Abstract
Sleep disruption appears to be a core component of Alzheimer's disease (AD) and its pathophysiology. Signature abnormalities of sleep emerge before clinical onset of AD. Moreover, insufficient sleep facilitates accumulation of amyloid-β (Aβ), potentially triggering earlier cognitive decline and conversion to AD. Building on such findings, this review has four goals, evaluating: (i) associations and plausible mechanisms linking NREM sleep disruption, Aβ, and AD, (ii) a role for NREM sleep disruption as a novel factor linking cortical Aβ to impaired hippocampus-dependent memory consolidation, (iii) the potential diagnostic utility of NREM sleep disruption as a new biomarker of AD, and (iv) the possibility of sleep as a new treatment target in aging, affording preventative and therapeutic benefits.
Keywords: Sleep, Alzheimer's disease, Amyloid-β, Aging, Cognitive decline
Alzheimer's Disease and the Emerging Interaction with Sleep
Alzheimer's disease (AD) is one of the largest public health and economic challenges of the 21st century. One in ten adults over the age of 65 suffer from AD, representing a worldwide epidemic. As a result, there is a pressing need to develop sensitive biomarkers facilitating early detection, and effective treatment interventions1. Only by achieving both can the goals of prevention and therapeutic intervention be accomplished, the former before disease onset1. One emerging candidate that may fulfill all of these objectives is sleep. In this review, we evaluate evidence linking sleep disturbance with AD and its pathophysiology, especially amyloid-β (Aβ) pathology. We further outline the cognitive consequences of sleep disruption as a novel mechanistic conduit potentially contributing to cognitive decline associated with AD pathophysiology. Finally, we explore the potential of sleep to serve as both a biomarker of AD, and a new therapeutic and preventative strategy for lowering AD risk.
Sleep, Aβ, and Alzheimer’s Disease
Sleep in aging
A physiological hallmark of advancing age is the decline of sleep, wherein NREM slow wave sleep (SWS) declines are particularly significant2. These impairments begin in midlife, and in many older adults 75 years of age or older less than 10% of SWS time remains2. Similar reductions in the quality of SWS are observed, measurable in the electroencephalographic (EEG) signature of slow wave activity (SWA; ~0.5–4.5Hz)3, 4. These age-related decreases in NREM SWS quantity and quality are paralleled by increasing amounts of time spent awake at night, with sleep becoming more fragmented2. The prevalence of primary sleep disorders, including insomnia and sleep apnea, also increases with advancing age5, further impairing the restorative quality of sleep.
Importantly, however, sleep disruption is not uniformly observed across older adults of equivalent age5. Marked differences in the ability to generate sleep, including NREM SWS, exist2, 5. Similar variability is observed in the prevalence of sleep disorders5–7. This has lead to the suggestion that underlying pathological factors, such as those associated with abnormal aging and AD, may partially determine the type and severity of sleep deterioration in later life, and with it, the cognitive faculties supported by sleep4, 8.
Sleep in abnormal aging
Impairments of sleep structure are markedly exaggerated in those with mild cognitive impairment (MCI), and those suffering from AD9–11, relative to cognitively normal older adults. Analogous sleep impairments are present in older adults at highest biological risk for developing AD, such as carriers of the APOE4 allele, the most prominent genetic risk factor for late onset AD9. Additionally, the decline in physiological NREM sleep quality, specifically slow wave oscillatory activity, is accelerated in AD patients relative to age-matched controls10.
Indicating clinical and etiological relevance, the magnitude of sleep disruption progresses in unison with the severity of AD symptomatology and pathology6, 10, 12. For example, tau and Aβ protein levels measured in cerebrospinal fluid (CSF) predict the degree of reduced SWS time in AD patients, together with decreases in sleep efficiency and REM sleep12. Sleep disturbance also appears to be among the earliest observable symptoms of AD, present before and soon after MCI and AD diagnosis9, 10, 13–16. Beyond sleep disruption, clinical sleep disorders are strongly co-morbid with MCI and AD. Over 60% of patients with MCI and AD have at least one clinical sleep disorder6, 7, with sleep apnea and insomnia being most common. Furthermore, APOE4+ genotype is known to significantly increase the risk of developing sleep apnea17.
The physiological decline of sleep, particularly NREM sleep quantity and quality, is therefore a common feature of advancing age, yet the onset, severity, and nature of these impairments are all significantly accelerated in those with AD and those at highest risk for AD. Although these sleep disturbances have long been considered a robust symptom of AD, new evidence indicates that this relationship between AD and sleep disruption may be causal and bi-directional, representing an integral part of the disease and potentially its treatment.
Bidirectional links between sleep and Aβ pathology
Insomnia and sleep apnea are not only more prominent in AD, but conversely increase the risk of developing MCI and AD15, 16, suggesting a reciprocal relationship between sleep disturbance and AD pathophysiology. Furthermore, individuals with sleep apnea convert to MCI and AD at a younger age18. In contrast, successfully treating sleep disturbance can delay the age of onset into MCI18 and improve cognitive function in AD19, 20. While additional evidence is required, these findings point to a potential causal and bidirectional link between sleep disorders and AD. As this reciprocal model would further predict, older adults with superior sleep quality have a significantly lower risk of developing MCI and AD, and also maintain cognitive function for longer13, 14. Together, these findings indicate that healthier quality of sleep in later life may confer resilience to AD.
The bidirectional link between sleep disturbance and Aβ pathology is observed before clinical onset of AD, and can occur independent of insomnia or apnea12, 14, 21–23. This indicates that the association between sleep and Aβ pathology is not just a consequence of a primary sleep disorder, or end-stage neurodegeneration. Instead, emerging evidence links specific sleep deficits to the defining pathological features of AD: Aβ and tau pathology. Both subjective and objective measures of poor sleep correlate with the severity of cortical Aβ burden, CSF measures of Aβ, and phospohorylated tau in CSF12, 14, 21–23. Such sleep-Aβ associations have been reported in cognitively normal older adults, MCI patients, and those diagnosed with AD12, 21–23. Raising biomarker potential, the relationship between NREM sleep disruption and Aβ may be anatomically and neurophysiologically unique. First, associations with Aβ are selectively observed in the low frequency range of NREM SWA below 1Hz21, unlike the more general age-related decline in broader SWA from 1–4Hz linked to grey matter atrophy4. Second, the signature association with <1Hz NREM SWA correlates most significantly with Aβ in medial prefrontal cortex21—one of the earliest sites to accumulate Aβ24.
Rodent models further support a connection between Aβ and NREM sleep (Fig. 1A–F). Experimentally increasing cortical Aβ causally fragments NREM sleep25, 26, while experimentally decreasing NREM sleep and increasing wake time escalates Aβ production and corresponding cortical deposition25. Conversely, NREM sleep promotes the clearance of extracellular Aβ that accumulates during wakefulness27. Therefore, NREM sleep represents one critical pathway through which the brain appears to manage Aβ levels: sleep’s absence contributes to the aggregation of Aβ, while the presence of NREM sleep proactively reduces Aβ burden. Within this proposed framework, disrupted NREM SWS and excess wakefulness increases Aβ aggregation, which itself impairs NREM SWS, resulting in a vicious cycle accelerating AD progression28.
