Abstract
Investigations into the physiology of Xenopus laevis have the potential to greatly accelerate biomedical research, especially concerning neural plasticity and sensory systems, but are limited by the lack of available information on behavioral learning in this species. Here, we attempt to lay the foundations for a behavioral assay in Xenopus that can be used in conjunction with biological manipulations. We tested cohorts of Xenopus tadpoles across four light-mediated active-avoidance experiments, using either wavelength or intensity as the salient discriminative cue. In the wavelength task, we determine a baseline learning rate and characterize retention of learning, identifying active extinction effects as far more potent than the passage of time in the loss of behavior. In the intensity task, we examine the effects of varying differences between the discriminative stimuli on acquisition and extinction and identify a critical range of intensity differences where learning changes. The results of our experiments demonstrate that Xenopus is a tractable model organism for cognitive research and can learn a variety of associative tasks in the laboratory settings. These data reveal new aspects of the Xenopus larval visual processing system and facilitate future research between cognitive methods and biological/chemical manipulations to study mechanisms of brain structure and function.
Keywords: Xenopus, Learning, Wavelength, Intensity
Introduction
Xenopus laevis is a popular model organism in many areas of biomedical research, with its development and physiology well characterized in the literature (Beck and Slack 2001; Harland and Grainger 2011; Pratt and Khakhalin 2013a; Wallingford et al. 2010). However, despite the model's popularity, there remains a lack of information on this species’ cognitive capacities, and this gap severely limits the use of Xenopus in biomedical and translational research. Especially in the areas of development and regeneration (two areas where Xenopus has made significant contributions), behavioral tests are necessary to examine cognitive consequences of sensory and neural manipulations, but no such methods have been established for this species.
Xenopus was previously considered intractable to learning in the laboratory setting, and the research community had largely abandoned attempts to train both tadpoles and frogs due to a number of documented difficulties (Boice 1970; Mcgill 1960; Thompson and Boice 1975). Studies have characterized basic behaviors including mating (Hayes et al. 2010; Tobias et al. 2004), swimming (Elepfandt 1985, 1986; Katz et al. 1981), and feeding (Avilaj and Frye 1978; Gouchie et al. 2008). Additionally, its sensory systems are highly studied, especially with regard to vision, with past experiments using spectrophotometry and electroretinography to determine how the Xenopus eye responds to specific wavelengths (Rohlich and Szel 2000; Witkovsky et al. 1981). However, few investigations examined how visual information directs behavior, and therefore, the literature is incomplete in characterizing Xenopus vision (Dong et al. 2009; Khakhalin et al. 2014; McKeown et al. 2013; Pratt et al. 2008; Viczian and Zuber 2014).
Our laboratory has recently developed a method for training Xenopus tadpoles in an associative light learning assay. Using an automated system, we have shown that with the correct stimuli Xenopus can learn an active-avoidance task that requires the discrimination of two different light wavelengths (Blackiston et al. 2010; Blackiston and Levin 2012). Additionally, a follow-up study, where blinded Xenopus tadpoles learned the same task using transplanted eyes grafted onto the body during embryonic stages, highlighted the importance of establishing more complex behavioral assays in Xenopus for conjunctive use with biological manipulations (Blackiston and Levin 2013). Here, we have more fully characterized learning and memory extinction in the wavelength discrimination task, extended our behavioral method to include intensity discrimination, and started an exploration into the developmental underpinnings of learning. These findings contribute to the repertoire of a behavioral assay in Xenopus laevis that can be integrated into a number of other fields to advance biomedical research to new heights.
Methods
Animal husbandry
A total of 310 Xenopus laevis tadpoles were used across the four experiments described below. No tadpoles were used in more than one experiment. Xenopus embryos were maintained according to standard procedures (Sive et al. 2000) in 0.1× Marc's Modified Ringer (MMR), pH 7.80, and the embryonic stages were determined using standard guidelines (Nieuwkoop and Faber 1967). Throughout development and testing, individuals were housed at 16 °C under a 12:12-h light/dark cycle. Animals were reared in 100 × 25 mm Petri dishes, with a maximum of 30 individuals per dish. Beginning at stage 46, tadpoles were fed once per day with Sera micron diet at the beginning of the light cycle by vortexing 0.025 g of food into 10 ml of 0.1× MMR. Previous data indicated that hunger interferes with learning in Xenopus (Blackiston and Levin 2012), so all testing sessions began shortly after feeding and additional food was provided in the behavioral testing chambers. Tadpoles were tested no more than two times and were then euthanized with a solution of 0.5 % tricaine mesylate. All individuals in this experiment were tested at Nieuwkoop and Faber stage 48, except those in experiment 4, which were Nieuwkoop and Faber stage 47. The Institutional Animal Care and Use Committees (IACUC) and Tufts University Department of Laboratory Animal Medicine (DLAM) approved all procedures involving animal experimentation under protocol M2014-70.
