Abstract
Since its discovery, the protein regulated in development and DNA damage 1 (REDD1) has been implicated in the cellular response to various stressors. Most notably, its role as a repressor of signaling through the central metabolic regulator, the mechanistic target of rapamycin in complex 1 (mTORC1) has gained considerable attention. Not surprisingly, changes in REDD1 mRNA and protein have been observed in skeletal muscle under various physiological conditions (e.g., nutrient consumption and resistance exercise) and pathological conditions (e.g., sepsis, alcoholism, diabetes, obesity) suggesting a role for REDD1 in regulating mTORC1-dependent skeletal muscle protein metabolism. Our understanding of the causative role of REDD1 in skeletal muscle metabolism is increasing mostly due to the availability of genetically modified mice in which the REDD1 gene is disrupted. Results from such studies provide support for an important role for REDD1 in the regulation of mTORC1 as well as reveal unexplored functions of this protein in relation to other aspects of skeletal muscle metabolism. The goal of this work is to provide a comprehensive review of the role of REDD1 (and its paralog REDD2) in skeletal muscle during both physiological and pathological conditions.
Keywords: muscle mass, RTP801, DDIT4, dig2
maintaining skeletal muscle mass is of critical importance to many groups of people, ranging from athletes trying to accrete muscle protein to individuals trying to minimize protein loss with catabolic diseases such as cancer, sarcopenia, HIV/AIDS, sepsis, alcoholism, and diabetes. It is widely accepted that an increase in muscle mass and strength enhances physical performance in young, healthy individuals (16, 47, 97), and maintenance of skeletal muscle mass and strength in catabolic states is associated with decreased mortality (56, 80, 91, 94, 107). Moreover, in many catabolic conditions, current nutritional and pharmacological interventions are unable to prevent or reverse the erosion of muscle mass, thus presenting a barrier to improved patient care (122, 123). Therefore, a more complete understanding of how skeletal muscle mass is regulated is central to minimize its loss and/or speed recovery following disease in which muscle mass is compromised.
The protein regulated in development and DNA damage 1 (REDD1) has been identified as a key regulator of skeletal muscle mass. For example, skeletal muscle-specific overexpression of REDD1 via electroporation reduces muscle fiber cross-sectional area (CSA) (35), and using mice with a global disruption of the REDD1 gene [hereafter referred to as REDD1 knockout (KO) mice] has implicated REDD1 as an important protein in muscle metabolism under both physiological and pathological conditions. As a thorough phenotypic description of the skeletal muscle from mice with the global disruption of the REDD1 gene has been presented elsewhere (12), it will not be reiterated here. However, it should be noted that basal muscle mass was not different in these animals (12). Despite the growing realization of the diverse actions of REDD1, a comprehensive review of the literature pertaining to its importance specifically in skeletal muscle is lacking.
Regulation of Skeletal Muscle Mass and Protein Synthesis
Skeletal muscle mass is determined by the net balance between rates of muscle protein synthesis and protein degradation (60). These two processes wax and wane throughout the diurnal cycle in response to various external stimuli (e.g., nutrient availability and exercise), and when in relatively long-term balance, muscle mass is maintained (41, 44). Muscle mass is increased when the rate of protein synthesis exceeds the rate of protein degradation for a sustained period of time (45); conversely, enhancing protein degradation relative to protein synthesis leads to a loss of muscle (65). Although stimuli such as resistance exercise and nutrient consumption shift the balance in favor of net protein accretion in healthy individuals (29, 45, 48, 49), the magnitude of this shift is either blunted or nonexistent in various disease states and is associated with a loss of muscle mass (41, 74, 95).
The shift in protein metabolism toward net synthesis following an anabolic stimulus (e.g., nutrients or resistance exercise) requires signaling through the mechanistic target of rapamycin in complex 1 (mTORC1), as pharmacological inhibition of this kinase using rapamycin negates stimulation of protein synthesis (8, 27, 29, 45). Activation of mTORC1 regulates cap-dependent mRNA translation through at least two known downstream substrates, termed the 70-kDa ribosomal protein S6 kinase-1 (p70S6K1) and the eukaryotic initiation factor 4E (eIF4E)-binding protein-1 (4E-BP1) (23, 113). Phosphorylation of these substrates leads to the formation and activation of the 48S preinitiation complex and subsequent cap-dependent translation (25, 53). A detailed description of this specific process has been reviewed elsewhere (46, 69, 102).
Regulation of mTORC1 Signaling
Signaling through mTORC1 is increased by various stimuli including hormones and nutrients that converge on at least two known proximal mTORC1 effectors, the small GTPase ras homolog enriched in brain (Rheb) and the Ras-related small GTP-binding proteins (RagA-D) (Fig. 1) (22). Rheb bound to GTP will activate mTORC1, whereas Rheb bound to GDP will not (76). Hormones such as insulin and insulin-like growth factor I (IGF-I) increase the GTP binding state of Rheb by activating kinases such as Akt (aka protein kinase B, PKB) and ERK, causing phosphorylation and inhibition of the tuberous sclerosis complex (TSC1/2), which functions as a GTPase activating protein (GAP) toward Rheb (43). Conversely, TSC1/2 GAP activity is increased following phosphorylation by AMP-activated protein kinase (AMPK) under conditions of energy stress (71). In contrast, amino acids activate mTORC1 through the concerted action of several guanine nucleotide exchange factors (GEFs) and GAPs that act to modulate the GTP/GDP loading status of the Rag proteins (6, 7, 110). Thus, by promoting the accumulation of RagA/B in the GTP-bound form and/or RagC/D in the GDP-bound form, amino acids are thought to recruit mTORC1 to the lysosomal/late endosomal membrane to interact with Rheb (22). Although the requirement for the guanine nucleotide binding status of RagA/B and RagC/D in the activation of mTORC1 has been questioned (93), it is apparent that the Rag proteins indeed associate with mTORC1 in an amino acid-sensitive fashion (67, 110). There are several excellent reviews for readers interested in a more detailed analysis of mTORC1 signaling (46, 54, 69, 73, 77).
Fig. 1.
Regulation of mRNA translation initiation by the mechanistic target of rapamycin complex 1(mTORC1) signaling pathway.
Regulated in Development and DNA Damage 1 (REDD1)
REDD1 (aka RTP801, DDIT4, dig2) was first identified as a novel hypoxia-inducible factor (HIF)-1-responsive protein upregulated by hypoxia and a target gene of the p53 and p63 transcription factors (33, 112). Following its discovery, the relationship between REDD1 and mTORC1 was identified in Drosophila. Expression of Scylla, the Drosophila homolog of REDD1, repressed phosphorylation of S6 kinase (S6K), a known substrate of the target of rapamycin (TOR), the Drosophila ortholog of mTOR (109). These results were later confirmed in mammalian cells (17).
In addition to regulating mTORC1 signaling, there is also evidence that REDD1 protein stability and REDD1 expression are altered by changes in mTORC1 signaling. For example, increased signaling through mTORC1 in cultured cells stabilized the REDD1 protein, as evidenced by an increase in its half-life (120). Conversely, treatment with rapamycin to inhibit mTORC1 reduced the half-life of REDD1 (120). An additional study showed that REDD1 mRNA and protein expression were increased either 3 h following injection of IGF-I into the gastrocnemius or after constant infusion of insulin into rats (39). Further analysis in C2C12 myotubes illustrated that the IGF-I mediated induction of REDD1 mRNA expression occurred 2 h after treatment, while REDD1 protein expression was increased by 3 h (39). This effect is likely due to induction of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) following prolonged, elevated mTORC1 signaling and protein synthesis (2). Stimulation of the UPR activates the PKR-like ER kinase (PERK), which in turn, phosphorylates the eukaryotic initiation factor 2 (eIF2) on the α-subunit (52). Phosphorylation of eIF2α generally suppresses global rates of protein synthesis but paradoxically increases the translation of a specific subset of mRNAs, including the transcription factor activating transcription factor 4 (ATF4) (5). Increased ATF4 expression is sufficient to promote transcription of REDD1, which is presumed to repress mTORC1 in an attempt to alleviate ER stress (62, 131). Thus, it appears that changes in mTORC1 signaling alter REDD1 protein stability, and prolonged stimulation of mTORC1 can increase REDD1 expression through activation of the UPR.
Despite the known repressive effect of REDD1 on mTORC1 signaling, its mechanism(s) of action is not fully defined. Based on studies using Drosophila, it was first proposed that Scylla, acted upstream of TSC1/2 to repress TOR as overexpression of Scylla in cells without a functional TSC1/2 failed to repress TOR signaling (109). Similarly, overexpression of REDD1 in mammalian cells did not reduce mTORC1 signaling in the absence of a functional TSC1/2 complex (17). Based on these findings, and the observation that both REDD1 and TSC2 bind to 14-3-3 proteins in response to hypoxia, a model was proposed in which REDD1 competes with TSC2 for binding to 14-3-3 proteins and that increased REDD1 expression leads to the dissociation of 14-3-3 proteins from TSC2 (26). In this model, dissociation of TSC2 from 14-3-3 proteins allows it to interact with TSC1 to form a functional TSC1/2 complex, and subsequently repress mTORC1 activity (Fig. 2A). Evidence supporting this model was also provided by a study using a tetracycline-inducible model system in U2OS human bone cells to acutely increase REDD1 expression (26). However, a subsequent study using both functional and structural-based analyses concluded that it is likely that REDD1 does not directly interact with 14-3-3 proteins, thus questioning the validity of the first model (125). More recently, an alternative mechanism was proposed based on data using an acute tetracycline-inducible model system in HEK293 cells to assess the repressive actions of REDD1 on mTORC1 signaling independently of external cellular stressors (e.g., hypoxia) or long-term changes in its expression levels in the days prior to the experiment (e.g., transient transfection of REDD1). This work showed that REDD1 repressed signaling through mTORC1 by reducing the phosphorylation of Akt (Thr308) through recruitment of protein phosphatase-2A (PP2A) (24). Decreasing Akt activation reduced the phosphorylation and inhibition of TSC2, thereby increasing GAP activity toward Rheb and suppressing mTORC1 signaling (Fig. 2B) (24). Although a similar inducible model was employed in both mechanistic studies, Akt phosphorylation was not assessed in the earlier report, precluding direct comparison and integration of the two models.
Fig. 2.

Depictions of two proposed models (A and B) for the repressive action of REDD1 (regulated in development and DNA damage 1) toward mTORC1.
Despite the obvious differences in these models, they are similar in that both show an interaction of TSC2 with 14-3-3 proteins following REDD1 induction. However, the initial report suggested that TSC2 binding to 14-3-3 was reduced following induction of REDD1, while the later did not. Though both used the same pan 14-3-3 antibody for immunoprecipitation, possible reasons for the discrepant findings include the type of cells used (U2OS human bone cells vs. HEK293 cells), or the type of detergent used in the immunoprecipitation buffer Nonidet P-40 (NP-40) vs. 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). Regardless of the uncertainty by which REDD1 represses mTORC1 signaling, the studies are consistent in that REDD1 represses mTORC1 upstream of Rheb (17, 24, 26, 109). Indeed, REDD1 failed to blunt mTORC1 signaling in cells expressing constitutively active Rheb (which resists the GAP activity of TSC1/2), whereas it was able to effectively repress mTORC1 activity in cells expressing constitutively active variants of the Rag proteins (which are resistant to amino acid deprivation) (24).
In vivo, the two proposed models by which REDD1 represses mTORC1 signaling may not be mutually exclusive, and the mode by which REDD1 represses mTORC1 may be dependent on the physiological/pathological state. An example of nonmutual exclusivity is provided by glucocorticoid-treated wild-type (WT) mice and REDD1 KO mice where glucocorticoids effectively increased REDD1 in the muscle at the same time that there was a strong trend for both reduced Akt (Thr308) phosphorylation and increased TSC2 binding to 14-3-3 only in WT mice, consistent with elements of both proposed models (12). In contrast, others have shown that, despite increased REDD1 mRNA and repressed mTORC1 signaling, phosphorylation of Akt (Thr308) was elevated in skeletal muscle of tumor-bearing mice (103). These data support the conclusion that the two proposed mechanisms of action may not be mutually exclusive and that the mode by which REDD1 represses mTORC1 signaling may be specific to the physiological/pathological condition.
