg PG, Philipson BT. Lactate levels in the rabbit cornea and aqueous humor subsequent to non-gas permeable contact lens wear. Cornea 4: 173–176, 1985. [PubMed] [Google Scholar]
- 8.Bonanno J, Guan Y, Jelamskii S, Kang X. Apical and basolateral CO2-HCO3− permeability in cultured bovine corneal endothelial cells. Am J Physiol Cell Physiol 277: C545–C553, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. I. Na/H exchange in the absence and presence of HCO3−. Invest Ophthalmol Vis Sci 33: 3058–3067, 1992. [PubMed] [Google Scholar]
- 10.Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. II. Na:HCO3 cotransport and Cl/HCO3 exchange. Invest Ophthalmol Vis Sci 33: 3068–3079, 1992. [PubMed] [Google Scholar]
- 11.Bosoi CR, Zwingmann C, Marin H, Parent-Robitaille C, Huynh J, Tremblay M, Rose CF. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol 60: 554–560, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Deitmer JW, Theparambil SM, Ruminot I, Becker HM. The role of membrane acid/base transporters and carbonic anhydrases for cellular pH and metabolic processes. Front Neurosci 8: 430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diecke FP, Ma L, Iserovich P, Fischbarg J. Corneal endothelium transports fluid in the absence of net solute transport. Biochim Biophys Acta 1768: 2043–2048, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikstein S, Maurice DM. The active control of corneal hydration. Isr J Med Sci 8: 1523–1528, 1972. [PubMed] [Google Scholar]
- 15.Doughty MJ, Maurice D. Bicarbonate sensitivity of rabbit corneal endothelium fluid pump in vitro. Invest Ophthalmol Vis Sci 29: 216–223, 1988. [PubMed] [Google Scholar]
- 16.Fischbarg J, Lim J. Role of cations, anions, and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J Physiol 241: 647–675, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischbarg J, Lim J, Bourguet J. Adenosine stimulation of fluid transport across rabbit corneal endothelium. J Membr Biol 35: 95–112, 1977. [DOI] [PubMed] [Google Scholar]
- 18.Grillon E, Farion R, Fablet K, De Waard M, Tse CM, Donowitz M, Remy C, Coles JA. The spatial organization of proton and lactate transport in a rat brain tumor. PLoS One 6: e17416, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halestrap AP. Monocarboxylic acid transport. Compr Physiol 3: 1611–1643, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Hallerdei J, Scheibe RJ, Parkkila S, Waheed A, Sly WS, Gros G, Wetzel P, Endeward V. T tubules and surface membranes provide equally effective pathways of carbonic anhydrase-facilitated lactic acid transport in skeletal muscle. PLoS One 5: e15137, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Hamann S, Kiilgaard JF, la Cour M, Prause JU, Zeuthen T. Cotransport of H+, lactate, and H2O in porcine retinal pigment epithelial cells. Exp Eye Res 76: 493–504, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Hedbys B, Dohlman C. A new method for the determination of the swelling pressure of the corneal stroma in vitro. Exp Eye Res 2: 122–129, 1963. [DOI] [PubMed] [Google Scholar]
- 23.Hedbys B, Mishima S. The thickness-hydration relationship of the cornea. Exp Eye Res 5: 221–228, 1966. [DOI] [PubMed] [Google Scholar]
- 24.Hedbys B, Mishima S, Maurice D. The imbibition pressure of the corneal stroma. Exp Eye Res 2: 99–111, 1963. [DOI] [PubMed] [Google Scholar]
- 25.Hervy S, Thomas SR. Inner medullary lactate production and urine-concentrating mechanism: a flat medullary model. Am J Physiol Renal Physiol 284: F65–F81, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hodson S, Miller F. The bicarbonate ion pump in the endothelium which regulates the hydration of rabbit cornea. J Physiol 263: 563–577, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jelamskii S, Sun XC, Herse P, Bonanno JA. Basolateral Na+-K+-2Cl- cotransport in cultured and fresh bovine corneal endothelium. Invest Ophthalmol Vis Sci 41: 488–495, 2000. [PubMed] [Google Scholar]