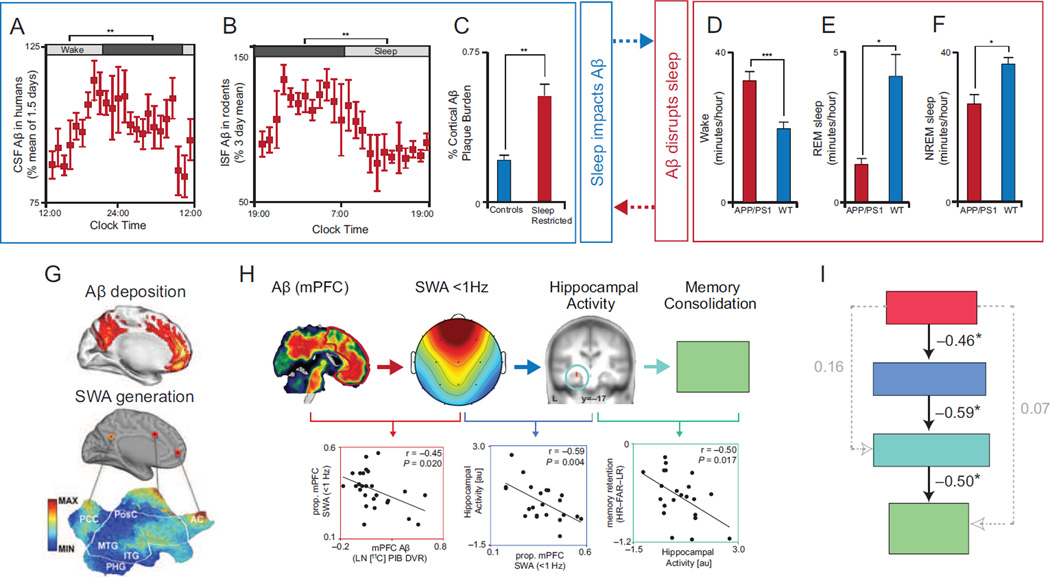
Figure 1.
Reciprocal relationship between Aβ and Sleep, and their influence on hippocampus-dependent memory consolidation. CSF Aβ in humans (a) and ISF Aβ in rodents (b) rise during wake and fall during sleep, and sleep restricting APP/PS1 mutant rodents results in higher cortical Aβ plaque burden (c; adapted from25). Further, APP/PS1 mutant rodents (red bars) exhibit increased wake time (d) and reduced REM (e) and NREM (f) sleep time relative to wild type rodents (blue bars; adapted from26). These findings represent a reciprocal relationship between sleep and Aβ: sleep and sleep disturbance can influence Aβ accumulation (a–c, blue box), while Aβ aggregation can disrupt sleep and increases wake time (d–f, red box). A potential mechanism underlying disrupted NREM SWS by Aβ pathology is the aggregation of Aβ (g, top sagittal brain slice adapted from48) within the same medial prefrontal cortical nodes critical for the electrical source generation of NREM slow waves (g, bottom sagittal brain slice adapted from47). Indeed, medial prefrontal Aβ burden predicts the degree of disrupted <1Hz NREM SWA (h, red scatter plot, adapted from21). The disruption of <1Hz NREM SWA by Aβ, in turn, is associated with impaired sleep-dependent consolidation of hippocampus-dependent memory. Disrupted <1Hz NREM SWA is associated with reduced overnight development of hippocampus-independent retrieval (h, blue scatter plot), that normally fosters superior memory stabilization and thus remembering (h, turquoise scatter plot). These interactions are further supported by structural equation modeling, which revealed that the only significant path linking Aβ pathology to impaired hippocampus-dependent memory was through its intermediary disruption of <1Hz NREM SWA (i, adapted from21). While the relationship between Aβ and NREM SWA is likely to be bidirectional, the strongest link between Aβ and memory was through its association with NREM SWA. Abbreviations: Aβ, amyloid-β protein; CSF, cerebrospinal fluid; ISF, interstitial fluid; APP/PS1, amyloid precursor protein and presenilin 1 mutant rodents; SWA, slow wave activity; MAX, maximum; MIN, minimum; PCC, posterior cingulate cortex; PosC, post-central gyrus; MTG, medial temporal gyrus; ITG, inferior temporal gyrus; PHG, parahippocampal gyrus; AC, anterior cingulate gyrus; mPFC, medial prefrontal cortex; prop., proportion; LN, natural logarithm; DVR, distribution volume ratio; L, left hemisphere; HR, hit rate; FAR, false alarm rate; LR, lure rate; au, arbitrary units; and HC, hippocampus.
Though NREM sleep associations with Aβ are most prominent, of note are emerging links between Aβ and REM sleep (detailed in Box 1). Moreover, evidence for the impact of tau pathology on sleep is rapidly growing, highlighting multifactorial mechanistic links between sleep disturbance and AD (described in Box 2).
Box 1. REM sleep, Aβ pathology, and Alzheimer's Disease.
Relationships between sleep, AD, and AD pathology extends beyond NREM SWS, and includes REM sleep disturbance. Patients with MCI and AD demonstrate reduced REM sleep amount, delayed REM sleep onset, and blunted rebound of REM sleep following selective deprivation9–12, 84, 85. MCI and AD patients both demonstrate reductions in the EEG quality of REM sleep86, 87; a feature that can even discriminate those with AD from cognitively normal older adults87. Moreover, Aβ correlates with reduced REM sleep amount in healthy older adults21 and patients with AD12. The selective degeneration of cholinergic projection neurons within the brainstem and basal forebrain (BF) may underlie aspects of this disruption88, 89. Both brainstem and BF cholinergic neurons regulate REM sleep90, 91, and BF cholinergic degeneration is an initial component of AD pathophysiological progression88. The degree of cortical Aβ burden correlates with the degree of BF atrophy in healthy older adults, MCI, and AD patients92. Aβ and tau have further been implicated in the degeneration of cholinergic neurons projecting from the BF to the cortex (see Box 2)92, 93.
REM sleep disruption in AD has cognitive and affective consequences. REM sleep disruption predicts worse Mini-Mental State Examination scores in MCI and AD patients, and neuropsychological impairment in older adults and MCI patients11, 12, 94. Disrupted REM sleep also predicts more severe longitudinal decline across multiple cognitive domains in older adults and AD patients84, 94. Another critical function of REM sleep is the regulation of emotional reactivity and mood states95, both of which are disturbed in AD. AD patients fail to show the normal enhancing effects of emotion on memory retention96, and express deficits in processing of complex emotional information97, both of which rely on REM sleep95. Furthermore, neuropsychiatric symptoms of AD, including depression, aggression, agitation, and anxiety98, are all observed in sleep-deprived individuals95. Moreover, depression and post-traumatic stress disorder (PTSD)—associated with REM sleep disturbance95—are risk factors for developing AD99, 100. Thus, REM sleep deficits may exacerbate psychiatric conditions common in AD patients101, pertinent considering the impact of these symptoms on caregiver burden and the likelihood of institutionalization102. While therapeutic interventions that selectively increase REM sleep are currently limited, cholinesterase inhibitors do increase REM sleep quality and duration, the success of which predicts the degree of memory improvement in AD patients20. Whether cholinesterase inhibitors offer similar benefits to the mood and emotional symptoms of AD remains a currently uninvestigated question.
Box 2. Sleep disturbance associated with tau pathology.
Tau-associated neurofibrillary tangles (NFT) are a central neuropathological component underlying AD and its symptoms50, 88, 103. The medial temporal lobe (MTL) accumulates NFT early in AD disease progression50. This regional aggregation is relevant considering the role of the hippocampus in generating ripples that are time-locked to the expression of NREM sleep spindles and slow waves which collectively support sleep-dependent memory processing53, 55.
Tau within the MTL diminishes the expression of hippocampal ripples in rodents, resulting in less temporally synchronized ripple events104. This desynchrony is, in part, due to loss of GABA-dependent inhibition that dictates patterned neural firing and thus governs neural oscillations104. Tau is further associated with abnormally long hyperpolarized down states and impaired depolarizing up states during NREM slow oscillations within the cortex105. Adding to reports in rodents, human studies have identified associations between CSF tau and diminished NREM SWS in patients with AD12. Moreover, AD patients have fewer NREM sleep spindles relative to healthy older adults, with the degree of spindle reduction predicting the severity of memory impairment106.