In addition to mTORC1 repression, REDD1 may function as a regulator of autophagic flux in an mTORC1-independent manner. Mouse embryonic fibroblasts (MEFs) lacking REDD1 were resistant to the induction of autophagy by stimuli including hypoxia and pharmacological mTORC1 inhibition (rapamycin and Torin2) (104). Based on these studies, it was proposed that REDD1 binds to the thioredoxin interacting protein (TXNIP) and inhibits the TRX1/2 thioredoxins, which function to reduce cellular levels of reactive oxygen species (ROS) (104). Thus, when the TRX1/2 proteins are inhibited, cellular ROS levels increase, thereby enhancing autophagic flux. Although this report described an mTORC1-independent role for REDD1 in the regulation of autophagy, it is still possible that REDD1 also promotes autophagy by inhibiting mTORC1 signaling.
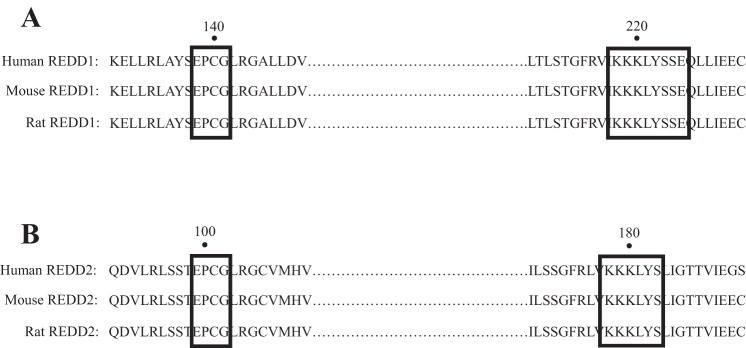
Structural analysis of REDD1 has led to a better understanding of the functional domains within the protein that mediate its repressive action on mTORC1. This analysis revealed that deletion of short stretches of amino acids within two segments of REDD1, comprising amino acids 85–193 and 207–225, completely prevented REDD1-mediated inhibition of mTORC1 (125). To obtain further insight into this region of the protein, the crystal structure of REDD1 amino acids 89–226 was solved (125). The analysis revealed that this portion of the protein folds into two α-helices sandwiched against four β-sheets, with β-sheets 1–3 forming a rare structural motif referred to as a psi loop. Interestingly, a search of the Protein Data Bank failed to identify other proteins with this structure, suggesting that the topology of REDD1 is unique. Mapping of conserved residues to the crystal structure revealed two segments of the protein, comprising amino acids 85–193 and 207–225 (Fig. 3) that are not contiguous in the primary sequence but are adjacent in the three-dimensional structure (125). Conservative mutations of individual amino acids within these regions, e.g., changing Cys140 to Ser, mildly impaired the repressive effects of REDD1 on mTORC1, while less conservative mutations, e.g., converting both Lys219 and Tyr222 to Ala, completely prevented its repressive actions (125). Solving the crystal structure of REDD1 also raised doubts regarding its ability to bind to the 14-3-3 proteins, a process believed to be involved in the REDD1-mediated repression of mTORC1 (26, 125). The location of the 14-3-3 consensus binding motif within the REDD1 sequence creates part of an α-helix and a subsequent loop structure, meaning that REDD1 would have to undergo a dramatic conformational change to permit binding to this location (125). Though this does not exclude the possibility that REDD1 might still bind 14-3-3 proteins, further functional analysis of the unique structure of REDD1 will likely lead to greater clarity as to how REDD1 suppresses mTORC1 among its other actions.
Fig. 3.
Sequence alignment of the proposed surface patch hotspot between REDD1 (A) and REDD2 (B). Numerical value indicates the amino acid position within the primary sequence.
Role of REDD1 During Physiological Conditions
REDD1 and fasting/refeeding.
A role for REDD1 in skeletal muscle metabolism during physiological conditions was first observed following changes in nutrient consumption. Here, REDD1 mRNA and protein abundance were increased in skeletal muscle following an overnight fast (18 h), and both were rapidly reduced 45 min after refeeding. Importantly, the changes in REDD1 mRNA and protein were negatively associated with signaling through mTORC1 (84). Although not demonstrating causality, these data suggested that REDD1 is a potentially important mediator of mTORC1 signaling by nutrients. This hypothesis was supported by data in a subsequent report using male WT mice and REDD1 KO mice subjected to fasting/refeeding (49). Here, the decrease in mTORC1 signaling and rate of protein synthesis in skeletal muscle following food deprivation was at least partially due to increased REDD1 protein, as both protein synthesis and mTORC1 signaling remained elevated in the tibialis anterior of food-deprived REDD1 KO mice (49). Moreover, the refeeding-induced turnover of REDD1 was required for the maximal nutrient-induced stimulation of mTORC1 signaling and protein synthesis, as WT mice exhibited a lesser response to feeding than REDD1 KO mice prior to the turnover of REDD1 (49). Together, these data showed that changes in REDD1 protein within skeletal muscle modulate mTORC1 signaling and rates of protein synthesis in response to nutrient availability.
Contrary to these findings in rodents, nutrient consumption (i.e., 30 g of whey protein) was insufficient to decrease REDD1 protein in the vastus lateralis of either young (male) or old (female and male) humans after a 10-h overnight fast (38). Although this finding clearly contrasts with those observations in rodents, the fact that REDD1 protein was nearly undetectable prior to nutrient consumption makes it difficult to conclude that nutrients do not decrease REDD1 in human skeletal muscle. Additionally, subjects in the human study consumed a drink enriched with protein, whereas the animals in the studies above ingested a chow diet consisting of all three macronutrients (i.e., carbohydrates, fats, and protein), of which only 18% was protein. This could suggest macronutrient-specific responses of REDD1. Last, the timing of the measurements in the human trial (i.e., 30 min post-refeeding) may not have been sufficient to observe a change as REDD1 protein was also unchanged 30 min post-refeeding in mice (49).
Though the increase in REDD1 mRNA and protein with fasting are likely the result of increased circulating glucocorticoids (discussed below), the factor(s) that leads to the rapid turnover of REDD1 protein following refeeding are unknown. The existing data support a potential role for changes in REDD1 gene transcription or REDD1 mRNA stability, as reductions in the relative abundance of REDD1 mRNA seem to parallel the decrease in REDD1 protein expression following refeeding (84). Should this prove to be true, an increase in nutrient availability (e.g., glucose or amino acids), a change in circulating hormones (e.g., insulin), or both may be responsible for the changes in REDD1 mRNA. However, further investigations are needed to confirm this speculation.
Several questions remain regarding REDD1 and nutrient consumption. For example, the data described above were derived from measurements in the gastrocnemius of rats and tibialis anterior of mice, which are composed of both slow- and fast-twitch fibers (55, 63). Whether REDD1 possesses a fiber type-specific effect with refeeding remains to be determined. Additionally, the role of REDD1 in the regulation of mTORC1 signaling and protein synthesis at later time points following refeeding (i.e., postprandial or postabsorptive) is also unknown. Understanding the role of REDD1 at these later time points is potentially important, as it was observed that phosphorylation of p70S6K1 (Thr389) was lower in the gastrocnemius of male REDD1 KO mice compared with WT mice when assessed in the freely fed state, suggesting an alternative metabolic function of REDD1 (134). Furthermore, the above-mentioned studies were performed in male rodents, and whether a similar REDD1 response occurs in skeletal muscle of female mice is unknown. Last, there is only one report in which REDD1 protein expression was measured in the skeletal muscle of humans shortly following refeeding, which makes it difficult to determine the relevance of REDD1 in the skeletal muscle of humans under different nutrient conditions. Thus, there remain many unanswered questions regarding the role of REDD1 in relation to nutrient availability in skeletal muscle metabolism.
REDD1 and resistance exercise.
A change in REDD1 mRNA following resistance exercise was first described in humans after a single bout of knee extensions at an intensity of 20% of the individuals' one-repetition maximum (1RM) under normal and blood flow-restricted conditions in the fasted state (30). Here, a decrease in REDD1 mRNA content in the vastus lateralis of young males was observed 3 h postexercise (30). At a similar time point postexercise, Greig et al. also found that REDD1 mRNA was decreased following 20 sets of 6 maximal isometric muscle contractions in young (26–30 yr of age), but not old (>75 yr of age) females (50). A differential age response of REDD1 mRNA following a bout of knee extensions at 70% of the subjects 1RM was also observed in young and old males. Here, only the old males experienced a decrease in REDD1 mRNA 6 h postexercise, whereas REDD1 mRNA was not altered in either young or old male subjects at 3 h postexercise (31). A lack of change in REDD1 mRNA 3 h postexercise in the vastus lateralis of young males and females has also been shown in response to concurrent knee extension resistance exercise at 65% of 1RM and stationary cycling exercise at 65% of V̇o2 peak (78). In all, these data suggest that changes in REDD1 mRNA are sensitive to the intensity of the exercise (20% vs. 75% of 1RM), the timing of measurement postexercise, the age of the subject, or a combination of these variables. However, whether the decrease (or lack thereof) in REDD1 mRNA was accompanied by concurrent alterations in REDD1 protein and changes in rates of protein synthesis or mTORC1 signaling was not reported in any of the above studies.
In humans, REDD1 protein was not altered in the vastus lateralis of young males 30 min following a bout of knee extensions (10 sets of 10 repetitions at 70% of their 3RM) (38). However, REDD1 protein abundance in the fasted, preexercise state in the young males was nearly undetectable, making it difficult to conclude whether REDD1 expression was actually altered (38). Also, only a single measurement was made 30 min postexercise (38). This time point postexercise may not have been sufficient to observe a change in REDD1 protein in young humans. Interestingly, this group found that REDD1 protein was increased in the vastus lateralis of older males and females 30 min following resistance exercise in association with blunted mTORC1 signaling relative to the young subjects (74). In a separate study, REDD1 protein was not altered immediately following, or at 1 or 3 h, after a single bout of knee extensions at 20% of 1RM under both normal or blood flow-restricted conditions in older subjects (42). Therefore, it remains to be determined whether REDD1 protein is altered in humans following resistance exercise and whether exercise intensity, age, or sex of the subject or the time of measurement influence these potential changes in REDD1 protein.
Resistance exercise can be partially mimicked in rodents by using several methods including electrically induced muscle contractions via stimulation of the sciatic nerve (4). Accordingly, it was shown that a single bout of electrically induced eccentric muscle contractions was sufficient to reduce REDD1 protein both 30 min and 4 h postexercise in the tibialis anterior (48) and at 4 h in the gastrocnemius of male mice (117). These findings are in contrast to a report that failed to show a change in REDD1 protein in the plantaris muscle of female Wistar rats 5 days after muscle overload produced by synergistic ablation (14) or those data described above in humans. Furthermore, REDD1 was shown to be increased in the plantaris of male mice 14 days after synergistic ablation-induced muscle overload (115). Either the type of anabolic stimuli (acute eccentric contraction vs. chronic overload), the intensity of the exercise (20% of 1RM vs. maximal eccentric contractions), or the time in which the measurements were made (hours vs. days) may have contributed to these divergent findings regarding REDD1 protein.
To gain a better understanding of the role of REDD1 in the contraction-induced activation of mTORC1 and protein synthesis, male WT mice and REDD1 KO mice underwent a single bout of electrically induced eccentric contractions of the tibialis anterior (48). As resistance exercise increases both mTORC1 signaling and protein synthesis, it was posited that the contraction-induced reduction in REDD1 protein would be permissive for both processes. Accordingly, in WT mice, REDD1 protein in skeletal muscle limited the stimulation of mTORC1 signaling and protein synthesis following muscle contractions, consistent with the notion described above where increased expression of REDD1 was associated with blunted activation of mTORC1 in the muscle of aged humans (48). Despite this, the precise role of the contraction-induced decrease in REDD1 protein remains unclear, as there was no interaction observed between contractions and genotype in relation to mTORC1 signaling in this study (48). The putative action of REDD1 would suggest that a reduction in REDD1 protein expression following contractions likely contributes to the increased signaling through mTORC1 and protein synthesis following muscle contractions, but confirmation of this hypothesis would require a model in which REDD1 protein is unchanged by muscle contractions. It is also unknown whether REDD1 has a role in the rate or the magnitude of muscle hypertrophy following a long-term overload stimulus (e.g., synergistic ablation or chronic resistance exercise training), as the events that occur immediately following a single bout of resistance exercise may not be representative of the events that occur with an increase in muscle mass resulting from repetitive training.
