Abstract
Background
Cardiometabolic diseases are increasing disproportionately in South Asia compared to other regions of the world despite high levels of vegetarianism. This unexpected discordance may be explained by differences in the healthfulness of vegetarian and non-vegetarian diets in South Asia versus the US.
Objective
(1) To compare the food group intake of vegetarians versus non-vegetarians in South Asia and the US and (2) to evaluate associations between vegetarianism and cardiometabolic disease risk factors (overweight/obesity, central obesity, diabetes, hypertension, high triglycerides, high LDL, low HDL, and high Framingham Heart Score).
Design
Using cross-sectional data from adults (20–69 years) in South Asia (CARRS 2010–2011; n=15,665) and the US (NHANES 2003–2006; n=2159), adherence to a vegetarian diet was assessed using food propensity questionnaires. Multivariable logistic regression was used to estimate odds ratios and predicted margins (e.g. adjusted prevalence of the outcomes).
Results
One-third (33.0%; n=4968) of adults in the South Asian sample were vegetarian in contrast to only 2.4% (n=59) in the US sample. Among South Asians, compared with non-vegetarians, vegetarians more frequently ate dairy, legumes, vegetables, fruit, desserts, and fried foods (all p<0.05). Among Americans, compared with non-vegetarians, vegetarians more frequently ate legumes, fruit, and whole grains, and less frequently ate refined cereals, desserts, fried foods, fruit juice, and soft drinks (all p<0.05). After adjustment for confounders (age, sex, education, tobacco, alcohol, and also city in CARRS), South Asian vegetarians were slightly less frequently overweight/obese compared to non-vegetarians – 49% (95% CI: 45%, 53%) versus 53% (51%, 56%), respectively – while US vegetarians were considerably less frequently overweight/obese compared to non-vegetarians: 48% (32%, 63%) versus 68% (65%, 70%), respectively. Furthermore, US vegetarians were less likely to exhibit central obesity compared to non-vegetarians: 62% (43%, 78%) versus 78% (76%, 80%), respectively.
Conclusions
There is greater divergence between vegetarian and non-vegetarian diets in the US compared to South Asia, and US vegetarians have more consistently healthier food group intakes compared to South Asian vegetarians. Vegetarians in both populations have a lower probability of overweight/obesity compared to non-vegetarians. The strength of this association may be stronger for US vegetarian diets, which were also protective against central obesity.
Keywords: vegetarianism, India, obesity, visceral adiposity, food groups
INTRODUCTION
The prevalence of cardiometabolic diseases such as diabetes (1, 2) and coronary heart disease (3, 4) is increasing disproportionately in South Asia compared to other regions of the world (5, 6) despite high levels of vegetarianism (7) – a phenomenon we refer to as the “South Asian Paradox.” For example, the age-adjusted prevalence of diabetes among adults in India increased from 6.7% in 2006 to 9.3% in 2014 (8, 9). In the US, these numbers were 7.8% and 10.8%, respectively (8, 9). Between 1990 and 2020, coronary heart disease is estimated to increase by 120–137% in developing countries compared to 30–60% in developed countries (6). In India, between 2000 and 2030, an estimated 35% of all cardiovascular disease (CVD) deaths will occur among individuals aged 35–64 years, compared to only 12% in the US (6).
The South Asian Paradox may be explained by differences in the relative healthfulness of vegetarian versus non-vegetarian diets in South Asia versus the US and Europe (7). While several studies, including randomized controlled trials, have documented protective effects of vegetarian diets on cardiometabolic disease risk factors in American and European populations [summarized in references (10–12)], few have evaluated these relationships in South Asian populations.
Two studies have explored these associations among South Asian immigrants to New Zealand and the US: the first found vegetarians had, on average, a lower body mass index (BMI) and waist circumference compared to non-vegetarians but no difference in insulin resistance after adjustment for BMI (13). The second study found lower insulin resistance among vegetarians compared to non-vegetarians but did not adjust for BMI (14). The few studies conducted among South Asians living in South Asia have reported mixed results: some observed that vegetarians were less likely to be overweight (15, 16) and have diabetes (16, 17) compared to non-vegetarians; while others have observed no difference in BMI (18, 19) but that vegetarians exhibit lower levels of circulating lipids and blood pressure compared to non-vegetarians (18).
There is an even greater paucity of data comparing vegetarian diets in South Asia to other populations. Exploring inter-country differences is an important first step in identifying points of intervention to improve diet quality and prevent disease. We therefore (1) assessed differences in food group intake of vegetarians versus non-vegetarians and (2) quantified the association of vegetarian dietary patterns with overweight/obesity, central obesity, diabetes, hypertension, dyslipidemia, and a composite CVD risk score within urban South Asian (India and Pakistan) and national US samples.
METHODS
Samples
Cross-sectional data on men and non-pregnant women (20–69 years) in urban South Asia were from the baseline survey of the CARRS (Centre for cArdiometabolic Risk Reduction in South-Asia) cohort, conducted in three cities in 2011: Chennai and New Delhi in India, and Karachi in Pakistan (20). Cross-sectional data on men and non-pregnant women (20–69 years) in the US were from NHANES (National Health and Nutrition Examination Survey) 2003–2004 and 2005–2006, which assessed more comparable dietary data to CARRS than more recent NHANES. Both CARRS (20) and NHANES used complex, multistage probability sampling to select representative samples of the target populations (for CARRS, each of the three cities, n=16,288, and for NHANES, the general US population, n=12,761 for 2003–2004 and n=12,862 for 2005–2006). The response rates for CARRS were 94.7% for questionnaire completion and 84.3% for bio-specimen collection. The response rates for interview and examination completion in NHANES 2003–2004 were 79% and 76%, respectively, and 80% and 77%, respectively, for NHANES 2005–2006.
Dietary Assessment
CARRS administered a 26-item food propensity questionnaire adapted from the INTERHEART study (21). In order to improve comparability between CARRS and NHANES, NHANES 139-item food propensity questionnaire data, collected only during the 2003–2004 and 2005–2006 survey cycles, were used. For both questionnaires, portion size information was not collected, only the frequency of consumption (never or less than once a month, per month, per week, or per day) over the past year, which was standardized to consumption per day and categorized into four categories: never consumed, consumed ≥1 time/month but <1 time/week, consumed ≥1 time/week but <1 time/day, and consumed daily.
More specifically for NHANES, the National Cancer Institute’s DietCalc software was used to convert raw frequencies from the food propensity questionnaire into average consumption per day. This software also imputed data in the case of inconsistent responses to stem and follow-up questions. Given that the NHANES food propensity questionnaire queried many more items (total of 139), multiple items were collapsed and summed within individuals in order to derive average daily frequencies for food groups that were consistent with those in CARRS. Similarly, for CARRS, because some items were added to make the instrument culturally appropriate, such as mithai (Indian sweets), select items were also collapsed and summed within individuals. In the end, this resulted in 18 food groups used for the analysis (Supplemental Table 1). As a sensitivity analysis, vegetables were further divided into leafy green vegetables, other raw vegetables, and cooked vegetables including potatoes but not fried potatoes. Cooked vegetables were even further divided into non-potato cooked vegetables and potatoes for NHANES; this distinction could not be made for CARRS given the items included on the CARRS food propensity questionnaire.
Six dietary patterns were defined as follows: non-vegetarian (no restrictions on animal-based products); vegan (eat meat, poultry, fish, eggs, and dairy never or <1 time/month); lacto-vegetarian (eat meat, poultry, fish, and eggs never or <1 time/month); lacto-ovo vegetarian (eat meat, poultry, and fish never or <1 time/month); pesco-vegetarian (eat meat and poultry never or <1 time/month); semi-vegetarian (eat meat, poultry, and fish ≥1 time/month but <1 time/week) (16, 22). Given the small sample size of vegetarian dietary patterns in NHANES, participants following any of the five vegetarian dietary patterns were combined into a single “vegetarian” group. The same was done for CARRS participants for the purposes of comparison, in addition to a subgroup analyses looking across different vegetarian dietary patterns within the CARRS sample. Thus, herein, unless otherwise indicated, the term “vegetarian” refers to all participants adhering to any of the five vegetarian dietary patterns.