In addition to tau disrupting sleep, sleep impacts tau accumulation. Preliminary evidence indicates that chronic sleep restriction may impair hippocampus-dependent memory and increase tau accumulation, particularly insoluable tau linked to NFT formation107, 108. Conversely, the glymphatic system may also promote tau clearance109. Mechanistically, this may help explain why older adults with superior sleep continuity have significantly less NFT pathology at autopsy14. It may further account for greater resilience against the detrimental impact of the APOE4 genotype on AD risk14, implicating sleep as a potential reserve factor countering AD pathophysiology.
Several critical questions emerge from the proposed vicious cycle linking tau and sleep disruption. For example, does MTL tau aggregation proportionally disrupt the expression of, and network interaction between, NREM sleep oscillations (ripples, spindles, and slow waves), thereby contributing to memory impairment? Additionally, what is the nature of relationships between sleep, Aβ, and tau pathology? Is the impact of Aβ and tau on sleep (and vice versa) inter-related or independent, and do these interactions forecast the progression of cognitive decline in aging and AD? Considering several reports that have linked NFT accumulation in the basal forebrain, brainstem, and hypothalamus with both NREM and REM sleep disruption, neuronal degeneration, and the cognitive functions sleep subserves20, 64, 88, 89, 110–112 (Box 3), another issue regards tau’s impact on sleep beyond its accumulation within the MTL and its association with NREM sleep oscillations.
Mechanisms of sleep disruption and Aβ
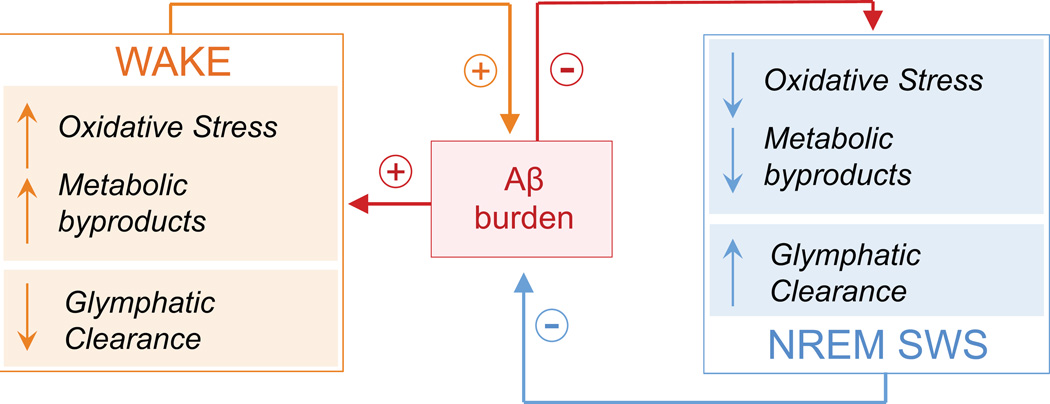
While a bidirectional relationship between NREM sleep disruption and Aβ pathology is likely, the underlying mechanism(s) are unclear. Some clues are emerging, however, implicating active, antagonistic mechanisms underlying the reciprocal relationship between NREM sleep and Aβ, as well as the facilitatory relationships between wake-dependent processes and Aβ production (Fig. 2).
Figure 2.
Proposed theoretical schematic of active mechanistic processes regulating the reciprocal nature between NREM sleep and wake with respect to Aβ burden. During wake (orange box), glymphatic Aβ clearance is low27, neurometabolic and neuronal spiking activity is high34, 42, and oxidative stress is high38, fostering higher Aβ burden (red box)25, 27, 28, 40. Aβ, in turn, promotes greater oxidative stress41, neuronal hyperexcitability42, and reduces glymphatic clearance through processes including cerebral amyloid angiopathy29 and, presumably, NREM sleep disturbance21, 26. Thus, a facilitatory process is created where Aβ may promote its own accumulation. During NREM sleep (blue box), glymphatic Aβ clearance is high27, neurometabolic rate is low34,35, and active cellular processes promote cellular restitution that reduces oxidative damage36. This balances both Aβ accumulation and the negative consequences of Aβ accumulation under conditions of healthy sleep. However, under pathological conditions, Aβ burden may actively disrupt NREM sleep21, 26. This disruption, alongside reduced Aβ clearance due to cerebral amyloid angiopathy29, theoretically creates an environment whereby NREM sleep can no longer successfully suppress Aβ accumulation. This once again exacerbates the vicious cycle, triggering greater Aβ aggregation and accelerating AD pathophysiological progression. Abbreviations: Aβ, amyloid-β protein; and NREM SWS, non-rapid-eye-movement slow wave sleep.
One recent discovery has described a sleep-dependent role for the glymphatic system in dictating Aβ clearance27. During NREM sleep, glial cells shrink by as much as 60%, facilitating a markedly increased flow of cerebrospinal fluid through interstitial space. The result is an enhanced clearance of extracellular toxins and metabolic detritus during NREM sleep. Extracellular Aβ is vacated by this mechanism at a two-fold faster rate during NREM SWS than during25, 27 wake (Fig. 2). Of relevance, Aβ clearance is reduced in AD29. The cause may, in part, be due to chronic sleep disruption and/or sleep-apnea induced hypoxia. Both can increase blood vessel stiffness by triggering chronic hypertension30–32, which alongside cerebral amyloid angiopathy29, reduces clearance efficiency32.
Beyond the role of NREM sleep in this model of Aβ regulation is an active impact of the waking brain state that further contributes to increases in Aβ25, in particular through its higher neurometabolic rate relative to NREM sleep (Fig. 2)33. Neurons consume greater levels of oxygen and ATP during wakefulness34, 35, while NREM sleep is associated with reduced oxygen consumption and the active replenishment of ATP levels34, 35. Waking therefore represents a state of higher oxygen, ATP, and glucose consumption, resulting in higher rates of metabolic distress36. Ergo, without sufficient NREM sleep to manage this waking burden, a higher risk for neurotoxic and oxidative consequences that promote AD pathophysiology is suffered36–38. Supporting this proposal, amplified neurometabolic activity results in increased amyloid precursor protein (APP) production and β and γ-secretase interactions, directly increasing Aβ production39. In addition, Aβ accumulation is promoted by oxidative stress40 and further promotes oxidative stress itself41. This is in direct contrast to NREM sleep, which actively regulates oxidative stress and promotes cellular repair in the face of accumulating cellular oxidative damage36, 38.
Through its increased metabolic activity, wakefulness may therefore promote both APP and β and γ-secretase interactions and the build-up of oxidative stress. Both of these processes cause Aβ to accumulate, with Aβ itself further potentiating its own production40, 41 (Fig. 2). In an otherwise healthy system, wake-dependent build-up of metabolic and oxidative byproducts is managed by NREM sleep, through at least two routes: (1) a sleep-dependent glymphatic response that promotes clearance of metabolic and neurotoxic waste, including Aβ27, and (2) active restorative cellular processes that mitigates the impact of accumulated oxidative stress, e.g. replenishment of ATP, repair of DNA damage35, 36. However, NREM sleep disturbance and/or sleep apnea-associated hypoxia30—both of which are more common in older adults, and especially those with MCI and AD—impairs this restorative process, leading to an escalation of Aβ. This Aβ aggregation, in turn, triggers increased sleep disruption through a positive feedback loop, and thus a vicious cycle ensues (Fig. 2). Further promoting this vicious cycle, increased Aβ burden enhances neuronal excitability, with chronic sleep loss exacerbating this hyperexcitability through epileptogenic mechanisms42. Thus, not only does sleep loss promote Aβ aggregation while Aβ aggregation promotes sleep loss, but sleep loss also magnifies the effect of Aβ aggregation on neuronal function. This magnification has the potential to facilitate neuronal hyperexcitability42, disrupt the impact of sleep on synaptic potentiation23, and even trigger nonlinear increases in Aβ accumulation, accelerating AD pathogenesis.