- 28.Jentsch T, Korbmacher C, Janicke I, Fischer D, Stahl F, Helbig H, Hollwede H, Cragoe E, Keller S, Wiederholt M. Regulation of cytoplasmic pH of cultured bovine corneal endothelial cells in the absence and presence of bicarbonate. J Membr Biol 103: 29–40, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Kenyon E, Yu K, La Cour M, Miller SS. Lactate transport mechanisms at apical and basolateral membranes of bovine retinal pigment epithelium. Am J Physiol Cell Physiol 267: C1561–C1573, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Kim D, Liao J, Hanrahan JW. The buffer capacity of airway epithelial secretions. Front Physiol 5: 188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y, Choi JW, Lee JH, Kim YS. Expression of lactate/H(+) symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and The Cancer Genome Atlas data analyses. Hum Pathol 46: 104–112, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Klier M, Schuler C, Halestrap AP, Sly WS, Deitmer JW, Becker HM. Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II. J Biol Chem 286: 27781–27791, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klyce S. Stromal lactate accumulation can account for corneal oedema osmotically following epithelial hypoxia in the rabbit. J Physiol (Lond) 321: 49–64, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klyce S, Dohlman C, Tolpin D. In vivo determination of corneal swelling pressure. Exp Eye Res 11: 220–229, 1970. [DOI] [PubMed] [Google Scholar]
- 35.Kuang K, Li Y, Yiming M, Sanchez JM, Iserovich P, Cragoe EJ, Diecke FP, Fischbarg J. Intracellular [Na+], Na+ pathways, and fluid transport in cultured bovine corneal endothelial cells. Exp Eye Res 79: 93–103, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Kuang K, Xu M, Koniarek J, Fischbarg J. Effects of ambient bicarbonate, phosphate and carbonic anhydrase inhibitors on fluid transport across rabbit endothelium. Exp Eye Res 50: 487–493, 1990. [DOI] [PubMed] [Google Scholar]
- 37.la Cour M, Lin H, Kenyon E, Miller SS. Lactate transport in freshly isolated human fetal retinal pigment epithelium. Invest Ophthalmol Vis Sci 35: 434–442, 1994. [PubMed] [Google Scholar]
- 38.Li J, Allen KT, Sun XC, Cui M, Bonanno JA. Dependence of cAMP meditated increases in Cl- and HCO(3)- permeability on CFTR in bovine corneal endothelial cells. Exp Eye Res 86: 684–690, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Sun XC, Bonanno JA. Role of NBC1 in apical and basolateral HCO3− permeabilities and transendothelial HCO3− fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol 288: C739–C746, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Nguyen TT, Bonanno JA. CD147 required for corneal endothelial lactate transport. Invest Ophthalmol Vis Sci 55: 4673–4681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liebovitch LS, Fischbarg J. Effects of inhibitors of passive Na+ and HCO3− fluxes on electrical potential and fluid transport across rabbit corneal endothelium. Curr Eye Res 2: 183–186, 1982. [DOI] [PubMed] [Google Scholar]
- 42.Lindinger MI, Leung MJ, Hawke TJ. Inward flux of lactate(-) through monocarboxylate transporters contributes to regulatory volume increase in mouse muscle fibres. PLoS One 8: e84451, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu C, Cheng Q, Nguyen T, Bonanno JA. Knockdown of NBCe1 in vivo compromises the corneal endothelial pump. Invest Ophthalmol Vis Sci 51: 5190–5197, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maurice DM. Passive ion fluxes cross the corneal endothelium. Cur Eye Res 4: 339–349, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Mayes KR, Hodson S. Local osmotic coupling to the active trans-endothelial bicarbonate flux in the rabbit cornea. Biochim Biophys Acta 514: 286–293, 1978. [DOI] [PubMed] [Google Scholar]
- 46.Mori S, Morishima S, Takasaki M, Okada Y. Impaired activity of volume-sensitive anion channel during lactacidosis-induced swelling in neuronally differentiated NG108-15 cells. Brain Res 957: 1–11, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TT, Bonanno JA. Bicarbonate, NBCe1, NHE, and carbonic anhydrase activity enhance lactate-H+ transport in bovine corneal endothelium. Invest Ophthalmol Vis Sci 52: 8086–8093, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen TT and Bonanno JA. Lactate-H(+) transport is a significant component of the in vivo corneal endothelial pump. Invest Ophthalmol Vis Sci 53: 2020–2029, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ovens MJ, Manoharan C, Wilson MC, Murray CM, Halestrap AP. The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein. Biochem J 431: 217–225, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riley M. Glucose and oxygen utilization by the rabbit cornea. Exp Eye Res 8: 193–200, 1969. [DOI] [PubMed] [Google Scholar]
- 51.Riley M, Winkler B, Czajkowski C, Peters M. The roles of bicarbonate and CO2 in transendothelial fluid movement and control of corneal thickness. Invest Ophthalmol Vis Sci 36: 103–112, 1995. [PubMed] [Google Scholar]