The factors that alter REDD1 protein following resistance exercise remain unknown. At least in animal studies, a change in REDD1 mRNA content does not seem to be the primary mechanism controlling the reduction in REDD1 protein following resistance exercise (48). These data suggest that resistance exercise either reduces the stability of the REDD1 protein, preferentially reduces its translation, or both. Though not known, it is likely that REDD1 protein expression would also decrease in those studies where REDD1 mRNA was reduced because of the short half-life (<5 min) of the REDD1 protein (68). It also remains to be determined why certain resistance exercise paradigms (type of contraction or intensity) differentially alter REDD1 mRNA expression. Thus, more work is necessary to understand the mechanism(s) by which REDD1 mRNA and protein expression are altered by resistance exercise, and importantly, whether findings from rodent models accurately translate to humans.
REDD1 and aerobic exercise.
The role of REDD1 in regulating the nutrient- and contraction-induced stimulation of mTORC1 and protein synthesis is relatively well explored, whereas there is a paucity of consistent data pertaining to its role during aerobic exercise. A single bout of treadmill aerobic exercise increases REDD1 mRNA and protein in the gastrocnemius of male rats as well as male and female mice (51, 89, 99). In mice, the increase in REDD1 protein appeared to coincide with repressed mTORC1 signaling, whereas in rats, mTORC1 signaling was increased in parallel with elevated REDD1 protein (51, 99). In humans, a single 20-min bout of submaximal aerobic exercise on a cycle ergometer at an intensity of 1.2 W/kg (∼50% of the subjects' V̇o2 max) failed to change REDD1 protein in the vastus lateralis, wheereas mTORC1 signaling was blunted (81). Potential explanations for these discordant results include sex (male vs. female), species (human vs. mouse vs. rat), type of exercise (treadmill vs. cycle ergometer), the source of the REDD1 antibody (Proteintech vs. Bio-Connect), the time that measurements were made (immediately vs. hours postexercise), and a difference in exercise acclimation (2 vs. 10 days for the animal studies). Thus, the influence of aerobic exercise on REDD1 expression and subsequent control of mTORC1 signaling requires further exploration.
Increased REDD1 protein in skeletal muscle following aerobic exercise may also influence autophagic flux and ATP production. For example, it was concluded that autophagic flux and ATP production were reduced in skeletal muscle of REDD1 KO mice following a single bout of treadmill aerobic exercise (2 h in duration) relative to WT mice (104). Here, exercise increased the expression of LC3 II in the muscle of WT mice, whereas the accumulation of LC3 II was reduced in REDD1 KO mice (104). Furthermore, it was presumed that the reduction in ATP production was due to impaired mitophagy throughout the life of these animals and subsequent accumulation of nonfunctional mitochondria (104). This conclusion was supported by the finding that mitochondrial DNA was greater in the muscle of mice lacking REDD1 (104). Whether the increase in autophagy was simply due to decreased mTORC1 signaling is unknown and requires further investigation. In all, these data support the idea that REDD1 is important for regulating skeletal muscle metabolism during and/or following a bout of aerobic exercise, but further work is needed to establish its importance with long-term aerobic exercise training.
Role of REDD1 During Pathological Conditions
REDD1 and glucocorticoids.
A sustained elevation of circulating glucocorticoids promotes muscle atrophy, in part by repressing the rate of protein synthesis (10). Evidence for a role of REDD1 in this repression was provided in a seminal report showing that dexamethasone increased REDD1 mRNA and protein in cultured L6 muscle cells and in the gastrocnemius of male rats (126). It was hypothesized that the glucocorticoid-mediated induction of REDD1 might contribute to dexamethasone-induced muscle atrophy, as mTORC1 function was impaired in association with the increase in REDD1 (127, 128). These data were the first to link glucocorticoids with REDD1 expression in skeletal muscle, as the prior report showing such an association was performed in lymphoid cells (128). However, this original study provided only associative and not causal evidence, thereby increasing the importance of later work that REDD1 KO mice to determine the link between REDD1 and glucocorticoid-induced muscle atrophy. As described therin, REDD1 KO mice (20-wk-old female) were resistant to the loss in muscle mass and muscle fiber CSA induced by 7 days of dexamethasone treatment compared with WT mice (12). Furthermore, the acute (5 h) dexamethasone-induced decrease in protein synthesis as well as phosphorylation of 4E-BP1 (Thr37/46) and ribosomal protein S6 (rpS6; Ser240/244) was offset in REDD1 KO mice (12). Phosphorylation of rpS6 is commonly used to imply mTORC1 activity, as it lies directly downstream from p70S6K1 and will often exhibit more robust changes than p70S6K1 (Thr389). Of note, this report also showed a main effect for glucocorticoids to increase the interaction of TSC2 with 14-3-3 even in the absence of REDD1 (12). These findings provided conclusive evidence that REDD1 is an important target of dexamethasone both acutely and chronically and that REDD1 is necessary for the reduction in protein synthesis and muscle mass following glucocorticoid treatment. It also highlights that the REDD1 mechanism of action is not fully defined.
The increase in REDD1 in response to glucocorticoids depends on the availability of the glucocorticoid receptor, as the receptor antagonist RU-486 attenuated the increase in REDD1 mRNA in differentiated L6 myotubes (135). As described above, nutrient deprivation (e.g., overnight fast) increased the expression of REDD1 protein in skeletal muscle of male rats, and pretreatment with the glucocorticoid receptor inhibitor RU-486 prevented this augmentation (84). Additionally, the serum corticosterone level and REDD1 mRNA content in skeletal muscle were increased in male mice after 90 min of confinement stress, and treatment with RU-486 prior to the stress prevented the increase in REDD1 (70). These studies highlight the importance of the glucocorticoid receptor in the induction of REDD1 mRNA in skeletal muscle in response to either nutrient or psychological stress.
Although glucocorticoids produce muscle atrophy in association with increased REDD1, anabolic hormones can effectively counter this result. After showing that testosterone effectively prevented glucocorticoid-induced muscle atrophy (18, 138), it was subsequently determined that coadministration of a supraphysiological dose of testosterone prevented the dexamethasone-induced increase in REDD1 mRNA and protein in the gastrocnemius of male rats (135). Just as pretreatment with RU-486 blocked the increase in REDD1 following dexamethasone, in vitro experiments showed that the androgen receptor must be functional for testosterone to prevent the dexamethasone-induced increase in REDD1 mRNA and protein (135). Moreover, REDD1 mRNA increased in the gastrocnemius after castration of male mice to remove endogenous testosterone, whereas hormone replacement with nandrolone decanoate once a week for 4 wk suppressed this increase (130).
Growth hormone (GH) may also impact REDD1 expression in response to dexamethasone. Lack of GH in GH-deficient spontaneous dwarf rats (abnormally spliced GH gene) attenuated the dexamethasone-induced increase in REDD1 mRNA in the extensor digitorum longus (EDL) muscle (90). Replacement of GH for 14 days using implanted osmotic minipumps restored the ability of dexamethasone to increase REDD1 expression (90). Future work is needed to determine whether REDD1 is a viable target for attenuating the negative impact of glucocorticoids on skeletal muscle health in humans and whether anabolic hormones are a potential countertherapy. Of additional importance will be to investigate whether anabolic steroid hormones have similar effects in females who do not normally have high circulating levels of these anabolic molecules. Furthermore, the interaction of estrogen in these experimental settings remains unknown despite sexual dimorphic responses being well known to occur in response to glucocorticoids and inflammatory diseases (106).
REDD1 and cancer cachexia.
The preferential loss of skeletal muscle mass in patients with cancer (termed cachexia) appears to be due in part to decreased protein synthesis and repressed mTORC1 signaling. Two independent laboratories have provided data indicating a potential role for REDD1 in the cachectic process induced by the implantation of Lewis lung carcinoma (LLC) cells. This murine model exhibits a loss of muscle mass between ∼28 and 35 days after LLC implantation in association with increased REDD1 mRNA in the gastrocnemius at the terminal time point (9, 103). The increase in REDD1 mRNA paralleled the decrease in mTORC1 signaling, suggesting that, at least during this late stage of cachexia, REDD1 may repress mTORC1 signaling (103). Though other studies show a progressive decrease in mTORC1 signaling as cachexia advances (129), it has yet to be determined whether REDD1 mRNA and protein are increased at earlier time points and whether the cachectic response could be ameliorated in REDD1 knockout mice. Such data would support REDD1 as a potential therapeutic target for cancer cachexia.
In addition to regulating mTORC1 signaling, REDD1 has been proposed to have mTORC1-independent effects on autophagy; however, this is yet to be confirmed in a model of cachexia. In lieu of this, it has been reported that there is a gradual increase in the expression of proteins related to autophagy (e.g., beclin-1, LC3B, and Atg7) as cachexia progresses in the ApcMin/+ mouse model of colon cancer (129). Although speculative, a gradual increase in REDD1 protein as cachexia progresses may promote autophagy, which would contribute to muscle loss, but additional work is required to conclusively establish this relationship.
In addition to induction of REDD1 mRNA due to tumor burden, there is also evidence that REDD1 mRNA is increased by chemotherapeutic drugs in the gastrocnemius of both male and female mice (6 and 20 wk of age) free of tumors (11). The increase in REDD1 mRNA in response to an acute injection of a combination of cyclophosphamide, doxorubicin, and fluorouracil was independent of glucocorticoid signaling, as deletion of the glucocorticoid receptor was not sufficient to offset this induction (11). Interestingly, muscle mass was spared from long-term chemotherapy-induced wasting in mice lacking a functional glucocorticoid receptor, but REDD1 was not measured at death, precluding any conclusion from being made pertaining to the relationship between REDD1 induction and muscle wasting in this catabolic model (11). Therefore, REDD1 mRNA induction by chemotherapeutics could be a transient response or it could be unrelated to the glucocorticoid-induced muscle loss. The role of REDD1 in skeletal muscle metabolism following chemotherapy treatment requires further investigation including whether the effects of chemotherapeutics would be additive to the increase in REDD1 expression observed in tumor bearing models.
The stimulus for increased REDD1 mRNA following tumor establishment is currently unknown, although glucocorticoids are hypothesized to play a role. Muscle-specific deletion of the glucocorticoid receptor in mice blunted, but did not eliminate, the tumor-induced increase in REDD1 mRNA (9). Whether the increase in glucocorticoids was due to a reduction in food consumption was not reported. This highlights the point that in vivo studies of REDD1 should always include detailed information on the nutritional status of the animal, both pre- and posttreatment, as nutritional differences can independently alter REDD1 and reduced nutrient intake has been observed in those with cancer (36, 49, 84). It was also not reported whether the reduction in REDD1 mRNA in the absence of a functional glucocorticoid receptor altered protein synthesis, mTORC1 signaling, autophagy, or other cellular processes. Thus, future work needs to determine what augments REDD1 mRNA and/or protein in skeletal muscle during cancer cachexia as well as chemotherapeutic treatment. It is also important to determine whether reducing glucocorticoid signaling will influence muscle metabolism in these catabolic conditions.
REDD1 and skeletal muscle disuse.
Skeletal muscle atrophy can occur with a reduction in muscular activity and/or weight-bearing activities including limb immobilization and extended bed rest (79). During these atrophic conditions, both the basal and nutrient-induced stimulation of protein synthesis and mTORC1 signaling are blunted (98). For example, 1, 2, and 3 days after male rats underwent unilateral hindlimb immobilization where the posterior muscles (e.g., gastrocnemius, soleus, and plantaris) were casted in the shortened position, protein synthesis and mTORC1 signaling were lower in the soleus muscle of the immobilized limb at baseline and after leucine stimulation (65). Coinciding with these early changes was an increase in REDD1 mRNA beginning 1 day post-immobilization and increasing further at days 2 and 3 (65). Although the increase in REDD1 mRNA occurred at these early time points, it had returned to values observed in the nonimmobilized muscle after 7 days (66). However, whether a concurrent change in REDD1 protein expression paralleled the changes in REDD1 mRNA was not reported, precluding a decisive conclusion from being made pertaining to whether the early increase in REDD1 acts to limit the magnitude of both the basal and nutrient-induced stimulation of mTORC1 and protein synthesis observed within 1 day of immobilization.
A subsequent study extended these initial findings by showing that the position in which the muscle is immobilized influences the induction of REDD1 mRNA. Previous work showed increased REDD1 mRNA when the soleus was immobilized in the shortened position (65); however, REDD1 was unaltered when the soleus was fixed in a stretched position (64). Similar to the finding of unaltered REDD1 mRNA in the lengthened position, protein synthesis and mTORC1 signaling were maintained when the soleus was immobilized in the stretched position (64). In all, changes in REDD1 as well as mTORC1 signaling and protein synthesis are dependent on the position in which the muscle is fixed during immobilization, likely due to the degree of stretch imposed on the muscle. Additionally, skeletal muscle can adapt to long-term changes in stretch by either adding or removing sarcomeres in series to achieve a new resting length (132). Since the degree of muscle stretch appears to alter REDD1 mRNA expression, it is unknown whether such changes (as well as mTORC1 signaling and protein synthesis) are due simply to the remodeling of muscle length, a reduction in muscle fiber CSA, or both.