Behavioral apparatus
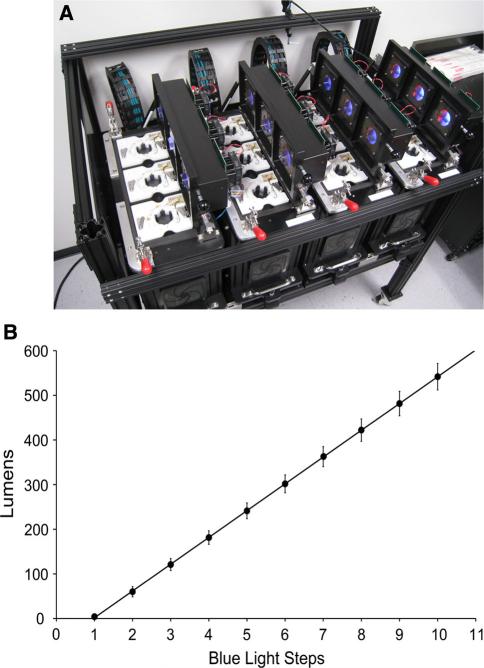
A previously documented custom-made automated training apparatus was utilized for all behavioral testing (Blackiston et al. 2010). The device consists of 12 independent and isolated chambers, with each containing a disposable Petri dish to house one subject at a time, an overhead light source to present stimuli, a machine vision (i.e., tracking) camera (Insight-Micro 1400, Cognex Corporation, Natick, MA, USA) to record positional data, and an electrode plate to administer punishment. Custom software controls each of these components and allows them interact with one another.
Light inside each chamber is provided by red and blue LEDs housed above the Petri dish (Osram Opto Semiconductors, Sunnyvale, CA, USA; blue LED, 470 nm, part no. LBW5SM; red LED, 635 nm, part no. LRG6SP). The lights are divided into four quadrants, with one red LED and one blue LED in each, and the apparatus's software can vary each light's state independently, regardless of quadrant or chamber. Opaque dividers between each quadrant prevent different stimuli from bleeding into one other. Xenopus eyes have been shown to contain at least three cone classes, and the apparatus’ LEDs overlap with Xenopus’ red and blue spectral sensitivity (Rohlich and Szel 2000). The intensities of the LEDs range between off and maximum in 15 even steps, but only the first 10 steps were utilized in this experiment. Lumen values for each intensity step were measured using an LX1010B light meter positioned 2 cm from the center of the illumination control module.
Tracking cameras below each chamber record positional data continuously throughout a behavioral session. These data serve two purposes: (1) they are matched with the presence of the S+ or S– light stimuli to determine when a tadpole should receive a punishing current, and (2) they provide the metrics that measure performance in behavioral tests. The cameras identify and track subjects using a subtraction algorithm which requires constant background light to function properly, so red light must be displayed at all times, and any blue light presentations are overlaid.
Punishment in behavioral sessions is provided by a set of six iridium oxide-coated titanium electrodes arranged circularly around the arena to deliver an AC current of 1.2 mA. Current is issued for a period of 100 ms, followed by 300 ms of shock inhibition to avoid an erratic swimming behavior that is sometimes observed with continuous current. These electric shock parameters were optimized in a previous study (Blackiston and Levin 2012).
The apparatus’ software ties each of these parameters together, allowing for precise behavioral training: light stimuli are presented to the subject, positional data are recorded by a tracking camera, and based on these data, AC current can be delivered based on specific behaviors. The software executes one experiment simultaneously in all 12 chambers, but responds to each subject's behavior independently, allowing the apparatus to test up to 12 subjects at a time in any given session.
Experiment 1: wavelength discrimination
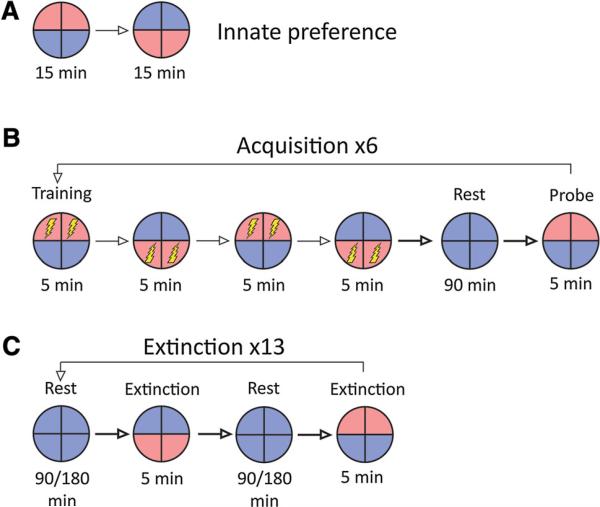
A basic light-mediated avoidance paradigm was created to test discrimination of different light wavelengths. Red light (635 nm, 49 lm) was designated as the S– (punishment condition) and blue light (470 nm, 422 lm) was designated as the S+ (safe condition). Each session began with a 30-min innate color preference test to determine whether subjects had a prior bias for either stimulus. During this test, the dish was illuminated half blue and half red, and no punishment was administered. The position of the lights was inverted (180° rotation) after 15 min to control for tadpoles that showed no movement.