- 52.Riley M, Winkler B, Starnes C, Peters M. Adenosine promotes regulation of corneal hydration through cyclic adenosine monophosphate. Invest Ophthalmol Vis Sci 37: 1–10, 1996. [PubMed] [Google Scholar]
- 53.Riley M, Winkler B, Starnes C, Peters M. Fluid and ion transport in corneal endothelium: insensitivity to modulators of Na-K-2Cl cotransport. Am J Physiol Cell Physiol 273: C1480–C1486, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Riley MV. Intraocular dynamics of lactic acid in the rabbit. Invest Ophthalmol 11: 600–607, 1972. [PubMed] [Google Scholar]
- 55.Riley MV, Winkler BS, Peters MI, Czajkowski CA. Relationship between fluid transport and in situ inhibition of Na(+)-K+ adenosine triphosphatase in corneal endothelium. Invest Ophthalmol Vis Sci 35: 560–567, 1994. [PubMed] [Google Scholar]
- 56.Roos A and Boron W. Intracellular pH. Physiol Rev 61: 297–432, 1981. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez JM, Cacace V, Kusnier CF, Nelson R, Rubashkin AA, Iserovich P, Fischbarg J. Net fluorescein flux across corneal endothelium strongly suggests fluid transport is due to electro-osmosis. J Membr Biol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez JM, Li Y, Rubashkin A, Iserovich P, Wen Q, Ruberti JW, Smith RW, Rittenband D, Kuang K, Diecke FP, Fischbarg J. Evidence for a central role for electro-osmosis in fluid transport by corneal endothelium. J Membr Biol 187: 37–50, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Schutte E, Schulz I, Reim M. Lactate and pyruvate levels in the aqueous humor and in the cornea after cyclodiathermy. Albrecht Von Graefes Arch Klin Exp Ophthalmol 185: 325–330, 1972. [DOI] [PubMed] [Google Scholar]
- 60.Stridh MH, Alt MD, Wittmann S, Heidtmann H, Aggarwal M, Riederer B, Seidler U, Wennemuth G, McKenna R, Deitmer JW, Becker HM. Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J Physiol 590: 2333–2351, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun XC, Bonanno JA. Expression, localization, and functional evaluation of CFTR in bovine corneal endothelial cells. Am J Physiol Cell Physiol 282: C673–C683, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun XC, Bonanno JA, Jelamskii S, Xie Q. Expression and localization of Na+-HCO3− cotransporter in bovine corneal endothelium. Am J Physiol Cell Physiol 279: C1648–C1655, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Sun XC, Li J, Cui M, Bonanno JA. Role of carbonic anhydrase IV in corneal endothelial HCO3− transport. Invest Ophthalmol Vis Sci 49: 1048–1055, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Svichar N, Chesler M. Surface carbonic anhydrase activity on astrocytes and neurons facilitates lactate transport. Glia 41: 415–419, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Takaichi K, Balkovetz DF, Van Meir E, Warnock DG. Cytosolic pH sensitivity of an expressed human NHE-1 Na+-H+ exchanger. Am J Physiol Cell Physiol 264: C944–C950, 1993. [DOI] [PubMed] [Google Scholar]
- 66.Tan-Allen KY, Sun XC, Bonanno JA. Characterization of adenosine receptors in bovine corneal endothelium. Exp Eye Res 80: 687–696, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas RC. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol 255: 715–735, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tosco M, Orsenigo MN, Gastaldi G, Faelli A. Protein kinase C regulation of rat jejunal transport system: mechanisms involved in lactate movement. Exp Physiol 87: 653–662, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Usui T, Seki G, Amano S, Oshika T, Miyata K, Kunimi M, Taniguchi S, Uwatoko S, Fujita T, Araie M. Functional and molecular evidence for Na(+)-HCO3− cotransporter in human corneal endothelial cells. Pflugers Arch 438: 458–462, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Wetzel P, Hasse A, Papadopoulos S, Voipio J, Kaila K, Gros G. Extracellular carbonic anhydrase activity facilitates lactic acid transport in rat skeletal muscle fibres. J Physiol 531: 743–756, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Li J, Xie Q, Bonanno JA. Molecular expression and functional involvement of the bovine calcium-activated chloride channel 1 (bCLCA1) in apical HCO3− permeability of bovine corneal endothelium. Exp Eye Res 83: 1215–1224, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Xie Q, Sun XC, Bonanno JA. Enhancement of HCO(3)(-) permeability across the apical membrane of bovine corneal endothelium by multiple signaling pathways. Invest Ophthalmol Vis Sci 43: 1146–1153, 2002. [PubMed] [Google Scholar]