Outcome Assessment
In both studies, trained study staff used standardized procedures to measure weight, height, waist circumference, and blood pressure. BMI was calculated as weight (kg) divided by height-squared (m2). Obesity including overweight was defined as BMI ≥25 kg/m2. This definition is consistent with the definition used by the Global Burden of Disease Study (23), previous analyses of the Demographic and Health Surveys (24), and the World Health Organization Report on Diet, Nutrition, and the Prevention of Chronic Diseases (25). In sensitivity models for CARRS, a definition of 23 kg/m2 was used given that the World Health Organization Expert Consultation identified this as a “potential health action point” for Asian populations (26) and that this definition is often used in regional analyses of overweight/obesity. Central obesity was defined as a waist-to-height ratio >0.5 (27) given evidence that this measure is more strongly associated with percentage body fat (measured by dual-energy X-ray absorptiometry) than is waist circumference alone (28). Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or treatment of previously diagnosed hypertension (29).
Fasting blood glucose, glycated hemoglobin A1c (HbA1c), triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL), and high-density lipoprotein (HDL) cholesterol were analyzed using standardized laboratory procedures for both studies. Diabetes was defined as fasting blood glucose ≥126 mg/dl, HbA1c ≥6.5%, or treatment of previously diagnosed diabetes with oral agents or insulin. High triglycerides were defined as triglycerides ≥150 mg/dl or specific treatment for this lipid abnormality (29). High LDL was defined as LDL ≥130 mg/dl (29). Low HDL was defined as HDL <40 mg/dl in males and <50 mg/dl in females (29).
The Framingham Heart Score was calculated as a composite measure of CVD risk (30), and has been used previously in CARRS (31). To improve compatibility with the original regression models (30), diabetes was defined as fasting blood glucose ≥126 mg/dl or treatment of previously diagnosed diabetes with oral agents or insulin when calculating this Score. Similarly, current tobacco use was restricted to current smoking. Participants with a Framingham Heat Score >10% were considered to have a high CVD risk score.
Statistical Analysis
Analyses were conducted using SUDAAN (RTI International, Research Triangle Park, NC) to account for complex survey design. Values given are weighted percent (un-weighted frequency) unless otherwise indicated. Differences in socio-demographic characteristics and dietary intake between vegetarians and non-vegetarians within each sample were evaluated using F-statistics and Wald chi-square statistics with Satterthwaite correction (32). Sensitivity analyses were also conducted specifying the food groups continuously and testing for differences in means between vegetarians and non-vegetarians within each sample.
Multivariable logistic regression was used to estimate the association between adhering to vegetarian dietary patterns and exhibiting overweight/obesity, central obesity, diabetes, hypertension, high triglycerides, high LDL, low HDL, and high CVD risk score adjusted for confounders. Separate models were run for each outcome and for the CARRS and NHANES samples. Potential confounders were evaluated by assessing their association with the exposure (vegetarian diet) and outcomes (cardiometabolic disease risk factors). Variables associated with both the exposure and outcome and not thought to be on the causal pathway were included in the final adjustment set (33). These included age, sex, education, tobacco use (smoking and smokeless tobacco in CARRS; smoking in NHANES), alcohol consumption, and also city in CARRS. As a sensitivity analysis, the relationships using continuous measures for outcomes (BMI, waist-to-height ratio, fasting blood glucose, HbA1c, SBP, DBP, triglycerides, LDL, HDL, and Framingham Heart Score) were modeled using multivariable linear regression. To improve interpretability of the results, we also calculated predicted margins (e.g. adjusted prevalence or adjusted mean levels of the outcomes among vegetarians and non-vegetarians).
RESULTS
One-third (33.0%; n=4968) of adults in the South Asian sample were vegetarian. In contrast, only 2.4% (n=59) of adults in the US sample were vegetarian, representing an approximate 14-fold difference in the prevalence of vegetarianism in South Asians versus Americans.
Among South Asian vegetarians, the most common form of vegetarianism was semi-vegetarianism (eat meat, poultry, and fish <1 time/week): 40.0% of vegetarians followed this particular pattern (Table 1). Of the remaining patterns, 4.0% of vegetarians were pesco-vegetarians (restrict meat and poultry), 10.6% were lacto-ovo vegetarians (restrict meat, poultry, and fish), 36.6% were lactovegetarians (restrict meat, poultry, fish, and eggs), and 8.8% were vegan (restrict all animal products). The most common vegetarian dietary pattern in the US sample was also semi-vegetarian: 48.6% of vegetarians followed this particular pattern. Of the remaining patterns, 24.6% of vegetarians were pesco-vegetarians, 5.0% were lacto-ovo vegetarians, 18.0% were lacto-vegetarians, and 3.8% were vegan.
TABLE 1.
Comparison of the socio-demographic characteristics of vegetarian and non-vegetarian adults (20–69 years) participating in CARRS (South Asia; n=15,665) and NHANES (US; n=2159) †
| South Asia |
US |
|||
|---|---|---|---|---|
| Non- vegetarian (n=10,697) |
Vegetarian ‡ (n=4968) |
Non- vegetarian (n=2100) |
Vegetarian ‡ (n=59) |
|
| Age | ||||
| 20–29 years | 22.5 (2244) | 14.7 (658) * | 16.5 (349) | 21.2 (11) |
| 30–39 years | 31.3 (3197) | 27.7 (1187) * | 20.5 (404) | 21.3 (12) |
| 40–49 years | 27.0 (2887) | 29.9 (1451) * | 25.8 (473) | 22.1 (14) |
| 50–59 years | 13.0 (1574) | 19.2 (1006) * | 21.3 (379) | 22.6 (9) |
| 60–69 years | 6.2 (795) | 8.5 (666) * | 15.8 (495) | 12.8 (13) |
| Sex | ||||
| Male | 48.5 (5179) | 43.5 (2193) * | 47.9 (1035) | 31.7 (22) * |
| Female | 51.5 (5518) | 56.5 (2775) * | 52.1 (1065) | 68.3 (37) * |
| City | ||||
| Chennai | 52.6 (5718) | 17.0 (990) * | - | - |
| Delhi | 19.5 (1702) | 72.9 (3411) * | - | - |
| Karachi | 27.9 (3277) | 10.1 (567) * | - | - |
| Race/ethnicity | ||||
| Non-Hispanic white | - | - | 73.2 (1076) | 53.6 (20) * |
| Other | - | - | 26.8 (1024) | 46.4 (39) * |
| Country of birth | ||||
| US | - | - | 86.8 (1706) | 63.0 (33) * |
| Other | - | - | 13.2 (394) | 37.0 (26) * |
| Education | ||||
| < High school | 21.1 (2365) | 18.8 (1003) * | 14.5 (476) | 21.9 (21) * |
| High school to some college | 64.3 (6843) | 55.3 (2760) * | 58.7 (1182) | 33.4 (21) * |
| College degree | 14.5 (1488) | 25.9 (1205) * | 26.8 (442) | 44.8 (17) * |
| Tobacco use§ | ||||
| Never | 73.4 (7830) | 79.3 (3924) * | 49.3 (1059) | 67.8 (37) * |
| Used to but not current | 1.7 (189) | 1.4 (88) * | 24.5 (510) | 18.9 (13) * |
| Current | 25.0 (2677) | 19.3 (956) * | 26.2 (531) | 13.4 (9) * |
| Alcohol use | ||||
| Never | 82.1 (8790) | 87.6 (4355) * | 10.8 (261) | 12.4 (8) |
| Moderate ║ | 9.8 (1026) | 9.4 (447) * | 62.7 (1246) | 71.9 (38) |
| Heavy ¶ | 8.1 (881) | 3.0 (166) * | 26.5 (518) | 15.6 (12) |
| Sedentary time†† | ||||
| <5 hours/day | 37.9 (4280) | 50.2 (2449) * | 80.1 (1648) | 89.2 (47) * |
| ≥5 hours/day | 62.1 (6417) | 49.8 (2519) * | 19.9 (452) | 10.8 (12) * |
| Vegetarian dietary pattern | ||||
| Semi-vegetarian | - | 40.0 (2017) | - | 48.6 (33) |
| Pesco-vegetarian | - | 4.0 (193) | - | 24.6 (11) |
| Lacto-ovo vegetarian | - | 10.6 (507) | - | 5.0 (2) |
| Lacto-vegetarian | - | 36.6 (1802) | - | 18.0 (8) |
| Vegan | - | 8.8 (449) | - | 3.8 (5) |
Values presented are weighted percent (un-weighted frequency).
Participants adhering to any one of the following five vegetarian dietary patterns: vegan (restrict meat, poultry, fish, eggs, and dairy); lacto-vegetarian (restrict meat, poultry, fish, and eggs); lacto-ovo vegetarian (restrict meat, poultry, and fish); pesco-vegetarian (restrict meat and poultry); or semi-vegetarian (eat meat, poultry, and fish ≥1 time/month but <1 time/week).