Nevertheless, numerous questions remain unresolved. For example, the precise mechanism(s) through which Aβ disrupts NREM sleep physiology, specifically within slow oscillation frequency range (<1Hz), is unknown. One tenable candidate that we offer is Aβ-disruption of frontal NMDA and GABAA receptor function that underlies NREM slow oscillation expression in cortical regions known to accumulate Aβ early43–45. The low frequency (<1Hz) slow oscillations of NREM sleep are governed by NMDA and GABAA receptor activity, the former dictating a cellular UP state of cortical excitation, the latter the DOWN state involving prolonged hyperpolarization44. Any perturbation in their function, such as that caused by Aβ43, 44, could result in a selective reduction in NREM slow oscillation generation. Three lines of evidence tentatively support this possibility. First, NREM slow oscillations are impaired in rodent models of AD, with higher Aβ levels associated with increased UP state activity through NMDA-dependent Ca2+ influx, and thus reduced DOWN state duration through GABAA-dependent Cl− influx46. Second, pharmacologically blocking cortical NMDA receptors decreases the incidence of the NREM slow oscillation while hastening its frequency44. Third, NMDA receptor function is disrupted in AD, particularly within the frontal lobe—the same regions in which NREM slow oscillations are predominantly generated43, 47. While preliminary, these findings implicate an influence of Aβ on GABA and NMDA receptor function that may underlie the selective impairment of frontal NREM SWA expression in the <1Hz frequency range in older adults. Although more empirical evidence is required, this hypothesis offers at least one, receptor-dependent, pathway through which Aβ pathology impairs the qualitative expression of NREM slow oscillations, resulting in a sleep state more vulnerable to fragmentation.
The Role of Sleep Disruption in AD and Aβ -dependent Cognitive Decline
Individuals with higher cortical Aβ burden have proportionally worse hippocampus-dependent memory21, 48–52. While Aβ aggregates significantly within specific medial and lateral prefrontal, posterior cingulate, and precuneus cortical regions48, 50, all of which generate NREM slow oscillations47 (Fig. 1G), Aβ does not accumulate substantively within the hippocampus until relatively late in AD. How then does a largely cortical-based pathology engender a sub-cortical, hippocampus-dependent memory impairment? While tauopathy and synaptic loss undoubtedly play critical roles50, it remains possible that Aβ pathology influences hippocampus-dependent memory indirectly, through intermediary factor(s) including disturbed NREM sleep.
Mounting basic and translational evidence suggests that NREM sleep disruption represents one potential factor brokering the influence of cortical Aβ on impaired, hippocampus-dependent long-term memory consolidation. First, NREM sleep causally enhances episodic memory consolidation in healthy adults through the coordinated interaction of three associated oscillations: (1) hippocampal ripples, (2) cortical slow oscillations (<1 Hz), and (3) thalamo-cortical sleep spindles53–55. Cortical NREM slow oscillations coordinate a time-locked expression of sleep spindle and ripple events, with hippocampal ripples nested in temporal synchrony within the troughs of the sleep spindle oscillation53. Through this interaction, the hippocampus and neocortex are proposed to engage in a coordinated dialogue, allowing memory representations to become increasingly cortically-dependent and hippocampally-independent—a transformation that offers resistance to interference and minimizes forgetting55. This innate physiological system can be experimentally manipulated. Stimulation methods in humans that causally enhance <1Hz slow oscillations and sleep spindles, as well as their coupling, enhance overnight memory consolidation and associated next-day retention54, 56. Conversely, both sleep deprivation and the selective deprivation of slow waves impairs episodic memory57,58. It is therefore possible that any pathological disruption of this set of coordinated NREM oscillations—such as that associated with Aβ and/or tau pathology—could impair numerous aspects of sleep-dependent memory processing that contribute to cognitive decline in aging, including those of initial encoding and subsequent offline consolidation4, 8, 21. Consistent with this prediction, cognitive impairment in MCI and AD is associated with quantitative measures of poor sleep quality, particularly the deterioration of NREM sleep11, 12, 21. Moreover, CSF Aβ, tau, and orexin levels correlate with both sleep and cognitive measures, suggesting that sleep may be linked to both disease pathology and the memory decline associated with that pathology (further orexin details in Box 3)12. The degree of disruption in slow wave activity further predicts the severity of memory impairment in both healthy and Aβ+ older adults4, 21. Perhaps most compelling are recent findings demonstrating that the severity of Aβ burden within medial prefrontal cortex significantly predicts the degree of impairment in <1Hz NREM SWA generation21 (Fig. 1H). Moreover, this reduced <1Hz NREM SWA generation was further associated with impaired overnight memory consolidation (and thus retention), together with impoverished hippocampal-neocortical memory transformation. Finally, structural equation models revealed that the association between cortical β-amyloid pathology and impaired hippocampus-dependent memory consolidation statistically depended on the degree of diminished <1Hz NREM SWA (Fig. 1I). An important next challenge will be to understand if and how AD-related sleep disruption impacts memory processing before and beyond consolidation, since sleep has been associated with all key stages of long-term memory: encoding8, integration59, reconsolidation (post-retrieval)60, and retrieval of long-term memory61.
Box 3. The role of orexin in Alzheimer's Disease.
The hypothalamic orexin system contributes to the regulation of sleep and wake states. Degeneration of the orexin system in AD has long been recognized110–112. However, hypothalamic orexin dysfunction may actively contribute to AD pathophysiology; a possibility supported by the finding that individuals carrying a polymorphism of an orexin receptor gene show increased AD risk113. Nevertheless, controversies remain. Some studies reported higher orexin levels in AD12, 114. Others, in contrast, reported either no difference115–117, or lower orexin levels110. A potential explanation is that orexin changes across AD stages are not linear. In early stages12, 114, orexin levels may increase in response to orexinergic neurodegeneration. In later AD stages, degeneration of orexinergic neurons may overtake compensation110. In MCI and early AD, higher orexin levels predicted longer sleep latency, more fragmented sleep, and shorter REM sleep duration12, while lower orexin levels in late stage AD predicted more fragmented daytime wakefulness116. Whether increased orexin is unique to AD, separating it from dementias such as frontotemporal and Lewy body dementia, remains unclear. While some data supports this differential distinction117, 118, there is also evidence that hypothalamic tau burden predicts the severity of orexin neurodegeneration independent of dementia type112.
While the precise pathway(s) through which alteration of orexin impacts AD remain unclear, Aβ and tau are implicated. Orexin levels predict CSF Aβ114 and tau levels12, 115 in AD, though tau relationships are not AD specific112, 115. In rodents, orexin infusion increases Aβ levels, while Aβ levels decreased following the blockage of orexin receptors25, 83. Aβ levels are also reduced in orexin knockout mice25, 83. However, orexin knockout mice slept more, and sleep deprivation still increased Aβ deposition83. Thus, orexin alters Aβ through its impact on sleep/wake behavior.
AD pathophysiology may therefore induce hypothalamic orexin neurodegeneration, while orexinergic degeneration, and the sleep-wake dysfunction associated with it, may instigate AD pathophysiology. Why tau pathology preferentially accumulates within hypothalamic orexin neurons, and how tau triggers increased orexin levels remains unknown. Another perplexing finding is that patients with narcolepsy, who have profound orexingic system degeneration, do not show an elevated risk for AD119. This despite the fact that CSF tau and Aβ levels are altered in narcoleptic patients120, with two thirds of narcoleptic patients having tau pathology119. Such disparity may suggest that the neurobiology of narcolepsy is more indicative of a general increased risk for tauopathies, rather than AD specifically, though a more complex mechanistic explanation may emerge.