Next, subjects entered the acquisition phase. This phase was arranged in blocks, each consisting of a training trial, a rest trial, and a probe trial. In training trials, the dish was illuminated half S+ (blue) and half S– (red). When tadpoles occupied the S– half of the dish, current was delivered as punishment to train subjects to avoid this stimulus. These trials lasted 20 min, and the position of the lights was inverted every 5 min. Next, in rest trials, the entire dish was illuminated with the S+ for 90 min, and no shocks were delivered. Finally, in probe trials, the dish was lit half S+ and half S–, but no current was delivered. These trials lasted 5 min, and the position of the lights was always opposite the final position in the previous training trial (before the rest period) to prevent false detection of avoidance for tadpoles that did not move during the rest period. All tadpoles received six of these acquisition blocks consecutively.
Immediately following acquisition, tadpoles entered the extinction phase to test for duration of memory. This phase was also arranged in blocks, with each block consisting of two rest trials and two extinction trials (order: rest–extinction–rest–extinction). In rest trials, the entire dish was illuminated with the S+ and no punishment was delivered. The length of the rest trials varied to test the effect of time on loss of acquisition: the tadpoles were divided into two groups, with one group receiving 90-min extinction rest trials (henceforth referred to as the 90-min group) and the other receiving 180-min extinction rest trials (henceforth referred to as the 180-min group).
In extinction trials, the dish was illuminated half S+ and half S–, and no punishment was administered. The color orientation in extinction trials was always opposite to the final presentation in the previous extinction trial (or, in the case of the first extinction trial, the previous probe trial) to control for tadpoles that did not move during rest trials. These trials lasted 5 min. Each extinction block was organized with 2 pairs of rest and extinction trials to include both inversions of the light positions in the extinction trials. Each individual received 13 blocks of extinction testing, and each block consisted of two extinction probes, so the tadpoles in this experiment received 26 total extinction probes per session.
Experiment 2: intensity discrimination
To determine how well tadpoles can distinguish between two intensities of the same wavelength, rather than to separate wavelengths, individuals were trained in the paradigm above using a combination of two blue intensity values. The intensity of the blue S+ was set to 542 lm, and the blue S– stimuli was one of six lower intensities ranging from 60 to 363 lm in increasing increments of approximately 60 lm. Red light is required in the device for image processing; therefore, red light was present at all times in this experiment at 49 lm, and all blue stimuli were overlaid. The net intensity of each stimulus in this experiment was equal to the intensity of the overlaid blue light plus 49 lm. Each session tested one of the six S– stimuli against the fixed S+.
Behavioral sessions proceed similar to those in experiment 1, consisting of an innate preference test, an acquisition phase, and an extinction phase. The innate preference test and acquisition phase were identical to those in experiment 1, with the exception of the stimuli, which now consisted of two blue lights of differing intensity instead of two lights with different wavelengths. In extinction, all tadpoles were given three blocks of testing and received 90-min rest periods.
Experiment 3: wavelength discrimination with minimum intensity variation
An additional discrimination test was developed in which the red (S–) and blue (S+) light were set to similar intensities to determine whether tadpoles could differentiate between the two wavelengths in the absence of brightness differences. Background red light was presented at 140 lm, and blue light was overlaid on half of the dish at 70 lm (net blue intensity = 210 lm, percent difference over red = 50 %). Behavioral sessions consisted of an innate preference test and an acquisition phase, and all methods for these phases were identical to those in experiment 1.
Experiment 4: wavelength discrimination in younger tadpoles
Tadpoles in experiment 1 were tested during stage 48; this experiment examined acquisition of the wavelength discrimination in tadpoles at stage 47. Behavioral sessions consisted of an innate preference test and an acquisition phase. All behavioral methods in this experiment were identical to those in experiment 1, except that no food was present in the device at the time of testing (as these tadpoles were too young to eat, and unconsumed food created errors in the tracking software).
Data analysis and statistics
All positional data during behavioral trials were written to a log file, which was parsed and analyzed over 5-min time blocks. Performance in the avoidance task was quantified by measuring the percent of time a subject spent in the S– region during probe and extinction trials. In innate tests, a tadpole was considered to have an innate color preference if its time spent in the S– region was >70 % or <30 %. These individuals were excluded from further analyses, as a strong innate preference for one color or the other would bias the proceeding learning results. In extinction tests, tadpoles were considered to have lost acquisition learning when their time spent in S– regions was greater than or equal to 50 %, or chance performance.
Statistical tests were performed in R version 3.2.3 (The R Foundation for Statistical Computing) and GraphPad Prism version 6.05 (GraphPad Software, LaJolla, CA, USA). Acquisition was measured using one-tailed paired-sample t tests comparing performance in the innate test (pre-training) to performance in the last three acquisition probes (post-training). Averaging the final three probes as a measure of acquisition reduced variability compared to examining only the final probe. In extinction analysis, a log-rank test for trend (χ2) determined significant hierarchies among the different extinction rates.