In NHANES, includes only smoking. In CARRS, includes both smoking and smokeless tobacco.
In CARRS, defined as self-reported “occasional” alcohol use. In NHANES, defined as ≤2 drinks per day on days when participant drinks alcohol.
In CARRS, defined as self-reported “regular” alcohol use. In NHANES, defined as >2 drinks per day on days when participant drinks alcohol.
In CARRS, defined as all time spent sitting or lying down (not including sleeping). In NHANES defined as screen time (computer + TV).
P-value <0.05 from Wald chi-square statistics with Satterthwaite correction comparing non-vegetarians and vegetarians within samples.
Compared with non-vegetarians, vegetarians in the South Asian sample were significantly older (p<0.0001), more likely to be female (p=0.0001), from Delhi (p<0.0001), have a college degree (p=0.0007), and less likely to use tobacco (p=0.001) or alcohol (p<0.0001) and to be sedentary for ≥5 hours per day (p<0.0001) (Table 1). South Asian vegetarians were also significantly more likely to be Hindu, Buddhist, Sikh, or Jain versus Muslim or Christian (p<0.0001, data not shown). Similarly, compared with non-vegetarians, US vegetarians were significantly more likely to be female (p=0.04) and have a college degree (p=0.04), and less likely to use tobacco (p=0.04) and to be sedentary for ≥5 hours per day (p=0.02). US vegetarians were significantly more likely to report a race/ethnicity other than “non-Hispanic white” (p=0.02) and being born outside the US (p=0.007).
Food group intake differences between vegetarians and non-vegetarians in South Asia and the US
Among South Asians, compared with non-vegetarians, vegetarians were significantly less likely to eat eggs (p<0.0001), refined cereals (p<0.0001) and coffee (p<0.0001), and more likely to eat dairy (p=0.0001), legumes (p<0.0001), vegetables (p<0.0001), fruit (p=0.002), desserts (p<0.0001), fried foods (p=0.0001), and fruit juice (p=0.0001) (Table 2). The biggest difference was in legumes: 33.9% versus 10.6% of vegetarians and non-vegetarians, respectively, reported eating legumes daily. Within vegetables, the biggest difference was seen for other (i.e. non-leafy green) raw vegetables: 30.5% of vegetarians reported eating them daily versus only 11.5% of non-vegetarians.
TABLE 2.
Comparison of food group intakes of vegetarian and non-vegetarian adults (20–69 years) participating in CARRS (South Asia; n=15,665) and NHANES (US; n=2159) †
| South Asia |
US |
|||
|---|---|---|---|---|
| Non-vegetarian (n=10,697) |
Vegetarian ‡ (n=4968) |
Non-vegetarian (n=2100) |
Vegetarian ‡ (n=59) |
|
| Meat | ||||
| Never or <1/mo | 18.2 (1962) | 71.6 (3522) * | 0.3 (9) | 55.0 (27) * |
| ≥1/mo but <1/wk | 24.7 (2683) | 28.4 (1446) * | 2.8 (58) | 45.0 (32) * |
| ≥1/wk but <1/dy | 55.3 (5868) | - | 64.0 (1312) | - |
| ≥1/dy | 1.8 (184) | - | 32.9 (721) | - |
| Poultry | ||||
| Never or <1/mo | 4.2 (499) | 62.4 (3079) * | 2.8 (64) | 70.8 (40) * |
| ≥1/mo but <1/wk | 11.3 (1203) | 37.6 (1889) * | 19.5 (404) | 29.2 (19) * |
| ≥1/wk but <1/dy | 82.8 (8817) | - | 65.3 (1346) | - |
| ≥1/dy | 1.7 (178) | - | 12.3 (286) | - |
| Fish & shellfish | ||||
| Never or <1/mo | 21.7 (2283) | 76.8 (3781) * | 15.8 (349) | 50.4 (32) * |
| ≥1/mo but <1/wk | 23.2 (2493) | 20.4 (1051) * | 43.1 (905) | 30.7 (19) * |
| ≥1/wk but <1/dy | 53.3 (5723) | 2.6 (128) * | 39.1 (797) | 17.8 (7) * |
| ≥1/dy | 1.8 (198) | 0.2 (8) * | 1.9 (49) | 1.1 (1) * |
| Eggs | ||||
| Never or <1/mo | 10.0 (1095) | 51.4 (2563) * | 22.9 (513) | 63.0 (39) * |
| ≥1/mo but <1/wk | 11.9 (1311) | 16.4 (824) * | 36.4 (709) | 12.9 (9) * |
| ≥1/wk but <1/dy | 64.2 (6910) | 29.1 (1433) * | 37.2 (807) | 24.1 (11) * |
| ≥1/dy | 13.8 (1381) | 3.1 (148) * | 3.5 (71) | 0 (0) * |
| Milk & milk products | ||||
| Never or <1/mo | 31.0 (3391) | 26.0 (1326) * | 0.8 (23) | 7.2 (7) |
| ≥1/mo but <1/wk | 10.0 (1092) | 9.7 (471) * | 2.9 (78) | 4.8 (5) |
| ≥1/wk but <1/dy | 27.2 (2901) | 22.6 (1122) * | 25.7 (578) | 27.3 (16) |
| ≥1/dy | 31.7 (3313) | 41.8 (2049) * | 70.6 (1421) | 60.7 (31) |
| Nuts | ||||
| Never or <1/mo | 62.3 (6645) | 57.6 (2872) * | 24.5 (572) | 34.4 (27) |
| ≥1/mo but <1/wk | 17.6 (1914) | 18.7 (931) * | 26.6 (562) | 20.2 (12) |
| ≥1/wk but <1/dy | 17.0 (1827) | 16.9 (828) * | 42.3 (836) | 32.0 (14) |
| ≥1/dy | 3.1 (311) | 6.8 (337) * | 6.6 (130) | 13.4 (6) |
| Legumes | ||||
| Never or <1/mo | 10.2 (1125) | 4.7 (254) * | 34.7 (687) | 28.3 (17) * |
| ≥1/mo but <1/wk | 5.9 (699) | 4.8 (245) * | 36.2 (700) | 17.4 (9) * |
| ≥1/wk but <1/dy | 73.3 (7824) | 56.6 (2832) * | 24.7 (569) | 26.3 (18) * |
| ≥1/dy | 10.6 (1049) | 33.9 (1637) * | 4.3 (144) | 28.1 (15) * |
| Fruit | ||||
| Never or <1/mo | 14.0 (1481) | 9.7 (561) * | 2.4 (44) | 3.4 (4) * |
| ≥1/mo but <1/wk | 18.3 (1980) | 15.7 (822) * | 10.4 (206) | 1.5 (1) * |
| ≥1/wk but <1/dy | 50.3 (5495) | 51.0 (2486) * | 46.4 (902) | 40.7 (22) * |
| ≥1/dy | 17.3 (1741) | 23.5 (1099) * | 40.9 (948) | 54.4 (32) * |
| Total vegetables | ||||
| Never or <1/mo | 0.5 (56) | 0.8 (45) * | 0.2 (4) | 1.5 (2) |
| ≥1/mo but <1/wk | 0.8 (94) | 0.5 (28) * | 0.3 (7) | 0.7 (1) |
| ≥1/wk but <1/dy | 35.9 (3979) | 25.1 (1316) * | 12.9 (287) | 14.1 (13) |
| ≥1/dy | 62.8 (6568) | 73.6 (3579) * | 86.7 (1802) | 83.7 (43) |
| Leafy greens | ||||
| Never or <1/mo | 9.3 (981) | 4.9 (271) * | 11.9 (292) | 22.2 (18) |
| ≥1/mo but <1/wk | 15.3 (1676) | 11.4 (578) * | 20.0 (439) | 8.4 (9) |
| ≥1/wk but <1/dy | 70.4 (7511) | 71.2 (3512) * | 60.0 (1186) | 58.6 (27) |