Disrupted sleep therefore could be a novel, yet clinically underappreciated, mechanistic conduit through which cortical Aβ contributes to hippocampus-dependent cognitive decline in the initial stages of AD progression. However, this same disruption of NREM SWA, integral to AD pathophysiology, offers new translational opportunities, the diagnostic and therapeutic aspects of which we outline in the remaining sections.
Sleep Disruption as an Early Diagnostic Biomarker of AD Risk
There is urgent need to identify and develop early biomarkers that determine which individuals are at greatest risk for developing AD, motivated by at least two goals: (1) offering the chance for preventative measures, pre-disease onset, and (2) allowing nascent treatment intervention, early in the disease process1, 50. Several lines of evidence now suggest that selective diminutions of NREM sleep quality may serve both of these goals, potentially representing a novel, non-invasive, relatively inexpensive, and potentially specific biomarker of AD pathology. First, disruptions of NREM SWS have been detected at early stages of those declining into AD, before clinical onset9, 11, 21. Second, the degree of sleep disruption is exaggerated in individuals with a genetic risk for developing AD, i.e. APOE4+ older adults9. Third, even in healthy older adults without mild cognitive impairment, subjective and objective measures of sleep quality significantly predict the degree of existing cortical Aβ burden21–23. Fourth, reduced <1Hz NREM SWA predicts Aβ in medial prefrontal cortex21—one of the earliest cortical sites to accumulate Aβ pathology24. Of note, this association is independent of the general age-related reductions in SWA (1–4Hz) associated with grey matter loss4.
Frequency-specific quantitative EEG measures of NREM sleep, particularly that in the <1 Hz signature range, therefore offer signs of being an early biomarker of Aβ burden. Alongside other established biomarkers50, sleep EEG may therefore aid in identifying an individual's risk for developing AD years or even decades before onset of clinical symptoms. However, before this can be accepted, rigorous examination of this NREM spectral EEG signature must be undertaken. Specifically, its diagnostic utility must be characterized beyond its ability to distinguish between otherwise healthy Aβ+/− older adults. For example, while sleep disturbance is present among many other psychiatric and neurological conditions5, 62, it remains unclear to what degree this selective <1Hz NREM SWA disturbance is also present. Targeted examinations in a variety of clinical populations will ultimately determine the accuracy of sleep EEG for differential diagnosis, and for assessing clinical risk regarding the development of specific medical conditions, including AD.
In addition to quantitative EEG measures of NREM sleep, wrist actigraphy-measured sleep fragmentation and sleep efficiency may be independent or additive candidate biomarkers of AD pathology and risk. Actigraphy-defined low sleep efficiency and high sleep fragmentation in older adults predicts higher CSF-measured Aβ42 levels, declining cognitive status, and higher risk for developing AD within six years13, 63. Additionally, the degree of actigraphy-measured sleep fragmentation in aging and AD tracks the magnitude of neuronal degeneration within hypothalamic sleep regulatory regions, with AD patients showing the greatest sleep fragmentation and neuronal degeneration64. In contrast, individuals with more consolidated measures of actigraphy-determined sleep exhibit superior cognitive function, reduced risk for developing MCI or AD, and a reduced impact of APOE4+ genotype on both cognitive outcomes and AD risk14. These findings are consistent with rodent models with high Aβ production, which express a phenotype of marked NREM sleep fragmentation26. Therefore, the association between Aβ and actigraphy-measured sleep fragmentation, in conjunction with EEG-assessed deficits in <1Hz NREM SWA, may offer more meaningful sleep-related diagnostic utility in individuals at risk for developing AD. This hypothesis will need to be tested in future clinical investigations. Nevertheless, as actigraphy devices become more accessible, and if corresponding accuracy in tracking sleep quality sufficiently improves, the ramifications of this biomarker proposal could scale dramatically. However, in order to evaluate this possibility at a population scale, current mass-marketed wearable actigraphy devices will need to substantially improve upon currently reported accuracy, which appears to be low65.
Treatment Implications—Sleep Intervention as Preventative and Therapeutic
Unlike many other consequences of AD pathology, such as structural brain atrophy or reductions in cerebral blood flow, sleep is a modifiable factor, and thus a treatable target54, 56, 66. This is especially important considering that Aβ-related sleep disruption may impair hippocampus-dependent memory, thus contributing to cognitive decline21 (Fig. 3A). Therapeutic interventions that restore NREM slow wave sleep quantity and/or quality offer at least two new treatment possibilities. First, NREM sleep enhancement in mid- to late-life may deliver a preventative benefit that reduces AD risk, in part, through improved Aβ clearance27 and/or enhanced cellular restitution processes to combat accumulated oxidative stress36. While sleep enhancement should benefit all older adults, it may prove especially efficacious in high-vulnerable populations, such as APOE4+ individuals, which express marked sleep deficits14. Second, sleep restoration may help minimize the degree of cognitive decline in those with already extant Aβ pathology through two non-mutually exclusive mechanistic pathways: (i) increased Aβ clearance and cellular restitution, or (ii) enhance long-term memory consolidation that helps counteract cognitive decline associated with AD pathophysiology.
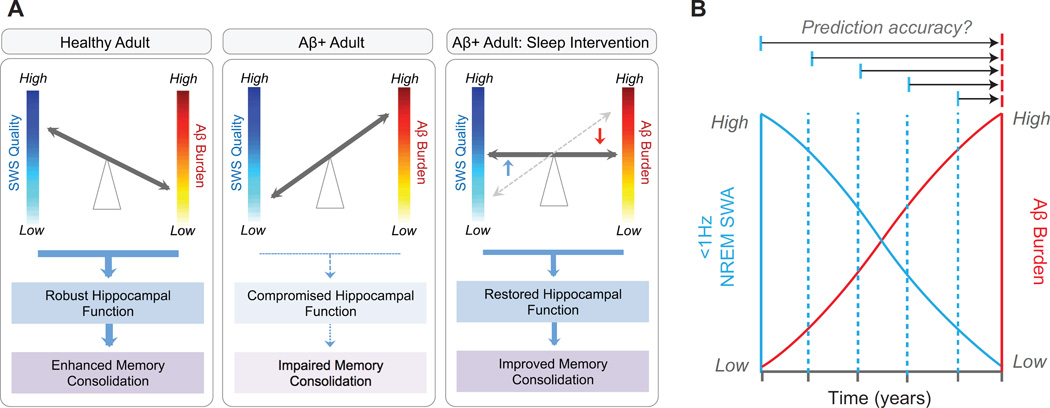
Figure 3.
Proposed consequences of the reciprocal relationship between Aβ, <1Hz NREM SWA and memory functioning under different circumstances, and the potential utility of sleep as a novel biomarker. In healthy older adults with low Aβ burden (a, left panel), NREM SWS quality is high, thereby facilitating hippocampus-dependent memory consolidation. In Aβ+ older adults (a, middle panel), NREM SWS quality is low, resulting in compromised memory consolidation. However, should NREM SWS quality be rescued through therapeutic sleep intervention in Aβ+ older adults (a, right panel), memory consolidation should be improved through two non-mutually exclusive pathways: i) by minimizing the negative impact of Aβ burden on sleep-dependent memory processing, and/or ii) through facilitating greater glymphatic Aβ clearance. (b) Since <1Hz NREM SWA is associated with Aβ burden in healthy older adults before MCI or AD onset, it is possible that this measure may offer diagnostic utility as a noninvasive biomarker of Aβ burden and AD risk. Longitudinal studies have the ability to examine the diagnostic potential of <1Hz NREM SWA, not only as a static, surrogate marker of current Aβ burden, but as a predictive biomarker that forecasts Aβ accumulation or risk of conversion to AD years in advance (multiple horizontal arrow tests). Abbreviations: Aβ, amyloid-β protein; Aβ + adult, an older adult with Aβ pathology; SWS, slow wave sleep; and NREM SWA, non-rapid-eye-movement slow wave activity.