Similarly to muscle immobilization, prolonged bed rest in humans also negatively impacts protein synthesis and mTORC1 signaling with implications for REDD1 involvement. In a cohort of young (mean 22 yr old) and older (mean 66 yr old) males and females, 5 days of bed rest increased REDD1 mRNA in the vastus lateralis only in the younger subjects. Baseline measurements taken prior to bedrest indicated that older subjects had greater REDD1 mRNA that did not coincide with blunted amino acid-induced stimulation of mTORC1 or mixed muscle protein synthesis. Amino acid-induced phosphorylation of p70S6K1 (Thr389) was blunted in both groups following bedrest, whereas phosphorylation of 4E-BP1 (Thr37/46) was increased appropriately in both groups (121). Despite similar alteration in amino acid-induced stimulation of mTORC1 signaling following bed rest, mixed muscle protein synthesis was increased only in the young subjects despite increased REDD1 mRNA (121). Following a post-bed rest 8-wk rehabilitation program consisting of high-intensity eccentric contractions, REDD1 mRNA in young subjects was no longer increased relative to baseline measures, and REDD1 mRNA was reduced in older subjects to values below those observed in the young subjects (121). Furthermore, the amino acid-induced phosphorylation of p70S6K1 (Thr389) was restored following rehabilitation in both the young and old participants, and the amino acid-induced rate of mixed muscle protein synthesis was again increased in the old subjects (121). These data suggest that the increase in REDD1 mRNA in older subjects at baseline was not associated with blunted stimulation of mTORC1 or protein synthesis by nutrients and that the bed rest-induced resistance to amino acid-induced phosphorylation of p70S6K1 and protein synthesis in older subjects was independent of changes in REDD1 mRNA. The fact that REDD1 mRNA increased in young subjects following bed rest in association with blunted amino acid-induced phosphorylation of p70S6K1 might suggest an age-specific effect of REDD1 on mTORC1 regulation with disuse. Thus, more work is needed to understand the precise role of REDD1 in skeletal muscle during disuse as well as with age.
REDD1 and hypoxia.
A loss of muscle mass may also be seen in response to chronic hypoxia, which can be produced physiologically (e.g., high altitude) or result from an underlying disease state such as chronic obstructive pulmonary disorder (COPD). Though REDD1 was first identified as a hypoxia-inducible factor in nonmuscle cells, it has since been shown that hypoxia is sufficient to increase REDD1 mRNA and protein in skeletal muscle of animals and humans alike (13, 14, 20, 35). Furthermore, the loss of muscle mass during hypoxia may be due to an impairment in mTORC1 activity and protein synthesis, as REDD1 protein is required for the hypoxia-induced decrease in the phosphorylation of p70S6K1 (Thr389) in MEFs (13). Mechanistically, it was proposed that the hypoxia-mediated reduction in mTORC1 signaling was due to REDD1 sequestering 14-3-3 proteins preventing their association with TSC2, thereby enabling formation of a functional TSC1/2 complex and inhibition of mTORC1 kinase activity (Fig. 2) (26). While evidence of this mechanism was elucidated in nonmuscle cells in culture, similar results were reported in the soleus of male rats housed at simulated altitude (i.e., hypoxic conditions) for ∼2 wk (35). Here, atrophy of the soleus (decrease in weight and CSA) was associated with an increase in REDD1 protein, increased REDD1 binding to 14-3-3, and decreased phosphorylation of Akt (Ser473 and Thr308), mTOR (Ser2448), and rpS6 (Ser235/236) (35). However, in humans, hypoxia increased REDD1 protein without a concomitant decrease in the phosphorylation of Akt (Ser473), p70S6K1 (Thr389), or 4E-BP1 (Thr37/46) (81). Interestingly, in this latter study, the induction of REDD1 was associated with increased autophagy in the hypoxic state, consistent with the emerging role of REDD1 in the regulation of autophagy (81, 104). These data are in contrast to earlier human work in which simulated hypoxia increased REDD1 mRNA as well as phosphorylation of p70S6K1 (Thr389) and Akt (Ser473) compared with the time-matched normoxic condition (20). These studies highlight the lack of consistency of the effects of increased REDD1 on mTORC1 substrates within human muscle; however, the hypoxia protocols did differ, and feeding status at the time of biopsy was not clearly specified in either study (20, 81). Last, in COPD patients, a decrease in the phosphorylation of Akt (Thr308), p70S6K1 (Thr389), and GSK3β (Ser9) was observed. Though REDD1 protein content was not directly assessed, a nonsignificant (38%) increase in 14-3-3/REDD1 binding was reported (35).
As with other atrophic conditions, therapies to counter the loss of muscle mass associated with chronic hypoxia are highly desirable. Muscle overload may be a viable therapeutic intervention to offset the loss of muscle mass, as overload of the plantaris muscle in female rats effectively prevented the hypoxia-induced increase in REDD1 protein expression (14). Furthermore, the relative phosphorylation (singular) of p70S6K1 (Thr389) and 4E-BP1 (Thr37/46) was not altered by hypoxia in the overloaded muscle. Therefore, resistance exercise or an alternative method to activate skeletal muscle synthetic pathways may overcome or attenuate the effects of a hypoxic environment, including enhanced REDD1, on skeletal muscle.
Overall, there is strong evidence that REDD1 protein and mRNA are increased under hypoxic conditions in skeletal muscle, similar to other tissues. Although an increase in REDD1 would likely lead to muscle atrophy through a reduction in mTORC1 substrate phosphorylation and protein synthesis, the studies presented here do not convincingly support such a model, as mTORC1 substrate phosphorylation rarely changed coordinately with REDD1 expression. Therefore, more work is required to elucidate the role of REDD1 on mTOR activity after hypoxia as well as other physiological consequences of the increase in REDD1 expression under in vivo conditions.
REDD1 and alcohol.
Both acute and chronic alcohol intoxication decrease the rate of protein synthesis and mTORC1 signaling in skeletal muscle and over time can lead to a decline in muscle mass. Despite being a known repressor of anabolic signaling via mTORC1, whether REDD1 contributes to the aforementioned chronic alcohol-induced decrease in mTORC1 in the skeletal muscle of rats in unclear (72). Consumption of an alcohol-containing diet (36% of total calories) for 14 wk, which decreased gastrocnemius weight and whole body lean mass, did not increase REDD1 mRNA or protein at this terminal time point compared with pair-fed controls (72). However, a transient increase in REDD1 at an earlier time point(s) contributing to the loss in muscle mass cannot be excluded. Similarly, in a male mouse model of short-term (2 wk) daily alcohol intake (∼20 g·day−1·kg body wt−1) no change in REDD1 protein level was detected in the plantaris muscle (115).
Due to the prolonged time needed to produce alcohol-related muscle loss in rodents, models of binge drinking are commonly used as they mimic the physiological effects of chronic alcohol intake on skeletal muscle (118). In male rats, acute alcohol intoxication (i.e., binge drinking) increased REDD1 mRNA in the gastrocnemius in a time-dependent manner and mirrored the peak in blood alcohol concentration (BAC) at 1 and 4 h postadministration (72). The increase in REDD1 mRNA by acute alcohol intoxication occurred independently of sex, nutritional status (fasted/fed), or age [young (2 mo)/adult (12 mo)] (72).
To investigate the mechanism by which alcohol increased REDD1 mRNA/protein in skeletal muscle of rats, 4-methylpyrazole (4-MP) was injected in vivo to inhibit the oxidative metabolism of alcohol by blocking alcohol dehydrogenase activity. Administration of 4-MP prior to acute alcohol intoxication did not blunt the increase in REDD1 mRNA (72). Treatment with the nonmetabolizable alcohol tert-butanol also increased REDD1 mRNA, and together these data suggested that the ethanol-induced increase in REDD1 mRNA was independent of its metabolism. However, in direct contrast, perfusion of the isolated hindlimb of rats with alcohol to completely bypass hepatic metabolism did not increase REDD1 mRNA or protein in muscle, thereby suggesting that when alcohol is given in vivo the increase in REDD1 is mediated via production of a secondarily produced unidentified mediator. Although alcohol intoxication can increase plasma corticosterone, which upregulates REDD1 mRNA, pretreatment with the glucocorticoid receptor inhibitor RU-486 did not abrogate the alcohol-induced increase in REDD1 mRNA, indicating a glucocorticoid-independent mechanism (72).
In several of these experiments, the alcohol-induced increase in REDD1 was associated with a decrease in the rate of skeletal muscle protein synthesis; however, whether this was a causal link remained unknown until experiments were performed in REDD1 KO mice. An acute dose of alcohol was administered to female REDD1 KO mice via ip injection (3 g/kg body wt), and protein synthesis was determined in the gastrocnemius (116). Alcohol reduced protein synthesis and rpS6 (Ser240/244) phosphorylation equally in WT and REDD1 KO mice, although signaling through mTORC1 [i.e., phosphorylation of p70S6K1 (Thr389) and 4E-BP1 (Ser65)] was unaltered (116). These results suggest that the effect of alcohol on protein synthesis and phosphorylation of rps6 (Ser240/244) is REDD1 and mTORC1 independent. Although this mechanistic work did not find significant effects of REDD1 on anabolic pathways altered by alcohol, it did reveal a possible alternative role of REDD1 in the regulation of ubiquitin-proteasome-mediated proteolysis during acute alcohol intoxication, which will be important to explore in the future (116).
One caveat of the aforementioned work performed in REDD1 KO mice was that acute alcohol intoxication did not significantly increase REDD1 mRNA in female WT mice. Moreover, another previous study in male mice did not show an acute alcohol-induced increase in REDD1 protein in the gastrocnemius (117). It should be noted, though, that the male mice were fasted for 18 h prior to receiving alcohol, which may have significantly increased REDD1 so that the alcohol could not further augment REDD1 expression. To our knowledge, no human data exist measuring REDD1 expression following acute alcohol intake to reconcile these contrasting findings between rats and mice, making further experimentation necessary to better establish this relationship.
Overall, it remains to be determined how the acute administration of an intoxicating dose of alcohol affects REDD1 expression in skeletal muscle and whether in rat and human models this is linked to the decrease in skeletal muscle protein synthesis and the progressive loss of muscle. Furthermore, as REDD1 was not elevated following chronic alcohol intake in association with a loss of muscle mass, the time course and relevance of the acute increase in its expression need to be evaluated further.
REDD1 and sepsis.
The potential role of REDD1 in the catabolic condition of sepsis is not yet well defined despite previous observations showing decreases in mTORC1 signaling in skeletal muscle of experimental animal models (40). Using cecal ligation and puncture (CLP) as an acute mouse model of sepsis, it was shown that REDD1 protein was increased in the gastrocnemius of male mice along with a decrease in protein synthesis independent of changes in mTORC1 signaling (119). Interestingly, the increase in REDD1 was not offset by a bout of stimulated muscle contractions (∼18 h post-sepsis induction and 2 h prior to muscle collection), showing that sepsis rendered REDD1 unresponsive to this significant anabolic stimulus compared with the control condition in which REDD1 protein was reduced by muscle contractions (119).
Further investigation of the potential relationship between enhanced skeletal muscle expression of REDD1 protein during sepsis and the reduction in protein synthesis was performed using female REDD1 KO mice. Disruption of the REDD1 gene offset the sepsis-induced decrease in mTORC1 signaling [phosphorylation of p70S6K1 (Thr389), 4E-BP1 (Ser65), rpS6 (Ser240/244)] and protein synthesis (114). In addition to mediating the sepsis-related modulation of mTORC1 signaling, REDD1 also appeared to contribute to the sepsis-induced increase in autophagy. In mice lacking REDD1, the sepsis-induced decrease in the phosphorylation of ULK1 (Ser757) and p62 protein was completely offset, as was the increased ratio of LC3 II to LC3 I in the gastrocnemius. As described earlier in this review, there is mounting evidence that REDD1 is integral in the regulation of autophagic flux within skeletal muscle and future work should strive to elucidate this mechanism and its physiological role more completely.
In summary, sepsis increased REDD1 expression in the gastrocnemius muscle of both male and female mice, and this was resistant to anabolic stimulation prior to tissue collection. Furthermore, the relationship between REDD1 expression during sepsis, mTORC1 signaling, and sex remains to be determined, as in female mice mTORC1 signaling was suppressed whereas in males at a similar time point signaling remained mostly unaffected. Therefore, whether this is a response related to the sex of the animal or just an isolated experimental observation (that mTORC1 was not altered by sepsis) requires further attention.