| ≥1/dy | 5.0 (529) | 12.5 (607) * | 8.2 (183) | 10.9 (5) |
| Other raw vegetables | ||||
| Never or <1/mo | 43.4 (4831) | 19.5 (1078) * | 9.4 (225) | 11.3 (14) |
| ≥1/mo but <1/wk | 7.6 (858.0) | 8.0 (422.0) * | 16.8 (372) | 11.2 (8) |
| ≥1/wk but <1/dy | 37.5 (3849) | 42.1 (2054) * | 58.0 (1152) | 58.5 (29) |
| ≥1/dy | 11.5 (1159) | 30.5 (1414) * | 15.8 (351) | 19.1 (8) |
| Cooked vegetables | ||||
| Never or <1/mo | 6.5 (698) | 7.7 (386) * | 0.4 (9) | 2.1 (3) |
| ≥1/mo but <1/wk | 4.2 (457) | 7.4 (396) * | 1.2 (35) | 3.2 (3) |
| ≥1/wk but <1/dy | 41.7 (4569) | 32.4 (1637) * | 26.2 (530) | 34.6 (21) |
| ≥1/dy | 47.6 (4973) | 52.4 (2549) * | 72.2 (1526) | 60.1 (32) |
| Potatoes | ||||
| Never or <1/mo | - | - | 10.5 (271) | 22.0 (21) |
| ≥1/mo but <1/wk | - | - | 36.0 (773) | 41.9 (22) |
| ≥1/wk but <1/dy | - | - | 51.3 (1003) | 32.6 (14) |
| ≥1/dy | - | - | 2.2 (53) | 3.6 (2) |
| Whole grains | ||||
| Never or <1/mo | 13.6 (1346) | 11.9 (605) * | 8.1 (177) | 12.4 (13) |
| ≥1/mo but <1/wk | 10.4 (1039) | 10.4 (566) * | 11.8 (277) | 16.1 (13) |
| ≥1/wk but <1/dy | 29.1 (3197) | 20.3 (1047) * | 55.1 (1119) | 29.0 (16) |
| ≥1/dy | 46.8 (5115) | 57.4 (2750) * | 25.1 (527) | 42.4 (17) |
| Tea | ||||
| Never or <1/mo | 12.1 (1166) | 8.2 (438) * | 28.8 (619) | 30.6 (24) |
| ≥1/mo but <1/wk | 0.3 (32) | 0.3 (12) * | 23.2 (502) | 23.8 (14) |
| ≥1/wk but <1/dy | 1.6 (162) | 1.6 (76) * | 27.1 (552) | 19.6 (9) |
| ≥1/dy | 86.0 (9337) | 89.9 (4442) * | 20.9 (427) | 26.1 (12) |
| Coffee | ||||
| Never or <1/mo | 66.8 (7238) | 75.0 (3738) * | 33.0 (702) | 42.7 (32) |
| ≥1/mo but <1/wk | 3.5 (349) | 6.8 (310) * | 8.0 (190) | 16.5 (7) |
| ≥1/wk but <1/dy | 4.7 (506) | 7.2 (337) * | 12.2 (270) | 6.9 (4) |
| ≥1/dy | 25.0 (2604) | 11.0 (583) * | 46.7 (938) | 33.9 (16) |
| Pickles | ||||
| Never or <1/mo | 44.5 (4671) | 38.1 (2004) * | 56.9 (1266) | 76.1 (46) * |
| ≥1/mo but <1/wk | 16.1 (1793) | 18.6 (900) * | 26.7 (524) | 13.4 (7) * |
| ≥1/wk but <1/dy | 30.3 (3297) | 32.8 (1581) * | 15.4 (290) | 10.5 (6) * |
| ≥1/dy | 9.1 (936) | 10.6 (483) * | 1.0 (20) | 0 (0) * |
| Refined cereals | ||||
| Never or <1/mo | 3.4 (366) | 6.3 (328) * | 0.1 (3) | 3.4 (2) * |
| ≥1/mo but <1/wk | 3.6 (383) | 7.4 (379) * | 0.7 (19) | 11.2 (5) * |
| ≥1/wk but <1/dy | 27.7 (3027) | 43.2 (2090) * | 33.7 (677) | 48.8 (28) * |
| ≥1/dy | 65.3 (6921) | 43.0 (2171) * | 65.5 (1401) | 36.6 (24) * |
| Desserts | ||||
| Never or <1/mo | 26.1 (2960) | 17.1 (903) * | 1.4 (35) | 7.0 (8) * |
| ≥1/mo but <1/wk | 26.0 (2759) | 21.3 (1105) * | 9.4 (232) | 34.5 (19) * |
| ≥1/wk but <1/dy | 36.9 (3782) | 45.2 (2192) * | 55.3 (1106) | 35.5 (19) * |
| ≥1/dy | 11.0 (1196) | 16.4 (768) * | 33.9 (727) | 22.9 (13) * |
| Fried foods | ||||
| Never or <1/mo | 39.5 (4544) | 28.4 (1544) * | 6.5 (166) | 36.3 (23) * |
| ≥1/mo but <1/wk | 26.4 (2819) | 30.5 (1549) * | 25.1 (516) | 31.9 (20) * |
| ≥1/wk but <1/dy | 29.2 (2884) | 35.4 (1664) * | 59.0 (1204) | 30.4 (15) * |
| ≥1/dy | 5.0 (450) | 5.6 (211) * | 9.3 (214) | 1.4 (1) * |
| Fruit juice | ||||
| Never or <1/mo | 53.2 (5759) | 53.9 (2706) * | 7.4 (136) | 5.1 (6) * |
| ≥1/mo but <1/wk | 23.4 (2545) | 20.2 (1005) * | 20.8 (366) | 11.6 (6) * |
| ≥1/wk but <1/dy | 20.3 (2087) | 19.5 (947) * | 47.2 (989) | 68.8 (36) * |
| ≥1/dy | 3.2 (306) | 6.4 (310) * | 24.7 (609) | 14.5 (11) * |
| Cold beverages | ||||
| Never or <1/mo | 36.2 (4014) | 38.8 (2015) * | 6.1 (138) | 10.2 (7) * |
| ≥1/mo but <1/wk | 24.9 (2738) | 22.2 (1134) * | 12.1 (245) | 41.7 (20) * |
| ≥1/wk but <1/dy | 33.5 (3416) | 35.3 (1655) * | 36.6 (782) | 38.3 (22) * |
| ≥1/dy | 5.5 (529) | 3.7 (164) * | 45.1 (935) | 9.8 (10) * |
Values presented are weighted percent (un-weighted frequency).
Participants adhering to any one of the following five vegetarian dietary patterns: vegan (restrict meat, poultry, fish, eggs, and dairy); lacto-vegetarian (restrict meat, poultry, fish, and eggs); lacto-ovo vegetarian (restrict meat, poultry, and fish); pesco-vegetarian (restrict meat and poultry); or semi-vegetarian (eat meat, poultry, and fish ≥1 time/month but <1 time/week).
P-value <0.01 from Wald chi-square statistics with Satterthwaite correction comparing non-vegetarians and vegetarians within samples.
There were several notable differences in food group intake across vegetarian dietary patterns within the South Asian sample (Supplemental Figure 1). While lacto- and lacto-ovo vegetarians tended to have the healthiest diets – highest intakes of nuts, legumes, fruit, leafy green vegetables and other raw vegetables, and among the lowest intakes of refined cereals – they also had unhealthy aspects, such as the highest intakes of desserts, fried foods, and fruit juice.
Among Americans, compared with non-vegetarians, vegetarians were significantly less likely to eat eggs (p=0.0001), refined cereals (p=0.01), desserts (p=0.01), fried foods (p=0.0001), fruit juice (p=0.03), and cold beverages (including diet and non-diet soft drinks) (p=0.0001), and more likely to eat legumes (p=0.02), whole grains (p=0.05), and fruit (p=0.002) (Table 2). The biggest differences were in refined cereals and cold beverages: 36.6% of vegetarians reported eating refined cereals daily compared to 65.5% of non-vegetarians; and only 9.8% of vegetarians reported drinking cold beverages daily compared to 45.1% of non-vegetarians. Within vegetables, vegetarians were marginally less likely (p=0.09) to eat potatoes weekly, and marginally more likely (p=0.07) to eat leafy green vegetables daily.