Currently, there are several promising methods for achieving a NREM SWA enhancement benefit, particularly <1Hz NREM SWA. Several non-pharmacological methods represent the most tenable candidates for NREM sleep enhancement. Among the most well studied is transcranial direct current stimulation (tDCS) in the <1Hz range, which can double the overnight sleep-dependent memory benefit in young adults56. A few reports have successfully enhanced <1Hz NREM SWA and memory consolidation in young and older adults56, 66, patients with temporal lobe epilepsy67, individuals with attention deficit hyperactivity disorder68, and patients with schizophrenia69. A similar effect has also been reported in rodents70. Nevertheless, it is important to note that some failures to enhance <1Hz NREM SWA and associated memory consolidation have been described in young and older adults as well71, 72, suggesting that further refinement of the technique is required before this method can be recommended.
Other, more non-invasive, non-pharmacological methods include auditory closed-loop stimulation during NREM SWS that significantly enhances <1Hz NREM SWA and improves overnight hippocampus-dependent memory consolidation54. Preliminary findings in older adults have reported similar improvements in <1Hz NREM SWA using this same method73. Another method, kinesthetic stimulation during sleep—through slow, rhythmic bed rocking—has been shown to significantly increase low frequency NREM SWA in young adults, though no memory assessments were made74. Whether older adults would show similar low frequency NREM SWA enhancement, and whether such sleep improvement transacts a functional memory benefit, remains untested.
A limitation of all of these methods is that none of them have been tested for long-term efficacy. It remains unknown if any could foster enhanced NREM SWA and cognition for a sustained period. An alternative in this regard is cognitive behavioral therapy for chronic insomnia (CBT-i); a non-pharmacological, non-invasive method that can successfully enhance long-term sleep quality and cognitive outcomes in patients suffering from chronic insomnia75. As insomnia is more prominent in aging, MCI, and AD5, 7, and increases the risk for developing AD15, CBT-i is another candidate opportunity for intervention. However, it remains unknown whether CBT-i improves physiological sleep oscillations, including <1Hz NREM SWA, relevant for cognition and AD-pathology regulation.
Pharmacological methods for selective NREM sleep enhancement have so far proved less promising in the context of aging and cognition. Although multiple GABA-targeting hypnotic and anti-convulsive drugs that increase NREM SWA in a dose-dependent manner exist76–79, they often fail to trigger any corresponding sleep-dependent memory benefit in the elderly, and many even have amnestic effects77, 78, 80, 81. Moreover, such medications have actually been associated with an increased rather than lowering of dementia risk82. Two related mechanisms may explain these outcomes. First, many GABA-targeting drugs trigger faster frequency increases in NREM sleep spectral power, rather than enhancing slow frequencies that support memory and are disrupted by Aβ pathology77–79. Indeed, older adults expressing faster frequency NREM SWA (>1Hz) demonstrate significantly worse overnight hippocampus-dependent memory consolidation, highlighting the importance of attention to the slow frequencies in the context of AD therapy21. Current GABA-targeting medications may therefore enhance sleep EEG features that are not only non-optimal for memory consolidation, but counter to it. A second explanation is that many of these medications alter sleep spindles and their coupling with NREM slow waves77, with fewer spindles predicting worse memory81. Since the coupling between slow waves and sleep spindles is known to be critical for promoting hippocampal-neocortical communication that supports memory consolidation53–55, enhancing NREM SWA at the expense of sleep spindles and/or spindle-slow wave coupling may not promote memory consolidation, and may even disrupt sleep-dependent memory processing.
Little is currently known regarding the impact of non-GABA-targeting sleep medications on enhancing sleep-dependent memory in elderly populations at risk for dementia. For example, alterations in the orexin system have been implicated in both rodent models of AD and in human patients with AD12, 25, 83 (see Box 3). Nevertheless, it remains unclear whether therapies targeting orexin ameliorate sleep disruption in AD or in individuals at risk for developing AD, and whether such sleep improvement offers cognitive benefits.
Although considerably more research is necessary, it appears tenable that older adults and those with AD are permissive to sleep intervention. Indeed, treatment of sleep apnea in AD patients improves some cognitive outcomes19. Moreover, sleep apnea treatment before onset into MCI significantly delays the age of onset into MCI18. One goal of future research programs will be to determine whether experimentally enhancing NREM sleep—on its own, or in combination with other intervention and life-style factors—offers AD prophylaxis, limits AD progression upon development, and/or ameliorates disease symptomatology.
Concluding Remarks
As evidence for causal, bi-directional links between sleep disturbance and AD pathophysiology continues to grow, new key questions are emerging. We close by outlining a select few that, to us, appear pressing and potentially transformative.
First, most studies examining the relationship between sleep and AD pathology have used cross-sectional designs. No study to date has gathered longitudinal sleep EEG recordings alongside measures of AD pathophysiology and sleep-dependent memory. Such data are not only critical to establish the impact of sleep disturbance on AD risk within a given individual over time, but also to tease apart the directionality of these sleep-AD relationships and their relationship with varied stages of memory processing and retention. Furthermore, longitudinal designs offer a powerful test of the biomarker utility of sleep disturbance as an accurate forecasting tool of AD risk and AD pathological progression. Thus, longitudinal studies examining the diagnostic utility of sleep disturbance to forecast features of AD are now imperative. Such a scheme, outlined in Fig. 3B, include predictive changes in AD pathological burden, AD risk, conversion to MCI or AD, and/or the cognitive decline associated with AD.
Second, there is a need to systematically compare the relative impact of distinct sleep disorders and the varied signatures of sleep disturbance on AD risk. For example, are patients with sleep apnea, relative to otherwise healthy older adults or older adults with insomnia, at greater AD risk because they suffer from both chronic intermittent hypoxia and disrupted NREM slow oscillation expression? Furthermore, do co-morbid sleep disorders interact with other clinical risk factors, such as genetics, depression, cardiovascular disease, immune deficiencies or diabetes, to accelerate AD onset and/or progression? Is the sleep fragmentation associated with AD a symptom of co-morbid sleep disorders, such as sleep apnea or insomnia? While older adults at risk for AD can still show increased sleep fragmentation without having sleep apnea or insomnia, it remains unclear how much either diagnosed or undiagnosed sleep disorders explain this symptom.
A third unresolved question is whether specific electrophysiological signatures of sleep disruption, such as decreases in <1Hz NREM SWA, are unique to Aβ pathology, or if they similarly track tau pathological burden. If so, characterizing how the interaction of these factors leads to deficits in sleep-dependent learning, memory, and plasticity will be essential to obtain a complete understanding of the role of sleep in AD. The recent development of tau PET imaging in vivo in humans, combined with existing PET-amyloid imaging, now makes answering these questions viable.
Finally, there is urgent need for therapeutic sleep interventions and innovations that enhance sleep in the elderly and those with AD. A first step would be to focus on those aspects of sleep known to be especially impacted by AD pathology, and have functional cognitive consequences, such as NREM slow oscillations, sleep spindles, REM sleep, and sleep continuity. Moreover, given the multifaceted nature of sleep disturbance associated with AD, examining combinatorial approaches that target multiple underlying mechanisms of sleep disturbance in AD may be most effective. Clinical trials will then need to determine whether such targeted sleep improvement reduces AD risk, delays AD onset, slows AD pathophysiological progression, or alleviates the cognitive decline associated with AD.
Should any of the above be true, it would require that medical practice be more diligent in inquiring about, diagnosing, and treating sleep difficulties across the lifespan, especially in the elderly. More generally, such findings would argue for improved public health policies highlighting the critical need for sufficient quality sleep throughout adulthood—a memorandum that may lower dementia risk and maintain cognitive health across the population.
Highlights.
A bidirectional, causal interaction exists between NREM sleep and Aβ pathophysiology that may contribute to Alzheimer’s disease (AD) risk and progression.