Results
Experiment 1: tadpoles demonstrate memory following an associative wavelength-learning assay
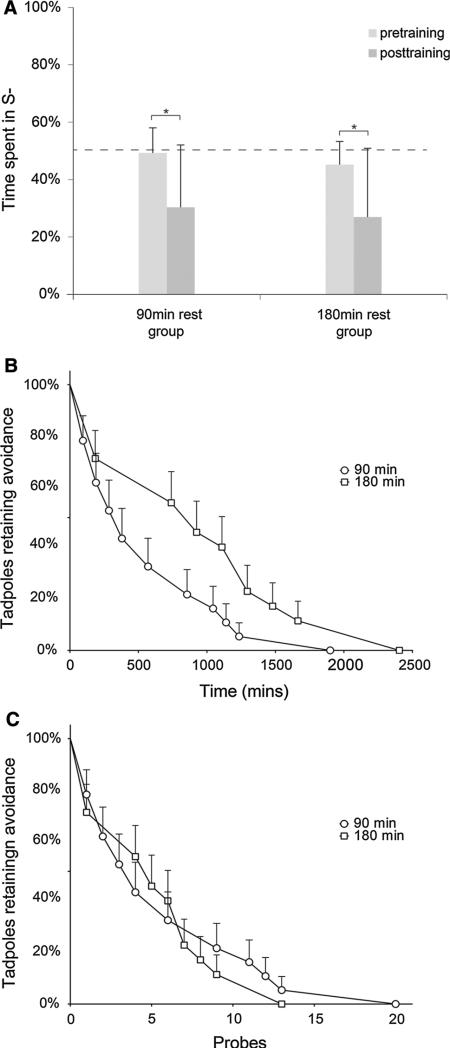
To assess both the acquisition and extinction of learning in Xenopus tadpoles, a custom automated training apparatus was utilized to train tadpoles in a visual avoidance task (Fig. 1). Subjects were divided into two groups; the initial training was identical for all animals, but during the extinction phase one group was given a 90-min rest in between extinction probes, and the other 180 min (Fig. 2). In the acquisition phase of this experiment, both groups of tadpoles demonstrated significant improvement in avoidance performance (Fig. 3a) when trained to avoid red light (S–) by pairing the wavelength with 1.2 mA current [90 min: t(24) = 3.66, p < 0.001; 180 min: t(24) = 3.84, p < 0.001]. Additionally, these results confirmed that tadpoles of both groups could learn to associate a wavelength of light with punishment and allowed further comparisons of extinction rates.
Fig. 1.
Automated behavioral apparatus used in all behavior trials. a The device consists of 12 individual chambers illuminated above by blue and red LEDs. The light presentation is divided into four quadrants. Motion tracking cameras below the dish record animal position, and electrodes along the walls of the environment can deliver a current to punish behavior. b Lumen values of the blue LEDs at each of the 12 possible steps, which can be specified through custom software (only values 2–10 were used in the present study)
Fig. 2.
Training regime used in Xenopus light discrimination tasks. a Individuals begin with an assessment for innate preferences in which they are presented with two wavelengths in the absence of punishment. b During acquisition, animals are pulsed with a 1.2-mA current when entering the S– half of the environment (red in the diagram). Following training, individuals are given a rest period in the S+ stimulus, followed by a probe for learning. c During extinction trials, the S– and S+ stimuli are presented in opposite halves of the dish in the absence of current (color figure online)
Fig. 3.
Tadpoles learn to discriminate between red and blue light, and extinction drives loss of learned behavior more strongly than the passage of time. a During initial training, both groups of tadpoles (n = 25 for 90- and 180-min group) learn to avoid the punishing S– color (red. b Following training, after being presented with the S– stimulus in the absence of shock, tadpoles appear to lose the avoidance behavior more quickly when given 90-min rests between probes compared to 180 min. c When both the 90- and 180-min data sets are plotted over total probes, decay rates are indistinguishable, indicating that extinction, and not the passage of time, is driving the loss in performance. Asterisks indicate p ≤ 0.05. Error bars indicate one standard deviation
Extinction effects were modeled as a survival curve: a tadpole was considered to have lost learning of the trained avoidance behavior when blue light (S+) preference crossed the extinction threshold of 50 %. By aggregating extinction performance across tadpoles, a decay function was built representing the loss of learning in each experimental group. In this analysis, the dependent variable was the percent of tadpoles retaining red light (S–) avoidance behavior at a given time point (Fig. 3b). Comparison of extinction performance over time elapsed in the 90- versus 180-min groups revealed a significant difference in learning decay [Mantel–Cox log-rank test, χ2(1) = 4.945, p = 0.026, Fig. 3b]. This initially suggested that tadpoles retained color learning for a longer time when given 180-min rests compared to 90-min rests between extinction probes; however, it was also possible that the duration of rests was not responsible for the loss of learning, and it was instead an effect of extinction [repeated trials presenting the red (S–) wavelength without punishment] that was driving down performance. If this were the case, similar extinction patterns between the two groups would be observed when analyzing the data over probes elapsed instead of time elapsed because this hypothesis proposes that loss of learning is a function of extinction probes and has little relationship to time. To test this, the 90- and 180-min groups were compared over number of probes elapsed (Fig. 3c), and no significant differences were observed [Mantel–Cox log-rank test, χ2(1) = 0.0356, p > 0.05]. These results reveal that Xenopus learning decay is being driven primarily by extinction effects from neutral probes and not memory degradation over time. Overall, the results indicate that Xenopus tadpoles can learn to discriminate between red and blue light reliably, and loss of this learning is driven most readily by active extinction through repeated presentation of red light in the absence of punishment. In addition, given reports which describe difficulty in training Xenopus tadpoles, the current visual assay is novel in that it results in both strong learning and memory, in an avoidance task.