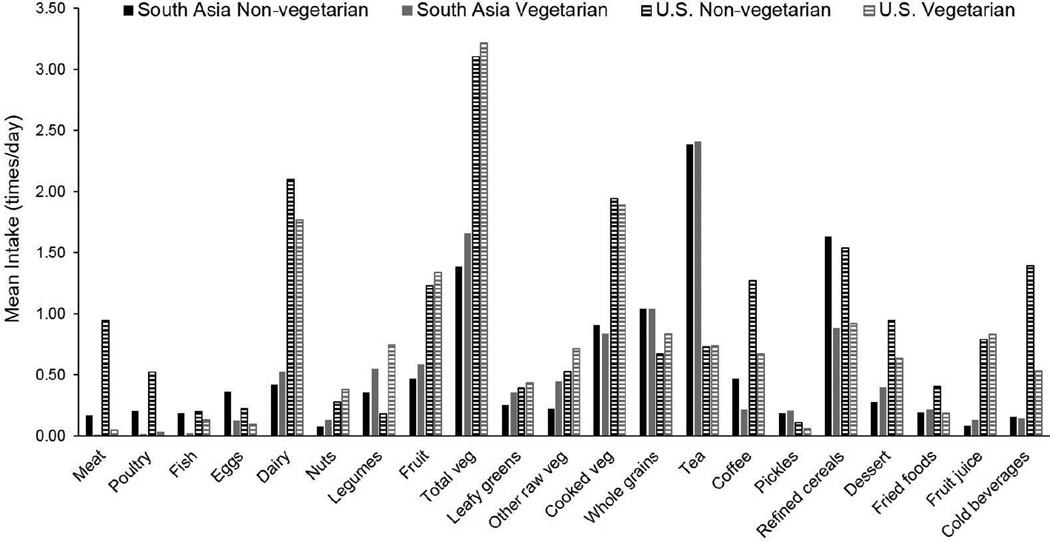
Vegetarian diets tended to be more divergent from non-vegetarian diets in the US compared to South Asia (Figure 1). This was especially true for meat, poultry, legumes, whole grains, and cold beverages. Nonetheless, the direction of differences between vegetarians and non-vegetarians was similar between the South Asian and US samples with three notable exceptions: South Asian vegetarians had higher dairy, dessert, and fried food intakes compared to South Asian non-vegetarians whereas US vegetarians had lower dairy, dessert, and fried food intakes compared to US non-vegetarians.
FIGURE 1.
Mean intake of food groups (times/day) according to vegetarian status among adults (20–69 years) participating in CARRS (South Asia; n=15,665) and NHANES (US; n=2159).
Association between vegetarian dietary patterns and cardiometabolic disease risk factors in South Asia and the US
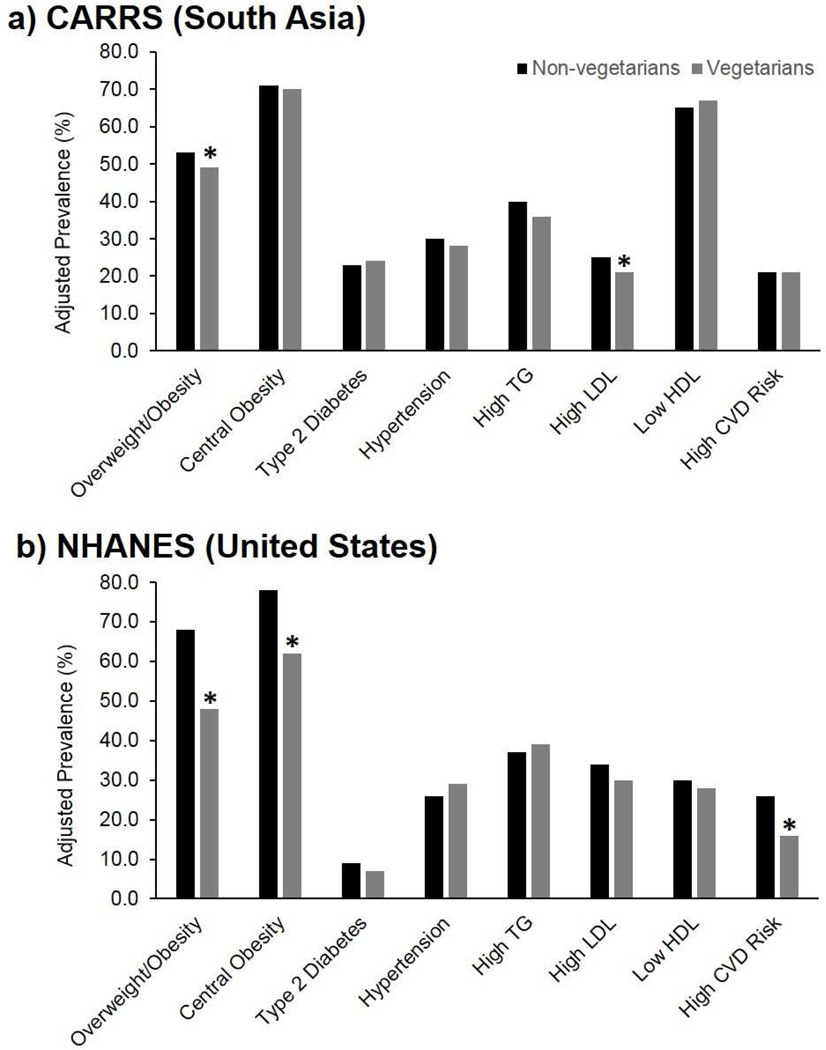
After adjustment for socio-demographic and behavioral confounders, both South Asian and US vegetarians were significantly less likely to exhibit overweight/obesity compared to non-vegetarians: adjusted OR (95% CI), 0.83 (0.72, 0.96) and 0.41 (0.21, 0.81), respectively (Table 3). In terms of predicted marginal probabilities, 49% (95% CI: 45%, 53%) of vegetarians versus 53% (51%, 56%) of non-vegetarians were overweight/obese in the South Asian sample, and 48% (32%, 63%) of vegetarians versus 68% (65%, 70%) of non-vegetarians were overweight/obese in the US sample (Figure 2). US vegetarians were also significantly less likely to have central obesity (0.41 [0.17, 0.99]) and less likely to have a high CVD risk score (0.24 [0.09, 0.64]) compared to non-vegetarians. These associations were not significant in the South Asian sample, but South Asian vegetarians were significantly less likely to have high LDL (0.80 [0.71, 0.90]).
TABLE 3.
Association of a vegetarian dietary pattern with overweight/obesity, central obesity, diabetes, hypertension, high triglycerides, high high-density lipoprotein cholesterol (LDL), low high-density lipoprotein cholesterol (HDL), and high cardiovascular disease (CVD) risk score among adults (20–69 years) participating in CARRS (South Asia; n=15,665) and NHANES (US; n=2159) †
| South Asia | US | |||
|---|---|---|---|---|
| Unadjusted | Adjusted ‡ | Unadjusted | Adjusted ‡ | |
| Overweight/obesity § | 0.98 (0.79, 1.20) |
0.83 (0.72, 0.96) |
0.38 (0.19, 0.77) |
0.41 (0.21, 0.81) |
| Central obesity ║ |
1.22 (1.03, 1.45) |
0.95 (0.81, 1.11) |
0.40 (0.18, 0.90) |
0.41 (0.17, 0.99) |
| Diabetes ¶ |
1.57 (1.28, 1.92) |
1.04 (0.86, 1.27) |
0.64 (0.24, 1.68) |
0.75 (0.29, 1.96) |
| Hypertension †† |
1.33 (1.14, 1.55) |
0.93 (0.81, 1.07) |
0.99 (0.50, 1.97) |
1.19 (0.58, 2.44) |
| High triglycerides ‡‡ | 1.04 (0.89, 1.22) |
0.85 (0.71, 1.02) |
0.87 (0.43, 1.77) |
1.09 (0.55, 2.17) |
| High LDL §§ | 0.88 (0.77, 1.00) |
0.80 (0.71, 0.90) |
0.85 (0.42, 1.73) |
0.85 (0.42, 1.70) |
| Low HDL ║║ |
0.78 (0.70, 0.88) |
1.10 (0.98, 1.24) |
0.85 (0.37, 1.96) |
0.90 (0.39, 2.08) |
| High CVD risk score ¶¶ |
1.38 (1.17, 1.63) |
0.92 (0.76, 1.11) |
0.32 (0.15, 0.69) |
0.24 (0.09, 0.64) |
Values presented are Odds Ratios (95% Confidence Intervals) from multivariable logistic regression.
Adjusted for age, sex, education, tobacco, alcohol, and also city in CARRS.
Body mass index ≥25 kg/m2.
Waist-to-height ratio >0.5.
Fasting blood glucose ≥126 mg/dl, glycated hemoglobin A1c ≥6.5%, or medication.
Systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or medication.
Triglycerides ≥150 mg/dl or medication.
LDL ≥130 mg/dl.