REDD1 and diabetes.
Uncontrolled diabetes leads to a loss in muscle mass, and accordingly, diabetes alters REDD1 expression in skeletal muscle (96). For instance, induction of type 1 diabetes, using alloxan in male rats, increased REDD1 protein and mRNA above those values observed in nondiabetic rats following 18 h of food deprivation (84). Moreover, REDD1 was differentially modulated in diabetic rats following refeeding, as REDD1 protein and mRNA in skeletal muscle of diabetic rats remained 20-fold higher than the values observed in nondiabetic controls (84). Furthermore, refeeding increased the phosphorylation of p70S6K1 (Thr389) over 200% in the gastrocnemius of nondiabetic rats, whereas no increase was observed in diabetic rats, possibly due to the sustained elevation in REDD1 protein (84).
REDD1 protein was also increased in the triceps surae muscle complex of male mice following streptozotocin (STZ)-induced type 1 diabetes (57). REDD1 protein was elevated after 1 wk of STZ and remained elevated at 3 and 5 wk following diabetes onset (57). This change in REDD1 protein paralleled a loss in triceps surae muscle mass and impairment in mTORC1 signaling (57). Thus, these data confirm that diabetes promotes an increase in REDD1 expression within skeletal muscle; however, whether REDD1 is required for the diabetes-induced loss of muscle mass is unknown.
Investigation of the factor(s) that promote an increase in REDD1 mRNA and protein in the diabetic state have yielded conflicting results. In the initial study performed in male diabetic rats, REDD1 mRNA in muscle positively correlated with levels of circulating glucocorticoids following 18 h of food deprivation (84). Administration of RU-486 to block the glucocorticoid receptor abolished this relationship, suggesting that changes in REDD1 mRNA following fasting in diabetic rats were due to elevated glucocorticoids (84). However, in STZ-induced diabetic mice, it was suggested that the increase in REDD1 protein was mediated by p63, which, as described above (33), is one of the initial transcription factors discovered to increase REDD1 expression during hypoxia (33). However, dissecting the relative contribution of either p63 or glucocorticoids to the increase in REDD1 is not possible, as these were not simultaneously compared. Additional factors that may complicate analyzing the contribution of each include the species (rats vs. mice), feeding status (18 h food deprivation vs. freely fed), and the method used to induce diabetes (alloxan vs. STZ). Thus, the factors that increase REDD1 mRNA and protein in the skeletal muscle during diabetes remain poorly defined.
REDD1 and obesity.
Recent work supports an emerging role for REDD1 in the regulation of skeletal muscle metabolism and insulin action in obesity (32, 100, 108, 133, 134). It was initially observed that under fasting conditions skeletal muscle REDD1 mRNA and/or protein were higher in the gastrocnemius of male obese mice compared with lean mice. This was unexpectedly associated with reduced formation of the TSC1/2 complex and increased mTORC1 activation as assessed by a reduction in the binding of raptor with mTOR (28). Additional analysis showed that REDD1 protein was elevated in the triceps surae muscle complex of obese male ob/ob mice compared with the values observed in the muscle of lean mice in both the fasted and fed states (134). Furthermore, male mice lacking REDD1 were partially resistant to weight gain induced by 2 mo of high-fat feeding, supporting a relationship between REDD1 and obesity development (134).
Insulin insensitivity and anabolic resistance have been observed in skeletal muscle from obese individuals and may be associated with the obesity-induced increase in REDD1 (133). Furthermore, REDD1 protein was increased to a greater magnitude following a 2-h euglycemic-hyperinsulinemic clamp in skeletal muscle of male and female obese type 2 diabetics compared with lean, healthy male and female subjects (133). This increase in REDD1 expression following insulin infusion may serve to limit an already hyperactive mTORC1 or act to induce insulin resistance, as the change in REDD1 corresponded with suppression of changes in the phosphorylation of Akt (Ser473), p70S6K1 (Thr389), ERK1/2 (Thr202/Tyr204), and MEK1/2 (Ser217/221) in the obese type 2 diabetics relative to the lean group (133). Hyperactive mTORC1 may also contribute to obesity-related insulin resistance, as p70S6K1 can phosphorylate and inhibit insulin receptor substrate-1 (15). Additionally, mTORC1 can phosphorylate the growth factor receptor-bound protein 10 leading to feedback inhibition of phosphatidylinositol 3-kinase (136). Thus, findings from these studies propose a REDD1-regulated mechanism in skeletal muscle of obese individuals that may contribute to skeletal muscle and whole body insulin resistance.
The physiological reason for the increase in REDD1 in skeletal muscle during obesity is unknown; however, it may be due to hyperactive mTORC1 signaling. As previously mentioned, signaling through mTORC1 regulates REDD1 stability, and since mTORC1 is constitutively active in the muscle of these individuals, this may partially explain the increase in REDD1 protein. The increase in REDD1 protein may also be due to ER stress associated with hyperactive mTORC1 signaling as ER stress increases the expression of the transcription factor, ATF4, which in turn promotes transcription of the REDD1 gene. Alternatively, circulating levels of glucocorticoids are increased during obesity, and this could also augment REDD1 (1, 86, 133, 137). Thus, the factors that increase REDD1 in skeletal muscle during obesity remain to be fully determined, as does the physiological significance of its increase and its role in the development of insulin resistance at the level of the skeletal muscle.
REDD2
An early report of public databases which identified REDD1 (RTP801) as a novel hypoxia-inducible factor also discovered two overlapping mouse EST clones containing an open reading frame substantially similar to RTP801 (111). In silico analysis revealed a new mouse gene product (designated RTP801L for RTP801-like; DDIT4L; REDD2) that displayed 57% sequence identity to both rat and human RTP801. Subsequently, Northern blot analysis showed REDD2 mRNA was induced in a dose-dependent manner in macrophages incubated with oxidized LDL or the iron chelator desferrioxamine, the latter being a chemical mimetic of hypoxia (19). REDD2 mRNA was increased in various nonmuscle cell lines by different stressors, including osmotic stress (92), ROS (59), and radiation (37) as well as immunomodulatory agents such as endotoxin (TLR4 agonists) and zymosan (TLR2 agonist) (58). However, REDD2 mRNA did not appear to change in C2C12 myotubes treated with CoCl2, a hypoxia mimetic, although the sample size in that particular study was relatively small (101). A systematic evaluation of the effect of hypoxia and related studies on mechanism of action on REDD2 mRNA and protein in skeletal muscle per se is lacking.
Work by Brugarolas et al. (13) and Corradetti et al. (17) provided the first strong data indicating that REDD2, like its paralog REDD1, regulates cellular and metabolic functions in part as a negative regulator of mTORC1. These cell-based studies (e.g., HEK-293 and MEFs) showed that overexpression of REDD2 inhibits p70S6K1 and 4E-BP1 phosphorylation, and conversely the silencing of REDD2 reverses this inhibition. Work by several independent laboratories using in vitro overexpression and silencing methods have provided compelling evidence that REDD2 negatively regulates mTORC1 signal transduction via a TSC2-dependent mechanism (13, 17, 87). While these studies indicate similarities in the regulation of mTORC1 by REDD1 and REDD2, other studies have shown selective mechanisms regulating REDD1, but not REDD2 (85).
To our knowledge, the crystal structure of REDD2 has not been described. However, sequence alignment of the REDD2 amino acid sequence with REDD1 shows an overall identity of only 36%, and most of the NH2-terminal 50 amino acids present in REDD1 are missing in REDD2. However, multispecies comparison of the human, mouse, and rat amino acid sequences of the two proteins revealed that all but two of the amino acids within the segments of REDD1 that were identified as being critical for its repressive action on mTORC1 (125) are conserved (Fig. 3). Moreover, the spacing between these two regions in REDD1 and REDD2 is also conserved; i.e., they are separated by 75 and 76 amino acids, respectively. Similar to REDD1, these two well-conserved regions within the REDD2 sequence may also be important for the mTORC1-repressive function of the protein.
Basal condition.
As outlined in previous sections of this review, the control of mTOR in skeletal muscle plays a central role in maintaining protein homeostasis in this tissue during both health and disease. In this regard, Northern blotting revealed a preferential distribution of REDD2 (e.g., SMHS1) in striated muscle, both skeletal and cardiac, as opposed to other tissues such as liver, lung, spleen, and kidney (101), an observation confirmed by others (87). The only significant discrepancy between these two studies involved REDD2 expression in the heart that was observed in the former but not the latter study. Microarray analysis of human tissues found that REDD2 mRNA expression was nearly 2:1 for heart having lower expression than skeletal muscle (124). REDD2 mRNA is also seen in vastus lateralis (i.e., mixed fiber type muscle) from humans, and there is no apparent difference in expression level between young (∼30 yr) and old (∼70 yr) men, findings that are internally consistent with the similar rates of muscle protein synthesis observed in these two groups (31). In a subsequent study, the same group reported no age effect on REDD2 mRNA expression using experimental groups that contained equal numbers of female and male volunteers (121). However, an increase in REDD2 mRNA has been reported in the soleus from 9- and 18-mo-old male rats compared with 2-mo-old animals (66). These two studies are not necessarily contradictory, as REDD2 may well be lower in adolescent humans, similar to the 2-mo-old rats, than in adults. The expression of REDD2 mRNA was relatively higher in the predominantly fast-twitch gastrocnemius compared with the soleus muscle which is composed of primarily slow-twitch fibers. Finally, in the masseter muscle, which is composed of relatively small-diameter fibers with a propensity for apoptosis, REDD2 was sixfold higher than in the tibialis anterior muscle, composed of fibers with a larger CSA (34). It is noteworthy that expression profiling of adult murine skeletal muscle showed that REDD2 mRNA exhibits a strong circadian rhythm, with expression being highest during the dark phase (82). Hence, studies of REDD2 should clearly report the time of day at which they are conducted.
Catabolic states.
Studies have reported dynamic changes in REDD2 mRNA under a variety of catabolic and anabolic conditions. Multiple studies from independent laboratories have generally reported the upregulation of REDD2 mRNA during catabolic conditions. The consequence of such a change can be seen under conditions where REDD2 is overexpressed in C2C12 myoblasts and leads to a decreased phosphorylation of p70S6K1 (and presumably mTORC1 activity) under basal conditions, and also prevents the typical increase in p70S6K1 phosphorylation seen in response to the anabolic stimuli of leucine or muscle stretch (87). Under in vivo conditions, REDD2 mRNA was increased in the soleus (but not the gastrocnemius) from female rats in response to disuse atrophy imposed by 2 wk of hindlimb suspension (101). Using a different model of disuse in rats (e.g., casting), the increase in REDD2 mRNA in the soleus was shown to occur as early as 24 h after immobilization, to persist for at least 3 days (65), and occurred only in 2- and 9-mo-old rats, not 18-mo-old animals (66). Such increases in REDD2 are unlikely to be mediated by overt hypoxia, as global muscle blood flow is unlikely to be decreased (83). Of interest, this increase in REDD2 mRNA was only seen when the soleus was immobilized in the shortened position, keeping the muscle in the stretched position during the 3-day immobilization decreased REDD2 mRNA compared with the noncasted contralateral muscle (64). This differential regulation of the REDD2 in the shortened vs. stretched position was consistent with the changes in muscle protein synthesis. Similarly, increased REDD2 mRNA was reported in human vastas lateralis after 5 days of bed rest (121).
In contradistinction, a decrease in muscle REDD2 mRNA has been reported in other preclinical models of muscle atrophy. For example, microarray analysis of gene expression in three models of genetic muscle atrophy indicate a consistent reduction in REDD2 regardless of the model (88), and REDD2 protein content was decreased in the soleus from CAPN3 knockout mice which causes limb-girdle muscular dystrophy type 2A (61). However, mTORC1 signaling and the rate of muscle protein synthesis per se were not directly determined in these studies, and it is possible that muscle protein synthesis in these models may actually be increased, a somewhat counterintuitive response similar to that observed in denervation-induced muscle atrophy (21). Many catabolic conditions are characterized by an elevation in circulating glucocorticoids that decrease muscle protein synthesis in an mTORC1-dependent manner. Thus, it was expected that the potent synthetic glucocorticoid dexamethasone would increase REDD2 mRNA in muscle. However, muscle REDD2 mRNA was decreased in gastrocnemius from rats and in L6 myotubes 4 h after treatment with dexamethasone, which also inhibited mTORC1-dependent protein synthesis (126). The reason for this apparent divergent effect of dexamethasone has not been elucidated. In large part, data interpretation for all of the above-mentioned studies as well as many of those described below is limited by the lack of a validated REDD2 antibody necessary to assess changes in REDD2 protein content.