Experiment 2: tadpoles learn in an associative intensity-learning assay
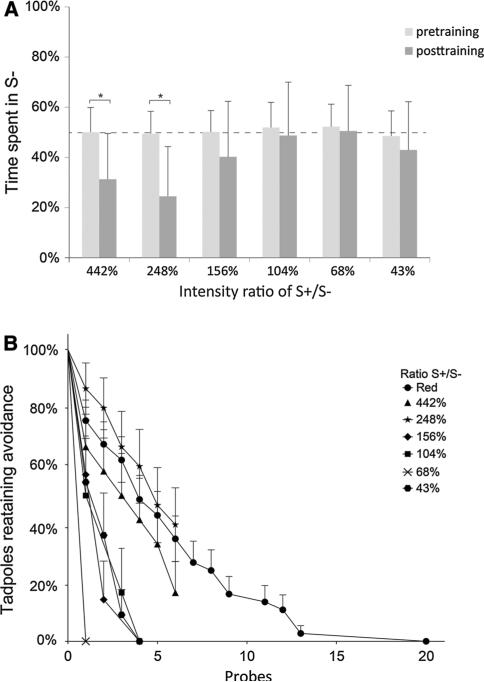
While the first experiment tested Xenopus tadpoles in a wavelength-based task, it is possible that individuals were using intensity differences between the two colors to make decisions rather than the wavelengths themselves. To specifically examine Xenopus tadpole's intensity discrimination ability, individuals were tested in a separate task pairing varying intensities of the same wavelength. In this assay, half of the dish was always illuminated with 542 lm blue light (S+), and the other half of the dish was illuminated with S– blue light with relative differences of 442, 248, 156, 104, 68.4, or 43.4 % compared to the S+, to determine whether cohorts of tadpoles could distinguish between the two intensities as they became more similar. Results from intensity discrimination trials reveal that learning rates are significant at ratio differences of 248 % or greater, corresponding to 442 and 248 % S– conditions [t(15) = 3.81, p < 0.001 and t(18) = 4.61, p < 0.001, respectively], but degrade to non-significant levels at intensity differences less than 248 % [156 %: t(15) = 1.55, p > 0.05; 104 %: t(16) = –0.52, p > 0.05; 68.4 %: t(13) = 0.34, p > 0.05; and 43.4 %: t(18) = 1.24, p > 0.05, Fig. 4a]. These results indicate a threshold value of intensity, below which tadpoles have difficulty distinguishing in an associative learning assay.
Fig. 4.
Tadpoles have difficulty discriminating blue intensities of less than a 248 % difference. a When trained to distinguish between a blue S+ intensity (540 lm) and a variety of S– intensities, tadpoles were able to learn at S– ratio differences of 442 and 248 %, but not 156, 104, 68.4, and 43.4 %. b Comparison of extinction curves comparing post-acquisition performance across different light conditions. As the margin of difference between the S+ and S– decreases, the slope of the curve increases (the number of probes needed to extinguish the behavior decreases), indicating that learning weakens as discrimination becomes more difficult. Asterisks indicate p ≤ 0.05. Error bars indicate 1 standard deviation. Sample size is 16 (442 %), 19 (248 %), 16 (156 %), 17 (104 %), 14 (68.4 %), 19 (43.4 %), respectively
To determine whether extinction rates in the intensity trial were similar to the wavelength test in experiment 1, extinction was again modeled as a survival curve and analyzed over additional probes in the absence of punishment (Fig. 4b). As no differences were noted between the 90- and 180-min rest periods in the results of experiment 1, 90-min rest periods were used here. Results indicate that retention is the strongest among the intensities with the greatest differences, as a log-rank test for trend showed the order (displayed left–right in Fig. 4a) to be significant [χ2(1) = 19.77, p < 0.001]. Overall, these results indicate that Xenopus tadpoles are able to differentiate between different intensities of the same wavelength of light, but discrimination weakens as the intensities become more similar. Similarly, the weakness of learning at similar intensities is paired with weaker extinction when tadpoles are subjected to repeated probes in the absence of punishment.
Experiment 3: tadpoles learn a wavelength-mediated task when intensity differences are minimized
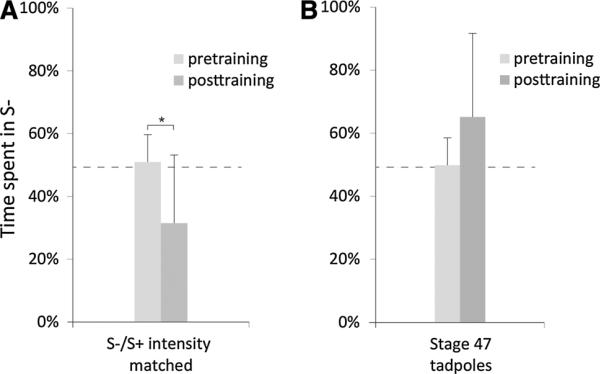
Given the results of experiment 2, it is possible that the tadpoles in experiment 1 were choosing between the red (S–) and blue (S+) light not by wavelength, but rather by the large intensity differences between the two (as the blue light in our assay was 420 lm and the red light was 49 lm, a 760 % difference). To rule out this possibility, cohorts of tadpoles were tested in an additional assay where the intensity of red and blue light were matched as closely as possible with a 50 % intensity difference between the two (S– red: 140 lm, S+ blue: 210 lm, below the 248 % S+/S– ratio threshold found in experiment 2). In this test, significant learning was observed [Fig. 5a; t(20) = 4.12, p < 0.001] demonstrating that Xenopus tadpoles can correctly identify wavelengths of light in the absence of strong intensity differences.