HDL ≤40 mg/dl in males and ≤50 mg/dl in females.
Framingham Heart Score >10%.
FIGURE 2.
Predicted marginal probabilities (e.g. prevalence adjusted for age, sex, education, alcohol, tobacco, and also city in CARRS) of cardiometabolic disease risk factors among adults (20–69 years) participating in a) CARRS (South Asia; n=15,665) and b) and NHANES (US; n=2159) according to vegetarian status. Overweight/obesity defined as body mass index ≥25 kg/m2. Central obesity defined as waist-to-height ratio >0.5. Diabetes defined as fasting blood glucose ≥126 mg/dl, glycated hemoglobin A1c ≥6.5%, or medication. Hypertension defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or medication. High triglycerides defined as triglycerides ≥150 mg/dl or medication. High LDL defined as LDL ≥130 mg/dl. Low HDL defined as HDL ≤40 mg/dl in males and ≤50 mg/dl in females. High CVD risk score defined as Framingham Heart Score >10%. Abbreviations: CVD, cardiovascular disease; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TG, triglycerides. * P-value <0.05 comparing vegetarians and non-vegetarians.
In sensitivity models using a BMI cut-point of 23 kg/m2 in the South Asian sample, the association with overweight/obesity was attenuated: adjusted OR (95% CI), 0.88 (0.76, 1.02). When the outcomes were modeled continuously, both South Asian and US vegetarians had a significantly lower adjusted mean BMI compared to non-vegetarians: 25.22 (95% CI: 24.80, 25.65) kg/m2 versus 25.62 (25.34, 25.89) kg/m2, respectively in the South Asian sample, and 26.22 (24.49, 27.95) kg/m2 versus 28.90 (28.46, 29.35) kg/m2, respectively in the US sample. Vegetarians in both samples also had significantly lower adjusted mean triglycerides compared to non-vegetarians: 139.30 (134.15, 144.45) mg/dl versus 148.90 (144.57, 153.23) mg/dl, respectively in the South Asian sample, and 126.96 (111.71, 142.21) mg/dl versus 142.35 (136.64, 148.06) mg/dl, respectively in the US sample. In the US sample, but not the South Asian sample, vegetarians had significantly lower adjusted mean waist-to-height ratio compared to non-vegetarians: 0.55 (0.52, 0.57) versus 0.58 (0.57, 0.59), respectively. In the South Asian sample, but not the US sample, vegetarians had significantly lower adjusted mean DBP and LDL compared to non-vegetarians: 80.66 (79.98, 81.34) mmHg versus 81.75 (81.18, 82.31) mmHg, respectively, and 106.3 (104.8, 107.8) mg/dl versus 110.2 (108.8, 111.5) mg/dl, respectively.
In sensitivity models adjusted for dairy, desserts, and fried food intake in the South Asian sample – the three food groups that differed in direction between samples – results were consistent (data not shown). After adjustment for sedentary time, the OR (95% CI) for central obesity in the US sample was slightly attenuated: 0.43 (0.18, 1.02). All other results were consistent with further adjustment for sedentary time and, in the US sample, for race/ethnicity (data not shown).
When vegetarians in the South Asian sample were further divided, a semi-vegetarian dietary pattern was found to be associated with significantly lower odds of overweight/obesity (adjusted OR [95% CI], 0.78 [0.68, 0.91]), lower odds of high triglycerides (0.82 [0.69, 0.97]), and lower odds of high LDL (0.82 [0.69, 0.98]) compared to a non-vegetarian dietary pattern. In terms of predicted marginal probabilities, 48% (43%, 52%) of semi-vegetarians versus 53% (51%, 56%) of non-vegetarians were overweight/obese, 35% (32%, 39%) of semi-vegetarians versus 40% (37%, 42%) of non-vegetarians had high triglycerides, and 22% (20%, 25%) of semi-vegetarians versus 25% (24%, 27%) of non-vegetarians had high LDL (Supplemental Figure 2). A lacto-ovo vegetarian dietary pattern was associated with higher odds of low HDL (1.37 [1.08, 1.74]), and a lacto-vegetarian dietary pattern was associated with lower odds of high LDL (0.77 [0.65, 0.92]). None of the other vegetarian dietary patterns were significantly associated with any of the outcomes.
DISCUSSION
The prevalence of any type of vegetarianism in this nationally representative US sample was substantially lower than that of the urban South Asian sample: 2.4% versus 33.0%. This proportion, defined objectively using food propensity data from the past year, is similar to the proportion of NHANES participants self-identifying as vegetarian in the 2007–2010 NHANES surveys (34). A vegetarian diet was strongly associated with a lower probability of overweight/obesity and central obesity among US adults. In contrast, a vegetarian diet was only weakly associated with a lower probability of overweight/obesity and not significantly associated with central obesity among South Asian adults. This may reflect the greater divergence between vegetarian and non-vegetarian diets in the US, particularly with respect to meat, poultry, legumes, whole grains, and cold beverages. It may also reflect the more consistently healthier food group intakes of vegetarians in the US: for example, whereas South Asian vegetarians had higher intakes of desserts and fried foods compared to non-vegetarians, US vegetarians had lower intakes of these food groups.
The motivations underlying participants’ decisions to avoid meat and poultry differ between the US and South Asia (35). In the US, individuals typically choose to become vegetarian later in life for varying reasons including animal welfare, environmental sustainability, and personal health – and these reasons can evolve over time (36). In contrast, vegetarians in South Asia more often adhere to a vegetarian diet for traditional, familial, religious, or spiritual reasons (37). Differences in these underlying factors may partially explain the differences we observed in the association between vegetarianism and cardiometabolic health between South Asian and US adults: there may be more residual confounding by other healthful behaviors among US vegetarians compared to South Asian vegetarians as US vegetarians are more likely to choose a vegetarian lifestyle for nutritional reasons, thus may be more health conscious overall.
Non-vegetarians in the South Asian sample tended to eat less meat than non-vegetarians in the US sample (adjusted mean intake of 0.17 times per day in South Asian non-vegetarians versus 0.95 times per day in US non-vegetarians). Therefore the contrast in dietary intake of meat was smaller between vegetarians and non-vegetarians in South Asia compared to the US Given that reduced meat intake has been implicated as a key driver of the health benefits of a vegetarian diet in US and European populations given its association with obesity (38) and diabetes (39–41), this may partially explain the lower magnitude of association in the South Asian versus US sample.
Perhaps because of the higher overall prevalence of vegetarianism in South Asia, there was greater heterogeneity within “vegetarians” in the South Asian versus US sample, and we were able to explore differences in health outcomes according to different types of vegetarian dietary patterns. We did not observe a “dose-response” relationship between increasing strictness of vegetarian dietary patterns (e.g. non-vegetarian to semi-vegetarian to pesco-vegetarian and so forth) and cardiometabolic disease risk factors in this sample, suggesting that animal-product intake may not be a key driver of observed cardiometabolic health benefits of vegetarian diets in South Asia. This is consistent with ecological data demonstrating that while cardiometabolic diseases such as diabetes and CVD have increased dramatically over the past 30–40 years in India, annual per capita meat, poultry, and fish intake has only increased by about 1 kg (7).
The health benefits of vegetarian diets observed in this study may stem from higher intakes of vegetables and legumes among vegetarians compared to non-vegetarians. These food groups have been associated with a decreased risk of obesity, diabetes, hypertension, dyslipidemia, and CVD in previous studies (42, 43). The contribution of legumes (i.e. pulses) to total caloric intake in India has remained relatively constant since 1993–1994: around 5% in rural areas and 6% in urban areas (44). In contrast, the contribution of dairy products has increased by about 1% in both rural and urban areas (44). Policies and interventions encouraging the consumption of pulses, particularly for non-vegetarians, may prove to be beneficial in India.
South Asian vegetarians were also slightly more likely to consume tea at least once daily compared to non-vegetarians (90% versus 86%). Results of randomized controlled trials and prospective cohort studies have indicated that tea is protective against stroke (45, 46), reduces blood pressure (including systolic and diastolic) (47), and leads to improvements in flow-mediated dilation of the brachial artery (a marker of endothelial function) (48); effects likely due to high levels of polyphenols. In addition, South Asian vegetarians were slightly more likely to consume pickled vegetables at least once daily compared to non-vegetarians (11% versus 9%). While pickled vegetable consumption has been associated with increased risk of esophageal (49) and gastric cancers (50), largely in epidemiological studies conducted in Chinese, Japanese, and Korean populations, more research is needed to understand potential cardiometabolic effects of this food group.