Anabolic states.
Anabolic stimuli have generally been reported to decrease REDD2, which is consistent with the role of this protein as a negative regulator of mTOR-dependent protein synthesis in skeletal muscle. Resistance exercise has a strong anabolic response in muscle. Although there is one report to the contrary (89), which shows REDD2 is not acutely altered by exercise in rats, all other studies show a generalized decrease in REDD2 after exercise. For example, REDD2 mRNA was markedly decreased in the biceps brachii of young men 24 h after completion of resistance exercise; however, this decrease was observed only in males and not in females (75). Although this study posited that such a sexual dimorphic difference may in part explain the increased muscle growth in males vs. females, this supposition remains to be tested. Resistance exercise, either alone or in combination with essential amino acid ingestions, can decrease REDD2 mRNA in as rapidly as 3–4 h (3, 31, 78). Exercise, muscle reloading, and administration of the anabolic steroid nandrolone are all able to decrease the elevation of REDD2 seen in response to various types of muscle atrophy in rats and humans (66, 105, 121). Finally, another anabolic stimulus, IGF-I, also decreased REDD2 mRNA in both myotubes and rat gastrocnemius, a change that was associated with increased rates of protein synthesis (39). In contrast to these relatively consistent findings, synergistic ablation of the gastrocnemius, which leads to a compensatory hypertrophy of the soleus muscle, did not alter REDD2 mRNA (101).
Although there is a solid foundation of basic information on modulators of REDD2 mRNA, there is a paucity of data pertaining to changes at the protein level. However, because of the tissue-specific localization of REDD2 to skeletal (and possibly cardiac) muscle, which is more limited than that of REDD1, there is a need for a more complete understanding of how this protein is regulated, how precisely it inhibits mTORC1-mediated events, and its role relative to REDD1 in growth and loss of muscle protein. Hence, both in vitro mechanistic studies and in vivo studies designed to elucidate the physiological relevance of described mechanisms will be required.
Conclusion and Future Directions
The evidence is convincing that REDD1 mRNA/protein content is altered in skeletal muscle during a variety of physiological and pathological conditions (Tables 1 and 2; Fig. 4). In general, the expression of REDD1 is increased during cachexia, hypoxia, glucocorticoid administration, sepsis, acute alcohol intoxication, diabetes, acute aerobic exercise, nutrient deprivation, and obesity. Conversely, REDD1 expression is generally decreased by acute resistance exercise and refeeding/nutrient consumption. Although eloquent cell-based studies have clearly demonstrated that REDD1 is a repressor of mTORC1 signaling, a change in REDD1 does not always correlate with a change in mTORC1 signaling under in vivo conditions. For example, a comprehensive summary of pathological conditions (Table 1) and physiological conditions (Table 2) clearly shows a lack of consistency regarding changes in REDD1 and mTORC1 signaling. This apparent discrepancy may be due to a variety of factors, including the model used (human vs. murine), sex (male vs. female), condition (pathological vs. physiological), nutrient status (fasted, refed, or freely fed), time point analyzed, or if the input to mTORC1 was downstream of REDD1 or in a parallel pathway. It is also possible that REDD1 functions to alter other metabolic processes in the muscle that are only now beginning to be elucidated following the creation of REDD1 KO mice. For example, REDD1 appears to regulate atrogene expression during alcohol intoxication, insulin signaling during obesity, and autophagy during aerobic exercise and sepsis. An important next step will be to determine the role of REDD1 in skeletal muscle during other pathological/physiological conditions.
Table 1.
Changes in REDD1 expression and mTORC1 signaling during pathological conditions
| Pathological Condition | Feeding Status at Death | Species/Sex | Muscle | Change in REDD1 | mTORC1 Signaling Associated with Change in REDD1 Expression | Reference |
|---|---|---|---|---|---|---|
| Hypoxia | Fasted (4 h) | humans/males | Vastus lateralis | ↑ mRNA at 4 h ↔ mRNA at 1 h | ↑ p70S6K1 (Thr389) | 20 |
| Hypoxia | Fed | humans/unknown | Vastus lateralis | ↑ protein | ↔ p70S6K1 (Thr389); ↔ 4E-BP1 (Thr37/46) | 81 |
| Hypoxia | NR | rats/male | Soleus | ↑ protein | ↓ mTOR (Ser2448); ↓ rpS6 (Ser235/236); ↔ 4E-BP1 (Thr36/47) | 35 |
| Hypoxia | NR | rats/female | Plantaris | ↑ protein | ↔ p70S6K1 (Thr389); ↔ rpS6 (Ser240/244); ↔ 4E-BP1 (Thr70) | 14 |
| Cancer cachexia | NR | mice/male | Gastrocnemius | ↑ mRNA | ↓ 4E-BP1 (Thr37/46); ↓ rpS6 (Ser235/236) | 103 |
| Cancer cachexia | NR | mice/male and female | Gastrocnemius | ↑ mRNA | NR | 9 |
| Immobilization | ON fasted and oral gavage with leucine | rats/male | Soleus | ↑ mRNA | ↓ S6K1 hyperphosphorylation; ↓ 4E-BP1 Hyperphosphroylation; ↓ 4E-BP1 (Ser65); ↓ p70S6K1 (Thr389) | 65 |
| Immobilization | ON fasted | rats/male | Soleus | ↑ mRNA | ↓ p70S6K1 (Thr389) | 64 |
| Bed rest | Refed | humans/male and female/old and young | Vastus lateralis | ↑ mRNA at baseline in old; ↑ mRNA with bed rest in young; ↓ mRNA following rehabilitation in old | ↑ p70S6K1 (Thr389) and ↑4E-BP1 (Thr37/46) at baseline in old and young with amino acid stimulation; ↔ p70S6K1 (Thr389) and ↑4E-BP1 (Thr37/46) after bed rest in old and young with amino acid stimulation; ↑ p70S6K1 (Thr389) and ↑4E-BP1 (Thr37/46) after rehabilitation with amino acid stimulation | 121 |
| Diabetes | Fasted (18 h) refed (45 min) | rats/male | Gastrocnemius | ↑ mRNA and protein after fast and refeeding | ↓ p70S6K1 (Thr389) and S6K1 hyperphosphorylation | 84 |
| Diabetes | NR | mice/male | Triceps surae complex | ↑ protein | ↓ p70S6K1 (Thr389) | 57 |
| Diabetes/obesity | Fasted (10–12 h) | humans/male and female | Vastus lateralis | ↔ protein | ↑ p70S6K1 (Thr389); ↓ 4E-BP1 (Thr37/46) | 133 |
| Obesity | Fasted/Fed | mice/male | Gastrocnemius | ↑ mRNA and protein in fasted and fed state | ↑ 4E-BP1 (Thr37/46); ↑ 4E-BP1 hyperphosphorylation; ↑ p70S6K1 (Thr389) in fasted state | 134 |
| Dexamethasone | Fasted | rats/male | Gastrocnemius | ↑ mRNA and protein | NR | 126 |
| Dexamethasone | NR | rats/male | Gastrocnemius | ↑ mRNA and protein | ↑ 4E-BP1 total | 135 |
| Dexamethasone + testosterone | NR | rats/male | Gastrocnemius | ↔ mRNA and protein | ↔ 4EBP1 total | 135 |
| Dexamethasone | NR | GH-deficient SDR rats | EDL and soleus | Soleus: ↔ mRNA EDL: ↔ mRNA | Soleus: ↔ 4E-BP1 (Thr37/46); ↔p70S6K1 (Thr389) No report in EDL | 90 |
| Dexamethasone | NR | rats/male | Gastrocnemius | ↑ mRNA | NR | 72 |
| Dexamethasone (acute and chronic) | NR | mice/female | Gastrocnemius | ↑ mRNA + protein | ↓ 4E-BP1 (Thr37/46); ↓ rpS6 (Ser240/244) | 12 |
| Castration | NR | mice/male | Gastrocnemius | ↑ mRNA | ↓MPS, ↓mTOR (Ser2448); ↓p70S6K1 (Thr389); ↓4E-BP1 (Thr37/46) | 130 |
| Acute alcohol | ON fasted | male/rats | Gastrocnemius and soleus | Gastroc: ↑ mRNA; Soleus: ↔ mRNA | ↓ 4E-BP1 (Thr37/46) | 72 |
| Acute alcohol | ON fasted | female/rats | Gastrocnemius | ↑ mRNA | ↓phosphorylated mTOR and 4E-BP1 (sites not specified) | 72 |
| Acute alcohol | Freely fed | rats/male | Gastrocnemius | ↑ mRNA | NR | 72 |
| Chronic alcohol | Freely fed | rats/male | Gastrocnemius | ↔ mRNA or protein | ↓ 4E-BP1 (Thr37/46) | 72 |
| Isolated limb-alcohol perfusion | ON fasted | rats/male | Gastrocnemius | ↔ mRNA or protein | ↓ 4E-BP1 (Thr37/46); ↓mTOR (Ser2448) | 72 |
| 2-wk Moderate alcohol | Freely fed | mice/male | Plantaris | ↔ protein | ↔ p70S6K1 (Thr389); ↔ 4EBP1 (Thr37/46); ↔ mTOR (Ser2448); ↔ MPS; ↔ rpS6 (Ser240/244) | 115 |
| Acute alcohol | ON fasted | mice/male | Gastrocnemius | ↔ protein | ↔ p70S6K1 (Thr389); ↔ rpS6 (Ser240/244); ↔ 4E-BP1 (Ser65) | 117 |
| Acute alcohol | Freely fed | mice/female | Gastrocnemius | ↔ mRNA | ↓rpS6 (Ser240/244); ↔p70S6K1 (Thr389); ↔mTOR (Ser2481); ↔4E-BP1 (Ser65) | 116 |
| Sepsis | Fasted (24 h) | mice/male | Gastrocnemius | ↑ protein | ↔p70S6K1 (Thr389); ↔ rpS6 (Ser240/244); ↔ mTOR (Ser2481); ↔ 4E-BP1 (Ser65) | 119 |
| Sepsis | Fasted (24 h) | mice/female | Gastrocnemius | ↑ mRNA and protein | ↓p70S6K1 (Thr389); ↓rpS6 (Ser240/244); ↓mTOR (Ser2481); ↓4E-BP1 (Ser65); ↓ULK1 (Ser757) | 114 |
NR, not reported; ON, overnight.
Table 2.