Fig. 5.
Tadpoles can distinguish wavelengths when intensities are matched, but not at earlier developmental stages. a When the intensities of red and blue light are matched as closely as possible in the device (140 lm S– and 210 lm S+ , a 50 % difference), tadpoles still spend significantly less time in the S– half of the dish after training. b Younger stage 47 tadpoles are unable to learn in the standard color training assay, as the time spent in the S– half of the dish does not decrease following training. Asterisks indicate p ≤ 0.05. Error bars indicate one standard deviation. Sample size is 21 for intensity training and 31 for stage 47 training
Experiment 4: tadpole associative learning ability is stage specific
To date, all of the Xenopus learning assays described have used Nieuwkoop and Faber stage 48 individuals, as anecdotal evidence suggests that younger tadpoles have difficulty learning in associative tasks. To verify this hypothesis, stage 47 tadpoles were tested in the red (S–)/blue (S+) assay described in experiment 1. Compared to stage 48 tadpoles which show a decrease in the time spent in the S– half of the dish following training, stage 47 tadpoles did not show a significant change in S– preference before and after training [t(30) = –3.05, p = 0.997, Fig. 5b]. Further, tadpoles younger than stage 47 could not be tested as they do not swim regularly nor feed until stage 47. These data are the first to specifically examine the stage at which associative learning appears in Xenopus tadpoles and suggest that developmental changes within the brain and/or central nervous system between stages 47 and 48 are critical for visually mediated behaviors.
Discussion
Our results in the wavelength tasks (experiments 1 and 3) are consistent with the findings of Blackiston and Levin (2012, 2013) that Xenopus tadpoles can learn a discriminative task mediated by different wavelengths, specifically 470-nm blue light and 635-nm red light. In addition, by matching the brightness levels between the two training colors, we have determined that individuals can correctly distinguish between wavelengths even in the absence of intensity differences. The development of a replicable behavioral method here is especially significant given that Xenopus was previously considered intractable to laboratory learning. The wavelengths used here are an ideal starting point for examining tadpole perception because they are at the far opposite ends of Xenopus’ visible spectrum (Rohlich and Szel 2000; Witkovsky et al. 1981). Future experiments should evaluate more values on the visible spectrum to more completely characterize Xenopus vision.
Xenopus also showed robust memory in our task, as there were no differences in retention when individuals were given 90- or 180-min rest periods between tests. Our data clearly show that, within the scope of our selected values, time is not an influencing factor in loss of learning, and exposure to extinction trials primarily drives loss of learning. This is reasonable given that avoidance learning tends to be very resistant to degradation, and extinction is generally more powerful than the passage of time in decreasing discriminative responding (Goodrick 1968). Rodent studies have investigated the impact of age on active-avoidance learning and retention and found impairments in younger rats at short-term retention intervals (Feigley and Spear 1970; Klein and Spear 1969). Unfortunately, there have been no successful active-avoidance studies in grown Xenopus frogs, so there is no directly comparable data. It is possible (and reasonable to hypothesize, given the literature in other species) that time has different effects at larger values and in individuals of different ages, so future research should examine whether Xenopus tadpoles and frogs can retain learning for periods of time longer than 180 min.
Our results in the intensity task are entirely novel findings in Xenopus and show that tadpoles can discriminate between a range of intensities of a 470-nm blue light wave. It was not surprising to find that learning generally decreased as the intensity difference decreased because the discriminative stimuli became more and more similar. Extinction of the intensity learning follows a strikingly similar pattern to acquisition, in that extinction rates become steeper as the training intensities become more similar. Overall, this learning retention evaluation shows that learning of smaller intensity differences is less robust, which, in conjunction with the acquisition results, allows us to conclude that decreasing intensity differences are an effective method for increasing overall task difficulty in Xenopus. A key observation in all of the phases of the intensity experiment is that learning significantly drops off at intensity differences of 248 % or less. This seems to suggest a critical range of difference ratios where Xenopus’ just noticeable difference of light intensity lies. Our current behavioral device has a limited range of intensity values for both blue and red light, and future studies would benefit from a more fine-scale examination of intensity discrimination in Xenopus.