There were mixed health associations for those adhering to a vegetarian dietary pattern: while semi-vegetarians had lower levels of overweight/obesity, high triglycerides, and high LDL compared to non-vegetarians, lacto-ovo vegetarians had higher levels of low HDL. This observation of mixed health associations is consistent with past studies. In a small study conducted in two outpatient clinics in New Delhi, India, vegetarians with diabetes had a smaller waist circumference compared to non-vegetarians, but the two groups did not differ with respect to any other measures (19). An analysis of the most recent Indian National Family Health Survey (2005–2006) also found substantial heterogeneity across vegetarian diets with respect to overweight/obesity: while pesco-vegetarians had a lower prevalence of overweight/obesity compared to non-vegetarians, lacto-vegetarians had a higher prevalence of overweight/obesity compared to non-vegetarians (16). In that same study, after adjustment for confounders, only lacto-, lacto-ovo, and semi-vegetarian diets (but not vegan or pesco-vegetarian diets) were found to be protective against self-reported diabetes relative to non-vegetarian diets (16).
While we used food propensity questionnaire data from NHANES to improve comparability of results to CARRS, which used a food propensity questionnaire, the different lengths of the questionnaire (139 versus 26 items in NHANES versus CARRS, respectively) may have resulted in misclassification of the exposure, especially for CARRS participants. These differences in diet assessment instruments prohibited us from directly comparing CARRS and NHANES using statistical testing. Furthermore, given that the food propensity questionnaires were qualitative (e.g. did not include portion size estimates), we were not able to calculate nutrient intakes, thus comparisons were limited to the frequency of consumption of food groups. A substantial advantage of focusing on food groups is that the results of this analysis may be more readily translatable into prevention interventions. Furthermore, past studies of vegetarian diets in NHANES have relied on a single, 24-hour dietary recall or self-reported adherence to a vegetarian diet (34, 51)– both of which would likely overestimate the prevalence of vegetarianism in the US and misclassify participants who are not vegetarian as vegetarian. By using food propensity data reflecting the past year, we were able to more objectively and accurately capture vegetarianism. Nonetheless, this definition reduced our sample size, limiting our power to detect associations with dichotomous outcomes such as diabetes and high triglycerides, in addition to limiting our ability to look at different types of vegetarian dietary patterns as we did for CARRS.
Another limitation is that due to differences in data collection over time in NHANES, food propensity data were only available in the 2003–2004 and 2005–2006 surveys. Thus, the data on US vegetarians was approximately 5 years older than that for South Asian vegetarians. In addition, the relative contribution of whole grains versus refined grains to the diets of participants in CARRS may have been overestimated due to the inclusion of roti in Delhi and all items made with maida (naan, roti, chapati, etc.) in Karachi as “whole grains.” Further adjustment for city in the comparison of whole grain and refined grain intake between vegetarians and non-vegetarians did not change the results, but this limitation is worth noting for future studies of dietary intake in South Asia. Finally, while the composite CVD risk score used in this analysis, the Framingham Heart Score, may underestimate CVD risk in South Asian populations, analyses suggest that it defines a significantly greater proportion of high-risk individuals compared to other composite CVD risk scores (for example, SCORE) in ethnically diverse samples (52, 53).
There is greater divergence between vegetarian and non-vegetarian diets in the US compared to South Asia, and US vegetarian diets have more consistently healthier food group intakes compared to South Asian vegetarian diets. Vegetarians had a lower probability and lower mean levels of several cardiometabolic disease risk factors in both South Asian and US populations. The strength of this cross-sectional association may be stronger for US vegetarian diets. Prospective studies are needed to confirm these observations, in addition to interventions testing improvements in the healthfulness of South Asian vegetarian diets including an emphasis on limiting fried foods, and the importance of nutrient-dense fruits, nuts, seeds, and whole grains.
Supplementary Material
Highlights.
This study is the first to explore vegetarian diets in both the US and South Asia
We use large representative samples & define vegetarianism with food propensity data
Vegetarians had a lower probability of overweight/obesity in both populations
The strength of this association may be stronger for US vegetarian diets
Results inform lifestyle interventions and policies for chronic disease prevention
Acknowledgments
The CARRS (Centre for cArdiometabolic Risk Reduction in South-Asia) cohort was funded by the National Heart, Lung, and Blood Institute at the National Institutes of Health (HHSN2682009900026C) and the Oxford Health Alliance Vision 2020 of the UnitedHealth Group (Minneapolis, MN, USA). Additional support was provided by the Fogarty International Center and the Eunice Kennedy Shriver National Institute of Child Health & Human Development at the National Institutes of Health (1 D43 HD065249), and the Emory Global Health Institute. None of the aforementioned funding sources had a role in the design, analysis, or writing of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None.
Authorship: All authors were involved in the conception and development of the research plan (study question and analysis); LMJ conducted the statistical analysis and wrote the paper; all other authors contributed to editing and revising the paper; LMJ has primary responsibility for final content. All authors read and approved the final manuscript.
Ethical Standards Disclosure: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Emory University Institutional Review Board, the Public Health Foundation of India Ethics Review Committee, the Aga Khan University Ethics Review Committee, and the Madras Diabetes Research Foundation Ethics Review Committee. Written informed consent was obtained from all subjects.
REFERENCES
- 1.Anjana R, Pradeepa R, Deepa M, et al. Prevalence of Diabetes and Prediabetes (Impaired Fasting Glucose and/or Impaired Glucose Tolerance) in Urban and Rural India: Phase I Results of the Indian Council of Medical Research–India Diabetes (ICMR–Indiab) Study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, et al. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Wang H, Lozano R, et al. Global, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R. Burden of Coronary Heart Disease in India. Indian Heart J. 2004;57:632–638. [PubMed] [Google Scholar]
- 5.Gaziano T, Reddy KS, Paccaud F, et al. Cardiovascular Disease. Chapter 33. In: Jamison DT, Breman JG, Measham AR, et al., editors. Source: Disease Control Priorities in Developing Countries. 2nd. Washington (DC): World Bank; 2006. 2006. [PubMed] [Google Scholar]
- 6.Leeder S, Raymond S, Greenberg H, et al. A Race against Time: The Challenge of Cardiovascular Disease in Developing Economies. 2004. New York: Trustees of Columbia University; 2012. 2012. [Google Scholar]
- 7.Singh PN, Arthur KN, Orlich MJ, et al. Global Epidemiology of Obesity, Vegetarian Dietary Patterns, and Noncommunicable Disease in Asian Indians. Am J Clin Nutr. 2014;100:359S–364S. doi: 10.3945/ajcn.113.071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Diabetes Federation. Diabetes Atlas. Seventh. Brussels: International Diabetes Federation; 2015. [Google Scholar]
- 9.International Diabetes Federation. Diabetes Atlas. Third. Brussels: International Diabetes Federation; 2006. [Google Scholar]
- 10.Yokoyama Y, Nishimura K, Barnard ND, et al. Vegetarian Diets and Blood Pressure: A Meta-Analysis. JAMA Int Med. 2014;174:577–587. doi: 10.1001/jamainternmed.2013.14547. [DOI] [PubMed] [Google Scholar]