The disruption of NREM sleep may represent a novel pathway through which cortical Aβ impairs hippocampus-dependent memory consolidation.
The disruption of NREM sleep physiology offers potential diagnostic utility in the form of a non-invasive biomarker of Aβ pathology, AD risk, and/or AD pathophysiological progression.
Evidence implicates sleep disturbance as a consequence and cause of AD progression; one that is modifiable, offering preventative and therapeutic treatment potential.
Outstanding Questions Box.
Longitudinally, what is the impact of sleep disturbance on AD risk and cognitive decline associated with AD, and what are the directionality of these relationships?
What is the contribution of distinct sleep disorders and age-associated sleep disturbance to AD risk and AD pathophysiology?
Are there distinct sleep biomarkers that predict unique aspects of AD patholophysiology and AD risk, and further track AD progression?
Are there therapeutic sleep interventions and innovations that enhance sleep in the elderly and those with AD, offering preventative and therapeutic value, respectively?
Acknowledgments
This work was supported by awards R01–AG031164(MPW), R01–AG054019(MPW), R01–AG034570(WJ) and F32–AG039170(BAM), from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sperling RA, et al. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm133. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 3.Carrier J, et al. Sleep slow wave changes during the middle years of life. Eur J Neurosci. 2011;33:758–766. doi: 10.1111/j.1460-9568.2010.07543.x. [DOI] [PubMed] [Google Scholar]
- 4.Mander BA, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitiello MV. Recent Advances in Understanding Sleep and Sleep Disturbances in Older Adults: Growing Older Does Not Mean Sleeping Poorly. Current Directions in Psychological Science. 2009;18:316–320. [Google Scholar]
- 6.Ancoli-Israel S, et al. Dementia in institutionalized elderly: relation to sleep apnea. J Am Geriatr Soc. 1991;39:258–263. doi: 10.1111/j.1532-5415.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 7.Guarnieri B, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord. 2012;33:50–58. doi: 10.1159/000335363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mander BA, et al. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb Cortex. 2014;24:3301–3309. doi: 10.1093/cercor/bht188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hita-Yanez E, et al. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE epsilon4 genotype. Curr Alzheimer Res. 2012;9:290–297. doi: 10.2174/156720512800107609. [DOI] [PubMed] [Google Scholar]
- 10.Prinz PN, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 11.Westerberg CE, et al. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. J Int Neuropsychol Soc. 2012:1–11. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liguori C, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71:1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 13.Lim AS, et al. Sleep Fragmentation and the Risk of Incident Alzheimer's Disease and Cognitive Decline in Older Persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim AS, et al. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70:1544–1551. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osorio RS, et al. Greater risk of Alzheimer's disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaffe K, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadotani H, et al. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–2890. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 18.Osorio RS, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancoli-Israel S, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moraes WdS, et al. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep. 2006;29:199–205. doi: 10.1093/sleep/29.2.199. [DOI] [PubMed] [Google Scholar]
- 21.Mander BA, et al. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spira AP, et al. Self-reported Sleep and beta-Amyloid Deposition in Community-Dwelling Older Adults. JAMA Neurol. 2013 doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sprecher KE, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sepulcre J, et al. In vivo characterization of the early states of the amyloid-beta network. Brain. 2013;136:2239–2252. doi: 10.1093/brain/awt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JE, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roh JH, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju YE, et al. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller RO, et al. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimers Res Ther. 2009;1:6. doi: 10.1186/alzrt6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93:1778–1794. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 31.Knutson KL, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyrtsos CR, Baras JS. Modeling the Role of the Glymphatic Pathway and Cerebral Blood Vessel Properties in Alzheimer's Disease Pathogenesis. PLoS One. 2015;10:e0139574. doi: 10.1371/journal.pone.0139574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchsbaum MS, et al. Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci. 1989;45:1349–1356. doi: 10.1016/0024-3205(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 34.Braun AR, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 35.Dworak M, et al. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everson CA, et al. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014;37:1929–1940. doi: 10.5665/sleep.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villafuerte G, et al. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev. 2015;2015:234952. doi: 10.1155/2015/234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, et al. Neuronal activity and secreted amyloid beta lead to altered amyloid beta precursor protein and presenilin 1 interactions. Neurobiol Dis. 2013;50:127–134. doi: 10.1016/j.nbd.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misonou H, et al. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry. 2000;39:6951–6959. doi: 10.1021/bi000169p. [DOI] [PubMed] [Google Scholar]
- 41.Yatin SM, et al. In vitro and in vivo oxidative stress associated with Alzheimer's amyloid beta-peptide (1–42) Neurobiol Aging. 1999;20:325–330. doi: 10.1016/s0197-4580(99)00056-1. discussion 339–342. [DOI] [PubMed] [Google Scholar]
- 42.Tabuchi M, et al. Sleep interacts with abeta to modulate intrinsic neuronal excitability. Curr Biol. 2015;25:702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurup P, et al. Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steriade M, et al. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulrich D. Amyloid-beta Impairs Synaptic Inhibition via GABAA Receptor Endocytosis. J Neurosci. 2015;35:9205–9210. doi: 10.1523/JNEUROSCI.0950-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busche MA, et al. Rescue of long-range circuit dysfunction in Alzheimer's disease models. Nat Neurosci. 2015;18:1623–1630. doi: 10.1038/nn.4137. [DOI] [PubMed] [Google Scholar]
- 47.Murphy M, et al. Source modeling sleep slow waves. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elman JA, et al. Neural compensation in older people with brain amyloid-beta deposition. Nat Neurosci. 2014;17:1316–1318. doi: 10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jack CR, Jr, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattsson N, et al. Brain structure and function as mediators of the effects of amyloid on memory. Neurology. 2015;84:1136–1144. doi: 10.1212/WNL.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mormino EC, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staresina BP, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015;18:1679–1686. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ngo HV, et al. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–553. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Diekelmann S, Born J. The memory function of sleep. Nature Reviews. Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 56.Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 57.Gais S, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Der Werf YD, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 59.Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nat Rev Neurosci. 2010;11:218. doi: 10.1038/nrn2762-c1. author reply 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker MP, et al. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 61.Dumay N. Sleep not just protects memories against forgetting, it also makes them more accessible. Cortex. 2016;74:289–296. doi: 10.1016/j.cortex.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Gagnon J-F, et al. Neurobiology of sleep disturbances in neurodegenerative disorders. Current pharmaceutical design. 2008;14:3430–3445. doi: 10.2174/138161208786549353. [DOI] [PubMed] [Google Scholar]
- 63.Ju YE, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim AS, et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain. 2014;137:2847–2861. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J, Finkelstein J. Consumer sleep tracking devices: a critical review. Stud Health Technol Inform. 2015;210:458–460. [PubMed] [Google Scholar]
- 66.Westerberg CE, et al. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Del Felice A, et al. Slow-oscillatory Transcranial Direct Current Stimulation Modulates Memory in Temporal Lobe Epilepsy by Altering Sleep Spindle Generators: A Possible Rehabilitation Tool. Brain Stimul. 2015;8:567–573. doi: 10.1016/j.brs.2015.01.410. [DOI] [PubMed] [Google Scholar]