The results from testing younger tadpoles provide an important building block for future experiments integrating developmental and cognitive manipulations. In multiple studies, we have shown that stage 48 tadpoles are able to learn in a variety of conditions, but in our most established learnable task (wavelength discrimination), stage 47 tadpoles were unable to learn to avoid red light. These findings show a drastic difference in learning ability across developmental stages and suggest that the ability to learn in a light-mediated assay develops over the course of only several days. This period also overlaps with a number of critical developmental events in the visual system of Xenopus tadpoles. Tectal neurons of the tadpole brain show significant changes in response properties between stages 46 and 48 (both field sharpness and temporal profiles), and avoidance behavior improves during this period as well; stage 46 tadpoles show weak performance, while stage 48/49 show strong performance (Dong et al. 2009). Further, the peripheral nuclear layer of the optic tectum begins to develop at stage 47 and is fully developed by stage 48 (Nieuwkoop and Faber 1994). These findings suggest a number of developmental events likely drive the learning differences we report here between stage 47 and 48 tadpoles.
One limitation of our training method with stage 48 tadpoles, which perform well in many conditions here, is that we have been unable to achieve learning with counterbalanced punishment assignments (where blue light is the punishing S– condition and red is the S+). This is odd given our results clearly show that the tadpoles can discriminate between the wavelengths, but for some reason this discrimination is only apparent in one of the two possible punishment setups. We believe this may indicate a functional asymmetry in learning, where animals can learn in one set of conditions but not in the reverse set, even though they can discriminate between the stimuli. Similar results have been reported in insects and chicks (Blackiston et al. 2011; Osorio et al. 1999), though the mechanism underlying these observations remains unclear.
This study was the first in-depth analysis of light-mediated associative learning in Xenopus, so a number of direct and significant conclusions can be drawn from the data concerning basic learning abilities: Xenopus tadpoles in developmental stage 48 are able to discriminate between both red and blue light and different intensities of blue light, and when acquisition is widely successful, the learning is quite robust to retention and extinction effects. Extinction is much more powerful in reducing discriminative responding than the passage of time, suggesting a highly developed learning system in this underdeveloped organism. However, this highly developed system likely develops at a specific period in developmental time–space, as tadpoles only a couple of days younger are unable to learn the same task.
These data represent a crucial step toward overcoming a significant barrier in the field. Xenopus remain a well-established model for developmental biology: the species has been used to examine fundamental pathways underlying gastrulation, neurulation, eye generation, and neural specification (Gilbert 2013), in addition to testing models of human disorders including epilepsy, autism, and cancer (Chernet et al. 2014; Pratt and Khakhalin 2013b). However, while a number of powerful tools exist to alter the genetics and molecular pathways of Xenopus embryos in a way that mimics human disorders, few tools exist to determine the cognitive outcomes or neurological parallels between amphibian and human data. Further, whether we are to develop molecular, pharmaceutical, or genetic treatments for biomedical contexts, such cognitive tools will be necessary to determine whether both behavior and brain function are restored following interventions. As such, we hope that the data presented here provide another step toward future work bridging the gap between genetic development and the behavioral results of organism-wide CNS function.
Acknowledgments
ML gratefully acknowledges the support of The G. Harold and Leila Y. Mathers Charitable Foundation. DB was supported by National Institutes of Health (5T32DE007327-09). We also thank Erin Switzer for animal husbandry support.
References
- Avilaj VL, Frye PG. Feeding behavior in the African clawed frog (Xenopus laevis Daudin). Herpetologica. 1978;33:152–161. [Google Scholar]
- Beck CW, Slack JM. An amphibian with ambition: a new role for Xenopus in the 21st century. Genome Biol. 2001;2:1029. doi: 10.1186/gb-2001-2-10-reviews1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, Levin M. Aversive training methods in Xenopus laevis: general principles. Cold Spring Harb Protoc. 2012 doi: 10.1101/pdb.top068338. doi:10.1101/pdb.top068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, Levin M. Ectopic eyes outside the head in Xenopus tadpoles provide sensory data for light-mediated learning. J Exp Biol. 2013;216:1031–1040. doi: 10.1242/jeb.074963. doi:10.1242/jeb.074963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Shomrat T, Nicolas CL, Granata C, Levin M. A second-generation device for automated training and quantitative behavior analyses of molecularly-tractable model organisms. PLoS One. 2010;5:e14370. doi: 10.1371/journal.pone.0014370. doi:10.1371/journal.pone.0014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Briscoe AD, Weiss MR. Color vision and learning in the monarch butterfly, Danaus plexippus (Nymphalidae). J Exp Biol. 2011;214:509–520. doi: 10.1242/jeb.048728. doi:10.1242/jeb.048728. [DOI] [PubMed] [Google Scholar]