- 11.Tonstad S, Butler T, Yan R, et al. Type of Vegetarian Diet, Body Weight, and Prevalence of Type 2 Diabetes. Diabetes Care. 2009;32:791–796. doi: 10.2337/dc08-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnard ND, Levin SM, Yokoyama Y. A Systematic Review and Meta-Analysis of Changes in Body Weight in Clinical Trials of Vegetarian Diets. J Acad Nutr Diet. 2015;115:954–969. doi: 10.1016/j.jand.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Gammon CS, von Hurst PR, Coad J, et al. Vegetarianism, Vitamin B12 Status, and Insulin Resistance in a Group of Predominantly Overweight/Obese South Asian Women. Nutrition. 2012;28:20–24. doi: 10.1016/j.nut.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Gadgil MD, Anderson CAM, Kandula NR, et al. Dietary Patterns in Asian Indians in the United States: An Analysis of the Metabolic Syndrome and Atherosclerosis in South Asians Living in America Study. J Acad Nutr Diet. 2014;114:238–243. doi: 10.1016/j.jand.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose K, Bisai S, Sadhukhan S, et al. Undernutrition among Adult Bengalees of Dearah, Hooghly District, West Bengal, India: Relationship with Educational Status and Food Habit. Anthropol Anz. 2009;67:121–128. doi: 10.1127/0003-5548/2009/0016. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal S, Millett CJ, Dhillon PK, et al. Type of Vegetarian Diet, Obesity and Diabetes in Adult Indian Population. Nutr J. 2014;13 doi: 10.1186/1475-2891-13-89. 89-2891-2813-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayanamurthy MR, Baghel RK, Siddalingappa H. Prevalence and Factors Influencing Type 2 Diabetes Mellitus in Rural Mysore. Int J Diabetes Dev Ctries. 2014:1–4. [Google Scholar]
- 18.Shridhar K, Dhillon PK, Bowen L, et al. The Association between a Vegetarian Diet and Cardiovascular Disease (CVD) Risk Factors in India: The Indian Migration Study. PLoS One. 2014;9:e110586. doi: 10.1371/journal.pone.0110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colles SL, Singh S, Kohli C, et al. Dietary Beliefs and Eating Patterns Influence Metabolic Health in Type 2 Diabetes: A Clinic-Based Study in Urban North India. Indian J Endocrinol Metab. 2013;17:1066. doi: 10.4103/2230-8210.122626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair M, Ali MK, Ajay VS, et al. CARRS Surveillance Study: Design and Methods to Assess Burdens from Multiple Perspectives. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-701. 701-2458-2412-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal R, Anand S, Ounpuu S, et al. Dietary Patterns and the Risk of Acute Myocardial Infarction in 52 Countries: Results of the INTERHEART Study. Circulation. 2008;118:1929–1937. doi: 10.1161/CIRCULATIONAHA.107.738716. [DOI] [PubMed] [Google Scholar]
- 22.Orlich MJ, Fraser GE. Vegetarian Diets in the Adventist Health Study 2: A Review of Initial Published Findings. Am J Clin Nutr. 2014;100:353S–358S. doi: 10.3945/ajcn.113.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng M, Fleming T, Robinson M, et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults During 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaacks LM, Slining MM, Popkin BM. Recent Underweight and Overweight Trends by Rural-Urban Residence among Women in Low- and Middle-Income Countries. J Nutr. 2015;145:352–357. doi: 10.3945/jn.114.203562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization and Food and Agriculture Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint Who/FAO Expert Consultation. Geneva: World Health Organization; 2003. [Google Scholar]
- 26.Nishida C, Consultation WE. Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 27.Ashwell M, Hsieh SD. Six Reasons Why the Waist-to-Height Ratio Is a Rapid and Effective Global Indicator for Health Risks of Obesity and How Its Use Could Simplify the International Public Health Message on Obesity. Int J Food Sci Nutr. 2005;56:303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 28.Flegal KM, Shepherd JA, Looker AC, et al. Comparisons of Percentage Body Fat, Body Mass Index, Waist Circumference, and Waist-Stature Ratio in Adults. Am J Clin Nutr. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chobanian A. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 30.D’Agostino RB, Vasan RS, Pencina MJ, et al. General Cardiovascular Risk Profile for Use in Primary Care the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 31.Anand S, Shivashankar R, Ali MK, et al. Prevalence of Chronic Kidney Disease in Two Major Indian Cities and Projections for Associated Cardiovascular Disease. Kidney Int. 2015;88:178–185. doi: 10.1038/ki.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao JN, Scott AJ. The Analysis of Categorical Data from Complex Sample Surveys: Chi-Squared Tests for Goodness of Fit and Independence in Two-Way Tables. J Am Stat Assoc. 1981;76:221–230. [Google Scholar]
- 33.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34.Juan W, Yamini S, Britten P. Food Intake Patterns of Self-Identified Vegetarians among the U.S. Population, 2007–2010; 38th National Nutrient Databank Conference; 2014. [Google Scholar]
- 35.Ruby MB, Heine SJ, Kamble S, et al. Compassion and Contamination. Cultural Differences in Vegetarianism. Appetite. 2013;71:340–348. doi: 10.1016/j.appet.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Ruby MB. Vegetarianism. A Blossoming Field of Study. Appetite. 2012;58:141–150. doi: 10.1016/j.appet.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Caplan P. Crossing the Veg/Non-Veg Divide: Commensality and Sociality among the Middle Classes in Madras/Chennai. South Asia: Journal of South Asian Studies. 2008;31:118–142. [Google Scholar]
- 38.Rouhani MH, Salehi-Abargouei A, Surkan PJ, et al. Is There a Relationship between Red or Processed Meat Intake and Obesity? A Systematic Review and Meta-Analysis of Observational Studies. Obes Rev. 2014;15:740–748. doi: 10.1111/obr.12172. [DOI] [PubMed] [Google Scholar]
- 39.Pan A, Sun Q, Bernstein AM, et al. Changes in Red Meat Consumption and Subsequent Risk of Type 2 Diabetes Mellitus: Three Cohorts of Us Men and Women. JAMA Int Med. 2013;173:1328–1335. doi: 10.1001/jamainternmed.2013.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnard N, Levin S, Trapp C. Meat Consumption as a Risk Factor for Type 2 Diabetes. Nutrients. 2014;6:897–910. doi: 10.3390/nu6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Keogh J, Clifton P. A Review of Potential Metabolic Etiologies of the Observed Association between Red Meat Consumption and Development of Type 2 Diabetes Mellitus. Metabolism. 2015;64:768–779. doi: 10.1016/j.metabol.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Rebello CJ, Greenway FL, Finley JW. A Review of the Nutritional Value of Legumes and Their Effects on Obesity and Its Related Co-Morbidities. Obes Rev. 2014;15:392–407. doi: 10.1111/obr.12144. [DOI] [PubMed] [Google Scholar]
- 43.Boeing H, Bechthold A, Bub A, et al. Critical Review: Vegetables and Fruit in the Prevention of Chronic Diseases. Eur J Nutr. 2012;51:637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ministry of Statistics and Programme Implementation, Government of India. New Delhi: Ministry of Statistics and Programme Implementation. New Delhi: Government of India; 2014. Nutritional Intake in India, 2011–12. [Google Scholar]
- 45.Shen L, Song LG, Ma H, et al. Tea Consumption and Risk of Stroke: A Dose-Response Meta-Analysis of Prospective Studies. Journal of Zhejiang University Science B. 2012;13:652–662. doi: 10.1631/jzus.B1201001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arab L, Liu W, Elashoff D. Green and Black Tea Consumption and Risk of Stroke: A Meta-Analysis. Stroke. 2009;40:1786–1792. doi: 10.1161/STROKEAHA.108.538470. [DOI] [PubMed] [Google Scholar]
- 47.Yarmolinsky J, Gon G, Edwards P. Effect of Tea on Blood Pressure for Secondary Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr Rev. 2015;73:236–246. doi: 10.1093/nutrit/nuv001. [DOI] [PubMed] [Google Scholar]
- 48.Ras RT, Zock PL, Draijer R. Tea Consumption Enhances Endothelial-Dependent Vasodilation; a Meta-Analysis. PLoS One. 2011;6:e16974. doi: 10.1371/journal.pone.0016974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islami F, Ren JS, Taylor PR, et al. Pickled Vegetables and the Risk of Oesophageal Cancer: A Meta-Analysis. Br J Cancer. 2009;101:1641–1647. doi: 10.1038/sj.bjc.6605372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HJ, Lim SY, Lee JS, et al. Fresh and Pickled Vegetable Consumption and Gastric Cancer in Japanese and Korean Populations: A Meta-Analysis of Observational Studies. Cancer Sci. 2010;101:508–516. doi: 10.1111/j.1349-7006.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farmer B, Larson BT, Fulgoni Iii VL, et al. A Vegetarian Dietary Pattern as a Nutrient-Dense Approach to Weight Management: An Analysis of the National Health and Nutrition Examination Survey 1999–2004. J Am Diet Assoc. 2011;111:819–827. doi: 10.1016/j.jada.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 52.Kanjilal S, Rao VS, Mukherjee M, et al. Application of Cardiovascular Disease Risk Prediction Models and the Relevance of Novel Biomarkers to Risk Stratification in Asian Indians. Vasc Health Risk Manag. 2008;4:199–211. doi: 10.2147/vhrm.2008.04.01.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhopal R, Fischbacher C, Vartiainen E, et al. Predicted and Observed Cardiovascular Disease in South Asians: Application of Finrisk, Framingham and Score Models to Newcastle Heart Project Data. J Public Health (Oxf) 2005;27:93–100. doi: 10.1093/pubmed/fdh202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.