- 68.Prehn-Kristensen A, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7:793–799. doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 69.Goder R, et al. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144:153–154. doi: 10.1016/j.schres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Binder S, et al. Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats. Brain Stimul. 2014;7:508–515. doi: 10.1016/j.brs.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Eggert T, et al. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimul. 2013;6:938–945. doi: 10.1016/j.brs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Sahlem GL, et al. Oscillating Square Wave Transcranial Direct Current Stimulation (tDCS) Delivered During Slow Wave Sleep Does Not Improve Declarative Memory More Than Sham: A Randomized Sham Controlled Crossover Study. Brain Stimul. 2015;8:528–534. doi: 10.1016/j.brs.2015.01.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Papalambros PA, et al. 1134: Acoustic Stimulation increases slow wave activity in older adults. Sleep. 2015;38:A402. [Google Scholar]
- 74.Bayer L, et al. Rocking synchronizes brain waves during a short nap. Curr Biol. 2011;21:R461–R462. doi: 10.1016/j.cub.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 75.Trauer JM, et al. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 76.Bettica P, et al. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. 2012;37:1224–1233. doi: 10.1038/npp.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feld GB, et al. Slow wave sleep induced by GABA agonist tiagabine fails to benefit memory consolidation. Sleep. 2013;36:1317–1326. doi: 10.5665/sleep.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vienne J, et al. Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory. Sleep. 2012;35:1071–1083. doi: 10.5665/sleep.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walsh JK, et al. Enhancing slow wave sleep with sodium oxybate reduces the behavioral and physiological impact of sleep loss. Sleep. 2010;33:1217–1225. doi: 10.1093/sleep/33.9.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hall-Porter JM, et al. The effect of two benzodiazepine receptor agonist hypnotics on sleep-dependent memory consolidation. J Clin Sleep Med. 2014;10:27–34. doi: 10.5664/jcsm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mednick SC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shih HI, et al. An increased risk of reversible dementia may occur after zolpidem derivative use in the elderly population: a population-based case-control study. Medicine (Baltimore) 2015;94:e809. doi: 10.1097/MD.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roh JH, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J Exp Med. 2014;211:2487–2496. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moe KE, et al. Symposium: Cognitive processes and sleep disturbances: Sleep/wake patterns in Alzheimer's disease: relationships with cognition and function. J Sleep Res. 1995;4:15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 85.Reynolds CF, 3rd, et al. Rapid eye movement sleep deprivation as a probe in elderly subjects. Arch Gen Psychiatry. 1990;47:1128–1136. doi: 10.1001/archpsyc.1990.01810240048009. [DOI] [PubMed] [Google Scholar]
- 86.Brayet P, et al. Quantitative EEG of Rapid-Eye-Movement Sleep: A Marker of Amnestic Mild Cognitive Impairment. Clin EEG Neurosci. 2015 doi: 10.1177/1550059415603050. [DOI] [PubMed] [Google Scholar]
- 87.Hassainia F, et al. Quantitative EEG and statistical mapping of wakefulness and REM sleep in the evaluation of mild to moderate Alzheimer's disease. Eur Neurol. 1997;37:219–224. doi: 10.1159/000117446. [DOI] [PubMed] [Google Scholar]
- 88.Mesulam M, et al. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55:815–828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- 89.Mufson EJ, et al. Neurofibrillary tangles in cholinergic pedunculopontine neurons in Alzheimer's disease. Ann Neurol. 1988;24:623–629. doi: 10.1002/ana.410240506. [DOI] [PubMed] [Google Scholar]
- 90.Lee MG, et al. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saper CB, et al. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 92.Kerbler GM, et al. Basal forebrain atrophy correlates with amyloid beta burden in Alzheimer's disease. Neuroimage Clin. 2015;7:105–113. doi: 10.1016/j.nicl.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geula C, et al. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:309–318. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song Y, et al. Relationships Between Sleep Stages and Changes in Cognitive Function in Older Men: The MrOS Sleep Study. Sleep. 2015;38:411–421. doi: 10.5665/sleep.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kensinger EA, et al. Effects of Alzheimer disease on memory for verbal emotional information. Neuropsychologia. 2004;42:791–800. doi: 10.1016/j.neuropsychologia.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 97.Torres B, et al. Facial expression recognition in Alzheimer's disease: a longitudinal study. Arq Neuropsiquiatr. 2015;73:383–389. doi: 10.1590/0004-282X20150009. [DOI] [PubMed] [Google Scholar]
- 98.Zhao QF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: Systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. doi: 10.1016/j.jad.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 99.Cherbuin N, et al. Dementia risk estimates associated with measures of depression: a systematic review and meta-analysis. BMJ Open. 2015;5:e008853. doi: 10.1136/bmjopen-2015-008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yaffe K, et al. Posttraumatic Stress Disorder and Risk of Dementia Among US Veterans. Archives of General Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Snowdon MB, et al. Longitudinal association of dementia and depression. Am J Geriatr Psychiatry. 2015;23:897–905. doi: 10.1016/j.jagp.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Craig D, et al. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer's disease. Am J Geriatr Psychiatry. 2005;13:460–468. doi: 10.1176/appi.ajgp.13.6.460. [DOI] [PubMed] [Google Scholar]
- 103.Schöll M, et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron. doi: 10.1016/j.neuron.2016.01.028. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Witton J, et al. Disrupted hippocampal sharp-wave ripple-associated spike dynamics in a transgenic mouse model of dementia. J Physiol. 2014 doi: 10.1113/jphysiol.2014.282889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menkes-Caspi N, et al. Pathological tau disrupts ongoing network activity. Neuron. 2015;85:959–966. doi: 10.1016/j.neuron.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 106.Rauchs Gr, et al. Is there a link between sleep changes and memory in Alzheimer's disease? Neuroreport. 2008;19:1159. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di Meco A, et al. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer's disease with plaques and tangles. Neurobiol Aging. 2014;35:1813–1820. doi: 10.1016/j.neurobiolaging.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 108.Rothman SM, et al. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical AÎ2 and pTau in a mouse model of Alzheimer's disease. Brain Research. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iliff JJ, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fronczek R, et al. Hypocretin (orexin) loss in Alzheimer's disease. Neurobiol Aging. 2012;33:1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 111.Ishii T. Distribution of Alzheimer's neurofibrillary changes in the brain stem and hypothalamus of senile dementia. Acta neuropathologica. 1966;6:181–187. doi: 10.1007/BF00686763. [DOI] [PubMed] [Google Scholar]
- 112.Kasanuki K, et al. Neuropathological investigation of hypocretin expression in brains of dementia with Lewy bodies. Neuroscience letters. 2014;569:68–73. doi: 10.1016/j.neulet.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 113.Gallone S, et al. Is HCRTR2 a Genetic Risk Factor for Alzheimer's Disease? Dementia and Geriatric Cognitive Disorders. 2014;38:245–253. doi: 10.1159/000359964. [DOI] [PubMed] [Google Scholar]
- 114.Dauvilliers YA, et al. Hypocretin and brain beta-amyloid peptide interactions in cognitive disorders and narcolepsy. Frontiers in Aging Neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deuschle M, et al. Hypocretin in cerebrospinal fluid is positively correlated with Tau and pTau. Neuroscience letters. 2014;561:41–45. doi: 10.1016/j.neulet.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 116.Friedman LF, et al. In Alzheimer disease, increased wake fragmentation found in those with lower hypocretin-1. Neurology. 2007;68:793–794. doi: 10.1212/01.wnl.0000256731.57544.f9. [DOI] [PubMed] [Google Scholar]
- 117.Wennstrom M, et al. Altered CSF orexin and alpha-synuclein levels in dementia patients. J Alzheimers Dis. 2012;29:125–132. doi: 10.3233/JAD-2012-111655. [DOI] [PubMed] [Google Scholar]
- 118.Coban A, et al. Reduced Orexin-A Levels in Frontotemporal Dementia: Possible Association With Sleep Disturbance. American Journal of Alzheimers Disease and Other Dementias. 2013;28:606–611. doi: 10.1177/1533317513494453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scammell TE, et al. Coexistence of narcolepsy and Alzheimer's disease. Neurobiol Aging. 2012;33:1318–1319. doi: 10.1016/j.neurobiolaging.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heier MS, et al. Increased cerebrospinal fluid levels of nerve cell biomarkers in narcolepsy with cataplexy. Sleep Medicine. 2014;15:614–618. doi: 10.1016/j.sleep.2014.02.005. [DOI] [PubMed] [Google Scholar]