- Boice R. Avoidance learning in active and passive frogs and toads. J Comp Physiol Psychol. 1970;70:154. [Google Scholar]
- Chernet BT, Fields C, Levin M. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front Physiol. 2014;5:519. doi: 10.3389/fphys.2014.00519. doi:10.3389/fphys.2014.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, et al. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J Neurophysiol. 2009;101:803–815. doi: 10.1152/jn.90848.2008. doi:10.1152/jn.90848.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elepfandt A. Naturalistic conditioning reveals good learning in a frog (Xenopus Laevis). Naturwissenschaften. 1985;72:492–493. doi:10.1007/Bf00441080. [Google Scholar]
- Elepfandt A. Wave frequency recognition and absolute pitch for water-waves in the clawed frog Xenopus laevis. J Comp Physiol Sens Neural Behav Physiol. 1986;158:235–238. [Google Scholar]
- Feigley DA, Spear NE. Effect of age and punishment condition on long-term retention by the rat of active- and passive-avoidance learning. J Comp Physiol Psychol. 1970;73:515–526. doi: 10.1037/h0030234. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental biology. 10th edn. Sinauer Associates, Inc.; Sunderland: 2013. [Google Scholar]
- Goodrick CL. Learning, retention, and extinction of a complex maze habit for mature-young and senescent Wistar albino rats. J Gerontol. 1968;23:298–304. doi: 10.1093/geronj/23.3.298. [DOI] [PubMed] [Google Scholar]
- Gouchie GM, Roberts LF, Wassersug RJ. Effects of available cover and feeding schedule on the behavior and growth of the juvenile African clawed frog (Xenopus laevis). Lab Anim (NY) 2008;37:165–169. doi: 10.1038/laban0408-165. doi:10.1038/laban0408-165. [DOI] [PubMed] [Google Scholar]
- Harland RM, Grainger RM. Xenopus research: metamorphosed by genetics and genomics trends in genetics. TIG. 2011;27:507–515. doi: 10.1016/j.tig.2011.08.003. doi:10.1016/j.tig.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, et al. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc Natl Acad Sci USA. 2010;107:4612–4617. doi: 10.1073/pnas.0909519107. doi:10.1073/pnas.0909519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Potel MJ, Wassersug RJ. Structure and mechanisms of Schooling in tadpoles of the clawed frog, Xenopus laevis. Anim Behav. 1981;29:20–33. [Google Scholar]
- Khakhalin AS, Koren D, Gu J, Xu H, Aizenman CD. Excitation and inhibition in recurrent networks mediate collision avoidance in Xenopus tadpoles. Eur J Neurosci. 2014;40:2948–2962. doi: 10.1111/ejn.12664. doi:10.1111/ejn.12664. [DOI] [PubMed] [Google Scholar]
- Klein SB, Spear NE. Influence of age on short-term retention of active-avoidance learning in rats. J Comp Physiol Psychol. 1969;69:583–589. doi: 10.1037/h0028225. [DOI] [PubMed] [Google Scholar]
- Mcgill TE. Response of the leopard frog to electric shock in an escape-learning situation. J Comp Physiol Psychol. 1960;53:443–445. [Google Scholar]
- McKeown CR, Sharma P, Sharipov HE, Shen WH, Cline HT. Neurogenesis is required for behavioral recovery after injury in the visual system of Xenopus laevis. J Comp Neurol. 2013;521:2262–2278. doi: 10.1002/cne.23283. doi:10.1002/cne.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin). North-Holland Publishing Company; Amsterdam: 1967. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Pub; New York: 1994. [Google Scholar]
- Osorio D, Jones CD, Vorobyev M. Accurate memory for colour but not pattern contrast in chicks. Curr Biol. 1999;9:199–202. doi: 10.1016/s0960-9822(99)80089-x. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Khakhalin AS. Modeling human neurodevelopmental disorders in the Xenopus tadpole: from mechanisms to therapeutic targets. Dis Models Mech. 2013a;6:1057–1065. doi: 10.1242/dmm.012138. doi:10.1242/dmm.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Khakhalin AS. Modeling human neurodevelopmental disorders in the Xenopus tadpole: from mechanisms to therapeutic targets. Dis Model Mech. 2013b;6:1057–1065. doi: 10.1242/dmm.012138. doi:10.1242/dmm.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci. 2008;11:467–475. doi: 10.1038/nn2076. doi:10.1038/nn2076. [DOI] [PubMed] [Google Scholar]
- Rohlich P, Szel A. Photoreceptor cells in the Xenopus retina. Microsc Res Tech. 2000;50:327–337. doi: 10.1002/1097-0029(20000901)50:5<327::aid-jemt2>3.3.co;2-g. doi:10.1002/1097-0029(20000901)50:5<327:AID-JEMT2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- Thompson PA, Boice R. Attempts to train frogs—review and experiments. J Biol Psychol. 1975;17:3–13. [Google Scholar]
- Tobias ML, Barnard C, O'Hagan R, Horng SH, Rand M, Kelley DB. Vocal communication between male Xenopus laevis. Anim Behav. 2004;67:353–365. doi: 10.1016/j.anbehav.2003.03.016. doi:10.1016/j.anbehav.2003.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viczian AS, Zuber ME. A simple behavioral assay for testing visual function in Xenopus laevis. J Vis Exp JoVE. 2014 doi: 10.3791/51726. doi:10.3791/51726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Liu KJ, Zheng Y. Xenopus. Curr Biol. 2010;20:R263–R264. doi: 10.1016/j.cub.2010.01.012. doi:10.1016/j.cub.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Yang CY, Ripps H. Properties of a blue-sensitive rod in the Xenopus retina. Vis Res. 1981;21:875–883. doi: 10.1016/0042-6989(81)90188-7. doi:10.1016/0042-6989(81)90188-7. [DOI] [PubMed] [Google Scholar]