Changes in REDD1 expression and mTORC1 signaling during physiological conditions
| Physiological Condition | Feeding Status at Death | Species/Sex | Muscle | Change in REDD1 | mTORC1 Signaling Associated with Change in REDD1 Expression | Ref. |
|---|---|---|---|---|---|---|
| Nutrient availability | Freely fed, fasted (18 h/refed (45 min) | rats/male | Gastrocnemius | ↑ mRNA and protein with 18 h fast; ↓ mRNA and protein after 45 min refeeding | ↓ S6K1 hyperphosphorylation and p70S6K1 (Thr389) after fasting; ↑ S6K1 hyperphosphorylation and p70S6K1 (Thr389) after refeeding | 84 |
| Nutrient availability | Fasted (16 h)/refed (30 min)/refed (75 min) | mice/male | Tibialis anterior | ↔ protein after 30 min refeeding; ↓ protein after 75 min refeeding | ↑ p70S6K1 (Thr389) and ↑ 4E-BP1 (Ser65) after 30 min refeeding though blunted relative to values observed in REDD1-null mice. ↑ p70S6K1 (Thr389) and ↑ 4E-BP1 (Ser65) after 75 min refeeding equal to values observed in REDD1-null mice. | 49 |
| Resistance exercise | ON fasted | humans/male (young and old) | Vastus lateralis | ↓ mRNA in old 6 h post exercise; ↔ mRNA in young | NR | 31 |
| Resistance exercise | ON fasted | humans/male | Vastus lateralis | ↓ mRNA | NR | 30 |
| Resistance exercise | NR | humans/female (young and old) | Lateral quadriceps | ↓ mRNA in young; ↔ mRNA in old | NR | 50 |
| Resistance exercise | ON fasted | humans/males (old) | Vastus lateralis | ↓ mRNA; ↔ protein (Occurred under both control and blood flow restricted conditions) | ↑ p70S6K1 (Thr389); ↔ 4E-BP1 (Thr37/46) | 42 |
| Resistance exercise | Amino acid ingestion | humans/males and females (old and young) | Vastus lateralis | ↔ protein (young); ↑ protein (old) | ↑ p70S6K1 (Thr389): blunted in aged muscle | 38 |
| Resistance exercise and aerobic exercise | NR | humans/male and female | Vastus lateralis | ↔ mRNA | NR | 78 |
| Muscle contractions | Fasted/refed | mice/male | Tibialis anterior | ↓ protein | ↑ p70S6K1 (Thr389); ↑ 4E-BP1 (Ser65) | 48 |
| Muscle contractions | Fasted (∼24 h) | mice/male | Gastrocnemius | ↓ protein | ↑ p70S6K1 (Thr389); ↑ p70S6K1 (Thr421/Ser424); ↑ rpS6 (Ser240/244); ↑ 4E-BP1 (Ser65); ↑ 4E-BP1 (Thr37/46) | 117 |
| Muscle Contractions | Fasted (∼24 h) | mice/male | Gastrocnemius | ↔ protein | ↑ p70S6K1 (Thr389); ↑ p70S6K1 (Thr421/Ser424); ↑ rpS6 (Ser240/244); ↑ 4E-BP1 (Ser65) | 119 |
| Muscle overload | Freely fed | mice/male | Plantaris | ↑ protein | ↑ p70S6K1 (Thr389); ↑ 4E-BP1 (Thr37/46); ↑ mTOR (Ser2448) | 115 |
| Muscle overload | NR | rats/female | Plantaris | ↔ protein | ↑ p70S6K1 (Thr389); ↔ 4E-BP1 (Thr37/46); ↑ mTOR (Ser2448) | 14 |
| Aerobic exercise | Fasted (3 h) prior to exercise | mice/female | Gastrocnemius | ↑ protein | ↓ p70S6K1 (Thr389); ↓ S6K1 activity; ↓ 4E-BP1 (Thr37/46); ↓ rpS6 (Ser235/236); ↓ rpS6 (Ser240/244) | 99 |
| Aerobic exercise | 16-h fast | rats/male | Gastrocnemius | ↑ mRNA and protein | ↑ p70S6K1 (Thr389); ↑ mTOR (Ser2448); ↓ 4E-BP1 hyperphosphorylation | 51 |
| Aerobic exercise | NR | humans | Vastus lateralis | ↔ protein | ↓ p70S6K1 (Thr389); ↔ 4E-BP1 (Thr37/46) | 81 |
| Aerobic exercise | NR | mice | NR | ↑ mRNA | NR | 104 |
| Aerobic exercise | 16-h fast, glucose or BCAA refeeding | rats/male | Gastrocnemius | ↑ mRNA and protein | NR | 89 |
Fig. 4.

Conditions associated with altered REDD1 mRNA and protein expression and the known factors that alter REDD1 expression. Those conditions in green are associated with increased REDD1 mRNA and/or protein; those in red are associated with reduced REDD1 mRNA and/or protein. ER, endoplasmic reticulum; UPR, unfolded protein response; GR, glucocorticoid receptor; ATF4, activating transcription factor 4; HIF1α, hypoxia-inducible factor 1α; AR, androgen receptor.
In addition to uncovering the role of REDD1 during these conditions, it will be important to identify the factor(s) that regulate REDD1 expression. Though this generally remains elusive, it will be central to the future design of therapies to prevent or treat REDD1-centric skeletal muscle disease. Similarly, it is unknown what factor(s) and pathway(s) regulate REDD1 protein turnover, as the half-life of REDD1 is quite short (<5 min). In all, experiments to answer these and other questions represent the initial step(s) in the generation of new therapies specifically targeting REDD1 and/or REDD2 to treat pathological conditions in which skeletal muscle is negatively impacted.
GRANTS
This review was supported in part by Grants R01 GM-38036 (C. H. Lang), R37 AA-011290 (C. H. Lang), F32 AA-023422 (J. L. Steiner), R01 DK-15658 (S. R. Kimball), R01 DK-13499 (S. R. Kimball), and R01 DK-094141-03 (S. R. Kimball).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.S.G., J.L.S., C.H.L., and S.R.K. conception and design of research; B.S.G. and J.L.S. prepared figures; B.S.G., J.L.S., D.L.W., C.H.L., and S.R.K. drafted manuscript; B.S.G., J.L.S., D.L.W., C.H.L., and S.R.K. edited and revised manuscript; B.S.G., J.L.S., D.L.W., C.H.L., and S.R.K. approved final version of manuscript.
REFERENCES
- 1.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94: 2692–2701, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol 22: 274–282, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Apro W, Wang L, Ponten M, Blomstrand E, Sahlin K. Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 305: E22–E32, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv Nutr 3: 307–321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100–1106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150: 1196–1208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Braun TP, Grossberg AJ, Krasnow SM, Levasseur PR, Szumowski M, Zhu XX, Maxson JE, Knoll JG, Barnes AP, Marks DL. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J 27: 3572–3582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol 6: 12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun TP, Szumowski M, Levasseur PR, Grossberg AJ, Zhu XX, Agarwal A, Marks DL. Muscle Atrophy in Response to Cytotoxic Chemotherapy Is Dependent on Intact Glucocorticoid Signaling in Skeletal Muscle. Plos One 9: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britto FA, Begue G, Rossano B, Docquier A, Vernus B, Sar C, Ferry A, Bonnieu A, Ollendorff V, Favier FB. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 307: E983–E993, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18: 2893–2904, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaillou T, Koulmann N, Simler N, Meunier A, Serrurier B, Chapot R, Peinnequin A, Beaudry M, Bigard X. Hypoxia transiently affects skeletal muscle hypertrophy in a functional overload model. Am J Physiol Regul Integr Comp Physiol 302: R643–R654, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55: 2565–2582, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cormie P, McGuigan MR, Newton RU. Adaptations in athletic performance after ballistic power versus strength training. Med Sci Sports Exerc 42: 1582–1598, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem 280: 9769–9772, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Crawford BA, Liu PY, Kean MT, Bleasel JF, Handelsman DJ. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab 88: 3167–3176, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Cuaz-Perolin C, Furman C, Larigauderie G, Legedz L, Lasselin C, Copin C, Jaye M, Searfoss G, Yu KT, Duverger N, Negre-Salvayre A, Fruchart JC, Rouis M. REDD2 gene is upregulated by modified LDL or hypoxia and mediates human macrophage cell death. Arterioscler Thromb Vasc Biol 24: 1830–1835, 2004. [DOI] [PubMed] [Google Scholar]
- 20.D'Hulst G, Jamart C, Van Thienen R, Hespel P, Francaux M, Deldicque L. Effect of acute environmental hypoxia on protein metabolism in human skeletal muscle. Acta Physiol (Oxf) 208: 251–264, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Davis TA, Karl IE. Resistance of protein and glucose metabolism to insulin in denervated rat muscle. Biochem J 254: 667–675, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 286: 8287–8296, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis MD, Jefferson LS, Kimball SR. Role of p70S6K1-mediated phosphorylation of eIF4B and PDCD4 proteins in the regulation of protein synthesis. J Biol Chem 287: 42890–42899, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis MD, Coleman CS, Berg A, Jefferson LS, Kimball SR. REDD1 enhances protein phosphatase 2A-mediated dephosphorylation of Akt to repress mTORC1 signaling. Sci Signal 7: ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis MD, Shenberger JS, Stanley BA, Kimball SR, Jefferson LS. Hyperglycemia mediates a shift from Cap-dependent to Cap-independent translation via a 4E-BP1 dependent mechanism. Diabetes 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev 22: 239–251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 141: 856–862, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake JC, Alway SE, Hollander JM, Williamson DL. AICAR treatment for 14 days normalizes obesity-induced dysregulation of TORC1 signaling and translational capacity in fasted skeletal muscle. Am J Physiol Regul Integr Comp Physiol 299: R1546–R1554, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 40: 691–698, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol (1985) 106: 1403–1411, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dungan CM, Wright DC, Williamson DL. Lack of REDD1 reduces whole body glucose and insulin tolerance, and impairs skeletal muscle insulin signaling. Biochem Biophys Res Commun 453: 778–783, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, Haber DA. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell 10: 995–1005, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Evans M, Morine K, Kulkarni C, Barton ER. Expression profiling reveals heightened apoptosis and supports fiber size economy in the murine muscles of mastication. Physiol Genomics 35: 86–95, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Favier FB, Costes F, Defour A, Bonnefoy R, Lefai E, Bauge S, Peinnequin A, Benoit H, Freyssenet D. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am J Physiol Regul Integr Comp Physiol 298: R1659–R1666, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Fearon KC, Voss AC, Hustead DS, and Cancer Cachexia Study G. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr 83: 1345–1350, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Forrester HB, Li J, Leong T, McKay MJ, Sprung CN. Identification of a radiation sensitivity gene expression profile in primary fibroblasts derived from patients who developed radiotherapy-induced fibrosis. Radiother Oncol 111: 186–193, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Francaux M, Demeulder B, Naslain D, Fortin R, Lutz O, Caty G, Deldicque L. Aging Reduces the Activation of the mTORC1 Pathway after Resistance Exercise and Protein Intake in Human Skeletal Muscle: Potential Role of REDD1 and Impaired Anabolic Sensitivity. Nutrients 8: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost RA, Huber D, Pruznak A, Lang CH. Regulation of REDD1 by insulin-like growth factor-I in skeletal muscle and myotubes. J Cell Biochem 108: 1192–1202, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 26: 83–96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985) 108: 1199–1209, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11: 1457–1466, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Garlick PJ, Millward DJ, James WP. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J 136: 935–945, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589: 5485–5501, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon BS, Kelleher AR, Kimball SR. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol 45: 2147–2157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon BS, Moir GL, Davis SE, Witmer CA, Cummings DM. An investigation into the relationship of flexibility, power, and strength to club head speed in male golfers. J Strength Cond Res 23: 1606–1610, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Gordon BS, Steiner JL, Lang CH, Jefferson LS, Kimball SR. Reduced REDD1 expression contributes to activation of mTORC1 following electrically induced muscle contraction. Am J Physiol Endocrinol Metab 307: E703–E711, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon BS, Williamson DL, Lang CH, Jefferson LS, Kimball SR. Nutrient-induced stimulation of protein synthesis in mouse skeletal muscle is limited by the mTORC1 repressor REDD1. J Nutr 145: 708–713, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greig CA, Gray C, Rankin D, Young A, Mann V, Noble B, Atherton PJ. Blunting of adaptive responses to resistance exercise training in women over 75 y. Exp Gerontol 46: 884–890, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Hayasaka M, Tsunekawa H, Yoshinaga M, Murakami T. Endurance exercise induces REDD1 expression and transiently decreases mTORC1 signaling in rat skeletal muscle. Physiol Rep 2: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12: 703–719, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123: 569–580, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol 43: 1267–1276, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol 85: 1337–1341, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Huang CX, Tighiouart H, Beddhu S, Cheung AK, Dwyer JT, Eknoyan G, Beck GJ, Levey AS, Sarnak MJ. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int 77: 624–629, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulmi JJ, Silvennoinen M, Lehti M, Kivela R, Kainulainen H. Altered REDD1, myostatin, and Akt/mTOR/FoxO/MAPK signaling in streptozotocin-induced diabetic muscle atrophy. Am J Physiol Endocrinol Metab 302: E307–E315, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Iliev DB, Goetz GW, Mackenzie S, Planas JV, Goetz FW. Pathogen-associated gene expression profiles in rainbow trout macrophages. Comp Biochem Physiol Part D Genomics Proteomics 1: 416–422, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Imen JS, Billiet L, Cuaz-Perolin C, Michaud N, Rouis M. The regulated in development and DNA damage response 2 (REDD2) gene mediates human monocyte cell death through a reduction in thioredoxin-1 expression. Free Radic Biol Med 46: 1404–1410, 2009. [DOI] [PubMed] [Google Scholar]
- 60.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287: C834–C843, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Jaka O, Kramerova I, Azpitarte M, Lopez de Munain A, Spencer M, Saenz A. C3KO mouse expression analysis: downregulation of the muscular dystrophy Ky protein and alterations in muscle aging. Neurogenetics 13: 347–357, 2012. [DOI] [PubMed] [Google Scholar]
- 62.Jin HO, Seo SK, Woo SH, Kim ES, Lee HC, Yoo DH, An S, Choe TB, Lee SJ, Hong SI, Rhee CH, Kim JI, Park IC. Activating transcription factor 4 and CCAAT/enhancer-binding protein-beta negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress. Free Radic Biol Med 46: 1158–1167, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Kammoun M, Cassar-Malek I, Meunier B, Picard B. A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur J Histochem 58: 163–168, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelleher AR, Gordon BS, Kimball SR, Jefferson LS. Changes in REDD1, REDD2, and atrogene mRNA expression are prevented in skeletal muscle fixed in a stretched position during hindlimb immobilization. Physiol Rep 2: e00246, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab 304: E229–E236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelleher AR, Pereira SL, Jefferson LS, Kimball SR. REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: responses to aging, Eimmobilization, and remobilization. Am J Physiol Endocrinol Metab 308: E122–E129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10: 935–945, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimball SR, Do AN, Kutzler L, Cavener DR, Jefferson LS. Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J Biol Chem 283: 3465–3475, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimball SR, Jefferson LS. Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise. J Biol Chem 285: 29027–29032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumari R, Willing LB, Jefferson LS, Simpson IA, Kimball SR. REDD1 (regulated in development and DNA damage response 1) expression in skeletal muscle as a surrogate biomarker of the efficiency of glucocorticoid receptor blockade. Biochem Biophys Res Commun 412: 644–647, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet 14, Spec No 2: R251–R258, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Lang CH, Frost RA, Vary TC. Acute alcohol intoxication increases REDD1 in skeletal muscle. Alcohol Clin Exp Res 32: 796–805, 2008. [DOI] [PubMed] [Google Scholar]
- 73.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 122: 3589–3594, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50, Spec No: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 75.Liu D, Sartor MA, Nader GA, Gutmann L, Treutelaar MK, Pistilli EE, Iglayreger HB, Burant CF, Hoffman EP, Gordon PM. Skeletal muscle gene expression in response to resistance exercise: sex specific regulation. BMC Genomics 11: 659, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol 15: 702–713, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Ma XJM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009. [DOI] [PubMed] [Google Scholar]
- 78.MacNeil LG, Glover E, Bergstra TG, Safdar A, Tarnopolsky MA. The Order of Exercise during Concurrent Training for Rehabilitation Does Not Alter Acute Genetic Expression, Mitochondrial Enzyme Activity or Improvements in Muscle Function. Plos One 9: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marimuthu K, Murton AJ, Greenhaff PL. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. J Appl Physiol (1985) 110: 555–560, 2011. [DOI] [PubMed] [Google Scholar]
- 80.Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 809–813, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Masschelein E, Van Thienen R, D'Hulst G, Hespel P, Thomis M, Deldicque L. Acute environmental hypoxia induces LC3 lipidation in a genotype-dependent manner. FASEB J 28: 1022–1034, 2014. [DOI] [PubMed] [Google Scholar]
- 82.McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics 31: 86–95, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDonald KS, Delp MD, Fitts RH. Effect of hindlimb unweighting on tissue blood flow in the rat. J Appl Physiol (1985) 72: 2210–2218, 1992. [DOI] [PubMed] [Google Scholar]
- 84.McGhee NK, Jefferson LS, Kimball SR. Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1. J Nutr 139: 828–834, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michel G, Matthes HW, Hachet-Haas M, El Baghdadi K, de Mey J, Pepperkok R, Simpson JC, Galzi JL, Lecat S. Plasma membrane translocation of REDD1 governed by GPCRs contributes to mTORC1 activation. J Cell Sci 127: 773–787, 2014. [DOI] [PubMed] [Google Scholar]
- 86.Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A. Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab 295: E385–E392, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyazaki M, Esser KA. REDD2 is enriched in skeletal muscle and inhibits mTOR signaling in response to leucine and stretch. Am J Physiol Cell Physiol 296: C583–C592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mo K, Razak Z, Rao P, Yu Z, Adachi H, Katsuno M, Sobue G, Lieberman AP, Westwood JT, Monks DA. Microarray analysis of gene expression by skeletal muscle of three mouse models of Kennedy disease/spinal bulbar muscular atrophy. PLoS One 5: e12922, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murakami T, Hasegawa K, Yoshinaga M. Rapid induction of REDD1 expression by endurance exercise in rat skeletal muscle. Biochem Biophys Res Commun 405: 615–619, 2011. [DOI] [PubMed] [Google Scholar]
- 90.Nishida H, Ikegami A, Kaneko C, Kakuma H, Nishi H, Tanaka N, Aoyama M, Usami M, Okimura Y. Dexamethasone and BCAA Failed to Modulate Muscle Mass and mTOR Signaling in GH-Deficient Rats. PLoS One 10: e0128805, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oppert JM, Charles MA, Thibult N, Guy-Grand B, Eschwege E, Ducimetiere P. Anthropometric estimates of muscle and fat mass in relation to cardiac and cancer mortality in men: the Paris Prospective Study. Am J Clin Nutr 75: 1107–1113, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Ortells MC, Morancho B, Drews-Elger K, Viollet B, Laderoute KR, Lopez-Rodriguez C, Aramburu J. Transcriptional regulation of gene expression during osmotic stress responses by the mammalian target of rapamycin. Nucleic Acids Res 40: 4368–4384, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oshiro N, Rapley J, Avruch J. Amino Acids Activate Mammalian Target of Rapamycin (mTOR) Complex 1 without Changing Rag GTPase Guanyl Nucleotide Charging. J Biol Chem 289: 2658–2674, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 207: 534–540, 2009. [DOI] [PubMed] [Google Scholar]
- 95.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286: E321–E328, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, de Rekeneire N, Harris TB, Kritchevsky S, Tylavsky FA, Nevitt M, Cho YW, Newman AB, Health A, and Body Composition S. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 32: 1993–1997, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez-Gomez J, Rodriguez GV, Ara I, Olmedillas H, Chavarren J, Gonzalez-Henriquez JJ, Dorado C, Calbet JA. Role of muscle mass on sprint performance: gender differences? Eur J Appl Physiol 102: 685–694, 2008. [DOI] [PubMed] [Google Scholar]
- 98.Phillips SM, Glover EI, Rennie MJ. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J Appl Physiol (1985) 107: 645–654, 2009. [DOI] [PubMed] [Google Scholar]
- 99.Philp A, Schenk S, Perez-Schindler J, Hamilton DL, Breen L, Laverone E, Jeromson S, Phillips SM, Baar K. Rapamycin does not prevent increases in myofibrillar or mitochondrial protein synthesis following endurance exercise. J Physiol 593: 4275–4284, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pieri BL, Souza DR, Luciano TF, Marques SO, Pauli JR, Silva AS, Ropelle ER, Pinho RA, Lira FS, De Souza CT. Effects of physical exercise on the P38MAPK/REDD1/14-3-3 pathways in the myocardium of diet-induced obesity rats. Horm Metab Res 46: 621–627, 2014. [DOI] [PubMed] [Google Scholar]
- 101.Pisani DF, Leclerc L, Jarretou G, Marini JF, Dechesne CA. SMHS1 is involved in oxidative/glycolytic-energy metabolism balance of muscle fibers. Biochem Biophys Res Commun 326: 788–793, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Proud CG. mTORC1 signalling and mRNA translation. Biochem Soc Trans 37: 227–231, 2009. [DOI] [PubMed] [Google Scholar]
- 103.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130's role in Lewis lung carcinoma-induced cachexia. FASEB J 28: 998–1009, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S, Shirihai OS, Lee RT, Reed JC, Ellisen LW. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat Commun 6: 7014, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qin W, Pan J, Bauman WA, Cardozo CP. Differential alterations in gene expression profiles contribute to time-dependent effects of nandrolone to prevent denervation atrophy. BMC Genomics 11: 596, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quinn M, Ramamoorthy S, Cidlowski JA. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann NY Acad Sci 1317: 1–6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55: M168–M173, 2000. [DOI] [PubMed] [Google Scholar]
- 108.Regazzetti C, Dumas K, Le Marchand-Brustel Y, Peraldi P, Tanti JF, Giorgetti-Peraldi S. Regulated in development and DNA damage responses -1 (REDD1) protein contributes to insulin signaling pathway in adipocytes. PLoS One 7: e52154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev 18: 2879–2892, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, Kalinski H, Kamer I, Rozen A, Mor O, Keshet E, Leshkowitz D, Einat P, Skaliter R, Feinstein E. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol 22: 2283–2293, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, Kalinski H, Kamer I, Rozen A, Mor O, Keshet E, Leshkowitz D, Einat P, Skaliter R, Feinstein E. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol 22: 2283–2293, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Showkat M, Beigh MA, Andrabi KI. mTOR Signaling in Protein Translation Regulation: Implications in Cancer Genesis and Therapeutic Interventions. Mol Biol Int 2014: 686984, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steiner JL, Crowell KT, Kimball SR, Lang CH. Disruption of REDD1 gene ameliorates sepsis-induced decrease in mTORC1 signaling but has divergent effects on proteolytic signaling in skeletal muscle. Am J Physiol Endocrinol Metab 309: E981–E994, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steiner JL, Gordon BS, Lang CH. Moderate alcohol consumption does not impair overload-induced muscle hypertrophy and protein synthesis. Physiol Rep 3: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steiner JL, Kimball SR, Lang CH. Acute Alcohol-Induced Decrease in Muscle Protein Synthesis in Female Mice Is REDD-1 and mTOR-Independent. Alcohol Alcohol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steiner JL, Lang CH. Alcohol intoxication following muscle contraction in mice decreases muscle protein synthesis but not mTOR signal transduction. Alcohol Clin Exp Res 39: 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Metab 308: E699–E712, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Steiner JL, Lang CH. Sepsis attenuates the anabolic response to skeletal muscle contraction. Shock 43: 344–351, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tan CY, Hagen T. mTORC1 dependent regulation of REDD1 protein stability. PLoS One 8: e63970, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, Drummond MJ. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 593: 4259–4273, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas DR. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr 26: 389–399, 2007. [DOI] [PubMed] [Google Scholar]
- 123.Tisdale MJ. Cancer anorexia and cachexia. Nutrition 17: 438–442, 2001. [DOI] [PubMed] [Google Scholar]
- 124.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten Proteomics F. Tissue-based map of the human proteome. Science 347: 1260419, 2015. [DOI] [PubMed] [Google Scholar]
- 125.Vega-Rubin-de-Celis S, Abdallah Z, Kinch L, Grishin NV, Brugarolas J, and Zhang X. Structural analysis and functional implications of the negative mTORC1 regulator REDD1. Biochemistry (Mosc) 49: 2491–2501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem 281: 39128–39134, 2006. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z, Malone MH, Thomenius MJ, Zhong F, Xu F, Distelhorst CW. Dexamethasone-induced gene 2 (dig2) is a novel pro-survival stress gene induced rapidly by diverse apoptotic signals. J Biol Chem 278: 27053–27058, 2003. [DOI] [PubMed] [Google Scholar]
- 129.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One 6: e24650, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol 365: 174–186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whitney ML, Jefferson LS, Kimball SR. ATF4 is necessary and sufficient for ER stress-induced upregulation of REDD1 expression. Biochem Biophys Res Commun 379: 451–455, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williams PE, Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat 127: 459–468, 1978. [PMC free article] [PubMed] [Google Scholar]
- 133.Williamson DL, Dungan CM, Mahmoud AM, Mey JT, Blackburn BK, Haus JM. Aberrant REDD1-mTORC1 responses to insulin in skeletal muscle from type 2 diabetics. Am J Physiol Regul Integr Comp Physiol 309: R855–R863, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Williamson DL, Li Z, Tuder RM, Feinstein E, Kimball SR, Dungan CM. Altered nutrient response of mTORC1 as a result of changes in REDD1 expression: Effect of obesity versus REDD1 deficiency. J Appl Physiol (1985) 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu Y, Zhao W, Zhao J, Zhang Y, Qin W, Pan J, Bauman WA, Blitzer RD, Cardozo C. REDD1 is a major target of testosterone action in preventing dexamethasone-induced muscle loss. Endocrinology 151: 1050–1059, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332: 1322–1326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yuen KC, Chong LE, Riddle MC. Influence of glucocorticoids and growth hormone on insulin sensitivity in humans. Diabet Med 30: 651–663, 2013. [DOI] [PubMed] [Google Scholar]
- 138.Zhao W, Pan J, Zhao Z, Wu Y, Bauman WA, Cardozo CP. Testosterone protects against dexamethasone-induced muscle atrophy, protein degradation and MAFbx upregulation. J Steroid Biochem Mol Biol 110: 125–129, 2008. [DOI] [PubMed] [Google Scholar]